Hydrogen Induced Abrupt Structural Expansion at High Temperatures of a Ni32Nb28Zr30Cu10 Membrane for H2 Purification
Abstract
:1. Introduction
2. Results and Discussion
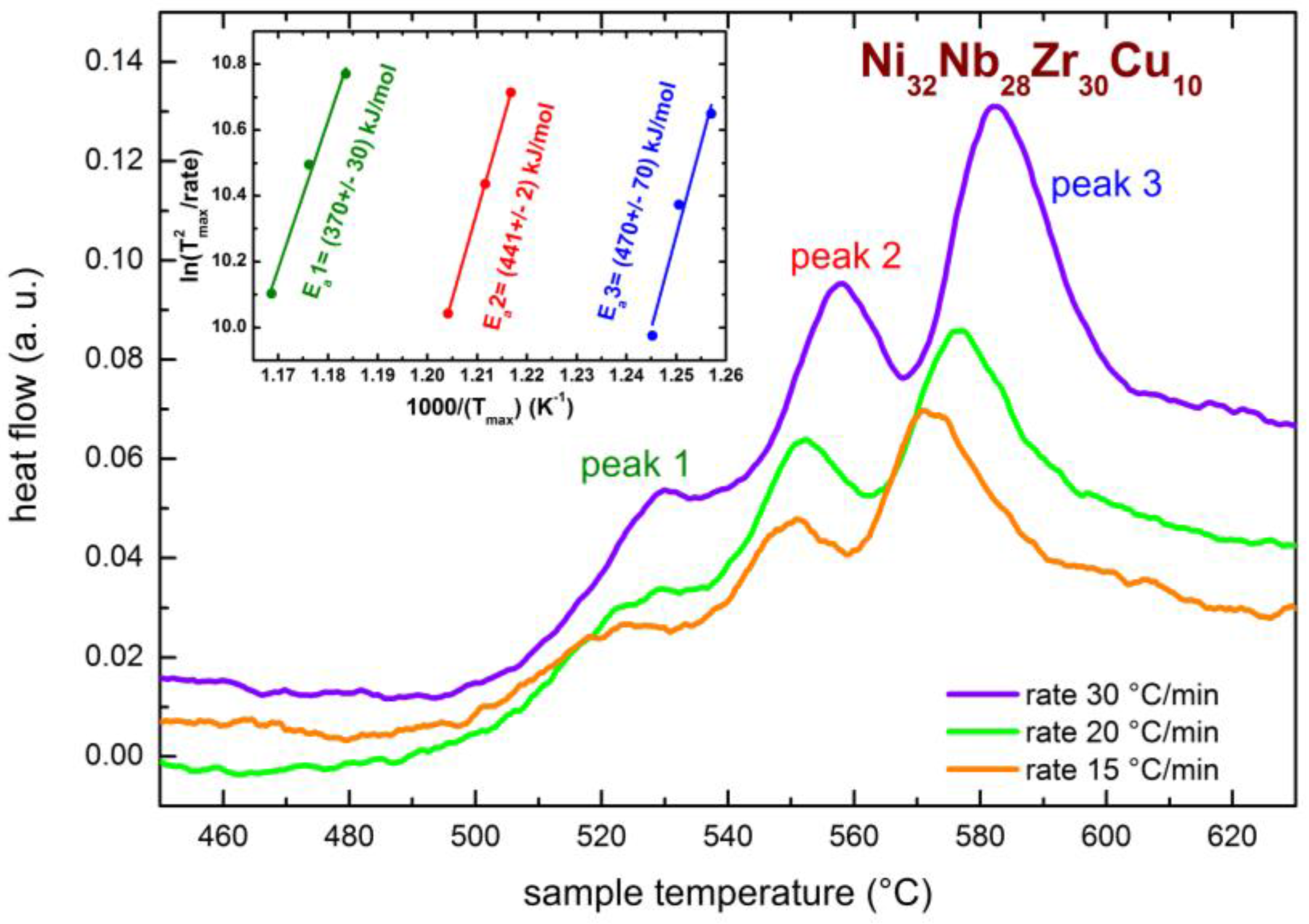
2.1. Preliminarily DTA Measurements
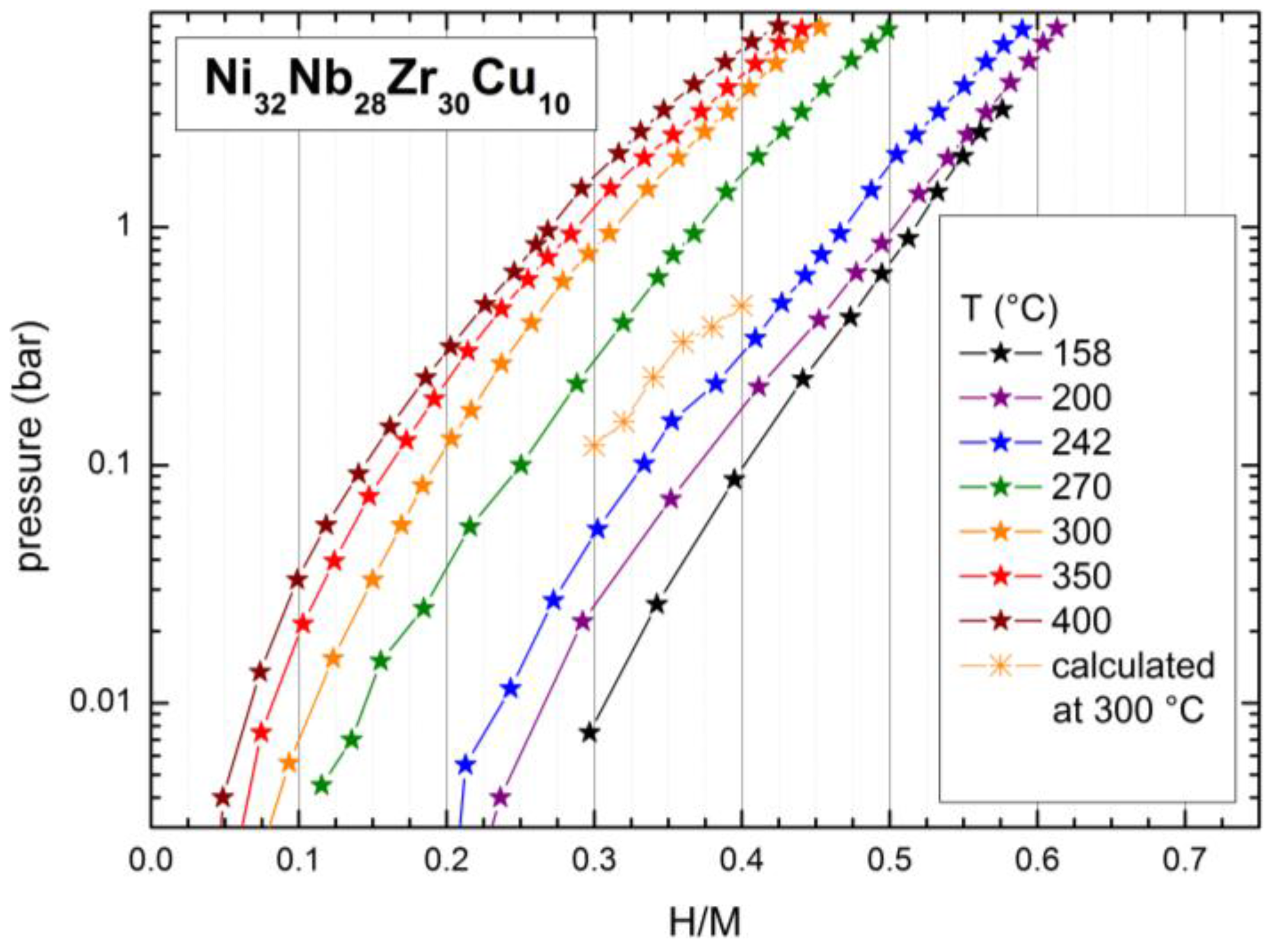
2.2. Hydrogen Absorption Properties
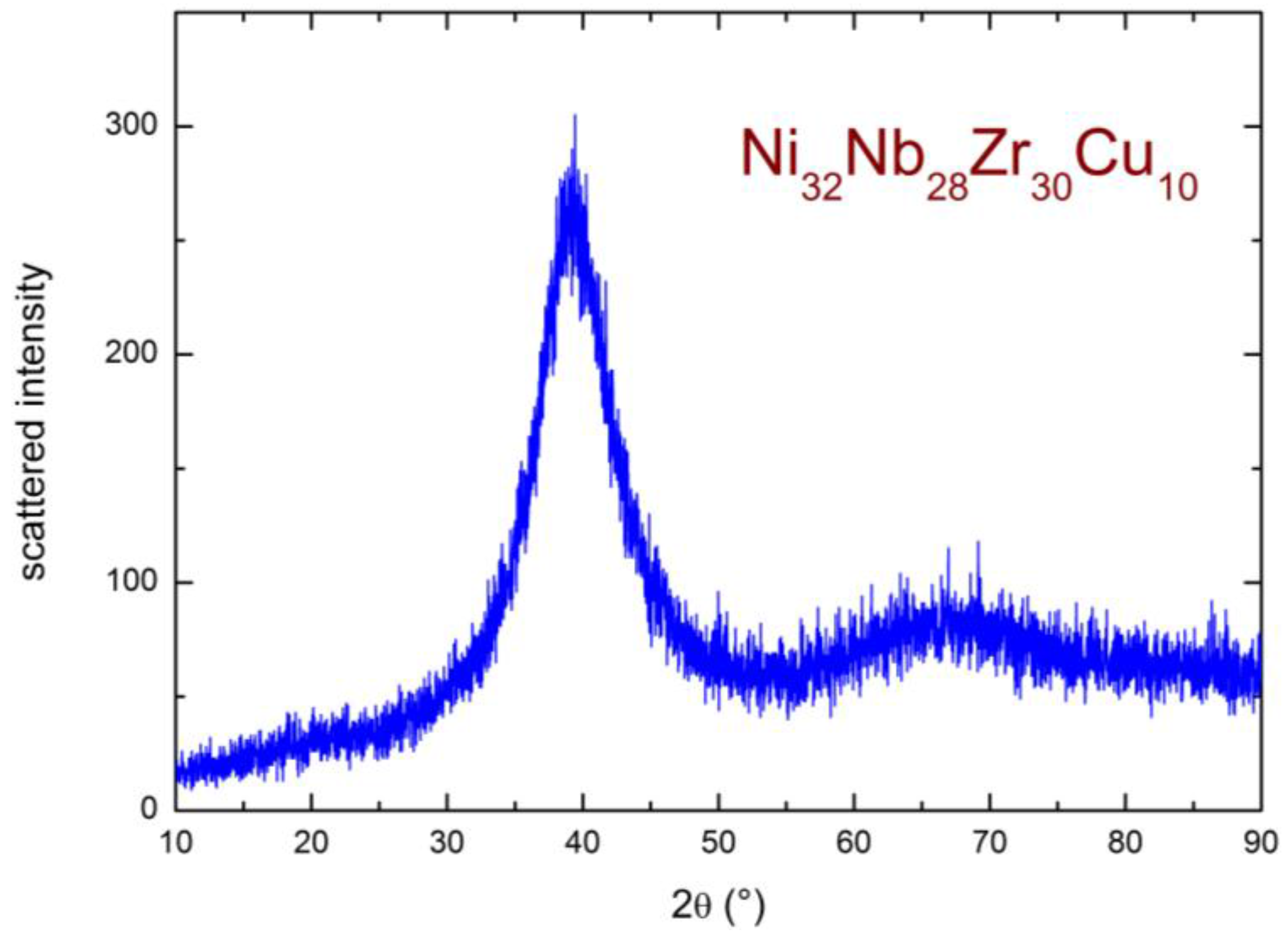
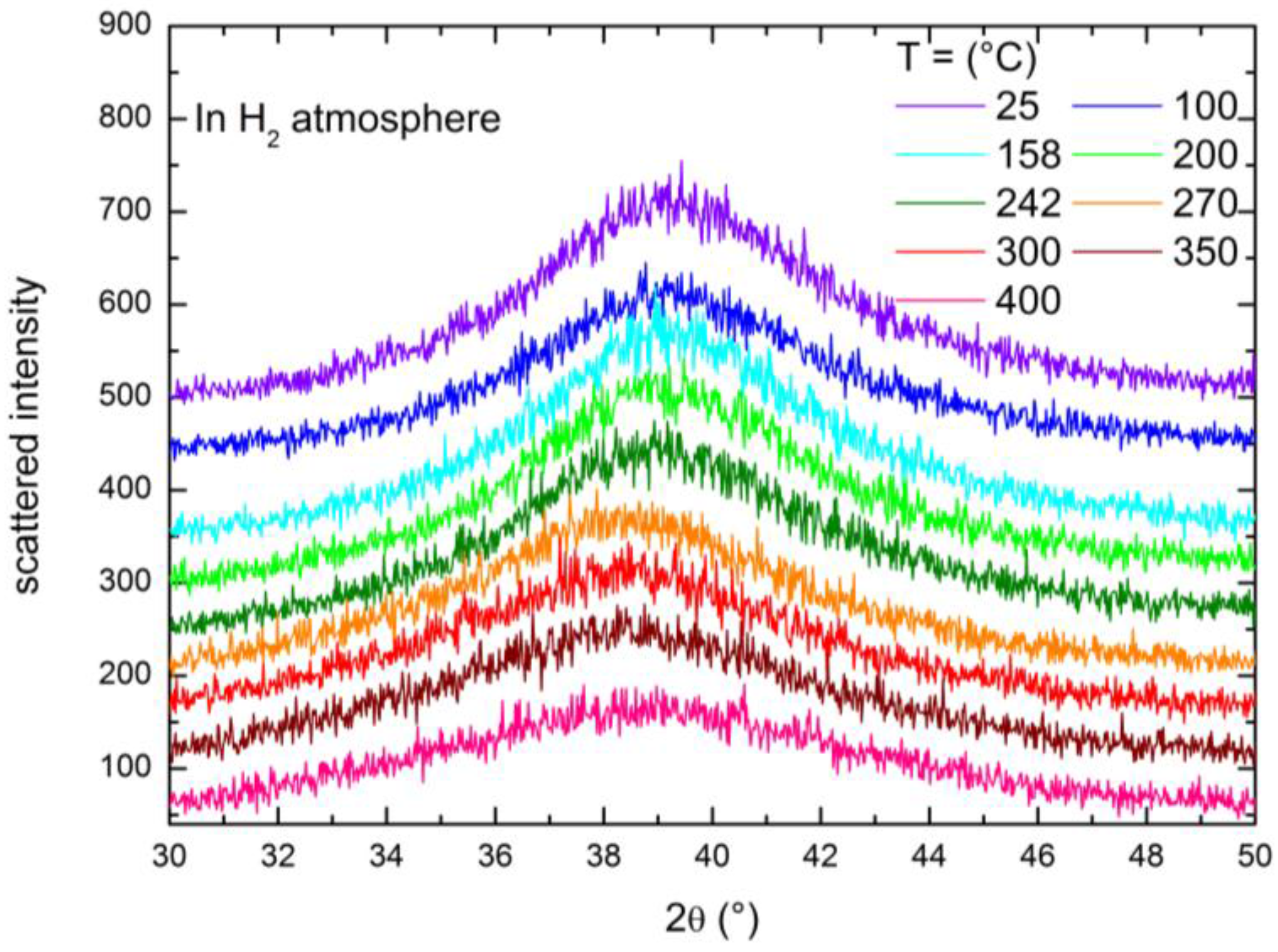
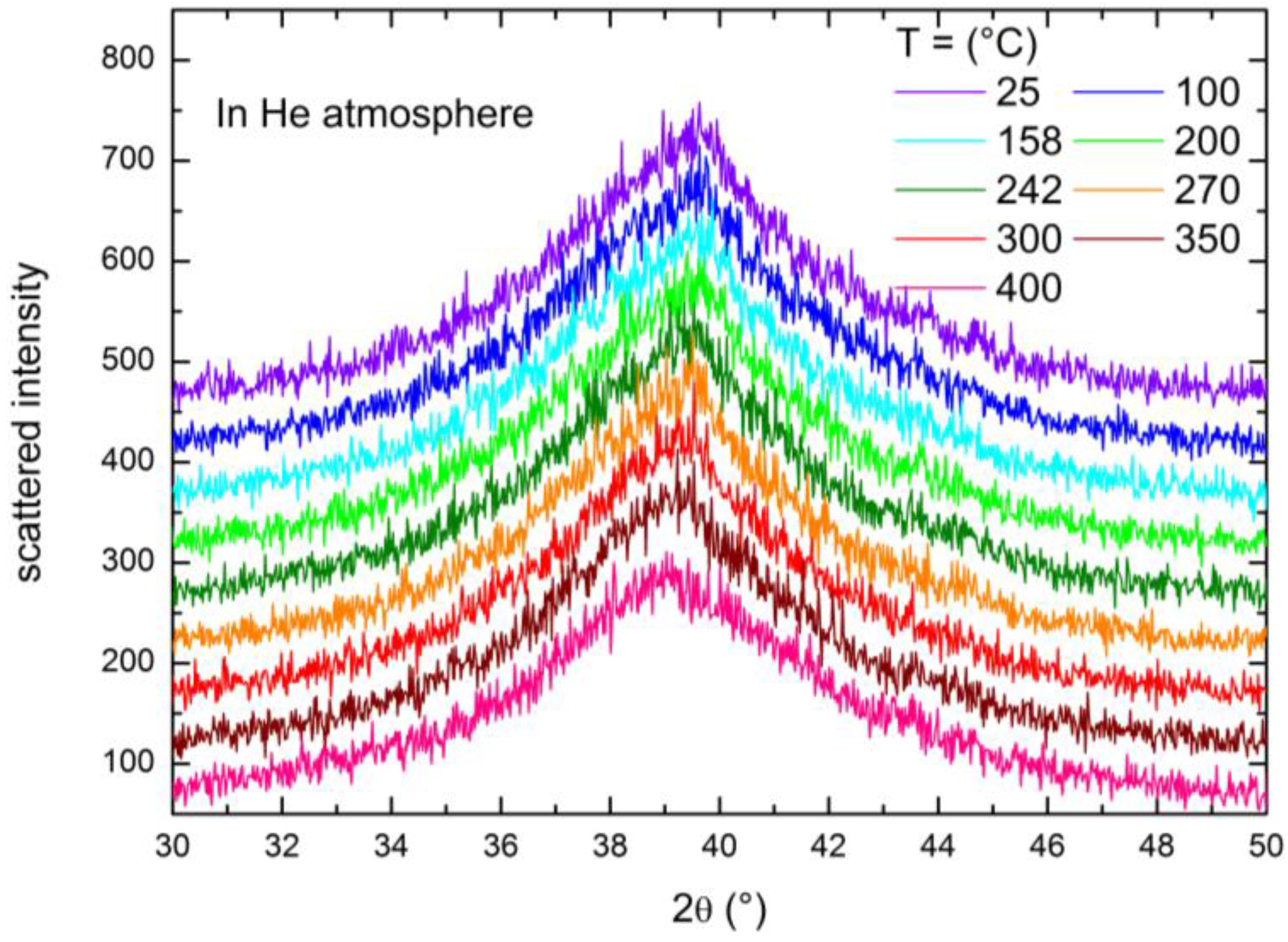
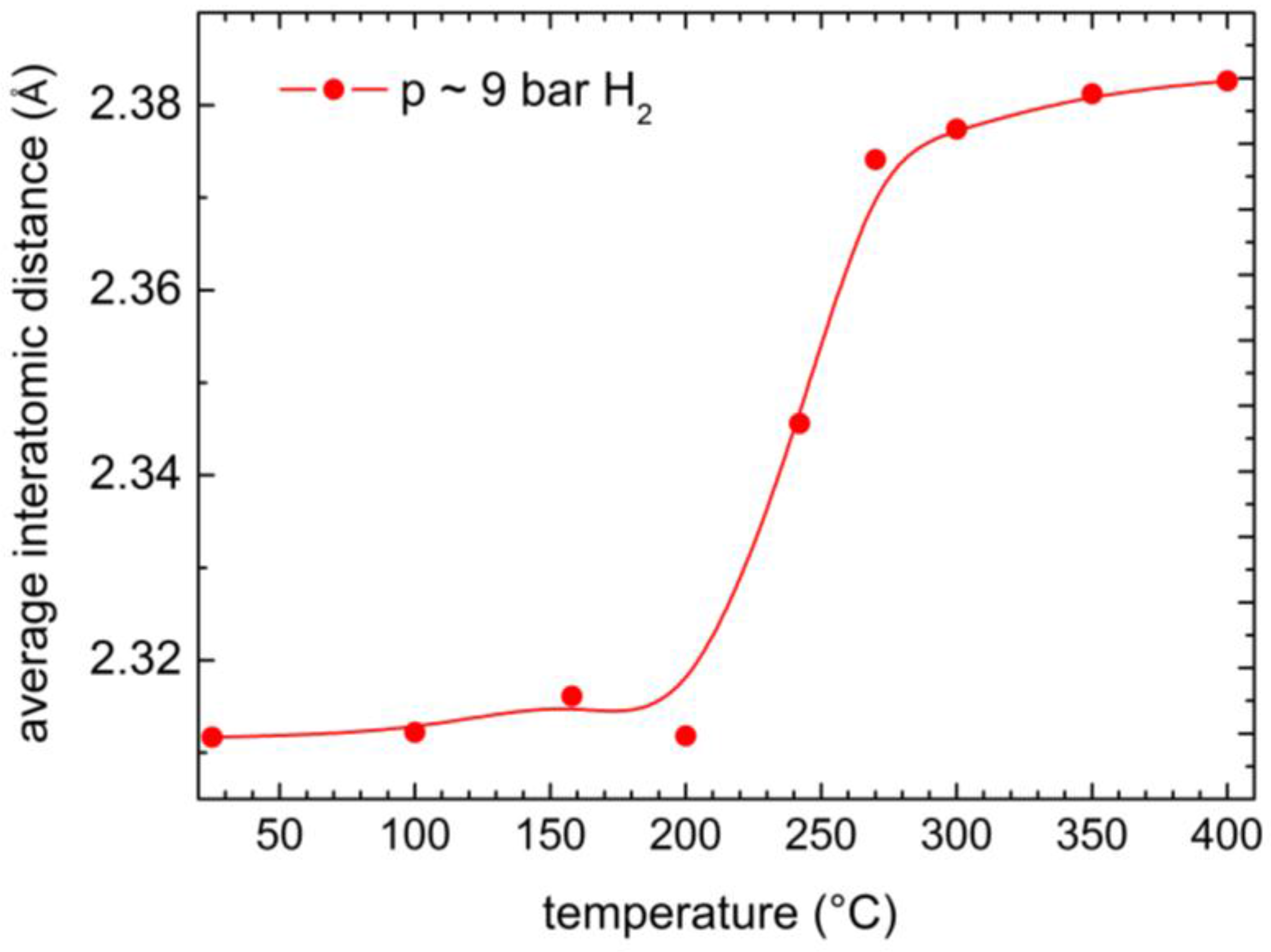
2.3. XRD Study of the High Temperature Evolution of the Structure
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ockwig, N.W.; Nenoff, T.M. Membranes for Hydrogen Separation. Chem. Rev. 2007, 107, 4078–4110. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.Q.; Diniz da Costa, J.C.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Paglieri, S.; Way, J.D. Innovations in palladium membrane research. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Tosti, S.; Bettinali, L.; Violante, V. Rolled thin Pd and Pd-Ag membranes for hydrogen separation and production. Int. J. Hydrog. Energy 2000, 25, 319–325. [Google Scholar] [CrossRef]
- Kanezashi, M.; Sano, M.; Yoshioka, T.; Tsuru, T. Extremely thin Pd-silica mixed-matrix membranes with nano-dispersion for improved hydrogen permeability. Chem. Commun. 2010, 46, 6171–6173. [Google Scholar] [CrossRef] [PubMed]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Yampolskii, Y. Polymer Gas Separation Membranes. Macromolecules 2012, 45, 3298–3311. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymer gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Susteinable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef]
- Steward, S.A. Review of Hydrogen Isotope Permeability through Metals; US National Laboratory Report: UCRL-53441; Lawrence Livermore National Lab.: Livermore, CA, USA, 1983.
- Sarker, S.; Chandra, D.; Hirscher, M.; Dolan, M.; Isheim, D.; Wermer, J.; Viano, D.; Baricco, M.; Udovic, T.J.; Grant, D.; et al. Developments in the Ni-Nb-Zr Amorphous Alloy Membranes. Appl. Phys. A 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Dolan, M.D.; Dave, N.C.; Ilyushechkin, A.Y.; Morpeth, L.D.; McLennan, K.G. Composition and operation of hydrogen-selective amorphous alloy membrane. J. Membr. Sci. 2006, 285, 30–55. [Google Scholar] [CrossRef]
- Hara, S.; Hatakeyma, N.; Itoh, N.; Kimura, H.-M.; Inoue, A. Hydrogen permeation through amorphous Zr36−xHfxNi64 alloy membranes. J. Membr. Sci. 2003, 211, 149–156. [Google Scholar] [CrossRef]
- Hara, S.; Sakaki, K.; Itoh, N.; Kimura, H.-M.; Asami, K.; Inoue, A. An amorphous alloy membrane without noble metals for gaseous hydrogen separation. J. Membr. Sci. 2000, 164, 289–294. [Google Scholar] [CrossRef]
- Hara, S.; Hatakeyma, N.; Itoh, N.; Kimura, H.-M.; Inoue, A. Hydrogen permeation through palladium-coated amorphous Zr-M-Ni (M = Ti, Hf) alloy membranes. Desalination 2002, 144, 115–120. [Google Scholar] [CrossRef]
- Yamaura, S.-I.; Shimpo, Y.; Okouchi, H.; Nishida, M.; Kajita, O.; Kimura, H.; Inoue, A. Hydrogen permeation characteristics of melt-spun Ni-Nb-Zr amorphous alloy membranes. Mater. Trans. 2003, 44, 1885–1890. [Google Scholar] [CrossRef]
- Yamaura, S.-I.; Sakurai, M.; Hasegawa, M.; Wakoh, K.; Shimpo, Y.; Nishida, M.; Kimura, H.; Matsubara, E.; Inoue, A. Hydrogen permeation and structural features of melt-spun Ni-Nb-Zr amorphous alloys. Acta Mater. 2005, 53, 3703–3711. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Pal, N.K.; Dolan, M.D.; Kim, S.-M.; Chien, W.-M.; Lamb, J.; Chandra, D.; Hubbard, K.M.; Moore, D.P. Hydrogen permeability, thermal stability and hydrogen embrittlement of Ni-Nb-Zr and Ni-Nb-Ta-Zr amorphous alloy membranes. J. Membr. Sci. 2011, 378, 42–50. [Google Scholar] [CrossRef]
- Kim, S.-M.; Chandra, D.; Pal, N.K.; Dolan, M.D.; Chien, W.-M.; Talekar, A.; Lamb, J.; Paglieri, S.N.; Flanagan, T.B. Hydrogen permeability and crystallization kinetics in amorphous Ni-Nb-Zr alloys. Int. J. Hydrog. Energy 2012, 37, 3904–3913. [Google Scholar] [CrossRef]
- Adibhatla, A.; Dolan, M.D.; Chien, W.; Chandra, D. Enhancing the catalytic activity of Ni-based amorphous alloy membrane surfaces. J. Membr. Sci. 2014, 463, 190–195. [Google Scholar] [CrossRef]
- Kim, S.-M.; Chien, W.-M.; Chandra, D.; Pal, N.K.; Talekar, A.; Lamb, J.; Dolan, M.D.; Paglieri, S.N.; Flanagan, T.B. Phase transformation and crystallization kinetics of melt-spun Ni60Nb20Zr20 amorphous alloy. J. Non-Cryst. Solids 2012, 358, 1165–1170. [Google Scholar] [CrossRef]
- Palumbo, O.; Brutti, S.; Trequattrini, F.; Sarker, S.; Dolan, M.; Chandra, D.; Paolone, A. Temperature Dependence of the Elastic Modulus of (Ni0.6Nb0.4)1−xZrx Membranes: Effects of Thermal Treatments and Hydrogenation. Energies 2015, 8, 3944–3954. [Google Scholar] [CrossRef]
- Fukubara, M.; Fujima, N.; Oji, H.; Inoue, A.; Emura, A. Structures of the icosahedral clusters in Ni-Nb-Zr-H glassy alloys determined by first-principles molecular dynamics calculation and XAFS measurements. J. Alloys Compd. 2010, 497, 182–187. [Google Scholar] [CrossRef]
- Fujima, N.; Hoshino, T.; Fukuhara, M. Local structures and structural phase change in Ni-Zr-Nb glassy alloys composed of Ni5Zr5Nb3 icosahedral clusters. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Fleury, E. High temperature mechanical properties of rapidly quenched Zr50Ni27Nb18Co5 amorphous alloy. Met. Mater. Int. 2009, 15, 701–711. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Park, S.O.; Kim, K.B.; Fleury, E.; Kim, D.H. Studies on hydrogen embrittlement in Zr- and Ni-based amorphous alloys. Mater. Sci. Eng. A 2007, 449–451, 920–923. [Google Scholar] [CrossRef]
- Yamaura, S.-I.; Simpo, Y.; Okouchi, H.; Nishida, M.; Kajita, O.; Inoue, A. The Effect of Additional Elements on Hydrogen permeation Properties of Melt-spun Ni-Nb-Zr amorphous alloys. Mater. Trans. 2004, 45, 330–333. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Pal, N.; Hulyalkar, M.; Sarker, S.; Chandra, D.; Flanagan, T.; Dolan, M.; Paolone, A. Hydrogen absorption properties of amorphous (Ni0.6Nb0.4−yTay)100−xZrx membranes. Prog. Nat. Sci. 2017, in press. [Google Scholar]
- Jayalakshmi, S.; Choi, Y.G.; Kim, Y.C.; Kim, Y.B.; Fleury, E. Hydrogenation properties of Ni-Nb-Zr-Ta amorphous ribbons. Intermetallics 2010, 18, 1988–1993. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Vasantha, V.S.; Fleury, E.; Gupta, M. Characteristics of Ni–Nb-based metallic amorphous alloys for hydrogen-related energy applications. Appl. Energy 2012, 90, 94–99. [Google Scholar] [CrossRef]
- Conic, D.; Gradisek, A.; Radakovic, J.; Iordoc, M.; Mirkovic, M.; Cebela, M.; Batalovi, K. Influence of Ta and Nb on the hydrogen absorption kinetics in Zr-based alloys. Int. J. Hydrogen Energy 2015, 40, 5677–5682. [Google Scholar] [CrossRef]
- Hao, S.; Sholl, D.S. Comparison of first principles calculations and experiments for hydrogen permeation through amorphous ZrNi and ZrNiNb films. J. Membr. Sci. 2010, 50, 402–409. [Google Scholar] [CrossRef]
- Karger, B.L.; Snyder, L.R.; Horvath, C. An Introduction to Separation Science; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Palumbo, O.; Paolone, A.; Rispoli, P.; Cantelli, R.; Autrey, T. Decomposition of NH3BH3 at sub-ambient pressures: A combined thermogravimetry–differential thermal analysis–mass spectrometry study. J. Power Sources 2010, 195, 1615–1618. [Google Scholar] [CrossRef]
- Palumbo, O.; Trequattrini, F.; Vitucci, F.M.; Bianchin, A.; Paolone, A. Study of the hydrogenation/dehydrogenation process in the Mg-Ni-C-Al system. J. Alloys Compd. 2015, 645, S239–S241. [Google Scholar] [CrossRef]
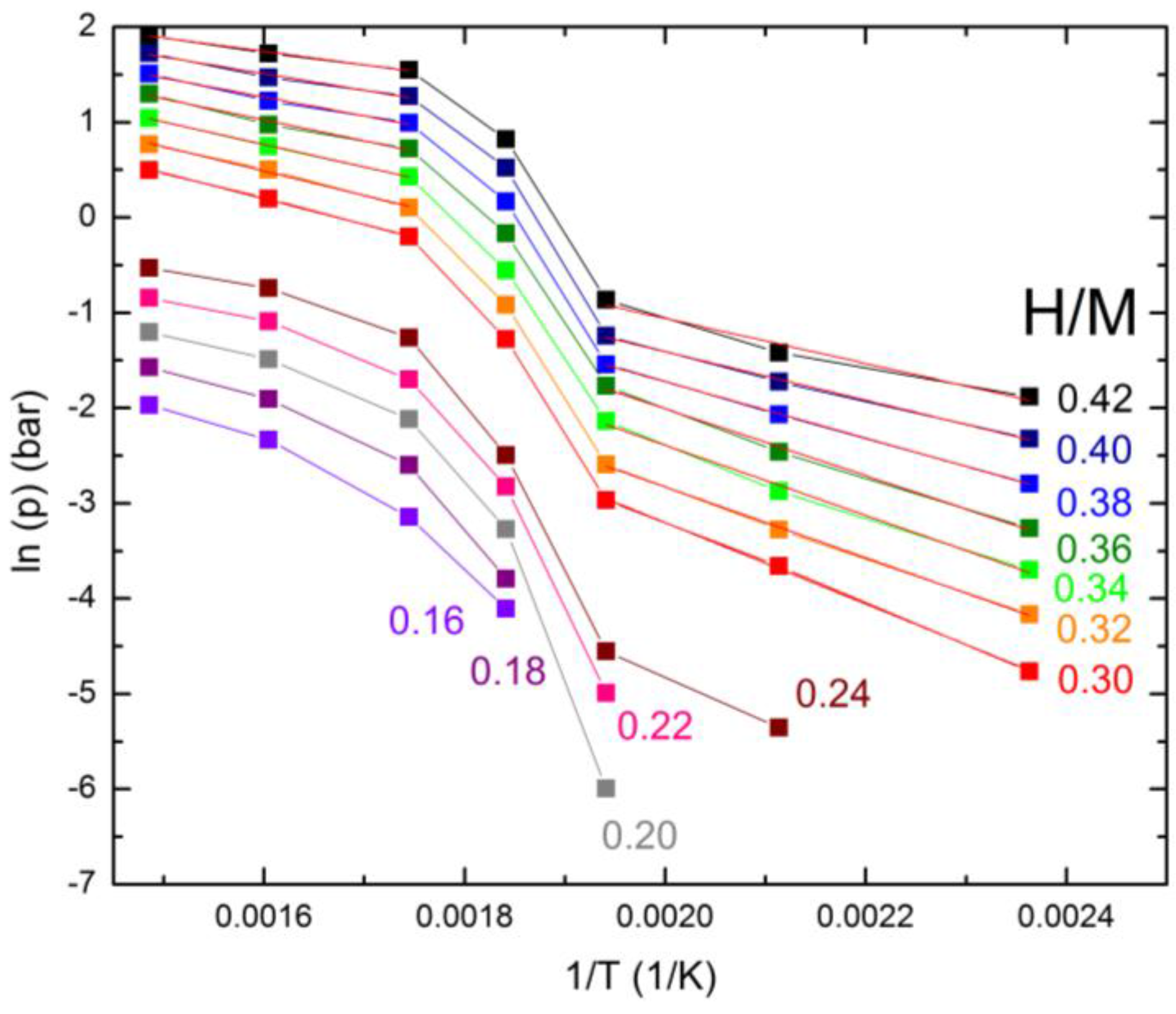
| ΔHhyd (kJ/mol) | H/M = 0.30 | H/M = 0.32 | H/M = 0.34 | H/M = 0.36 | H/M = 0.38 | H/M = 0.40 |
|---|---|---|---|---|---|---|
| low T region | 36 ± 1 | 31 ± 1 | 31 ± 1 | 29 ± 1 | 25 ± 1 | 21 ± 1 |
| high T region | 23 ± 1 | 21 ± 1 | 20 ± 1 | 18 ± 1 | 17 ± 1 | 15 ± 1 |
| T (°C) | Under Hydrogen (~9 bar) | Under Helium (~1 bar) | ||
|---|---|---|---|---|
| 2θ | Interatomic Distance (Å) | 2θ | Interatomic Distance (Å) | |
| 25 | 38.927 | 2.3117 | 39.287 | 2.2914 |
| 100 | 38.93 | 2.3122 | 39.235 | 2.2943 |
| 158 | 38.852 | 2.3161 | 39.221 | 2.2951 |
| 200 | 38.926 | 2.3118 | 39.223 | 2.295 |
| 242 | 38.343 | 2.3456 | 39.183 | 2.2973 |
| 270 | 37.866 | 2.3741 | 39.152 | 2.299 |
| 300 | 37.811 | 2.3774 | 39.127 | 2.3004 |
| 350 | 37.748 | 2.3812 | 39.059 | 2.3042 |
| 400 | 37.726 | 2.3826 | 39.207 | 2.2959 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, O.; Trequattrini, F.; Hulyalkar, M.; Sarker, S.; Pal, N.; Chandra, D.; Flanagan, T.; Dolan, M.; Paolone, A. Hydrogen Induced Abrupt Structural Expansion at High Temperatures of a Ni32Nb28Zr30Cu10 Membrane for H2 Purification. Membranes 2016, 6, 48. https://doi.org/10.3390/membranes6040048
Palumbo O, Trequattrini F, Hulyalkar M, Sarker S, Pal N, Chandra D, Flanagan T, Dolan M, Paolone A. Hydrogen Induced Abrupt Structural Expansion at High Temperatures of a Ni32Nb28Zr30Cu10 Membrane for H2 Purification. Membranes. 2016; 6(4):48. https://doi.org/10.3390/membranes6040048
Chicago/Turabian StylePalumbo, Oriele, Francesco Trequattrini, Madhura Hulyalkar, Suchismita Sarker, Narendra Pal, Dhanesh Chandra, Ted Flanagan, Michael Dolan, and Annalisa Paolone. 2016. "Hydrogen Induced Abrupt Structural Expansion at High Temperatures of a Ni32Nb28Zr30Cu10 Membrane for H2 Purification" Membranes 6, no. 4: 48. https://doi.org/10.3390/membranes6040048
APA StylePalumbo, O., Trequattrini, F., Hulyalkar, M., Sarker, S., Pal, N., Chandra, D., Flanagan, T., Dolan, M., & Paolone, A. (2016). Hydrogen Induced Abrupt Structural Expansion at High Temperatures of a Ni32Nb28Zr30Cu10 Membrane for H2 Purification. Membranes, 6(4), 48. https://doi.org/10.3390/membranes6040048








