New RO TFC Membranes by Interfacial Polymerization in n-Dodecane with Various co-Solvents
Abstract
:1. Introduction
2. Results and Discussion
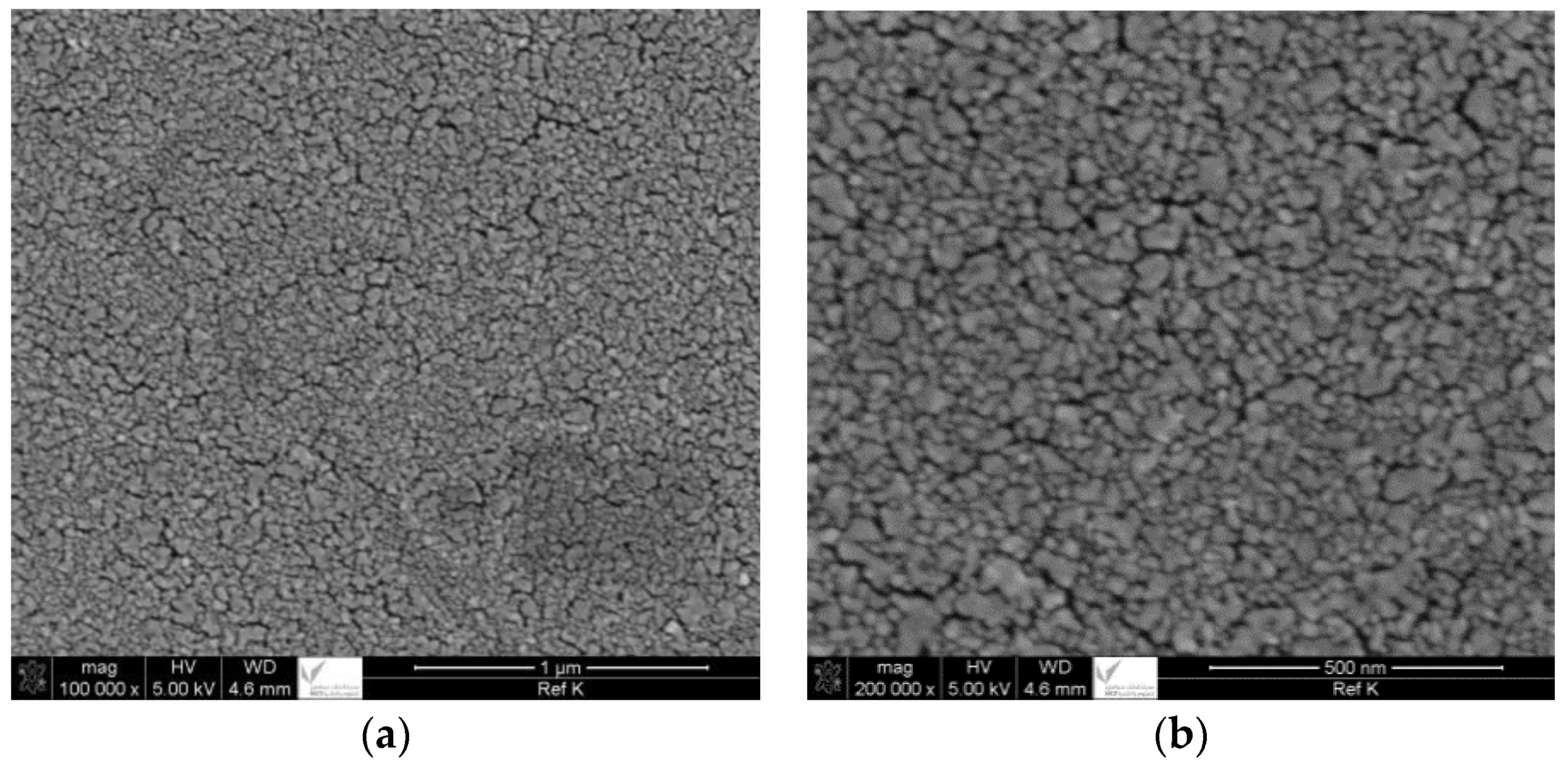
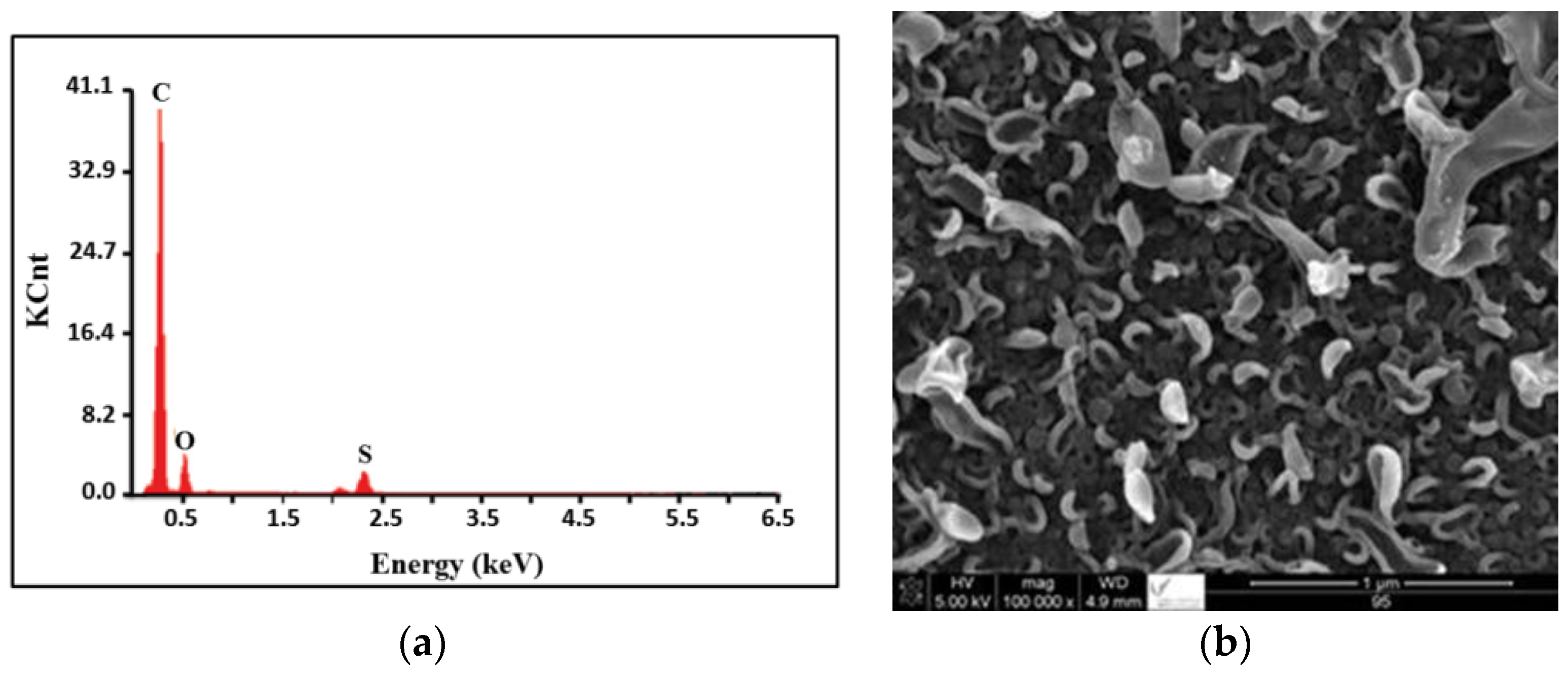
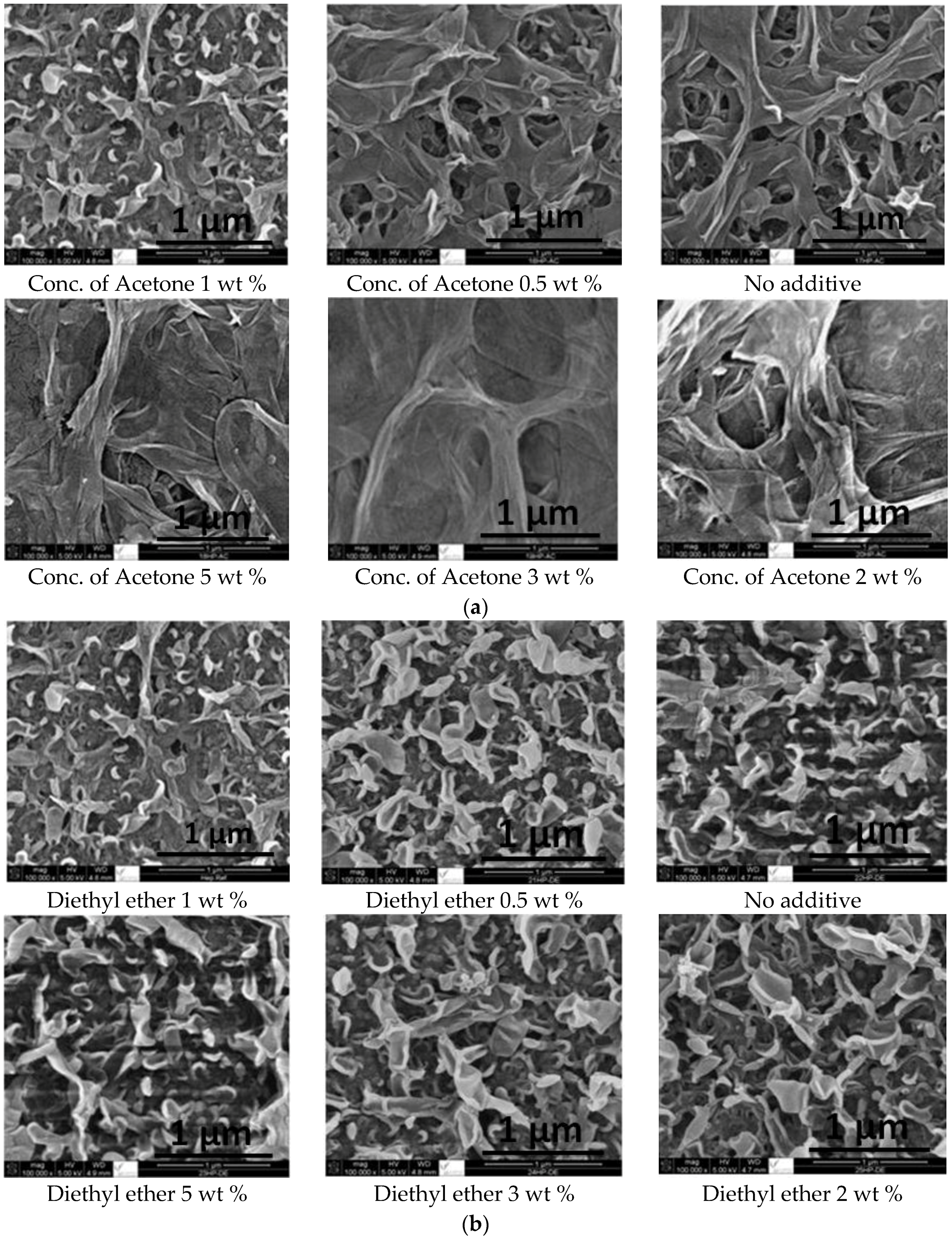
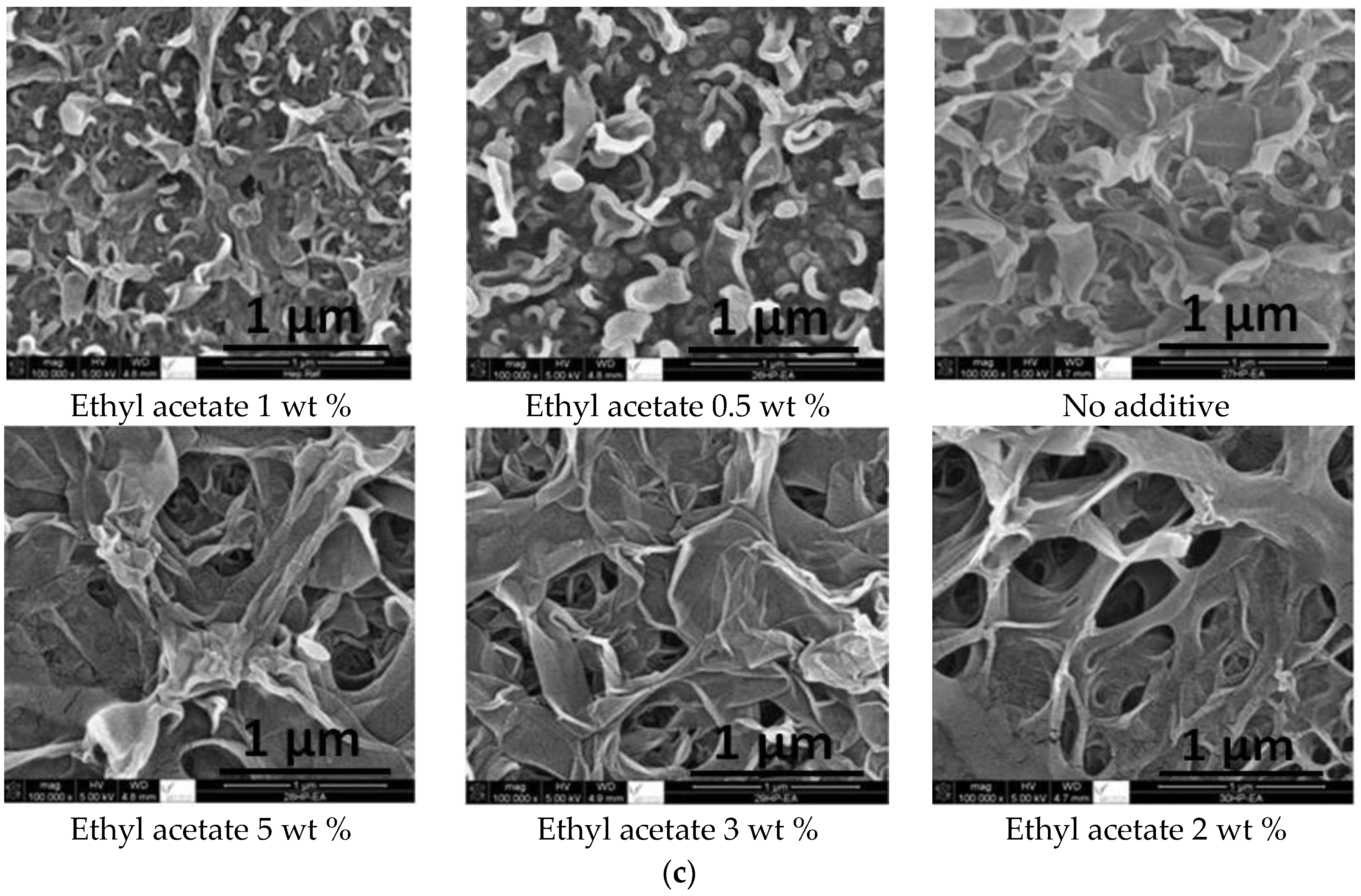
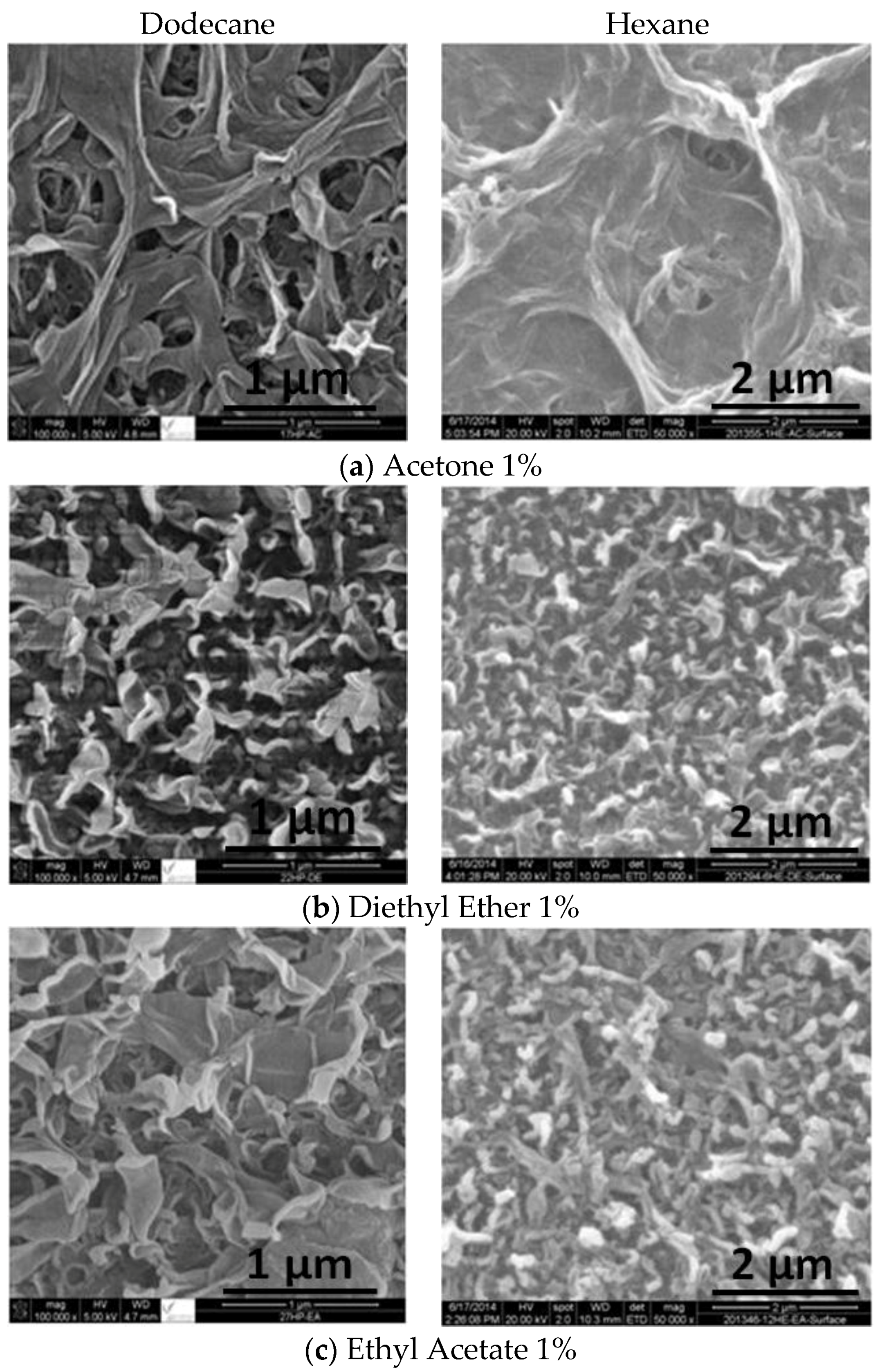
2.1. SEM and EDX Analysis
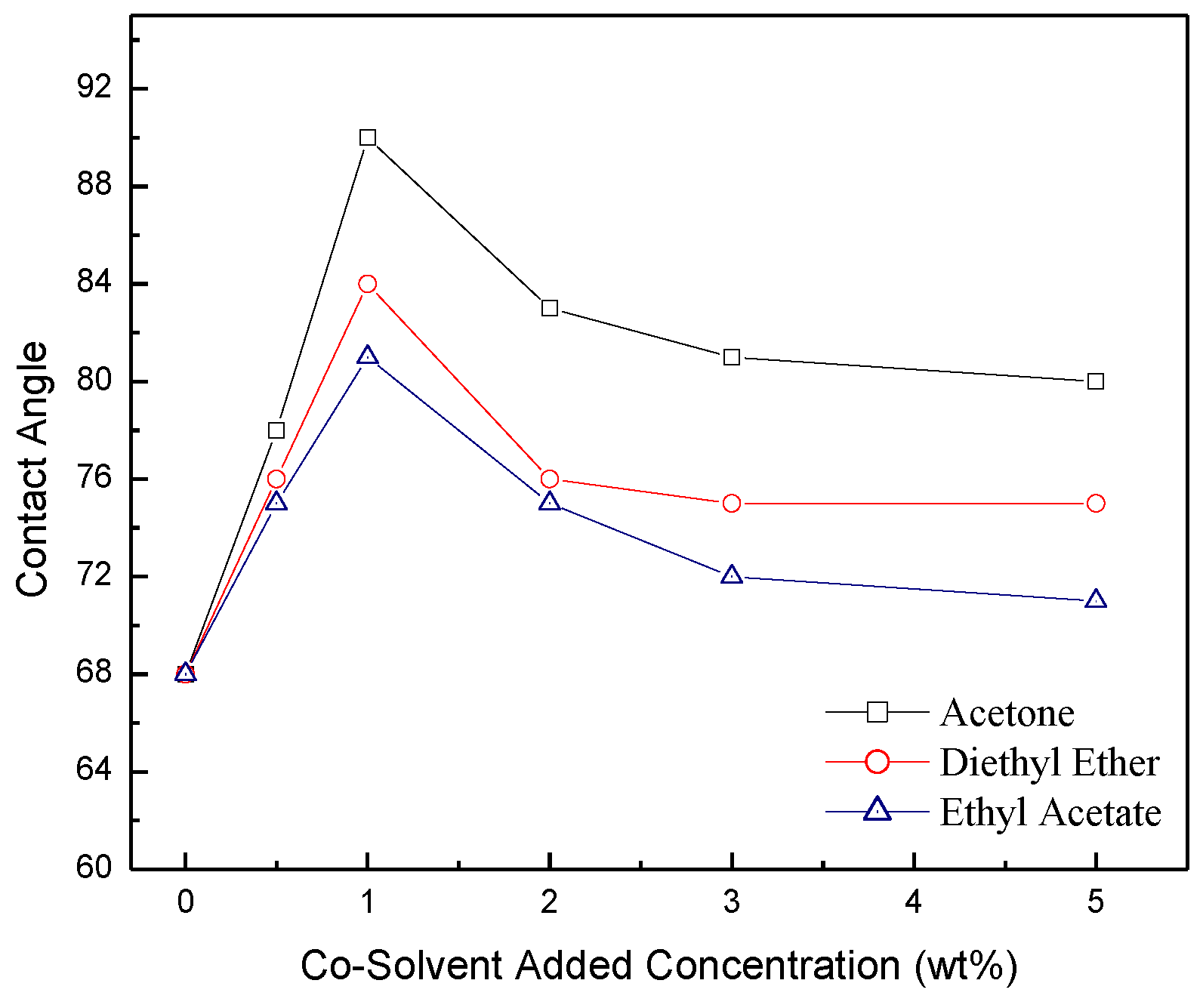
2.2. Contact Angle Measurements
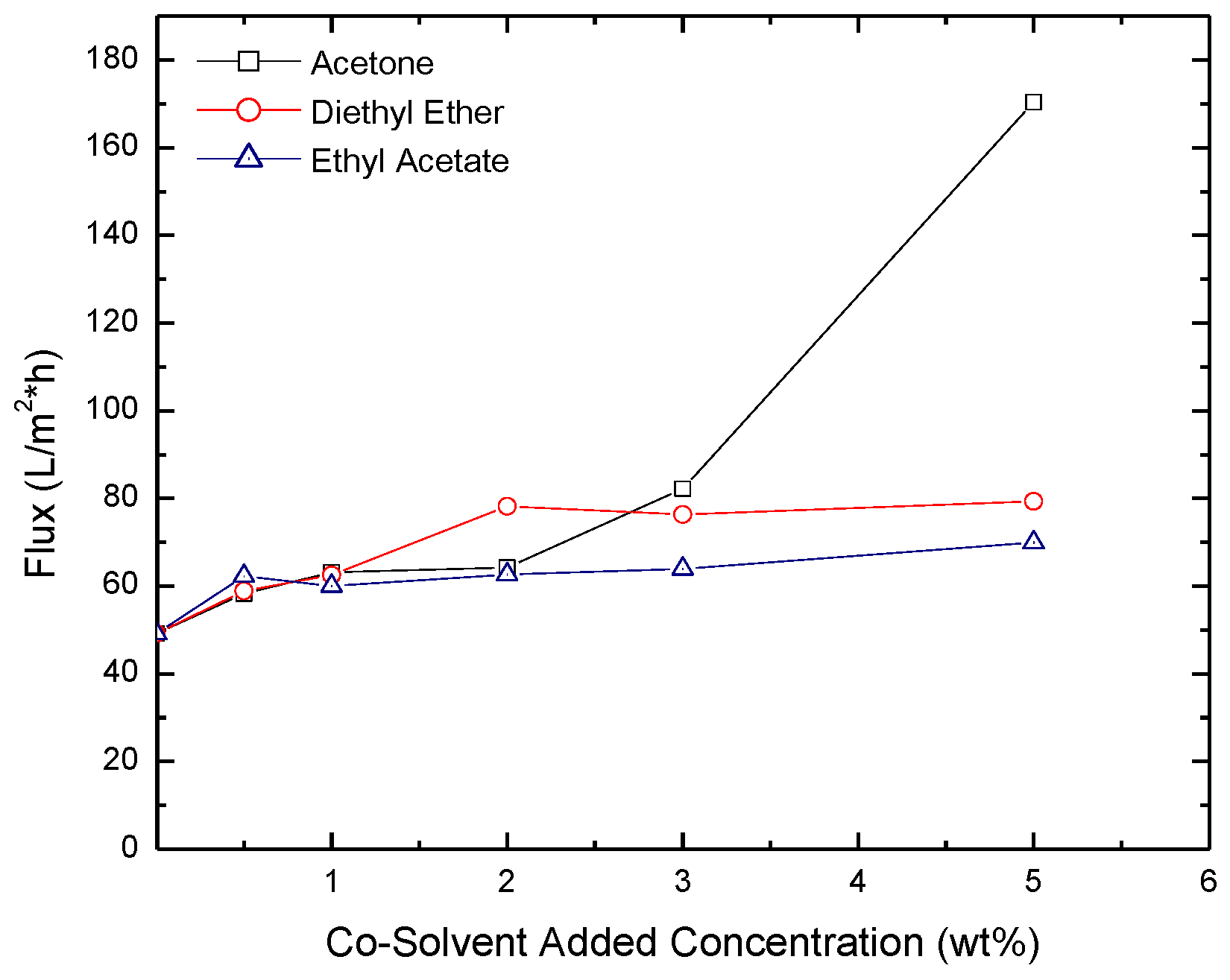
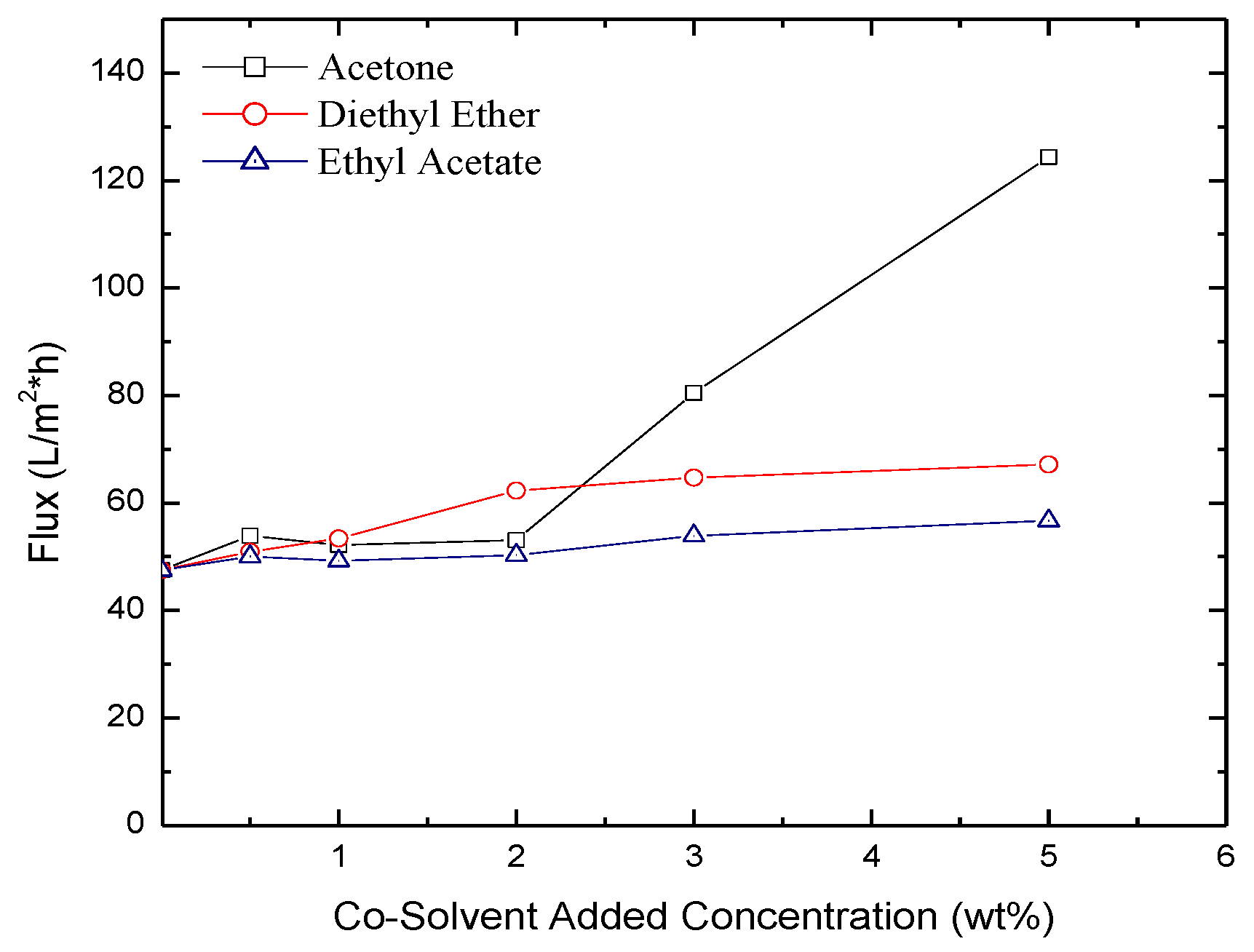
2.3. Permeate Flux
3. Experimental Section
3.1. Materials
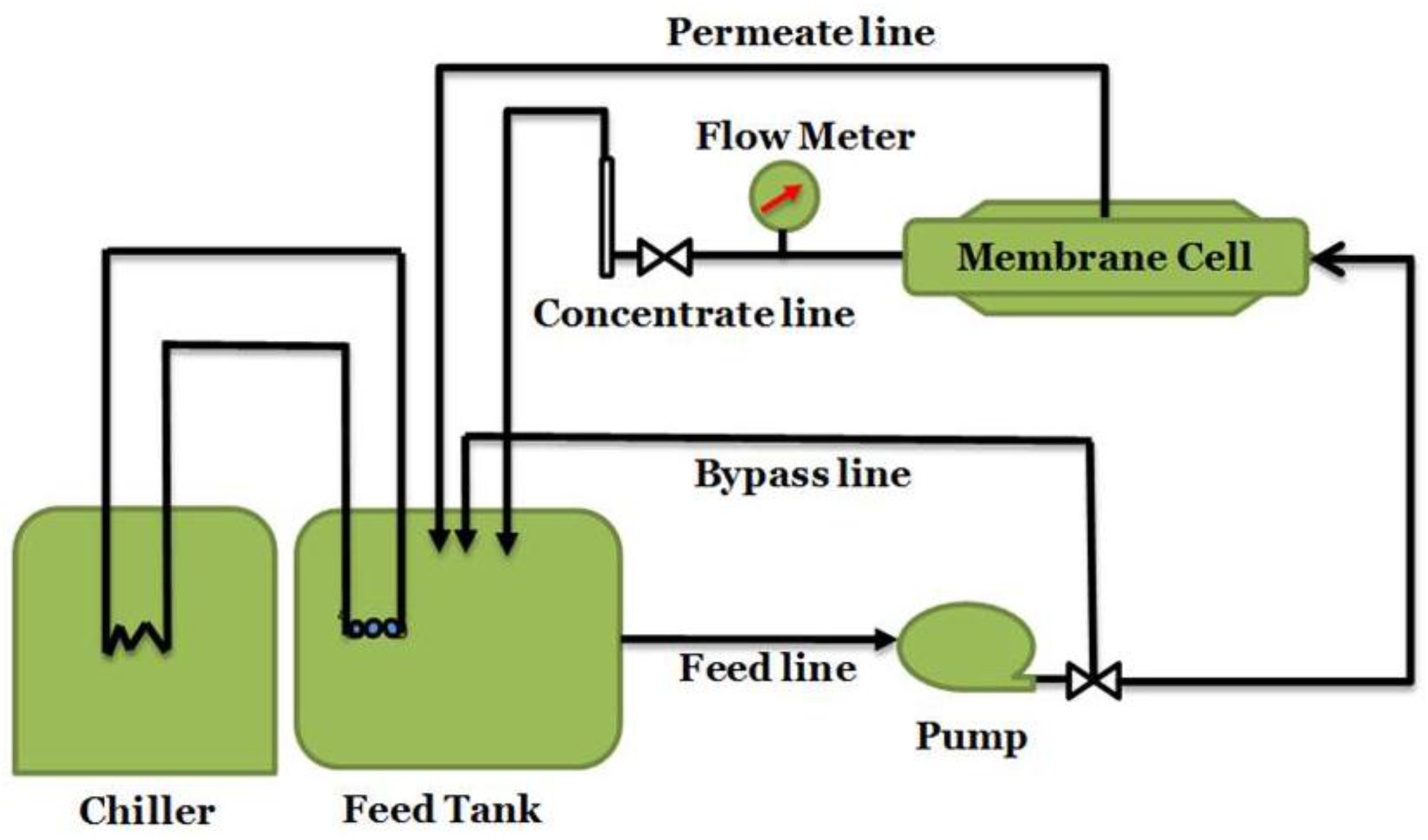
3.2. Methods
Preparation of Modified Polyamide Membranes
3.3. Characterization and Instrumentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van der Bruggen, B.; Vandecasteele, C.; van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Nicolaisen, B. Developments in membrane technology for water treatment. Desalination 2003, 153, 355–360. [Google Scholar] [CrossRef]
- Rautenbach, R.; Vossenkaul, K.; Linn, T.; Katz, T. Waste water treatment by membrane processes—New development in ultrafiltration, nanofiltration and reverse osmosis. Desalination 1997, 108, 247–253. [Google Scholar] [CrossRef]
- Garud, R.M.; Kore, S.V.; Kore, V.S.; Kulkarni, G.S. A short review on process and applications of reverse osmosis. Univers. J. Environ. Res. Technol. 2011, 1, 233–238. [Google Scholar]
- Obaid, M.; Tolba, G.M.K.; Motlak, M.; Fadali, O.A.; Khalil, K.A.; Almajid, A.A.; Kim, B.; Barakat, N.A.M. Effective polyslufone-amorphous SiO2 NPs electrospun nanofiber membrane for high flux oil/water separation. Chem. Eng. J. 2015, 279, 631–638. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Kong, J.; Li, K. Oil removal from oil-in-water emulsions using PVDF membranes. Sep. Purif. Technol. 1999, 16, 83–93. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-haired inorganic membranes with superhydrophilicity and underwater ultralow adhesive superoleophobicity for high-efficiency oil/water separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Dulneva, T.Y.; Kucheruk, D.D.; Goncharuk, V.V. Water purification of dyes by ceramic membranes modifed by Fe3+ hydroxocompounds. J. Water. Chem. Technol. 2015, 37, 85–89. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ismail, A.F.; Salim, R. Behaviours of natural organic matter in membrane filtration for surface water treatment—A review. Desalination 2006, 194, 211–231. [Google Scholar] [CrossRef]
- Mousa, H.A.; Al-Hitmi, S.A. Treatability of wastewater and membrane fouling. Desalination 2007, 217, 65–73. [Google Scholar] [CrossRef]
- Wintgens, T.; Melin, T.; Schäfer, A.; Khan, S.; Muston, M.; Bixio, D.; Thoeye, C. The role of membrane processes in municipal wastewater reclamation and reuse. Desalination 2005, 178, 1–11. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Darby, J.; Bourgeous, K.; McArdle, J.; Genest, P.; Tylla, M. Ultrafiltration as an advanced tertiary treatment process for municipal wastewater. Desalination 1998, 119, 315–321. [Google Scholar] [CrossRef]
- Ćwikła, J.; Konieczny, K. Treatment of sludge water with reverse osmosis. Environ. Prot. Eng. 2011, 37, 21–34. [Google Scholar]
- Cruver, J.E.; Nusbaum, I. Application of reverse osmosis to wastewater treatment. J. Water. Pollut. Control Fed. 1974, 46, 301–311. [Google Scholar] [PubMed]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, Q.; Zhang, X.; Kang, R.; Shi, S.; Cong, W. Membrane fouling caused by amino acid and calcium during bipolar membrane electrodialysis. J. Chem. Technol. Biotechnol. 2008, 83, 1551–1557. [Google Scholar] [CrossRef]
- AL-Sheetan, K.M.; Shaik, M.R.; Al-Hobaib, A.S.; Alandis, N.M. Characterization and evaluation of the improved performance of modified reverse osmosis membranes by incorporation of various organic modifiers and SnO2 nanoparticles. J. Nanomater. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Fang, Y.; Duranceau, S.J. Study of the effect of nanoparticles and surface morphology on reverse osmosis and nanofiltration membrane productivity. Membranes 2013, 3, 196–225. [Google Scholar] [CrossRef] [PubMed]
- AL-Hobaib, A.S.; AL-Sheetan, K.M.; El Mir, L. Effect of iron oxide nanoparticles on the performance of polyamide membrane for ground water purification. Mater. Sci. Semicond. Process. 2016, 42, 107–110. [Google Scholar] [CrossRef]
- Jin, L.M.; Yu, S.L.; Shi, W.X.; Yi, X.S.; Sun, N.; Ge, Y.L.; Ma, C. Synthesis of a novel composite nanofiltration membrane incorporated SiO2 nanoparticles for oily wastewater desalination. Polymer 2012, 53, 5295–5303. [Google Scholar] [CrossRef]
- Al-Hobaib, A.; AL-Sheetan, K.; Shaik, M.R.; Al-Andis, N.M.; Al-Suhybani, M.S. Characterization and evaluation of reverse osmosis membranes modified with Ag2O nanoparticles to improve performance. Nanoscale Res. Lett. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, Y.; Zhang, H.; Liu, J. Development of a molecular separation membrane for efficient separation of low-molecular-weight organics and salts. Desalination 2015, 359, 176–185. [Google Scholar] [CrossRef]
- Luo, R.-L.; Young, T.-H.; Sun, Y.-M. Structure formation and characterization of EVAL membranes with cosolvent of isopropanol and water. Polymer 2003, 44, 157–166. [Google Scholar] [CrossRef]
- Lalia, B.S.; Ahmed, F.E.; Shah, T.; Hilal, N.; Hashaikeh, R. Electrically conductive membranes based on carbon nanostructures for self-cleaning of biofouling. Desalination 2015, 360, 8–12. [Google Scholar] [CrossRef]
- Yan, H.; Ma, N.; Zhan, Z.; Wang, Z. Fabrication of zeolite NaA membranes on hollow fibers using nano-sized seeds exfoliated from mesoporous zeolite crystals. Microporous Mesoporous Mater. 2015, 215, 244–248. [Google Scholar] [CrossRef]
- Kazemimoghadam, M.; Mohammadi, T. Preparation of NaA zeolite membranes for separation of water/UDMH mixtures. Sep. Purif. Technol. 2006, 47, 173–178. [Google Scholar] [CrossRef]
- Ma, J.; Shao, J.; Wang, Z.; Yan, Y. Preparation of zeolite NaA membranes on macroporous alumina supports by secondary growth of gel layers. Ind. Eng. Chem. Res. 2014, 53, 6121–6130. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Y.; Sun, C.; Yu, S.; Liu, M.; Gao, C. Thin-film composite membranes formed by interfacial polymerization with natural material sericin and trimesoyl chloride for nanofiltration. J. Membr. Sci. 2014, 471, 381–391. [Google Scholar] [CrossRef]
- Eren, E.; Sarihan, A.; Eren, B.; Gumus, H.; Kocak, F.O. Preparation, characterization and performance enhancement of polysulfone ultrafiltration membrane using PBI as hydrophilic modifier. J. Membr. Sci. 2015, 475, 1–8. [Google Scholar] [CrossRef]
- Saffar, A.; Carreau, P.J.; Kamal, M.R.; Ajji, A. Hydrophilic modification of polypropylene microporous membranes by grafting TiO2 nanoparticles with acrylic acid groups on the surface. Polymer 2014, 55, 6069–6075. [Google Scholar] [CrossRef]
- Gohari, R.J.; Korminouri, F.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Chowdhury, M.N.K.; Halakoo, E.; Gohari, M.S.J. A novel super-hydrophilic PSf/HAO nanocomposite ultrafiltration membrane for efficient separation of oil/water emulsion. Sep. Purif. Technol. 2015, 150, 13–20. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Zhao, K.; Wu, B. Preparation and characterization of high-hydrophilic polyhydroxy functional PP hollow fiber membrane. Mater. Lett. 2015, 159, 189–192. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. [Google Scholar] [CrossRef]
- Mansourpanah, Y.; Madaeni, S.S.; Adeli, M.; Rahimpour, A.; Farhadian, A. Surface modification and preparation of nanofiltration membrane from polyethersulfone/polyimide blend—Use of a new material (polyethyleneglycol-triazine). J. Appl. Polym. Sci. 2009, 112, 2888–2895. [Google Scholar] [CrossRef]
- Kong, C.; Kanezashi, M.; Yamomoto, T.; Shintani, T.; Tsuru, T. Controlled synthesis of high performance polyamide membrane with thin dense layer for water desalination. J. Membr. Sci. 2010, 362, 76–80. [Google Scholar] [CrossRef]
- Hirose, M.; Ito, H.; Kamiyama, Y. Effect of skin layer surface structures on the flux behavior of RO membranes. J. Membr. Sci. 1996, 121, 209–215. [Google Scholar] [CrossRef]
- Hirose, M.; Ito, H.; Maeda, M.; Tanaka, K. Highly Permeate Composite Reverse Osmosis Membrane, Method of Producing the Same, and Method of Using the Same. U.S. Patent 5,614,099, 25 March 1997. [Google Scholar]
- Zhao, L.; Philip, C.-Y.; Chang, W.S.; Winston, H.O. High-flux reverse osmosis membranes incorporated with hydrophilic additives for brackish water desalination. Desalination 2012, 308, 225–232. [Google Scholar] [CrossRef]
- AL-Hobaib, A.; Alsuhybani, M.; AL-Sheetan, K.M.; Mousa, H.; Shaik, M.R. Modification of thin-film composite polyamide membrane with co-solvents to improve performance. Anal. Methods 2016. submitted. [Google Scholar]
- Kong, C.; Shintani, T.; Kamada, T.; Freger, V.; Tsuru, T. Co-solvent-mediated synthesis of thin polyamide membranes. J. Membr. Sci. 2011, 384, 10–16. [Google Scholar] [CrossRef]
- Kamada, T.; Ohara, T.; Shintani, T.; Tsuru, T. Optimizing the preparation of multi-layered polyamide membrane via the addition of a co-solvent. J. Membr. Sci. 2014, 453, 489–497. [Google Scholar] [CrossRef]
- Kamada, T.; Ohara, T.; Shintani, T.; Tsuru, T. Controlled surface morphology of polyamide membranes via the addition of co-solvent for improved permeate flux. J. Membr. Sci. 2014, 467, 303–312. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations, 3rd ed.; McGraw Hill: New York, NY, USA, 1980. [Google Scholar]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 1–20. [Google Scholar] [CrossRef]


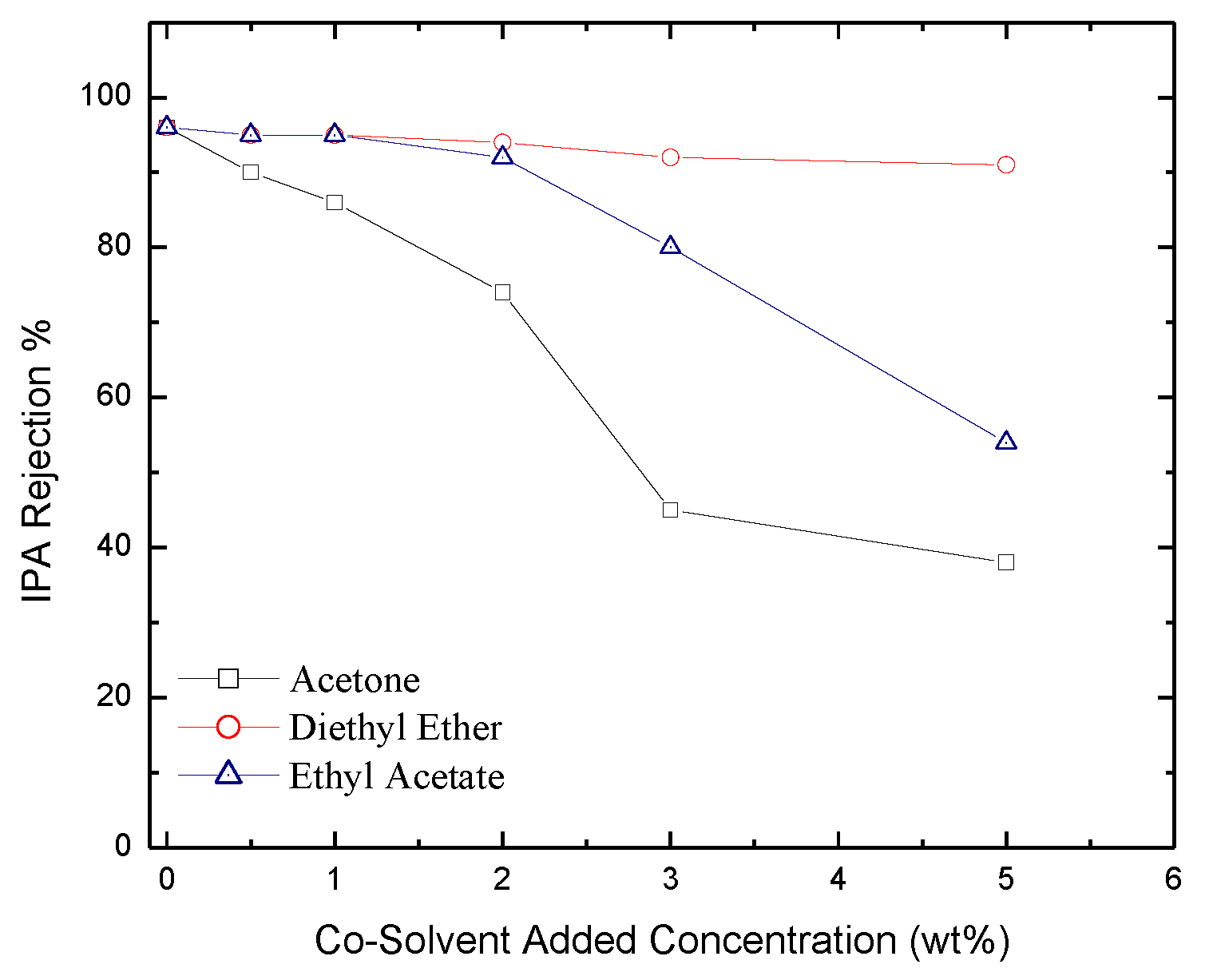
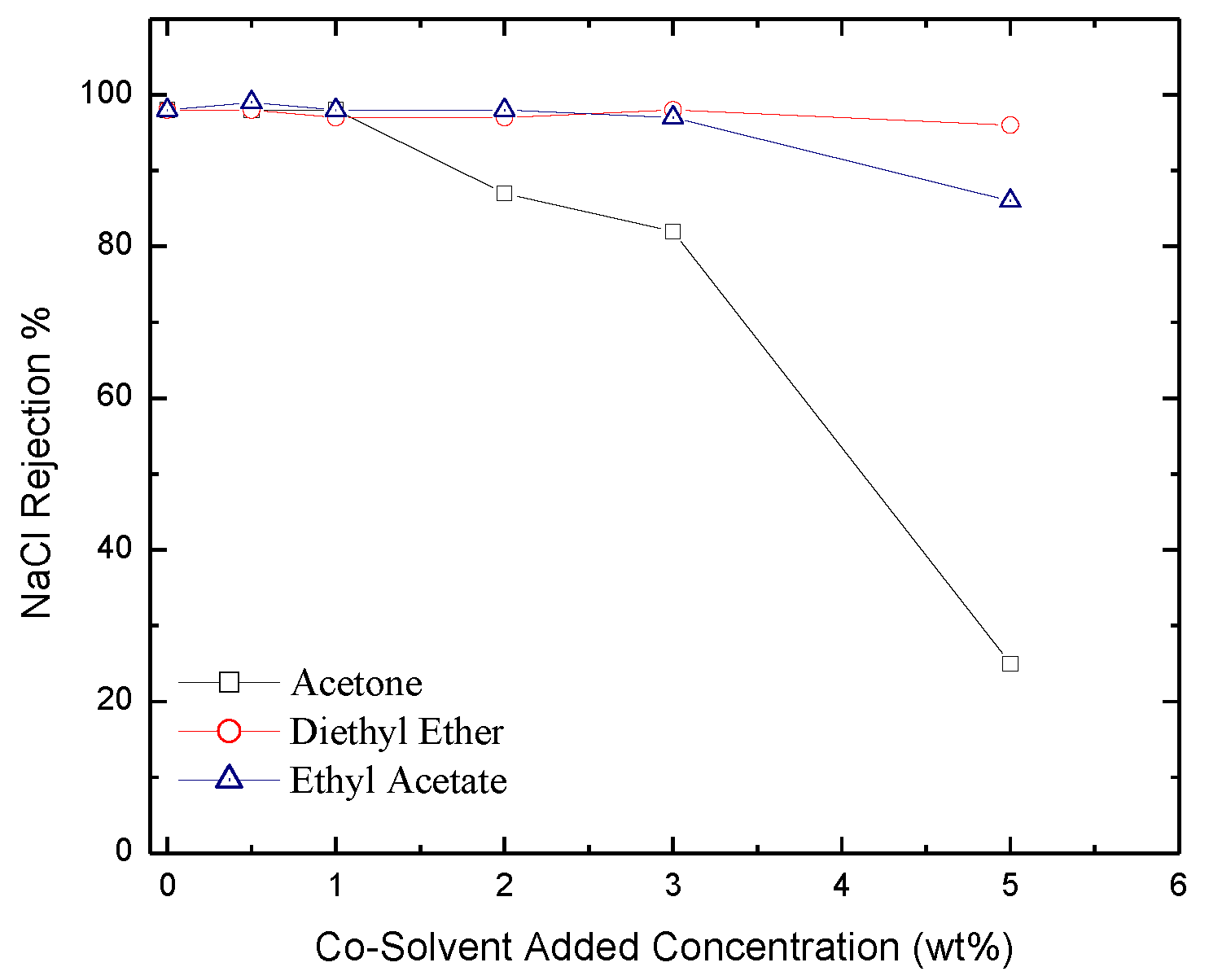
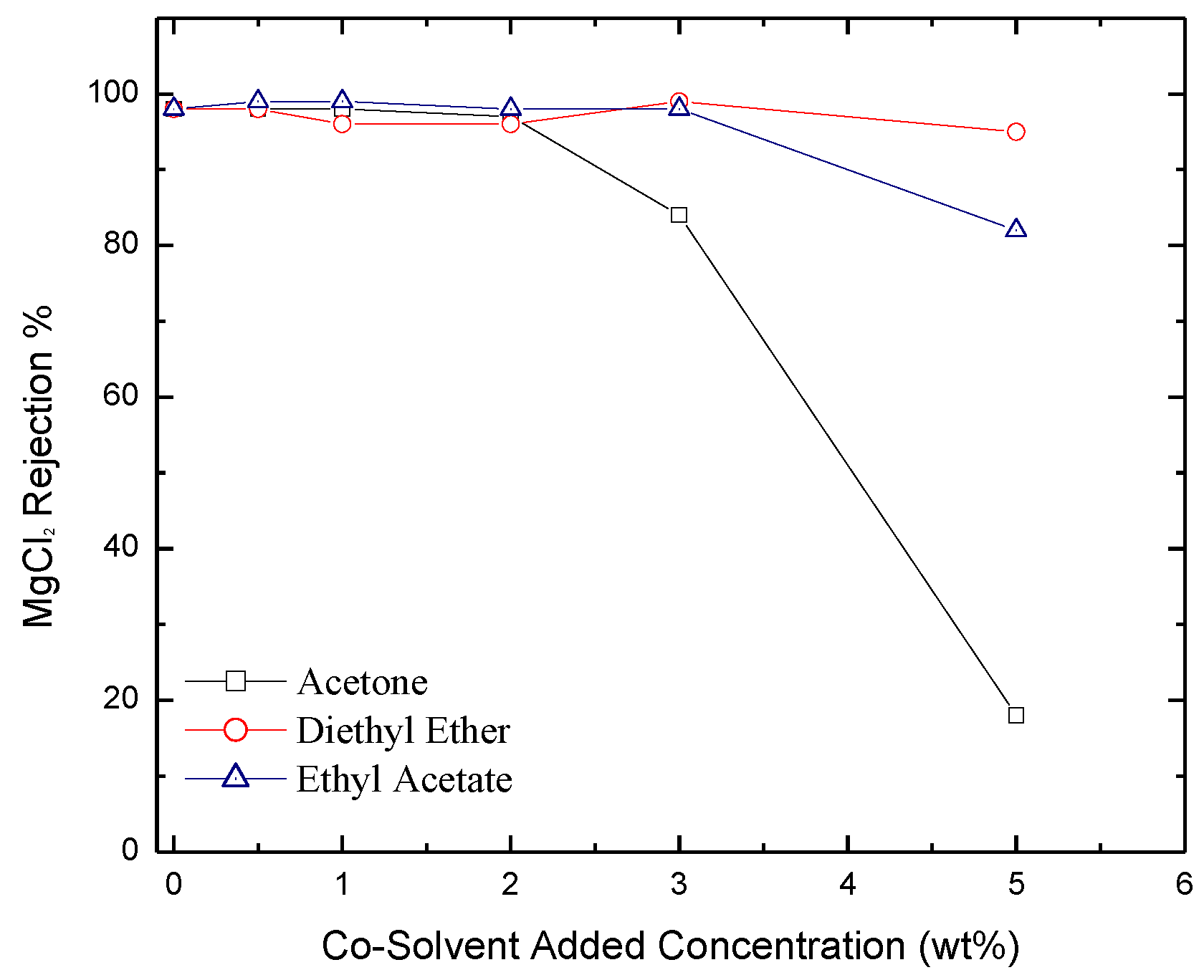
| Isopropanol (IPA) | ||||||||||||
| (wt %) | Co-Solvents Type | |||||||||||
| Acetone | Diethyl Ether | Ethyl Acetate | ||||||||||
| n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | |||||||
| F | S | F | S | F | S | F | S | F | S | F | S | |
| 0 | 26 | 95 | 49 | 96 | 26 | 95 | 49 | 96 | 27 | 95 | 49 | 96 |
| 0.5 | 47 | 88 | 59 | 90 | 41 | 95 | 59 | 95 | 37 | 95 | 63 | 95 |
| 1 | 51 | 83 | 64 | 86 | 46 | 93 | 63 | 95 | 40 | 92 | 62 | 95 |
| 2 | 66 | 70 | 65 | 74 | 53 | 91 | 79 | 94 | 44 | 91 | 63 | 92 |
| 3 | 76 | 42 | 83 | 45 | 54 | 91 | 77 | 92 | 48 | 80 | 64 | 80 |
| NaCl | ||||||||||||
| (wt %) | Co-Solvents Type | |||||||||||
| Acetone | Diethyl Ether | Ethyl Acetate | ||||||||||
| n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | |||||||
| F | S | F | S | F | S | F | S | F | S | F | S | |
| 0 | 26 | 99 | 47 | 99 | 26 | 99 | 47 | 98 | 27 | 98 | 47 | 98 |
| 0.5 | 39 | 96 | 53 | 99 | 36 | 96 | 51 | 98 | 34 | 98 | 50 | 99 |
| 1 | 41 | 93 | 53 | 98 | 41 | 96 | 54 | 98 | 38 | 97 | 50 | 98 |
| 2 | 56 | 83 | 54 | 97 | 45 | 92 | 63 | 87 | 42 | 97 | 51 | 98 |
| 3 | 67 | 37 | 81 | 97 | 42 | 80 | 65 | 82 | 44 | 98 | 54 | 97 |
| MgCl2 | ||||||||||||
| (wt %) | Co-Solvents Type | |||||||||||
| Acetone | Diethyl Ether | Ethyl Acetate | ||||||||||
| n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | n-Hexane | n-Dodecane | |||||||
| F | S | F | S | F | S | F | S | F | S | F | S | |
| 0 | 27 | 99 | 48 | 98 | 27 | 99 | 48 | 98 | 27 | 99 | 48 | 98 |
| 0.5 | 41 | 97 | 62 | 98 | 42 | 98 | 54 | 98 | 35 | 95 | 54 | 99 |
| 1 | 45 | 94 | 61 | 98 | 40 | 97 | 57 | 96 | 39 | 95 | 53 | 99 |
| 2 | 56 | 88 | 63 | 97 | 47 | 96 | 64 | 96 | 44 | 92 | 54 | 98 |
| 3 | 74 | 26 | 82 | 84 | 44 | 97 | 69 | 99 | 45 | 77 | 56 | 98 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hobaib, A.S.; Al-Suhybani, M.S.; Al-Sheetan, K.M.; Mousa, H.; Shaik, M.R. New RO TFC Membranes by Interfacial Polymerization in n-Dodecane with Various co-Solvents. Membranes 2016, 6, 24. https://doi.org/10.3390/membranes6020024
Al-Hobaib AS, Al-Suhybani MS, Al-Sheetan KM, Mousa H, Shaik MR. New RO TFC Membranes by Interfacial Polymerization in n-Dodecane with Various co-Solvents. Membranes. 2016; 6(2):24. https://doi.org/10.3390/membranes6020024
Chicago/Turabian StyleAl-Hobaib, Abdullah Sulaiman, Mohammed Sulaiman Al-Suhybani, Khalid Mohammed Al-Sheetan, Hasan Mousa, and Mohammed Rafi Shaik. 2016. "New RO TFC Membranes by Interfacial Polymerization in n-Dodecane with Various co-Solvents" Membranes 6, no. 2: 24. https://doi.org/10.3390/membranes6020024
APA StyleAl-Hobaib, A. S., Al-Suhybani, M. S., Al-Sheetan, K. M., Mousa, H., & Shaik, M. R. (2016). New RO TFC Membranes by Interfacial Polymerization in n-Dodecane with Various co-Solvents. Membranes, 6(2), 24. https://doi.org/10.3390/membranes6020024







