Abstract
In recent years, the use of biomimetic membranes that incorporate membrane proteins, i.e., biomimetic-hybrid membranes, has increased almost exponentially. Key membrane proteins in these systems have been aquaporins, which selectively permeabilize cellular membranes to water. Aquaporins may be incorporated into synthetic lipid bilayers or to more stable structures made of block copolymers or solid-state nanopores. However, translocation of aquaporins to these alien environments has adverse consequences in terms of performance and stability. Aquaporins incorporated in biomimetic membranes for use in water purification and desalination should also withstand the harsh environment that may prevail in these conditions, such as high pressure, and presence of salt or other chemicals. In this respect, modified aquaporins that can be adapted to these new environments should be developed. Another challenge is that biomimetic membranes that incorporate high densities of aquaporin should be defect-free, and this can only be efficiently ascertained with the availability of completely inactive mutants that behave otherwise like the wild type aquaporin, or with effective non-toxic water channel inhibitors that are so far inexistent. In this review, we describe approaches that can potentially be used to overcome these challenges.
1. General Features of Aquaporins
Aquaporins (AQPs) are integral membrane proteins that transport water through cellular membranes [1,2,3]. In humans, there are 13 different AQPs [4], from AQP0 to AQP12. The last 15 years have revealed important physiological roles for AQPs (reviewed in [5]), not surprisingly related to their water channel activity, e.g., in the urinary concentrating mechanism, glandular fluid secretion, or brain swelling. The architecture of AQPs is remarkably conserved from bacteria to humans [6,7,8,9,10,11,12]: the functional form is a homo-tetramer, where each AQP monomer has six transmembrane (TM) α-helical domains and functions independently as a water channel, e.g., human AQP1 [13] (Figure 1). Each monomer can transport as many as 3 billion water molecules per second, while rejecting all other solutes, including protons [14]. Such high permeabilities and selectivities depend on geometric and physico-chemical factors that are now beginning to be understood, as recently shown for a yeast aquaporin [15].

Figure 1.
General structure of aquaporins, with a tetrameric arrangement of water channels. This structure is for human AQP1.
The availability of functional AQP proteins of high purity has helped tremendously in the high-resolution X-ray structural determination of important AQPs: Escherichia coli orthodox AqpZ [16] and glycerol facilitator GlpF [17], archaeal AqpM [11], mammalian AQP0 [18], AQP1 [7], AQP2 [19], AQP4 [20] and AQP5 [9], as well as spinach SoPIP2;1 [21]. The use of solid-state NMR may also be useful for structural studies of AQPs in the near future, as suggested by high resolution spectra obtained for hAQP1 [22]. Recombinant AQP proteins are commonly expressed from several host organisms, including the bacterial E. coli [23], mammalian insect Spodoptera frugiperda Sf9 [24], yeast Pichia pastoris [25], and Saccharomyces cerevisiae [26]. Typically, a target AQP encoded by a host expression vector will be expressed in fusion with an affinity tag, commonly a polyhistidine (6His) tag. Once this recombinant AQP is produced from host cells, host lysis is closely followed by reconstitution of the recombinant AQP from host cell membranes into stabilizing biomimetic environments, i.e., detergent micelles. This will provide a suitable environment for downstream affinity purification of the recombinant AQP using nickel-nitrilotriacetic acid (Ni-NTA) chromatography.
The addition of recombinant fusion partners such as the maltose binding protein (MBP), and the optimization of codon usage has been shown to boost production levels of AqpZ to 200 mg/L of E. coli culture [27]. Similarly, protein yields of human AQPs expressed from P. pastoris can also be improved by codon optimization and clone selection [28]. In addition, high production of AQP proteins is also possible using cell-free (CF) expression system, a novel approach which mimics the natural cell cytoplasmic environment for protein synthesis. This holds advantages over traditional in vivo membrane protein expression in living cells, such as elimination of toxicity to host cell physiology due to membrane incorporation of recombinant proteins, and overloading of essential cellular protein-targeting machineries in order to overexpress a foreign protein [29]. CF extracts can be obtained from E. coli cells, wheat germs, rabbit reticulocytes, insect cells, and more recently, Chinese hamster ovary (CHO) cells [30]. In the CF system, newly synthesized AQP proteins can be incorporated directly into artificial hydrophobic environments, like detergent micelles [31] or synthetic liposomes [32]. By introducing isotopically-labelled or fluorescence-enhanced amino acids into the reaction mixture, efficient labelling of the target AQP is also possible.
2. Aquaporins in Biomimetic Membranes
The exquisite permeability and selectivity of aquaporins to water made them a unique component in the development of water filtration devices. Since a landmark paper in 2007 [33], development of desalination membranes based on AQPs (E. coli aquaporin Z, AqpZ) has attracted much attention globally. Research in this field has grown over the last few years, e.g., [34,35,36,37,38]. Recent reviews are available for aquaporin-based biomimetic membranes [39,40] and for biomimetic membranes in general [41].
For example, significant improvements have been achieved using AqpZ-based proteoliposomes in a cross-linked polyamide matrix by interfacial polymerization [38], but water permeability is still modest compared to their expected values. One possible cause is the negative impact of the chemicals used for interfacial polymerization on the activity of AQPs in lipid membranes (proteoliposomes). In addition, the loading amount of proteoliposome in the selective layer is relatively low. In addition, membrane defect minimization depends on chemical modification of the membrane, which may, in turn, have a negative impact on AQP function.
Amphiphilic block copolymers (BCPs) have also been used as substitutes of lipids. These assemble into bilayer-like structures [42] that have superior properties when compared to lipids, e.g., higher mechanical and chemical stability and low water and gas permeability. In addition, the geometric and chemical characteristics of these membranes can be customized. The use of different BCPs is an alternative strategy, but, so far, tests are limited to a single polymer type, polydimethylsiloxane (PDMS) hydrophobic block. Alternatively, functional AqpZ mutants could be screened for compatibility and insertion efficiency with a given polymer type. These mutants may also lead to higher expression levels, which is another fundamental challenge for large-scale applications. While high yields have been reported [43] in E. coli, these were obtained from a fusion form, which may require further purification steps.
Additionally, although AQPs other than AqpZ have been incorporated into BCP membranes with success [44], the requirements for compatibility between membrane protein and polymer are not well understood, therefore a rational basis for polymer selection does not exist.
A successful biomimetic membrane would require high levels of protein packing in membranes, compared to the low levels achieved so far for aquaporins in BCP membranes. For AqpZ in poly-(2-methyloxazoline)-block-poly-(dimethylsiloxane)-block-poly-(2-methyloxazoline) (PMOXA-PDMS-PMOXA) triblock copolymer membranes, a molar polymer to protein ratio of 50–100 was obtained, beyond which permeability decreased [33]. Optimization of these parameters can be achieved with the availability of robust and functional AQP mutants, which can withstand a wider range of chemical modifications, while at the same time increase shelf life and potentially increase insertion efficiency and packing. Another exciting achievement would be to obtain AQP mutants that can withstand cross-linking with lipids in order to stabilize membranes, without being denatured [45] while still retaining permeability and selectivity.
These AQP mutants should have increased stability while obviously retaining their function. Fortunately, it is not difficult to obtain stabilizing mutations in a membrane protein, while retaining function [46,47,48]. However, at present, it is impractical to engineer these mutants based only on theoretical principles and available detailed structural data [15]. Generic assays to select thermostable mutants of proteins have been recently reported [49], but these mutants have to be tested for function later, in a high-throughput manner. This is especially challenging for aquaporins, which do not have easily testable enzymatic or binding activities. In the following sections, we will provide an overview on the current assays used to study aquaporin function with special emphasis on a recently developed assay for the identification of functional AQP mutants and how these mutants can be further characterized for protein stability.
3. Functional Assays for Aquaporins
Currently known functional assays for AQPs described in the literature are summarized in Table 1, along with their ease of use and high-throughput capabilities.
3.1. Stopped-Flow Water Permeability Assay
The kinetics of cell volume changes in response to rapidly imposed osmotic gradients can be monitored to quantify water permeability through membranes containing AQPs. This can be performed using suspended cells (e.g., erythrocytes which express native AQP1), plasma membrane vesicles obtained from AQP-expressing cells, or artificial liposomes (of a defined size) reconstituted with purified AQP proteins (proteoliposomes) [50]. Typically, the rate of cell or vesicle swelling or shrinking due to rapid hypotonic or hypertonic osmotic challenges can be studied by following changes in light scattering intensity. Alternatively, vesicles can be pre-loaded with fluorophores such as carboxyfluorescein, where changes in intravesicular fluorescence caused by the self-quenching of entrapped carboxyfluorescein as a result of water efflux can be measured [51]. Water flow measurements require specialized instrumentation, i.e., the stopped-flow spectrometer [52]. For instance, the rapid mixing of a cell suspension with a hyperosmolar solution (containing a nonpermeant osmolyte such as sucrose) will generate an osmotic gradient to drive water efflux, and the rate of cell shrinkage is measured based on the change in intensity of 90° scattered light (λ = 500 nm), as a function of time, allowing for calculation of osmotic water permeability (Pf) [53].

Table 1.
Currently known aquaporins (AQP) water transport functional assays.
| Assay | System | Readout | Throughput | Characteristics | References |
|---|---|---|---|---|---|
| Stopped-flow water permeability assay | Suspended AQP-proteoliposomes, vesicles or cells (e.g., erythrocytes) | Light scattering or fluorescence changes | Low; about 10 samples per hour | Requires specialized instrumentation i.e., stopped-flow spectrometer | [ 52,53] |
| Transepithelial assay | Cell monolayers cultured on porous support | Dilution of indicator dye | Low; 12 wells per plate | Virtually free from artifacts Laborious to perform | [ 54] |
| Fluorescence-based assay | Cell monolayers cultured on solid support | Cytoplasmic fluorescence changes | Medium; 96-well plates | Potential artifacts related to interaction of compound with reporter fluorescence Easy to perform | [ 55–57] |
| Oocyte swelling assay | Oocytes from Xenopus laevis | Oocyte imaging | Low; about 2–5 samples per day | Prone to artifacts Technically challenging | [ 3] |
| Erythrocyte lysis assay | Erythrocytes | Cell lysis | High; 96-well plates | Only applicable for AQP1 | [ 58,59] |
| Yeast freeze-thaw assay | Yeast cells | Cell viability | High; 96-well plates | Generic Easy to perform | [ 60] |
3.2. Transepithelial Assay
Mammalian transepithelial assays measure water flux across a tight cell layer cultured on a porous support, with a typical transepithelial resistance of 1–2 kΩ·cm2 [54]. The cells under study can either be expressing endogenous AQPs, or to be stably transfected with plasmids encoding AQPs. In this assay, transepithelial water permeability driven by an osmotic gradient using membrane-impermeable solutes such as mannitol or sucrose is measured using a dye dilution method. Measurements of the rate of fluorescence changes of an indicator dye in the apical fluid volume enable a quantitative readout of water flux across the epithelial cell layer in order to compute the transepithelial osmotic water permeability coefficient (Pf). Although this method is sensitive and virtually free from artifacts, it requires expertise in the use of cells and is therefore recommended to be used as a secondary assay to verify hits obtained from primary high-throughput screening campaigns.
3.3. Fluorescence-Based Assays
Cell volume changes can be studied by monitoring changes in the intensity of fluorescence reporter dyes in fluorescently-labelled cells. In the calcein method, cells cultured on a solid support, such as a 96-multiwell fluorescence plate format, are loaded with membrane-permeable calcein-acetomethoxy derivate (calcein-AM), which gets trapped intracellularly due to cleavage by cellular esterases. The screening strategy is based on the kinetics of concentration-dependent self-quenching of calcein fluorescence in response to cell shrinkage. Upon rapid exposure to a hypertonic stimulus, water leaves the cells through AQPs, leading to changes in fluorescence which is directly proportional to changes in cell water volume [55]. Since the assay can be performed multiwell plate format, it has the potential for lab automation. A similar fluorescence-based method uses genetically-encoded, cytoplasmically expressed fluorescent proteins, i.e., a mutant yellow fluorescence protein, YFP-H148Q/V163S, whose fluorescence is quenched by chloride [56]. Using this fluorescence chloride sensor, osmotic water permeability can be measured from the kinetics of decreasing cytoplasmic chloride concentrations resulted from an osmotically induced cell swelling. In order for the kinetics of cell volume changes to be measured accurately, the osmotic response of the cells has to be sufficiently slow (~few seconds), while the time taken for solution mixing should be short relative to the time course of osmotic equilibration.
3.4. Oocyte Swelling Assay
The Xenopus laevis oocyte swelling assay is the original functional assay used to demonstrate that AQP1 functions as a water channel [3]. In this system, defolliculated oocytes are microinjected with in vitro-transcribed AQP1 capped RNA (cRNA) to induce expression of AQP1 at the oocyte membrane. After ~72 h incubation, the oocyte will be transferred from an isotonic medium to a hypotonic medium, and oocyte volume changes will be followed with video microscopy using sequential oocyte images photoed at 15 s intervals for a total of 5 min or until just before the rupture of oocyte membrane. The osmotic water permeability Pf can be determined from the time-course changes in relative oocyte volume [3].
3.5. Erythrocyte Lysis Assay
A high-throughput method, based on the lysis of erythrocytes, has previously identified inhibitors of the urea transporter B (UT-B) of nanomolar potency [58], and can be improvised to screen for AQP inhibitors [59]. Human erythrocytes, which express endogenous AQP1 and urea transporter UT-B, are first pre-loaded with the urea analog acetamide, which is transported by UT-B and equilibrates across the erythrocyte membrane but at a slightly slower rate compared to water. The dilution of these acetamide-loaded erythrocytes into an acetamide-free solution results in a rapid AQP-dependent influx of water, cell swelling and subsequent lysis. If the AQP is not functional, or in the presence of an AQP1 inhibitor, the rate of water influx is reduced, thereby allowing the dissipation of the osmotic gradient by efflux of acetamide. As a result, cell lysis is prevented. Extent of cell lysis can be quantified by single time point readout of near-infrared light absorbance at 710 nm wavelength.
3.6. Yeast Freeze-Thaw Assay
This assay is based on the protective effect of AQPs on yeast in response to rapid freezing challenge. This effect was observed in earlier studies of the baker’s yeast Saccharomyces cerevisiae, where a correlation was shown to exist between freeze-resistance and expression of yeast aquaporins AQY1 and AQY2; deletion of AQP-encoding genes made yeast more sensitive to freezing, whereas overexpression of AQPs (yeast AQY1/AQY2 or human hAQP1) improved their freeze-thaw resistance [61,62]. Apparently, AQPs permit a rapid efflux of water through the yeast membrane during freezing, reducing intracellular ice crystal formation and thereby rescuing the cell from damage upon thawing [61,62]. This has been the basis to develop a generic high-throughput assay [60] that is in principle applicable to AQPs of any organism as long as they transport water.
In this assay, yeast cells lacking native AQPs but overexpressing an AQP of choice are exposed to freeze-thawing. Only cells expressing functional water-permeable AQPs are rescued from the challenge. Yeast expressing inactive AQPs, or yeast expressing active AQPs but exposed to AQP inhibitors, are not protected which results in cell death (see schematic representation of this method in Figure 2). Identification of functional AQP mutants can be achieved by generating first a library of random AQP mutants, which are then tested for viability after a freeze-thawing challenge (Figure 3) [60].
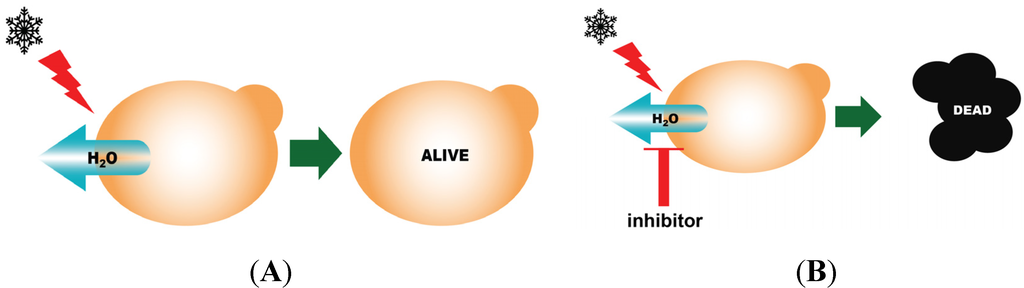
Figure 2.
Schematic representation of the yeast-based freeze-thaw assay. If the yeast expresses an active AQP, they will survive a freeze-thaw challenge (A); If an inactive AQP is expressed, or if an active AQP expressed is exposed to an AQP inhibitor, the protective effect is lost and the yeast will die (B).
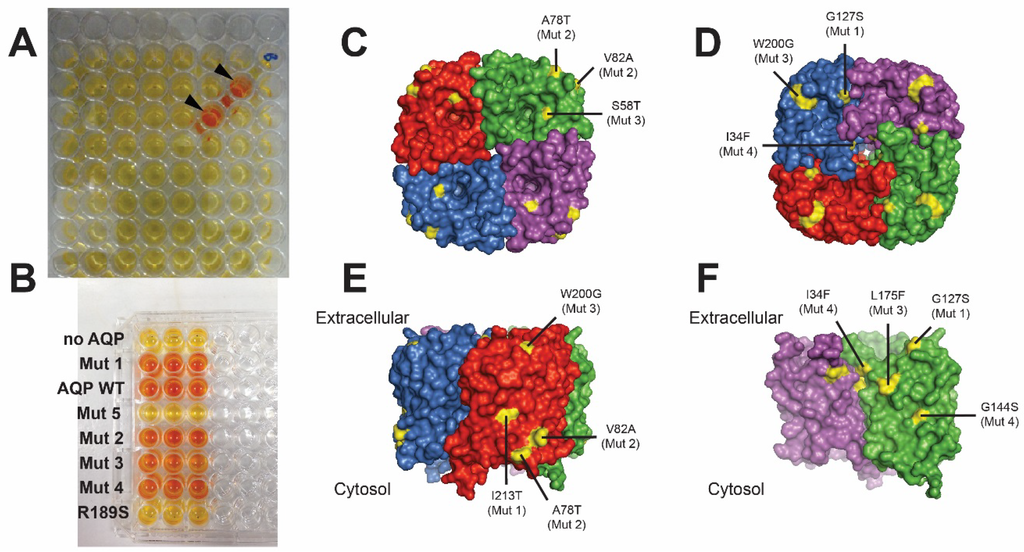
Figure 3.
Selection of functional AqpZ mutants. (A) Screening of a 96-well plate with yeast expressing AqpZ mutants, showing two wells with water-permeable AqpZ (arrows). Conversion of added reagents to orange colour indicates presence of viable cells; (B) Re-screening comparing yeast without AQP or expressing wild type AqpZ (WT), an inactive mutant (R189S) and several inactive/active mutants found; (C-F) Nine functional mutations (yellow), out of 160 mutants carrying 1–5 mutations each, shown on the structure of the AqpZ tetramer: view from (C) cytoplasmic and (D) extracellular side; (E,F) side view (E) and after removing two monomers (F). Results adapted from [60].
One further advantage of this method is that these mutants may then be tested for thermostability while still in the yeast, avoiding lengthy expression and purification steps. This is possible because native AqpZ is tetrameric in detergent sodium dodecyl sulfate (SDS) (unlike hAQP1, which is monomeric) but forms monomers when heated (Figure 4A). In contrast, native hAQP1 forms monomers in SDS at room temperature, hence hAQP1 mutants with higher stability may retain their tetrameric form at room temperature. Therefore, a Western blot analysis on yeast cell lysates can detect increased thermal stability in AqpZ and resistance to SDS denaturation in AQP1, bypassing purification steps. Following this workflow, only those promising mutants would be then analyzed in detail using downstream biophysical assays, e.g., analytical ultracentrifugation sedimentation velocity (SV) to determine monomer-tetramer association constants.
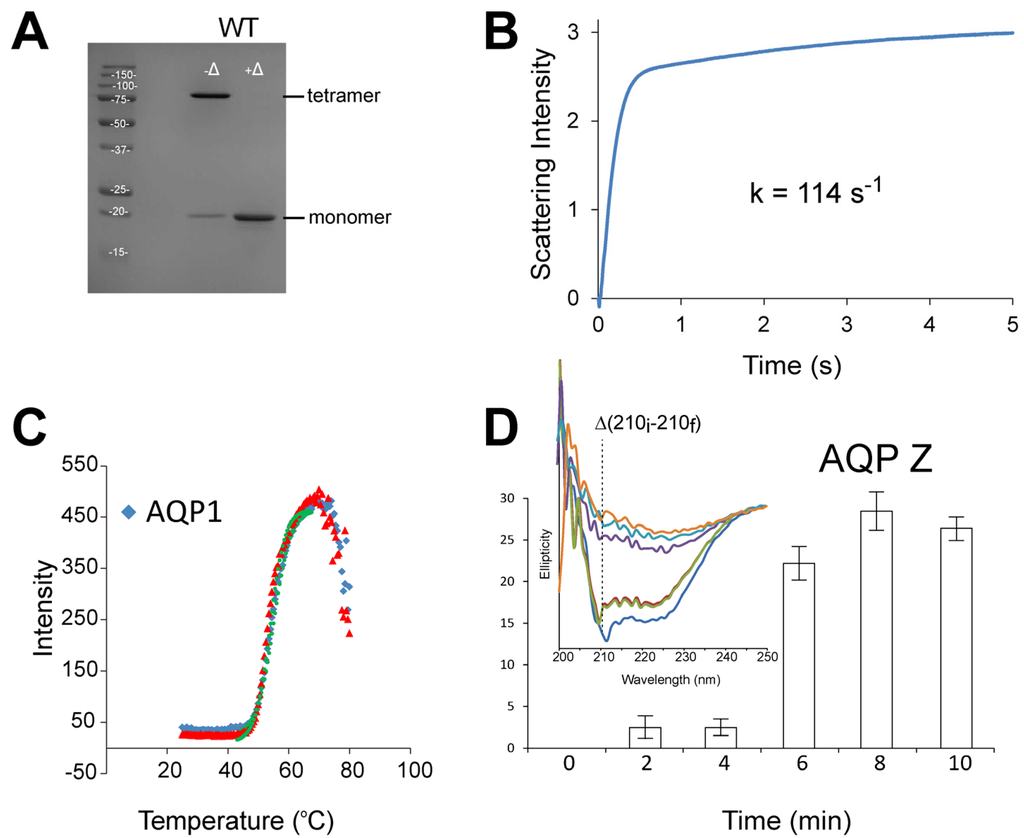
Figure 4.
Aquaporin thermostability and functional assays. (A) In SDS, AqpZ is tetrameric at room temperature −Δ but monomeric at higher temperature +Δ; (B) Stopped-flow water permeability assay, where light scattering is continuously recorded after mixing AqpZ proteoliposomes with a high sucrose solution; (C) TSAdata of hAQP1 for two sample repeats (blue and red) obtained using StarGazer-384. Data collected can be analysed using the Bioactive software (Harbinger Biotech), where the measured scattering intensities are plotted as a function of temperature and fitted to the Boltzmann equation by non-linear regression (green line). The temperature of aggregation (Tagg) is defined as the point of inflection (dotted lines)Shift of Tagg to a higher temperature indicates an increase in protein thermostability; (D) Unfolding of AqpZ, measured as decrease in ellipticity, when the protein is heated at constant temperature, e.g., 78 °C. In this case, the loss of α-helix content is measured at 210 nm, and expressed as the difference between the initial ellipticity at time zero (210i) and the ellipticity after a given time (210f).
4. Stability of Aquaporins
Assessment of protein thermal and chemical stability provides an indication of how the protein will behave under adverse conditions. The thermal stability of AQPs can be studied using the thermal shift assay (TSA) [60,63], which is based on differential static light scattering (DSLS). The latter can be measured using the StarGazer-384 (Harbinger Biotechnology, Toronto, Canada), without interference from detergents present in the system [64]. This assay estimates protein stability by monitoring protein aggregation and increase in scattering upon step-wise heating. Typically, samples are heated from 25 to 80 °C, at a rate of 1 °C per minute (Figure 4C). In this case, AQP mutants with enhanced thermostability would produce a higher protein’s aggregation temperature (Tagg) compared to the wild type. TSA can be easily scaled up to a 384-well format to simultaneously assess the thermostability of a large library of AQP mutants.
Native AqpZ shows a much higher Tagg than hAQP1 (>80 °C vs. ~55 °C), therefore the detection of thermal shift using this method is more difficult for AqpZ due to evaporation problems. Nevertheless, CD can be used instead to show that after brief exposure to a high temperature (78 °C) AqpZ starts unfolding in a few minutes (Figure 4D), allowing quantification of its stability. AQP thermal unfolding in different detergents and lipid environments has been previously followed using CD thermal denaturation assay, e.g., spinach AQP SoPIP2;1 [65], where it was found that the incorporation of the AQP into E. coli lipid membranes increased the melting temperature of the protein.
In addition, chemical stability of AQP mutants can be tested by exposure to a variety of environmental conditions, e.g., extreme pH or oxidants, or crosslinking with lipid-like molecules following established protocols [45].
5. Aquaporin Inhibitors
Selective inhibitors of AQPs serve as invaluable negative control tools to quantify water permeability through AQP-based biomimetic membranes, aside from the obvious benefits for drug discovery since AQPs are the new players in cancer biology [66], e.g., human AQP1 [67,68,69]. However, despite numerous reports describing the discovery of agents that can modulate water flux through AQPs, they are not suitable for drug discovery efforts, mainly due to their toxic side effects and/or lack of selectivity and potency [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84]. A discussion of various proposed AQP inhibitors and their molecular structures can be found in Figure 5 of reference [59]. Mercurial compounds, although notorious for their toxic properties, are the first blockers of water permeability through AQPs to be described in the literature. Inhibition of AQP1 by sulfhydryl-reactive mercurials such as mercury (II) chloride involves a covalent interaction of the Hg2+ with the Cys189 residue at the extracellular side, leading to a steric block of the water channel. Site-directed mutagenesis studies demonstrated that mutation in the Cys189 of AQP1 prevent inhibition by mercury in a Xenopus oocyte swelling assay [72], while other AQPs (e.g., AQP4) which lack the cysteine residue at that position are shown to be resistant to Hg2+ inhibition. Similarly, a mutant of AqpZ (T183C) engineered based on the known mercury-sensitive site of AQP1, rendered the channel sensitive to inhibition by Hg2+ [12]. Other heavy metals such as silver (as silver nitrate or silver sulfadiazine) [74], gold(III) (in compounds Auphen, Auterpy, Aubipy, AubipyMe and AubipyNH2) [85] and zinc (as zinc chloride) [79] are also able to inhibit several AQP isoforms. Additionally, other agents such as derivatives of loop diuretic bumetanide [80], TGN-020 (2-(nicotinamide)-1,3,4-thiadiazole) [86] and the general anesthetic propofol [87] have also been reported to inhibit AQPs.
Quaternary ammonium salts such as tetraethyl ammonium chloride (TEA), a known pore-occluding blocker of voltage-gated potassium ion channels [88], was reported to inhibit the water permeability through AQP1 [76,77,78]. Based on the resistance of an AQP1 mutant that carries a mutation at the Tyr186 site, the binding site for TEA+ ions was proposed to be through interactions with the Loop E pore region [76]. It has been described that acetazolamide (AZA), a pan-inhibitor of carbonic anhydrases, inhibits AQP1 in a Xenopus oocyte swelling assay [82], and also in human embryonic kidney (HEK293) cells transfected with AQP1 in a green fluorescence protein (GFP) fluorescence intensity assay [89]. In the latter study, the direct binding of AZA to AQP1 was also detected by biomolecular interaction analysis based on Surface Plasmon Resonance, with an equilibrium constant (KD) of ~1 × 10−4 M. However, despite these reported efficacies in blockage of water transport through AQP1-expressing Xenopus oocytes, the Verkman lab has demonstrated that neither TEA nor AZA could inhibit AQP1 in human erythrocyte stopped-flow and transepithelial water permeability measurements, even when tested at 10 mM (for TEA) or 2 mM (for AZA due to solubility limit) concentrations [70]. Later on, an improved Xenopus oocyte system also did not observe any effects of TEA or AZA on the water permeability of AQP1 [90]. It was suggested that the false positive results obtained previously could be due to secondary effects related to changes in membrane conductance and intracellular osmolarity throughout the time-course of oocyte swelling measurements, which are independent of direct interactions between AQP1 and the putative inhibitors.
A medium-throughput primary screening assay based on calcein self-quenching used mammalian cells expressing AQP1 and AQP4 to test a library of 3,575 compounds from the National Cancer Institute, including 418 FDA-approved drugs, for effects on water transport [57]. After a secondary evaluation of initial hits using stopped-flow water permeability assay on plasma membrane vesicles obtained from AQP4-transfected cells and rat erythrocytes expressing endogenous AQP1, the campaign yielded four hit compounds, NSC164914, NSC670229, NSC168597 and NSC301460, with EC50 values of ~27–49 µM when tested on rat erythrocytes.
More recently, the screening of a library of ~10,000 drug-like compounds against the human AQP1 using the yeast freeze-thaw assay has been completed, yielding putative inhibitors which can block water permeability through human erythrocytes [60]. The effectiveness of these hits on purified hAQP1 was also assessed using several biophysical methods to characterize binding, stabilization and inhibition of the protein. Two novel hAQP1 inhibitors, NSC670226 and NSC657298, were identified by this screening effort.
In a virtual screening effort by molecular docking of lead-like subset (1,000,000 compounds) against the extracellular part of hAQP1, 14 compounds from the top 50 list were experimentally in an hAQP1-cRNA injected Xenopus oocyte swelling assay [91]. In the same study, the inhibition by AZA produced an IC50 of 5.5 µM, although it has been previously. Three hit compounds showed reduction in osmotic swelling, with IC50 values of ~8–17 µM. Through molecular dynamics simulation studies, the Lys36 residue was observed to interact with these three compounds in some way. This was supported by frog oocyte swelling assays on a hAQP1-K36A mutant, which could not be inhibited by these hit compounds, but can still be inhibited by acetazolamide. Unfortunately, none of these three compounds were effective in inhibiting water transport when tested in a human erythrocyte stopped-flow water permeability assay. The authors suggested that such discrepancy could be due to lower protein expression levels in frog oocytes compared to that in human erythrocytes.
6. Conclusions
Many challenges must be overcome before the use of aquaporin-based biomimetic membranes can be considered mainstream. For example, systematic research is required to understand the interactions and compatibility between aquaporin and matrix materials. There is also a lack of understanding of the factors affecting long-term stability of biomimetic hybrid assemblies. In turn, these will determine scalability and production costs [41]. Despite these challenges, this promising area of research presents major opportunities for paradigm shift technologies in areas most critical to human health, quality of life and the environment.
Apart from constructing biomimetic membranes based on traditionally used E. coli AqpZ, other aquaporin isoforms may also be tested for higher permeability and/or better insertion efficiency in BCPs. However, given that aquaporins have adapted specifically to their natural environments, a more logical approach would be to create mutant forms of aquaporins adapted to these alien biomimetic environments. At the same time, these mutants could be selected for higher expression compared to the wild type. Robust mutants are expected to improve on the various aspects that are critical for these biomimetic membranes to be taken from its present small-scale format to much desired large-scale industrial applications. They are: extended shelf- and operational- life, resistance to chemicals, compatibility with synthetic polymers, as well as packing and production yields.
Protein thermal stability correlates with desirable features such as increased shelf life, robustness and enhanced crystallizability. Two main practical applications are proposed for these mutants: robust components of biomimetic membranes used for water purification and formation of protein crystals of superior X-ray diffraction properties. For the first application, the orthodox aquaporin from E. coli (AqpZ) is the current workhorse for biomimetic membrane development. Availability of robust mutants will accelerate the transition from small-scale format to large-scale industrial applications, via extended shelf- and operational- life, and possibly insertion efficiency and resistance to harsh chemical treatments.
Selective inhibitors, including naturally occurring toxins and organic molecules, have been identified and characterized for classical membrane proteins such as ion channels, membrane receptors and transporters. Unfortunately, specific efficient inhibitors are lacking. The progress in this field has been unexpectedly slow, owing to the poor druggability of aquaporins and the lack of gold-standard assays, in contrast to the electrophysiological assays that are available for ion channels. Nevertheless, continual advances in aquaporin-based research may eventually deliver a breakthrough in this area.
Acknowledgments
Jaume Torres acknowledges the funding of MOE Singapore Tier 1 grant RG150/14.
Author Contributions
Janet To wrote the manuscript; Jaume Torres wrote and supervised the organization of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Benga, G.; Popescu, O.; Borza, V.; Pop, V.I.; Muresan, A.; Mocsy, I.; Brain, A.; Wrigglesworth, J.M. Water permeability in human erythrocytes: Identification of membrane proteins involved in water transport. Eur. J. Cell Biol. 1986, 41, 252–262. [Google Scholar] [PubMed]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel m(r) 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in xenopus oocytes expressing red cell chip28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. More than just water channels: Unexpected cellular roles of aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Gonen, T.; Sliz, P.; Kistler, J.; Cheng, Y.; Walz, T. Aquaporin-0 membrane junctions reveal the structure of a closed water pore. Nature 2004, 429, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Han, B.-G.; Lee, J.K.; Walian, P.; Jap, B.K. Structural basis of water-specific transport through the aqp1 water channel. Nature 2001, 414, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Hiroaki, Y.; Tani, K.; Kamegawa, A.; Gyobu, N.; Nishikawa, K.; Suzuki, H.; Walz, T.; Sasaki, S.; Mitsuoka, K.; Kimura, K.; et al. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006, 355, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Horsefield, R.; Norden, K.; Fellert, M.; Backmark, A.; Tornroth-Horsefield, S.; Terwisscha Van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-resolution x-ray structure of human aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed]
- Tajkhorshid, E.; Nollert, P.; Jensen, M.Ã.; Miercke, L.J.W.; O’Connell, J.; Stroud, R.M.; Schulten, K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 2002, 296, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kozono, D.; Remis, J.; Kitagawa, Y.; Agre, P.; Stroud, R.M. Structural basis for conductance by the archaeal aquaporin aqpm at 1.68 ã. Proc. Natl. Acad. Sci. USA 2005, 102, 18932–18937. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Stroud, R.M. Structural basis of aquaporin inhibition by mercury. J. Mol. Biol. 2007, 368, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.R.; Ying, J.T.Y.; Darwis, D.; Soon, C.H.; Cornvik, T.; Torres, J.; Lescar, J. Crystallization and preliminary crystallographic analysis of human aquaporin 1 at a resolution of 3.28 angstrom. Acta Crystallogr. Sec. F Struct. Biol. Commun. 2014, 70, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Agre, P. The aquaporin water channels. Proc. Am. Thorac. Soc. 2006, 3, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom resolution x-ray structure details aquaporin-water interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.F.; Egea, P.F.; Robles-Colmenares, Y.; O’Connell Iii, J.D.; Stroud, R.M. Architecture and selectivity in aquaporins: 2.5 å x-ray structure of aquaporin z. PLoS Biol. 2003, 1. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Libson, A.; Miercke, L.J.W.; Weitzman, C.; Nollert, P.; Krucinski, J.; Stroud, R.M. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000, 290, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Harries, W.E.C.; Akhavan, D.; Miercke, L.J.W.; Khademi, S.; Stroud, R.M. The channel architecture of aquaporin 0 at a 2.2-å resolution. Proc. Natl. Acad. Sci. USA 2004, 101, 14045–14050. [Google Scholar] [CrossRef] [PubMed]
- Frick, A.; Eriksson, U.K.; De Mattia, F.; Öberg, F.; Hedfalk, K.; Neutze, R.; de Grip, W.J.; Deen, P.M.T.; Törnroth-Horsefield, S. X-ray structure of human aquaporin 2 and its implications for nephrogenic diabetes insipidus and trafficking. Proc. Natl. Acad. Sci. USA 2014, 111, 6305–6310. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.D.; Yeh, R.; Sandstrom, A.; Chorny, I.; Harries, W.E.C.; Robbins, R.A.; Miercke, L.J.W.; Stroud, R.M. Crystal structure of human aquaporin 4 at 1.8 a and its mechanism of conductance. Proc. Natl. Acad. Sci. USA 2009, 106, 7437–7442. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Fan, Y.; Munro, R.; Ladizhansky, V.; Brown, L.S. Yeast-expressed human membrane protein aquaporin-1 yields excellent resolution of solid-state mas nmr spectra. J. Biomol. NMR 2013, 55, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of aqpz, the e. Coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Werten, P.J.L.; Hasler, L.; Koenderink, J.B.; Klaassen, C.H.W.; de Grip, W.J.; Engel, A.; Deen, P.M.T. Large-scale purification of functional recombinant human aquaporin-2. FEBS Lett. 2001, 504, 200–205. [Google Scholar] [CrossRef]
- Nyblom, M.; Oberg, F.; Lindkvist-Petersson, K.; Hallgren, K.; Findlay, H.; Wikstrom, J.; Karlsson, A.; Hansson, O.; Booth, P.J.; Bill, R.M.; et al. Exceptional overproduction of a functional human membrane protein. Prot. Exp. Purif. 2007, 56, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bomholt, J.; Hélix-Nielsen, C.; Scharff-Poulsen, P.; Pedersen, P.A. Recombinant production of human aquaporin-1 to an exceptional high membrane density in saccharomyces cerevisiae. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Felgner, J.H.; Kumar, R.; Sridhar, C.N.; Wheeler, C.J.; Tsai, Y.J.; Border, R.; Ramsey, P.; Martin, M.; Felgner, P.L. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994, 269, 2550–2561. [Google Scholar] [PubMed]
- Öberg, F.; Sjöhamn, J.; Conner, M.T.; Bill, R.M.; Hedfalk, K. Improving recombinant eukaryotic membrane protein yields in pichia pastoris: The importance of codon optimization and clone selection. Mol. Membr. Biol. 2011, 28, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Junge, F.; Shirokov, V.A.; Durst, F.; Schwarz, D.; Dötsch, V.; Bernhard, F. Membrane protein expression in cell-free systems. Methods Mol. Biol. 2010, 601, 165–186. [Google Scholar] [PubMed]
- Brödel, A.K.; Sonnabend, A.; Kubick, S. Cell-free protein expression based on extracts from cho cells. Biotechnol. Bioeng. 2014, 111, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kai, L.; Kaldenhoff, R.; Lian, J.; Zhu, X.; Dötsch, V.; Bernhard, F.; Cen, P.; Xu, Z. Preparative scale production of functional mouse aquaporin 4 using different cell-free expression modes. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Hovijitra, N.T.; Wu, J.J.; Peaker, B.; Swartz, J.R. Cell-free synthesis of functional aquaporin z in synthetic liposomes. Biotechnol. Bioeng. 2009, 104, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein aquaporin z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhou, H.; Li, Y.; Jeyaseelan, K.; Armugam, A.; Chung, T.S. A novel method of aquaporinz incorporation via binary-lipid langmuir monolayers. Colloids Surf. B. Biointerfaces 2012, 89, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, R.; Tang, C.; Vararattanavech, A.; Zhao, Y.; Torres, J.; Fane, T. Preparation of supported lipid membranes for aquaporin z incorporation. Colloids Surf. B. Biointerfaces 2012, 94, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Wang, R.; Wicaksana, F.; Tang, C.Y.; Torres, J.; Fane, A.G. Preparation of high performance nanofiltration (nf) membranes incorporated with aquaporin z. J. Membr. Sci. 2014, 450, 181–188. [Google Scholar] [CrossRef]
- Xie, W.Y.; He, F.; Wang, B.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A.; Tong, Y.W. An aquaporin-based vesicle-embedded polymeric membrane for low energy water filtration. J. Mater. Chem. A 2013, 1, 7592–7600. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, C.Q.; Li, X.S.; Vararattanavech, A.; Shen, W.M.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and ro performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Kaufman, Y.; Freger, V. Supported biomimetic membranes for pressure-driven water purification. In On Biomimetics; Pramatarova, L.D., Ed.; In Tech Europe: Rijeka, Croatia, 2011. [Google Scholar]
- Tang, C.Y.; Zhao, Y.; Wang, R.; Helix-Nielsen, C.; Fane, A.G. Desalination by biomimetic aquaporin membranes: Review of status and prospects. Desalination 2013, 308, 34–40. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.Z.; Ding, S.H.; Cai, J.; Zhang, D.P.; Xu, Z.N.; Wang, X.N. Improving aquaporin z expression in escherichia coli by fusion partners and subsequent condition optimization. Appl. Microbiol. Biotechnol. 2009, 82, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Habel, J.E.O.; Shen, Y.X.; Meier, W.P.; Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 2012, 134, 18631–18637. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.F.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. Stabilization and immobilization of aquaporin reconstituted lipid vesicles for water purification. Colloid Surface B 2013, 102, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bowie, J.U. Building a thermostable membrane protein. J. Biol. Chem. 2000, 275, 6975–6979. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; Kummer, L.; Tremmel, D.; Pluckthun, A. Stabilizing membrane proteins through protein engineering. Curr. Opin. Chem. Biol. 2013, 17, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Bowie, J.U. Stabilizing membrane proteins. Curr. Opin. Struct. Biol. 2001, 11, 397–402. [Google Scholar] [CrossRef]
- Asial, I.; Cheng, Y.X.; Engman, H.; Dollhopf, M.; Wu, B.; Nordlund, P.; Cornvik, T. Engineering protein thermostability using a generic activity-independent biophysical screen inside the cell. Nat. Commun. 2013, 4, 2901. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Marrone, A.; Ciancetta, A.; Cobo, A.G.; Echevarria, M.; Moura, T.F.; Re, N.; Casini, A.; Soveral, G. Targeting aquaporin function: Potent inhibition of aquaglyceroporin-3 by a gold-based compound. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Zeidel, M.L.; Albalak, A.; Grossman, E.; Carruthers, A. Role of glucose carrier in human erythrocyte water permeability. Biochemistry 1992, 31, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Wilson, M.T. Stopped flow spectroscopy. In Spectrophotometry and Spectrofluorimetry; Gore, M., Ed.; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Van Hoek, A.N.; Verkman, A.S. Functional reconstitution of the isolated erythrocyte water channel chip28. J. Biol. Chem. 1992, 267, 18267–18269. [Google Scholar] [PubMed]
- Levin, M.H.; Sullivan, S.; Nielson, D.; Yang, B.; Finkbeiner, W.E.; Verkman, A.S. Hypertonic saline therapy in cystic fibrosis: Evidence against the proposed mechanism involving aquaporins. J. Biol. Chem. 2006, 281, 25803–25812. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.; Kiilgaard, J.F.; Litman, T.; Alvarez-Leefmans, F.J.; Winther, B.R.; Zeuthen, T. Measurement of cell volume changes by fluorescence self-quenching. J. Fluoresc. 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Galietta, L.J.V.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef]
- Mola, M.G.; Nicchia, G.P.; Svelto, M.; Spray, D.C.; Frigeri, A. Automated cell-based assay for screening of aquaporin inhibitors. Anal. Chem. 2009, 81, 8219–8229. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.H.; de La Fuente, R.; Verkman, A.S. Urearetics: A small molecule screen yields nanomolar potency inhibitors of urea transporter ut-b. FASEB J. 2007, 21, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- To, J.; Yeo, C.Y.; Soon, C.H.; Torres, J. A generic high-throughput assay to detect aquaporin functional mutants: Potential application to discovery of aquaporin inhibitors. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, A.; van Dijck, P.; Dumortier, F.; Teunissen, A.; Hohmann, S.; Thevelein, J.M. Aquaporin expression correlates with freeze tolerance in baker's yeast, and overexpression improves freeze tolerance in industrial strains. Appl. Environ. Microbiol. 2002, 68, 5981–5989. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, A.; Van Dijck, P.; Colavizza, D.; Thevelein, J.M. Aquaporin-mediated improvement of freeze tolerance of saccharomyces cerevisiae is restricted to rapid freezing conditions. Appl. Environ. Microbiol. 2004, 70, 3377–3382. [Google Scholar] [CrossRef] [PubMed]
- Vedadi, M.; Niesen, F.H.; Allali-Hassani, A.; Fedorov, O.Y.; Finerty, P.J.; Wasney, G.A.; Yeung, R.; Arrowsmith, C.; Ball, L.J.; Berglund, H.; et al. Chemical screening methods to identify ligands that promote protein stability, protein crystallization, and structure determination. Proc. Natl. Acad. Sci. USA 2006, 103, 15835–15840. [Google Scholar] [CrossRef] [PubMed]
- Senisterra, G.A.; Ghanei, H.; Khutoreskaya, G.; Dobrovetsky, E.; Edwards, A.M.; Prive, G.G.; Vedadi, M. Assessing the stability of membrane proteins to detect ligand binding using differential static light scattering. J. Biomol. Screen. 2010, 15, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, I.; Survery, S.; Ibragimova, S.; Hansen, J.S.; Kjellbom, P.; Helix-Nielsen, C.; Johanson, U.; Mouritsen, O.G. Structure and stability of the spinach aquaporin sopip2;1 in detergent micelles and lipid membranes. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Stigliano, C.; Sparaneo, A.; Rossi, A.; Frigeri, A.; Svelto, M. Inhibition of aquaporin-1 dependent angiogenesis impairs tumour growth in a mouse model of melanoma. J. Mol. Med. 2013, 91, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kim, J.K.; Verkman, A.S. Comparative efficacy of hgcl2 with candidate aquaporin-1 inhibitors dmso, gold, tea+ and acetazolamide. FEBS Lett. 2006, 580, 6679–6684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; van Hoek, A.N.; Biwersi, J.; Verkman, A.S. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel chip28k. Biochemistry 1993, 32, 2938–2941. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Jin Sup, J.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine 189 in the chip28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [PubMed]
- Huebert, R.C.; Vasdev, M.M.; Shergill, U.; Das, A.; Huang, B.Q.; Charlton, M.R.; LaRusso, N.F.; Shah, V.H. Aquaporin-1 facilitates angiogenic invasion in the pathological neovasculature that accompanies cirrhosis. Hepatology 2010, 52, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: Silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Lett. 2002, 531, 443–447. [Google Scholar] [CrossRef]
- Yukutake, Y.; Tsuji, S.; Hirano, Y.; Adachi, T.; Takahashi, T.; Fujihara, K.; Agre, P.; Yasui, M.; Suematsu, M. Mercury chloride decreases the water permeability of aquaporin-4-reconstituted proteoliposomes. Biol. Cell 2008, 100, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tetraethylammonium: Involvement of the loop e pore region. Mol. Pharmacol. 2000, 57, 1021–1026. [Google Scholar] [PubMed]
- Brooks, H.L.; Regan, J.W.; Yool, A.J. Inhibition of aquaporin-1 water permeability by tea. FASEB J. 1999, 13, A394. [Google Scholar]
- Detmers, F.J.M.; de Groot, B.L.; Müller, E.M.; Hinton, A.; Konings, I.B.M.; Sze, M.; Flitsch, S.L.; Grubmüller, H.; Deen, P.M.T. Quaternary ammonium compounds as water channel blockers: Specificity, potency, and site of action. J. Biol. Chem. 2006, 281, 14207–14214. [Google Scholar] [CrossRef] [PubMed]
- Yukutake, Y.; Hirano, Y.; Suematsu, M.; Yasui, M. Rapid and reversible inhibition of aquaporin-4 by zinc. Biochemistry 2009, 48, 12059–12061. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Ma, B.; Li, T.; Gao, J.W.; Yu, H.M.; Li, X.J. Acetazolamide inhibits aquaporin-1 protein expression and angiogenesis. Acta Pharmacol. Sin. 2004, 25, 812–816. [Google Scholar] [PubMed]
- Ma, B.; Xiang, Y.; Mu, S.M.; Li, T.; Yu, H.M.; Li, X.J. Effects of acetazolamide and anordiol on osmotic water permeability in aqp1-crna injected xenopus oocyte. Acta Pharmacol. Sin. 2004, 25, 90–97. [Google Scholar] [PubMed]
- Huber, V.J.; Tsujita, M.; Yamazaki, M.; Sakimura, K.; Nakada, T. Identification of arylsulfonamides as aquaporin 4 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.J.; Tsujita, M.; Kwee, I.L.; Nakada, T. Inhibition of aquaporin 4 by antiepileptic drugs. Biorg. Med. Chem. 2009, 17, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Ciancetta, A.; deAlmeida, A.; Marrone, A.; Re, N.; Soveral, G.; Casini, A. Aquaporin inhibition by gold(iii) compounds: New insights. ChemMedChem 2013, 8, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Huber, V.J.; Tsujita, M.; Nakada, T. Pretreatment with a novel aquaporin 4 inhibitor, tgn-020, significantly reduces ischemic cerebral edema. Neurol. Sci. 2011, 32, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Hayashi, M.K.; Aizu, S.; Yukutake, Y.; Takeda, J.; Yasui, M. A general anaesthetic propofol inhibits aquaporin-4 in the presence of Zn2+. Biochem. J. 2013, 454, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lang, D.G.; Ritchie, A.K. Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2+-activated K+ channels in a pituitary cell line. J. Physiol. 1990, 425, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, X.; Chang, Y.; Zhang, J.; Song, Q.; Yu, H.; Li, X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006, 350, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, R.; Zeuthen, T. Test of blockers of aqp1 water permeability by a high-resolution method: No effects of tetraethylammonium ions or acetazolamide. Pflugers Archiv. Eur. J. Physiol. 2008, 456, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; Zapater, C.; Krenc, D.; Haddoub, R.; Flitsch, S.; Beitz, E.; Cerdà, J.; de Groot, B.L. Discovery of novel human aquaporin-1 blockers. ACS Chem. Biol. 2013, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).