Potentialities of a Membrane Reactor with Laccase Grafted Membranes for the Enzymatic Degradation of Phenolic Compounds in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Enzyme and Chemicals
2.2. Preparation of the Active Membranes
2.3. Enzymatic Membrane Reactor (EMR)
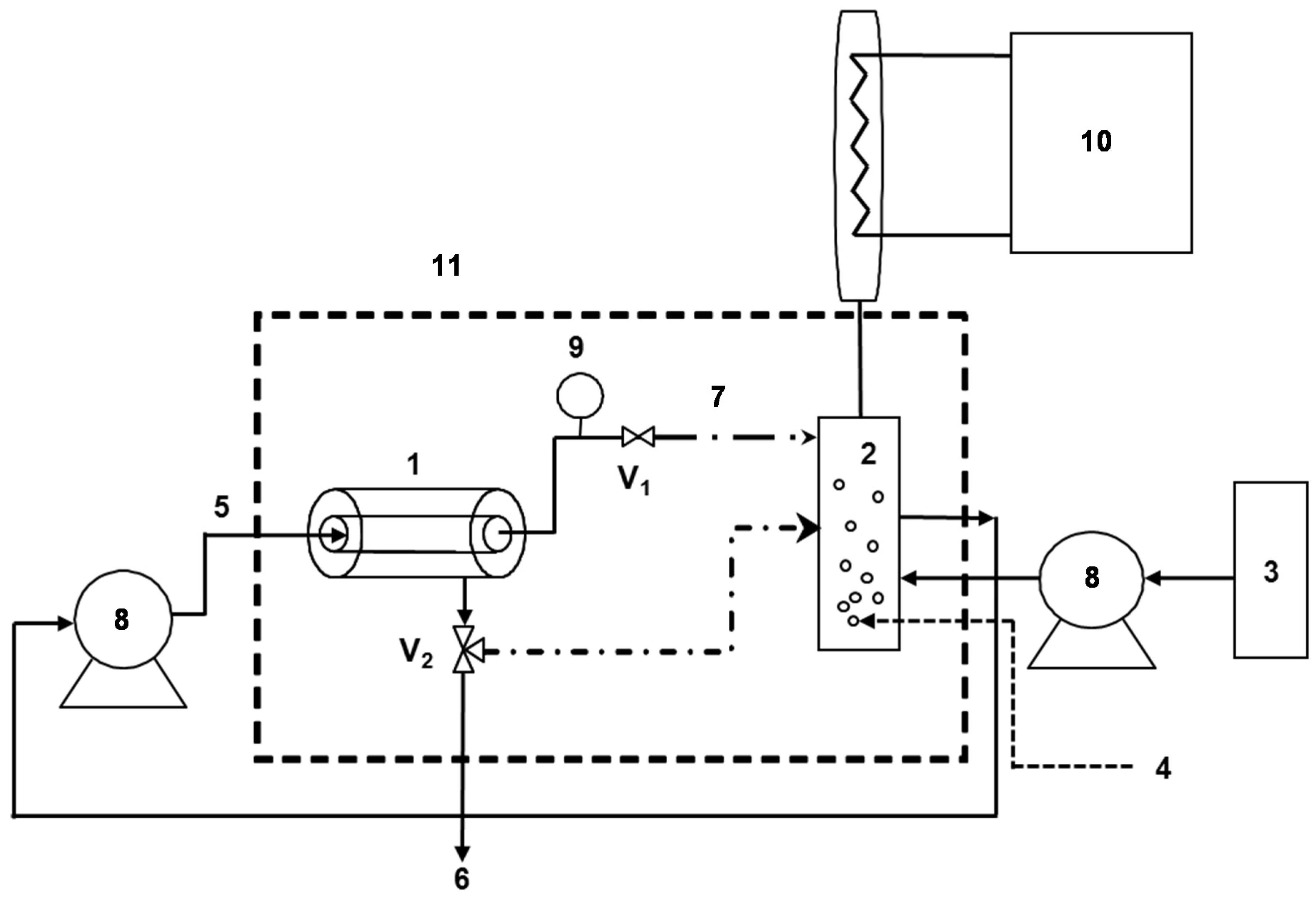
2.4. Phenolic Compounds Degradation in the EMR


2.5. Analysis
3. Results and Discussion
3.1. Degradation of Phenolic Compounds in the EMR
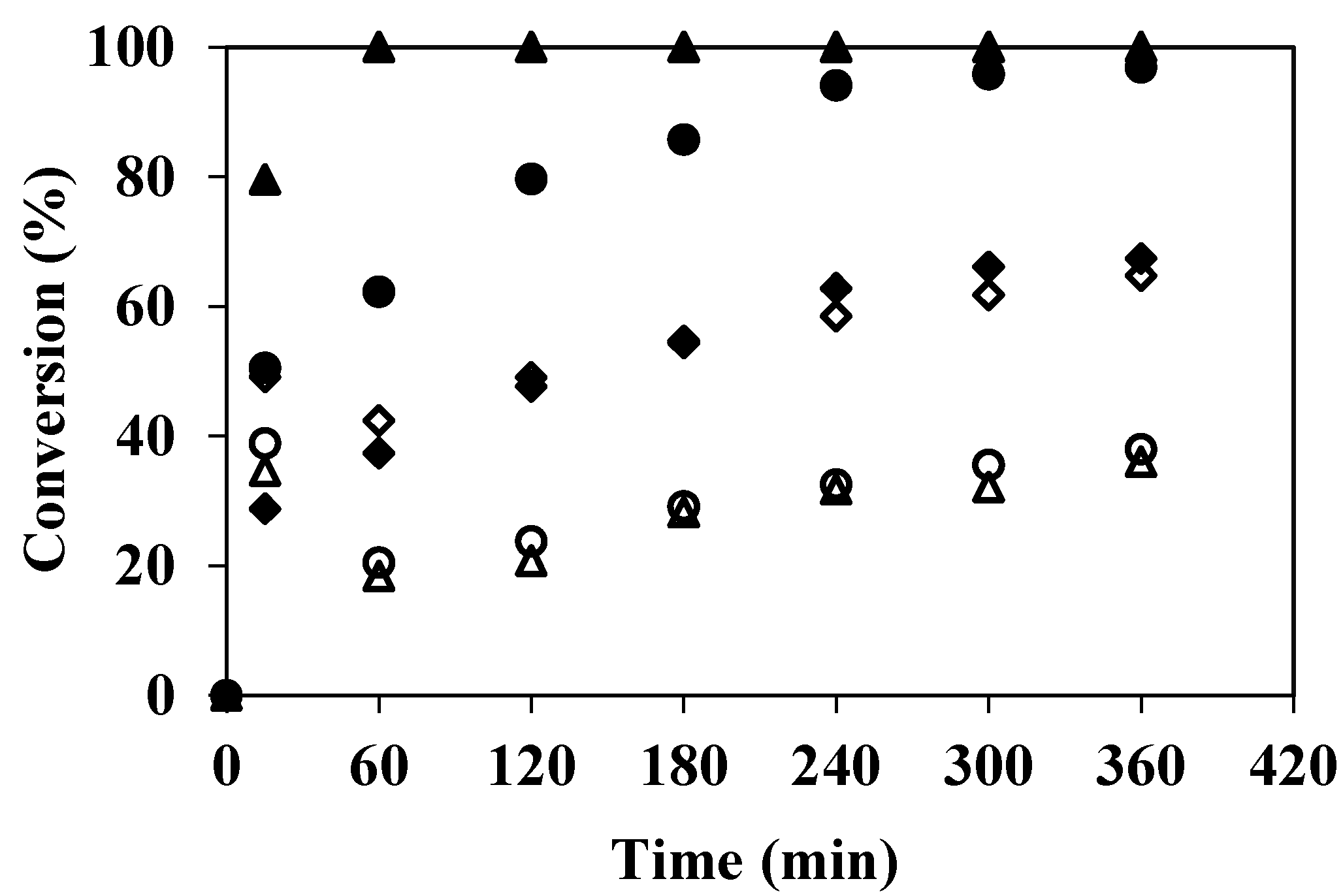
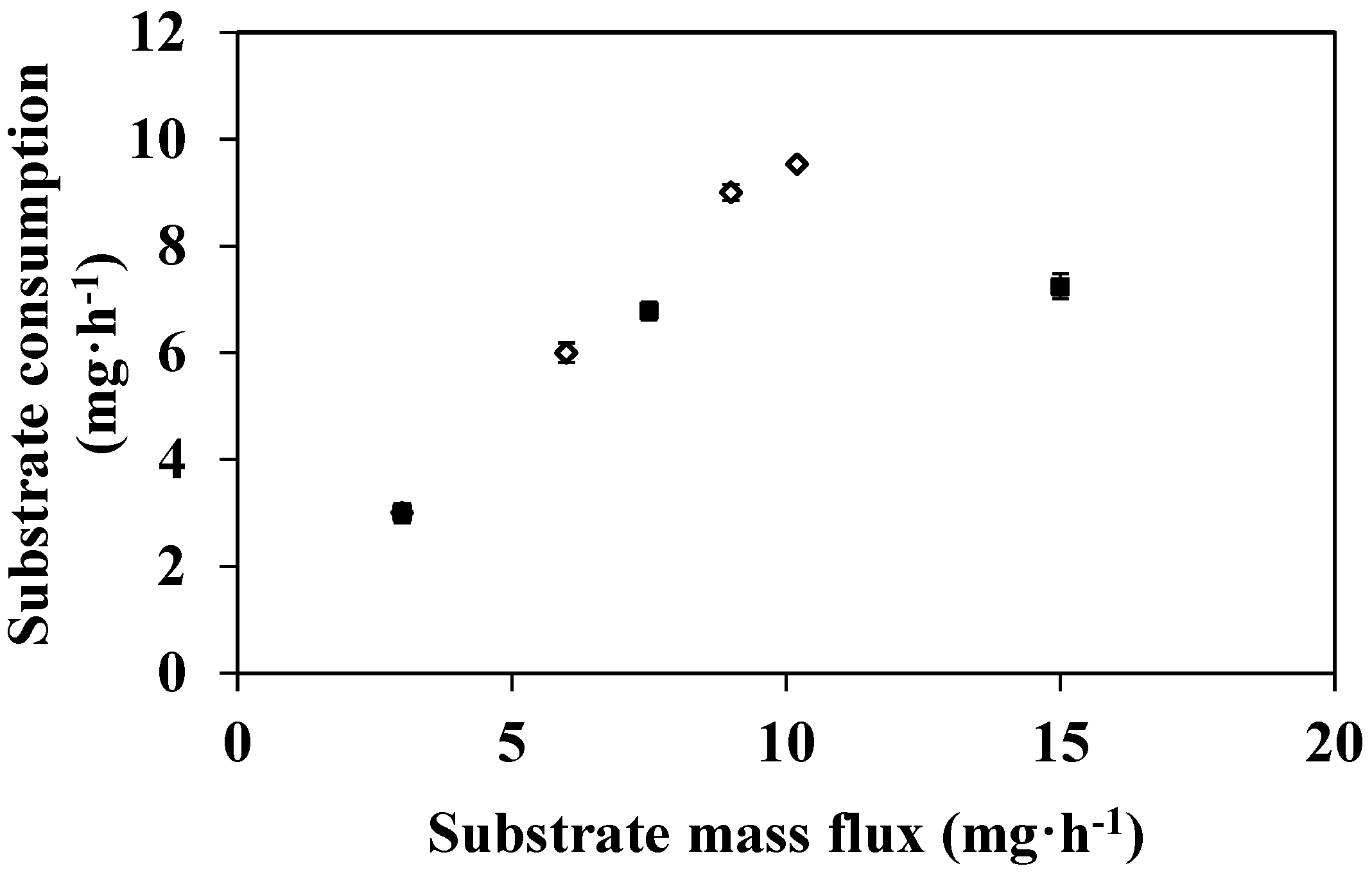
3.2. Feed Flux Effect on DMP Degradation

3.3. Effect of Substrate Concentration on DMP Degradation

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Rosa, M.A.; D’Annibale, A.; Gianfreda, L. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzyme Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Aktas, N.; Tanyolac, A. Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour. Technol. 2003, 87, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Canfora, L.; Iamarino, G.; Rao, M.A.; Gianfreda, L. Oxidative transformation of natural and synthetic phenolic mixtures by Trametes versicolor laccase. J. Agric. Food Chem. 2008, 56, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, S.; Nicell, J.A. Characterization of Trametes versicolor laccase for the transformation of aqueous phenol. Bioresour. Technol. 2008, 99, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Demarche, P.; Junghanns, C.; Nair, R.R.; Agathos, S.N. Harnessing the power of enzymes for environmental stewardship. Biotechnol. Adv. 2012, 30, 933–953. [Google Scholar] [CrossRef] [PubMed]
- Belleville, M.-P.; Paolucci, D.; Rios, G.M. Membrane Bioreactors and the Production of Food Ingredients in Separation, Extraction and Concentration Processes in the Food Beverage and Nutraceutical Industries; SSH, Rizvi, Ed.; Woodhead Publishing Limited: Cambridge, UK, 2010; pp. 314–337. [Google Scholar]
- Edwards, W.; Bownes, R.; Leukes, W.D.; Jacobs, E.P.; Sanderson, R.; Rose, P.D.; Burton, S.G. A capillary membrane bioreactor using immobilized polyphenol oxidase for the removal of phenols from industrial effluents. Enzyme Microb. Technol. 1999, 24, 209–217. [Google Scholar] [CrossRef]
- Edwards, W.; Leukes, W.D.; Rose, P.D.; Burton, S.G. Immobilization of polyphenol oxidase on chitosan-coated polysulphone capillary membranes for improved phenolic effluent bioremediation. Enzyme Microb. Technol. 1999, 25, 769–773. [Google Scholar] [CrossRef]
- Lante, A.; Crapisi, A.; Krastanov, A; Spettoli, P. Biodegradation of phenols by laccase immobilized in a membrane reactor. Process Biochem. 2000, 36, 51–58. [Google Scholar] [CrossRef]
- Jolivalt, C.; Brenon, S.; Caminade, E.; Mougin, C.; Pontié, M. Immobilization of laccase from Trametes versicolor on a modified PVDF microfiltration membrane: Characterization of the grafted support and application in removing a phenylurea pesticide in wastewater. J. Membr. Sci. 2000, 180, 103–113. [Google Scholar] [CrossRef]
- Erhan, E.; Keskinler, B.; Akay, G.; Algur, O.F. Removal of phenol from water by membrane-immobilized enzymes Part I. Dead-end filtration. J. Membr. Sci. 2002, 206, 361–373. [Google Scholar] [CrossRef]
- Ko, C.-H.; Chen, S.-S. Enhanced removal of three phenols by laccase polymerization with MF/UF membranes. Bioresour. Technol. 2008, 99, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Calabro, V.; Curcio, S.; De Paola, M.G.; Lorio, G. Optimization of membrane bioreactor performances during enzymatic oxidation of waste bio-polyphenols. Desalination 2009, 236, 30–38. [Google Scholar] [CrossRef]
- Palmieri, G.; Giardina, P.; Desiderio, B.; Marzullo, L.; Giamberini, M.; Sannia, G. A new enzyme immobilization procedure using copper alginate gel: Application to a fungal phenol oxidase. Enzyme Microb. Technol. 1994, 16, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang; Jiao, J.; Liu, M.; Hu, F.; Griffiths, B.S.; Li, H. Adsorption of Trametes versicolor laccase to soil iron and aluminumminerals: Enzyme activity, kinetics and stability studies. Colloid Surface B 2014, 114, 342–348. [Google Scholar] [CrossRef]
- De Cazes, M.; Belleville, M.-P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; de Gunzburg, J.; Barceló, D.; Sanchez-Marcano, J. Design and optimization of an enzymatic membrane reactor for the degradation of pharmaceutical micropollutants. Catal. Today 2014, 236, 146–152. [Google Scholar] [CrossRef]
- Belleville, M.-P.; Lozano, P.; Iborra, J.L.; Rios, G.M. Preparation of hybrid membranes for enzymatic reaction. Sep. Purif. Technol. 2001, 25, 229–233. [Google Scholar] [CrossRef]
- Gumí, T.; Albacete, J.F.-D.; Paolucci-Jeanjean, D.; Belleville, M.-P.; Rios, G.M. Study of the influence of the hydrodynamic parameters on the performance of an enzymatic membrane reactor. J. Membr. Sci. 2008, 311, 147–152. [Google Scholar] [CrossRef]
- Voudrias, E.L.; Larson, R.A.; Snoeyink, V.L. Effects of activated carbon on the reactions of free chlorine with phenols. Environ. Sci. Technol. 1985, 19, 441–449. [Google Scholar] [PubMed]
- Dodor, D.E.; Hwang, H.-M.; Ekunwe, S.I.N. Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from Trametes versicolor. Enzyme Microb. Technol. 2004, 35, 210–217. [Google Scholar]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Membrane Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Muktadirul Bari Chowdhury, A.K.M.; Akratos, C.S.; Vayenas, D.V.; Pavlou, S. Olive mill waste composting: A review. Int. Biodeter. Biodegr. 2013; 85, 108–119. [Google Scholar]
- Kudanga, T.; Nyanhongo, G.S.; Guebitz, G.M.; Burton, S. Potential applications of laccase-mediated coupling and grafting reactions: A review. Enzyme Microb. Tech. 2011, 48, 195–208. [Google Scholar] [CrossRef]
- Ba, S; Haroune, L.; Cruz-Morató, C.; Jacquet, C.; Touahar, I.E.; Bellenger, J.-P.; Legault, C.Y.; Jones, J.P.; Cabana, H. Synthesis and characterization of combined cross-linked laccase and tyrosinase aggregates transforming acetaminophen as a model phenolic compound in wastewaters. Sci. Total Environ. 2014, 487, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, J.M.; López-Muñoz, M.J.; Sotto, A. Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination 2010, 250, 829–832. [Google Scholar]
- Williams, M.E.; Hestekin, J.A.; Smothers, C.N.; Bhattacharyya, D. Separation of organic pollutants by reverse osmosis and nanofiltration membranes: Mathematical models and experimental verification. Ind. Eng. Chem. Res. 1999, 38, 3683–3695. [Google Scholar]
- Bódalo, A.; Gómez, E.; Hidalgo, A.M.; Gómez, M.; Murcia, M.D.; López, I. Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 2009, 245, 680–686. [Google Scholar] [CrossRef]
- Shuttleworth, K.L.; Bollag, J.-M. Soluble and immobilized laccase as catalysts for transformation of substituted phenols. Enzyme Microb. Technol. 1986, 8, 171–177. [Google Scholar] [CrossRef]
- Chivukula, M.; Renganathan, V. Phenolic Azo Dye Oxidation by Laccase from Pyricularia oryzae. Appl. Environ. Microb. 1995, 61, 4374–4377. [Google Scholar]
- Itoh, K.; Fujita, M.; Kumano, K.; Suyama, K.; Yamamoto, H. Phenolic acids affect transformations of chlorophenols by a Coriolus versicolor laccase. Soil Biol. Biochem. 2000, 32, 85–91. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Moreira, M.T.; Feijoo, G.; Lema, J.M.; Miyazaki, M. Improving the catalytic performance of laccase using a novel continuous-flow microreactor. Chem. Eng. J. 2013, 223, 497–506. [Google Scholar] [CrossRef]
- Katuri, K.P.; Mohan, S.V.; Sridhar, S.; Pati, B.R.; Sarma, P.N. Laccase-membrane reactors for decolorization of an acid azo dye in aqueous phase: Process optimization. Water Res. 2009, 43, 3647–3658. [Google Scholar] [PubMed]
- Riva, S. Laccases: Blue enzymes for green chemistry. Trends Biotechnol. 2006, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kurniawati, S.; Nicell, J.A. Variable stoichiometry during the laccase-catalysed oxidation of aqueous phenol. Biotechnol. Prog. 2007, 23, 389–397. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chea, V.; Paolucci-Jeanjean, D.; Sanchez, J.; Belleville, M.-P. Potentialities of a Membrane Reactor with Laccase Grafted Membranes for the Enzymatic Degradation of Phenolic Compounds in Water. Membranes 2014, 4, 678-691. https://doi.org/10.3390/membranes4040678
Chea V, Paolucci-Jeanjean D, Sanchez J, Belleville M-P. Potentialities of a Membrane Reactor with Laccase Grafted Membranes for the Enzymatic Degradation of Phenolic Compounds in Water. Membranes. 2014; 4(4):678-691. https://doi.org/10.3390/membranes4040678
Chicago/Turabian StyleChea, Vorleak, Delphine Paolucci-Jeanjean, José Sanchez, and Marie-Pierre Belleville. 2014. "Potentialities of a Membrane Reactor with Laccase Grafted Membranes for the Enzymatic Degradation of Phenolic Compounds in Water" Membranes 4, no. 4: 678-691. https://doi.org/10.3390/membranes4040678
APA StyleChea, V., Paolucci-Jeanjean, D., Sanchez, J., & Belleville, M.-P. (2014). Potentialities of a Membrane Reactor with Laccase Grafted Membranes for the Enzymatic Degradation of Phenolic Compounds in Water. Membranes, 4(4), 678-691. https://doi.org/10.3390/membranes4040678



