Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level
Abstract
:1. Introduction
2. Materials and Methods
2.1. Growth Media
2.1.1. Escherichia coli
2.1.2. Saccharomyces cerevisiae
2.2. Dry Mass of Microbial Suspensions
2.3. Suspensions Preparation for Crossflow Microfiltration
| Abbreviation | Saccharomyces cerevisiae (g L−1) | Escherichia coli (g L−1) |
|---|---|---|
| S1 | 6 | 0 |
| S2 | 6 | 0.15 |
| S3 | 6 | 0.3 |
| S4 | 8 | 0 |
| S5 | 10 | 0 |
| S6 | 10 | 0.15 |
| S7 | 10 | 0.30 |
2.4. Fundamentals of Microfiltration




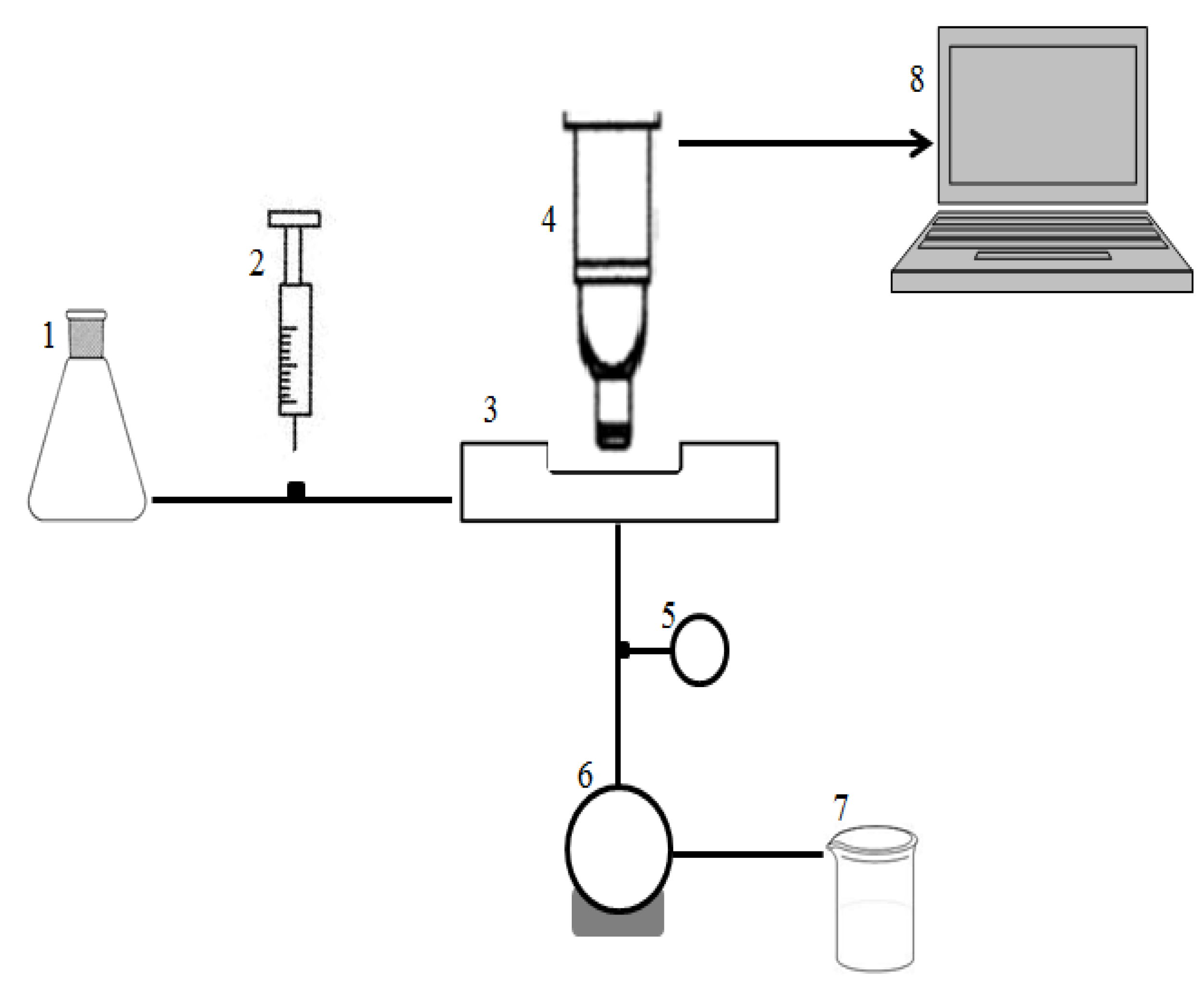
2.5. Crossflow Microfiltration Set-Up and Membrane
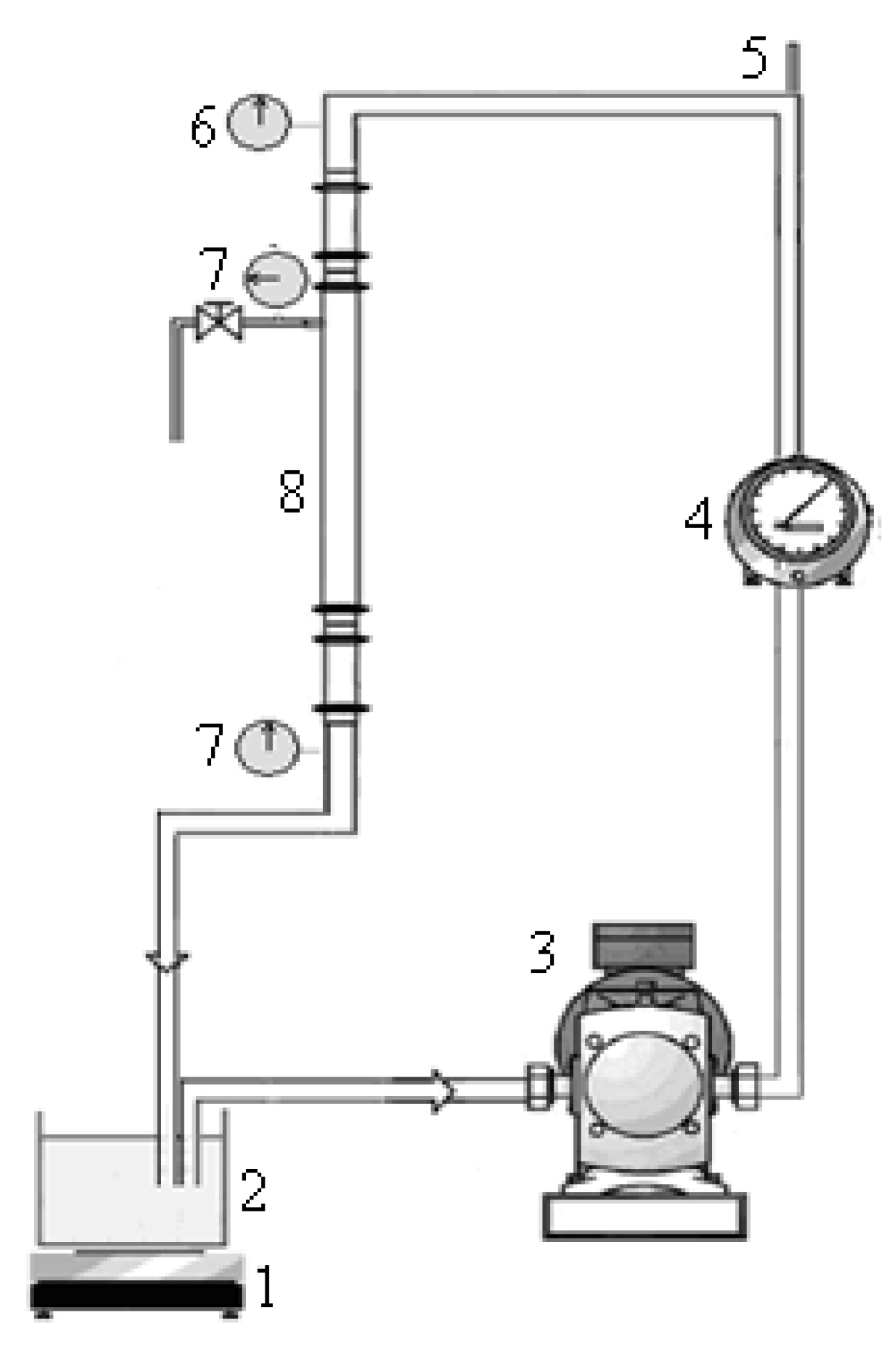



2.6. Membrane Regeneration
2.7. Scanning Electron Microscopy (SEM)
2.8. Confocal Laser Scanning Microscopy Analysis
2.8.1. Preparation of Microbial Suspensions
2.8.2. Microscopic Apparatus and Operating Conditions
2.9. Image Analysis
3. Results and Discussion
3.1. Microfiltration of Cells Suspensions
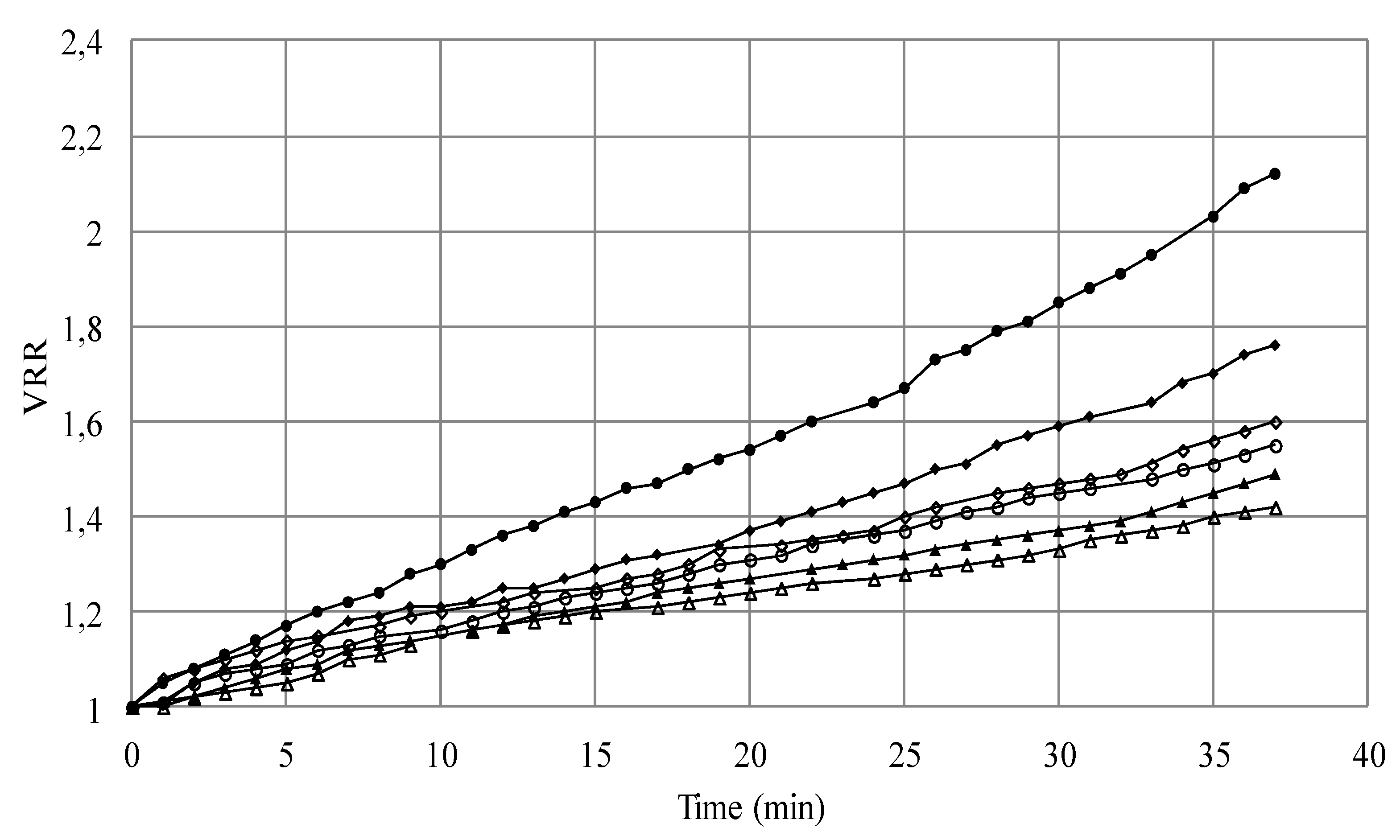
3.1.1. Microfiltration of Yeast Suspensions
 S1; ΔS4; □S5) versus time (TMP = 1.5 bar, U = 2.38 m/s, Re = 5950).
S1; ΔS4; □S5) versus time (TMP = 1.5 bar, U = 2.38 m/s, Re = 5950).
 S1; ΔS4; □S5) versus time (TMP = 1.5 bar, U = 2.38 m/s, Re = 5950).
S1; ΔS4; □S5) versus time (TMP = 1.5 bar, U = 2.38 m/s, Re = 5950). 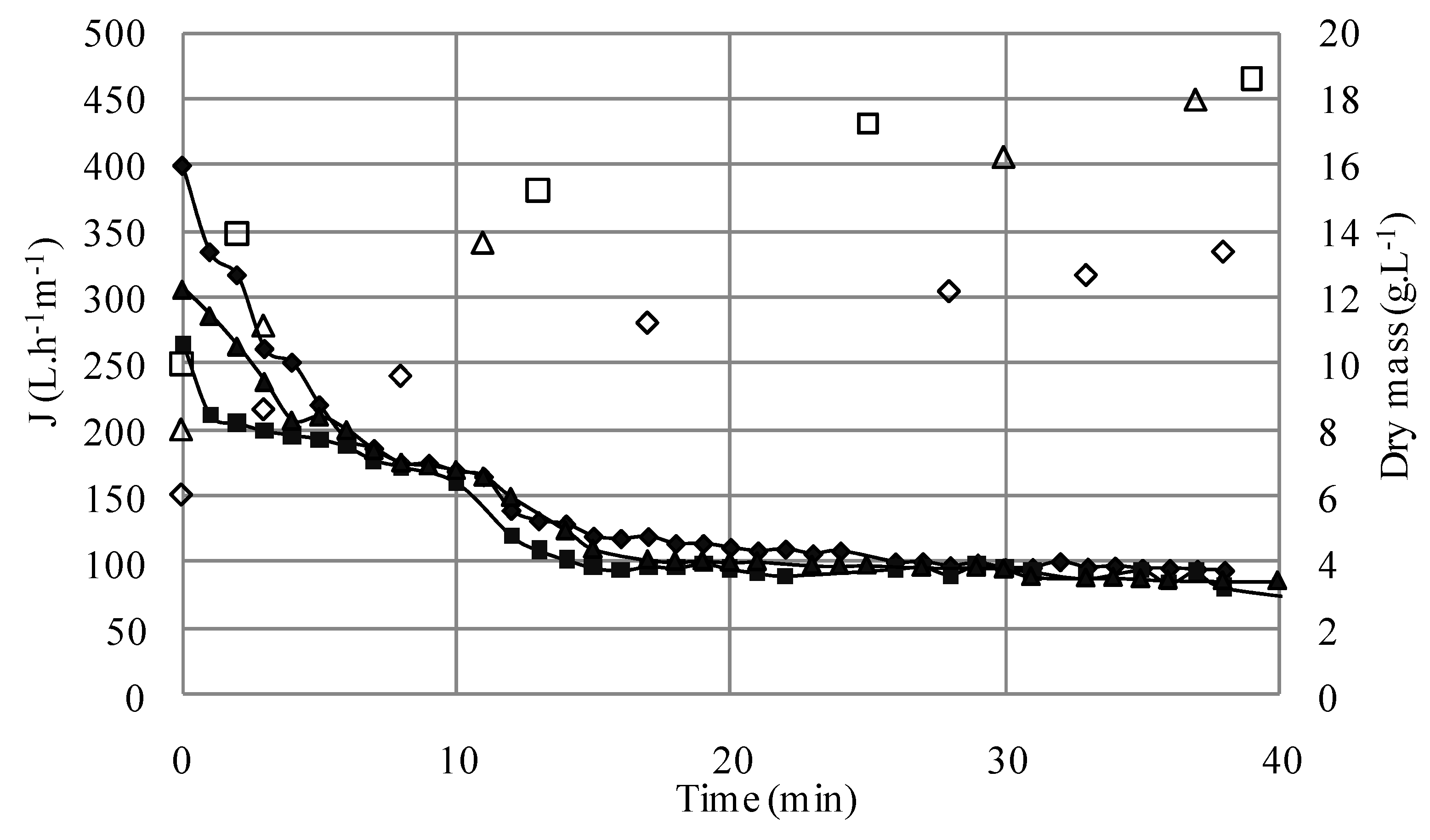
| Suspension | Resistances ×1012 (m−1) | Normalised resistances (%) | ||||
|---|---|---|---|---|---|---|
| Rt | Rf | Rif | Rrf | Rif/Rf | Rrf/Rf | |
| S1 | 5.79 | 5.34 | 4.01 | 1.33 | 75 | 25 |
| S2 | 6.40 | 5.95 | 2.83 | 3.12 | 48 | 52 |
| S3 | 6.49 | 6.04 | 2.39 | 3.65 | 40 | 60 |
| S4 | 6.24 | 5.78 | 2.67 | 3.12 | 46 | 54 |
| S5 | 6.63 | 6.18 | 1.65 | 4.53 | 27 | 73 |
| S6 | 6.72 | 6.27 | 2.51 | 3.76 | 38 | 62 |
| S7 | 7.68 | 7.23 | 2.84 | 4.39 | 40 | 60 |
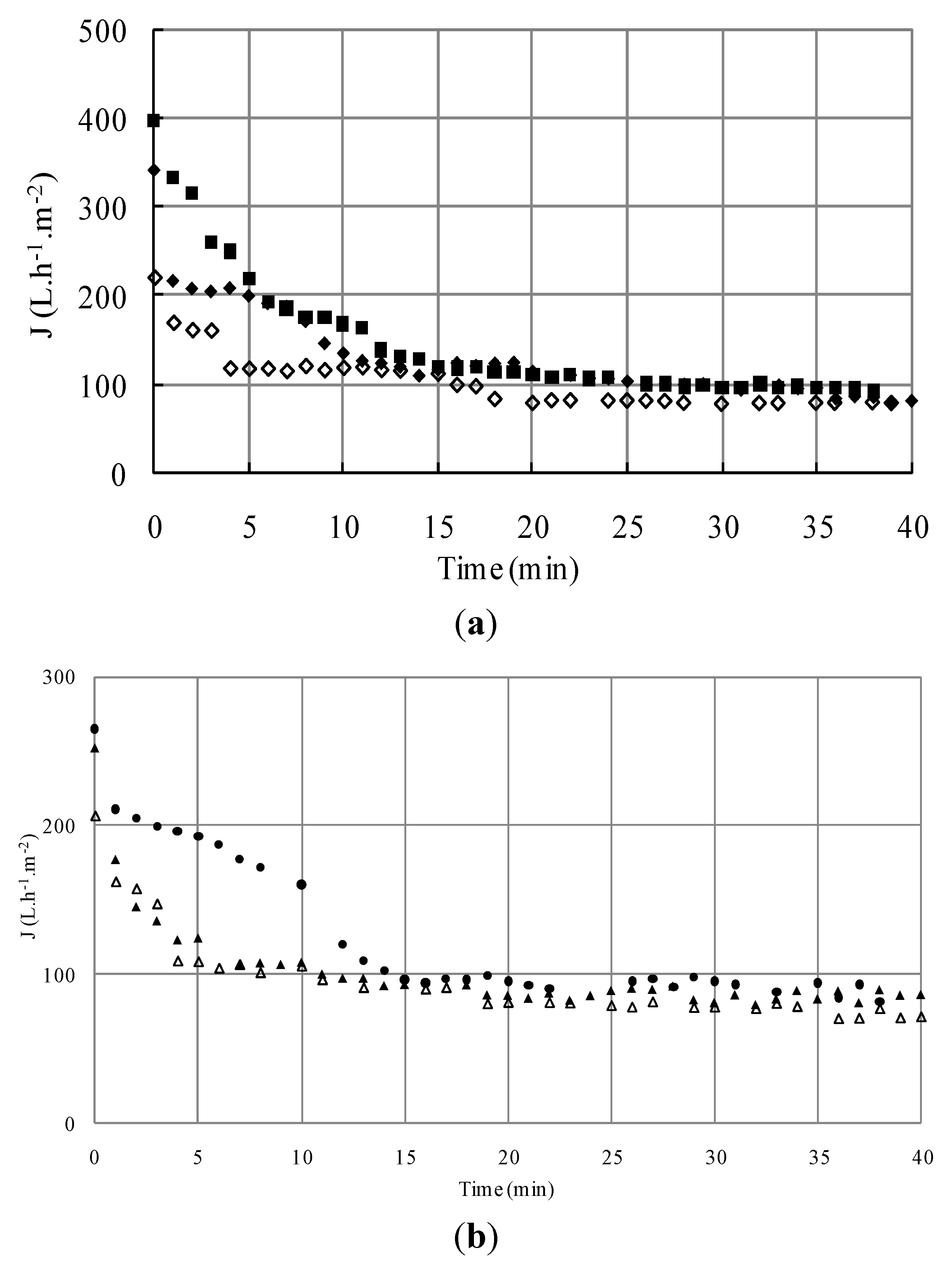
3.1.2. Microfiltration of Yeast and Bacteria Mixture
3.1.2.1. Effect of Initial Cell Concentration on Microfiltration Performances
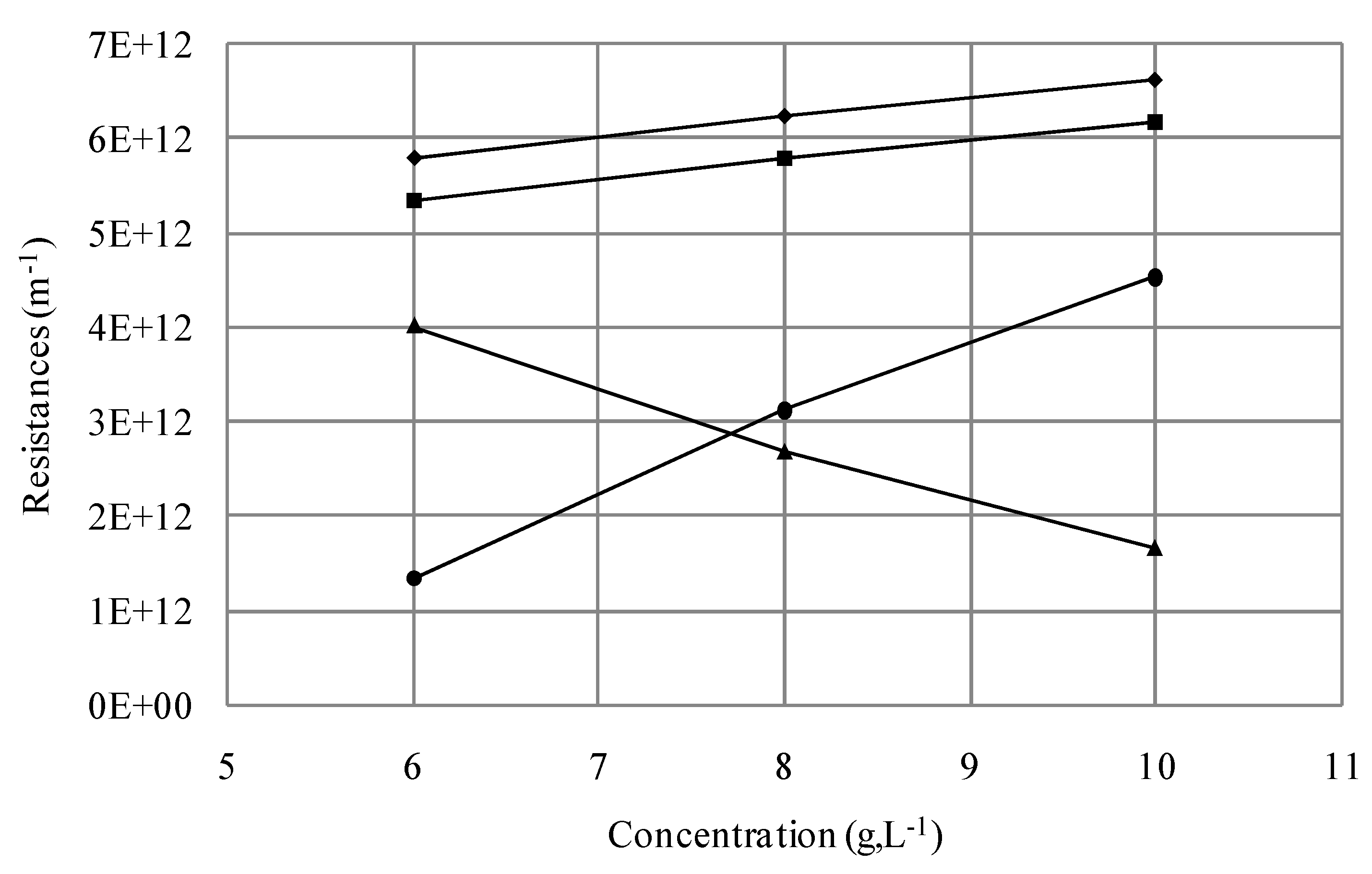
3.1.2.2. Effect of Initial Cell Concentration on Resistances
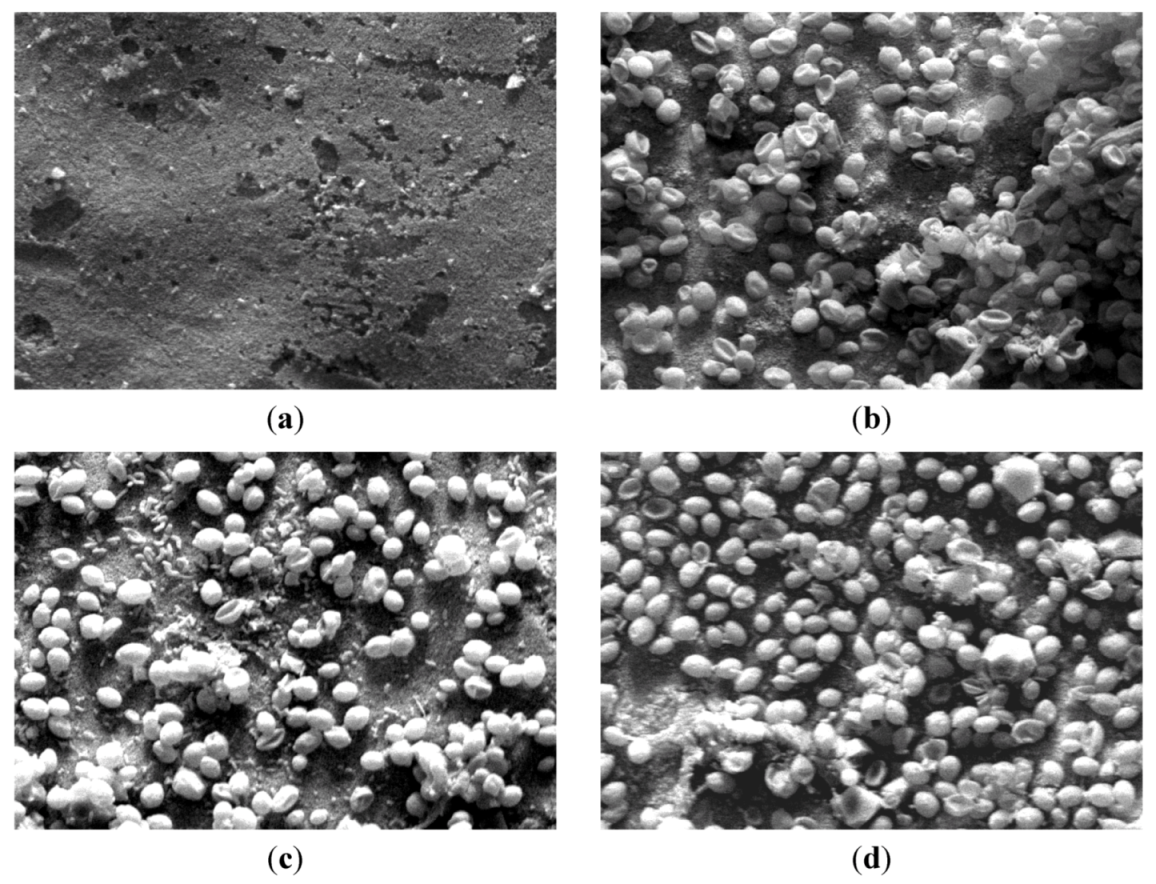
3.2. Filtration Chamber Design
3.2.1. Design Guidelines
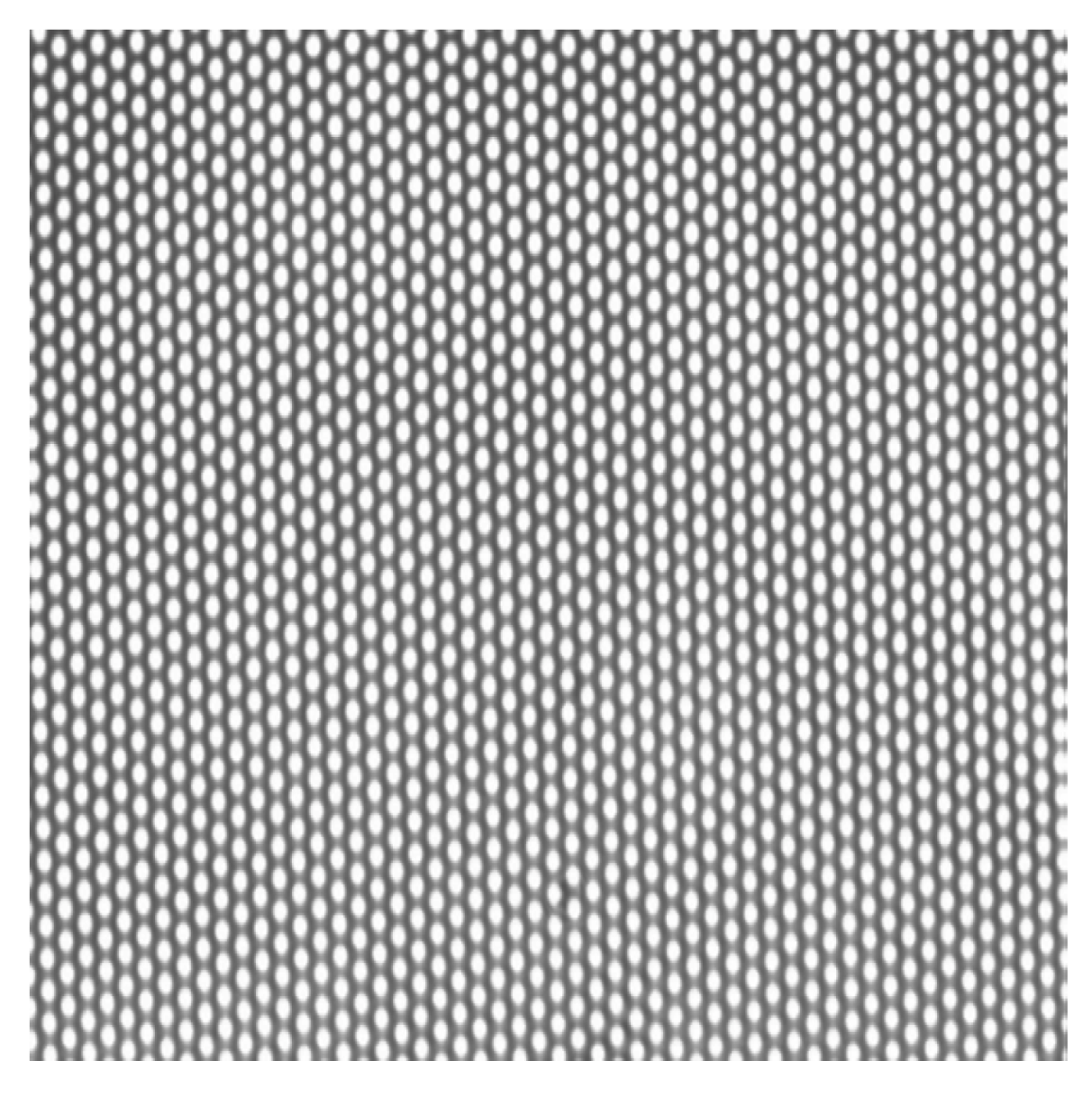
3.2.2. Direct Observation Chamber and Apparatus
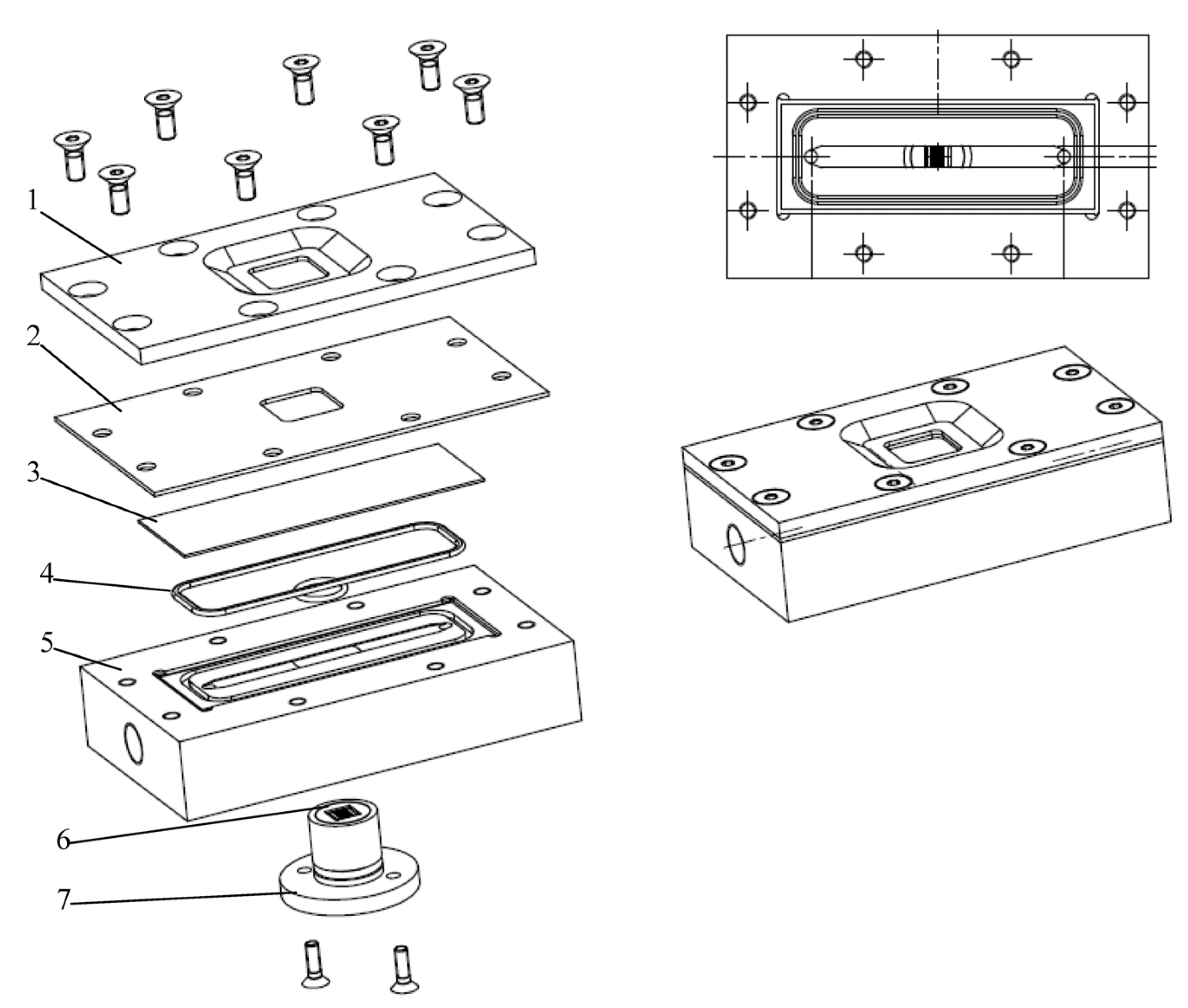
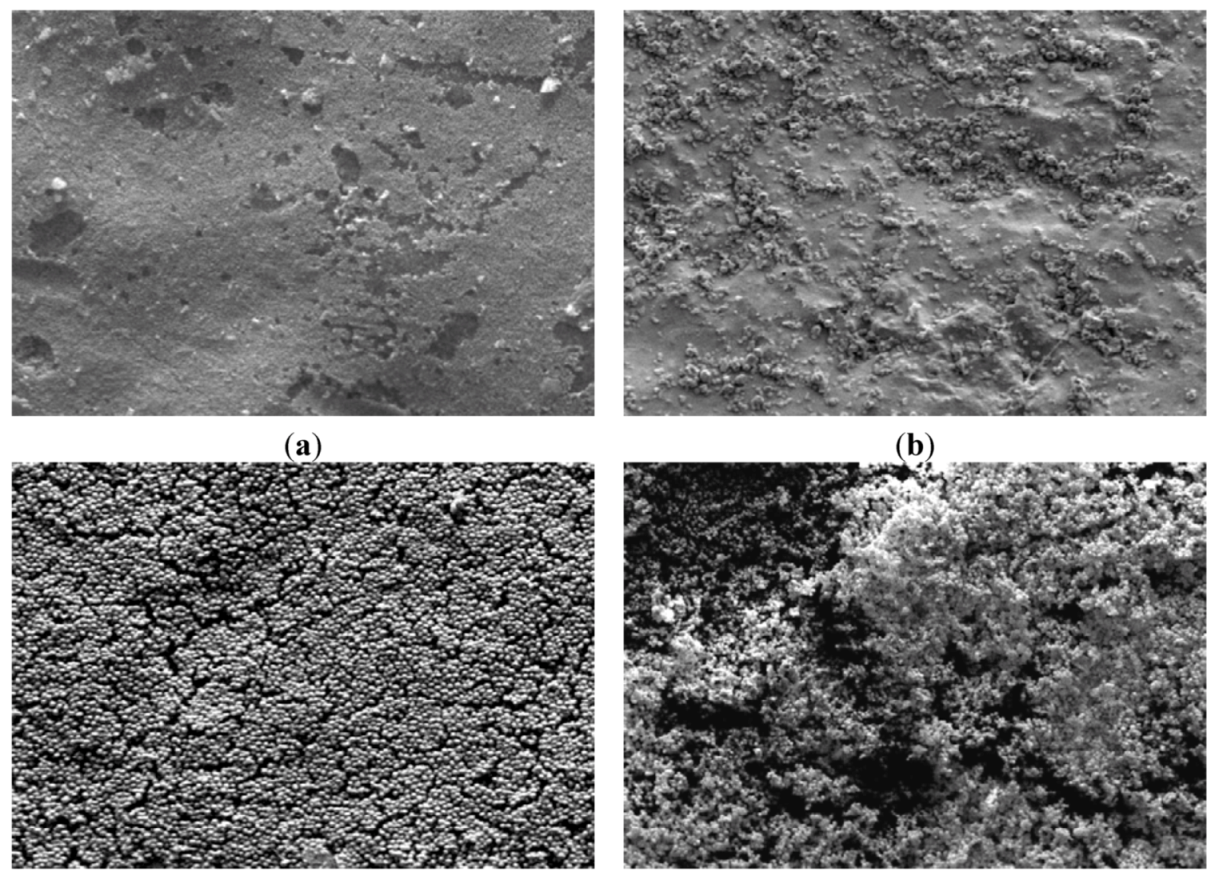
3.2.3. CLSM for Membrane Characterization and Preliminary Fouling Observations
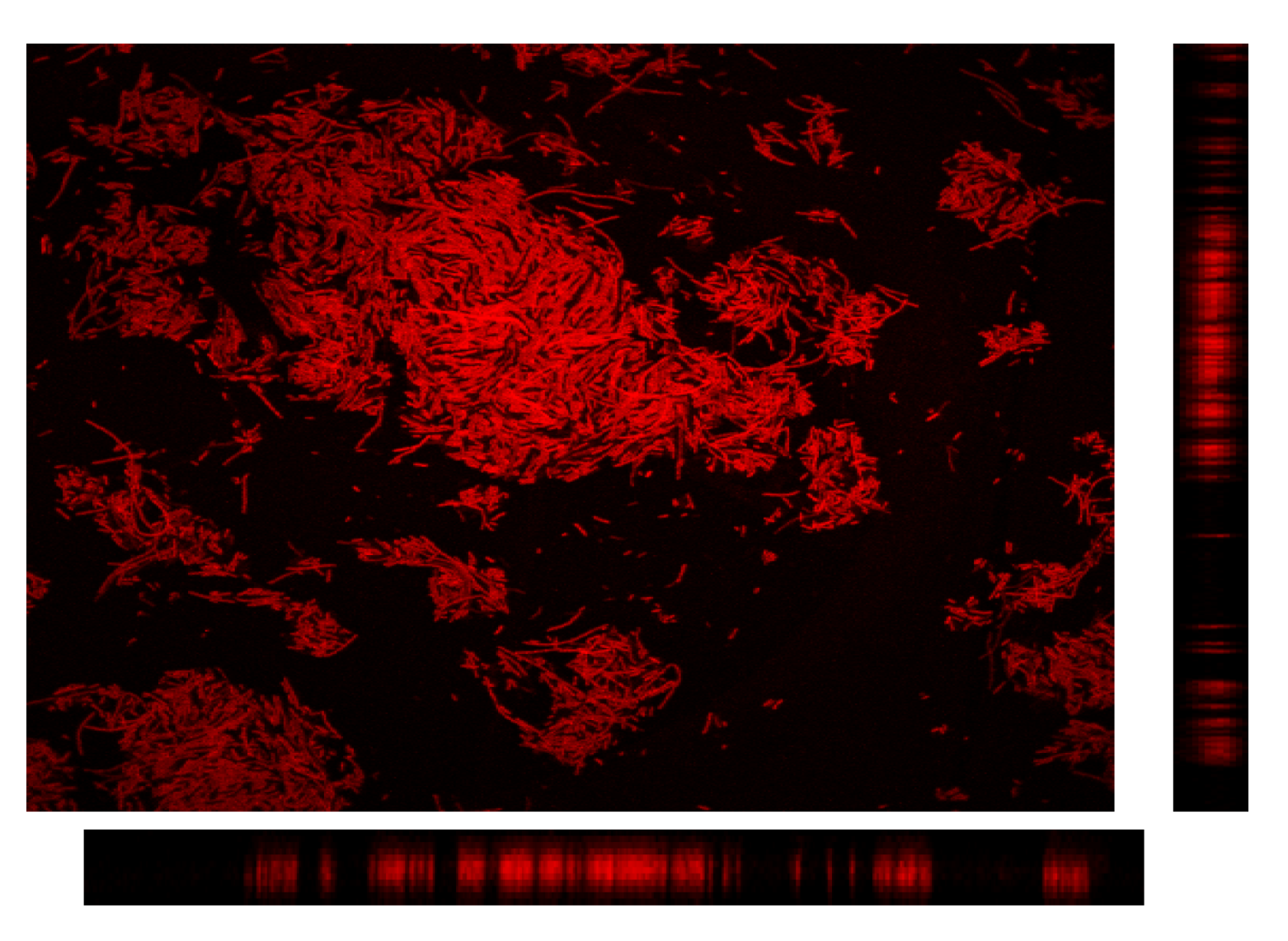
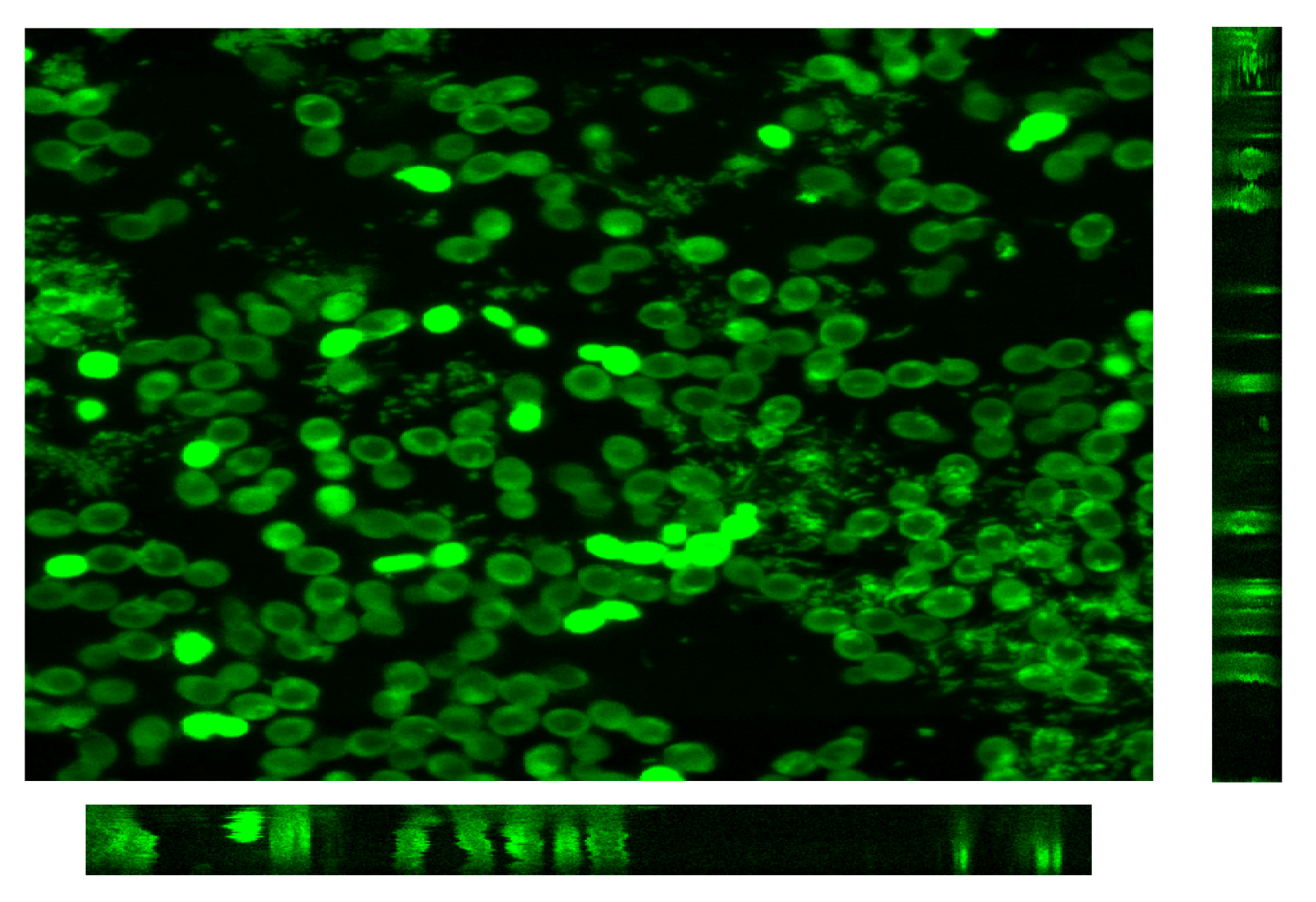
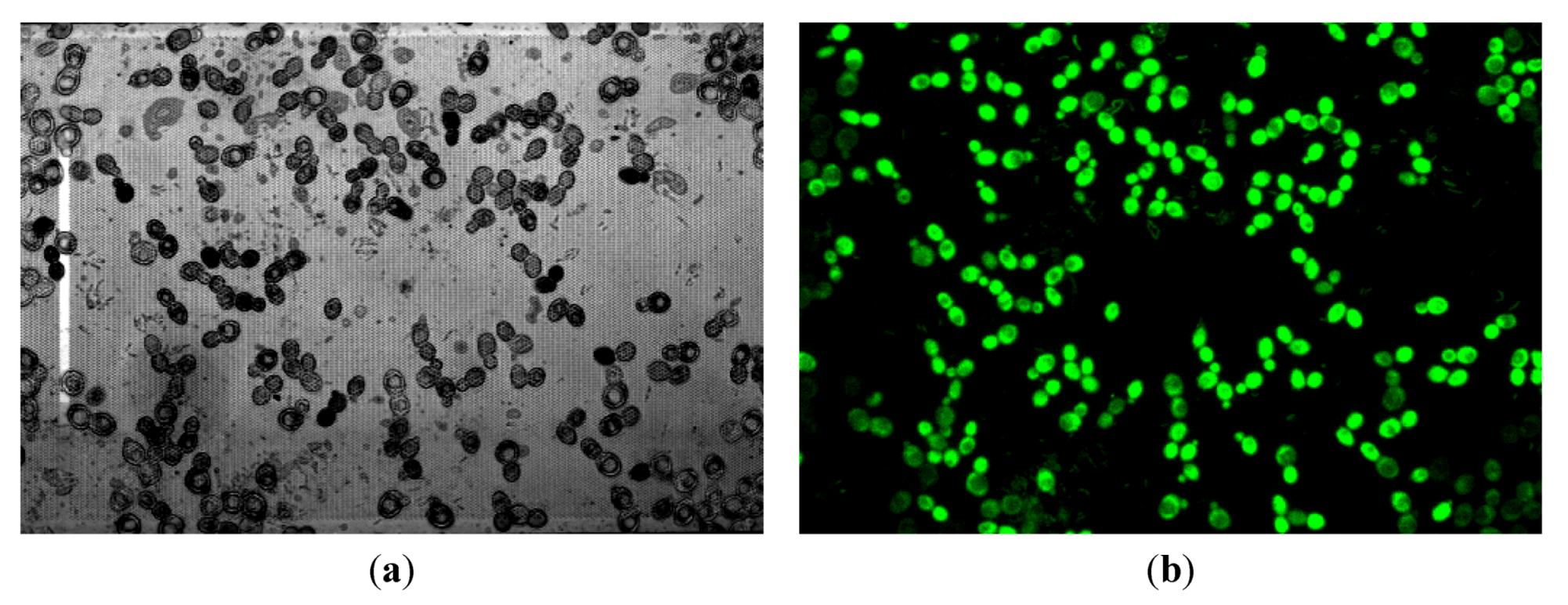
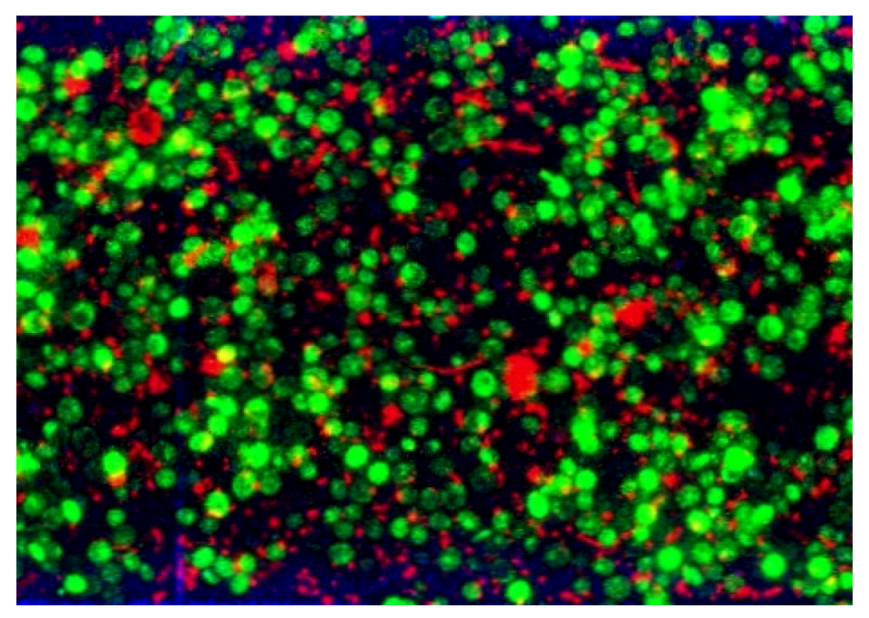
4. Conclusions and Perspectives
Acknowledgements
Nomenclature
| MF | microfiltration |
| UF | ultrafiltration |
| LB | Luria Bertani medium |
| YPD | Yeast Peptone Dextrose |
| VRR | volume reduction ratio |
| SEM | scanning electron microscopy |
| Re | Reynolds number |
| D | hydraulic diameter of the channel (m) |
| J | flux (L h−1 m−2) |
| J0 | flux of distilled water for the clean membrane (L h−1 m−2) |
| TMP | transmembrane pressure (bar) |
| Rt | the total resistance to flow (m−1) |
| Pf | pressure of the feed (bar) |
| Pr | pressure of the retentate (bar) |
| Pp | pressure of the filtrate (bar) |
| Rm | membrane intrinsic resistance of the clean membrane (m−1) |
| Rf | fouling resistance (m−1) |
| Rrf | reversible fouling resistance (m−1) |
| Rif | irreversible fouling resistance (m−1) |
| U | tangential velocity (m s−1) |
| V0 | feed initial volume (m3) |
| Vp | filtrate volume (m3) |
| VRR | volume reduction ratio |
| μ | dynamic viscosity (Pa s) |
| ρ | fluid density (kg m−3) |
| υ | υ = μ/ρ kinematic viscosity |
References
- Mota, M.; Teixeira, J.A.; Yelshin, A. Influence of cell-shape on the cake resistance in dead-end and cross-flow filtrations. Sep. Purif. Technol. 2002, 27, 137–144. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Carrère, H. Yeast cells, beer composition and mean pore diameter impacts on fouling and retention during cross-flow filtration of beer with ceramic membranes. J. Membr. Sci. 2002, 196, 39–57. [Google Scholar] [CrossRef]
- Daufin, G.; Escudier, J.P.; Carrère, H.; Berot, S.; Fillaudeau, L.; Decloux, M. Recent and emerging applications of membrane processes in food and dairy industry. Food Bioprod. Process. 2001, 79, 89–102. [Google Scholar] [CrossRef]
- Jin, J.; Chhatre, S.; Titchener-Hooker, N.J.; Bracewell, D.G. Evaluation of the impact of lipid fouling during the chromatographic purification of virus-like particles from Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2009, 85, 209–215. [Google Scholar]
- Chau, S.; Baldascini, H.; Hearle, D.; Hoare, M.; Titchener-Hooker, N.J. Effect of fouling on the capacity and breakthrough characteristics of a packed bed ion exchange chromatography column. Bioprocess. Biosys. Eng. 2006, 28, 405–414. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Fukushi, K. Effects of operational conditions on ultrafiltration membrane fouling. Desalination 2008, 229, 181–191. [Google Scholar] [CrossRef]
- Pelegrine, D.H.G.; Gasparetto, C.A. Whey proteins solubility as function of temperature and pH. Food Sci. Technol. 2005, 38, 77–80. [Google Scholar]
- Nigam, M.O.; Bansal, B.; Dong, C.X. Fouling and cleaning of whey protein concentrate fouled ultrafiltration membranes. Desalination 2008, 218, 313–322. [Google Scholar] [CrossRef]
- Foley, G. A review of factors affecting filter cake properties in dead-end microfiltration of microbial suspensions. J. Membr. Sci. 2006, 274, 38–46. [Google Scholar] [CrossRef]
- Foley, G.; McLoughlin, P.F.; Malone, D.M. Membrane fouling during constant flux crossflow microfiltration of dilute suspensions of active dry yeast. Sep. Sci. Technol. 1995, 30, 383–398. [Google Scholar] [CrossRef]
- Iskra, T.; Bolton, G.R.; Coffman, J.L.; Godavarti, R. The effect of protein A cycle number on the performance and lifetime of an anion exchange polishing step. Biotechnol. Bioeng. 2013, 110, 1142–1152. [Google Scholar] [CrossRef]
- Corbett, R.; Carta, G.; Iskra, T.; Gallo, C.; Godavarti, R.; Salm, J.R. Structure and protein adsorption mechanisms of clean and fouled tentacle-type anion exchangers used in a monoclonal antibody polishing step. J. Chromatogr. A 2013, 1278, 116–125. [Google Scholar] [CrossRef]
- Kuberkar, V.T.; Davis, R.H. Effects of added yeast on protein transmission and flux in cross-flow membrane microfiltration. Biotechnol. Prog. 1999, 15, 472–479. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, V. Reversibility of heterogeneous deposits formed from yeast and proteins during microfiltration. J. Membr. Sci. 2005, 265, 20–28. [Google Scholar] [CrossRef]
- Güll, C.; Czekaj, P.; Davis, R.H. Microfiltration of protein mixtures and the effects of yeast on membrane fouling. J. Membr. Sci. 1999, 155, 113–122. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Nakao, S.; Kimura, S. Macromolecule rejection with compressible and incompressible cake layer formed in crossflow microfiltration. J. Chem. Eng. Jpn. 1993, 26, 656–661. [Google Scholar] [CrossRef]
- Tanaka, T.; Kamimura, R.; Fujiwara, R.; Nakanishi, K. Crossflow filtration of yeast broth cultivated in molasses. Biotechnol. Bioeng. 1994, 43, 1094–1101. [Google Scholar] [CrossRef]
- Li, Y.; Shahbazi, A.; Kadzere, C.T. Separation of cells and proteins from fermentation broth using ultrafiltration. J. Food Eng. 2006, 75, 574–580. [Google Scholar] [CrossRef]
- Li, S.L.; Chou, K.S.; Lin, J.Y.; Yen, H.W.; Chu, I.M. Study on the microfiltration of Escherichia coli containing fermentation broth by a ceramic membrane filter. J. Membr. Sci. 1996, 110, 203–210. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ohmori, K.; Glatz, C.E. Harvest time effects on membrane cake resistance of Escherichia coli broth. J. Membr. Sci. 2001, 190, 93–106. [Google Scholar] [CrossRef]
- Boissier, B.; Lutin, F.; Moutounet, M.; Vernhet, A. Particles deposition during the cross-flow microfiltration of red wines-incidence of the hydrodynamic conditions and the yeast to fines ratio. Chem. Eng. Process. Process Intensif. 2008, 47, 276–286. [Google Scholar] [CrossRef]
- Mccarthy, A.A.; Walsh, P.K.; Foley, G. Experimental techniques for quantifying the cake mass, the cake and membrane resistances and the specific cake resistance during crossflow filtration of microbial suspensions. J. Membr. Sci. 2002, 201, 31–45. [Google Scholar] [CrossRef]
- Beaufort, S.; Alfenore, S.; Lafforgue, C. Use of fluorescent microorganisms to perform in vivo and in situ local characterization of microbial deposits. J. Membr. Sci. 2011, 369, 30–39. [Google Scholar] [CrossRef]
- Hwang, B.-K.; Lee, C.-H.; Chang, I.-S.; Drews, A.; Field, R. Membrane bioreactor: TMP rise and characterization of bio-cake structure using CLSM-image analysis. J. Membr. Sci. 2012, 419–420, 33–41. [Google Scholar] [CrossRef]
- Sun, C.; Fiksdal, L.; Hanssen-Bauer, A.; Rye, M.B.; Leiknes, T. Characterization of membrane biofouling at different operating conditions (flux) in drinking water treatment using confocal laser scanning microscopy (CLSM) and image analysis. J. Membr. Sci. 2011, 382, 194–201. [Google Scholar] [CrossRef]
- Günther, J.; Schmitz, P.; Albasi, C.; Lafforgue, C. A numerical approach to study the impact of packing density on fluid flow distribution in hollow fiber module. J. Membr. Sci. 2010, 348, 277–286. [Google Scholar] [CrossRef]
- Dufreche, J.; Prat, M.; Schmitz, P.; Sherwood, J.D. On the apparent permeability of a porous layer backed by a perforated plate. Chem. Eng. Sci. 2002, 57, 2933–2944. [Google Scholar] [CrossRef]
- Gassara, D.; Schmitz, P.; Ayadi, A.; Prat, M. Modelling the effect of particle size in microfiltration. Sep. Sci. Technol. 2008, 43, 1754–1770. [Google Scholar] [CrossRef]
- Kimura, K.; Hane, Y.; Watanabe, Y.; Amy, G.; Ohkuma, N. Irreversible membrane fouling during ultrafiltration of surface water. Water Res. 2004, 38, 3431–3441. [Google Scholar] [CrossRef]
- Kumar, M.S.; Madhu, G.M.; Roy, S. Fouling behaviour, regeneration options and on-line control of biomass-based power plant effluents using microporous ceramic membranes. Sep. Purif. Technol. 2007, 57, 25–36. [Google Scholar] [CrossRef]
- Image J Home Page. Available online: http://rsb.info.nih.gov/ij/ (accessed on 6 May 2013).
- Russotti, G.; Osawa, A.E.; Sitrin, R.D.; Buckland, B.C.; Adams, W.R.; Lee, S.S. Pilot-scale harvest of recombinant yeast employing microfiltration: A case study. J. Biotechnol. 1995, 42, 235–246. [Google Scholar]
- Kang, S.T.; Subramani, A.; Hoek, E.M.V.; Deshusses, M.A.; Matsumoto, M.R. Direct observation of biofouling in cross-flow microfiltration: Mechanisms of deposition and release. J. Membr. Sci. 2004, 244, 151–165. [Google Scholar] [CrossRef]
- Stewart, P.S.; Robertson, C.R. Microbial growth in a fixed volume: Studies with entrapped Escherichia coli. Appl. Microbiol. Biotechnol. 1989, 30, 34–40. [Google Scholar]
- McDonogh, R.M.; Fane, A.G.; Fell, C.J.D.; Flemming, H. The influence of polydispersity on the hydraulic behaviour of colloidal fouling layers on membranes perturbations on the behavior of the “ideal” colloidal layer. Colloids Surf. A. 1998, 138, 231–244. [Google Scholar]
- Lebleu, N.; Roques, C.; Aimar, P.; Causserand, C. Role of the cell-wall structure in the retention of bacteria by microfiltration membranes. J. Membr. Sci. 2009, 326, 178–185. [Google Scholar] [CrossRef]
- Johnson, W.P.; Martin, M.J.; Gross, M.J.; Logan, B.E. Facilitation of bacterial transport through porous media by changes in solution and surface properties. Colloids Surf. A 1996, 107, 263–271. [Google Scholar]
- Sharma, M.M.; Chang, Y.I.; Yen, T.F. Reversible and irreversible surface charge modification of bacteria for facilitating transport through porous media. Colloids Surf. 1985, 16, 193–206. [Google Scholar] [CrossRef]
- Mille, Y.; Beney, L.; Gervais, P. Viabilty of Escherichia coli after combined osmotic and thermal treatment: A plasma membrane implication. Biochim. Biophys. Acta 2002, 1567, 41–48. [Google Scholar] [CrossRef]
- Suchecka, T.; Piatkiewicz, W.; Sosnowski, T.R. Is the cell retention by MF membrane absolutely safe—A hypothetical model for cell deformation in a membrane pore. J. Membr. Sci. 2005, 250, 135–140. [Google Scholar] [CrossRef]
- Siu, S.C.; Boushaba, R.; Topoyassakul, V.; Graham, A.; Choudhury, S.; Moss, G.; Titchener-Hooker, N.J. Visualising fouling of a chromatographic matrix using confocal scanning laser microscopy. Biotechnol. Bioeng. 2006, 95, 714–723. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Fane, A.G.; Law, A.W.K. Critical flux and particle deposition of bidisperse suspensions during crossflow microfiltration. J. Membr. Sci. 2006, 282, 189–197. [Google Scholar] [CrossRef]
- Kuiper, S.; Brink, R.; Nijdam, W.; Krijnen, G.J.M.; Elwenspoek, M.C. Ceramic microsieves: Influence of perforation shape and distribution on flow resistance and membrane strength. J. Membr. Sci. 2002, 196, 149–157. [Google Scholar] [CrossRef]
- Brans, G.; van Dinther, A.; Odum, B.; Schroen, C.G.P.H.; Boom, R.M. Transmission and fractionation of micro-sized particle suspensions. J. Membr. Sci. 2007, 290, 230–240. [Google Scholar] [CrossRef]
- Van Rijn, C.J.M. Nano and Micro Engineered Membrane Technology; Membrane Science and Technology Series 10; Elsevier: Amsterdam, the Netherlands, 2004. [Google Scholar]
- Kuiper, S.; van Rijn, C.J.M.; Nijdam, W.; Raspe, O.; van Wolferen, H.; Krijnen, G.; Elwenspoek, M. Filtration of lager beer with microsieves: Flux, permeate, haze and in-line microscope observations. J. Membr. Sci. 2002, 196, 159–170. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hassan, I.B.; Ennouri, M.; Lafforgue, C.; Schmitz, P.; Ayadi, A. Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level. Membranes 2013, 3, 44-68. https://doi.org/10.3390/membranes3020044
Hassan IB, Ennouri M, Lafforgue C, Schmitz P, Ayadi A. Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level. Membranes. 2013; 3(2):44-68. https://doi.org/10.3390/membranes3020044
Chicago/Turabian StyleHassan, Ines Ben, Monia Ennouri, Christine Lafforgue, Philippe Schmitz, and Abdelmoneim Ayadi. 2013. "Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level" Membranes 3, no. 2: 44-68. https://doi.org/10.3390/membranes3020044
APA StyleHassan, I. B., Ennouri, M., Lafforgue, C., Schmitz, P., & Ayadi, A. (2013). Experimental Study of Membrane Fouling during Crossflow Microfiltration of Yeast and Bacteria Suspensions: Towards an Analysis at the Microscopic Level. Membranes, 3(2), 44-68. https://doi.org/10.3390/membranes3020044




