Biological Fuel Cells and Membranes
Abstract
:1. Introduction
- A primary fuel is used by a biofuel cell and generates a material such as hydrogen, which can be used as a secondary fuel within a conventional hydrogen/oxygen fuel cell.
- An organic fuel, such as glucose, is used in a biofuel cell and directly generates bioelectricity. This biofuel cell may contain enzymes or microorganisms.
- Photochemically active systems and biological moieties are used to harvest energy from sunlight and convert it to electrical energy.
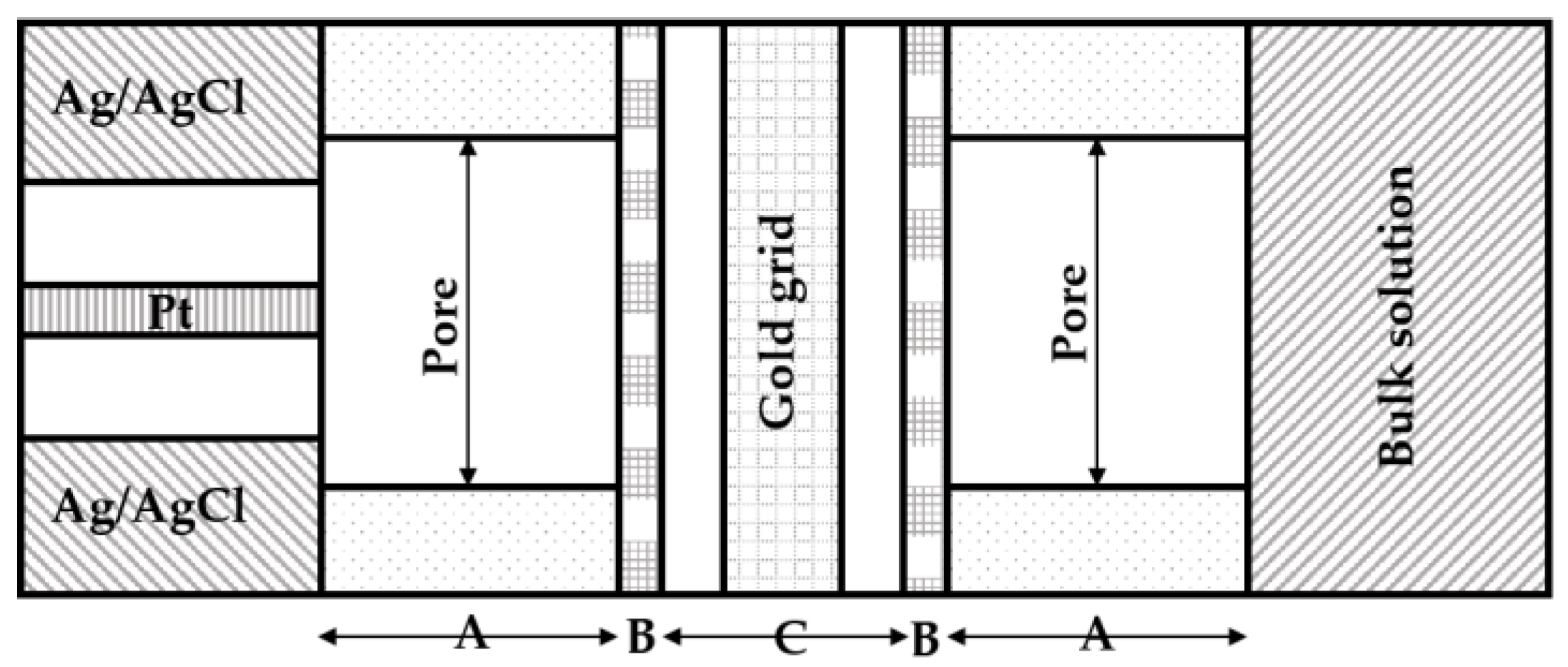
2. Enzymatic Biofuel Cell
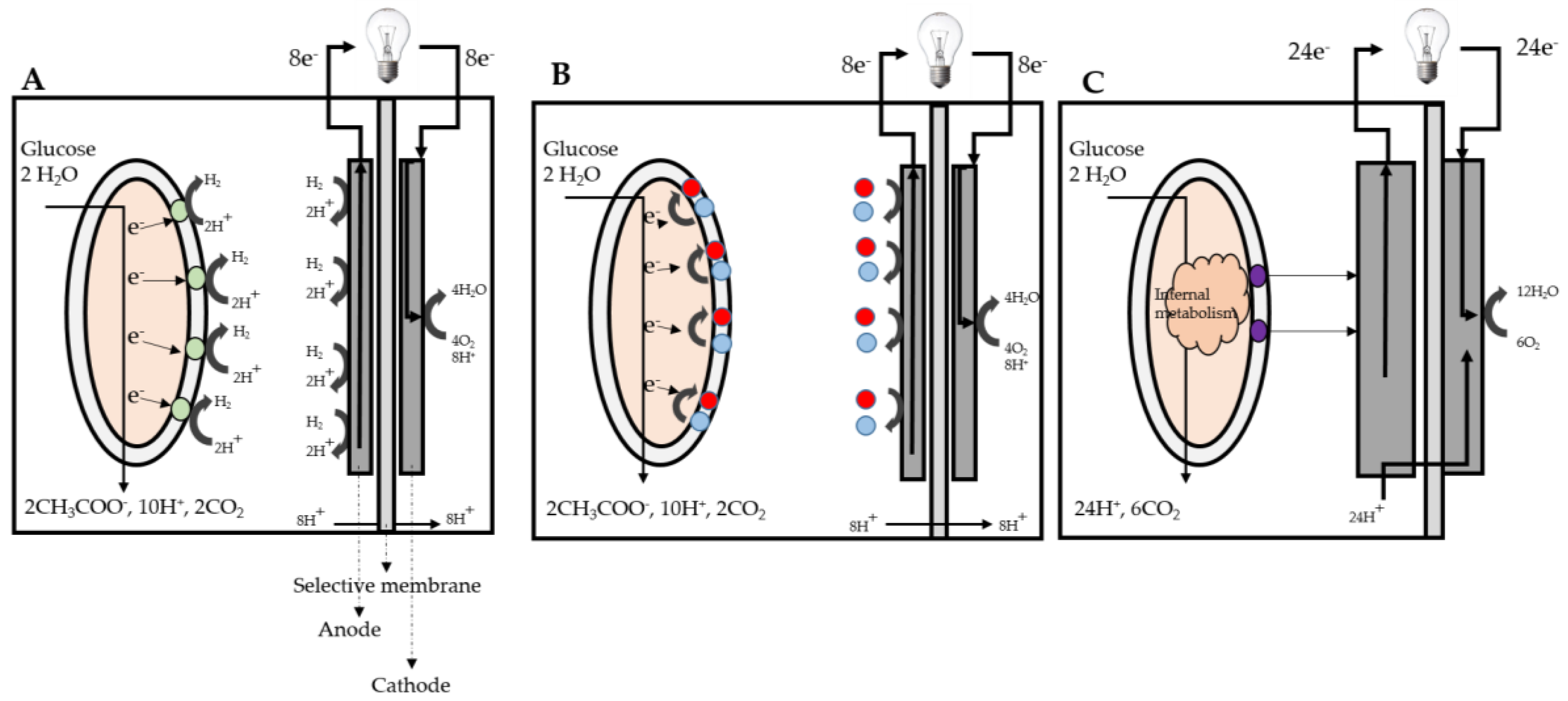
3. Microbial Fuel Cell
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, F.; Higson, S.P. Biofuel cells—Recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, M.; Zhao, F.; Liu, B.; Dong, S. A Low-Cost Biofuel Cell with pH-Dependent Power Output Based on Porous Carbon as Matrix. Chem. Eur. J. 2005, 11, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Calabrese Barton, S.; Gallaway, J.; Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Palmore, G.T.; Whitesides, G.M. Microbial and enzymatic biofuel cells. Enzym. Convers. Biomass Fuels Prod. 1994, 566, 271–290. [Google Scholar]
- Kano, K.; Ikeda, T. Bioelectrocatalysis, Powerful Means of Connecting Electrochemistry to Biochemistry and Biotechnology. Electrochem. Tokyo 2003, 71, 86–99. [Google Scholar]
- Katz, E.; Willner, I. A biofuel cell with electrochemically switchable and tunable power output. J. Am. Chem. Soc. 2003, 125, 6803–6813. [Google Scholar] [CrossRef] [PubMed]
- Kamitaka, Y.; Tsujimura, S.; Setoyama, N.; Kajino, T.; Kano, K. Fructose/dioxygen biofuel cell based on direct electron transfer-type bioelectrocatalysis. Phys. Chem. Chem. Phys. 2007, 9, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Palmore, G.T.; Bertschy, H.; Bergens, S.H.; Whitesides, G.M. A methanol/dioxygen biofuel cell that uses NAD+-dependent dehydrogenases as catalysts: Application of an electro-enzymatic method to regenerate nicotinamide adenine dinucleotide at low overpotentials. J. Electroanal. Chem. 1998, 443, 155–161. [Google Scholar] [CrossRef]
- Topcagic, S.; Minteer, S.D. Development of a membraneless ethanol/oxygen biofuel cell. Electrochim. Acta 2006, 51, 2168–2172. [Google Scholar] [CrossRef]
- Heineman, W.R.; Norris, B.J.; Goelz, J.F. Measurement of enzyme E. deg.’ values by optically transparent thin layer electrochemical cells. Anal. Chem. 1975, 47, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.R.; Richter, G.J.; Von Sturm, F.; Weidlich, E. The performance of glucose electrodes and the characteristics of different biofuel cell constructions. Bioelectrochem. Bioenerg. 1976, 3, 139–150. [Google Scholar] [CrossRef]
- Sakai, H.; Nakagawa, T.; Tokita, Y.; Hatazawa, T.; Ikeda, T.; Tsujimura, S.; Kano, K. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ. Sci. 2009, 2, 133–138. [Google Scholar] [CrossRef]
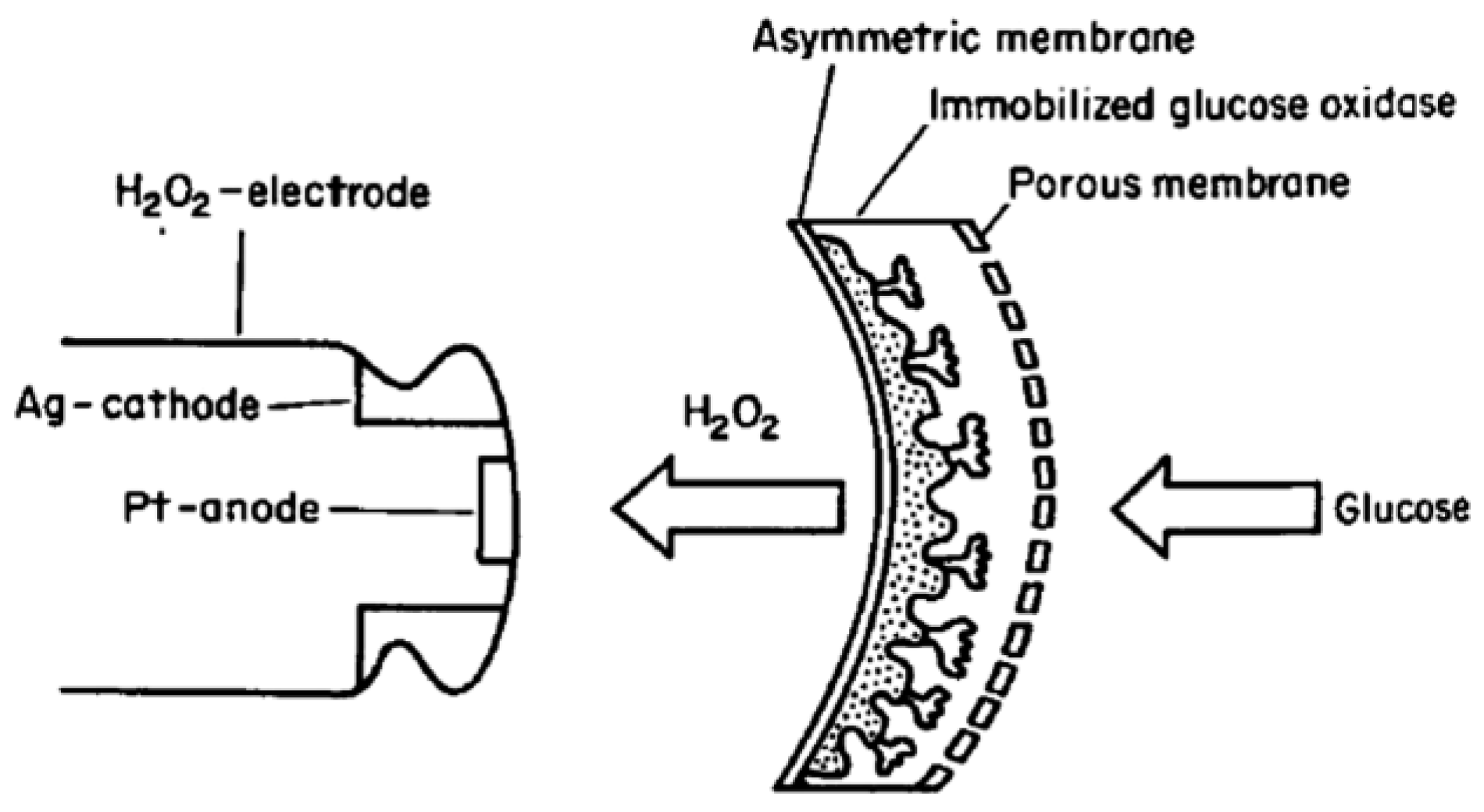
- Tsuchida, T.; Yoda, K. Immobilization of d-glucose oxidase onto a hydrogen peroxide permselective membrane and application for an enzyme electrode. Enzym. Microb. Technol. 1981, 3, 326–330. [Google Scholar] [CrossRef]
- Vos, K.D.; Hatcher, A.P.; Merten, U. Lifetime of cellulose acetate reverse osmosis membranes. Ind. Eng. Chem. Prod. Res. Dev. 1966, 5, 211–218. [Google Scholar] [CrossRef]
- Johnson, J.M.; Halsall, H.B.; Heineman, W.R. Potential-dependent enzymic activity in an enzyme thin-layer cell. Anal. Chem. 1982, 54, 1377–1383. [Google Scholar] [CrossRef]
- Persson, B.; Gorton, L.; Johansson, G. Biofuel anode for cell reactions involving nicotinamide adenine dinucleotide as a charge carrier. Bioelectrochem. Bioenerg. 1986, 16, 479–483. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Synthesis and characterization of a novel amphiphilic chitosan–polylactide graft copolymer. Carbohydr. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Han, H.Y.; Alakhov, V.Y.; Miller, D.W.; Kabanov, A.V. Effects of pluronic block copolymers on drug absorption in Caco-2 cell monolayers. Pharm. Res. 1998, 15, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Kazunori, K.; Masayuki, Y.; Teruo, O.; Yasuhisa, S. Block copolymer micelles as vehicles for drug delivery. J. Control. Release 1993, 24, 119–132. [Google Scholar] [CrossRef]
- Klotzbach, T.; Watt, M.; Ansari, Y.; Minteer, S.D. Effects of hydrophobic modification of chitosan and Nafion on transport properties, ion-exchange capacities, and enzyme immobilization. J. Membr. Sci. 2006, 282, 276–283. [Google Scholar] [CrossRef]
- Slaughter, G.; Kulkarni, T. A self-powered glucose biosensing system. Biosens. Bioelectron. 2016, 78, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Akers, N.L.; Hill, A.D.; Johnson, Z.C.; Minteer, S.D. Improving the environment for immobilized dehydrogenase enzymes by modifying Nafion with tetraalkylammonium bromides. Biomacromolecules 2004, 5, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, M.J.; Villigram, R.E.; Torrence, N.J.; Brancato, S.J.; Minteer, S.D. Effects of mixture casting Nafion® with quaternary ammonium bromide salts on the ion-exchange capacity and mass transport in the membranes. J. Membr. Sci. 2002, 205, 3–10. [Google Scholar] [CrossRef]
- Akers, N.L.; Moore, C.M.; Minteer, S.D. Development of alcohol/O2 biofuel cells using salt-extracted tetrabutylammonium bromide/Nafion membranes to immobilize dehydrogenase enzymes. Electrochim. Acta 2005, 50, 2521–2525. [Google Scholar] [CrossRef]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Skjåk-Braek, G.; Anthonsen, T.; Sandford, P.A. Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties, and Applications; Kluwer Academic Publishers: Norwell, MA, USA, 1989. [Google Scholar]
- Thomas, T.J.; Ponnusamy, K.E.; Chang, N.M.; Galmore, K.; Minteer, S.D. Effects of annealing on mixture-cast membranes of Nafion® and quaternary ammonium bromide salts. J. Membr. Sci. 2003, 213, 55–66. [Google Scholar] [CrossRef]
- Klotzbach, T.L.; Watt, M.; Ansari, Y.; Minteer, S.D. Improving the microenvironment for enzyme immobilization at electrodes by hydrophobically modifying chitosan and Nafion® polymers. J. Membr. Sci. 2008, 311, 81–88. [Google Scholar] [CrossRef]
- Willner, I.; Arad, G.; Katz, E. A biofuel cell based on pyrroloquinoline quinone and microperoxidase-11 monolayer-functionalized electrodes. Bioelectrochem. Bioenerg. 1998, 44, 209–214. [Google Scholar] [CrossRef]
- Katz, E.; Filanovsky, B.; Willner, I. A biofuel cell based on two immiscible solvents and glucose oxidase and microperoxidase-11 monolayer-functionalized electrodes. New J. Chem. 1999, 23, 481–487. [Google Scholar] [CrossRef]
- Palmore, G.T.; Kim, H.H. Electro-enzymatic reduction of dioxygen to water in the cathode compartment of a biofuel cell. J. Electroanal. Chem. 1999, 464, 110–117. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, F.; Guo, Z.; Chen, H.; Dong, S. Direct electron transfer and electrocatalysis of microperoxidase immobilized on nanohybrid film. J. Electroanal. Chem. 2005, 581, 1–10. [Google Scholar] [CrossRef]
- Potter, M.C. On the difference of potential due to the vital activity of microorganisms. Proc. Univ. Durham Phil. Soc. 1910, 3, 245–249. [Google Scholar]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Series B Contain. Pap. Biol. Character 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Allen, M.J. Chapter IX Cellular Electrophysiology. Methods Microbiol. 1972, 6, 247–283. [Google Scholar]
- Simons, E.L.; Cairns, E.J.; Surd, D.J. The performance of direct ammonia fuel cells. J. Electrochem. Soc. 1969, 116, 556–561. [Google Scholar] [CrossRef]
- Berk, R.S.; Canfield, J.H. Bioelectrochemical energy conversion. Appl. Microbiol. 1964, 12, 10–12. [Google Scholar] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.; Nießen, J.; Scholz, F. A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew. Chem. Int. Ed. 2003, 42, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.C.; Chang, I.S.; Kim, B.H.; Kim, M.; Jang, J.K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performannce of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Niessen, J.; Schröder, U.; Scholz, F. Exploiting complex carbohydrates for microbial electricity generation—A bacterial fuel cell operating on starch. Electrochem. Commun. 2004, 6, 955–958. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Kim, J.; Oh, S.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.; Buisman, C.J. Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 2006, 40, 5206–5211. [Google Scholar] [CrossRef] [PubMed]
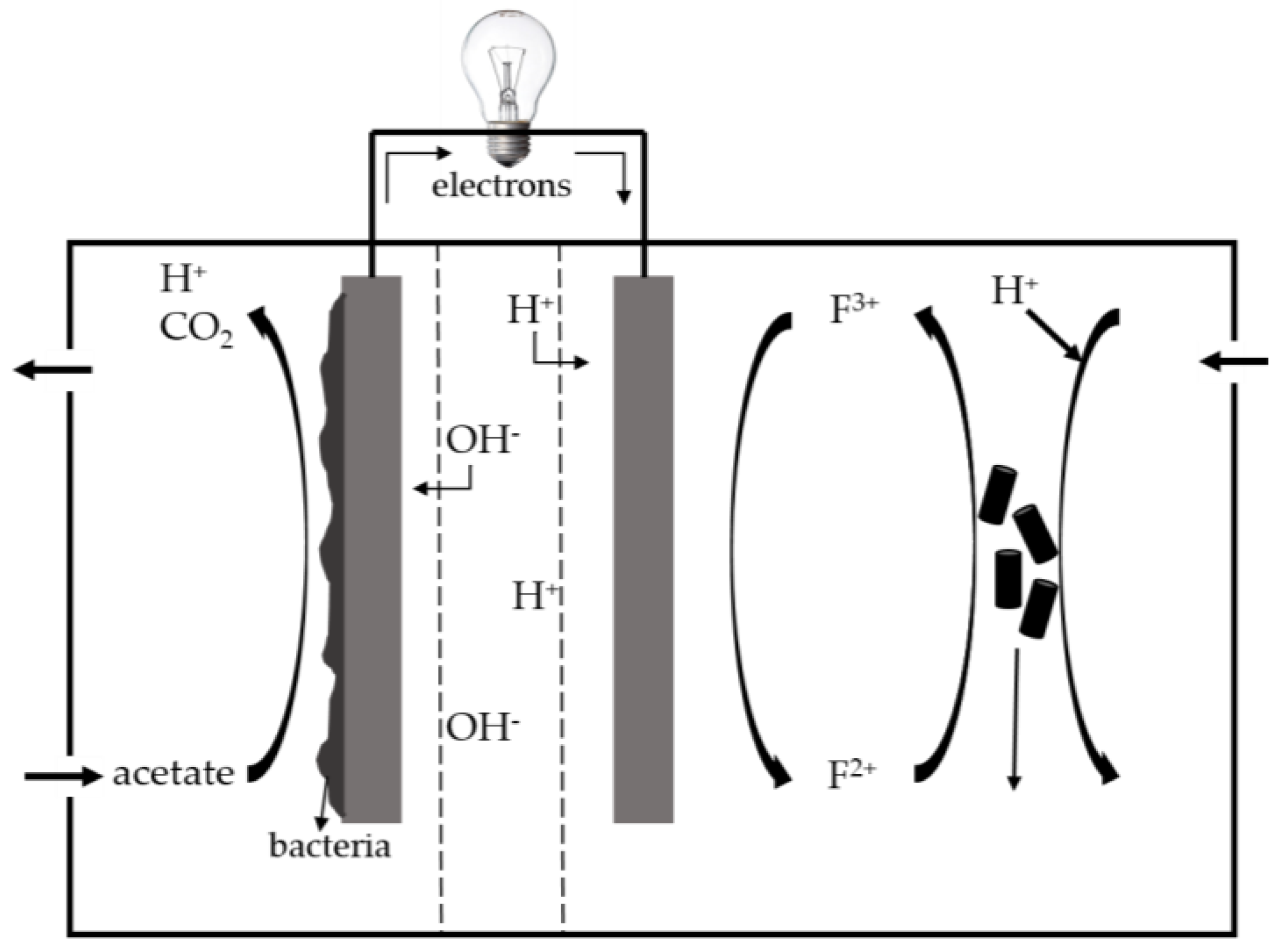
- Ter Heijne, A.; Hamelers, H.V.; De Wilde, V.; Rozendal, R.A.; Buisman, C.J. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.; Khanarian, G. Water dissociation in bipolar membranes: Experiments and theory. J. Membr. Biol. 1978, 38, 11–30. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Fernández Morales, F.J.; Kleerebezem, R.; Heijnen, J.J.; Van Loosdrecht, M.C. High-rate acidophilic ferrous iron oxidation in a biofilm airlift reactor and the role of the carrier material. Biotechnol. Bioeng. 2005, 90, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Cheng, S.; Oh, S.E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, Y.; Bi, Z.; Cao, Y. Improved performance of air-cathode single-chamber microbial fuel cell for wastewater treatment using microfiltration membranes and multiple sludge inoculation. J. Power Sources 2009, 187, 471–479. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Yaakob, Z.; Daud, W.R. New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems. Chem. Eng. J. 2012, 184, 82–89. [Google Scholar] [CrossRef]
- Lim, S.S.; Daud, W.R.; Jahim, J.M.; Ghasemi, M.; Chong, P.S.; Ismail, M. Sulfonated poly(ether ether ketone)/poly(ether sulfone) composite membranes as an alternative proton exchange membrane in microbial fuel cells. Int. J. Hydrog. Energy 2012, 37, 11409–11424. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yang, E.; Lee, M.Y.; Chae, K.J.; Kim, C.M.; Kim, I.S. Polydopamine coating effects on ultrafiltration membrane to enhance power density and mitigate biofouling of ultrafiltration microbial fuel cells (UF-MFCs). Water Res. 2014, 54, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, F.J.; de los Ríos, A.P.; Mateo-Ramírez, F.; Godínez, C.; Lozano-Blanco, L.J.; Moreno, J.I.; Tomás-Alonso, F. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 2015, 279, 115–119. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; De los Ríos, A.P.; Tomás-Alonso, F.; Gómez, D.; Rubio, M.; Víllora, G. Integrated reaction/separation processes for the kinetic resolution of rac-1-phenylethanol using supported liquid membranes based on ionic liquids. Chem. Eng. Process. Process Intensif. 2007, 46, 818–824. [Google Scholar] [CrossRef]
- Sleutels, T.H.; Ter Heijne, A.; Buisman, C.J.; Hamelers, H.V. Bioelectrochemical systems: An outlook for practical applications. ChemSusChem 2012, 5, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Comprehensive Study on Ceramic Membranes for Low-Cost Microbial Fuel Cells. ChemSusChem 2016, 9, 88–96. [Google Scholar] [CrossRef] [PubMed]
| Anode | Cathode | Membrane | Fuel | Power Output | Ref. |
|---|---|---|---|---|---|
| Ag/glucose oxidase (GOX) | Pt/peroxidase | Asymmetric acetyl cellulose | Glucose/H2O2 | – | [14] |
| Gold/galactose oxidase | Pt | Cellulose acetate and porous polycarbonate | Galactose | – | [16] |
| Graphite Felt/glucose dehydrogenase (GDH) | Simulated oxygen | d-3-hydroxybutyrate dehydrogenase (BDH) | Glucose | – | [17] |
| Au/glucose oxidase (GOX) | Au/microperoxidase | Glass frit | Glucose/H2O2 | 32 µW at 0.31 V vs. SCE or SCE | [30] |
| Au/GOX | Au/microperoxidase | H2O/CH2Cl2 interface | Glucose/cumene peroxide | 520 µW at 1 V vs. SCE | [31] |
| Graphite (formate/aldehyde/alcohol dehydrogenases soln.) | Pt | Nafion | MeOH/O2 | 670 µW·cm−2 at 0.49 V vs. SCE | [9] |
| Pt | C or Pt with laccase in solution | Nafion | H2/O2 | 42 µW·cm−2 at 0.61 V vs. SCE | [32] |
| Porous C/C nanotube/GOX | Porous C/C nanotube/laccase | Nafion | Glucose/O2 | 99.8 µW·cm−2 | [2,33] |
| Carbon felt/Nafion NBu4+ salt alcohol + aldehyde dehydrogenase | Pt/C | Tetrabutylammonium bromide/Nafion | MeOH/O2, EtOH/O2 | 1550 µW·cm−2, 2040 µW·cm−2 | [25] |
| Carbon/GDH | ELAT® (Woven carbon cloth gas diffusion layer with a carbon microporous layer) | Butyl-Chitosan | Glucose/NAD+ | 35 µW·cm−2, 0.699 v open circuit potential | [29] |
| Carbon/GDH | ELAT® | Octyl-Chitosan | Glucose/NAD+ | 17 µW·cm−2, 0.628 v open circuit potential | [29] |
| Membrane | COD Removal (%) | Coulombic Efficiency (%) | Maximum Power (mW/m2) |
|---|---|---|---|
| Nafion® | 90.7 | 4.44 | 157.9 |
| Ultrex® | 88.3 | 2.50 | 102.2 |
| [MTOA+][Cl−] | 89.1 | 2.06 | 103.9 |
| [omim+][NTf2−] | 81.3 | 2.74 | 72.1 |
| [omim+][BF4−] | 80.3 | 1.31 | 147.1 |
| [omim+][PF6−] | 27.3 | 18.60 | 215.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghassemi, Z.; Slaughter, G. Biological Fuel Cells and Membranes. Membranes 2017, 7, 3. https://doi.org/10.3390/membranes7010003
Ghassemi Z, Slaughter G. Biological Fuel Cells and Membranes. Membranes. 2017; 7(1):3. https://doi.org/10.3390/membranes7010003
Chicago/Turabian StyleGhassemi, Zahra, and Gymama Slaughter. 2017. "Biological Fuel Cells and Membranes" Membranes 7, no. 1: 3. https://doi.org/10.3390/membranes7010003
APA StyleGhassemi, Z., & Slaughter, G. (2017). Biological Fuel Cells and Membranes. Membranes, 7(1), 3. https://doi.org/10.3390/membranes7010003







