Separation Properties of Wastewater Containing O/W Emulsion Using Ceramic Microfiltration/Ultrafiltration (MF/UF) Membranes
Abstract
:1. Introduction
2. Experimental Section

3. Results and Discussion
3.1. Filtration Properties in Retentive Condition with UF Membrane (Nominal Pore Size 5 nm)
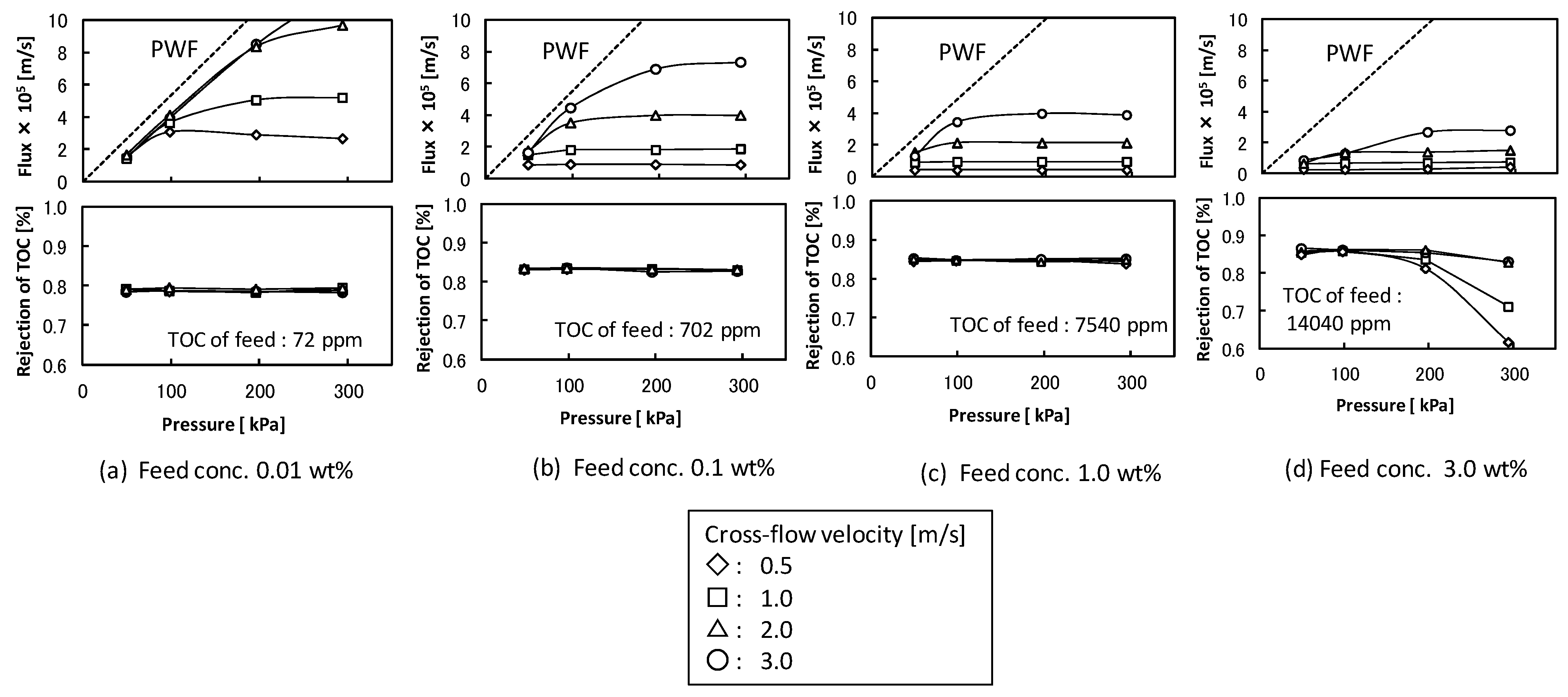

- at U = 0.5 m/s, K = 1.58 × 10−6 m/s
- at U = 1.0 m/s, K = 3.25 × 10−6 m/s
- at U = 2.0 m/s, K = 7.33 × 10−6 m/s
- at U = 3.0 m/s, K = 1.34 × 10−5 m/s


3.2. The Conditions of O/W Emulsion Permeate Through Membrane
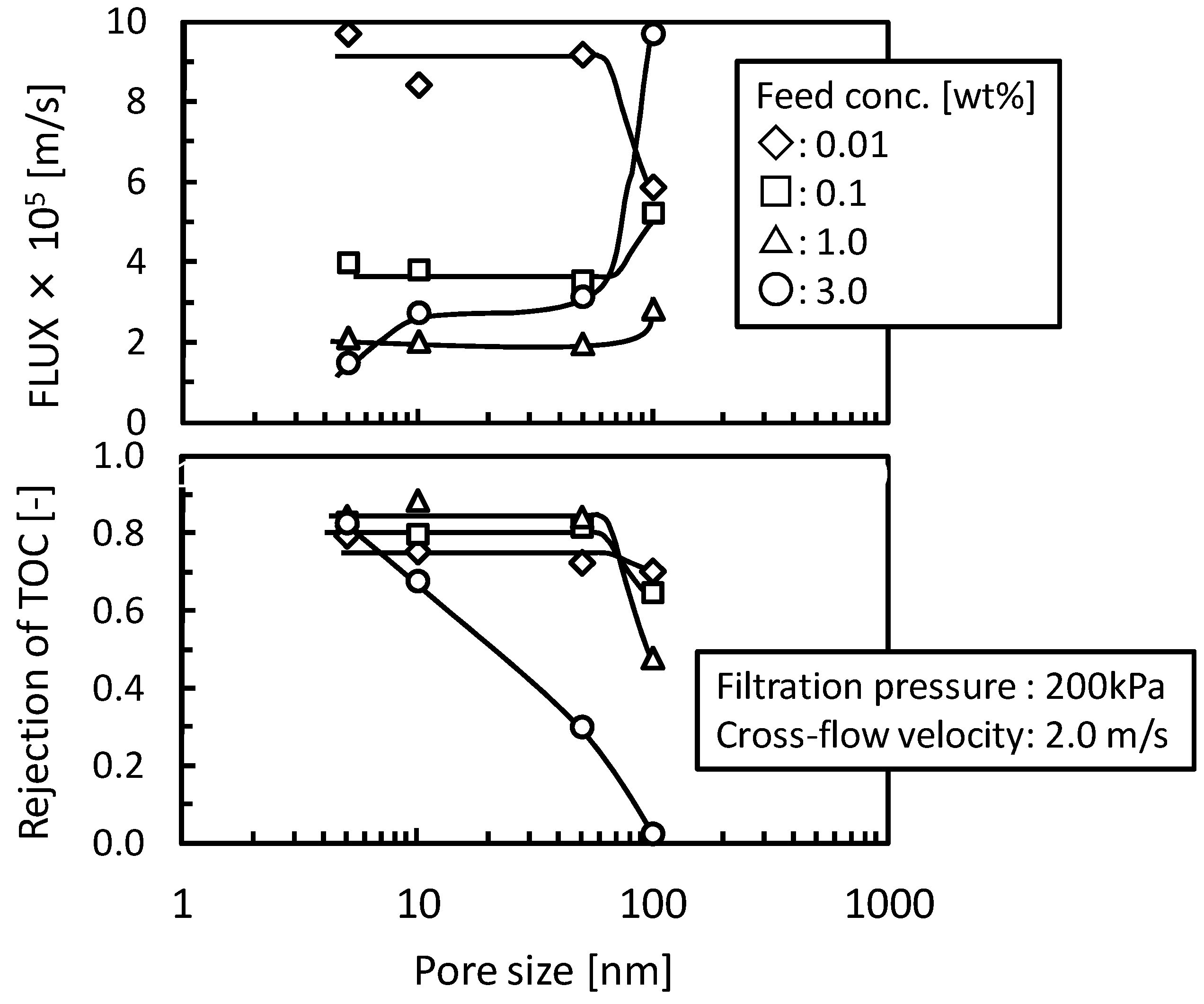
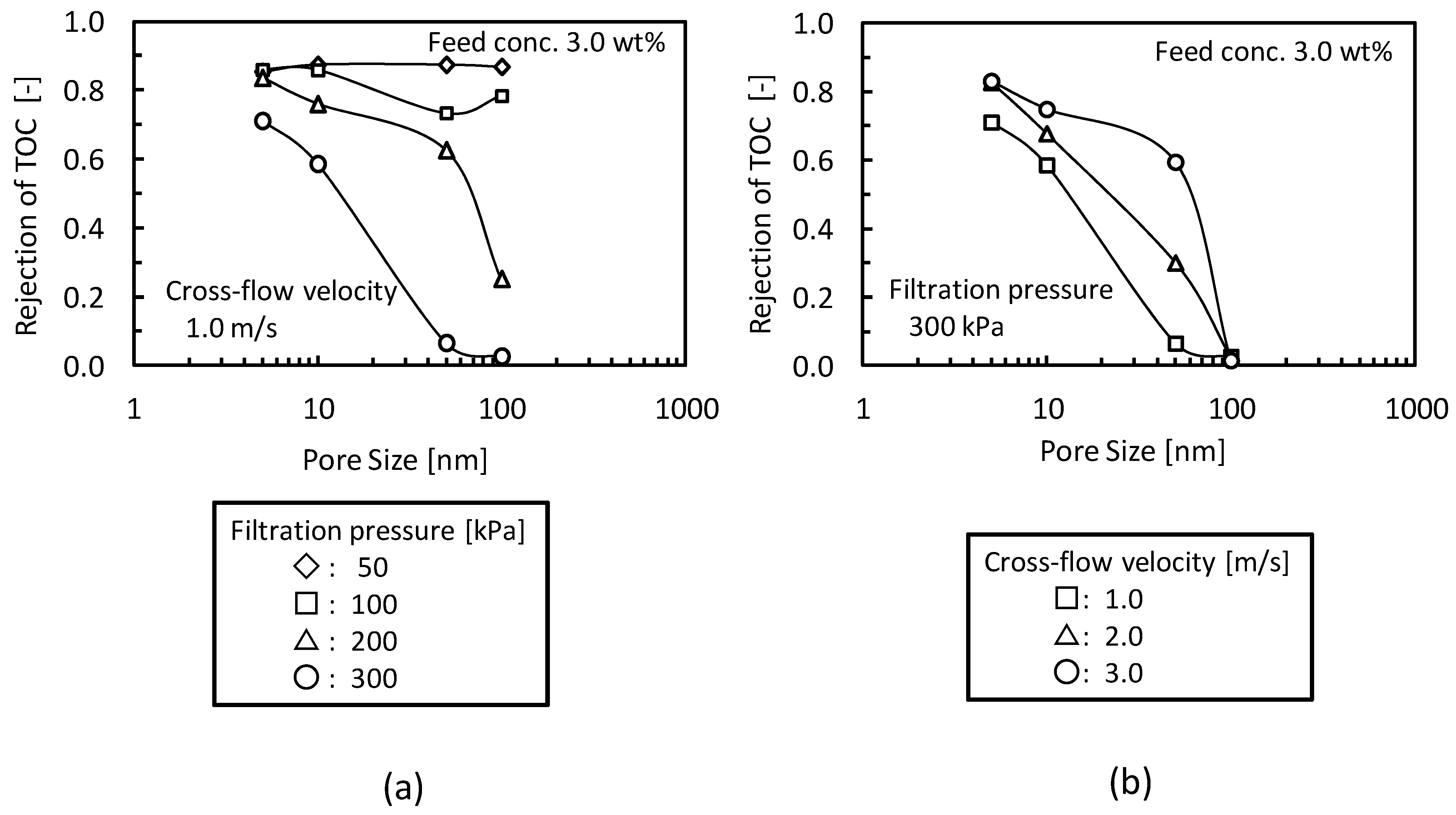
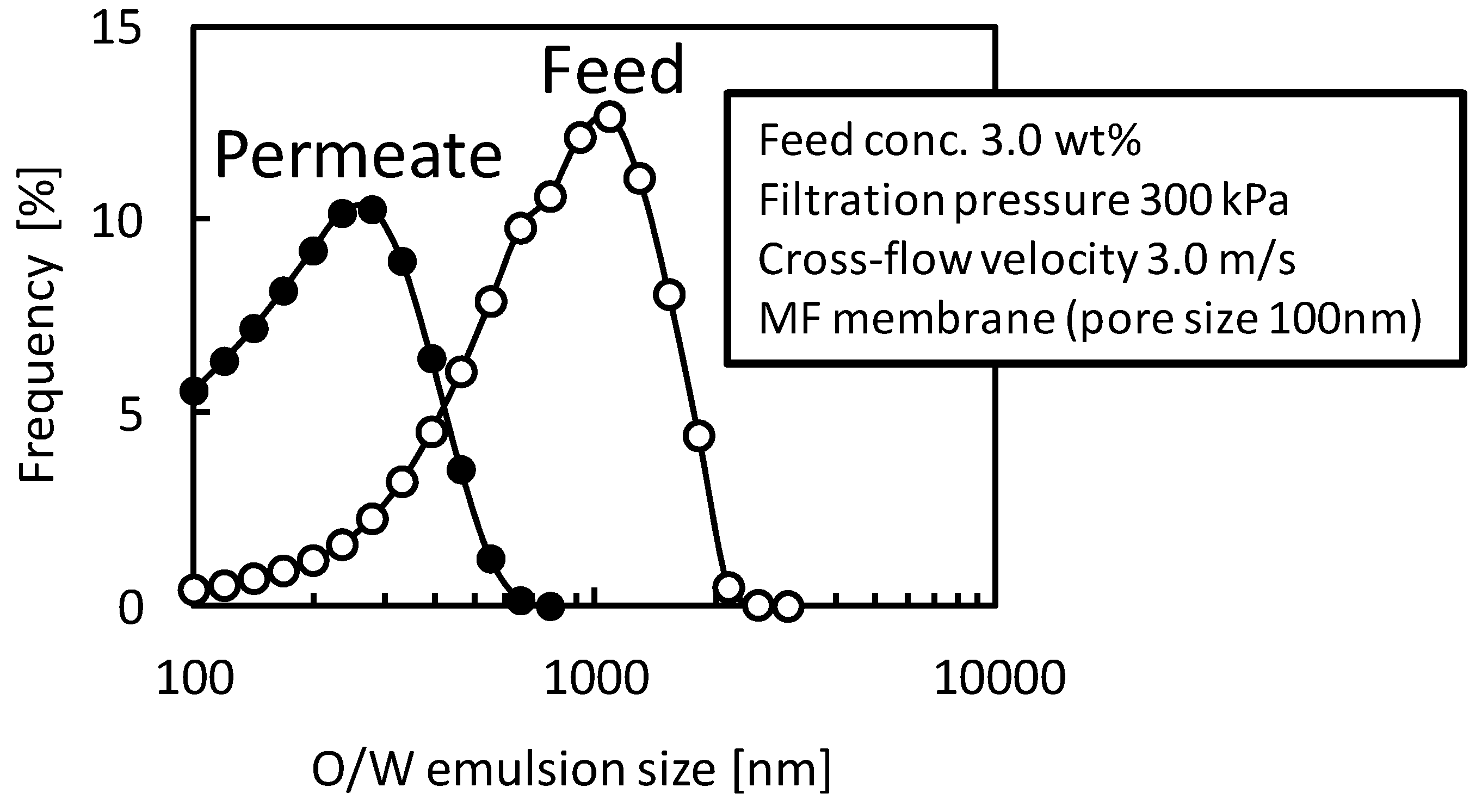
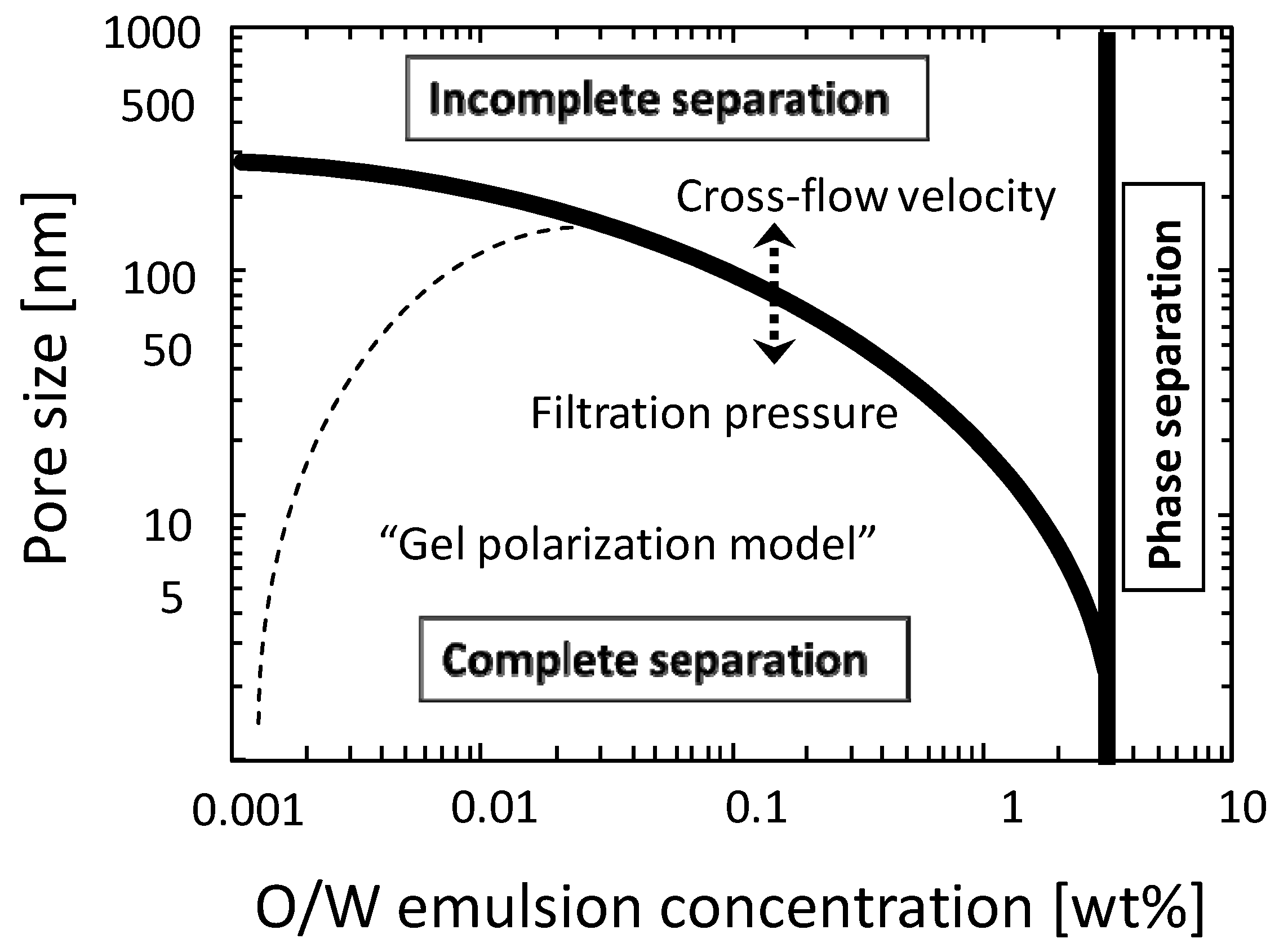
3.3. Addition of Machine Oil
3.4. Batch Concentration Filtration with UF Membrane (Pore Size 5 nm)

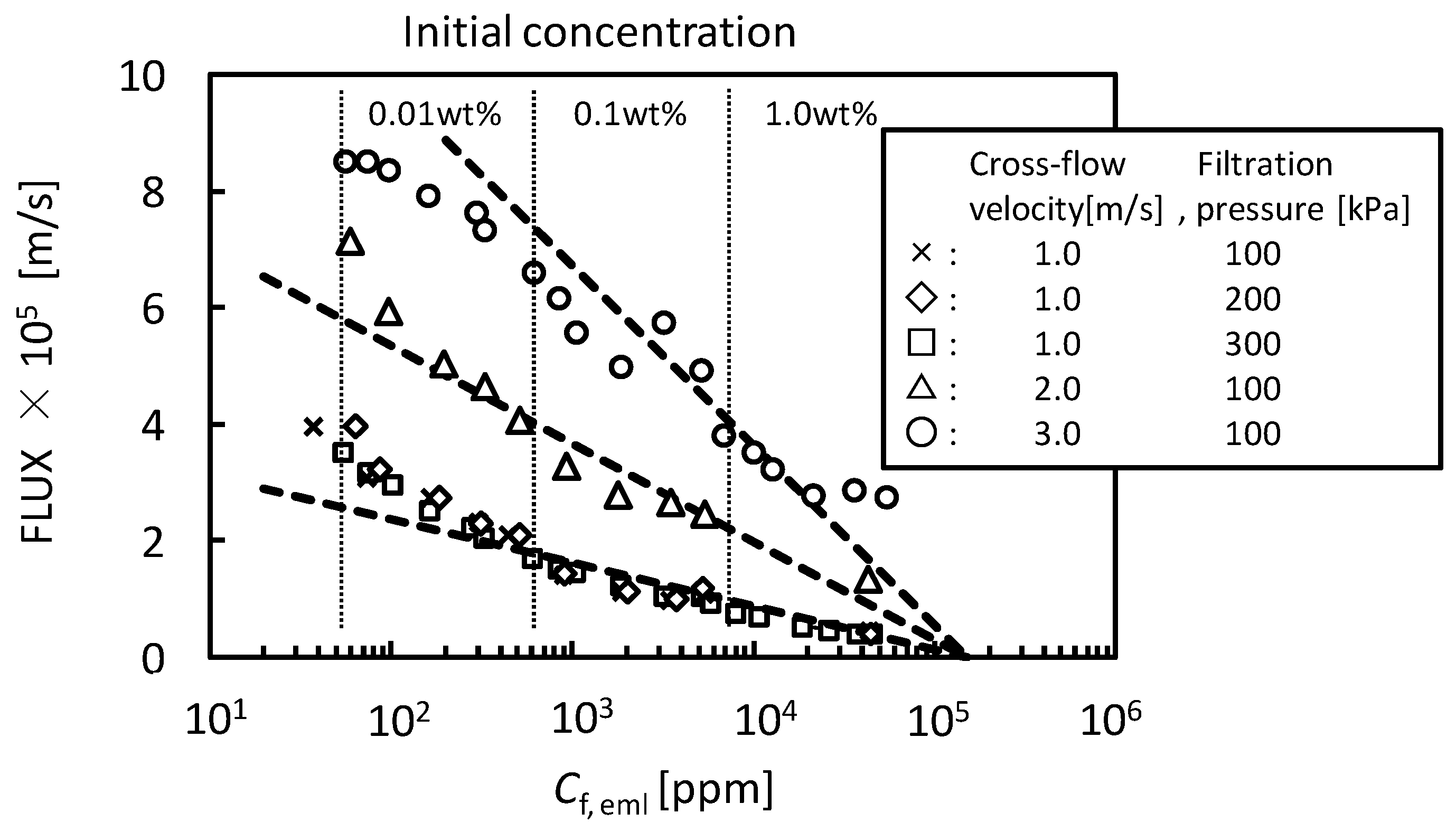
4. Conclusions
References
- Zaidi, A.; Simms, K.; Kok, S. The use of micro/ultrafiltration for the removal of oil and suspended solids from oilfield braines. Water Sci. Technol. 1992, 25, 163–176. [Google Scholar]
- Zeman, L.J.; Zydney, A.L. Microfiltration and Ultrafiltration; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Lin, S.H.; Lan, W.J. Waste oil/water emulsion treatment by membrane processes. J. Hazard. Mater. 1998, 59, 189–199. [Google Scholar] [CrossRef]
- Hilal, N.; Busca, G.; Hankins, N.; Mohammad, A.W. The use of ultrafiltration and nanofiltration membranes in the treatment of metal-working fluids. Desalination 2004, 167, 227–238. [Google Scholar] [CrossRef]
- Koltuniewicz, A.B.; Field, R.W.; Arnot, T.C. Cross-flow and dead-end microfiltration of oily-water emulsion. Part I: Experimental study and analysis of flux decline. J. Membr. Sci. 2006, 102, 193–207. [Google Scholar]
- López, R.V.; Elmaleh, S.; Ghaffor, N. Cross-flow ultrafiltration of hydrocarbon emulsions. J. Membr. Sci. 1995, 102, 55–64. [Google Scholar] [CrossRef]
- Li, H.-J.; Cao, Y.-M.; Qin, J.-J.; Jie, X.-M.; Wang, T.H.; Liu, J.H.; Yuan, Q. Development and characterization of anti-fouling cellulose hollow fiber UF membranes for oil-water separation. J. Membr. Sci. 2006, 279, 328–335. [Google Scholar] [CrossRef]
- Lipp, P.; Lee, C.H.; Fane, A.G.; Fell, C.J.D. A fundamental study of the ultrafiltration of oil-water emulsions. J. Membr. Sci. 1988, 36, 161–177. [Google Scholar] [CrossRef]
- Scott, K.; Jachuck, R.J.; Hall, D. Crossflow microfiltration of water-in-oil emulsions using corrugated membranes. Sep. Purif. Technol. 2001, 22–23, 431–441. [Google Scholar]
- Dal-Cin, M.M.; Lick, C.N.; Kumar, A.; Lealess, S. Dispersed phase back transport during ultrafiltration of cutting oil emulsions with a spinning membrane disc geometry. J. Membr. Sci. 1998, 141, 165–181. [Google Scholar] [CrossRef]
- Vatai, G.N.; Krstic, D.M.; Koris, A.K.; Gáspár, I.L.; Tekić, M.N. Ultrafiltration of oil-in-water emulsion: Comparison of ceramic and polymeric membranes. Desalin. Water Treat. 2009, 3, 162–168. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nakamura, K.; Matsumoto, K. Separation Properties of Wastewater Containing O/W Emulsion Using Ceramic Microfiltration/Ultrafiltration (MF/UF) Membranes. Membranes 2013, 3, 87-97. https://doi.org/10.3390/membranes3020087
Nakamura K, Matsumoto K. Separation Properties of Wastewater Containing O/W Emulsion Using Ceramic Microfiltration/Ultrafiltration (MF/UF) Membranes. Membranes. 2013; 3(2):87-97. https://doi.org/10.3390/membranes3020087
Chicago/Turabian StyleNakamura, Kazuho, and Kanji Matsumoto. 2013. "Separation Properties of Wastewater Containing O/W Emulsion Using Ceramic Microfiltration/Ultrafiltration (MF/UF) Membranes" Membranes 3, no. 2: 87-97. https://doi.org/10.3390/membranes3020087
APA StyleNakamura, K., & Matsumoto, K. (2013). Separation Properties of Wastewater Containing O/W Emulsion Using Ceramic Microfiltration/Ultrafiltration (MF/UF) Membranes. Membranes, 3(2), 87-97. https://doi.org/10.3390/membranes3020087



