Effects of Lipid Composition and Solution Conditions on the Mechanical Properties of Membrane Vesicles
Abstract
:1. Introduction
2. Results and Discussion
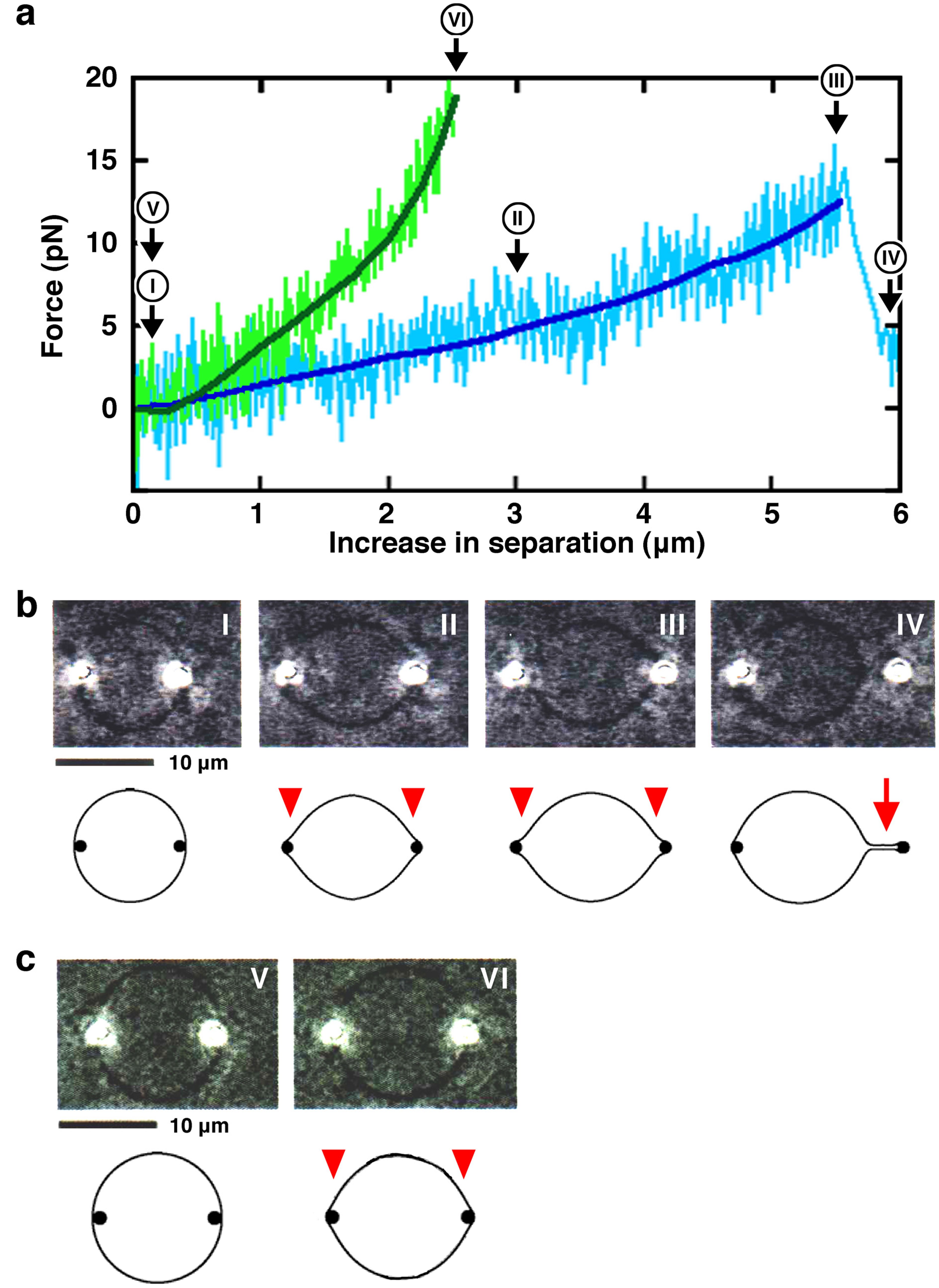
2.1. Effect of Lipid Composition

2.1.1. Effect of Acidic Phospholipids
2.1.2. Comparisons between PC and PE and Between PG and PA, the Effect of Substitution with a Small Head Phospholipid
2.1.3. Liposomes Made from Dimyristoylphosphatidylcholine (DMPC)
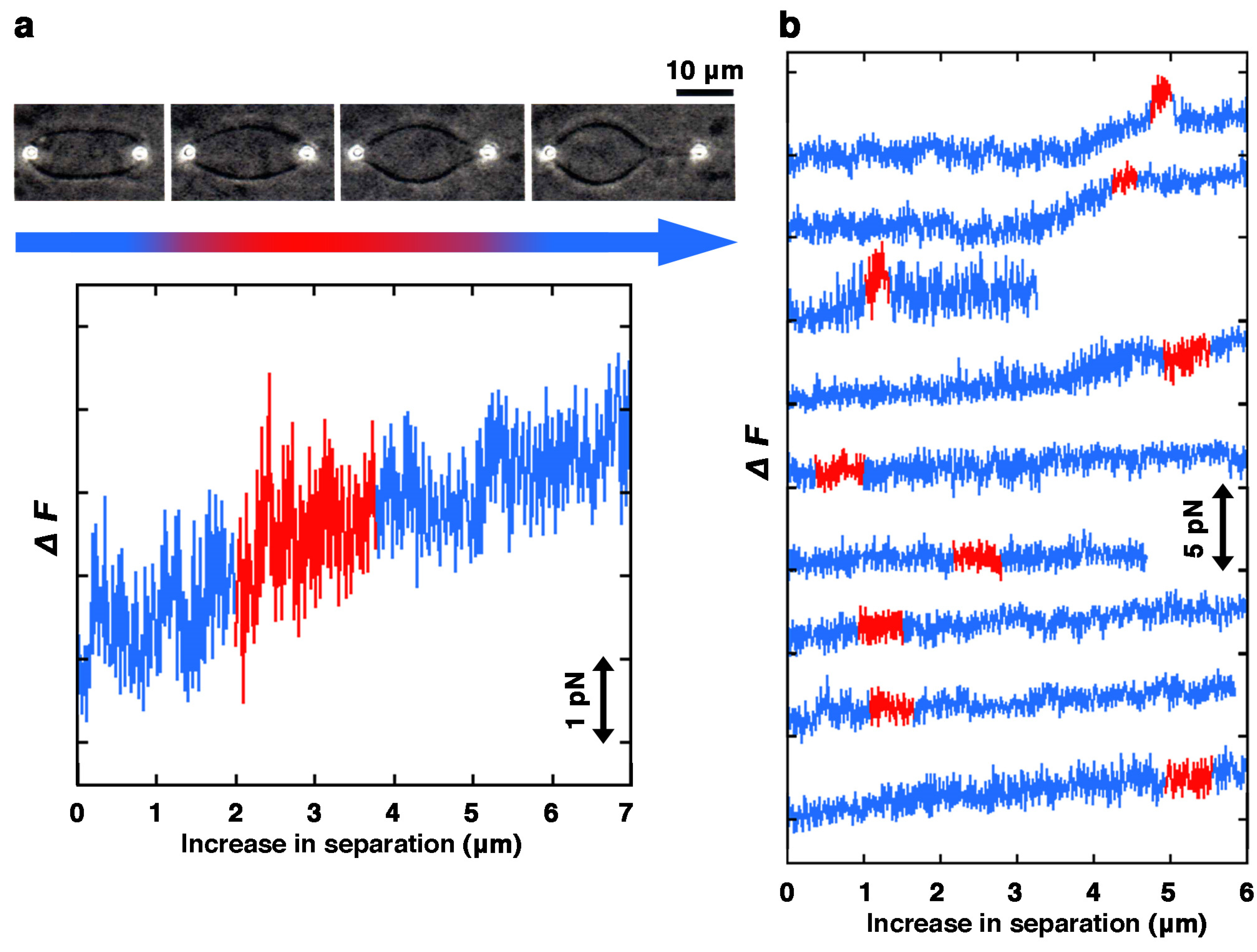
2.2. Effect of Solution Conditions
2.2.1. Effect of pH

2.2.2. Effect of Proteins in the Solution
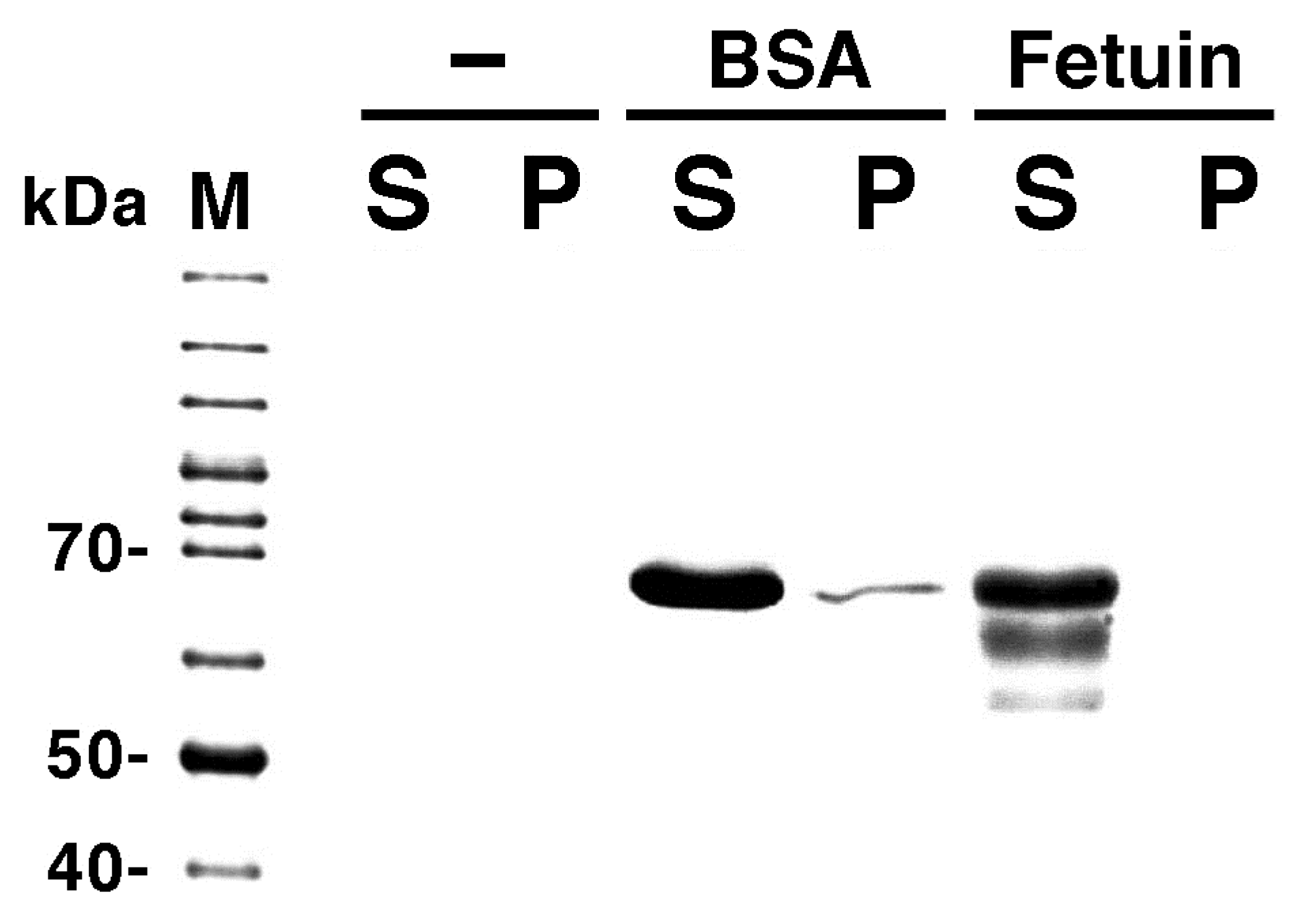
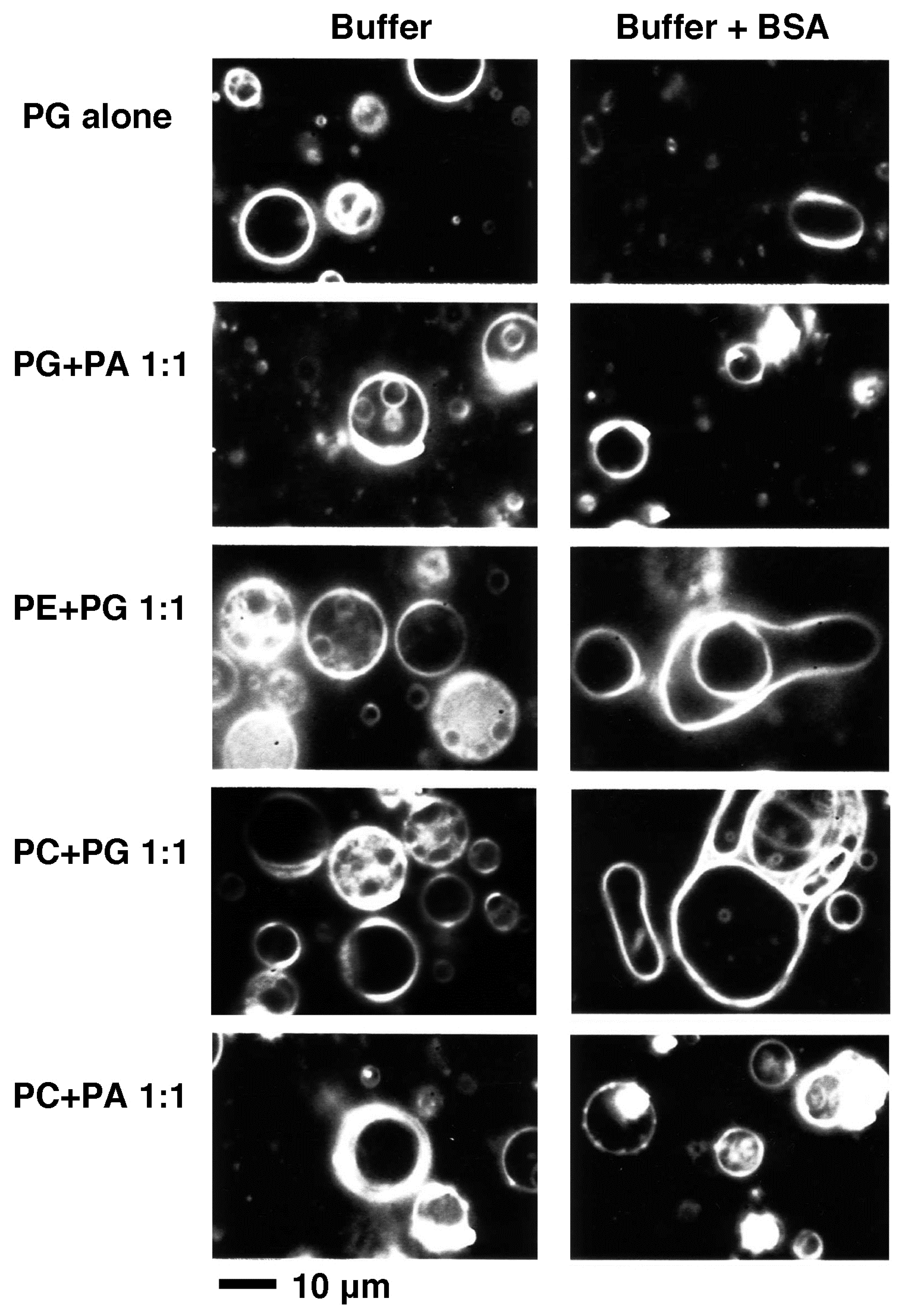
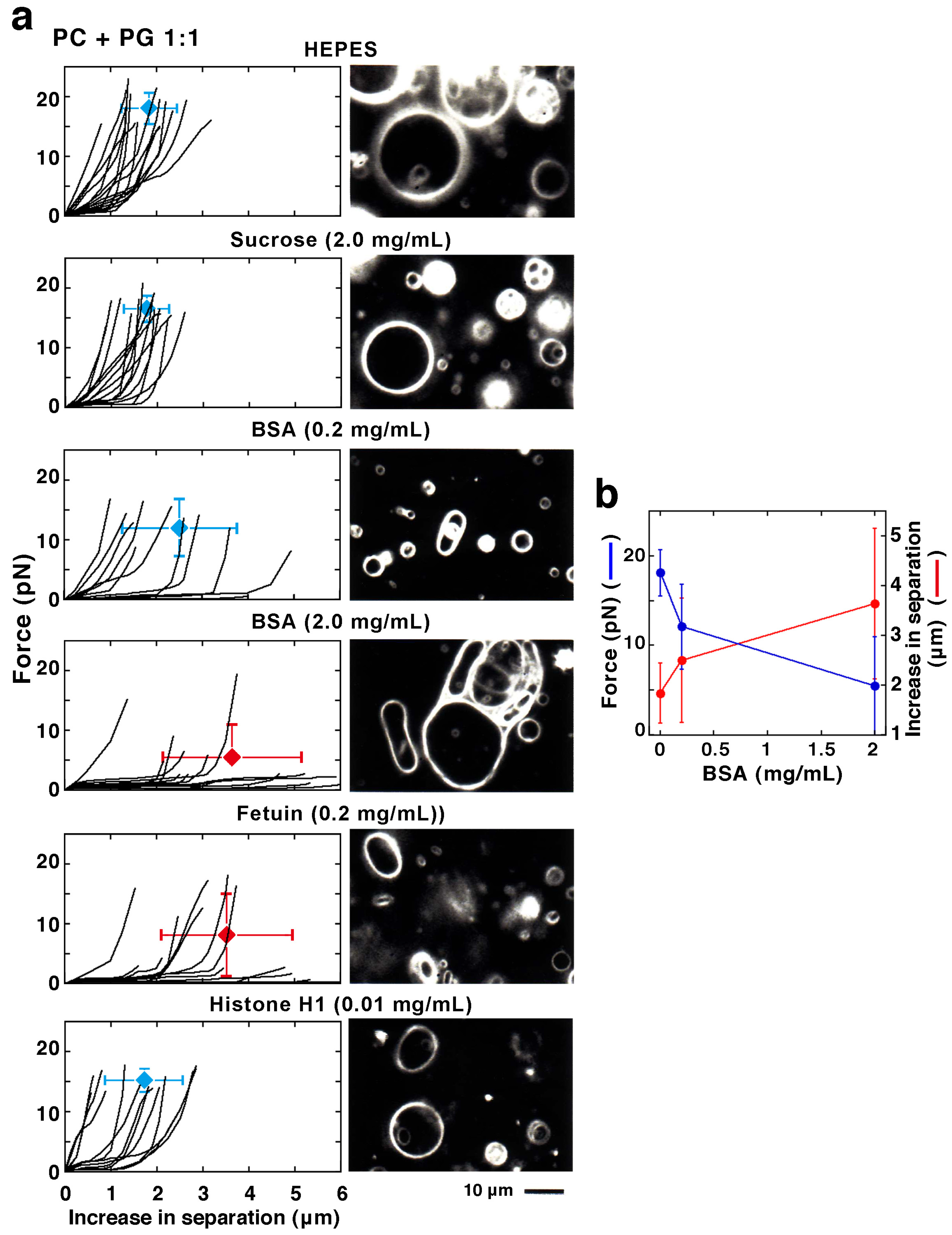
2.2.3. Effect of Membrane-Interacting Proteins in the Solution
2.3. Physiological Significance Related to Cytoskeletal Components
2.4. Possible Collaboration with Other Methods and Overcoming the Limitations of This Study
3. Experimental Section
3.1. Lipids and Other Materials
3.2. Preparation of Liposomes
3.3. Laser Tweezers
3.4. Cosedimentation Assay
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Conflicts of Interest
References and Notes
- Baumgart, T.; Hess, S.T.; Webb, W.W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Seguin, B.; Fried, E. Kinematics, material symmetry, and energy densities for lipid bilayers with spontaneous curvature. Biomech. Model Mechanobiol. 2013, 12, 997–1017. [Google Scholar] [CrossRef] [PubMed]
- Käs, J.; Sackmann, E. Shape transitions and shape stability of giant phospholipid-vesicles in pure water induced by area-to-volume changes. Biophys. J. 1991, 60, 825–844. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Hamada, T.; Takiguchi, K.; Homma, M. Dynamic behavior of giant liposomes at desired osmotic pressures. Langmuir 2009, 25, 11680–11685. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Miura, Y.; Ishii, K.; Araki, S.; Yoshikawa, K.; Vestergaard, M.; Takagi, M. Dynamic processes in endocytic transformation of a raft-exhibiting giant liposome. J. Phys. Chem. B 2007, 111, 10853–10857. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Imai, M.; Taniguchi, T. Shape deformation of ternary vesicles coupled with phase separation. Phys. Rev. Lett. 2008, 100, 148102. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Imai, M.; Taniguchi, T. Periodic modulation of tubular vesicles induced by phase separation. Phys. Rev. E 2010, 82, 051928. [Google Scholar] [CrossRef]
- Khalifat, N.; Puff, N.; Bonneau, S.; Fournier, J.-B.; Angelova, M.I. Membrane deformation under local pH gradient: Mimicking mitochondrial cristae dynamics. Biophys. J. 2008, 95, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Bitbol, A.-F.; Puff, N.; Sakuma, Y.; Imai, M.; Fournier, J.-B.; Angelova, M.I. Lipid membrane deformation in response to a local pH modification: theory and experiments. Soft Matter 2012, 8, 6073–6082. [Google Scholar] [CrossRef]
- Tanaka, T.; Tamba, Y.; Masum, S.M.; Yamashita, Y.; Yamazaki, M. La3+ and Gd3+ induce shape change of giant unilamellar vesicles of phosphatidylcholine. Biochim. Biophys. Acta Biomembranes 2002, 1564, 173–182. [Google Scholar] [CrossRef]
- Tanaka, T.; Yamazaki, M. Membrane fusion of giant unilamellar vesicles of neutral phospholipid membranes induced by La3+. Langmuir 2004, 20, 5160–5164. [Google Scholar] [CrossRef] [PubMed]
- Nomura, F.; Nagata, M.; Inaba, T.; Hiramatsu, H.; Hotani, H.; Takiguchi, K. Capabilities of liposomes for topological transformation. Proc. Natl. Acad. Sci. USA 2001, 98, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Hirabayashi, Y.; Ohta, T.; Takagi, M. Rhythmic pore dynamics in a shrinking lipid vesicle. Phys. Rev. E 2009, 80, 051921. [Google Scholar] [CrossRef]
- Hamada, T.; Hagihara, H.; Morita, M.; Vestergaard, M.C.; Tsujino, Y.; Takagi, M. Physicochemical profiling of surfactant-induced membrane dynamics in a cell-sized liposome. J. Phys. Chem. Lett. 2012, 3, 430–435. [Google Scholar] [CrossRef]
- Jones, N.M.; Chapman, D. Micells, Monolayers, and Biomembranes; Wiley-Liss, Inc.: New York, NY, USA, 1995. [Google Scholar]
- Kaneko, T.; Itoh, T.J.; Hotani, H. Morphological transformation of liposomes caused by assembly of encapsulated tubulin and determination of shape by microtubule-associated proteins (MAPs). J. Mol. Biol. 1998, 284, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Takiguchi, K.; Ishikawa, S.; Hotani, H. Morphogenesis of liposomes encapsulating actin depends on the type of actin-crosslinking. J. Mol. Biol. 1990, 287, 293–300. [Google Scholar] [CrossRef]
- Tamba, Y.; Ariyama, H.; Levadny, V.; Yamazaki, M. Kinetic pathway of antimicrobial peptide magainin 2-induced pore formation in lipid membranes. J. Phys. Chem. B 2010, 114, 12018–12026. [Google Scholar] [CrossRef] [PubMed]
- Shimanouchi, T.; Nishiyama, K.; Hiroiwa, A.; Vu, H.T.; Kitaura, N.; Umakoshi, H.; Kuboi, R. Growth behavior of Aβ protofibrils on liposome membranes and their membrane perturbation effect. Biochem. Eng. J. 2013, 71, 81–88. [Google Scholar] [CrossRef]
- Takahashi, T.; Nomura, F.; Yokoyama, Y.; Tanaka-Takiguchi, Y.; Homma, M.; Takiguchi, K. Multiple membrane interactions and versatile vesicle deformations elicited by melittin. Toxins 2013, 5, 637–664. [Google Scholar] [CrossRef] [PubMed]
- Wollert, T.; Wunder, C.; Lippincott-Schwartz, J.; Hurley, J.H. Membrane scission by the ESCRT-III complex. Nature 2009, 458, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wollert, T.; Hurley, J.H. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature 2010, 464, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Takiguchi, Y.; Kinoshita, M.; Takiguchi, K. Septin-mediated uniform bracing of phospholipid membranes. Curr. Biol. 2009, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Takiguchi, Y.; Itoh, T.; Tsujita, K.; Yamada, S.; Yanagisawa, M.; Fujiwara, K.; Yamamoto, A.; Ichikawa, M.; Takiguchi, K. Physicochemical analysis from real-time imaging of liposome tubulation reveals the characteristics of individual F-BAR domain proteins. Langmuir 2013, 29, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Cabré, E.J.; Sánchez-Gorostiaga, A.; Carrara, P.; Ropero, N.; Casanova, M.; Palacios, P.; Stano, P.; Jiménez, M.; Rivas, G.; Vicente, M. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J. Biol. Chem. 2013, 288, 26625–26634. [Google Scholar]
- Heinrich, V.; Božič, B.; Svetina, S.; Žekš, B. Vesicle deformation by an axial load: From elongated shapes to tethered vesicles. Biophys. J. 1999, 76, 2056–2071. [Google Scholar] [CrossRef]
- Hénon, S.; Lenormand, G.; Richert, A.; Gallet, F. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys. J. 1999, 76, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Daoa, M.; Limb, C.T.; Suresha, S. Mechanics of the human red blood cell deformed by optical tweezers. J. Mech. Phys. Solids 2003, 51, 2259–2280. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, C.; Li, J.; Lykotrafitis, G.; Zhang, S. One-particle-thick, solvent-free, coarse-grained model for biological and biomimetic fluid membranes. Phys. Rev. E 2010, 82, 011905. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, C.; Zhang, S. Dynamic shape transformations of fluid vesicles. Soft Matter 2010, 6, 4571–4579. [Google Scholar] [CrossRef]
- Dimova, R. Recent developments in the field of bending rigidity measurements on membranes. Adv. Coll. Interf. Sci. 2014, 208, 225–234. [Google Scholar] [CrossRef]
- Inaba, T.; Ishijima, A.; Honda, M.; Nomura, F.; Takiguchi, K.; Hotani, H. Formation and maintenance of tubular membrane projections require mechanical force, but their elongation and shortening do not require additional force. J. Mol. Biol. 2005, 348, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970, 24, 156–159. [Google Scholar] [CrossRef]
- Ashkin, A. Application of laser radiation pressure. Science 1980, 210, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Inaba, T.; Ishijima, A.; Takiguchi, K.; Hotani, H. Formation and maintenance of tubular membrane projections: Experiments and numerical calculations. BioSystems 2008, 93, 115–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, F.; Inaba, T.; Ishikawa, S.; Nagata, M.; Takahashi, S.; Hotani, H.; Takiguchi, K. Microscopic observations reveal that fusogenic peptides induce liposome shrinkage prior to membrane fusion. Proc. Natl. Acad. Sci. USA 2004, 101, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Andelman, D. Electrostatic properties of membranes: The Poisson-Boltzmann theory. In Handbook of Biological Physics: Structure and Dynamics of Membranes; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, the Netherland, 1995; Volume 1b, pp. 603–642. [Google Scholar]
- McPhee, C.I.; Zoriniants, G.; Langbein, W.; Borri, P. Measuring the lamellarity of giant lipid vesicles with differential interference contrast microscopy. Biophys. J. 2013, 105, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Miyazaki, M.; Ishiwata, S. Quantitative analysis of the lamellarity of giant liposomes prepared by the inverted emulsion method. Biophys. J. 2014, 107, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E. Physical basis of self-organization and function of membranes: Physics of vesicles. In Handbook of Biological Physics: Structure and Dynamics of Membranes; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, the Netherland, 1995; Volume 1a, pp. 491–519. [Google Scholar]
- Jones, M.N.; Chapman, D. Micells, Monolayers, and Biomembranes; Wiley-Liss: New York, NY, USA, 1995; pp. 102–116. [Google Scholar]
- Phase Transition Temperatures for Glycerophospholipids. Available online: http://avantilipids.com/index.php?option=com_content&id=1700&Itemid=419 (accessed on 24 July 2014).
- Dimova, R.; Pouligny, B.; Dietrich, C. Pretransitional effects in dimyristoylphosphatidylcholine vesicle membranes: Optical dynamometry study. Biophys. J. 2000, 79, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Boggs, J.M. Intermolecular hydrogen bonding between membrane lipids. In Biomembrane; Membrane Fluidity; Kates, M., Manson, L.A., Eds.; Plenum Press: New York, NY, USA, 1984; Volume 12. [Google Scholar]
- Denecke, B.; Gräber, S.; Schäfer, C.; Heiss, A.; Wöltje, M.; Jahnen-Dechent, W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. Biochem. J. 2003, 376, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cartellieri, S.; Hamer, O.; Helmholz, H.; Niemeyer, B. One-step affinity purification of fetuin from fetal bovine serum. Biotechol. Appl. Biochem. 2002, 35, 83–89. [Google Scholar] [CrossRef]
- Rytomma, M.; Kinnuen, K. Dissociation of cytochrome c from liposomes by histone H1. Comparison with basic peptides. Biochemistry 1996, 35, 4529–4539. [Google Scholar]
- Otto, J.J.; Kane, R.E.; Bryan, J. Redistribution of actin and fascin in sea urchin eggs after fertilization. Cell Motil. 1980, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Borisy, G.G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Kovar, D.R.; Pollard, T.D. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. USA 2004, 101, 14725–14730. [Google Scholar] [CrossRef] [PubMed]
- Dogterom, M.; Kerssemakers, J.W.J.; Romet-Lemonne, G.; Janson, M.E. Force generation by dynamic microtubules. Curr. Opin. Cell Biol. 2005, 17, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef]
- Mizushima-Sugano, J.; Maeda, T.; Miki-Nomura, T. Flexural rigidity of singlet microtubules estimated from statistical analysis of their contour length and end-to-end distances. Biochim. Biophys. Acta 1983, 755, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Toyoshima, N.; Hirakawa, N.; Okamoto, K.; Ishijima, A. A kinetic mechanism for the fast movement of Chara myosin. J. Mol. Biol. 2003, 328, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.M.; Finer, J.T.; Chu, S.; Spudich, J.A. Quantitative measurements of force and displacement using an optical trap. Biophys. J. 1996, 70, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Méléard, P.; Gerbeaud, C.; Pott, T.; Fernandez-Puente, L.; Bivas, I.; Marin, D.; Mitov, M.D.; Dufourcq, J.; Bothorel, P. Bending elasticities of model membranes: Influences of temperature and sterol content. Biophys. J. 1997, 72, 2616–2629. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Lyman, E.; Voth, G.A. Mechanism of membrane curvature sensing by amphipathic helix containing proteins. Biophys. J. 2011, 100, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P. Cell membranes and the cytoskeleton. In Handbook of Biological Physics: Structure and Dynamics of Membranes; Lipowsky, R., Sackmann, E., Eds.; Elsevier Science B.V.: Amsterdam, the Netherland, 1995; Volume 1b, pp. 805–849. [Google Scholar]
- Takiguchi, K.; Yamada, A.; Negishi, M.; Honda, M.; Tanaka-Takiguchi, Y.; Yoshikawa, K. Construction of cell-sized liposomes encapsulating actin and actin-crosslinking proteins. Methods Enzymol. 2009, 464, 31–53. [Google Scholar] [PubMed]
- Nishimura, K.; Hosoi, T.; Sunami, T.; Toyota, T.; Fujinami, M.; Oguma, K.; Matsuura, T.; Suzuki, H.; Yomo, T. Population analysis of structural properties of giant liposomes by flow cytometry. Langmuir 2009, 25, 10439–10443. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, N.; Ishijima, A.; Inaba, T.; Nomura, F.; Takeda, S.; Takiguchi, K. Effects of Lipid Composition and Solution Conditions on the Mechanical Properties of Membrane Vesicles. Membranes 2015, 5, 22-47. https://doi.org/10.3390/membranes5010022
Kato N, Ishijima A, Inaba T, Nomura F, Takeda S, Takiguchi K. Effects of Lipid Composition and Solution Conditions on the Mechanical Properties of Membrane Vesicles. Membranes. 2015; 5(1):22-47. https://doi.org/10.3390/membranes5010022
Chicago/Turabian StyleKato, Nobuhiko, Akihiko Ishijima, Takehiko Inaba, Fumimasa Nomura, Shuichi Takeda, and Kingo Takiguchi. 2015. "Effects of Lipid Composition and Solution Conditions on the Mechanical Properties of Membrane Vesicles" Membranes 5, no. 1: 22-47. https://doi.org/10.3390/membranes5010022
APA StyleKato, N., Ishijima, A., Inaba, T., Nomura, F., Takeda, S., & Takiguchi, K. (2015). Effects of Lipid Composition and Solution Conditions on the Mechanical Properties of Membrane Vesicles. Membranes, 5(1), 22-47. https://doi.org/10.3390/membranes5010022



