Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane
Abstract
:1. Introduction
2. Material and Method
2.1. Materials
2.2. Preparation of Ag/MWCNTs
2.3. Preparation of MWCNTs/PVDF-HFP Composite Membranes
2.4. Filtration Studies
2.5. Permeation Tests
2.5.1. Swellability Tests
2.5.2. Water Content and Porosity Measurements
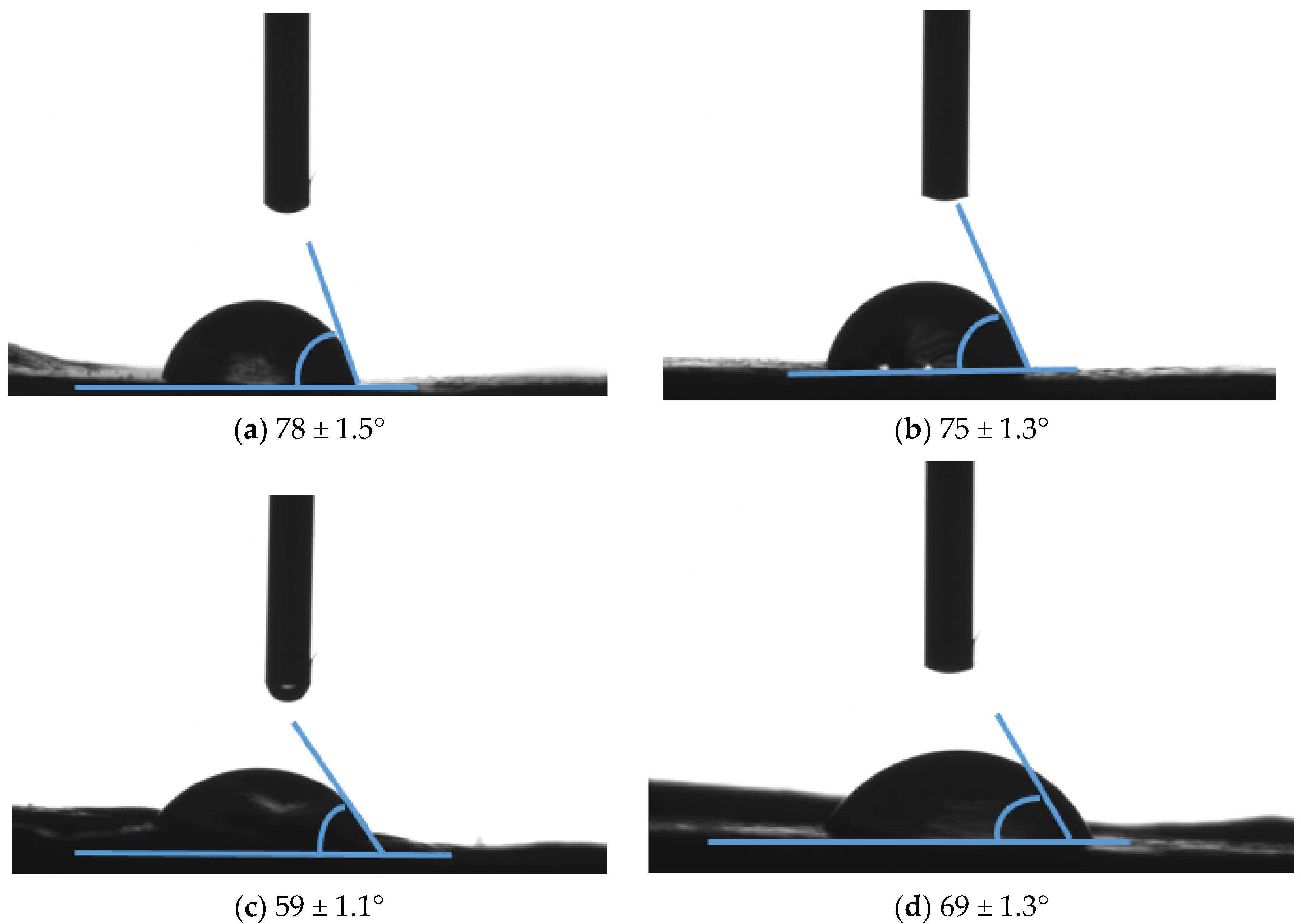
2.5.3. Contact Angle (Sessile-Drop Method)
2.6. Salt Rejection Tests
2.7. Membrane Characterisation
3. Results and Discussion
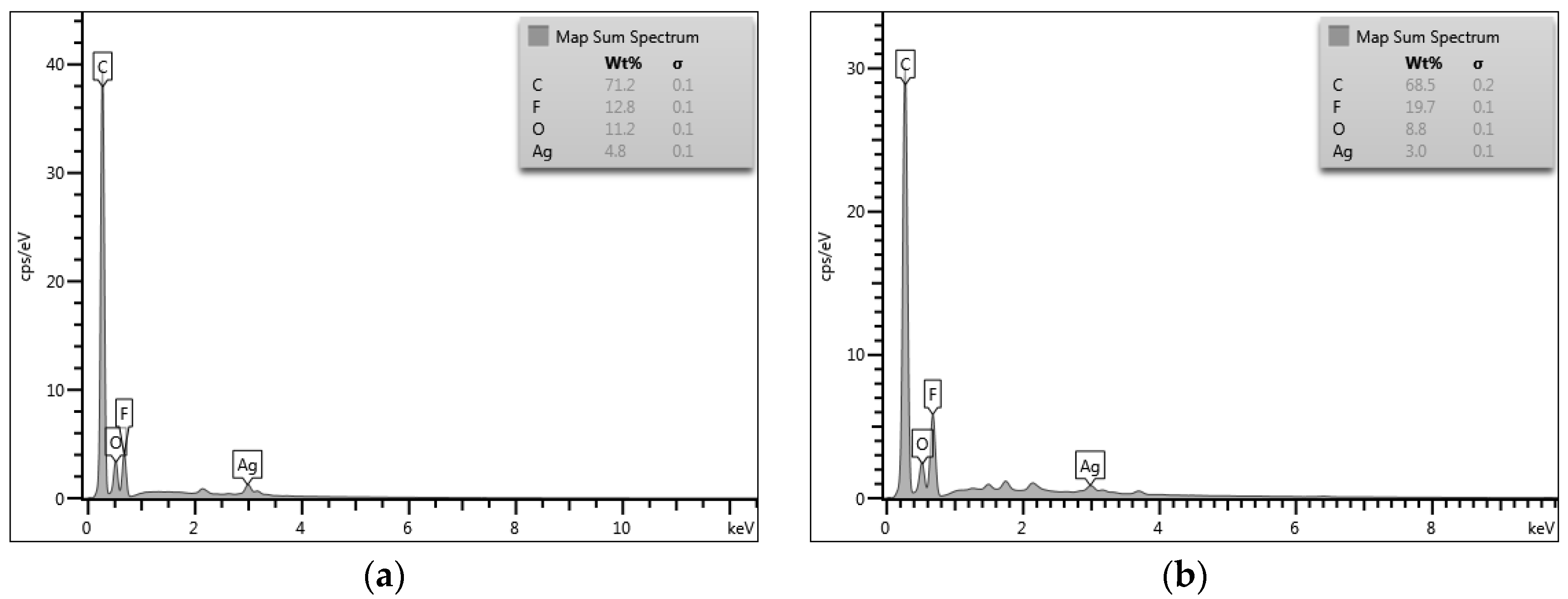
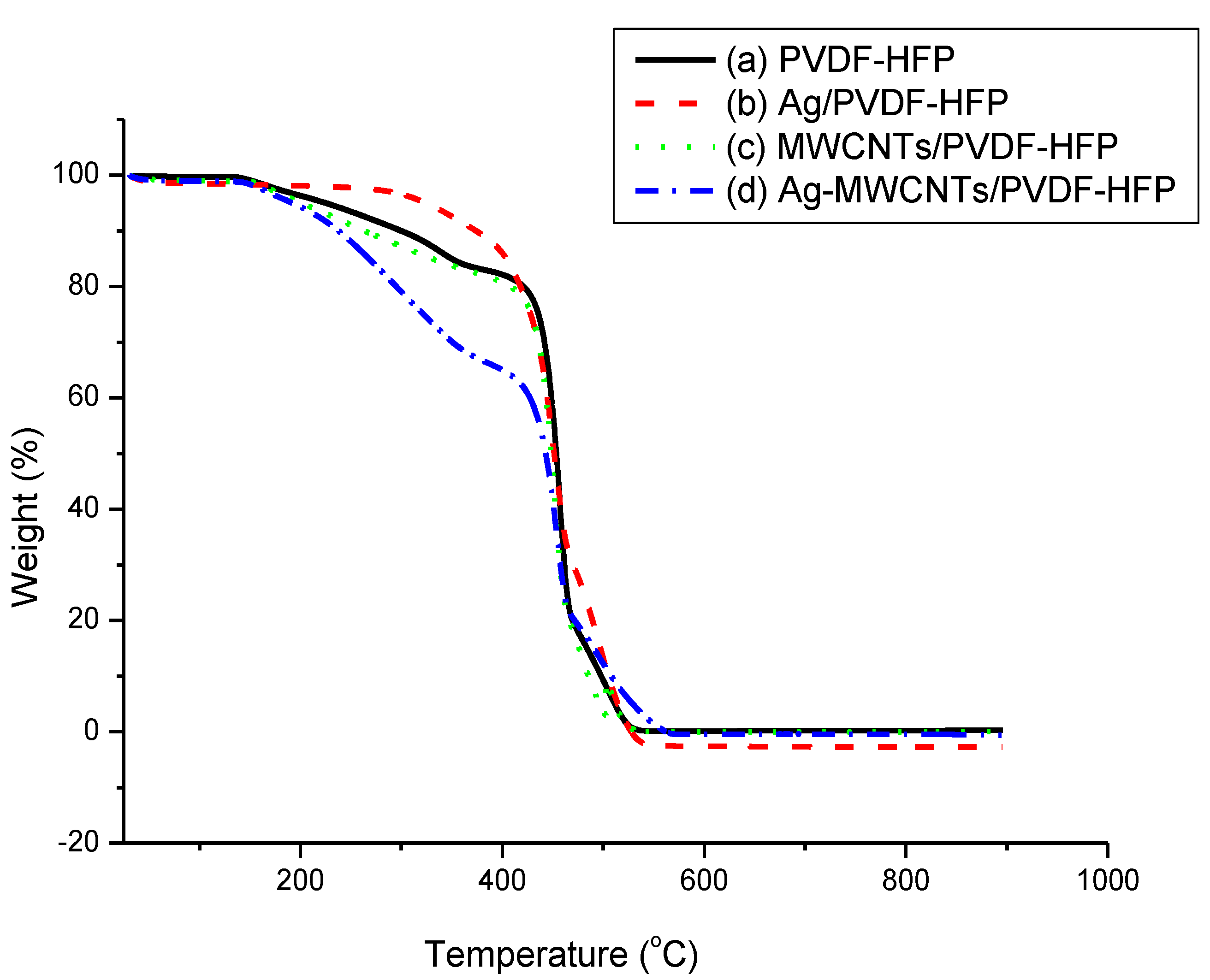
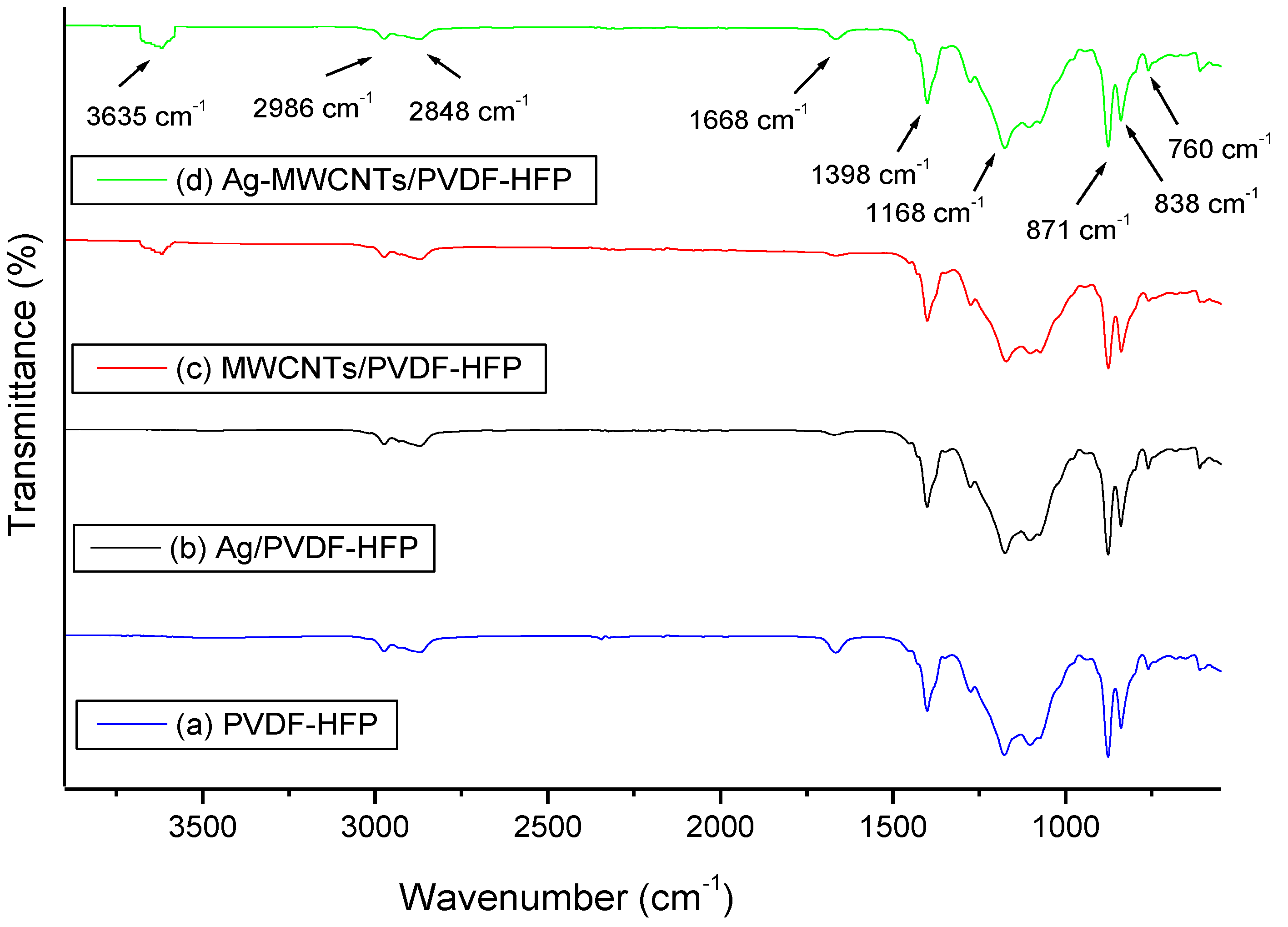
3.1. Thermal and Structural Properties of Composite Membranes
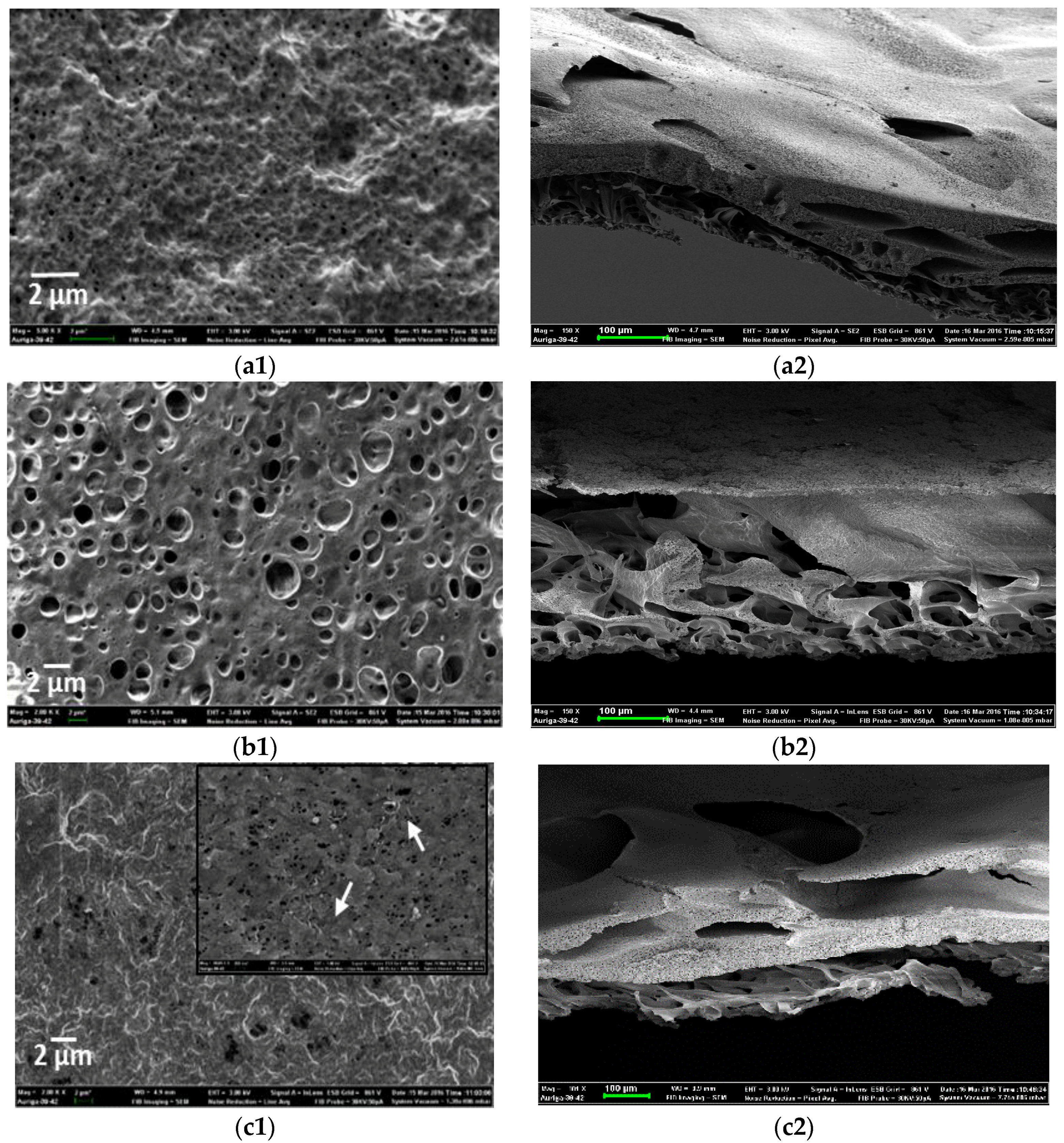
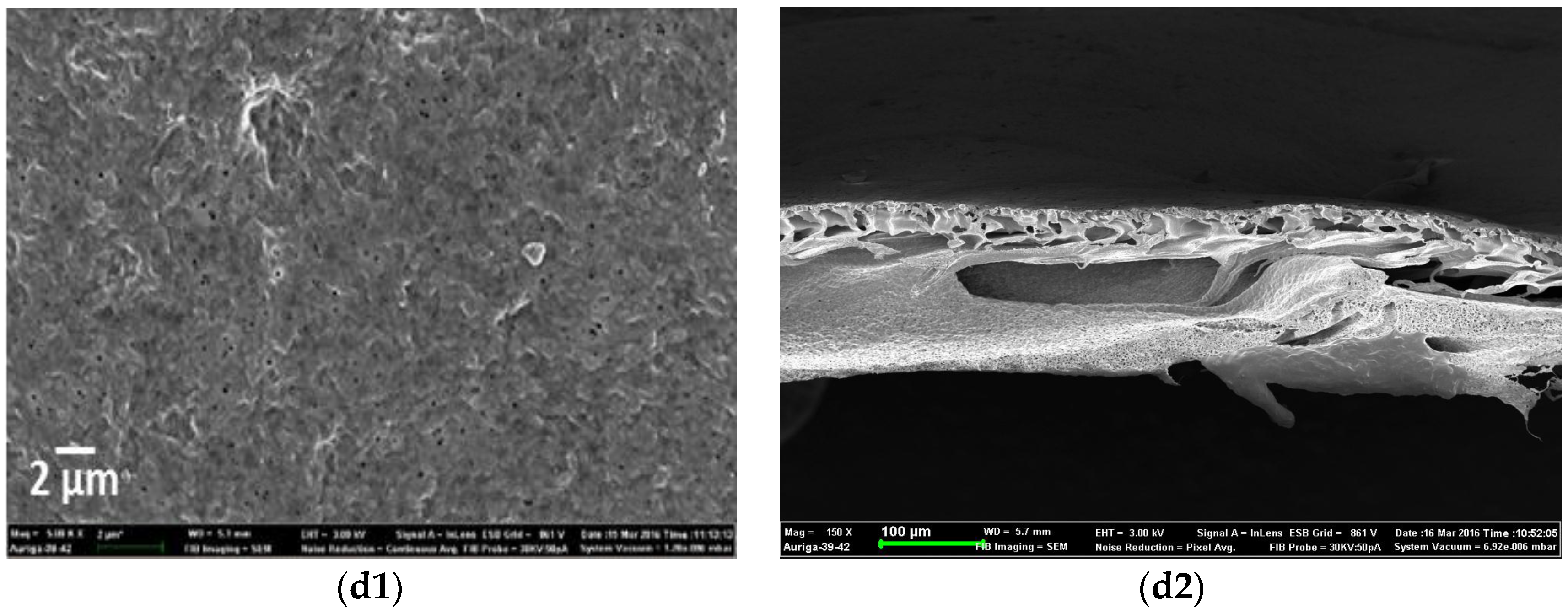
3.2. Morphology of PVDF-HFP Composite Membranes Doped with MWCNTs and Ag Nanoparticles
Physical Properties of Composite Membranes
3.3. Water Filtration Studies
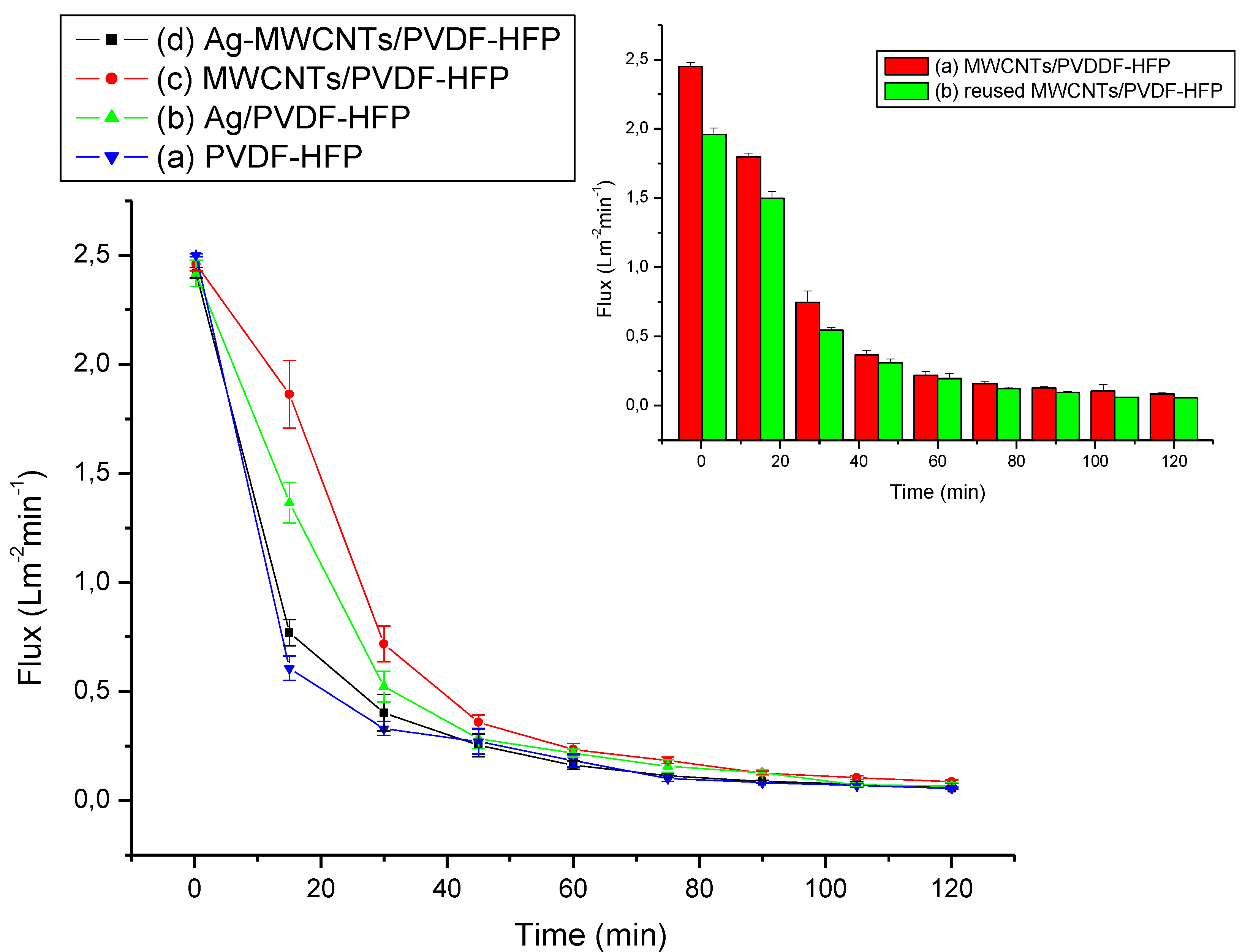
3.3.1. Pure Water Flux Measurement and Antifouling Performance
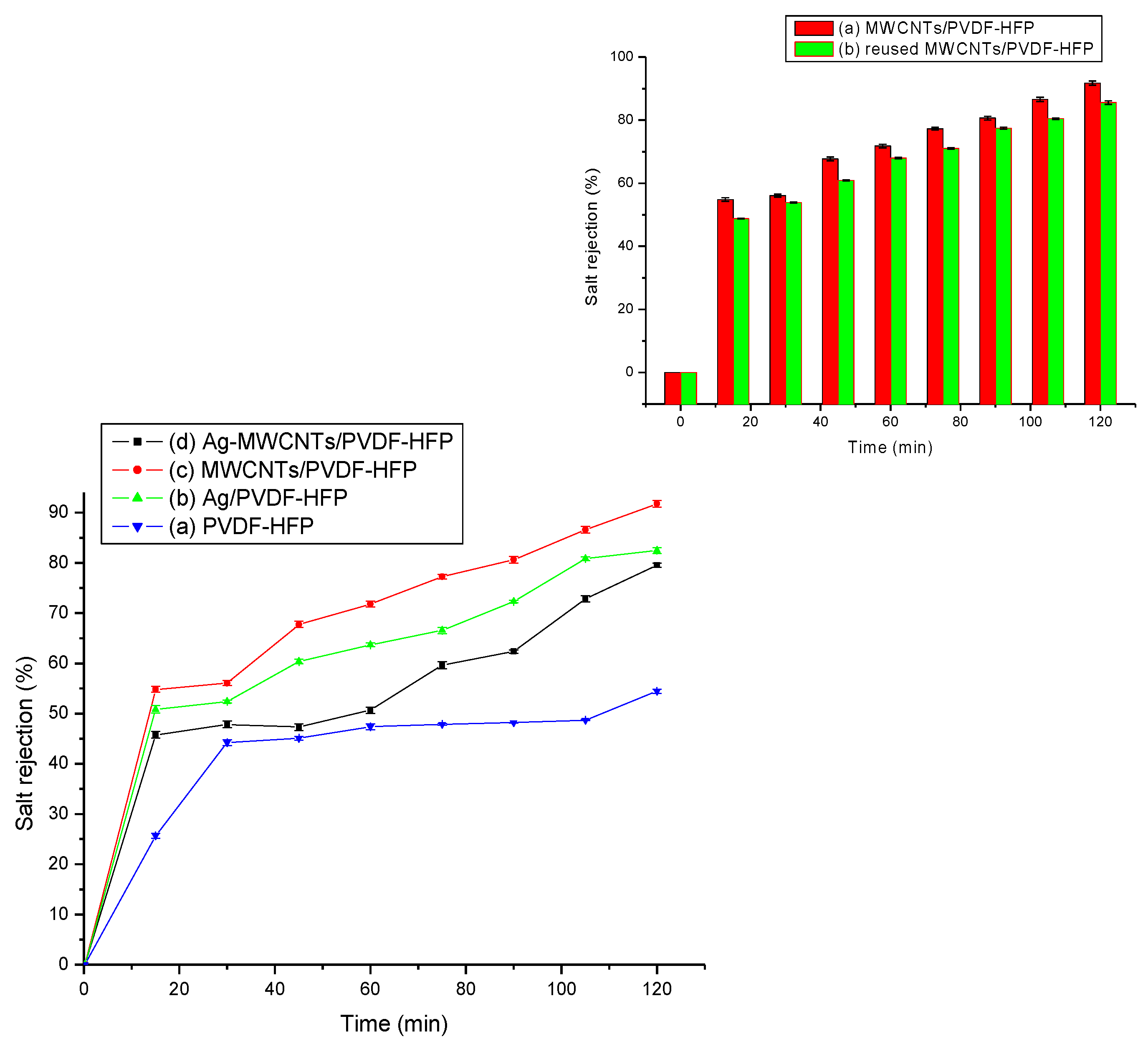
3.3.2. Salt Rejection Experiment
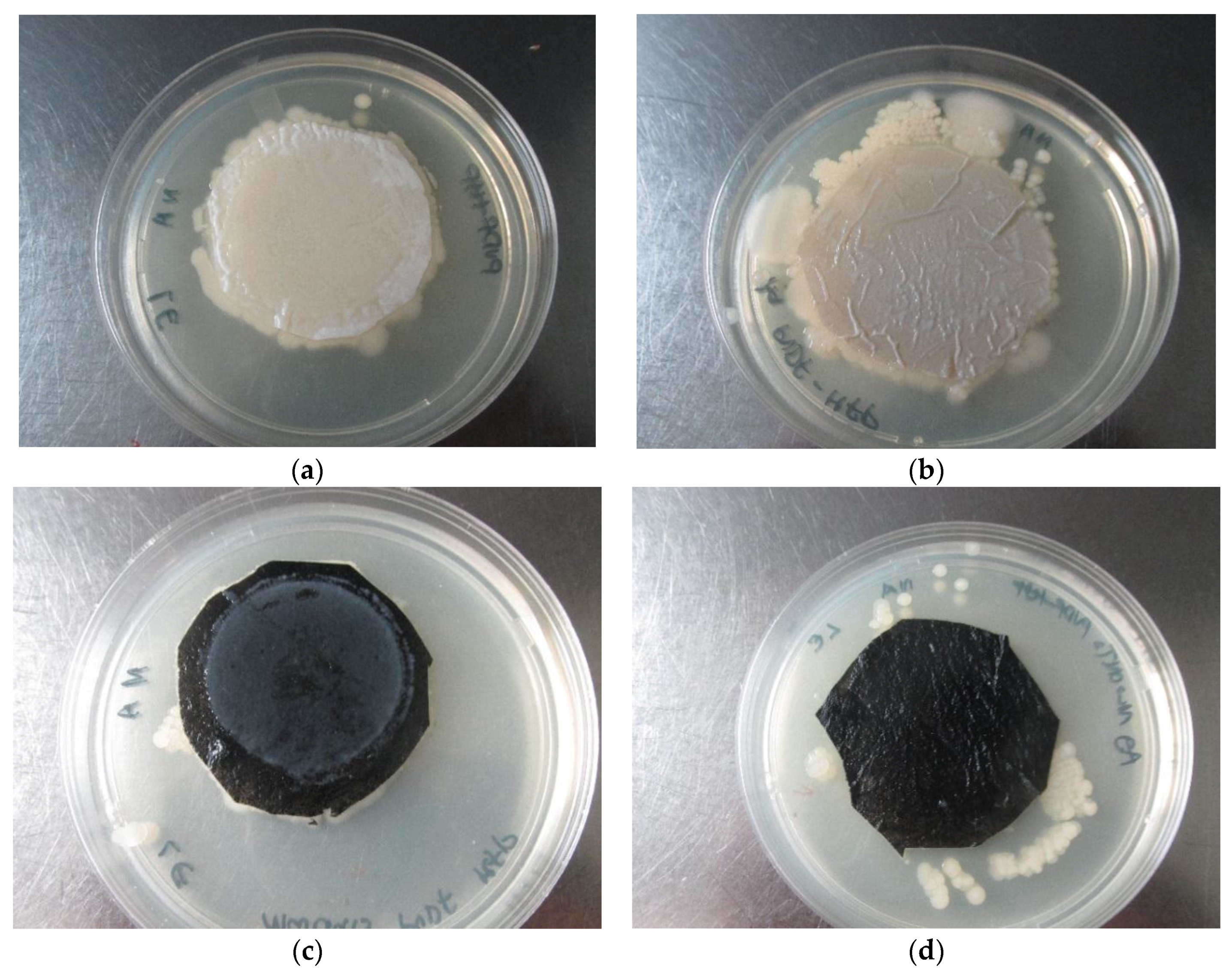
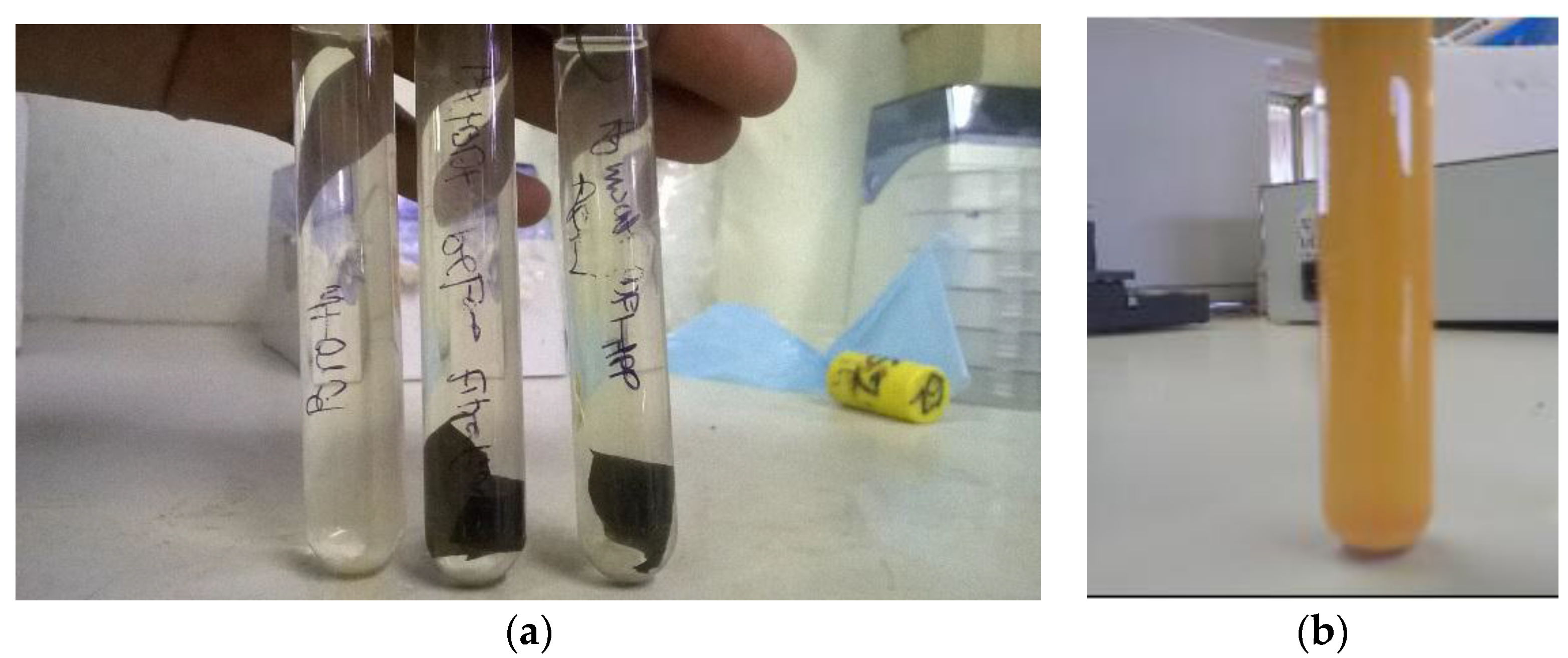
3.3.3. Effects of Membrane Structure on Microbial Load Reduction
3.3.4. Evaluation of Antibacterial and Non-Leaching Properties of PVDF-HFP Composite Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Reddy, C.D.; Voortman, W.J. Package Water Treatment Plant Selection. Water Res. Comm. Rep. 2005, 450, 3–7. [Google Scholar]
- Chan, C.L.; Zalifah, M.K.; Norrakiah, A.S. Microbiological and physiochemical quality of drinking water. Malays. J. Analyt. Sci. 2007, 11, 414–420. [Google Scholar]
- Abdel-Moety, N.M.; Al-Fassi, F.A.; Ali, M.A. Health aspects of virological water quality: An overview review. J. Appl Sci. Res. 2008, 4, 1205–1215. [Google Scholar]
- Havelaar, A.; Bartram, J. World Health Organization: Guidelines for drinking water quality. World Health Organ. 1996, 2, 29–31. [Google Scholar]
- Qu, X.L.; Brame, J.; Li, Q.; Alvarez, J.J.P. Nanotechnology for a safe and sustainable water supply: Enabling integrated water treatment and reuse. Acc. Chem. Res. 2013, 46, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Oshima, K.H.; Evans-Strickfaden, T.T.; Highsmith, A.K.; Ades, E.W. The use of a microporous polyvinylidene fluoride (PVDF) membrane filter to separate contaminating viral particles from biologically important proteins. Biologicals 1996, 24, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Stephan, A.M.; Kumar, S.G.; Renganathan, N.G.; Kulandainathan, M.A. Characterization of poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) electrolytes complexed with different lithium salts. Eur. Polym. J. 2005, 41, 15–21. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Xue, L. Fabrication and characterisation of antibacterial PVDF hollow fibre membrane by doping Ag-loaded zeolites. J. Membr. Sci. 2013, 437, 205–215. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Poly(vinylidene fluoride-co-hexafluoropropene) (PVDF-HFP) membranes for ethyl acetate removal from water. J. Hazard. Mater. 2008, 153, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, R.; Cao, Y.; Liang, D.T.; Tay, J.H. Fabrication of poly(vinylidene fluoride-co-hexafluropropylene) (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2007, 305, 215–225. [Google Scholar] [CrossRef]
- Choi, H.J.; Jegal, J.J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Su, F.; Miao, M. Effect of MWCNT dimension on the electrical percolation and mechanical properties of poly(vinylidenefluoride-hexafluoropropylene) based nanocomposite. Synth. Met. 2014, 191, 99–103. [Google Scholar] [CrossRef]
- Brady-Estevez, A.S.; Kang, S.; Elimelech, M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Santos, C.M.; Vergara, R.; Tria, M.C.R. Antimicrobial applications of electroactive PVK-SWNT nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Rananga, L.E.; Magadzu, T. Interaction of silver doped carbon nanotubes-cyclodextrin nanocomposites with Escherichia coli bacteria during water purification. Water Sci. Technol. 2014, 14, 367–375. [Google Scholar]
- Liao, C.; Yu, P.; Zhao, J.; Wang, L.; Luo, Y. Preparation and characterization of NaY/PVDF hybrid ultrafiltration membranes containing silver ions as antibacterial materials. Desalination 2011, 272, 59–65. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, X.; Wang, Z.; Gao, C. Antifouling and antibacterial improvement of surface-functionalized poly(vinylidene fluoride) membrane prepared via dihydroxyphenylalanine-initiated atom transfer radical graft polymerizations. J. Membr. Sci. 2012, 394–395, 107–119. [Google Scholar] [CrossRef]
- Li, J.H.; Shao, X.S.; Zhou, Q.; Li, M.Z.; Zhang, Q.Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387–388, 1–6. [Google Scholar] [CrossRef]
- Guzman, M.G.; Dille, J.; Godet, S. Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2008, 2, 91–98. [Google Scholar]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Bessadok, A.; Marais, S.; Gouanve, F.; Colasse, L.; Zimmerlin, I.; Roudesli, S.; Metayer, M. Effect of chemical treatments of Alfa (Stipa tenacissima) fibres on water-soption properties. Compos. Sci. Technol. 2007, 67, 685–697. [Google Scholar] [CrossRef]
- Liang, H.; Wan, L.; Xu, Z. Poly(vinylidene fluoride) Separators with Dual-asymmetric Structure for High-performance Lithium Ion Batteries. Chin. J. Polym. Sci. 2016, 12, 1423–1435. [Google Scholar] [CrossRef]
- Zheng, Q.Z.; Wang, P.; Yang, Y.N.; Cui, D.J. The relationship between porosity and kinetics parameter of membrane formation in PSF ultrafiltration membrane. J. Membr. Sci. 2006, 286, 7–11. [Google Scholar] [CrossRef]
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. Application of polysulfone/cyclodextrin mixed-matrix membranes in the removal of natural organic matter from water. Phys. Chem. Earth. 2014, 67–69, 71–78. [Google Scholar] [CrossRef]
- Kasim, N.; Mohammad, A.W.; Abdullah, S.R.S. Performance of membrane filtration in the removal of iron and manganese from Malaysia’s groundwater. Membr. Water Treat. 2016, 7, 277–296. [Google Scholar] [CrossRef]
- Rananga, L.E.; Magadzu, T. Comparative studies of silver doped carbon nanotubes and β-cyclodextrin for water disinfection. Dig. J. Nanomater. Bios. 2015, 10, 831–836. [Google Scholar]
- Malmonge, L.F.; Langiano, S.C.; Cordeiro, M.M.; Mattoso, L.H.C.; Malmonge, J.A. Thermal and mechanical properties of PVDF/PANI blends. Mater. Res. 2010, 13, 465–470. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Zulfiqar, M.; Munir, A. Study of the thermal-degradation of polychlorotrifluoroethylene, poly(vinylidene fluoride) and copolymers of chlorotrifluoroethylene and vinylidene fluoride. Polym. Degrad. Stabil. 1994, 43, 423–430. [Google Scholar] [CrossRef]
- Wang, W.Y.; Shi, J.Y.; Wang, J.L.; Li, Y.L.; Gao, N.N.; Liu, Z.X.; Lian, W.T. Preparation and characterization of PEG-g-MWCNTs/PSf nano-hybrid membranes with hydrophilicity and antifouling properties. RSC Adv. 2015, 5, 84746–84753. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini., S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Ho, C.C.; Zydney, A.L. A combined pore blockage and cake filtration model for protein fouling during microfiltration. J. Colloid Interf. Sci. 2000, 232, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Astaraee, R.S.; Mohammadi, T.; Kasiri, N. Analysis of BSA, dextran and humic acid foulingduring microfiltration, experimental and modelling. Food Bioprod. Process 2015, 94, 331–341. [Google Scholar] [CrossRef]
- Park, S.J.; Cheedrala, R.K.; Diallo, M.S.; Kim, C.; Kim, I.S.; Goddard, W.A. Nanofiltration membranes based on polyvinylidene fluoride nanofibrous scaffolds and crosslinked polyethyleneimine networks. J. Nanopart. Res. 2012, 14, 1884. [Google Scholar] [CrossRef]
- Zhu, H.; Szymczyk, A.; Balannec, B. On the salt rejection properties of nanofiltration polyamide membranes formed by interfacial polymerization. J. Membr. Sci. 2011, 379, 215–223. [Google Scholar] [CrossRef]

| Type of Membrane 1 | Swellability (%) | Water Content (%) | Porosity (%) | Fouling Resistance Rate 2 (L·m−2·min−2) |
|---|---|---|---|---|
| PVDF-HFP | 12 | 61 | 70 | 0.0233 ± 0.006 |
| Ag/PVDF-HFP | 13 | 67 | 82 | 0.0257 ± 0.0032 |
| Ag-MWCNTs/PVDF-HFP | 16 | 87 | 85 | 0.0376 ± 0.005 |
| MWCNTs/PVDF-HFP | 20 | 86 | 91 | 0.0455 ± 0.009 |
| Membrane | Pre-Filtration Colony Count (CFU/100 mL) | Post-Filtration Colony Count (CFU/100 mL) | % Microbial Load Reduction |
|---|---|---|---|
| PVDF-HFP | 150 | 50 | 67 |
| Ag-MWCNTs/PVDF-HFP | 150 | 0 | 100 |
| MWCNTs/PVDF-HFP | 150 | 0 | 100 |
| Ag/PVDF-HFP | 150 | 20 | 87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macevele, L.E.; Moganedi, K.L.M.; Magadzu, T. Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane. Membranes 2017, 7, 35. https://doi.org/10.3390/membranes7030035
Macevele LE, Moganedi KLM, Magadzu T. Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane. Membranes. 2017; 7(3):35. https://doi.org/10.3390/membranes7030035
Chicago/Turabian StyleMacevele, Lutendo E., Kgabo L. M. Moganedi, and Takalani Magadzu. 2017. "Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane" Membranes 7, no. 3: 35. https://doi.org/10.3390/membranes7030035
APA StyleMacevele, L. E., Moganedi, K. L. M., & Magadzu, T. (2017). Investigation of Antibacterial and Fouling Resistance of Silver and Multi-Walled Carbon Nanotubes Doped Poly(Vinylidene Fluoride-co-Hexafluoropropylene) Composite Membrane. Membranes, 7(3), 35. https://doi.org/10.3390/membranes7030035




