Membranes with Surface-Enhanced Antifouling Properties for Water Purification
Abstract
:1. Introduction
1.1. Membrane Technology
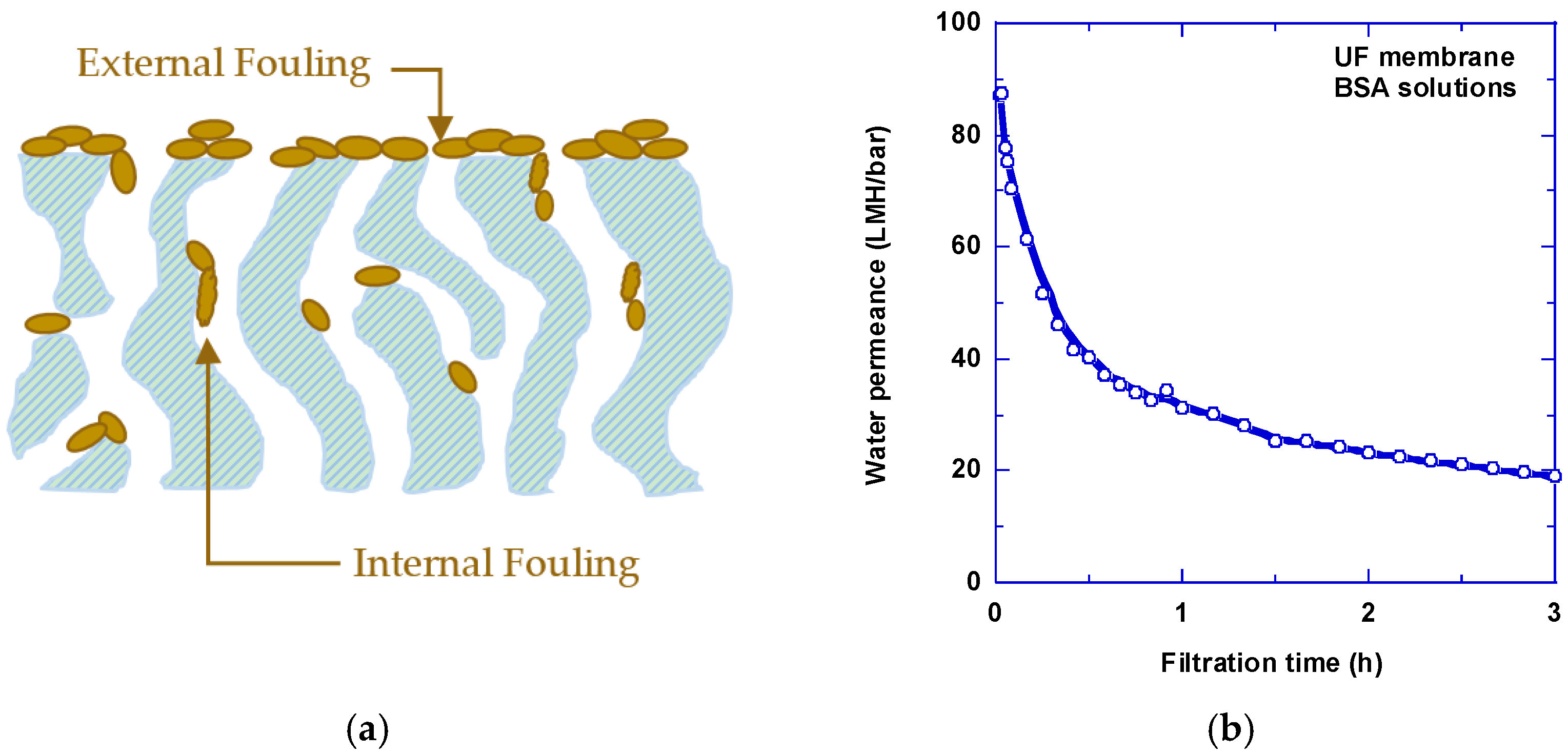
1.2. Membrane Fouling
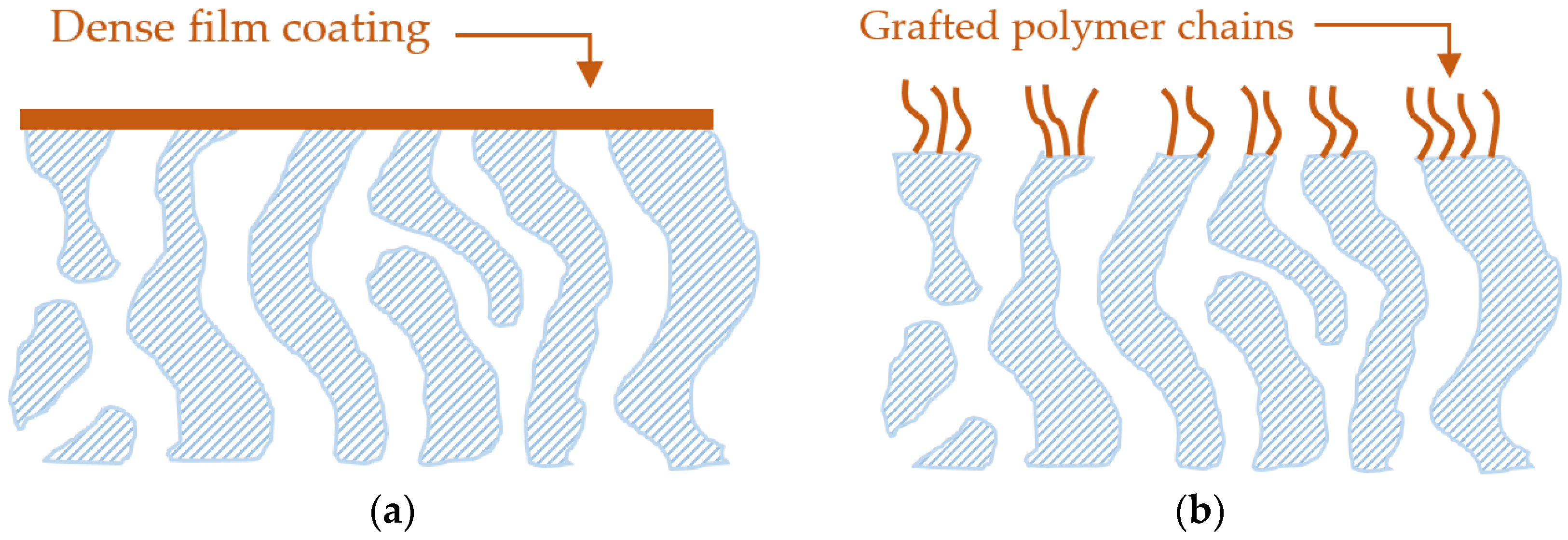
1.3. Surface Modification to Enhance Antifouling Properties
1.4. Outline of This Review
2. Membrane Surface Modification Using Hydrophilic Materials
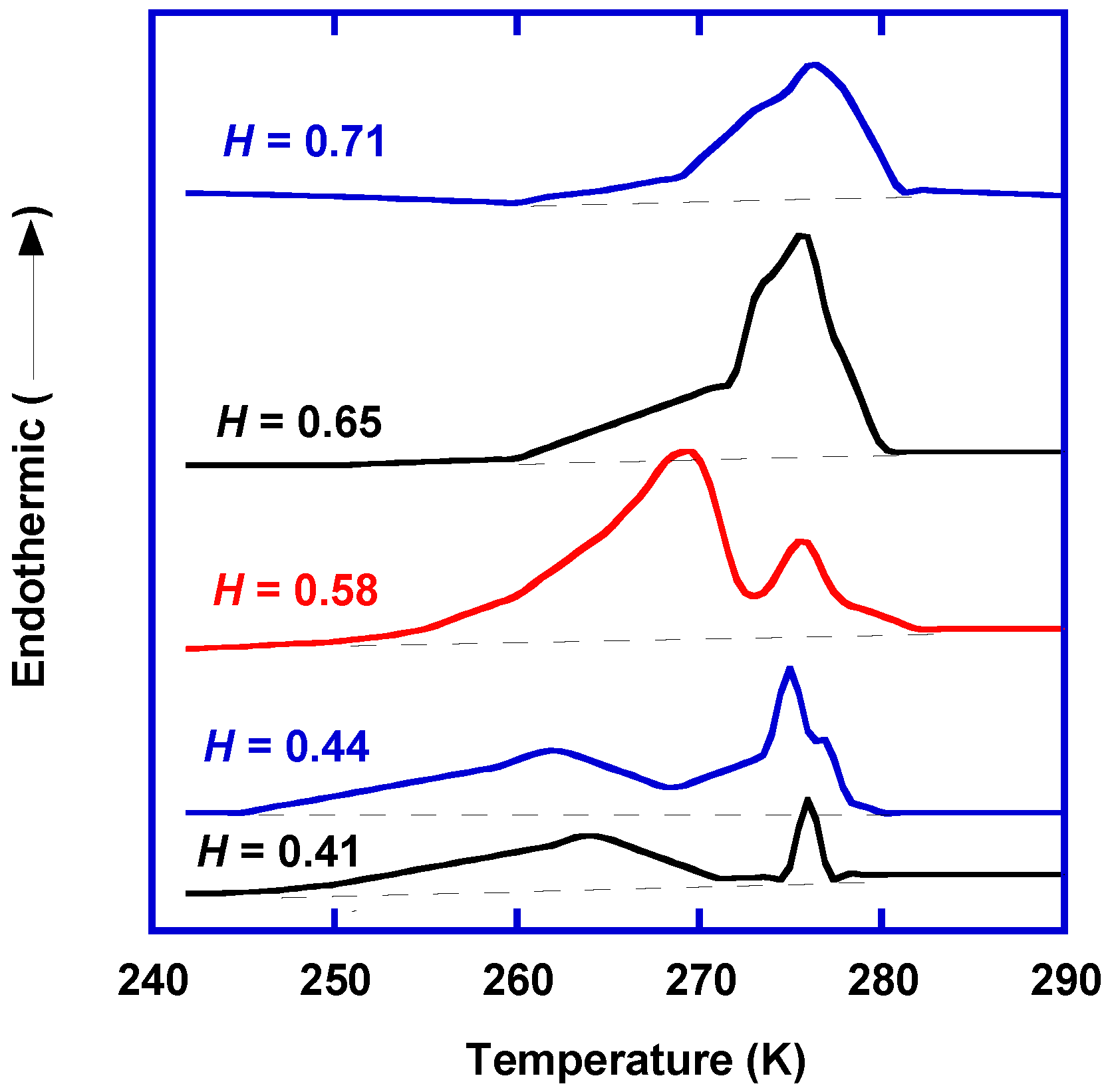
2.1. States of Water in Polymers
- (1)
- Free water does not interact with polymers via hydrogen bonding or van der Waals interactions, and therefore, it exhibits the same melting temperature as bulk water.
- (2)
- Freezable bound water forms due to weak interaction with polymers and/or capillary condensation in polymers, and therefore, its melting temperature is below 0 °C.
- (3)
- Nonfreezing water strongly interacts with hydrophilic sites of polymeric chains via hydrogen bonding, and thus, it does not crystallize below 0 °C.
2.2. Poly(ethylene glycol)-Based Coatings
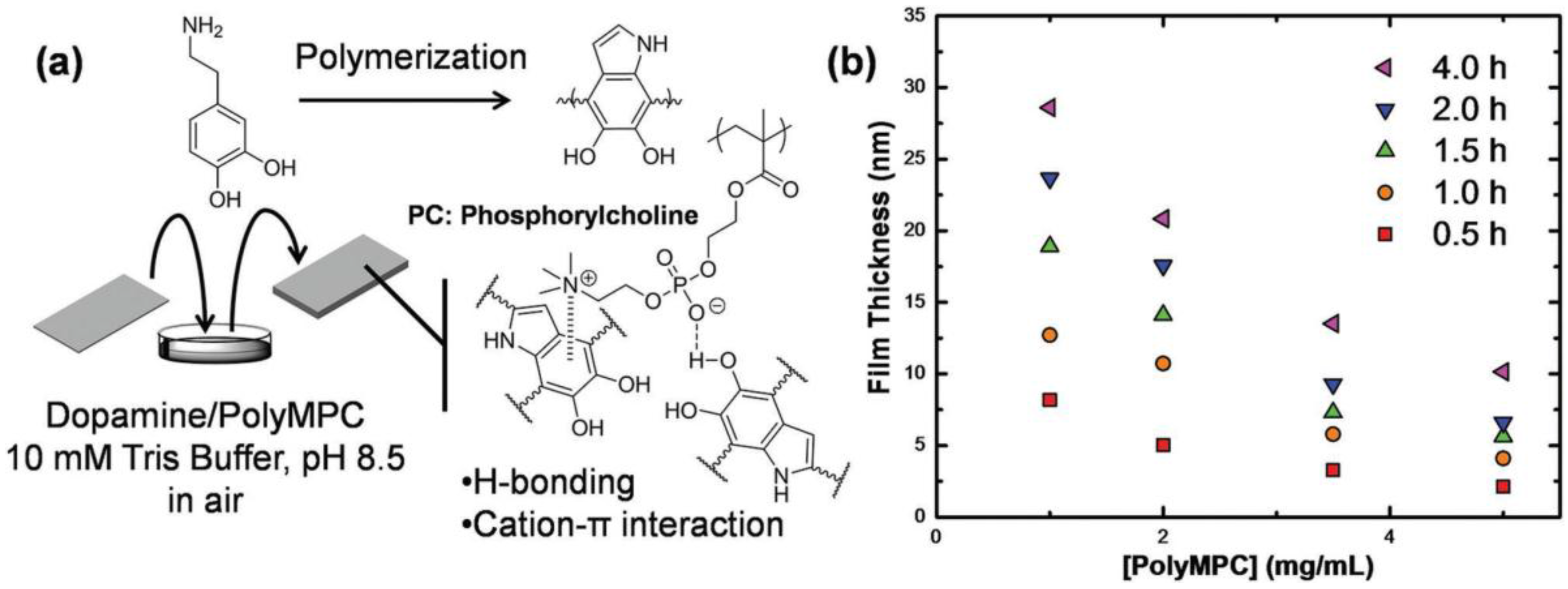
2.3. Polydopamine
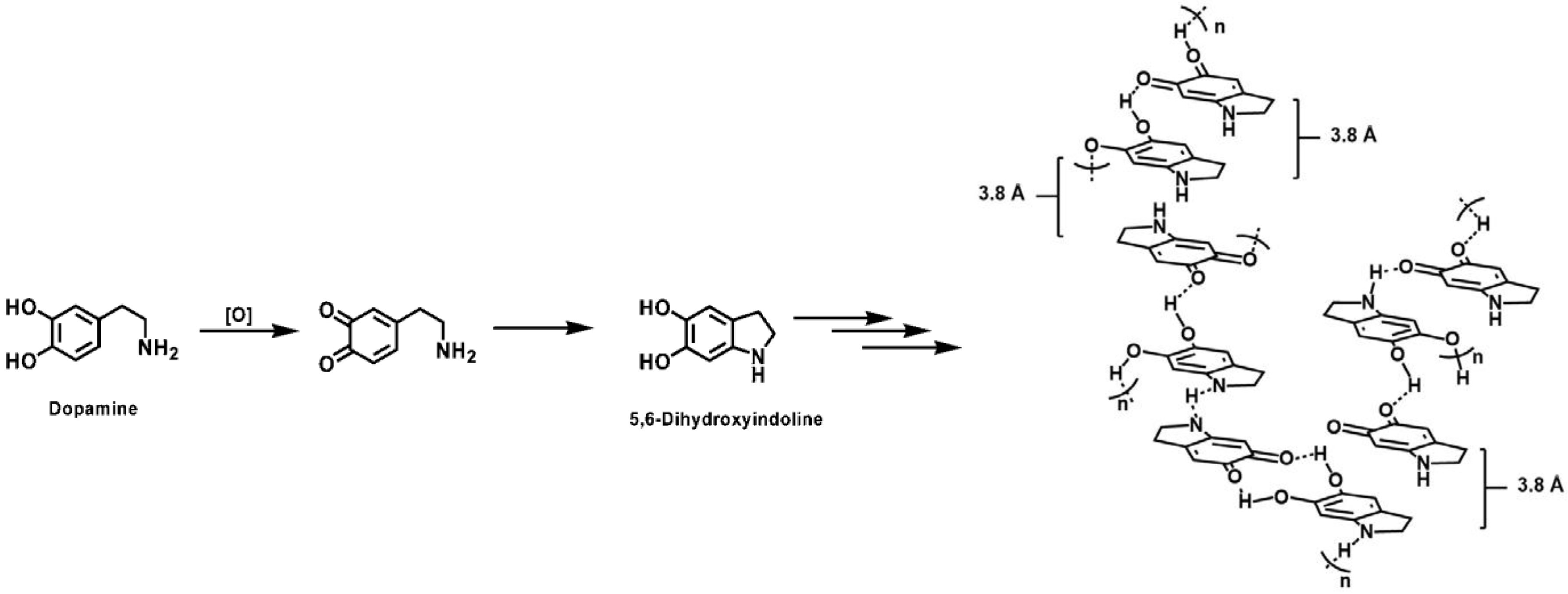
2.3.1. PDA Structure
2.3.2. PDA Coating on Membrane Surface for Water Purification
2.3.3. PDA as a Bio-Glue to Coat the Second Layer on Membranes
2.3.4. Dopamine-Like Materials
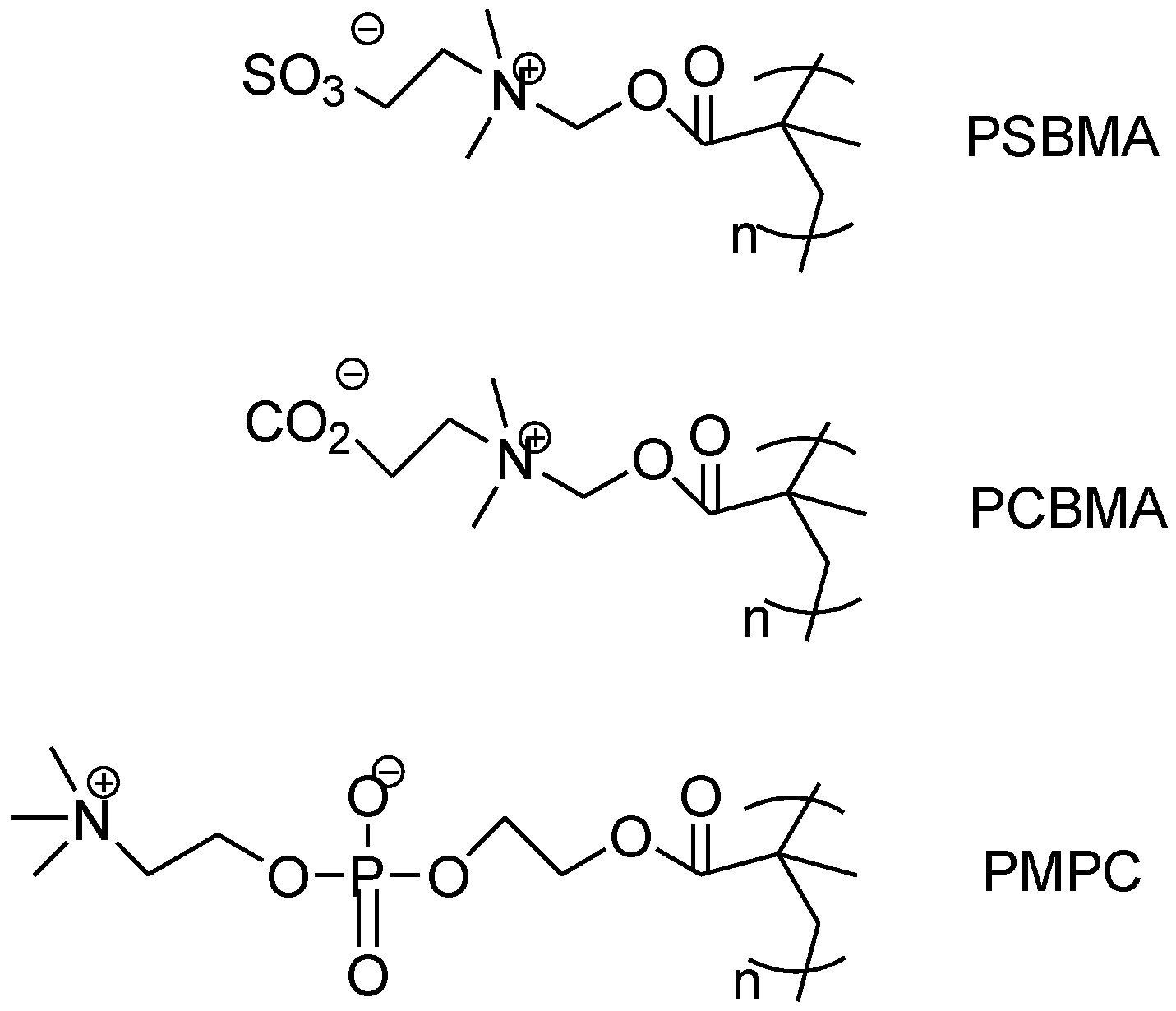
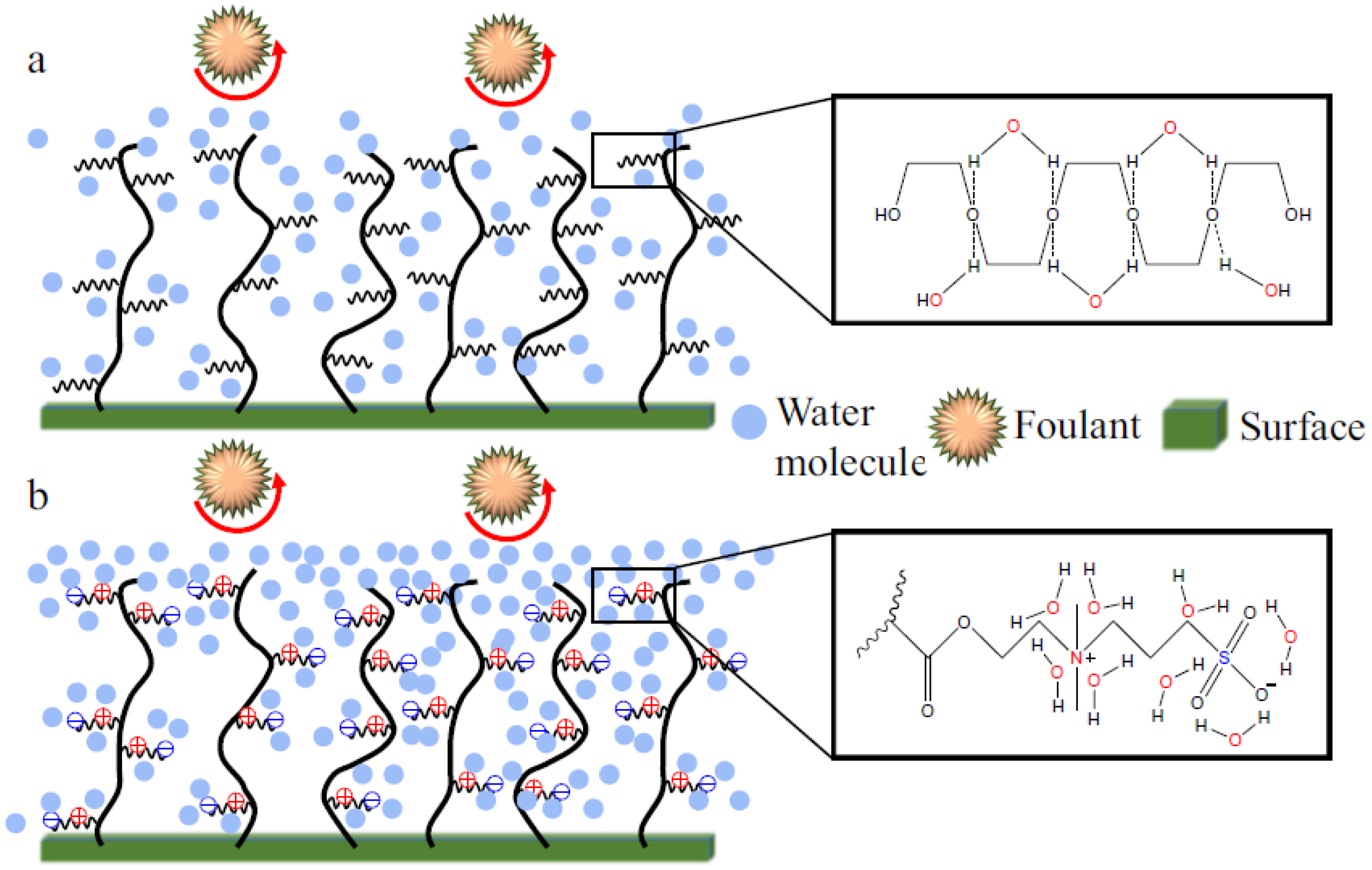
2.4. Zwitterionic Materials
2.4.1. Superhydrophilicity in Zwitterionic Materials
2.4.2. Surface Coating Using Dense Zwitterions
2.4.3. Surface Grafting of Zwitterions
3. Membrane Surface Modification Using Hydrophobic or Amphiphilic Materials
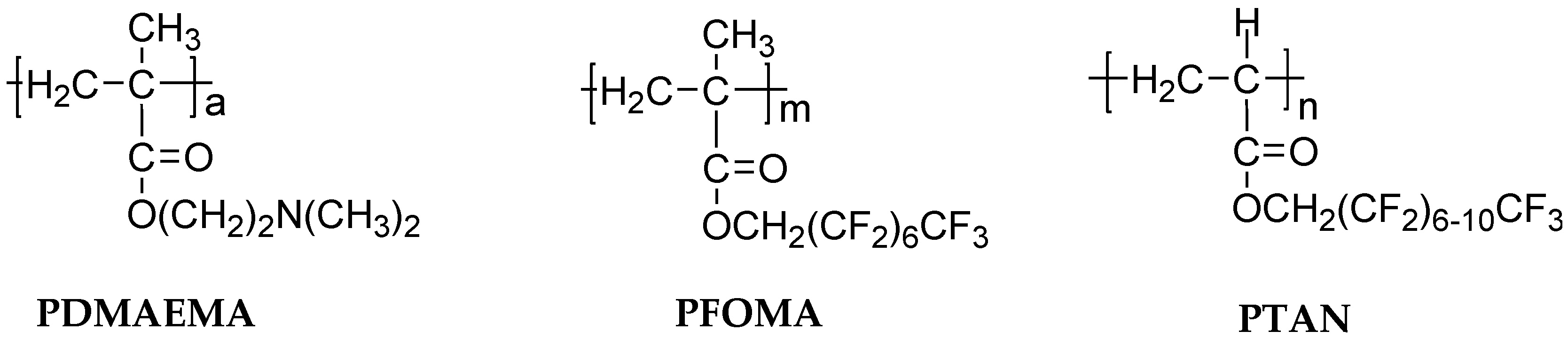
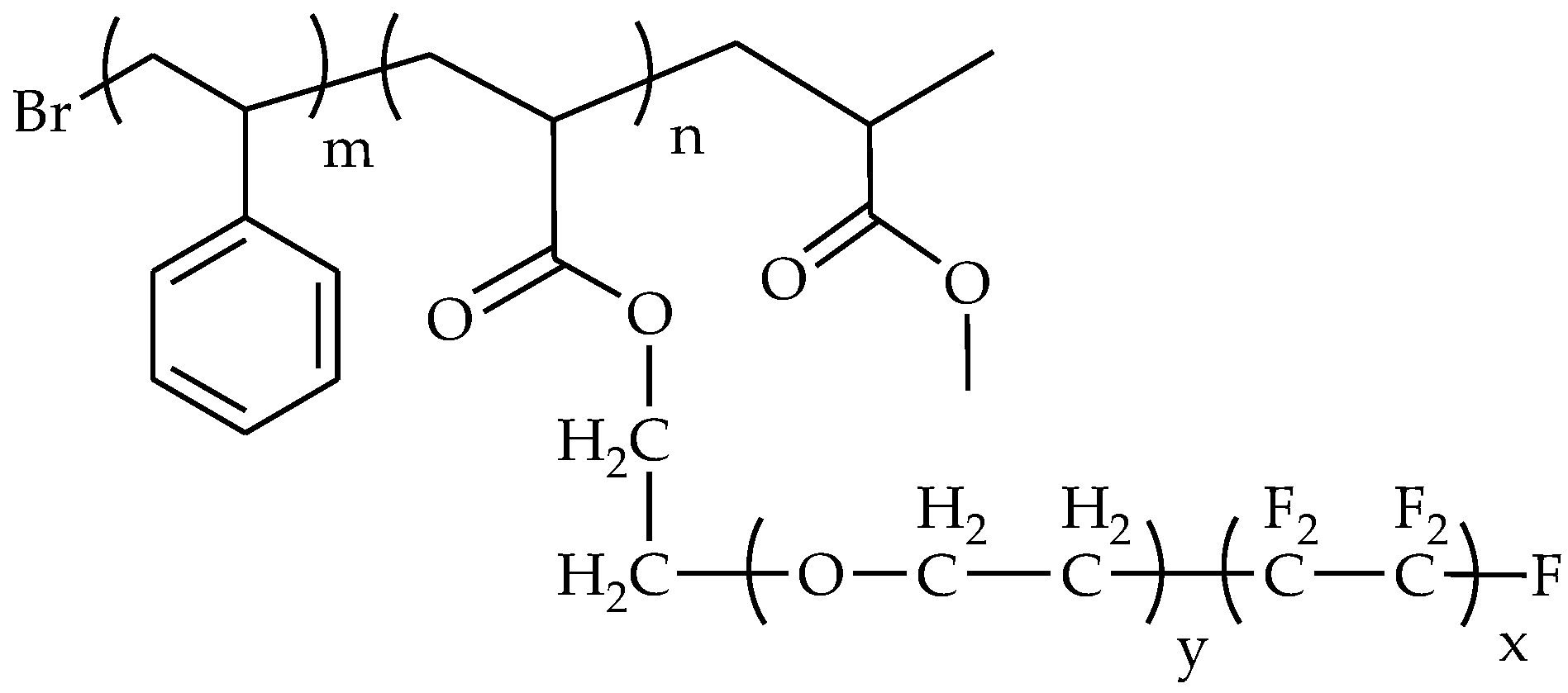
3.1. Fluoropolymers
3.2. Amphiphilic Polymers
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Miller, D.; Dreyer, D.; Bielawski, C.; Paul, D.; Freeman, B. Surface modification of water purification membranes: A review. Angew. Chem. Int. Ed. 2017, in press. [Google Scholar]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Geise, G.M.; Lee, H.-S.; Miller, D.J.; Freeman, B.D.; McGrath, J.E.; Paul, D.R. Water purification by membranes: The role of polymer science. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1685–1718. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Shahkaramipour, N.; Ramanan, S.N.; Fister, D.; Park, E.; Venna, S.R.; Sun, H.; Cheng, C.; Lin, H. Facile grafting of zwitterions onto membrane surface to enhance antifouling properties for wastewater reuse. ACS Appl. Mater. Interf. 2017, submitted. [Google Scholar]
- She, Q.H.; Wang, R.; Fane, A.G.; Tang, C.Y.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Vladkova, T. Surface engineering for non-toxic biofouling control. J. Univ. Chem. Technol. Metall. 2007, 42, 239–256. [Google Scholar]
- Li, Q.; Xu, Z.; Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 2007, 290, 173–181. [Google Scholar] [CrossRef]
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128. [Google Scholar] [CrossRef]
- Maruf, S.H.; Rickman, M.; Wang, L.; Mersch, J.; Greenberg, A.R.; Pellegrino, J.; Ding, Y.F. Influence of sub-micron surface patterns on the deposition of model proteins during active filtration. J. Membr. Sci. 2013, 444, 420–428. [Google Scholar] [CrossRef]
- Maruf, S.H.; Wang, L.; Greenberg, A.R.; Pellegrino, J.; Ding, Y.F. Use of nanoimprinted surface patterns to mitigate colloidal deposition on ultrafiltration membranes. J. Membr. Sci. 2013, 428, 598–607. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Mahon, H.I.; Cotton, O. Permeation of water and sodium chloride through cellulose acetate. J. Appl. Polym. Sci. 1967, 11, 2041–2065. [Google Scholar] [CrossRef]
- Higuchi, A.; Iijima, T. Dsc investigation of the states of water in poly(vinyl-alcohol) membranes. Polymer 1985, 26, 1207–1211. [Google Scholar] [CrossRef]
- Hirata, Y.; Miura, Y.; Nakagawa, T. Oxygen permeability and the state of water in nafion((r)) membranes with alkali metal and amino sugar counterions. J. Membr. Sci. 1999, 163, 357–366. [Google Scholar] [CrossRef]
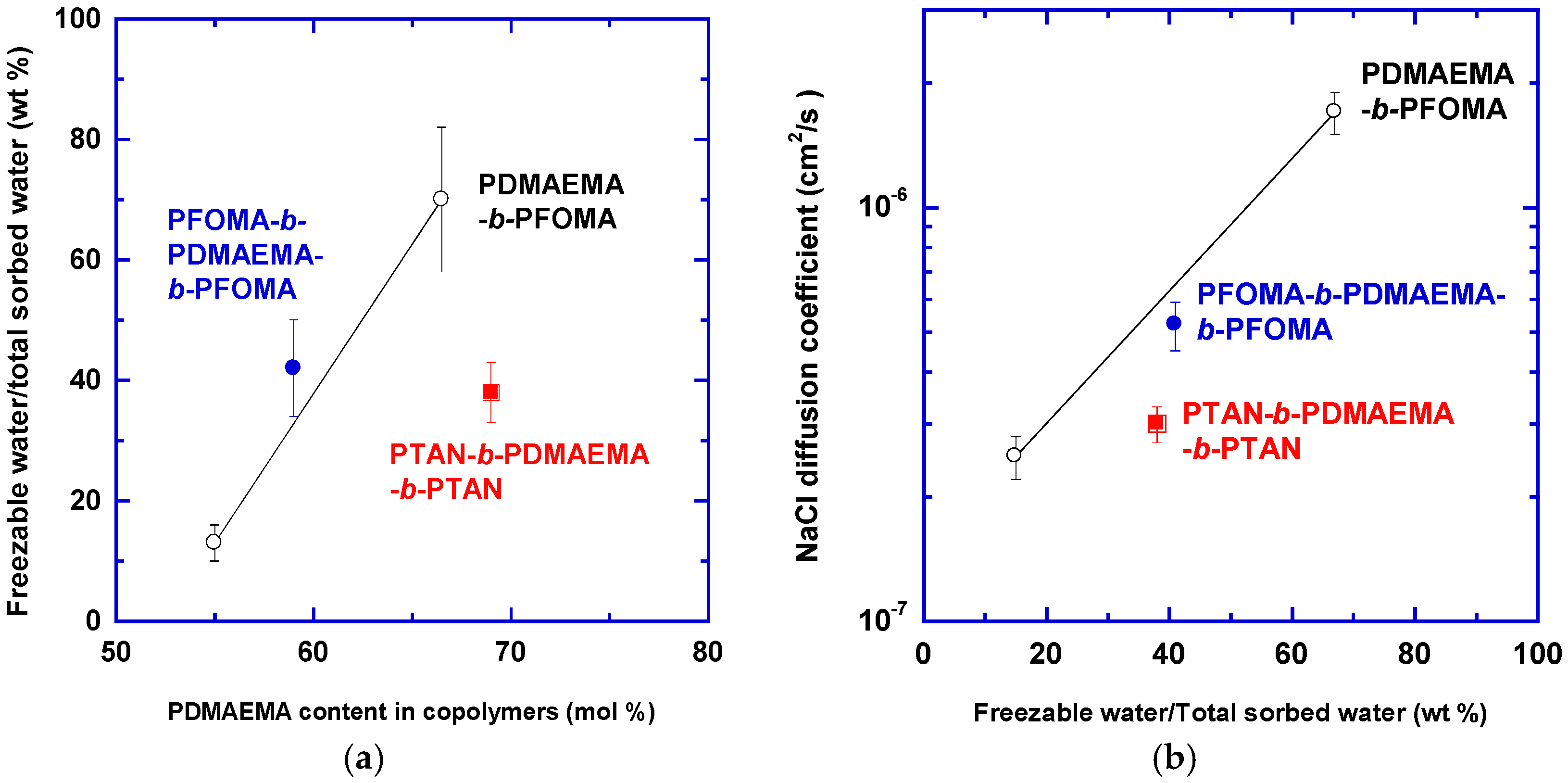
- Nagai, K.; Tanaka, S.; Hirata, Y.; Nakagawa, T.; Arnold, M.E.; Freeman, B.D.; Leroux, D.; Betts, D.E.; DeSimone, J.M.; DiGiano, F.A. Solubility and diffusivity of sodium chloride in phase-separated block copolymers of poly(2-dimethylaminoethyl methacrylate), poly (1,1′-dihydroperfluorooctyl methacrylate) and poly(1,1,2,2-tetrahydroperfluorooctyl acrylate). Polymer 2001, 42, 9941–9948. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Relationship between hydrogen-bonding and bound water in polyhydroxystyrene derivatives. Polymer 1983, 24, 871–876. [Google Scholar] [CrossRef]
- Jeon, S.I.; Lee, J.H.; Andrade, J.D.; de Gennes, P.G. Protein—Surface interactions in the presence of polyethylene oxide. J. Colloid Interf. Sci. 1991, 142, 149–158. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, K.; Lin, H. Impregnated membranes for water purification using forward osmosis. Ind. Eng. Chem. Res. 2015, 54, 12354–12366. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Ciobanu, I.; Ishida, K.P.; Ng, A.; Reinhard, M. Effects of polyether–polyamide block copolymer coating on performance and fouling of reverse osmosis membranes. J. Membr. Sci. 2006, 280, 762–770. [Google Scholar] [CrossRef]
- Louie, J.S.; Pinnau, I.; Reinhard, M. Effects of surface coating process conditions on the water permeation and salt rejection properties of composite polyamide reverse osmosis membranes. J. Membr. Sci. 2011, 367, 249–255. [Google Scholar] [CrossRef]
- Ju, H.; McCloskey, B.D.; Sagle, A.C.; Wu, Y.H.; Kusuma, V.A.; Freeman, B.D. Crosslinked poly(ethylene oxide) fouling resistant coating materials for oil/water separation. J. Membr. Sci. 2008, 307, 260–267. [Google Scholar] [CrossRef]
- Ju, H.; McCloskey, B.D.; Sagle, A.C.; Kusuma, V.A.; Freeman, B.D. Preparation and characterization of crosslinked poly(ethylene glycol) diacrylate hydrogels as fouling-resistant membrane coating materials. J. Membr. Sci. 2009, 330, 180–188. [Google Scholar] [CrossRef]
- Sagle, A.C.; van Wagner, E.M.; Ju, H.; McCloskey, B.D.; Freeman, B.D.; Sharma, M.M. Peg-coated reverse osmosis membranes: Desalination properties and fouling resistance. J. Membr. Sci. 2009, 340, 92–108. [Google Scholar] [CrossRef]
- Nunes, S.P.; Sforça, M.L.; Peinemann, K.-V. Dense hydrophilic composite membranes for ultrafiltration. J. Membr. Sci. 1995, 106, 49–56. [Google Scholar] [CrossRef]
- Lin, H.; Kai, T.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. The effect of cross-linking on gas permeability in cross-linked poly(ethylene glycol diacrylate). Macromolecules 2005, 38, 8381–8393. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Wei, T.; Chang, Y. Surface-initiated atom transfer radical polymerization from porous poly(tetrafluoroethylene) membranes using the C–F groups as initiators. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2076–2083. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Freeman, B.D. A bioinspired fouling-resistant surface modification for water purification membranes. J. Membr. Sci. 2012, 413, 82–90. [Google Scholar] [CrossRef]
- Waite, J.H.; Qin, X. Polyphosphoprotein from the adhesive pads of mytilus edulis. Biochemistry 2001, 40, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Ai, K.L.; Lu, L.H. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Perspectives on poly(dopamine). Chem. Sci. 2013, 4, 3796–3802. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- D’ischia, M.; Napolitano, A.; Pezzella, A. 5,6-dihydroxyindole chemistry: Unexplored opportunities beyond eumelanin. Eur. J. Org. Chem. 2011, 2011, 5501–5516. [Google Scholar] [CrossRef]
- Pezzella, A.; Iadonisi, A.; Valerio, S.; Panzella, L.; Napolitano, A.; Adinolfi, M.; d’Ischia, M. Disentangling eumelanin “black chromophore”: Visible absorption changes as signatures of oxidation state-and aggregation-dependent dynamic interactions in a model water-soluble 5,6-dihydroxyindole polymer. J. Am. Chem. Soc. 2009, 131, 15270–15275. [Google Scholar] [CrossRef] [PubMed]
- Kasemset, S.; Lee, A.; Miller, D.J.; Freeman, B.D.; Sharma, M.M. Effect of polydopamine deposition conditions on fouling resistance, physical properties, and permeation properties of reverse osmosis membranes in oil/water separation. J. Membr. Sci. 2013, 425, 208–216. [Google Scholar] [CrossRef]
- Miller, D.J.; Araújo, P.A.; Correia, P.B.; Ramsey, M.M.; Kruithof, J.C.; van Loosdrecht, M.C.; Freeman, B.D.; Paul, D.R.; Whiteley, M.; Vrouwenvelder, J.S. Short-term adhesion and long-term biofouling testing of polydopamine and poly (ethylene glycol) surface modifications of membranes and feed spacers for biofouling control. Water Res. 2012, 46, 3737–3753. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Huang, X.; Li, H.; Kasemset, S.; Lee, A.; Agnihotri, D.; Hayes, T.; Paul, D.R.; Freeman, B.D. Fouling-resistant membranes for the treatment of flowback water from hydraulic shale fracturing: A pilot study. J. Membr. Sci. 2013, 437, 265–275. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.-J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interf. 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Jia, H.; Qiao, S.; Jiang, Z.; Wang, J.; Wang, B.; Zhong, Y. Bioinspired fabrication of high performance composite membranes with ultrathin defect-free skin layer. J. Membr. Sci. 2009, 341, 279–285. [Google Scholar] [CrossRef]
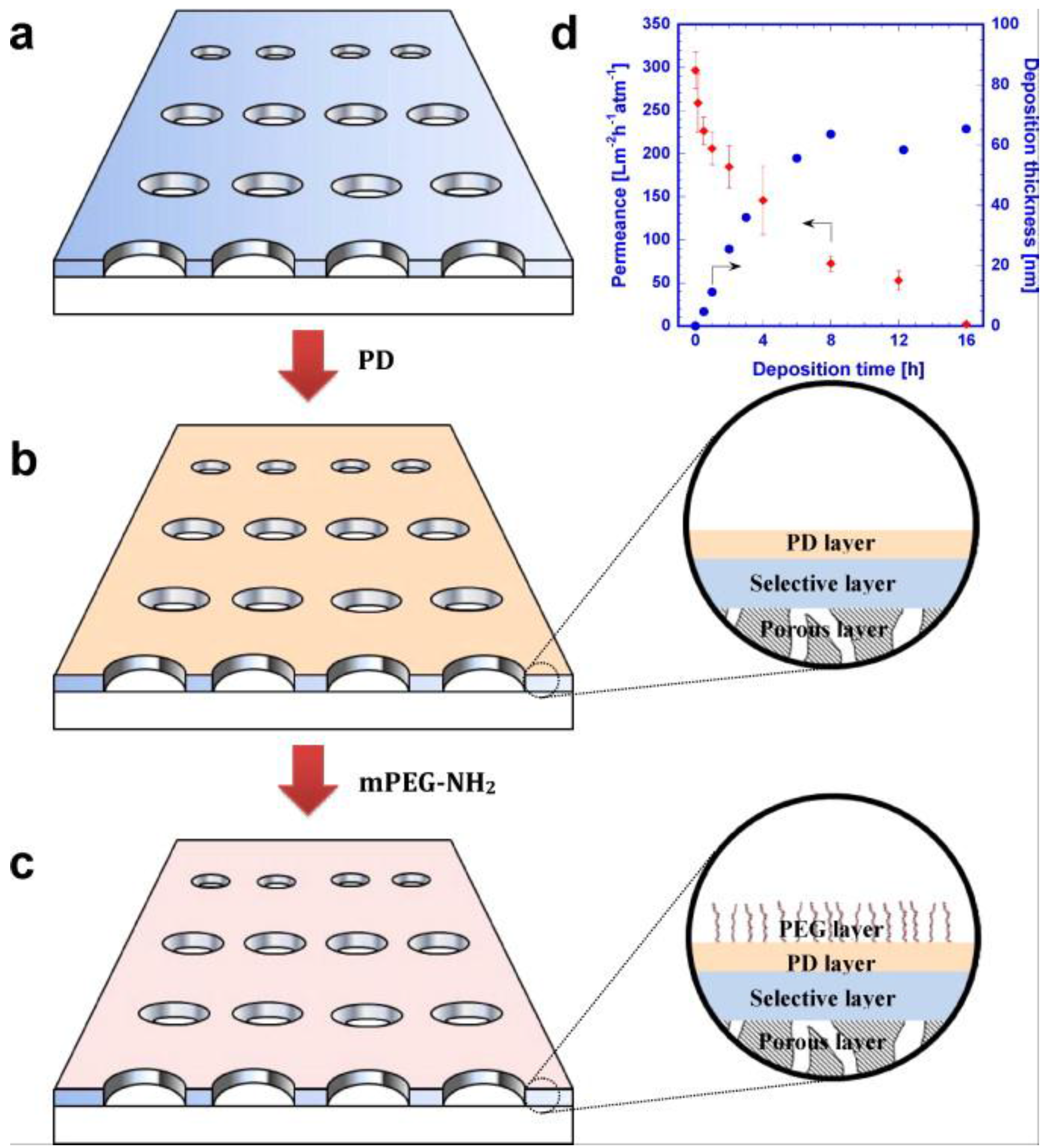
- Miller, D.J.; Paul, D.R.; Freeman, B.D. An improved method for surface modification of porous water purification membranes. Polymer 2014, 55, 1375–1383. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Wang, L.; Paul, D.R.; Freeman, B.D. Constant flux crossflow filtration evaluation of surface-modified fouling-resistant membranes. J. Membr. Sci. 2014, 452, 171–183. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Ostaszewski, B.L.; Weihofen, A.; Schlossmacher, M.G.; Selkoe, D.J. Dopamine covalently modifies and functionally inactivates parkin. Nat. Med. 2005, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Bellona, C.; Drewes, J.E. Fouling of nanofiltration and reverse osmosis membranes during municipal wastewater reclamation: Membrane autopsy results from pilot-scale investigations. J. Membr. Sci. 2010, 353, 111–121. [Google Scholar] [CrossRef]
- Zhang, R.; Su, Y.; Zhao, X.; Li, Y.; Zhao, J.; Jiang, Z. A novel positively charged composite nanofiltration membrane prepared by bio-inspired adhesion of polydopamine and surface grafting of poly (ethylene imine). J. Membr. Sci. 2014, 470, 9–17. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Braeken, L.; Luis, P.; Wang, X.-L.; Van der Bruggen, B. Novel binding procedure of TiO2 nanoparticles to thin film composite membranes via self-polymerized polydopamine. J. Membr. Sci. 2013, 437, 179–188. [Google Scholar] [CrossRef]
- Anderson, T.H.; Yu, J.; Estrada, A.; Hammer, M.U.; Waite, J.H.; Israelachvili, J.N. The contribution of dopa to substrate–peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv. Funct. Mater. 2010, 20, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
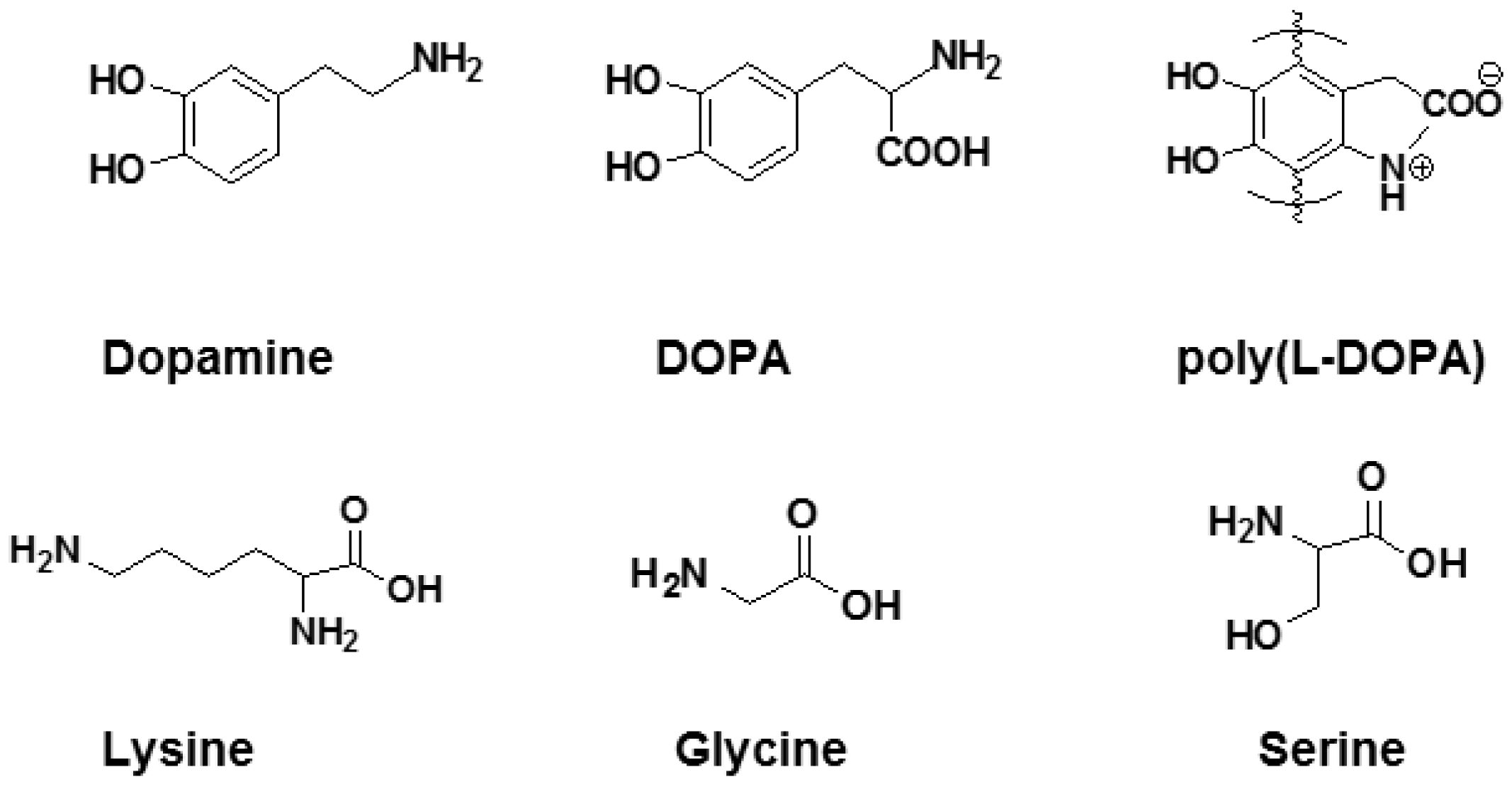
- Dalsin, J.L.; Lin, L.; Tosatti, S.; Vörös, J.; Textor, M.; Messersmith, P.B. Protein resistance of titanium oxide surfaces modified by biologically inspired mpeg-dopa. Langmuir 2005, 21, 640–646. [Google Scholar] [CrossRef]
- Azari, S.; Zou, L. Using zwitterionic amino acid l-DOPA to modify the surface of thin film composite polyamide reverse osmosis membranes to increase their fouling resistance. J. Membr. Sci. 2012, 401, 68–75. [Google Scholar] [CrossRef]
- Shi, Q.; Su, Y.; Chen, W.; Peng, J.; Nie, L.; Zhang, L.; Jiang, Z. Grafting short-chain amino acids onto membrane surfaces to resist protein fouling. J. Membr. Sci. 2011, 366, 398–404. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Chen, S.; Zhang, Z.; Jiang, S. Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir 2006, 22, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Liu, J.; Ng, S.; Luo, S.; Guo, R.; Cheng, C.; Lin, H. Transport properties of small molecules in zwitterionic polymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1924–1934. [Google Scholar] [CrossRef]
- Wu, J.; Lin, W.; Wang, Z.; Chen, S.; Chang, Y. Investigation of the hydration of nonfouling material poly (sulfobetaine methacrylate) by low-field nuclear magnetic resonance. Langmuir 2012, 28, 7436–7441. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Ramanan, S.; Lin, H. Synthesis of hydrogels with antifouling properties as membranes for water purification. J. Vis. Exp. 2017, in press. [Google Scholar]
- Ni, L.; Meng, J.Q.; Geise, G.M.; Zhang, Y.F.; Zhou, J. Water and salt transport properties of zwitterionic polymers film. J. Membr. Sci. 2015, 491, 73–81. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Jung, Y.; Han, S.; Tak, T.; Kwon, Y.-N. Surface modification of SWRO membranes using hydroxyl poly (oxyethylene) methacrylate and zwitterionic carboxylated polyethyleneimine. J. Membr. Sci. 2015, 486, 97–105. [Google Scholar] [CrossRef]
- Bengani, P.; Kou, Y.M.; Asatekin, A. Zwitterionic copolymer self-assembly for fouling resistant, high flux membranes with size-based small molecule selectivity. J. Membr. Sci. 2015, 493, 755–765. [Google Scholar] [CrossRef]
- Yang, R.; Xu, J.; Ozaydin-Ince, G.; Wong, S.Y.; Gleason, K.K. Surface-tethered zwitterionic ultrathin antifouling coatings on reverse osmosis membranes by initiated chemical vapor deposition. Chem. Mater. 2011, 23, 1263–1272. [Google Scholar] [CrossRef]
- Yang, R.; Gleason, K.K. Ultrathin antifouling coatings with stable surface zwitterionic functionality by initiated chemical vapor deposition (ICVD). Langmuir 2012, 28, 12266–12274. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Moni, P.; Gleason, K.K. Ultrathin zwitterionic coatings for roughness-independent underwater superoleophobicity and gravity-driven oil–water separation. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Razi, F.; Sawada, I.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. The improvement of antibiofouling efficiency of polyethersulfone membrane by functionalization with zwitterionic monomers. J. Membr. Sci. 2012, 401, 292–299. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, Q.; Luan, S.; Song, L.; Yang, H.; Shi, H.; Jin, J.; Li, X.; Yin, J.; Stagnaro, P. Improved biocompatibility and antifouling property of polypropylene non-woven fabric membrane by surface grafting zwitterionic polymer. J. Membr. Sci. 2011, 369, 5–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Chao, T.; Chen, S.; Jiang, S. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, X.; Li, P.; Li, L.; Shen, J. Surface-initiated raft polymerization of sulfobetaine from cellulose membranes to improve hemocompatibility and antibiofouling property. Polym. Chem. 2013, 4, 5074–5085. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Enhanced antibiofouling of ro membranes via polydopamine coating and polyzwitterion immobilization. Desalination 2014, 337, 23–30. [Google Scholar] [CrossRef]
- Chang, C.C.; Kolewe, K.W.; Li, Y.; Kosif, I.; Freeman, B.D.; Carter, K.R.; Schiffman, J.D.; Emrick, T. Underwater superoleophobic surfaces prepared from polymer zwitterion/dopamine composite coatings. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cation—π interaction: Its role and relevance in chemistry, biology, and material science. Chem. Rev. 2012, 113, 2100–2138. [Google Scholar] [CrossRef] [PubMed]
- Sin, M.-C.; Chen, S.-H.; Chang, Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 2014, 46, 436–443. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, Y.; Higuchi, A.; Shih, Y.-J.; Li, P.-T.; Chen, W.-Y.; Tsai, E.-M.; Hsiue, G.-H. Bioadhesive control of plasma proteins and blood cells from umbilical cord blood onto the interface grafted with zwitterionic polymer brushes. Langmuir 2012, 28, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Imbrogno, J.; Belfort, G.; Wang, X.L. Making polymeric membranes antifouling via “grafting from” polymerization of zwitterions. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, H.; Kilduff, J.E.; Langer, R.; Anderson, D.G.; Belfort, G. High-throughput membrane surface modification to control nom fouling. Environ. Sci. Technol. 2009, 43, 3865–3871. [Google Scholar] [CrossRef] [PubMed]
- Callow, M.E.; Fletcher, R.L. The influence of low surface energy materials on bioadhesion—A review. Int. Biodeterior. Biodegrad. 1994, 34, 333–348. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Y.; Wang, C.; Wang, S.; Müller-Steinhagen, H. Effect of surface free energy on the adhesion of biofouling and crystalline fouling. Chem. Eng. Sci. 2005, 60, 4858–4865. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling release coatings: A nontoxic alternative to biocidal antifouling coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef] [PubMed]
- Tsibouklis, J.; Stone, M.; Thorpe, A.A.; Graham, P.; Nevell, T.G.; Ewen, R.J. Inhibiting bacterial adhesion onto surfaces: The non-stick coating approach. Int. J. Adhes. Adhes. 2000, 20, 91–96. [Google Scholar] [CrossRef]
- Gao, J.; Yan, D.; Ni, H.; Wang, L.; Yang, Y.; Wang, X. Protein-resistance performance enhanced by formation of highly-ordered perfluorinated alkyls on fluorinated polymer surfaces. J. Colloid Interf. Sci. 2013, 393, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, J.C.; Rolland, J.P.; DeSimone, J.M.; Callow, M.E.; Finlay, J.A.; Callow, J.A. Contact angle analysis, surface dynamics, and biofouling characteristics of cross-linkable, random perfluoropolyether-based graft terpolymers. Macromolecules 2006, 39, 2521–2528. [Google Scholar] [CrossRef]
- Hu, Z.; Finlay, J.A.; Chen, L.; Betts, D.E.; Hillmyer, M.A.; Callow, M.E.; Callow, J.A.; DeSimone, J.M. Photochemically cross-linked perfluoropolyether-based elastomers: Synthesis, physical characterization, and biofouling evaluation. Macromolecules 2009, 42, 6999–7007. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Zhao, X.; Zhang, R.; Zhao, J.; Fan, X.; Jiang, Z. Surface fluorination of polyamide nanofiltration membrane for enhanced antifouling property. J. Membr. Sci. 2014, 455, 15–23. [Google Scholar] [CrossRef]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The antifouling and fouling-release perfomance of hyperbranched fluoropolymer (HBFP)-poly(ethylene glycol) (PEG) composite coatings evaluated by adsorption of biomacromolecules and the green fouling alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin-Ince, G.; Matin, A.; Khan, Z.; Zaidi, S.M.J.; Gleason, K.K. Surface modification of reverse osmosis desalination membranes by thin-film coatings deposited by initiated chemical vapor deposition. Thin Solid Films 2013, 539, 181–187. [Google Scholar] [CrossRef]
- Baxamusa, S.H.; Gleason, K.K. Random copolymer films with molecular-scale compositional heterogeneities that interfere with protein adsorption. Adv. Funct. Mater. 2009, 19, 3489–3496. [Google Scholar] [CrossRef]
- Krishnan, S.; Ayothi, R.; Hexemer, A.; Finlay, J.A.; Sohn, K.E.; Perry, R.; Ober, C.K.; Kramer, E.J.; Callow, M.E.; Callow, J.A.; et al. Anti-biofouling properties of comblike block copolymers with amphiphilic side chains. Langmuir 2006, 22, 5075–5086. [Google Scholar] [CrossRef] [PubMed]
- Pollack, K.A.; Imbesi, P.M.; Raymond, J.E.; Wooley, K.L. Hyperbranched fluoropolymer-polydimethylsiloxane-poly(ethylene glycol) cross-linked terpolymer networks designed for marine and biomedical applications: Heterogeneous nontoxic antibiofouling surfaces. ACS Appl. Mater. Interf. 2014, 6, 19265–19274. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahkaramipour, N.; Tran, T.N.; Ramanan, S.; Lin, H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes 2017, 7, 13. https://doi.org/10.3390/membranes7010013
Shahkaramipour N, Tran TN, Ramanan S, Lin H. Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes. 2017; 7(1):13. https://doi.org/10.3390/membranes7010013
Chicago/Turabian StyleShahkaramipour, Nima, Thien N. Tran, Sankara Ramanan, and Haiqing Lin. 2017. "Membranes with Surface-Enhanced Antifouling Properties for Water Purification" Membranes 7, no. 1: 13. https://doi.org/10.3390/membranes7010013
APA StyleShahkaramipour, N., Tran, T. N., Ramanan, S., & Lin, H. (2017). Membranes with Surface-Enhanced Antifouling Properties for Water Purification. Membranes, 7(1), 13. https://doi.org/10.3390/membranes7010013





