Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance
Abstract
:1. Introduction
2. Material and Methods
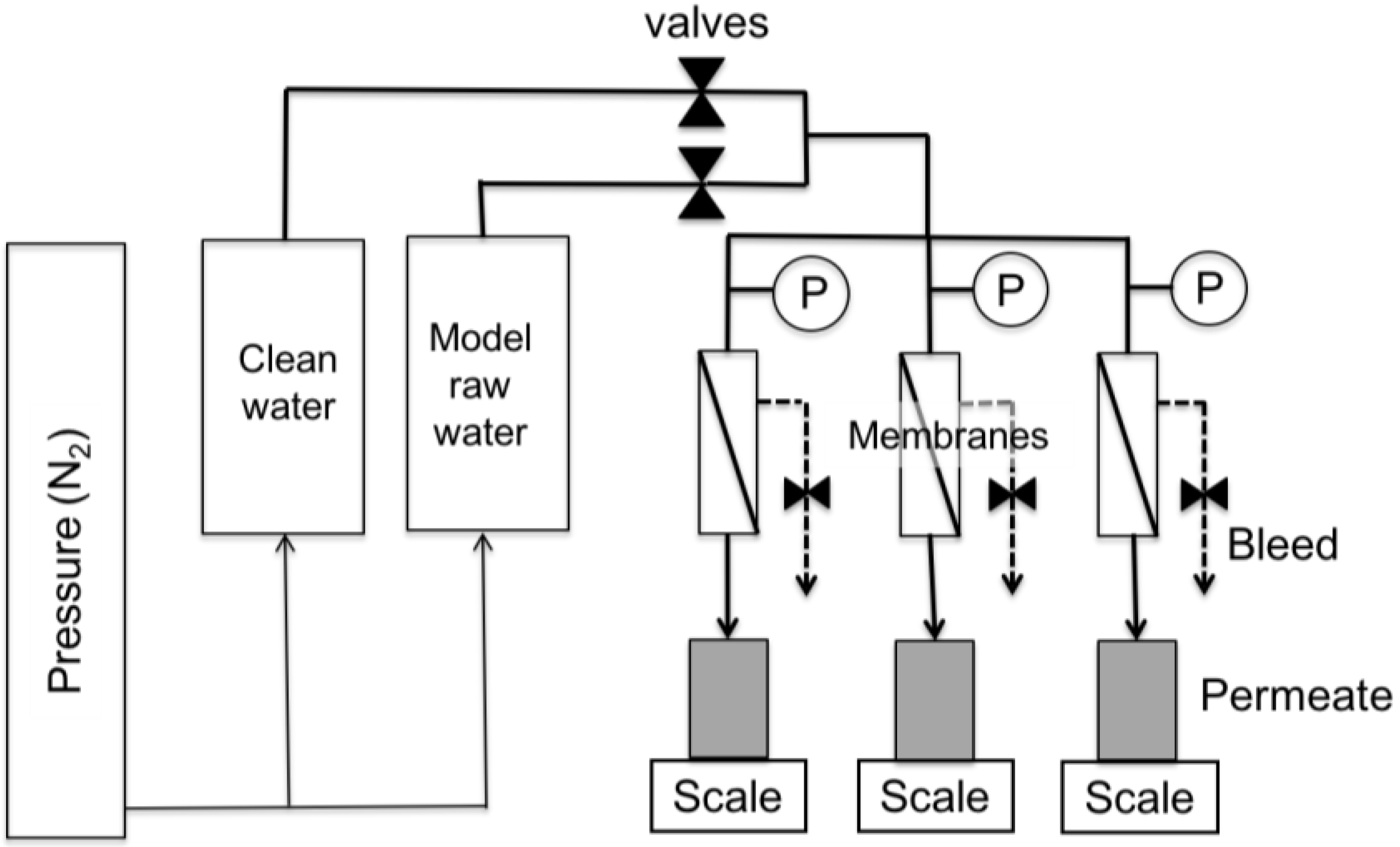
2.1. Experimental Approach
- Pre-clean water filtration phase (for a minimum of 3 h).
- Filtration phase with model raw waters (for approximately 3 h).
- Post-clean water filtration phase (for a minimum of 45 min).
2.2. Data Evaluation and Sample Analyses
3. Results
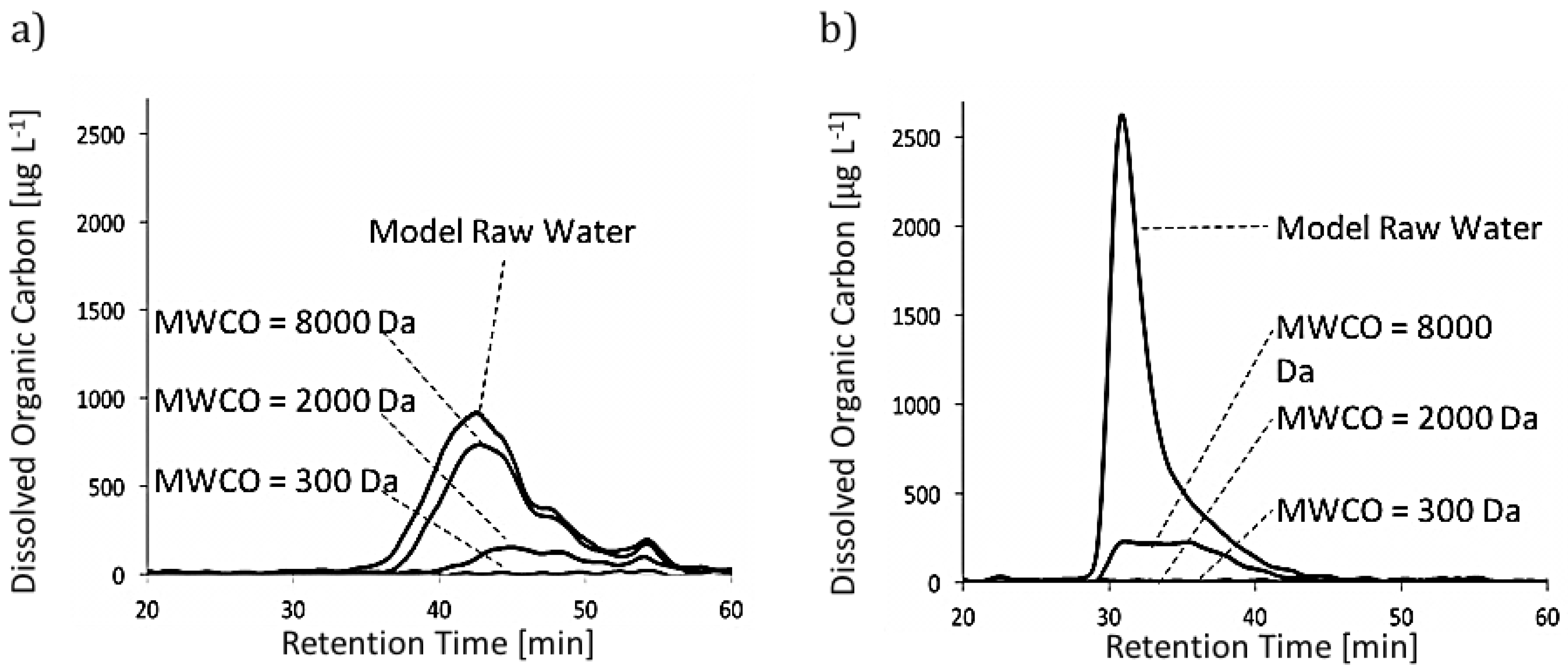
3.1. NOM Rejection
3.2. NOM Fouling and Concentration Polarization
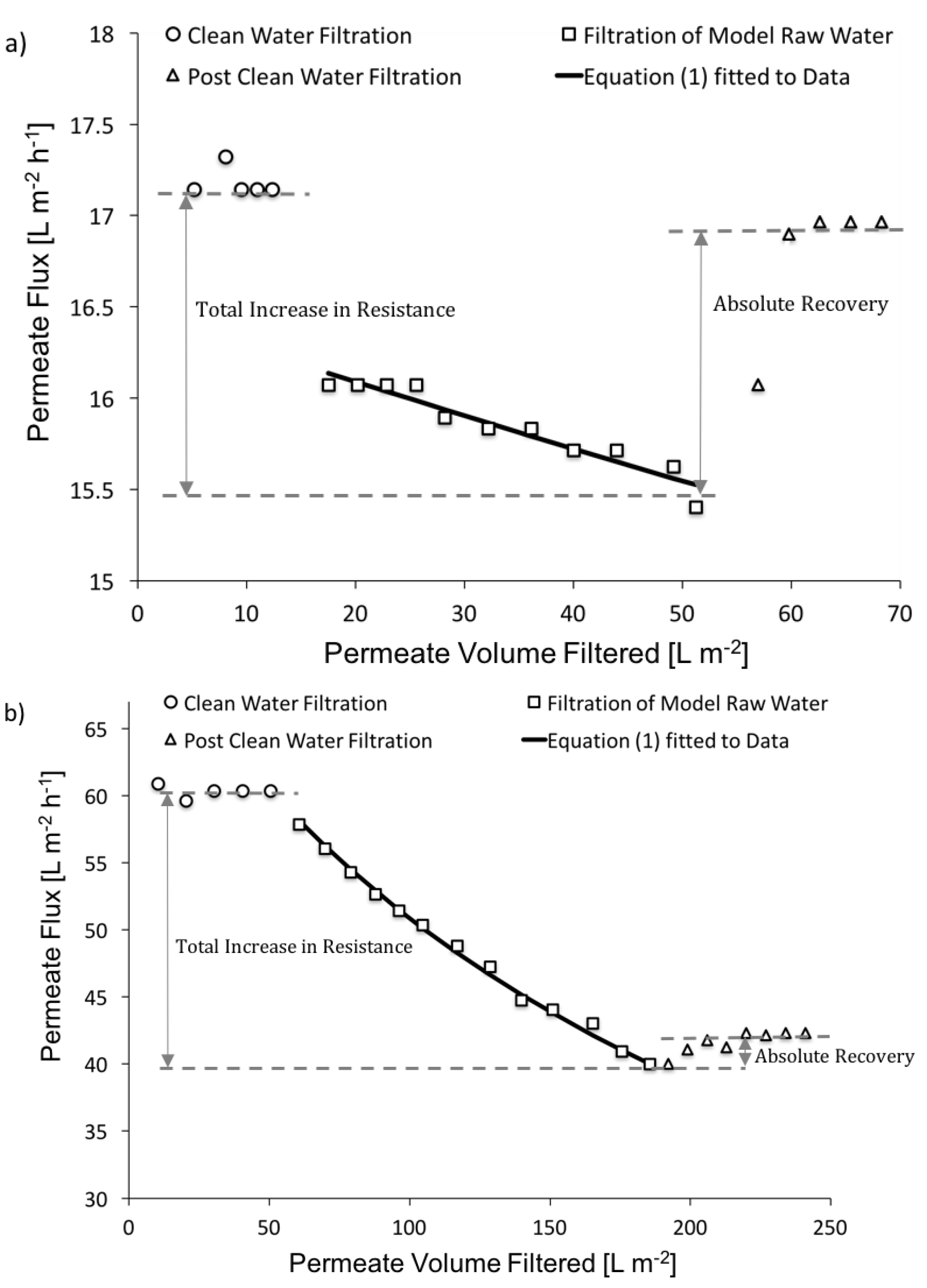
3.2.1. Contribution of Fouling to the Total Increase in Filtration Resistance
3.2.2. Contribution of CP to the Total Increase in Filtration Resistance
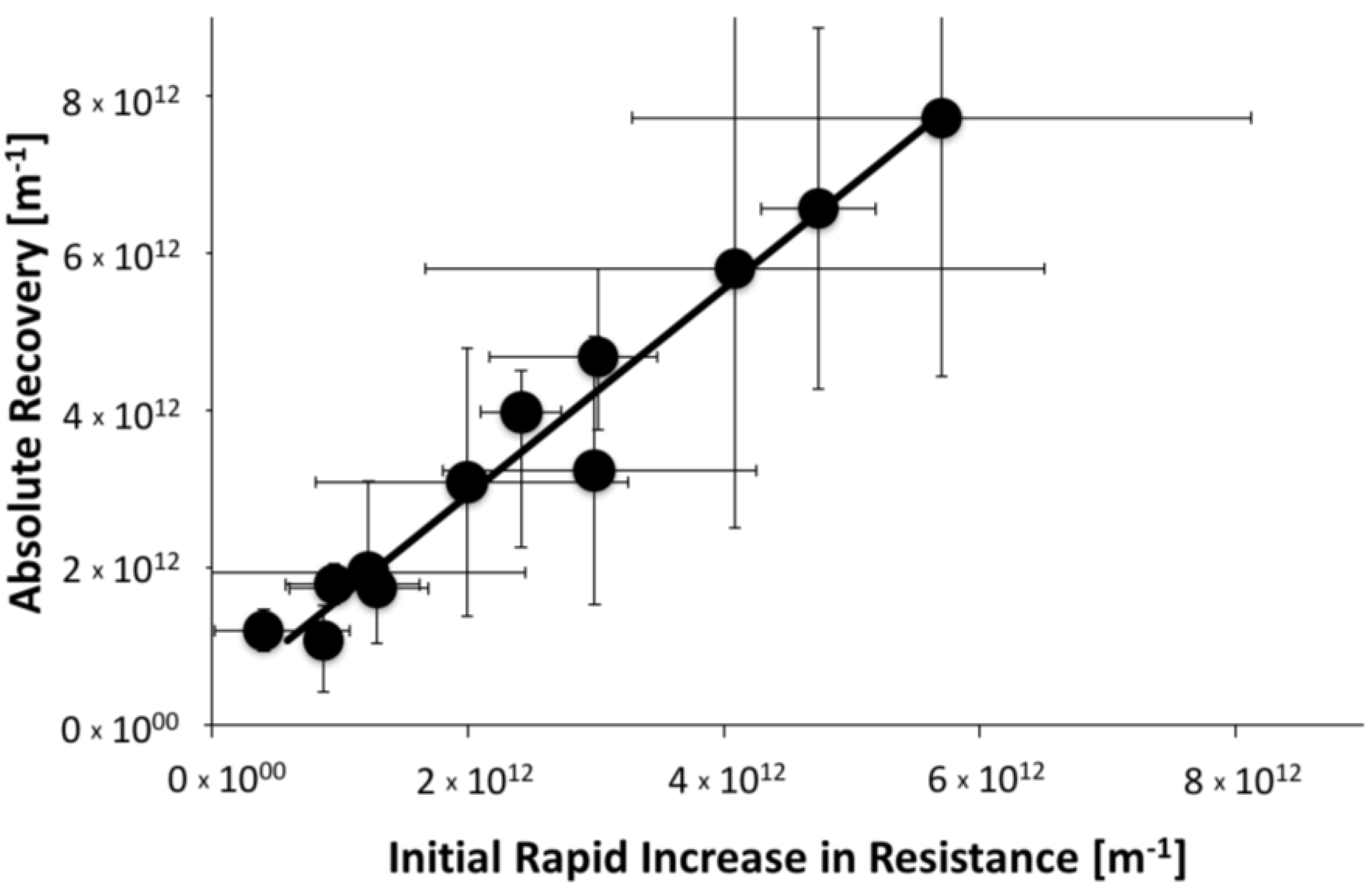
3.2.3. Overall Impact of Fouling and CP to the Increase in Resistance to Permeate Flow
4. Conclusions
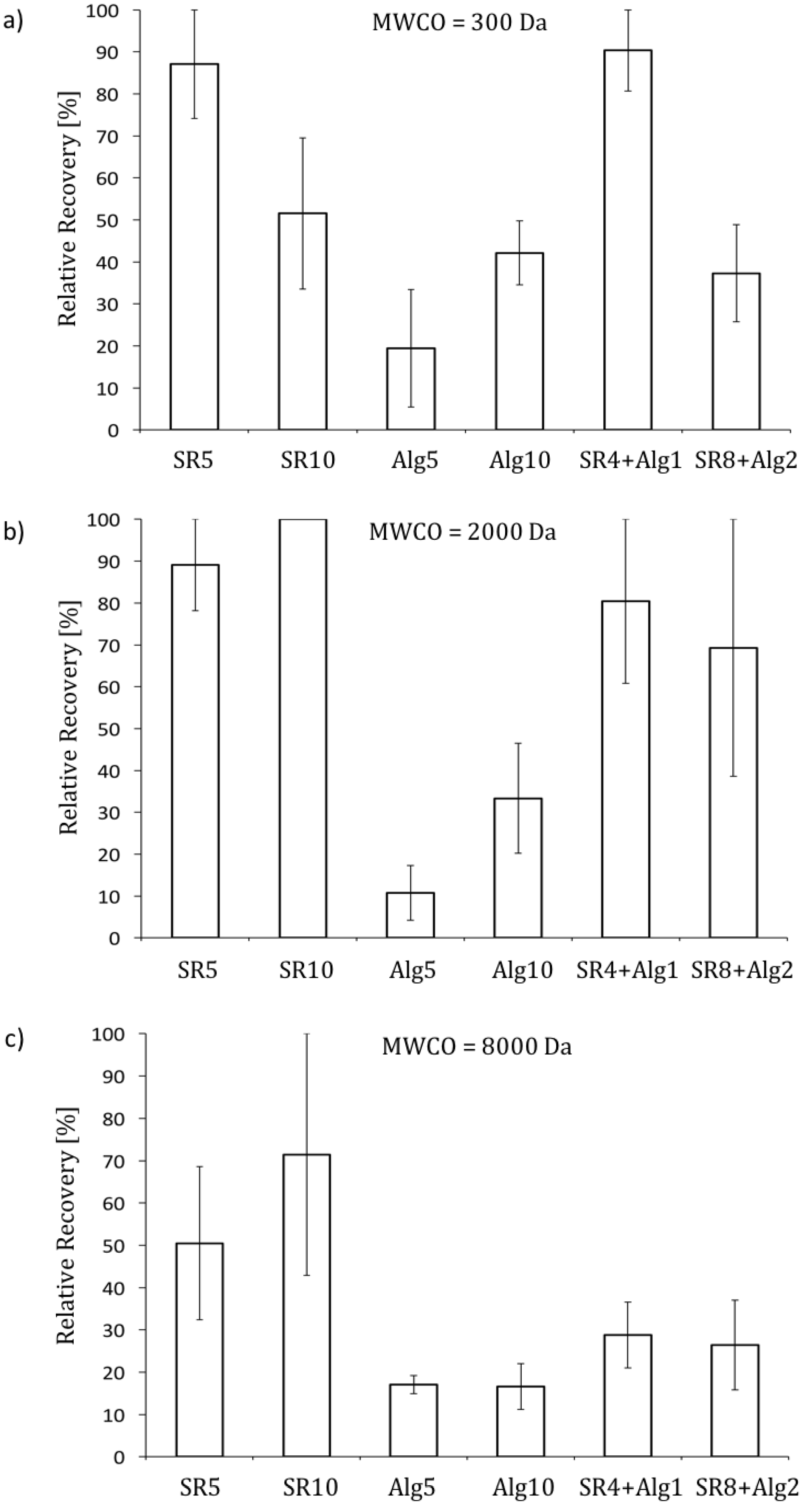
- Since NOM in most surface waters consists mainly of humic substances (i.e., lower molecular weight NOM), and because effective NOM removal is likely to be one of the main motivations for implementing NF and tight UF range membranes in drinking water treatment, membranes with lower MWCOs (i.e., <2000 Da) are recommended for this application.
- When filtering raw waters containing high concentrations of alginate (i.e., polysaccharides, high molecular weight NOM), the total increase in the filtration resistance is mainly dominated by fouling; when filtering raw waters containing predominantly humic substances (i.e., lower molecular weight NOM), the total increase in filtration resistance is mainly dominated by CP. The impact of CP is greatest for membranes with low MWCOs (i.e., <2000 Da).
- When filtering raw waters containing high concentrations of humic substances (i.e., 10 mg L−1) with the membrane with the lowest MWCO (i.e., 300 Da), the impact of CP became extensive and the increase in resistance was greater than that observed for any other experimental condition considered, likely due to the formation of a CP-induced cake/gel layer.
- Operations with control measures to continuously limit CP, such as cross-flow operation, should be considered in drinking water applications using NF and tight UF range membranes. Dead-end operation, which is typically used for drinking water production using UF, is not recommended because it cannot continuously limit CP.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gao, W.; Liang, H.; Ma, J.; Man, M.; Chen, Z.L.; Han, Z.S.; Li, G.B. Membrane fouling control in ultrafiltration technology for drinking water water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- ElHadidy, A.M.; Peldszus, S.; van Dyke, M.I. An evaluation of virus removal mechanisms by ultrafiltration membranes using MS2 and ϕX174 bacteriophage. Sep. Purif. Technol. 2013, 120, 215–223. [Google Scholar] [CrossRef]
- Song, H.; Shao, J.; He, Y.; Hou, J.; Chao, W. Natural organic matter removal and flux decline with charged ultrafiltration and nanofiltration membranes. J. Membr. Sci. 2011, 376, 179–187. [Google Scholar] [CrossRef]
- Lee, N.; Amy, G.; Croué, J.-P.; Buisson, H. Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res. 2004, 38, 4511–4523. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Wan, W.M.; Choi, Y. Comparison of membrane permeability and a fouling mechanism by pre-ozonation followed by membrane filtration and residual ozone in membrane cells. Desalination 2005, 178, 287–294. [Google Scholar] [CrossRef]
- Odegaard, H.; Osterhus, S.; Melin, E.; Eikebrokk, B. NOM removal technologies—Norwegian experiences. Drink. Water Eng. Sci. 2010, 3, 1–9. [Google Scholar] [CrossRef]
- Patterson, C.; Anderson, A.; Sinha, R.; Muhammad, N.; Pearson, D. Nanofiltration Membranes for Removal of Color and pathogens in Small Drinking Water Sources. J. Environ. Eng. 2012, 138, 48–57. [Google Scholar] [CrossRef]
- Sadmani, A.H.M.; Anwar, A.; Robert, C.; Bagley, D.M. Impact of natural water colloids and cations on the rejection of pharmaceutically active and endocrine disrupting compounds by nanofiltration. J. Membr. Sci. 2014, 450, 271–281. [Google Scholar] [CrossRef]
- Chin, A.; Bérubé, P. Removal of disinfection by-product precursors with ozone-UV advanced oxidation process. Water Res. 2005, 39, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Croue, J.-P.; Korshin, G.; Mark, B.M. Characterization of Natural Organic Matter in Drinking Water; AWWA Research Foundation (AWWARF): Denver, CO, USA, 1999. [Google Scholar]
- Hozalski, R.M.; Bouwer, E.J.; Goel, S. Removal of natural organic matter from drinking water supplies by ozone-biofiltration. Water Sci. Technol. 1999, 40, 157–163. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Stilwell, D. Modelling biological processes in water treatment: The integrated biofiltration model. J. Water Supply Res. Technol. AQUA 2002, 51, 1–14. [Google Scholar]
- Sarathy, S.; Mohseni, M. The fate of natural organic matter during UV/H2O2 advanced oxidation of drinking water. Can. J. Civ. Eng. 2009, 36, 160–169. [Google Scholar] [CrossRef]
- Wang, G.-S.; Hsieh, S.-T. Monitoring natural organic matter in water with scanning spectrophotometer. Environ. Int. 2001, 26, 205–212. [Google Scholar] [CrossRef]
- Bequet, S.; Remigy, J.C.; Rouch, J.C. From ultrafiltration to nanofiltration hollow fiber membranes: A continuous UV-photografting process. Desalination 2002, 144, 9–14. [Google Scholar] [CrossRef]
- Frank, M.; Bargeman, G.; Zwijnenburg, A. Capillary hollow fiber nanofiltration membranes. Sep. Purif. Technol. 2001, 22, 499–506. [Google Scholar] [CrossRef]
- Spruck, M.; Hoefer, G.; Fili, G.; Gleinser, D.; Ruech, A.; Schmidt-Baldassari, M.; Rupprich, M. Preparation and characterization of composite multichannel capillary membranes on the way to nanofiltration. Desalination 2013, 314, 28–33. [Google Scholar] [CrossRef]
- Verberk, J.Q.J.C.; van Dijk, J.C. Air Sparging in Capillary Nanofiltration. J. Membr. Sci. 2006, 284, 339–351. [Google Scholar] [CrossRef]
- Geraldes, V.; Semião, V.; de Pinho, M.N. Flow management in nanofiltration spiral wound modules with ladder-type spacers. J. Membr Sci. 2002, 203, 87–102. [Google Scholar] [CrossRef]
- Thorsen, T.; Flogstat, H. Nanofiltration in Drinking Water Treatment—Literature Review. Available online: https://www.techneau.org/fileadmin/files/Publications/Publications/Deliverables/D5.3.4b.pdf (accessed on 13 March 2017).
- Song, L.; Elimelech, M. Particle deposition onto a permeable surface in laminar flow. J. Colloid Interface Sci. 1995, 173, 165–180. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in cross-flow microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Summers, R.S.; Greenberg, A.R.; Hermandez, M.T. Extra-cellular Polysaccharides, soluble microbial products, and natural organic matter impact on nanofiltration flux decline. Environ. Sci. Technol. 2007, 41, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lee, N.; Young, T.; Amy, G.; Lozier, J.C.; Jacangelo, J.G. Natural organic matter fouling of low-pressure, hollow-fiber membranes: Effects of NOM source and hydrodynamic conditions. Water Res. 2007, 41, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Jermann, D.; Pronk, W.; Kägi, R.; Halbeisen, M.; Boller, M. Influence of interactions between NOM and particles on UF fouling mechanism. Water Res. 2008, 42, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.I.; Fane, A.G.; Waite, T.D. Nanofiltration of natural organic matter: Removal, fouling and influence of multivalent ions. Desalination 1998, 118, 109–122. [Google Scholar] [CrossRef]
- Zheng, X.; Ernst, M.; Jekel, M. Identification and quantification of major organic foulants in treated domestic wastewater affecting filterability in dead-end ultrafiltration. Water Res. 2009, 43, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Her, N.; Amy, G.; Park, H.-R.; Song, M. Characterizing algogenic organic matter (AOM) and evaluating associated NF membrane fouling. Water Res. 2004, 38, 1427–1438. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.I. Natural Organics Removal Using Membranes—Principles, Performance and Cost; Technomic Publishing: Lancaster, PA, USA, 2007. [Google Scholar]
- Zularisam, A.W.; Ismail, A.F.; Salim, M.R.; Sakinah, M.; Ozaki, H. The effects of natural organic matter (NOM) fractions on fouling characteristics and flux recovery of ultrafiltration membranes. Desalination 2007, 212, 191–208. [Google Scholar] [CrossRef]
- Winter, J.; Uhl, W.; Bérubé, P.R. Integrated Oxidation Membrane Filtration Process—NOM Rejection and Membrane Fouling. Water Res. 2016, 104, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Zumstein, J.; Buffle, J. Circulation of Pedogenic and Aquagenic Organic Matter in an Eutrophic Lake. Water Res. 1989, 23, 229–239. [Google Scholar] [CrossRef]
- Amy, G.; Her, N. Size exclusion chromatography (SEC) with multiple detectors: A powerful tool in treatment process selection and performance. Water Sci. Technol. 2004, 4, 19–24. [Google Scholar]
- Biber, M.V.; Guelacar, F.O.; Buffle, J. Seasonal Variations in Principal Groups of Organic Matter in a Eutrophic Lake using Pyrolysis/GC/MS. Environ. Sci. Technol. 1996, 30, 3501–3507. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Meylan, S.; Boller, M. Interplay of different NOM fouling mechanisms during ultrafiltration of drinking water production. Water Res. 2007, 41, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Laabs, C.; Amy, G.; Jekel, M. Understanding the Size and character of Fouling-Causing Substances from Effluent Organic Matter (EfOM) in Low Pressure Membrane filtration. Environ. Sci. Technol. 2006, 40, 4495–4499. [Google Scholar] [CrossRef] [PubMed]
- Wray, H.E.; Andrews, R.C.; Bérubé, P.R. Surface shear stress and membrane fouling when considering natural water matrices. Desalination 2013, 330, 22–27. [Google Scholar] [CrossRef]
- Jarusutthirak, C.; Amy, G.; Croue, J.-P. Fouling characteristics of wastewater effluent organic matter (EfOM) isolates on NF and UF membranes. Desalination 2002, 145, 247–255. [Google Scholar] [CrossRef]
- Seidel, A.; Elimelech, M. Coupling between chemical and physical interactions in natural organic matter (NOM) fouling of nanofiltration membranes: Implications for fouling control. J. Membr. Sci. 2002, 203, 245–255. [Google Scholar] [CrossRef]
- Sari, M.A.; Chellam, S. Surface water nanofiltration incorporating (electro) coagulation-microfiltration pretreatment: Fouling control and membrane characterization. J. Membr. Sci. 2013, 437, 249–256. [Google Scholar] [CrossRef]
- Cho, J.; Amy, G.; Pellegrino, J. Membrane filtration of natural organic matter: Initial comparison of rejection and flux decline characteristics with ultrafiltration and nanofiltration membranes. Water Res. 1999, 33, 2517–2526. [Google Scholar] [CrossRef]
- Her, N.; Amy, G.; Plottu-Pecheux, A.; Yoon, Y. Identification of nanofiltration foulants. Water Res. 2007, 41, 3936–3947. [Google Scholar] [CrossRef] [PubMed]
- Listiarini, K.; Chun, W.; Sun, D.D.; Leckie, J. Fouling mechanism and resistance analyses of systems containing sodium alginate, calcium, alum and their combination in dead-end fouling of nanofiltration membranes. J. Membr. Sci. 2009, 344, 244–251. [Google Scholar] [CrossRef]
- Mahlangu, T.O.; Thwala, J.M.; Mamba, B.B.; D’Haese, A.; Verliefde, A.R.D. Factors governing combined fouling by organic and colloidal foulants in cross-flow nanofiltration. J. Membr. Sci. 2015, 49, 53–62. [Google Scholar] [CrossRef]
- Schäfer, A.I.; Fane, A.G.; Waite, T.D. Nanofiltration: Principles and Applications; Elsevier: Oxford, UK; New York, NY, USA, 2005. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Van den Berg, G.B.; Smolders, C.A. Flux Decline in Ultrafiltration Processes. Desalination 1990, 77, 101–133. [Google Scholar] [CrossRef]
- Elimelech, M.; Bhattacharjee, S. A novel approach for modeling concentration polarization in crossflow membrane filtration based on the equivalence of osmotic pressure model and filtration theory. J. Membr. Sci. 1998, 145, 223–241. [Google Scholar] [CrossRef]
- Chin, Y.-P.; Aiken, G.; O’Loughlin, E. Molecular Weight, Polydispersity, and Spectroscopic Properties of Aquatic Humic Substances. Environ. Sci. Technol. 1994, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Her, N.; Amy, G.; Foss, D.; Cho, J.; Yoon, Y.; Kosenka, P. Optimization of Method for Detecting and Characterizing NOM by HPLC-Size Exclusion Chromatography with UV and On-Line DOC detection. Environ. Sci. Technol. 2002, 36, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.A.; Balz, A.; Abert, M.; Pronk, W. Characterisation of aquatic humic and non-humic matter with size-exclusion chromatograpgy-organic carbon detection-organic nitrogen detection (LC-OCD-OND). Water Res. 2011, 45, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.K.; Summers, S.R.; Roberts, P.V. Diffusion of Humic Acid in Dilute Aqueous Solution. J. Colloid Interface Sci. 1986, 110, 149–164. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chen, J.C.; Elimelech, M. Coupled model of concentration polarization and pore transport in cross-flow nanofiltration. AIChE J. 2001, 47, 2733–2745. [Google Scholar] [CrossRef]
- Brian, P.L.T. Concentration Polarization in Reverse Osmosis Desalination with Variable Flux and Incomplete Salt Rejection. Ind. Eng. Chem. Fundam. 1965, 4, 439–445. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Elimelech, M. Cake-Enhanced Concentration Polarization—A New Fouling Mechanism for Salt-Rejecting Membranes. Environ. Sci. Technol. 2003, 37, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, R.; Albrecht, R. Membrane Processes; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Yoon, Y.; Amy, G.; Cho, J.; Her, N. Effects of retained natural organic matter (NOM) on NOM rejection and membrane flux decline with nanofiltration and ultrafiltration. Desalination 2005, 173, 209–221. [Google Scholar] [CrossRef]
- Hincks, S.S.; Mackie, G.L. Effect of pH, calcium, alkalinity, hardness, and chlorophyll on the survival, growth, and reproductive success of zebra mussel (Dreissena polymarpha) in Ontario lakes. Can. J. Fish. Aquat. Sci. 1997, 54, 2049–2057. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws—Application to Power-Law Non-Newtonian Fluids. Chem. Eng. Res. Des. 1982, 60, 183–187. [Google Scholar]
| NOM Constituents | NOM Concentration [mg/L] | Fouling Coefficient kc [1011 m−1 m−3 m2] Average (Range) | ||
|---|---|---|---|---|
| MWCO = 300 Da | MWCO = 2000 Da | MWCO = 8000 Da | ||
| SRNOM | 5 | 0.7 (0.5–0.9) | 0.1 (0–0.1) | 0.1 (0.1–0.1) |
| 10 | 3.5 (3.5–3.5) | 0.6 (0–1.1) | 0.2 (0.2–0.2) | |
| Alginate | 5 | 1.1 (0.8–1.6) | 0.8 (0.5–1.1) | 0.8 (0.7–0.9) |
| 10 | 2.1 (2.0–2.2) | 2.1 (1.6–2.4) | 1.6 (1.5–1.7) | |
| SRNOM + Alginate | 4 + 1 | 0.7 (0.4–1.0) | 0.2 (0.1–0.3) | 0.2 (0.2–0.2) |
| 8 + 2 | 5.1 (4.7–5.5) | 1.2 (0.7–2.0) | 0.5 (0.5–0.5) | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, J.; Barbeau, B.; Bérubé, P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes 2017, 7, 34. https://doi.org/10.3390/membranes7030034
Winter J, Barbeau B, Bérubé P. Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes. 2017; 7(3):34. https://doi.org/10.3390/membranes7030034
Chicago/Turabian StyleWinter, Joerg, Benoit Barbeau, and Pierre Bérubé. 2017. "Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance" Membranes 7, no. 3: 34. https://doi.org/10.3390/membranes7030034
APA StyleWinter, J., Barbeau, B., & Bérubé, P. (2017). Nanofiltration and Tight Ultrafiltration Membranes for Natural Organic Matter Removal—Contribution of Fouling and Concentration Polarization to Filtration Resistance. Membranes, 7(3), 34. https://doi.org/10.3390/membranes7030034






