Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gas Permeation
| Sample | He permeation (mol·m−2·s−1·Pa−1) | N2 permeation (mol·m−2·s−1·Pa−1) | He/N2 permselectivity |
|---|---|---|---|
| Bare tube—new | 39.1 × 10−7 | 27.6 × 10−7 | 1.4 |
| Bare tube—dry | 30.5 × 10−7 | 24.6 × 10−7 | 1.2 |
| Membrane—new | 1.3 × 10−7 | 0.7 × 10−7 | 1.8 |
| Membrane—dry | 1.3 × 10−7 | 0.7 × 10−7 | 1.8 |
2.2. Desalination Performance


| NaCl concentration (mg·L−1) | Temperature (°C) | π (MPa) |
|---|---|---|
| 3000 | 21 | 0.23 |
| 3000 | 90 | 0.28 |
| 35,000 | 21 | 2.6 |
| 50,000 | 21 | 3.8 |
| 70,000 | 21 | 5.3 |
| 90,000 | 21 | 6.8 |
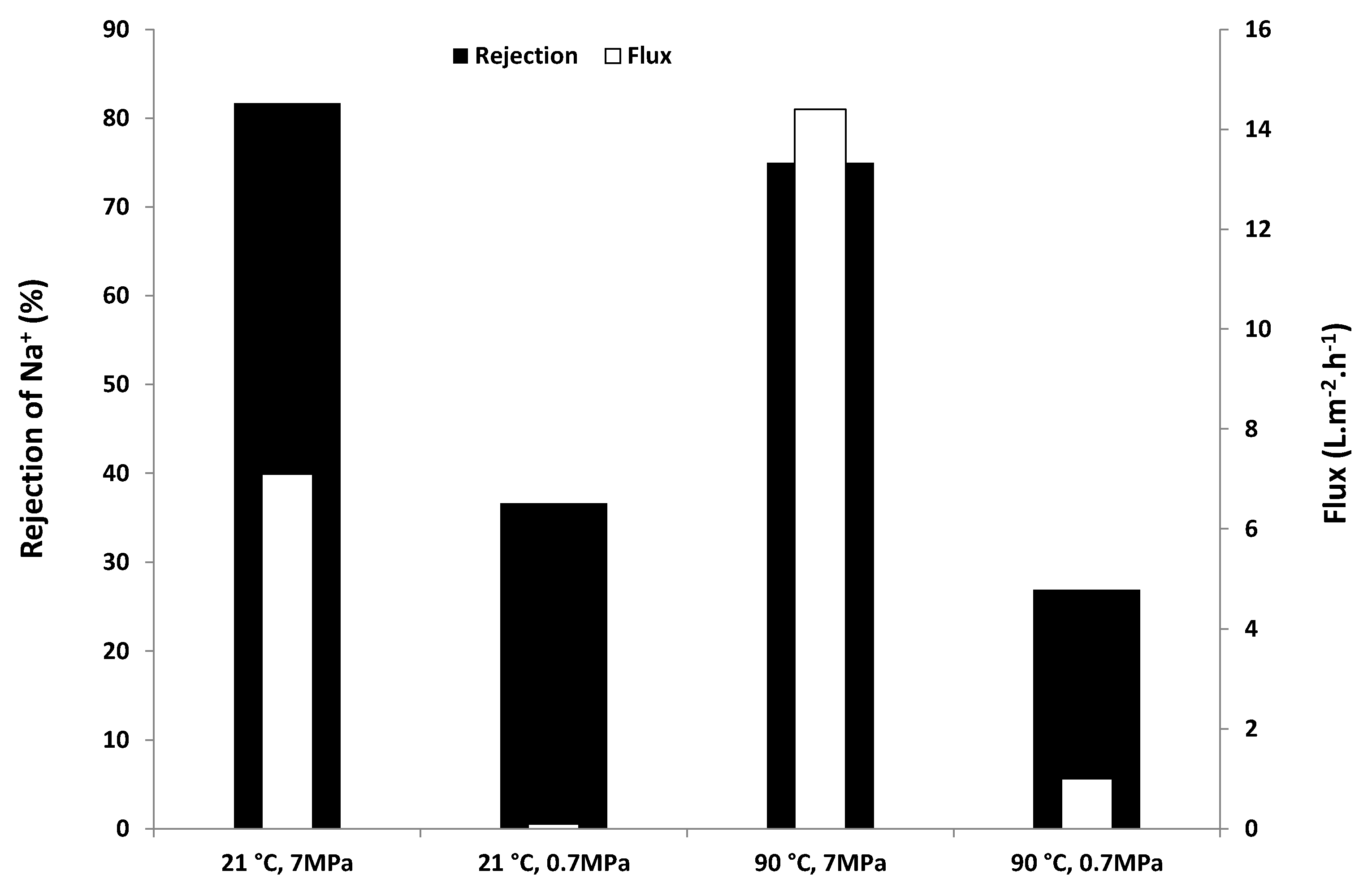

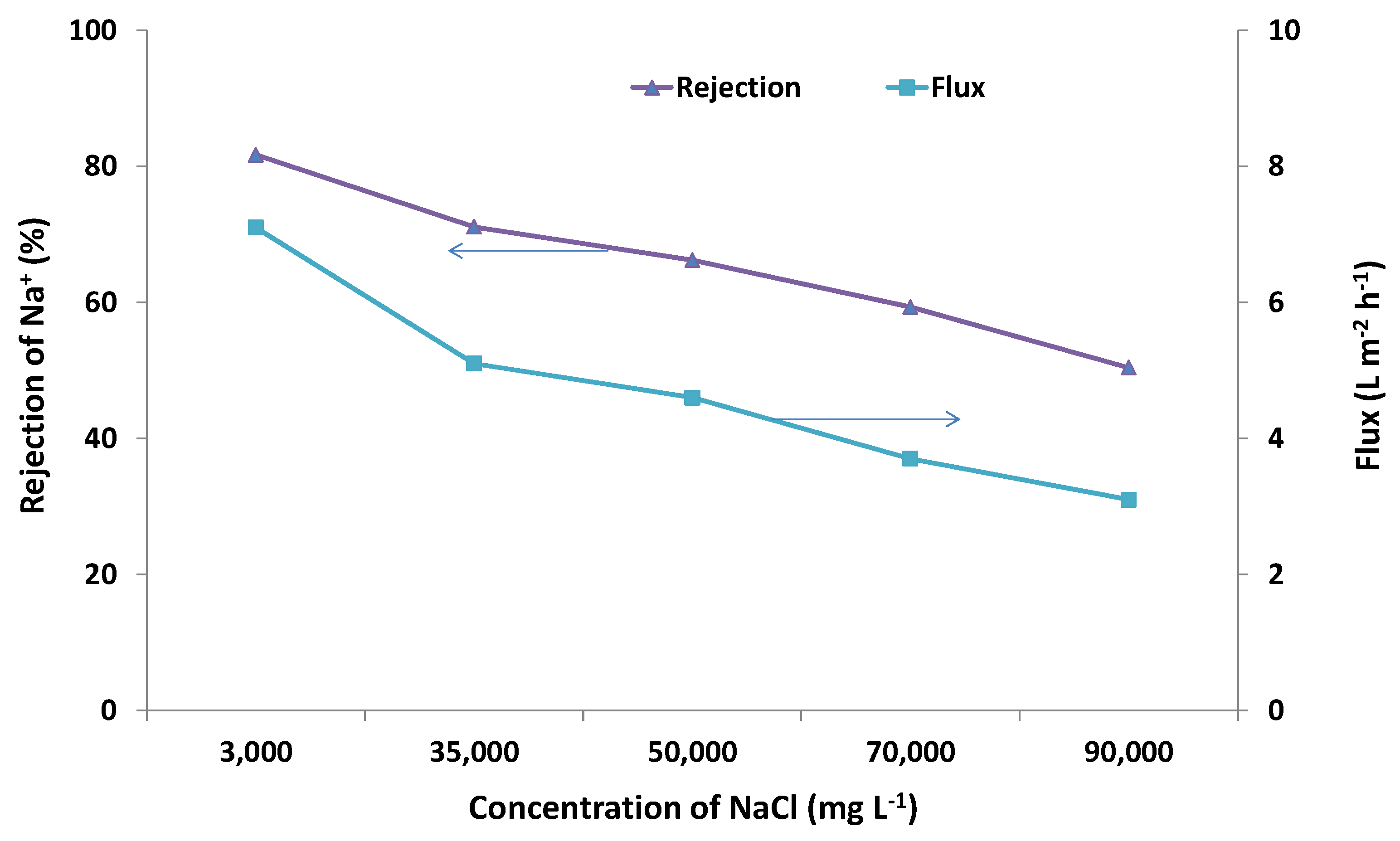
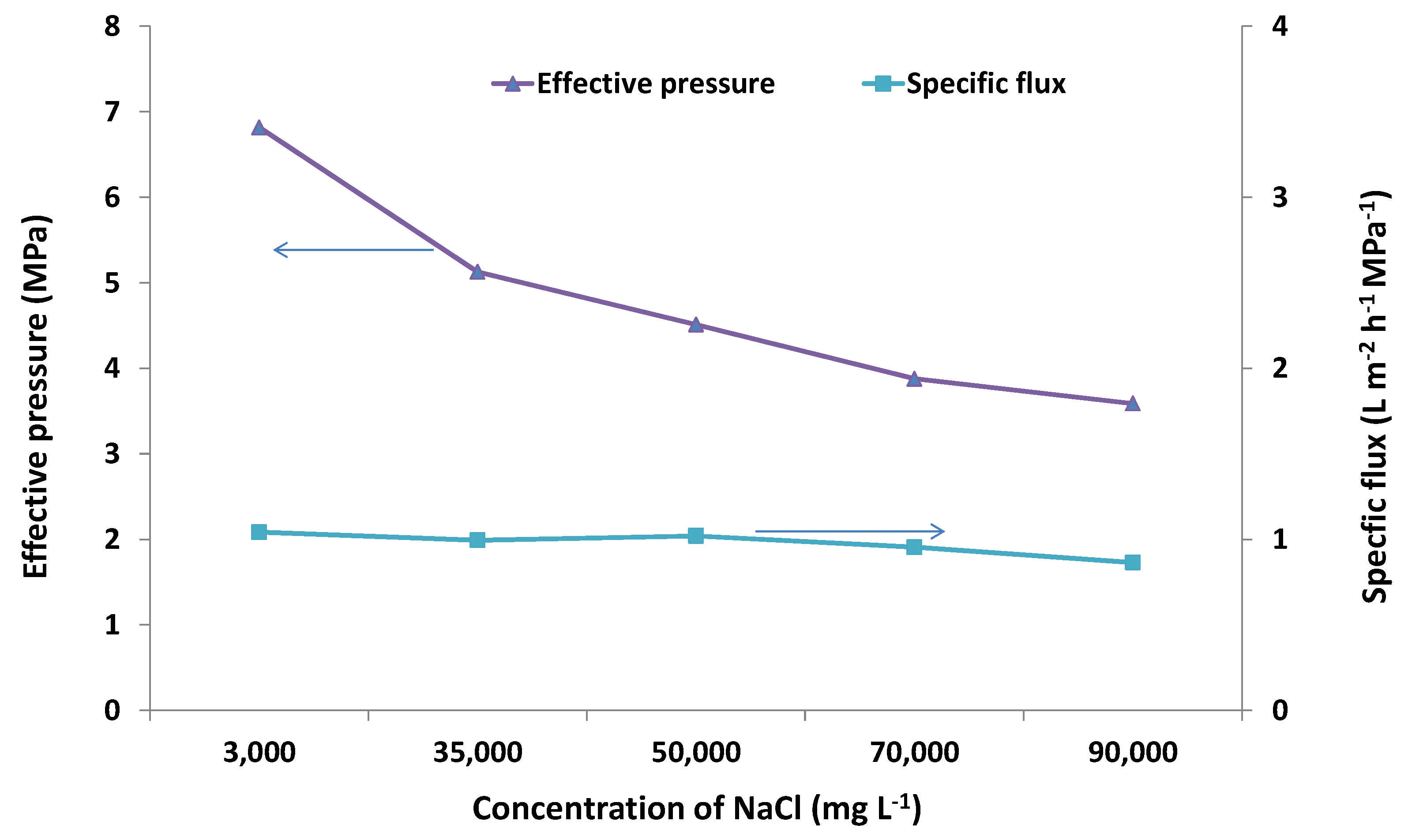
2.3. SEM

2.4. XPS
| Element | Bare tube (at %) | Original membrane (at %) | NaCl tested membrane (at %) |
|---|---|---|---|
| O 1s | 18.3 | 31.2 | 35.4 |
| C 1s | 54.8 | 40.8 | 33.2 |
| Al 2p | 15.9 | 2.0 | – |
| Si 2p | 3.0 | 22.7 | 24.7 |
| Ca 2p | 1.6 | 1.3 | 0.9 |
| N 1s | 2.4 | 2.0 | 2.7 |
| Cl 2p | 0.8 | – | 0.9 |
| Na 2s | 3.2 | – | 2.2 |
3. Experimental Section
3.1. Preparation of MFI-Type Zeolite Membrane
3.2. Characterisation
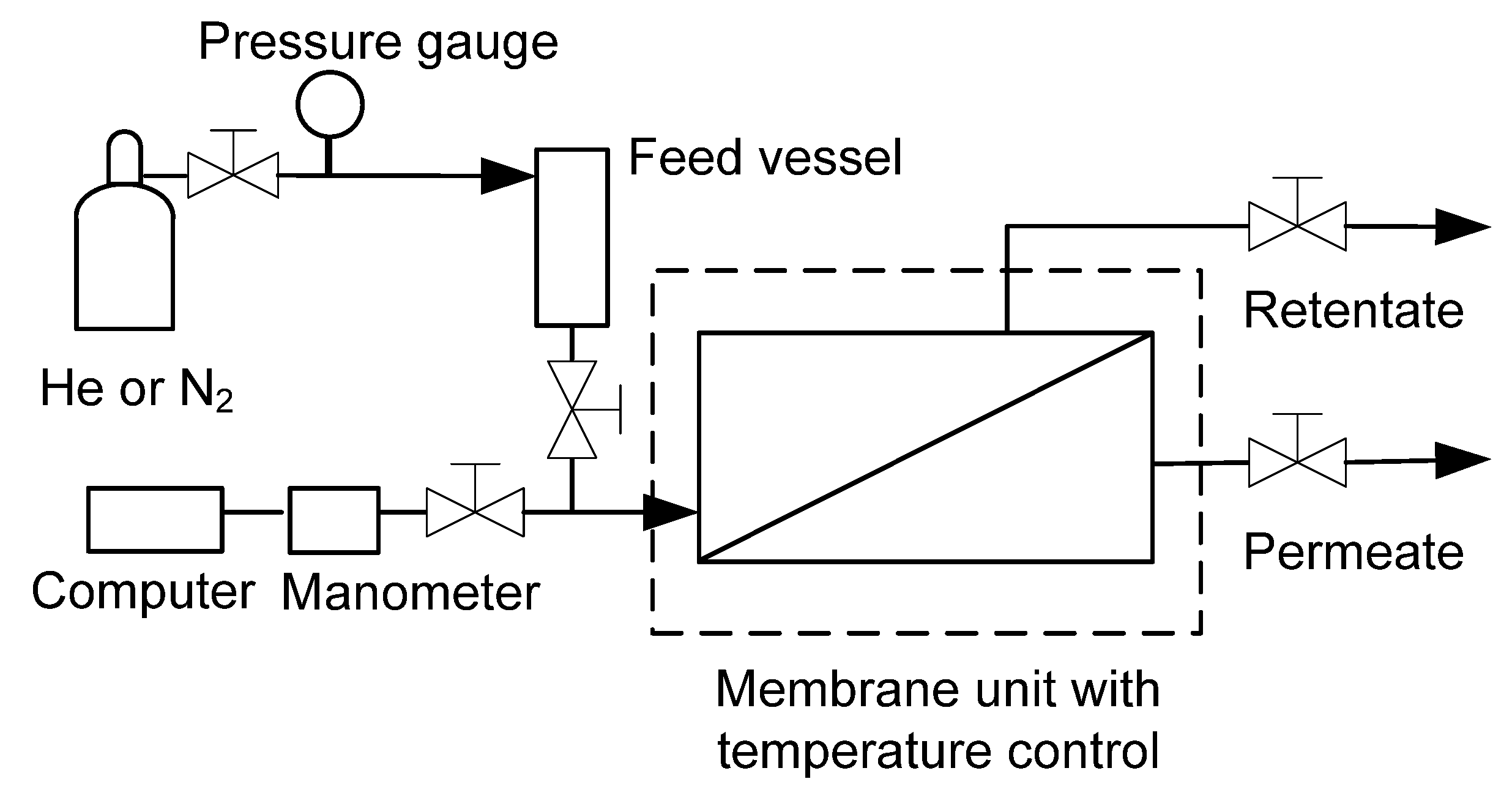
3.3. Desalination Test
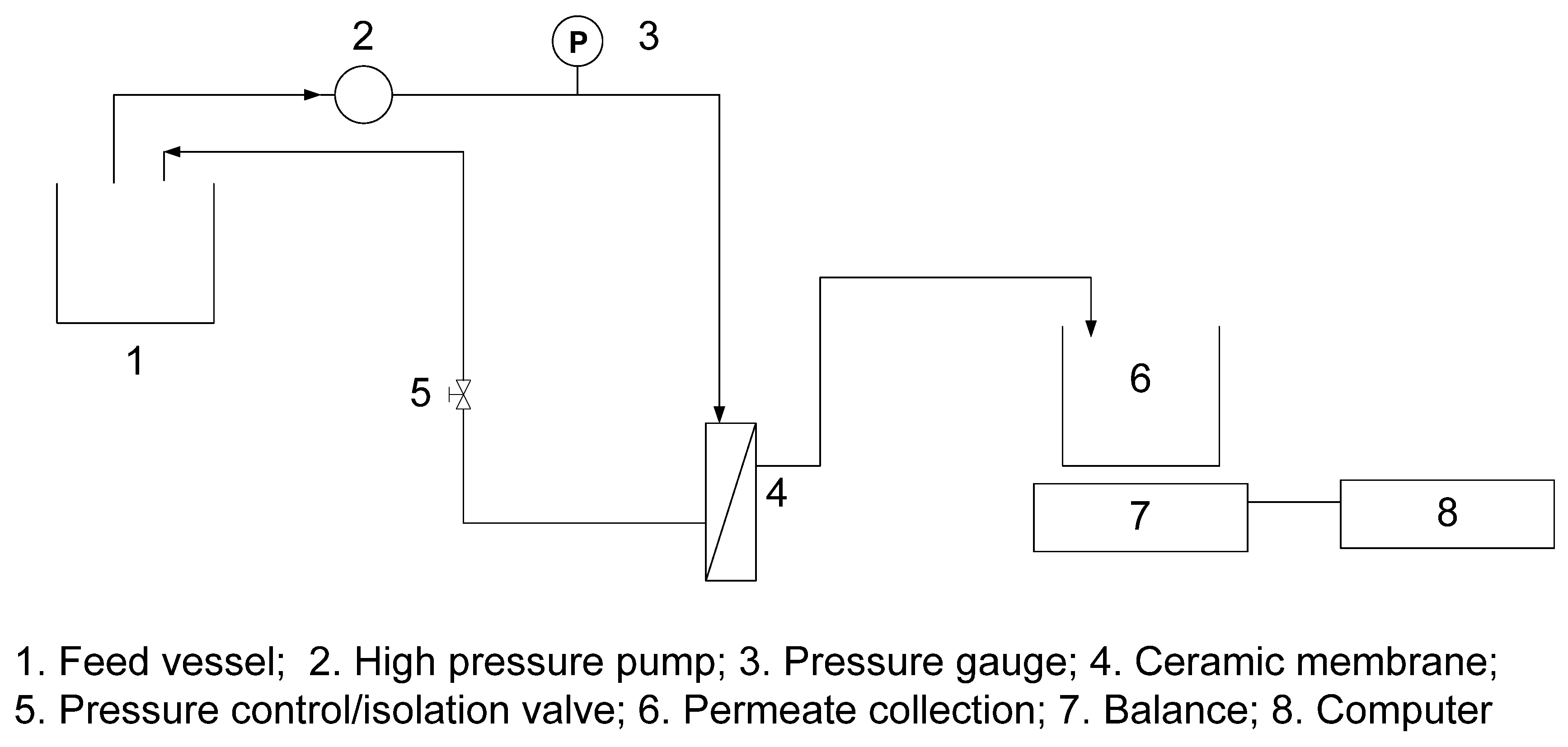
4. Conclusions
Acknowledgments
References
- Chesters, S.P.; Pena, N.; Gallego, S.; Fazel, M.; Armstrong, M.W.; del Vigo, F. Results from 99 Sea Water Reverse Osmosis (SWRO) Membrane Autopsies. In Proceedings of the IDA World Congress, Perth, Western Australia, Australia, 4–9 September 2011.
- NRC (National Research Council), Review of the Desalination and Water Purification Technology Roadmap; The National Academic Press: Washington, DC, USA, 2004.
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using naa zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Membr. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]
- Samuel de Lint, W.B.; Zivkovic, T.; Benes, N.E.; Bouwmeester, H.J.M.; Blank, D.H.A. Electrolyte retention of supported Bi-layered nanofiltration membranes. J. Membr. Sci. 2006, 277, 18–27. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Development of robust organosilica membranes for reverse osmosis. Langmuir 2011, 27, 13996–13999. [Google Scholar] [CrossRef]
- Li, L.X.; Dong, J.H.; Nenoff, T.M.; Lee, R. Desalination by reverse osmosis using mfi zeolite membranes. J. Membr. Sci. 2004, 243, 401–404. [Google Scholar] [CrossRef]
- Li, L.X.; Dong, J.H.; Nenoff, T.M.; Lee, R. Reverse osmosis of ionic aqueous solutions on a mfi zeolite membrane. Desalination 2004, 170, 309–316. [Google Scholar] [CrossRef]
- Li, L.X.; Dong, J.H.; Nenoff, T.M. Transport of water and alkali metal ions through mfi zeolite membranes during reverse osmosis. Sep. Purif. Technol. 2007, 53, 42–48. [Google Scholar]
- Duke, M.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, Y.S.; Diniz da Costa, J.C. Seawater desalination performance of mfi type membranes made by secondary growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- Kazemimoghadam, M.; Mohammadi, T. Synthesis of MFI zeolite membranes for water desalination. Desalination 2007, 206, 547–553. [Google Scholar] [CrossRef]
- Lin, J.; Murad, S. A computer simulation study of the separation of aqueous solutions using thin zeolite membranes. Mol. Phys. 2001, 99, 1175–1181. [Google Scholar] [CrossRef]
- Deng, Y.; Deng, C.; Qi, D.; Liu, C.; Liu, J.; Zhang, X.; Zhao, D. Synthesis of core/shell colloidal magnetic zeolite microspheres for the immobilization of trypsin. Adv. Mater. 2009, 21, 1377–1382. [Google Scholar] [CrossRef]
- Doocey, D.J.; Sharratt, P.N.; Cundy, C.S.; Plaisted, R.J. Zeolite-mediated advanced oxidation of model chlorinated phenolic aqueous waste: Part 2: Solid phase catalysis. Process. Saf. Environ. Prot. 2004, 82, 359–364. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Mao, Q.; Yue, J.; Wang, X. Novel nay zeolite-supported nanoscale zero-valent iron as an efficient heterogeneous fenton catalyst. Catal. Commun. 2010, 11, 937–941. [Google Scholar] [CrossRef]
- Coronas, J. Present and future synthesis challenges for zeolites. Chem. Eng. J. 2010, 156, 236–242. [Google Scholar] [CrossRef]
- Weller, M.T. Inorganic Materials; Oxford University Press Inc.: New York, NY, USA, 1994; pp. 71–81. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves—Structure, Chemistry, and Use; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Kaduk, J.A.; Faber, J. Crystal structure of zeolite Y as a function of ion exchange. Rigaku J. 1995, 12, 14–34. [Google Scholar]
- Lin, Y.S.; Duke, M.C. Recent progress in polycrystalline zeolite membrane research. Curr. Opin. Chem. Eng. 2013, 2, 209–216. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B. Database of zeolite structures. Available online: http://www.Iza-structure.org/databases/ (accessed on 11 July 2013).
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, X.; Liu, H.; Liu, S.; Yeung, K.L. Preparation and application of zeolite/ceramic microfiltration membranes for treatment of oil contaminated water. J. Membr. Sci. 2008, 325, 420–426. [Google Scholar] [CrossRef]
- Malekpour, A.; Millani, M.R.; Kheirkhah, M. Synthesis and characterization of a NAA zeolite membrane and its applications for desalination of radioactive solutions. Desalination 2008, 225, 199–208. [Google Scholar] [CrossRef]
- Li, L.; Liu, N.; McPherson, B.; Lee, R. Influence of counter ions on the reverse osmosis through MFI zeolite membranes: Implications for produced water desalination. Desalination 2008, 228, 217–225. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; McPherson, B.; Lee, R. Removal of organics from produced water by reverse osmosis using MFI-type zeolite membranes. J. Membr. Sci. 2008, 325, 357–361. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.H.; Lee, Y.J.; Jeong, N.C.; Yoon, K.B. Manual assembly of microcrystal monolayers on substrates. Angew. Chem. Int. Ed. Engl. 2007, 46, 3087–3090. [Google Scholar] [CrossRef]
- Yoo, W.C.; Stoeger, J.A.; Lee, P.-S.; Tsapatsis, M.; Stein, A. High-performance randomly oriented zeolite membranes using brittle seeds and rapid thermal processing. Angew. Chem. Int. Ed. Engl. 2010, 49, 8699–8703. [Google Scholar]
- Osmosis equation. Available online: http://www.chemteam.info/solutions/osmosis-equation.html (accessed on 30 May 2013).
- Fyfe, C.A.; Strobl, H.; Kokotailo, G.T.; Kennedy, G.J.; Barlow, G.E. Ultra-high-resolution/sup 29/Si solid-state mas nmr investigation of sorbate and temperature-induced changes in the lattice structure of zeolite ZSM-5. J. Am. Chem. Soc. 1988, 110, 3373–3380. [Google Scholar] [CrossRef]
- Chaplin, M. Water structure and science: Ion hydration and aqueous solutions of salts. Available online: http://www.lsbu.ac.uk/water/ions.html (accessed on 21 February 2013).
- Drobek, M.; Yacou, C.; Motuzas, J.; Julbe, A.; Ding, L.; Diniz da Costa, J.C. Long term pervaporation desalination of tubular mfi zeolite membranes. J. Membr. Sci. 2012, 415–416, 816–823. [Google Scholar]
- Zhu, B.; Zou, L.; Doherty, C.M.; Hill, A.J.; Lin, Y.S.; Hu, X.R.; Wang, H.T.; Duke, M. Investigation of the effects of ion and water interaction on structure and chemistry of silicalite MFI type zeolite for its potential use as a seawater desalination membrane. J. Mater. Chem. 2010, 20, 4675–4683. [Google Scholar]
- Zhu, B.; Doherty, C.M.; Hu, X.; Hill, A.J.; Zou, L.; Lin, Y.S.; Duke, M. Designing hierarchical porous features of ZSM-5 zeolites via Si/Al ratio and their dynamic behavior in seawater ion complexes. Microporous Mesoporous Mater. 2013, 173, 78–85. [Google Scholar] [CrossRef]
- Kerber, S.J.; Barr, T.L.; Mann, G.P.; Brantley, W.A.; Papazoglou, E.; Mitchell, J.C. The complementary nature of X-ray photoelectron spectroscopy and angle-resolved X-ray diffraction. Part I: Background and theory. J. Mater. Eng. Perform. 1998, 7, 329–333. [Google Scholar]
- X-ray photoelectron spectroscopy. Available online: http://en.wikipedia.org/wiki/X-ray_photoelectron_spectroscopy (accessed on 11 June 2013).
- Electron spectroscopy for chemical analysis (ESCA) aka X-ray photoelectron spectroscopy (XPS). Available online: http://www.foothills-analytical.com/ESCA.html (accessed on 11 June 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, B.; Kim, J.H.; Na, Y.-H.; Moon, I.-S.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane. Membranes 2013, 3, 155-168. https://doi.org/10.3390/membranes3030155
Zhu B, Kim JH, Na Y-H, Moon I-S, Connor G, Maeda S, Morris G, Gray S, Duke M. Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane. Membranes. 2013; 3(3):155-168. https://doi.org/10.3390/membranes3030155
Chicago/Turabian StyleZhu, Bo, Jun Hyun Kim, Yong-Han Na, Il-Shik Moon, Greg Connor, Shuichi Maeda, Gayle Morris, Stephen Gray, and Mikel Duke. 2013. "Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane" Membranes 3, no. 3: 155-168. https://doi.org/10.3390/membranes3030155
APA StyleZhu, B., Kim, J. H., Na, Y.-H., Moon, I.-S., Connor, G., Maeda, S., Morris, G., Gray, S., & Duke, M. (2013). Temperature and Pressure Effects of Desalination Using a MFI-Type Zeolite Membrane. Membranes, 3(3), 155-168. https://doi.org/10.3390/membranes3030155






