Clarification of Orange Press Liquors by PVDF Hollow Fiber Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hollow Fiber Membrane Preparation and Post-treatment
| Dope composition (wt.%) | PVDF/DMF/PVP K-17 25/40/35 |
|---|---|
| Dope flow rate (g/min) | 12 |
| Dope temperature (°C) | 115 |
| Bore fluid composition (wt.%) and flow rate (mL/min) | Fiber A: DMF 45%, 9 mL/min Fiber B: DMF 45%, 12 mL/min Fiber C: DMF 45%, 15 mL/min Fiber D: DMF 45%, 18 mL/min Fiber E: EtOH 45%, 18 mL/min Fiber F: EtOH 45%, 15 mL/min |
| Bore fluid temperature (°C) | 50 |
| Outer coagulant | Tap water at room temperature |
| Air gap (cm) | 25 |
| Spinneret dimensions (cm) | O.D./I.D. 1.6/0.6 |
| Post treatment | NaClO 4000 ppm pH 7 overnight |
2.2. PVDF Hollow Fibers Characterization
2.2.1. Morphology
2.2.2. Mechanical Properties
2.2.3. Void Fraction
2.2.4. Bubble Point and Average Pore Size
2.2.5. Pure Water Permeability
2.3. Orange Press Liquor
2.4. Experimental Set-Up and Procedures
2.5. Analytical Methods
2.5.1. Total Soluble Solids, pH and Total Suspended Solids
2.5.2. Total Antioxidant Activity
2.5.3. Total Phenol Content
2.5.4. Determination of Flavonoids
2.5.5. Statistical Analysis
3. Results and Discussion
3.1. Hollow Fiber Morphology
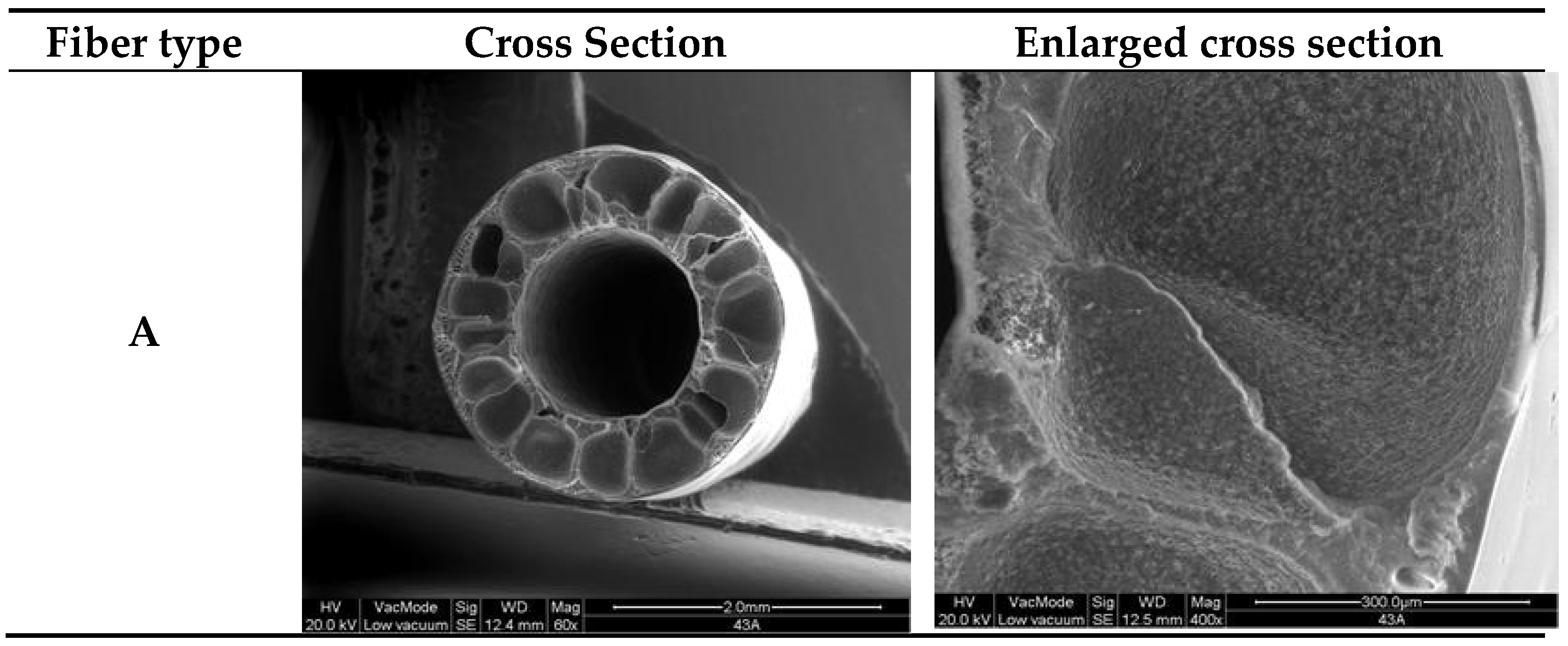
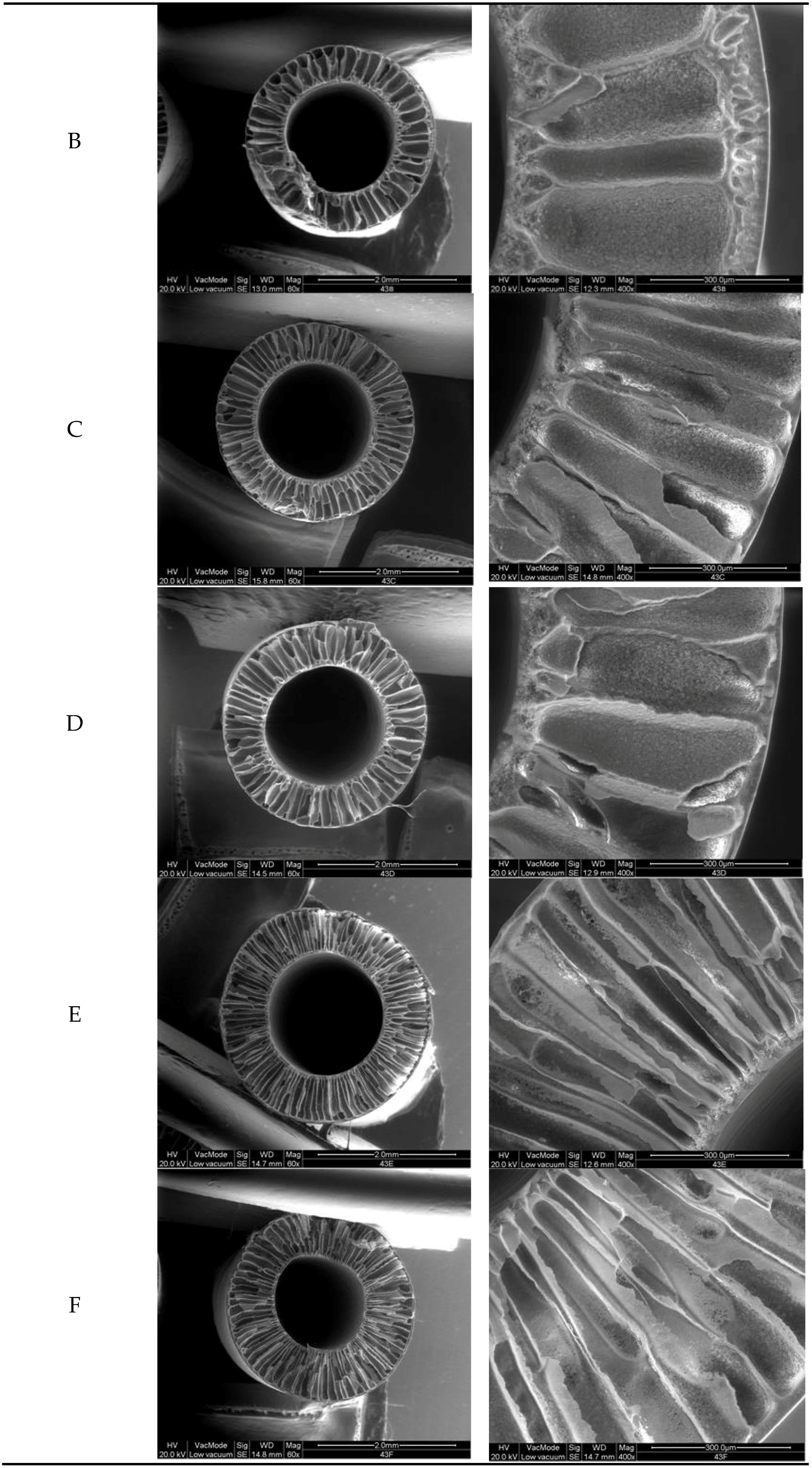
3.2. Fibers Mechanical Properties, Porosity, Pore Size and Pure Water Permeability
| Fiber Type | O.D. | I.D. | Thickness | Emod | εbreak | Porosity | Bubble Point | Pore Diameter | PWP * |
|---|---|---|---|---|---|---|---|---|---|
| (mm) | (mm) | (mm) | (N/mm²) | (%) | (%) | (bar) | (m) | (L/m2·h·bar) | |
| A | 2.60 ± 0.02 | 1.44 ± 0.04 | 0.58 ± 0.03 | 39.15 ± 2.94 | 79.71 ± 5.82 | 88.32 ± 1.23 | 1.45 | 0.31 | 340 |
| B | 2.73 ± 0.03 | 1.65 ± 0.01 | 0.54 ± 0.02 | 28.17 ± 4.50 | 122.12 ± 7.28 | 90.64 ± 0.21 | 0.98 | 0.14 | 207 |
| C | 2.87 ± 0.03 | 1.69 ± 0.03 | 0.59 ± 0.03 | 20.72 ± 0.50 | 109.87 ± 6.61 | 91.36 ± 0.79 | 0.67 | 0.17 | 369 |
| D | 2.98 ± 0.02 | 1.74 ± 0.02 | 0.62 ± 0.02 | 19.22 ± 1.45 | 98.55 ± 2.95 | 91.54 ± 0.34 | 0.49 | 0.22 | 530 |
| E | 3.10 ± 0.02 | 1.72 ± 0.05 | 0.69 ± 0.03 | 15.75 ± 1.44 | 119.41 ± 4.43 | 92.10 ± 0.65 | 0.48 | 0.12 | - |
| F | 2.79 ± 0.05 | 1.36 ± 0.06 | 0.71 ± 0.05 | 17.40 ± 1.32 | 122.90 ± 5.64 | 91.25 ± 0.39 | 0.37 | 0.14 | 345 |
3.3. Performance of HF Membranes in the Treatment of Orange Press Liquor
3.3.1. Effect of TMP on Permeate Flux
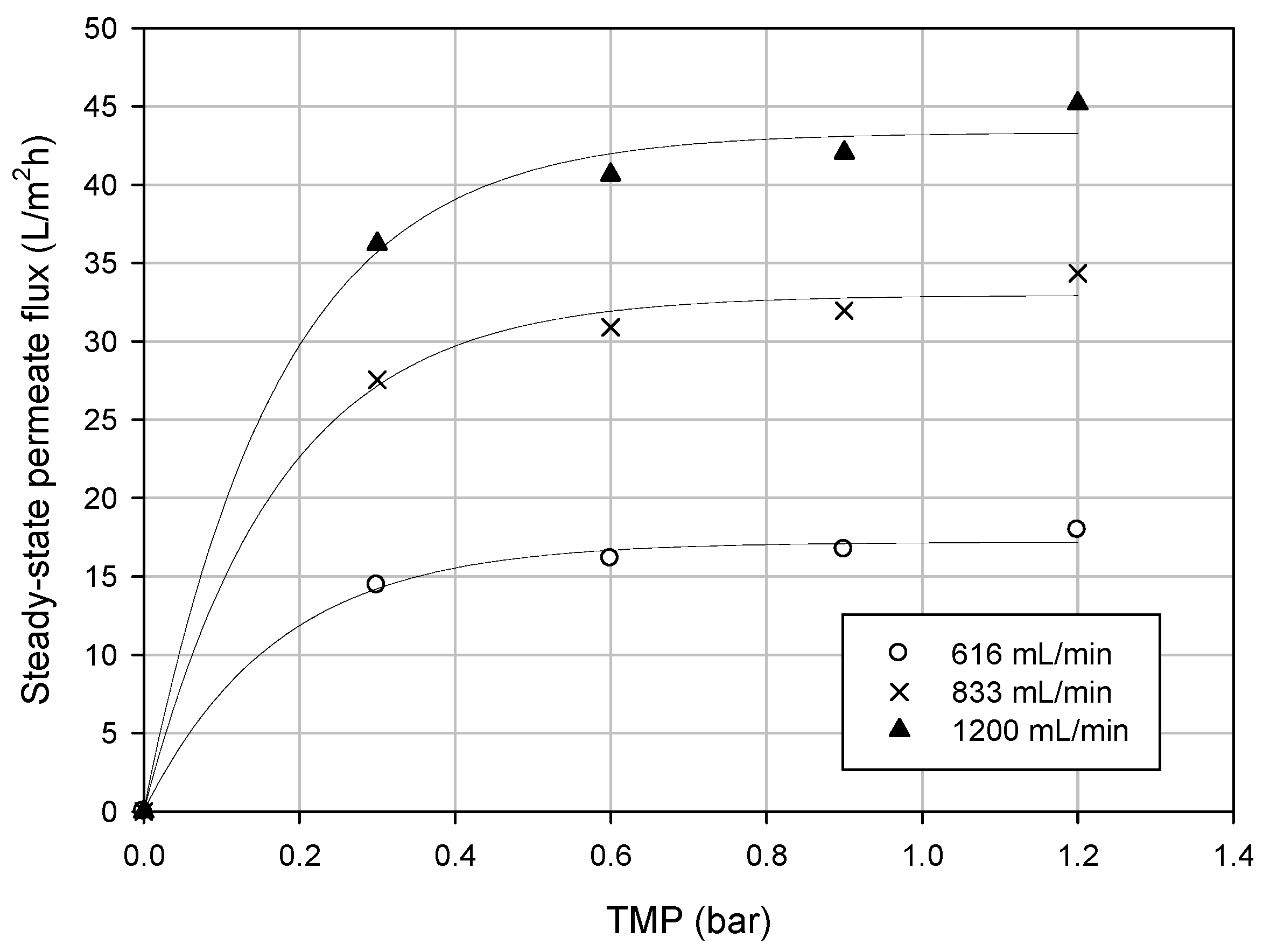
3.3.2. Experimental Runs According to the Batch Concentration Mode
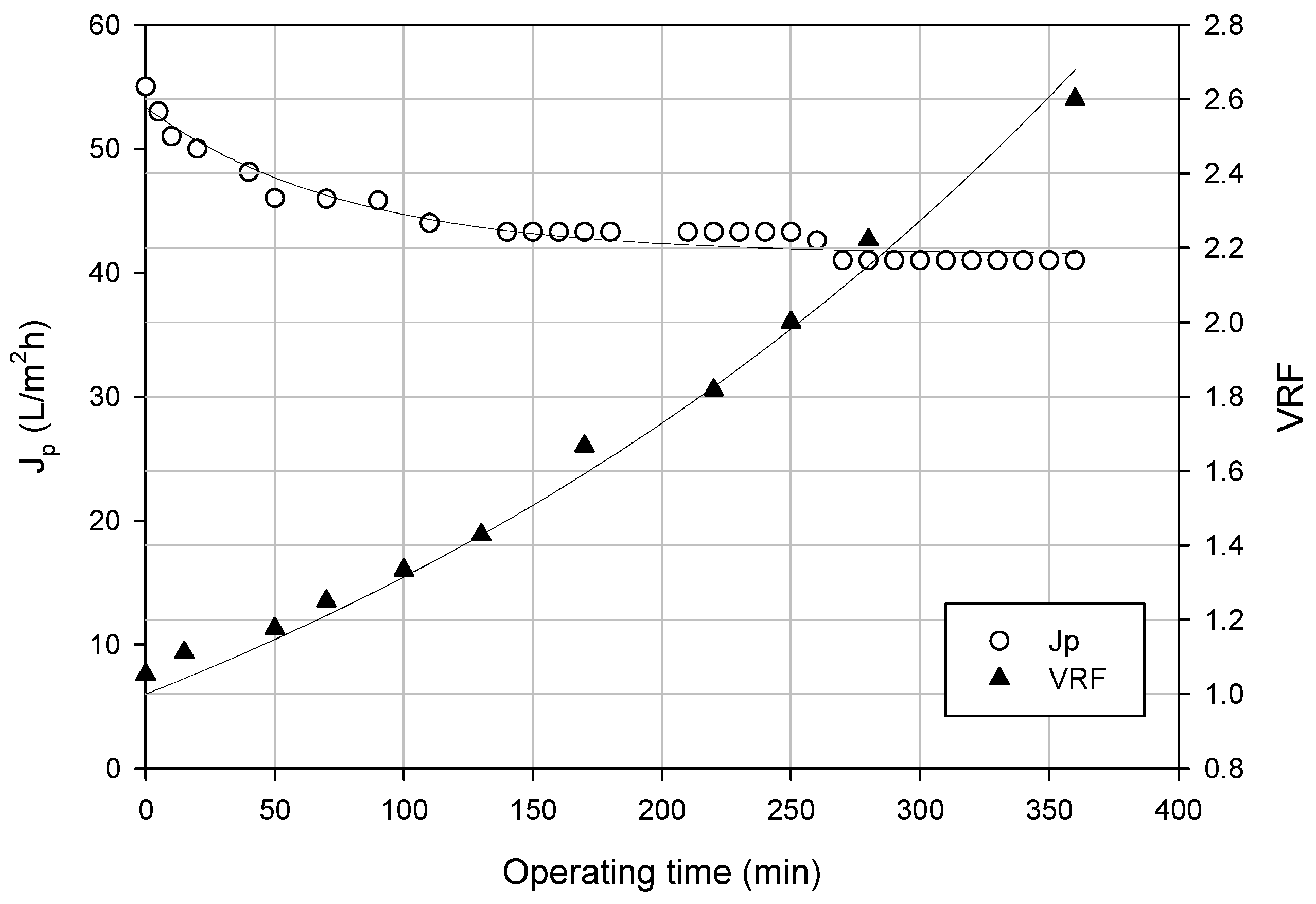
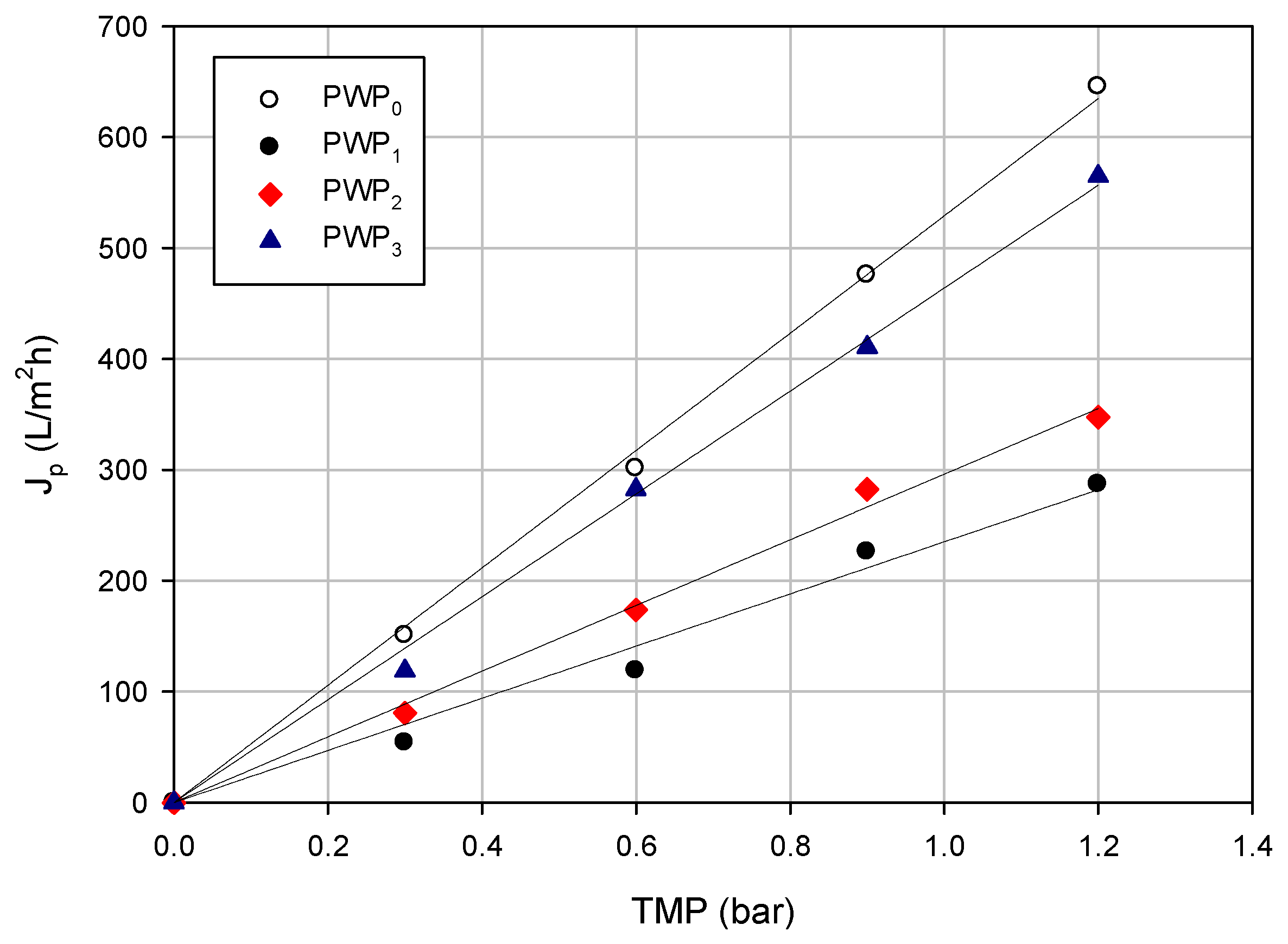
3.3.3. Analytical Results
| Parameters | Feed | Permeate | Retentate |
|---|---|---|---|
| Suspended solids (%) | 8.3 ± 0.2 a | - | 9.0 ± 0.5 a |
| TSS (°Brix) | 18.0 ± 0.1 a | 17.8 ± 0.2 a | 18.2 ± 0.4 a |
| pH | 3.5 ± 0.2 a | 3.5 ± 0.1 a | 3.4 ± 0.4 a |
| TAA (mM Trolox) | 21.4 ± 3.5 a | 21.1 ± 3.7 a | 18.4 ± 2.2 a |
| Total polyphenols (as GAE) (ppm) | 1217.3 ± 57.0 a | 1167.0 ± 30.6 a | 1346.6 ± 13.8 b |
| Neohesperidin (ppm) | 20.70 ± 0.41 a | 19.50 ± 0.39 a | 17.00 ± 0.34 b |
| Hesperidin (ppm) | 18.40 ± 0.36 a | 17.40 ± 0.34 b | 19.20 ± 0.38 a |
| Naringin (ppm) | 5.54 ± 0.11 a | 5.51 ± 0.11 a | 5.82 ± 0.11 b |

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Citrus fruit. Fresh and processed. Annual statistics, 2012. Available online: http://www.fao.org/fileadmin/templates/est/COMM_MARKETS_MONITORING/Citrus/Documents/CITRUS_BULLETIN_2012.pdf (accessed on 27 May 2013).
- Nikolic, J.A.; Cuperlovic, M.; Milijic, C.; Djordjevic, D.; Krsmanovic, J. The potential nutritive value for ruminants of some fibrous residues from the food processing industry. Acta. Vet-Beograd. 1986, 36, 13–22. [Google Scholar]
- Garcia-Castello, E.M.; Mayor, L.; Chorques, S.; Arguelles, A.; Vidal-Brotons, D.; Gras, M.L. Reverse osmosis concentration of press liquor from orange juice solid wastes: Flux decline mechanisms. J. Food Eng. 2011, 106, 199–205. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R. Dewatering press liquor derived from orange production by forward osmosis. J. Membr. Sci. 2011, 372, 97–101. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Bocco, A.; Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidant activity and phenolic composition of citrus peel and seed extract. J. Agric. Food Chem. 1998, 46, 2123–2129. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Caristi, C.; Leuzzi, U.; Gattuso, G. Flavonoid profile and radical-scavenging activity of mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem. 2011, 129, 417–422. [Google Scholar] [CrossRef]
- Marín, F.R.; Martínez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M.J. Changes in nutraceutical composition of lemon juices according to different industrial extraction systems. Food. Chem. 2002, 78, 319–324. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fallico, B.; Passerini, A.; Maccarone, E. Waste water from Citrus processing as a source of hesperidin by concentration on styrene-divinylbenzene resin. J. Agr. Food Chem. 2000, 48, 2291–2295. [Google Scholar] [CrossRef]
- Xu, G.H.; Ye, X.Q.; Chen, J.C.; Liu, D.H. Effect of Heat Treatment on the Phenolic Compounds and Antioxidant Capacity of Citrus Peel Extract. J. Agr. Food Chem. 2007, 55, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Oufedjikh, H.; Mahrouz, M.; Amiot, M.J.; Lacroix, M. Effect of γ-irradiation on phenolic compounds and phenylalanine ammonia-lyase activity during storage in relation to peel injury from peel of Citrus clementina Hort. Ex. Tanaka. J. Agr. Food Chem. 2000, 48, 559–565. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels II. Enzyme-assisted extraction method. Sep. Purif. Technol. 2006, 48, 189–196. [Google Scholar] [CrossRef]
- Drioli, E.; Di Profio, G.; Fontananova, E. Membrane separations for process intensification and sustainable growth. Fluid/Part. Sep. J. 2004, 16, 1–18. [Google Scholar]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Chung, T.S.; Qin, J.J.; Gu, J. Effect of shear rate within the spinneret on morphology, separation performance and mechanical properties of ultrafiltration polyethersulfone hollow fiber membranes. Chem. Eng. Sci. 2000, 55, 1077–1091. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Tasselli, F.; Conidi, C.; Drioli, E.; Timpone, R.; D’Avella, M.; Badalamenti, F.; Corleone, V. PAN hollow fibre membranes with triacetyl-b-cyclodextrin for the removal of pesticides from citrus essential oils. Sep. Purif. Technol. 2013, 116, 124–130. [Google Scholar] [CrossRef]
- Cassano, A.; Tasselli, F.; Conidi, C.; Drioli, E. Ultrafiltration of Clementine mandarin juice by hollow fibre membranes. Desalination 2009, 241, 302–308. [Google Scholar] [CrossRef]
- Tasselli, F.; Cassano, A.; Drioli, E. Ultrafiltration of kiwifruit juice using modified poly(ether ether ketone) hollow fibre membranes. Sep. Purif. Technol. 2007, 57, 94–102. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Clarification and concentration of pomegranate juice (Punica granatum L.) using membrane processes. J. Food Eng. 2011, 107, 366–373. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Timpone, R.; D’Avella, M.; Drioli, E. A membrane-based process for the clarification and the concentration of the cactus pear juice. J. Food Eng. 2007, 80, 914–921. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. A membrane-Based process for the valorization of the bergamot juice. Sep. Sci. Technol. 2013, 48, 537–546. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Membr. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Simone, S.; Macedonio, F.; Al-Jlil, S.A.; Al Shabonah, F.S.; Al-Romaih, H.S.; Al-Harbi, O.; Figoli, A.; Criscuoli, A. Novel PVDF hollow fiber membranes for vacuum and direct contact membrane distillation applications. Sep. Purif. Technol. 2013, 115, 27–38. [Google Scholar] [CrossRef]
- Figoli, A.; Simone, S.; Criscuoli, A.; Al-Jlil, S.A.; Al Shabouna, F.S.; Al-Romaih, H.S.; Di Nicolò, E.; Al-Harbi, O.A.; Drioli, E. Hollow fibers for seawater desalination from blends of PVDF with different molecular weights: Morphology, properties and VMD performance. Polymer 2014, 55, 1296–1306. [Google Scholar] [CrossRef]
- Feng, C.; Shi, B.; Li, G.; Wu, Y. Preparation and properties of microporous membrane from poly (vinylidene fluoride-co-tetrafluoroethylene) (F2.4) for membrane distillation. J. Membr. Sci. 2004, 237, 15–24. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J. Total antioxidant status in plasma and body fluids. Methods Enzymol. 1994, 234, 279–293. [Google Scholar] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Lin, D.; Chang, C.; Chang, C.; Chen, T.; Cheng, L. Fine structure of poly(vinylidene fluoride) membranes prepared by phase inversion from a Water/N-Methyl-2-pyrrollidone/poly(vinylidene fluoride system. J. Polym. Sci. Pt. B-Polym. Phys. 2004, 42, 830–842. [Google Scholar] [CrossRef]
- Lee, K.W.; Se, B.K.; Nam, S.T.; Han, M.J. Trade-off between thermodynamic enhancement and kinetic hindrance during phase inversion in the preparation of polysulfone membranes. Desalination 2003, 159, 289–296. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.S. Molecular elucidation of morphology and mechanical properties of PVDF hollow fiber membranes from aspects of phase inversion, crystallization and rheology. J. Membr. Sci. 2009, 340, 192–205. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.S.; Lai, J.Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Peng, N.; Chung, T.S.; Lai, J.Y. The rheology of Torlon® solutions and its role in the formation of ultra-thin defect-free Torlon® hollow fiber membranes for gas separation. J. Membr. Sci. 2009, 326, 608–617. [Google Scholar] [CrossRef]
- Tasselli, F.; Jansen, J.C.; Drioli, E. PEEKWC Ultrafiltration Hollow-Fiber Membranes: Preparation, Morphology, and Transport Properties. J. Appl. Polym. Sci. 2004, 91, 841–853. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Alfadul, S.; Al-Romaih, H.; Al-Shabouna, F.; Al-Harbi, O.A.; Drioli, E. Effect of selected spinning parameters on PVDF hollow fibers morphology for potential application in desalination by VMD. Desalination 2014, 344, 28–35. [Google Scholar] [CrossRef]
- Liu, Y.; Koops, G.H.; Strathmann, H. Characterization of morphology controlled polyethersulfone hollow fiber membranes by the addition of polyethylene glycol to the dope and bore liquid solution. J. Membr. Sci. 2003, 223, 187–199. [Google Scholar] [CrossRef]
- Vladisavljevic, G.T.; Vukosavljevic, P.; Bukvic, B. Permeate flux and fouling resistance in ultrafiltration of depectinized apple juice using ceramic membranes. J. Food Eng. 2003, 60, 241–274. [Google Scholar] [CrossRef]
- de Barros, S.T.D.; Andrade, C.M.G.; Mendes, E.S.; Peres, L. Study of fouling mechanism in pineapple juice clarification by ultrafiltration. J. Membr. Sci. 2003, 215, 213–224. [Google Scholar] [CrossRef]
- Carvalho, L.M.J.; Castro, I.M.; Silva, C.A.V. A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro- and ultra-filtration. J. Food Eng. 2008, 87, 447–454. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mohammad-Mousavi, S.; Aroujalian, A.; Navidbakhsh, M. Clarification of pomegranate juice by microfiltration with PVDF membranes. Desalination 2010, 264, 243–248. [Google Scholar] [CrossRef]
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.S.; Haruenkit, R.; Lojek, A.; Ciz, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tornberg, E.; Gekas, V. Clarification of high-added value products from olive mill wastewater. J. Food Eng. 2010, 99, 190–197. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simone, S.; Conidi, C.; Ursino, C.; Cassano, A.; Figoli, A. Clarification of Orange Press Liquors by PVDF Hollow Fiber Membranes. Membranes 2016, 6, 9. https://doi.org/10.3390/membranes6010009
Simone S, Conidi C, Ursino C, Cassano A, Figoli A. Clarification of Orange Press Liquors by PVDF Hollow Fiber Membranes. Membranes. 2016; 6(1):9. https://doi.org/10.3390/membranes6010009
Chicago/Turabian StyleSimone, Silvia, Carmela Conidi, Claudia Ursino, Alfredo Cassano, and Alberto Figoli. 2016. "Clarification of Orange Press Liquors by PVDF Hollow Fiber Membranes" Membranes 6, no. 1: 9. https://doi.org/10.3390/membranes6010009
APA StyleSimone, S., Conidi, C., Ursino, C., Cassano, A., & Figoli, A. (2016). Clarification of Orange Press Liquors by PVDF Hollow Fiber Membranes. Membranes, 6(1), 9. https://doi.org/10.3390/membranes6010009









