Light Responsive Polymer Membranes: A Review
Abstract
:1. Introduction
2. Photo-Switching Compounds and Mechanisms
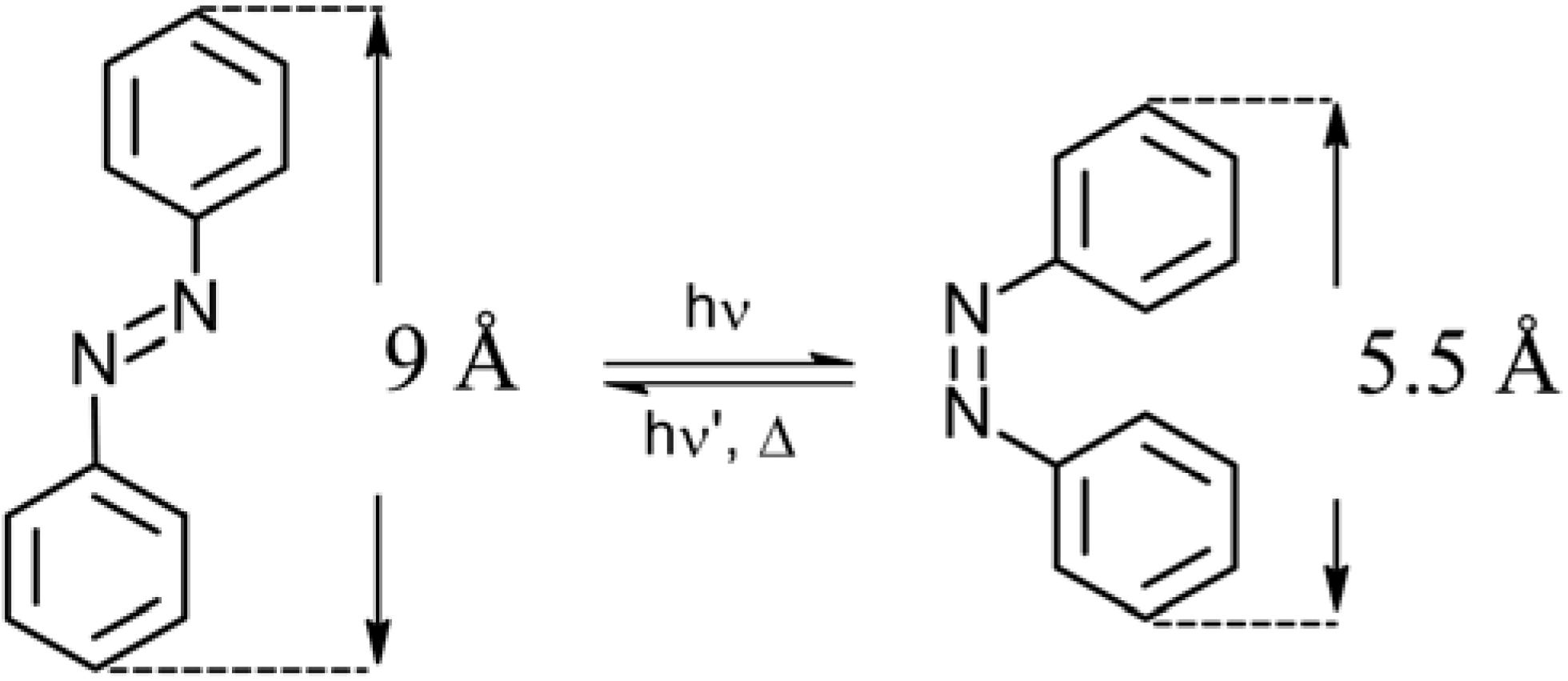
2.1. Azobenzene-Based Systems
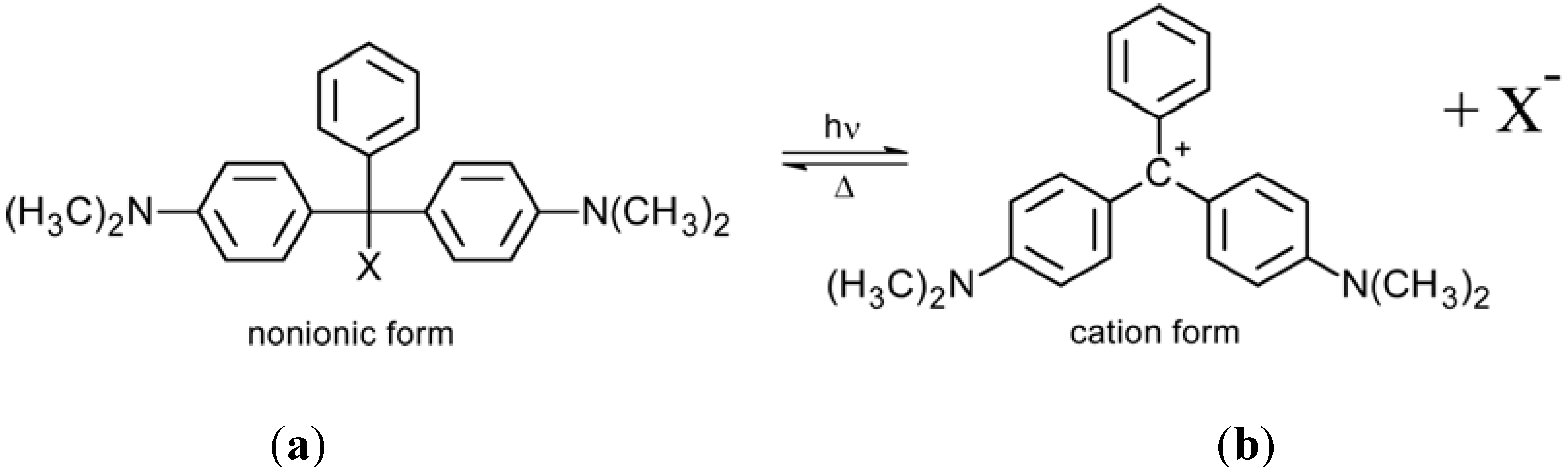
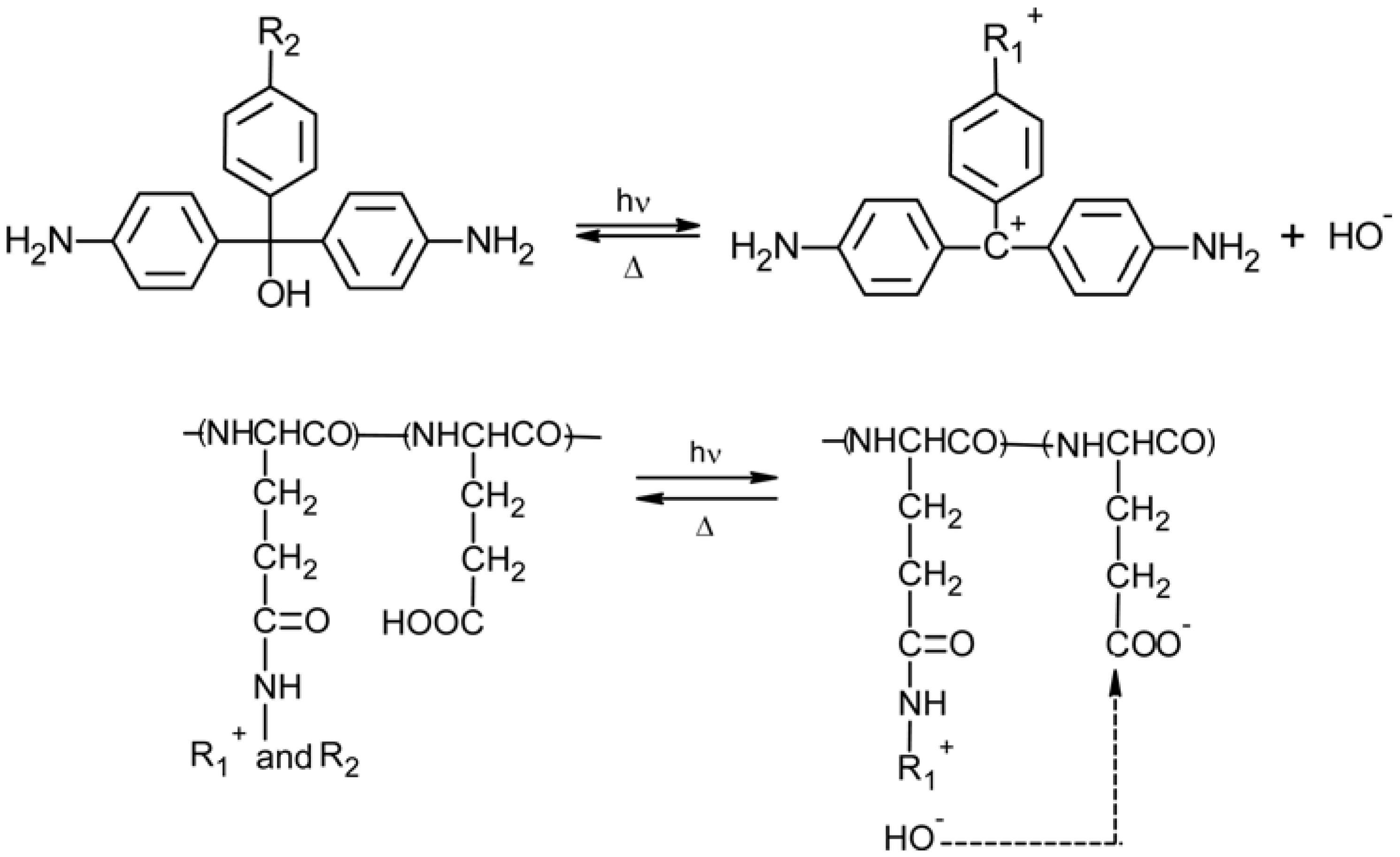
2.2. Triphenylmethane-Based Systems
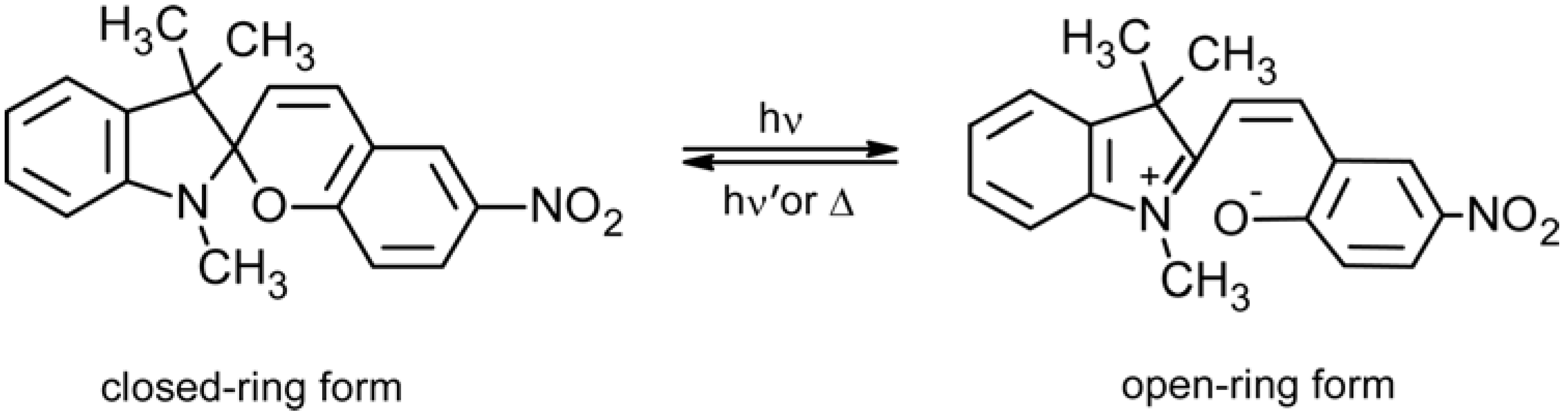
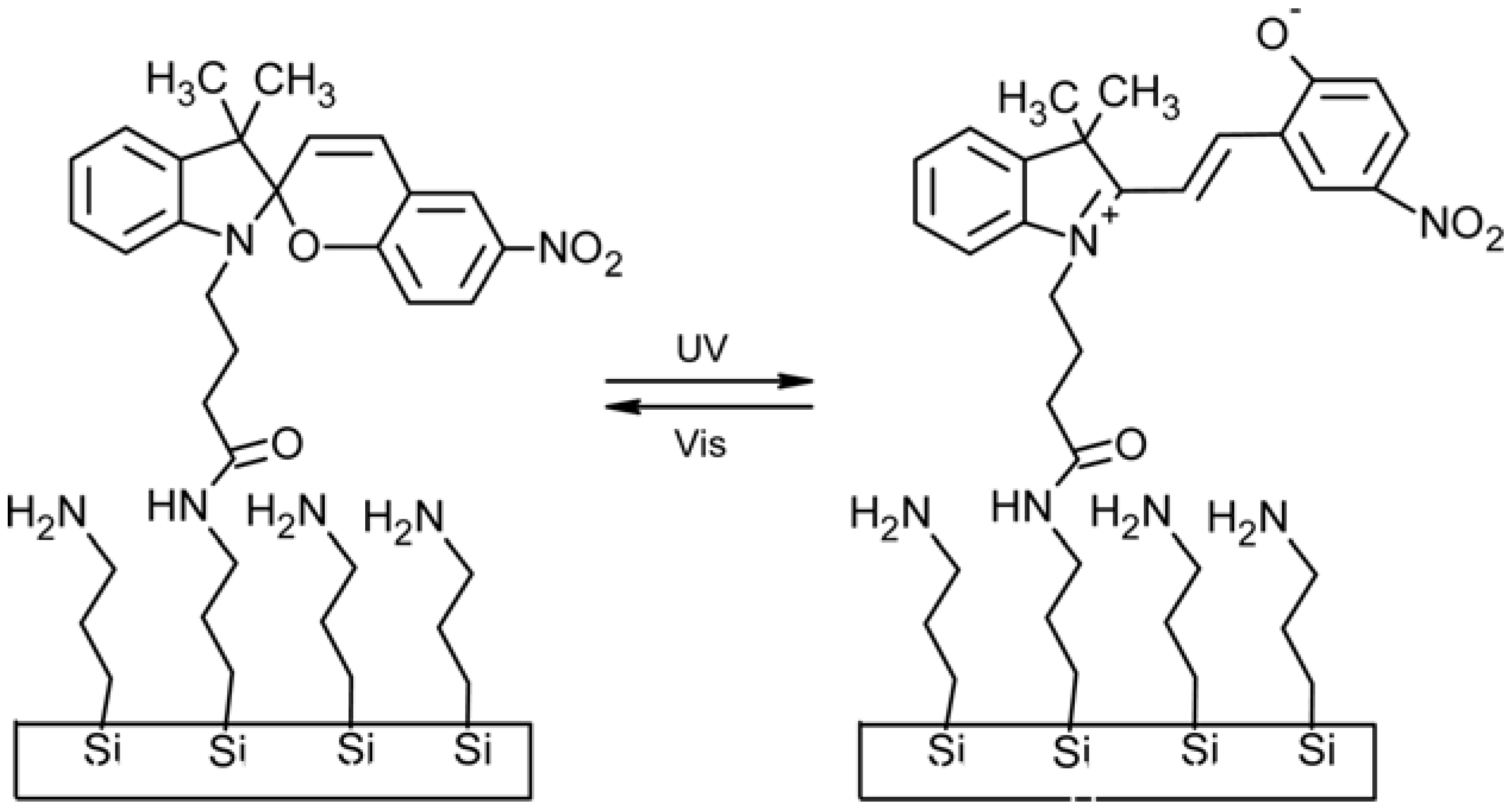
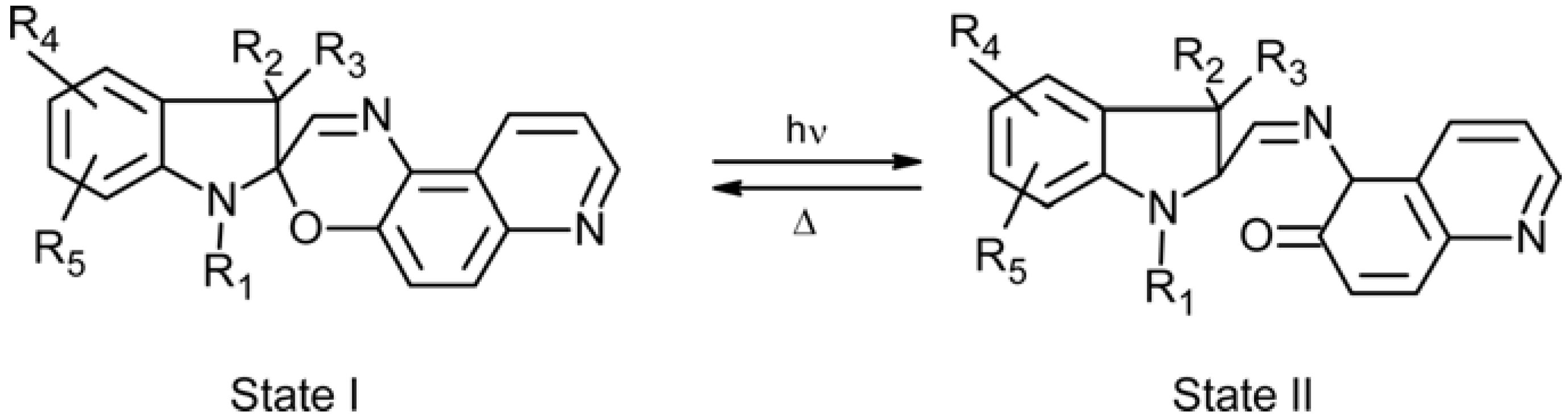
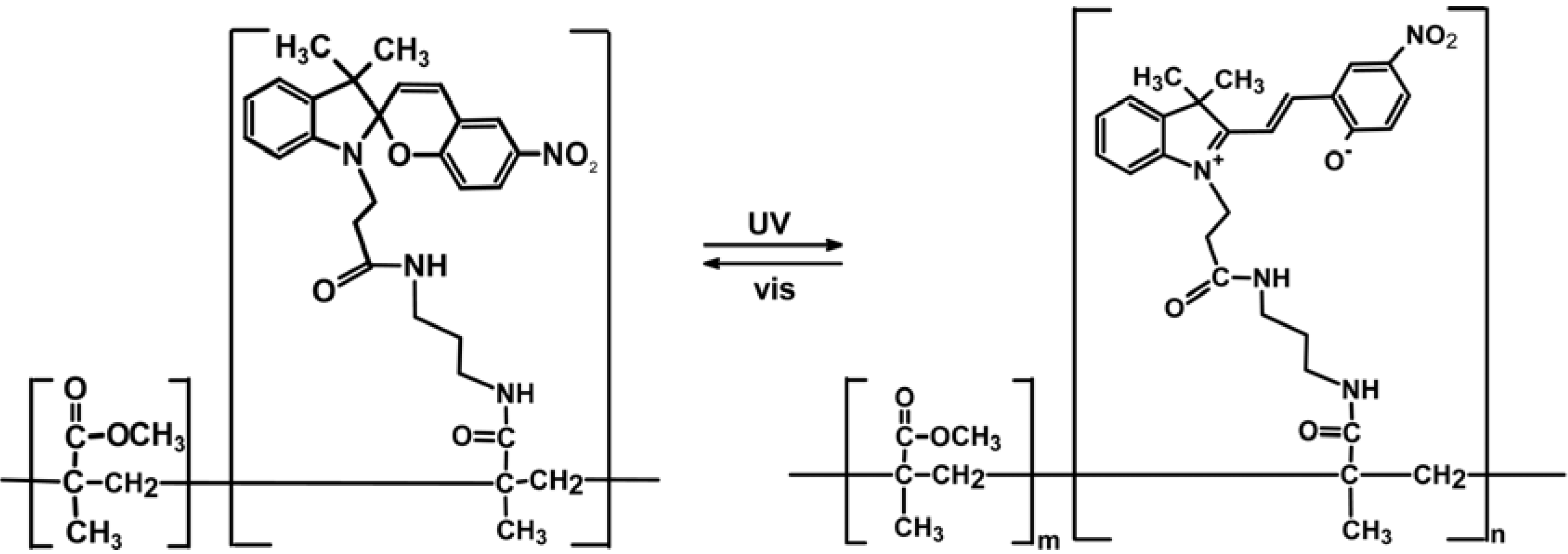
2.3. Spiropyran-Based Systems
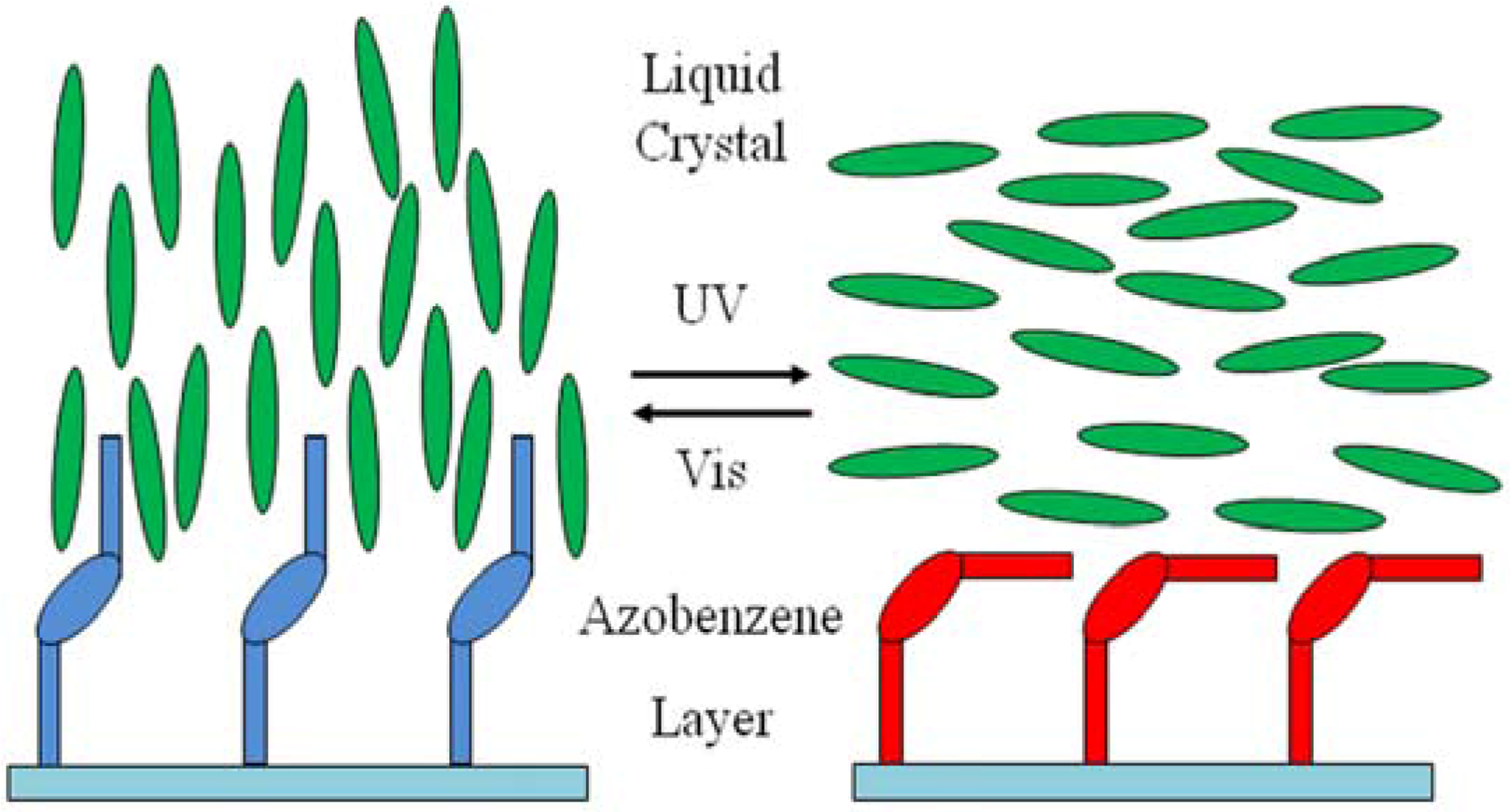
2.4. Photo-Pesponsive Liquid Crystal Devices
2.5. Photo-Responsive Polypeptide-Based Systems
3. Light-Switching Functions
| Technique | Property | Reference |
|---|---|---|
| UV–Vis spectroscopy | Isomerization | [51] |
| Ellipsometry | Variation of the average thickness of the sample in fair agreement with the calculated geometries of the molecules. | [52] |
| Surface plasmon resonance spectroscopy | Switching in real time under ambient conditions | [53] |
| Contact angle measurements | Switching wetting of the surfaces | [50] |
| Adsorption of molecules/particles from solution | Control of adsorption on surfaces | [50] |
| Atomic force microscopy | Switching in individual molecules | [52] |
| Kelvin probe measurements | Changes in the work function of functionalized surfaces | [54] |
| Measurements of electrical properties | Azobenzene switching controls electrical properties of SAMs | [50] |
| Electrochemical methods | Quantitative isomerization by cyclic voltammetry | [55] |
| Surface-enhanced Raman spectroscopy | Isomerization on the surface | [56] |
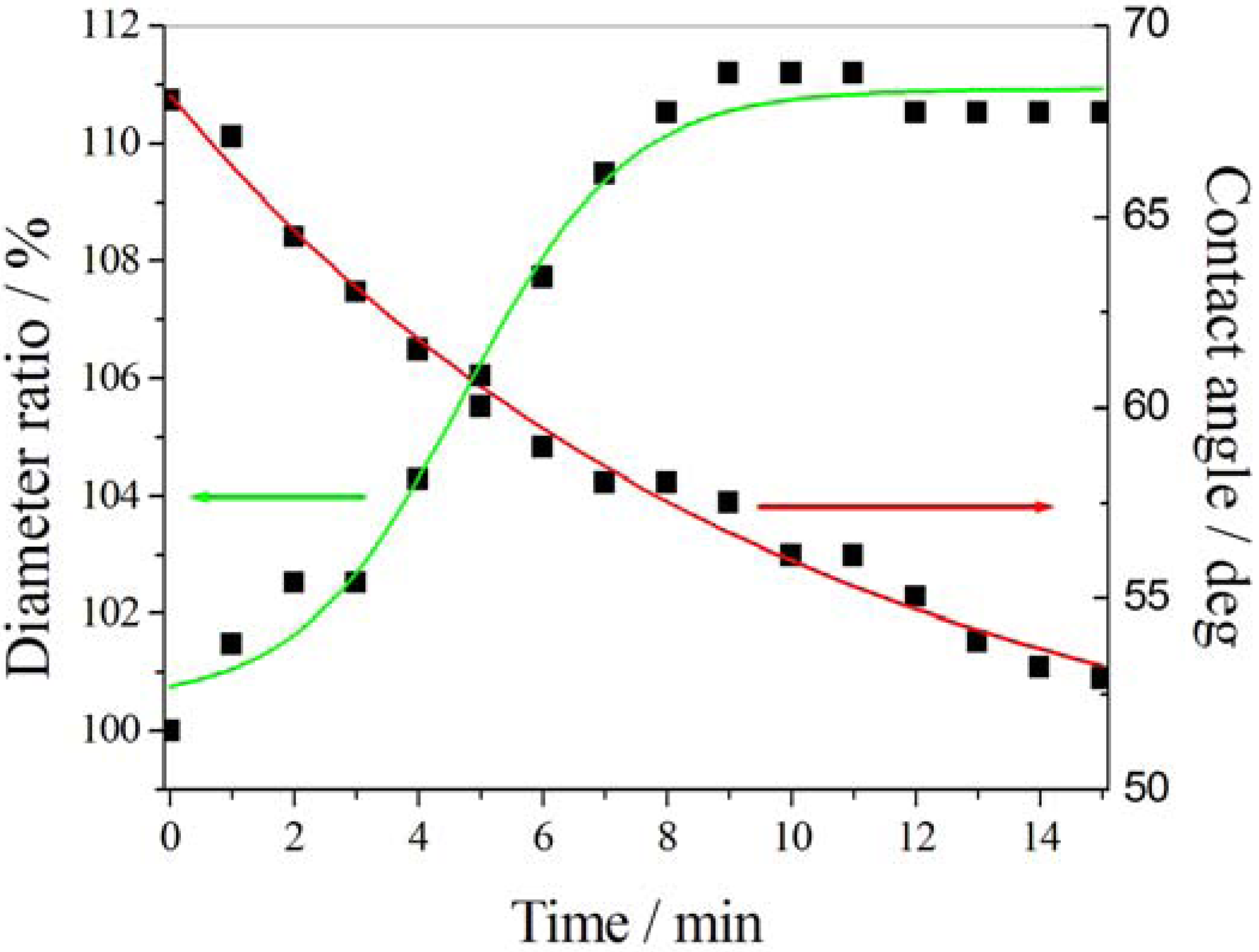
3.1. Light-Driven Wettability
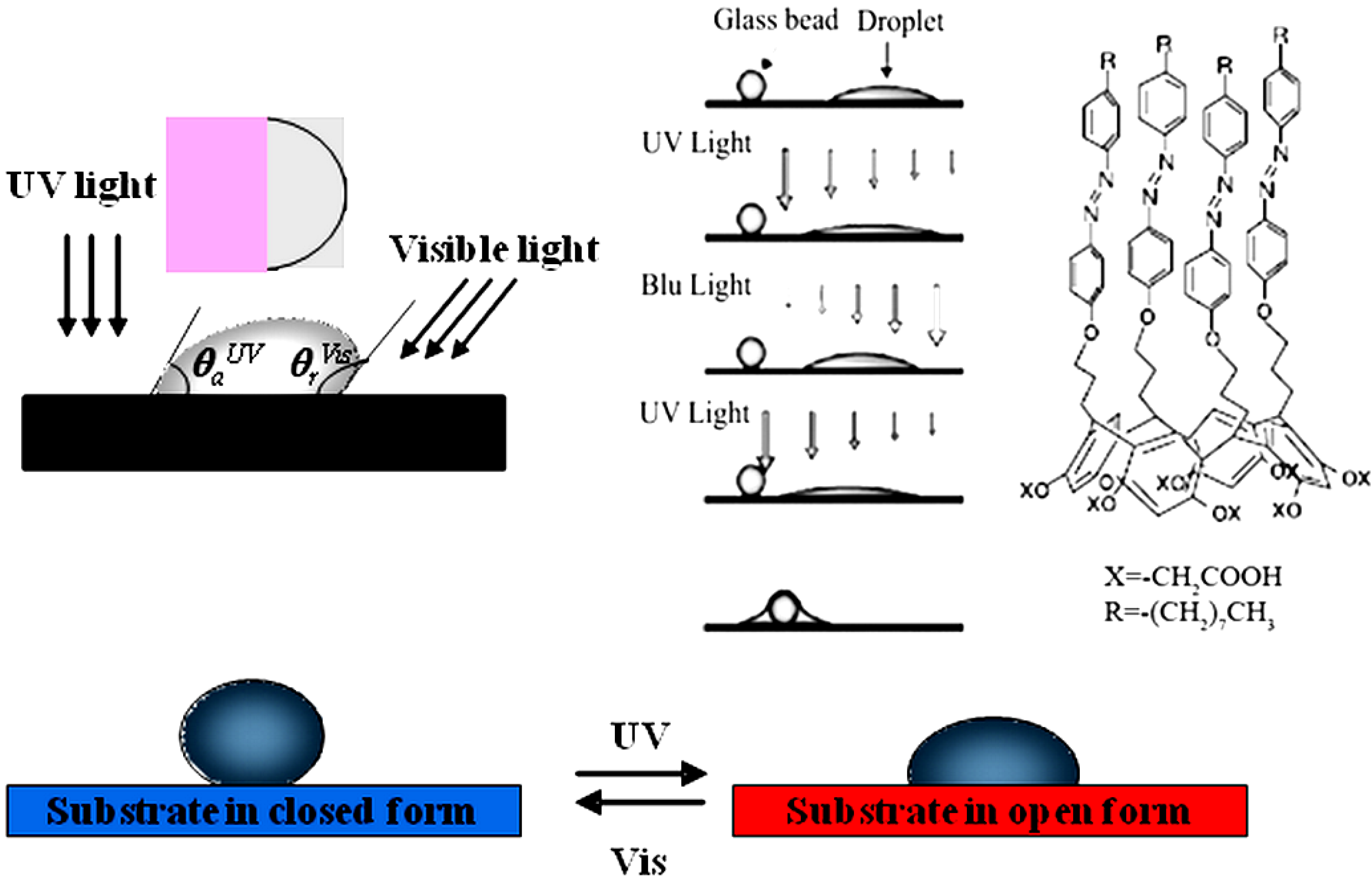



3.2. Light-Adaptative Membrane Gates
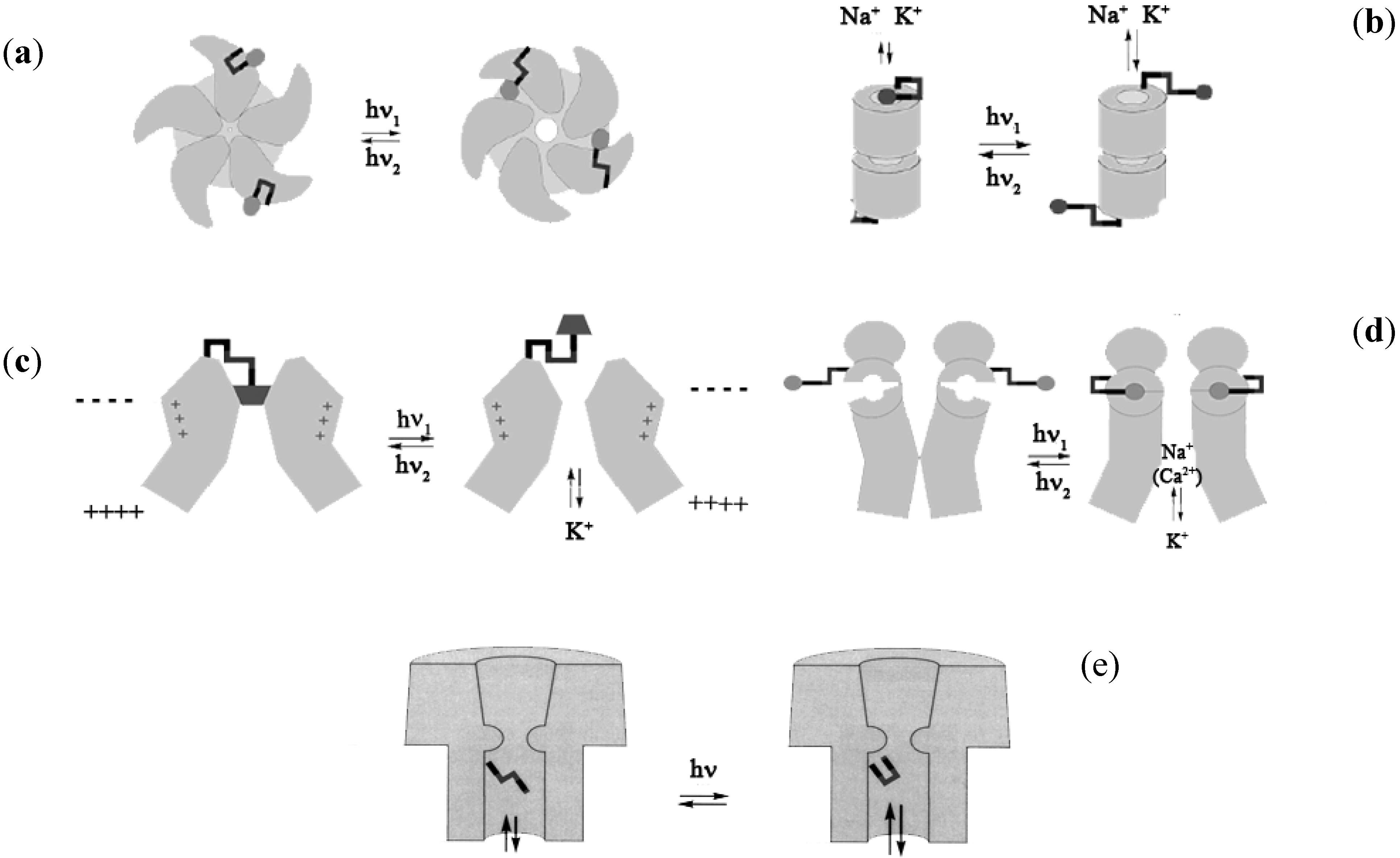
3.2.1. Photo-Controlled Ion Permeation through Membranes Gates

3.2.2. Photo-Controlled Organic Liquids Permeation through Membrane Gates
3.2.3. Permselectivity of Gases through Photo-Switching Membrane Gates
4. Photo-Responsive Membranes: Classes of Adaptive Materials and Their Applications
4.1. Photo-Responsive Polyelectrolyte Multilayer Membranes
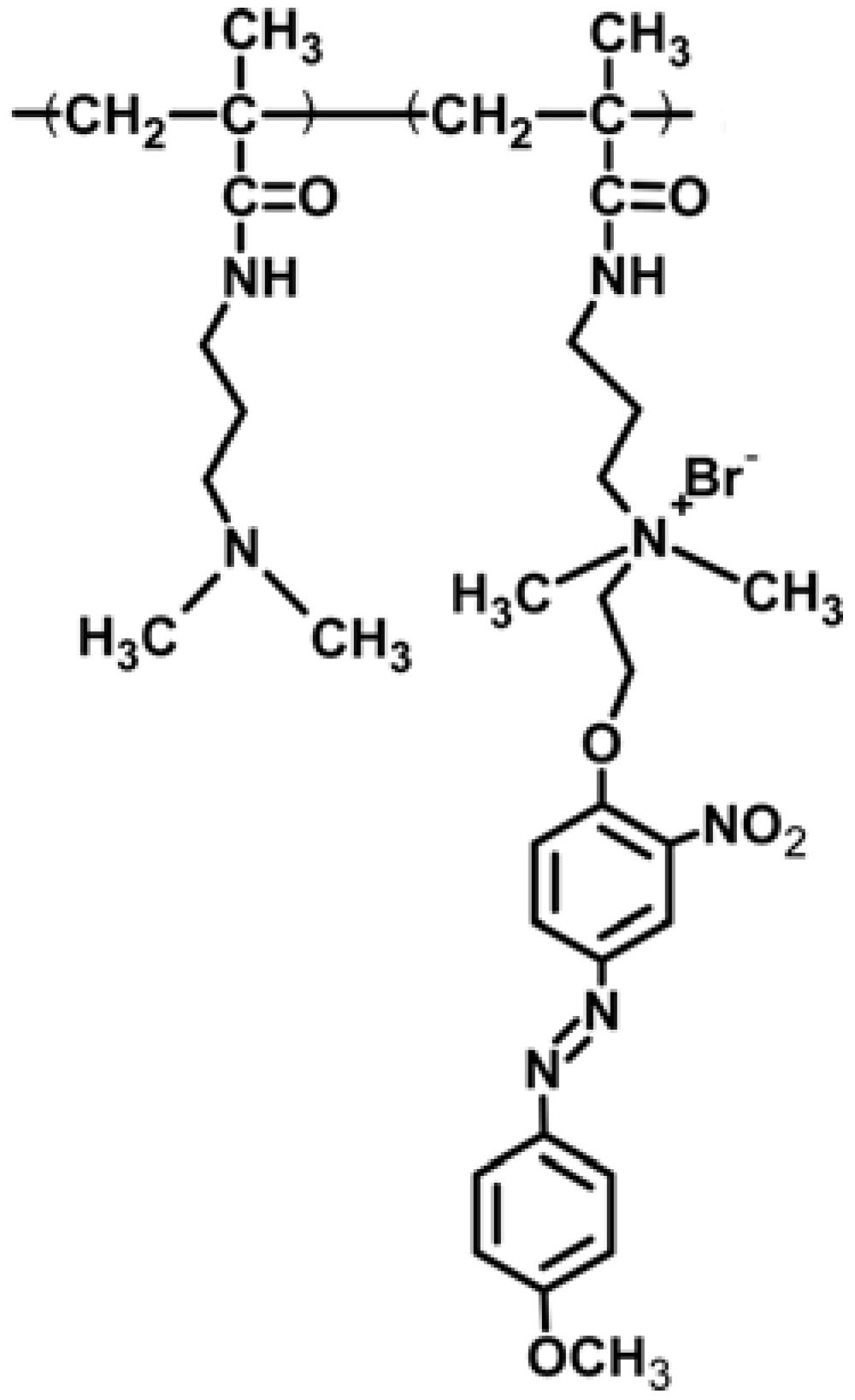
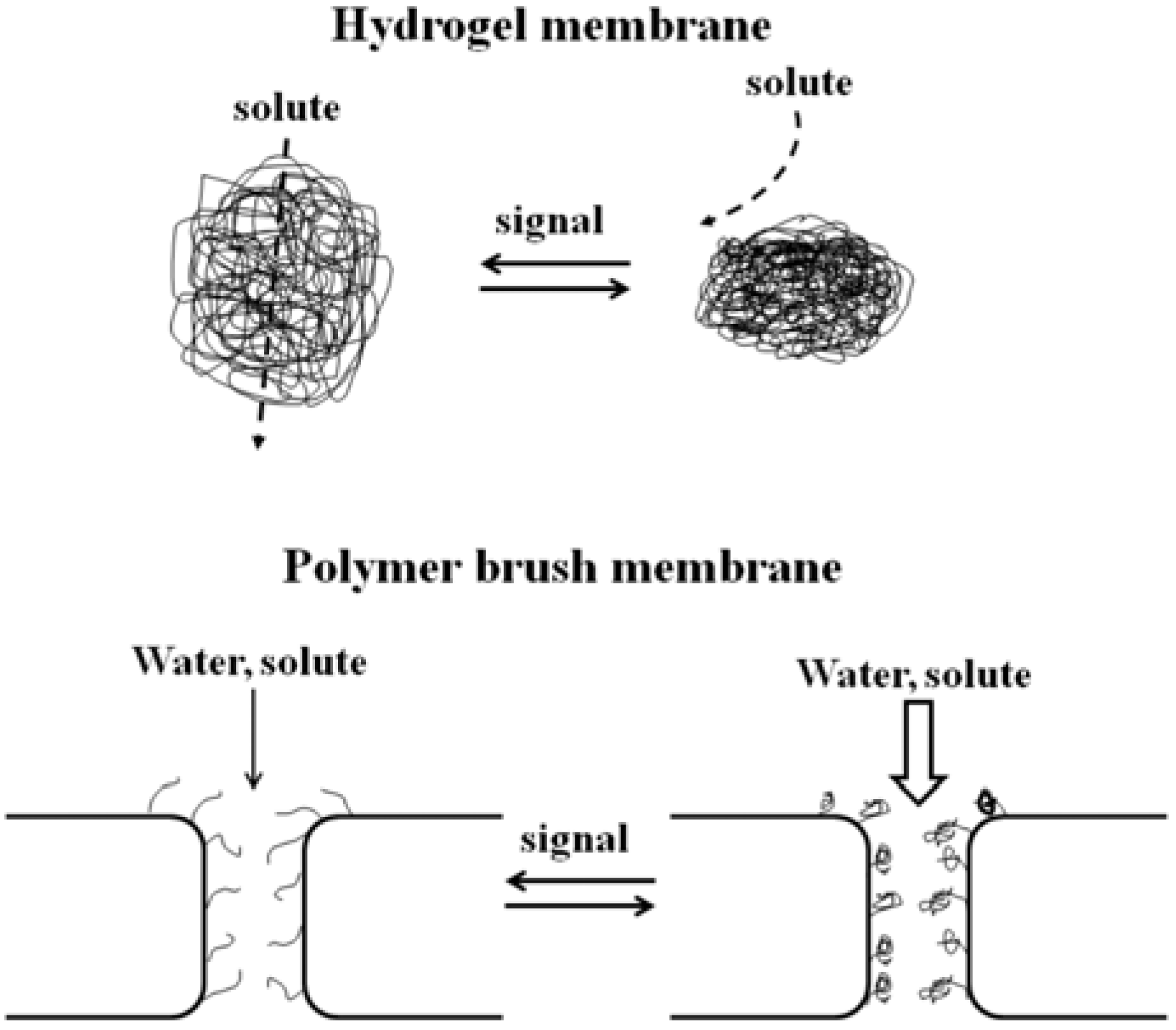
4.2. Photo-Responsive Polymer-Grafted Porous Membrane
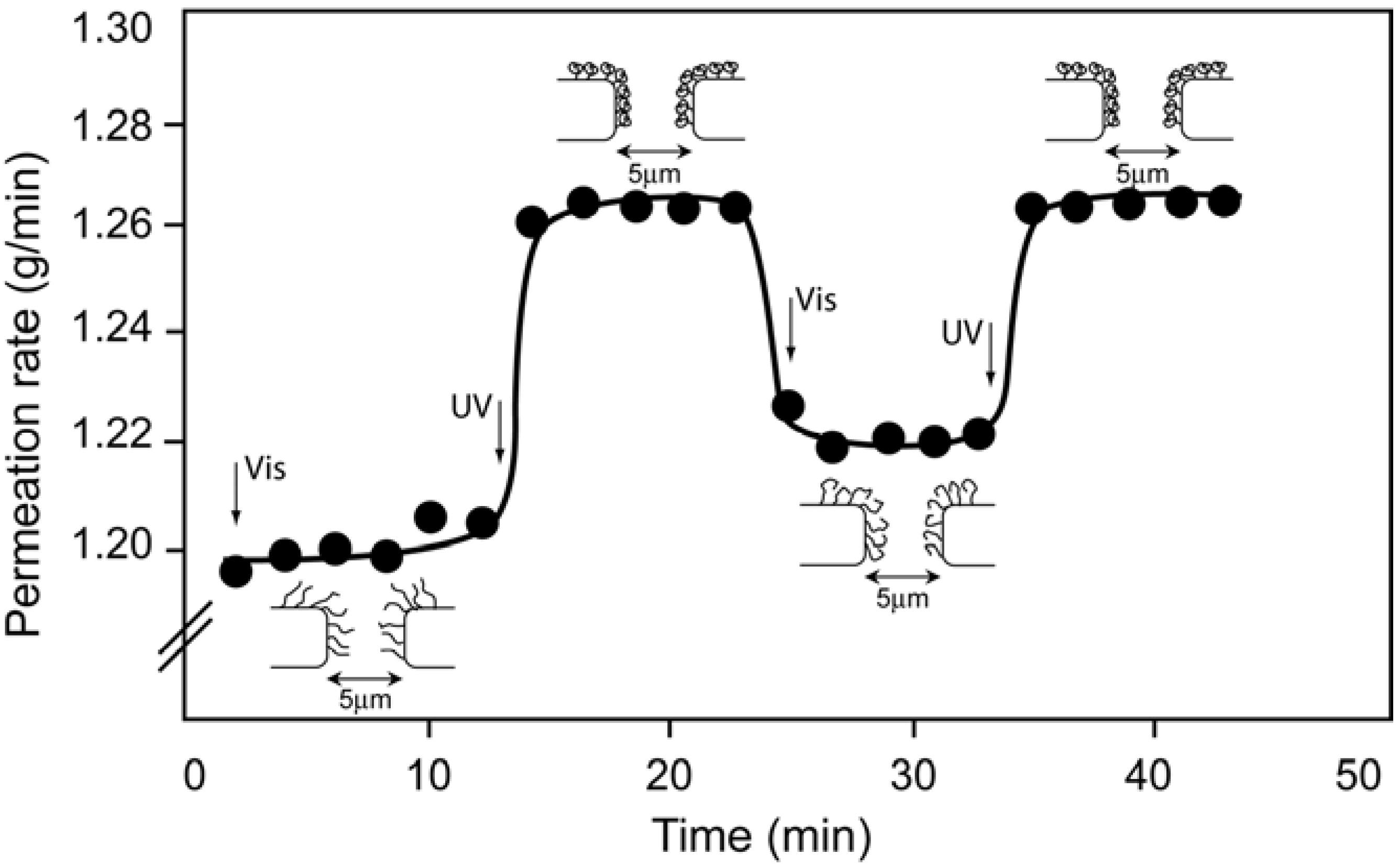
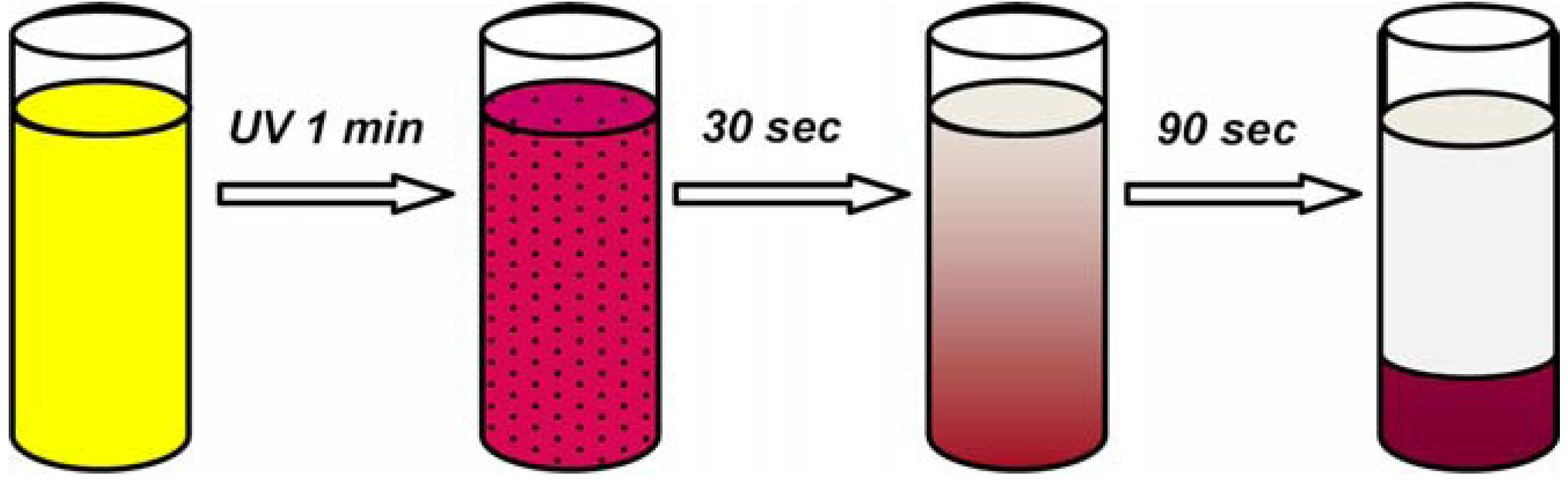
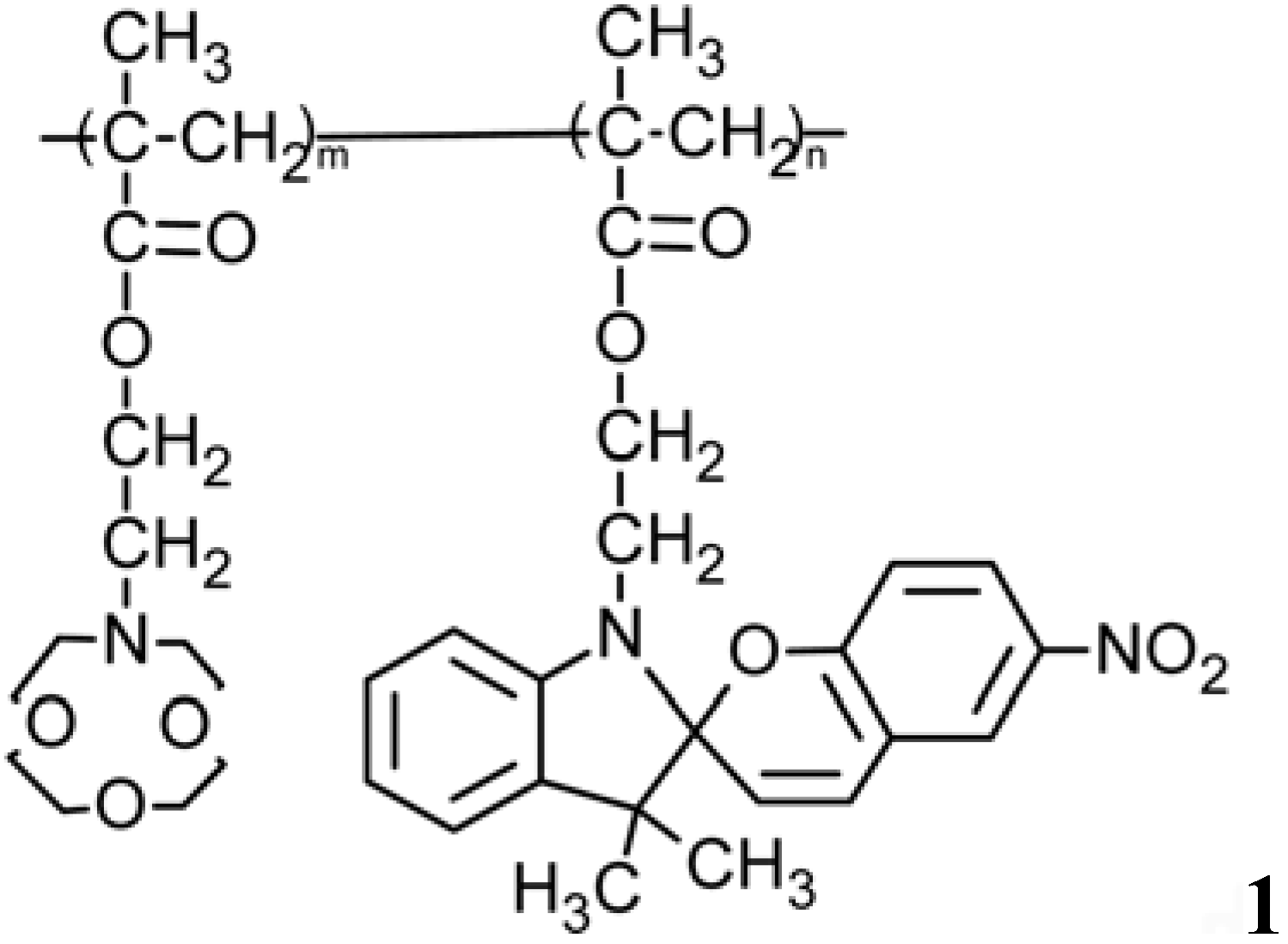
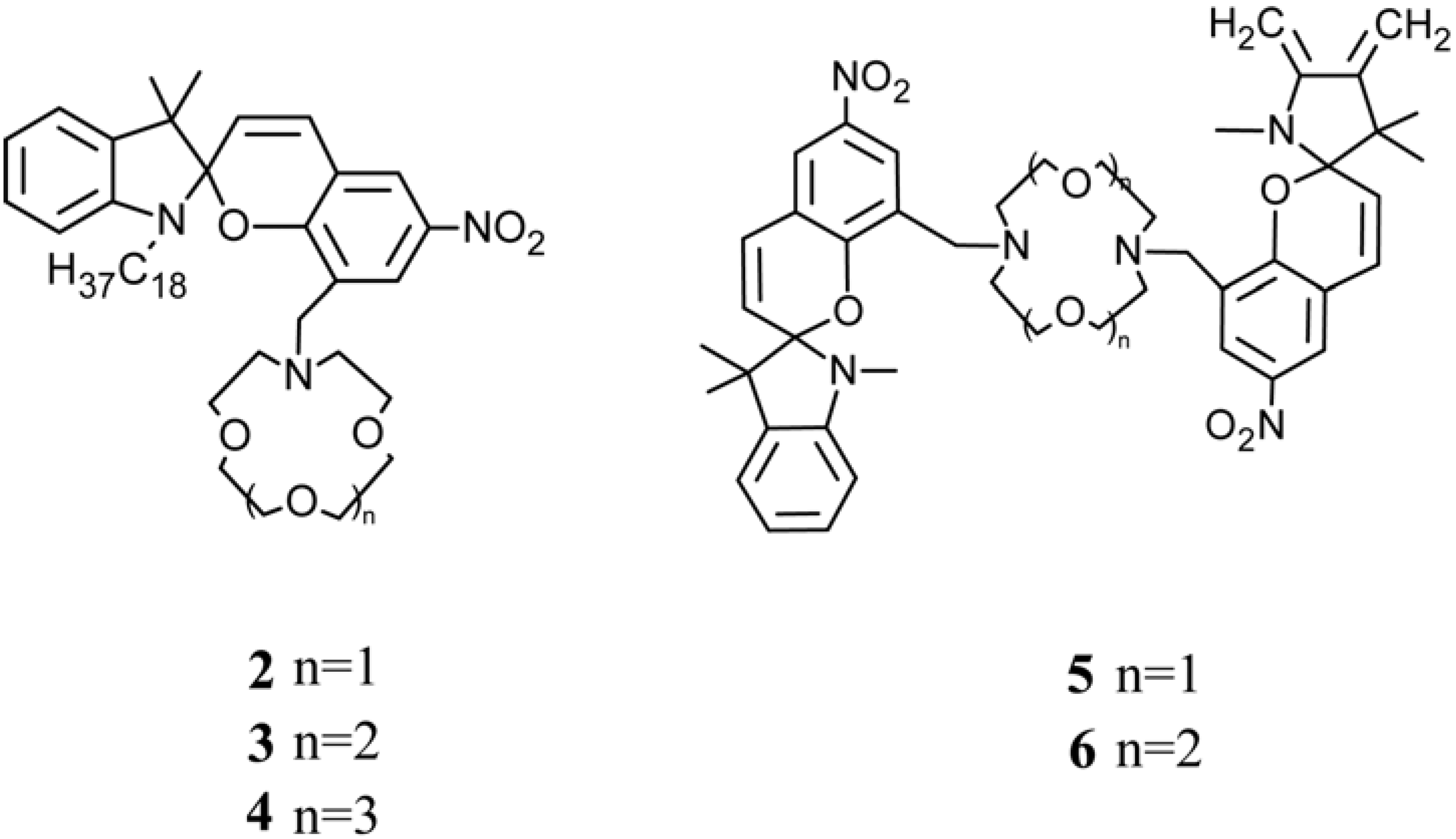
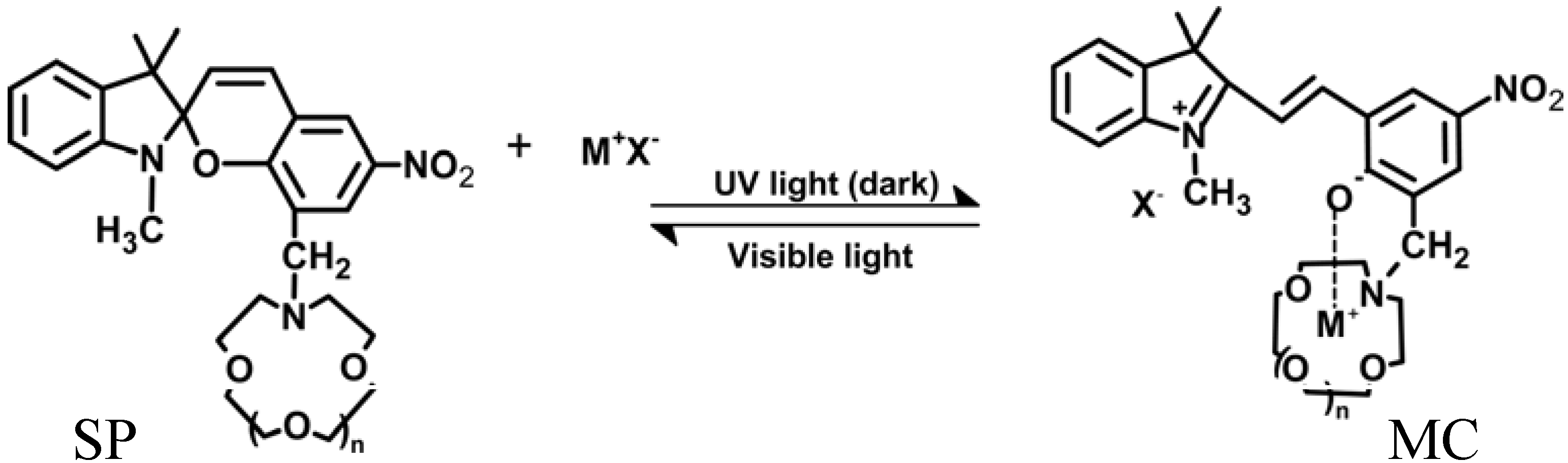
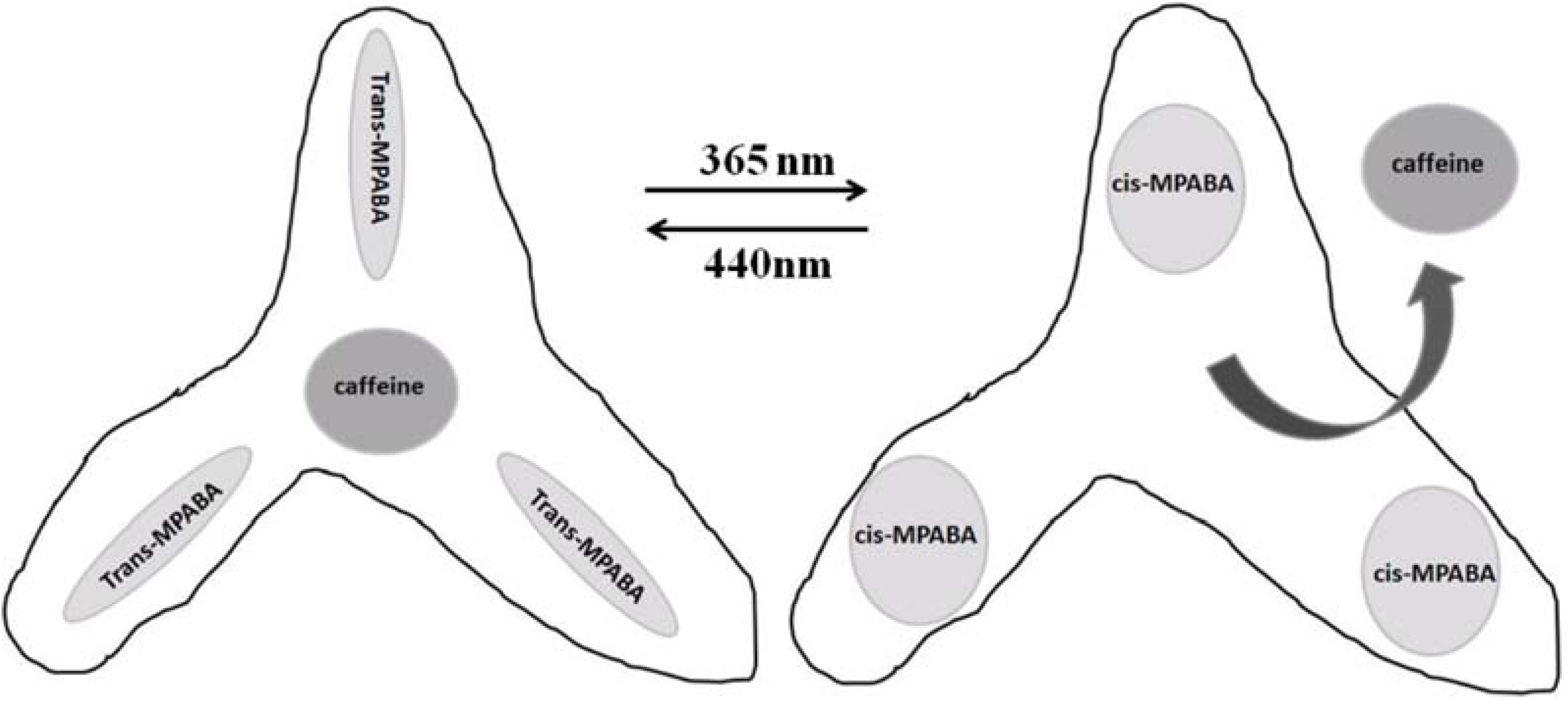
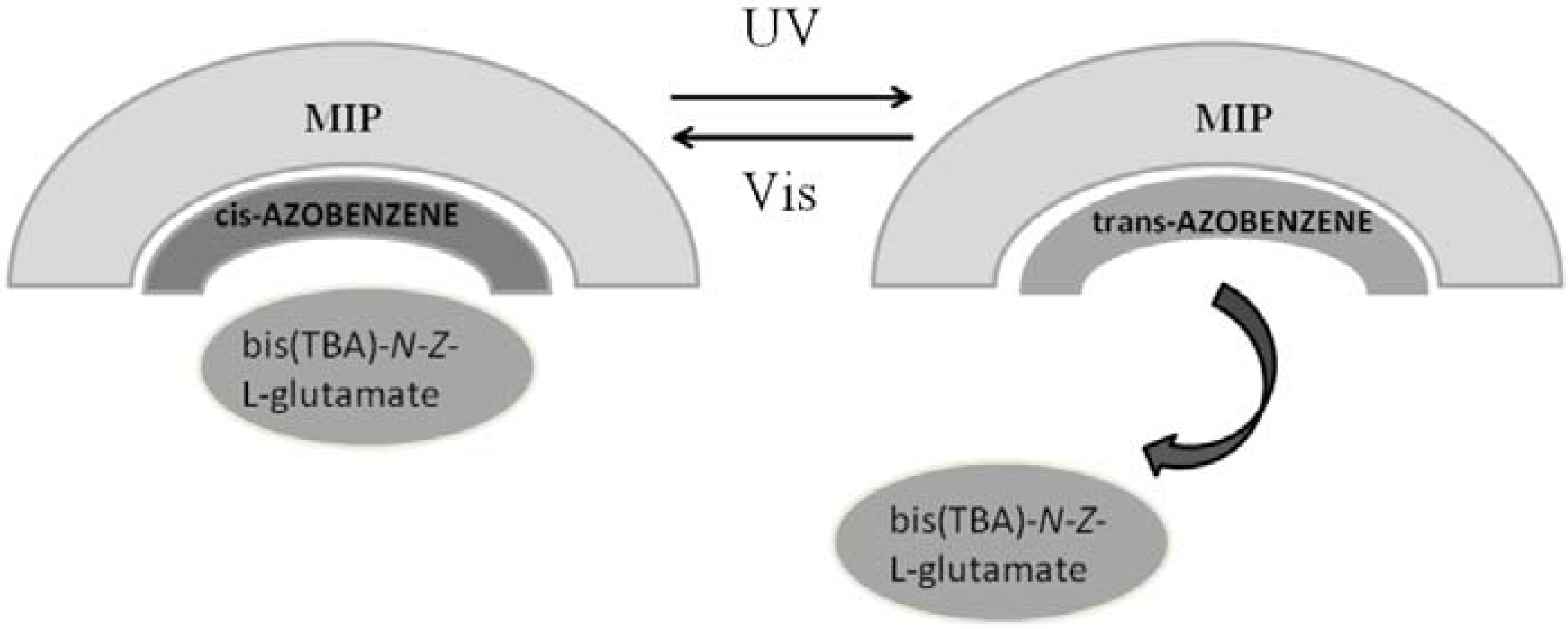
4.3. Photo-Responsive Molecularly Imprinted Membranes
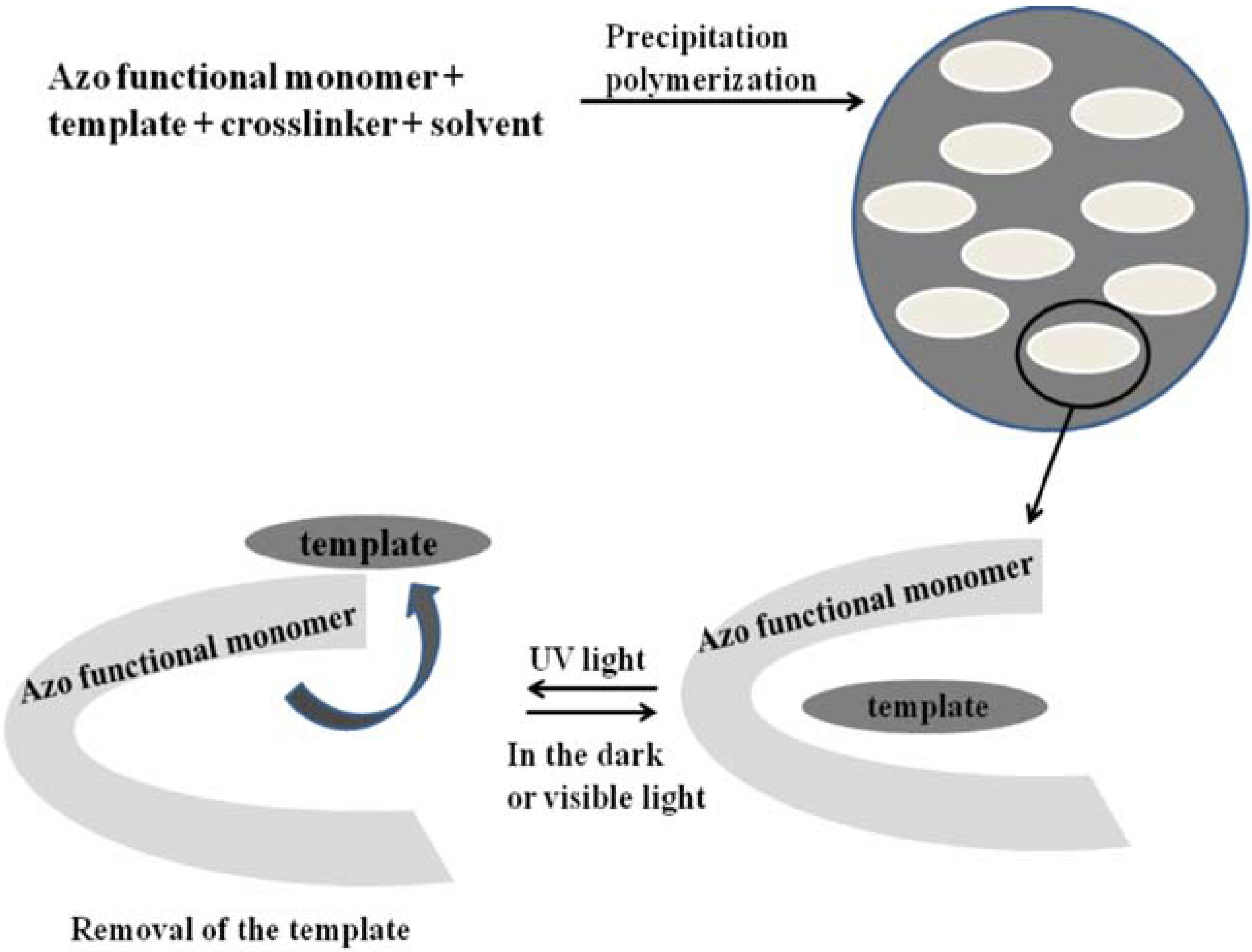
4.4. Photo-Responsive Hydrogel Membranes
4.5. Photo-Responsive Polymer Gels
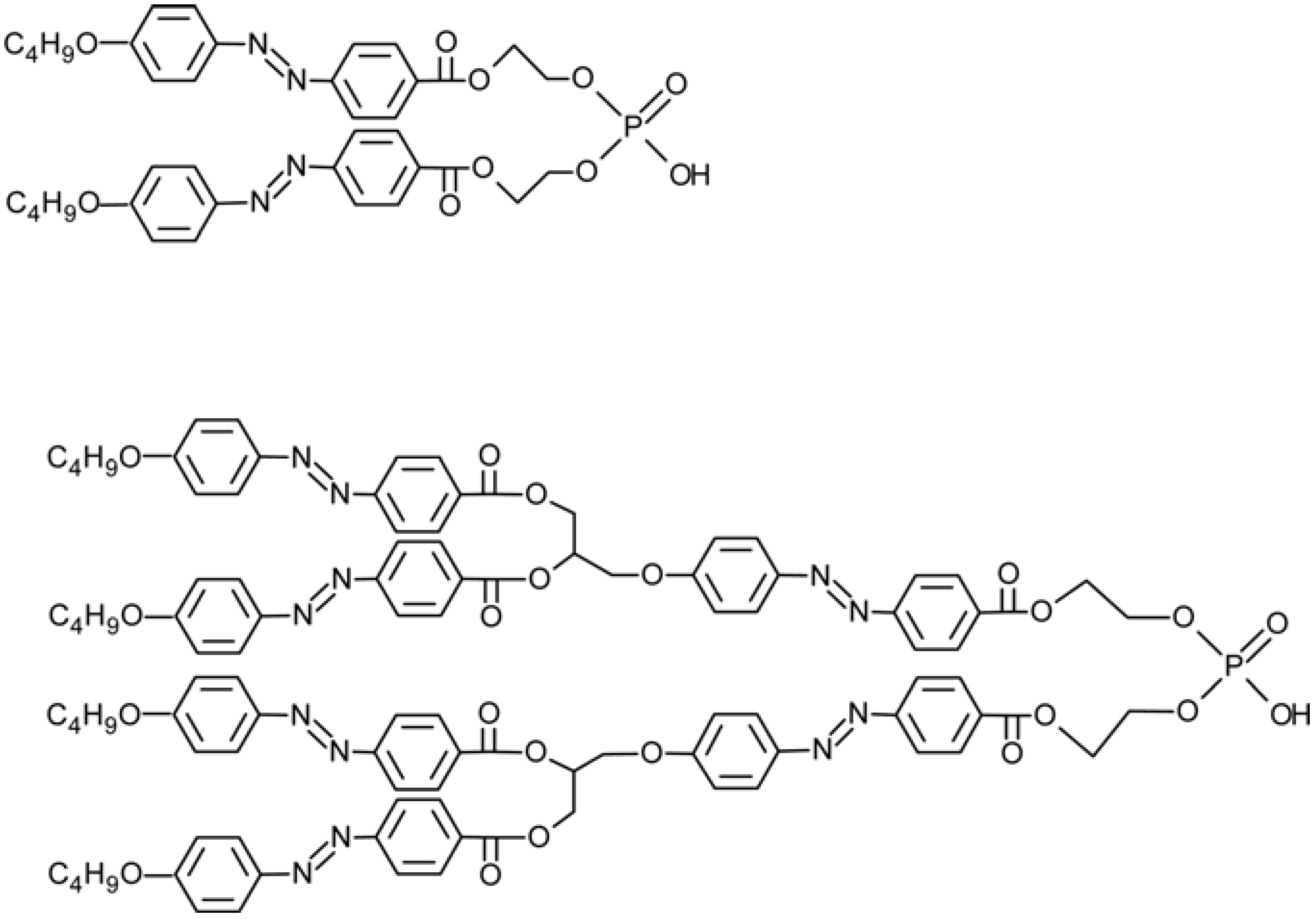
4.6. Photo-Responsive Dendrimers
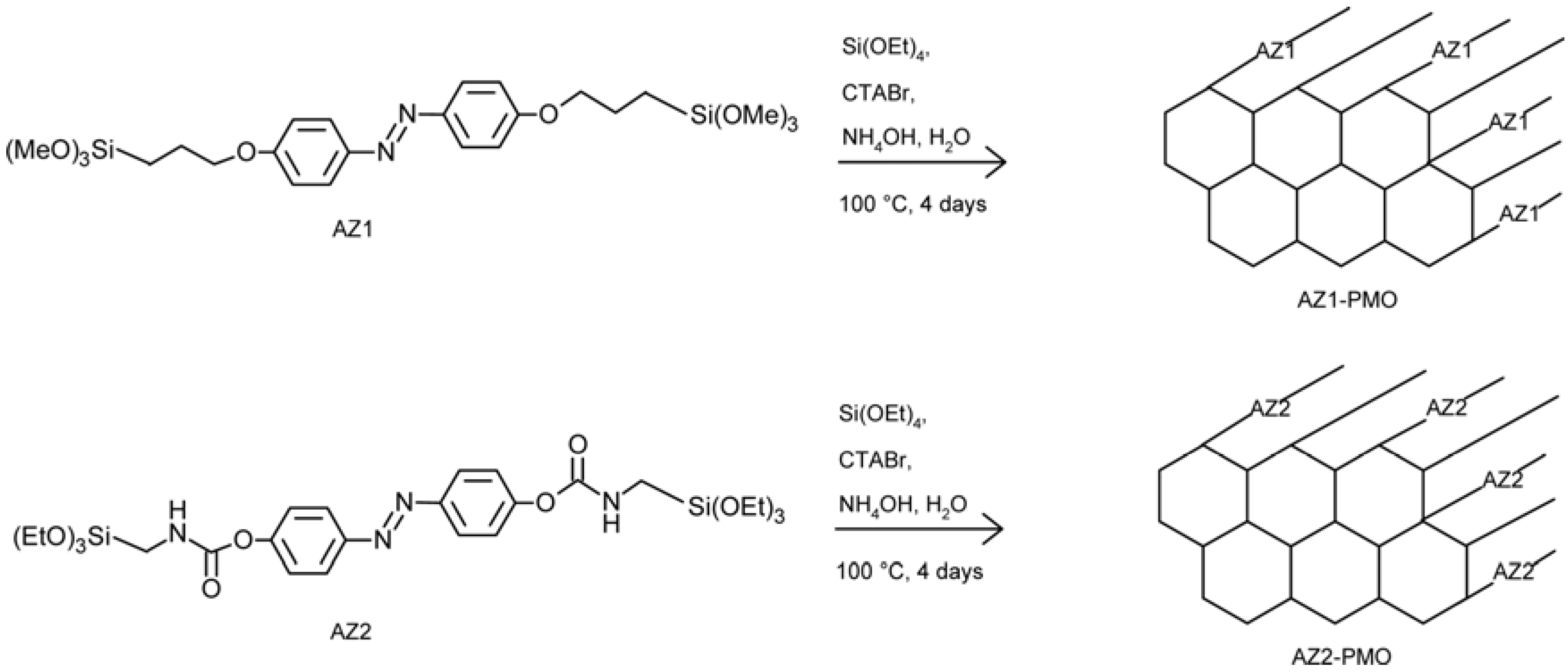
4.7. Photo-Responsive Nanoporous Silica Membranes
5. Photoresponsive Membrane Highlights in Technologically Sophisticated Applications
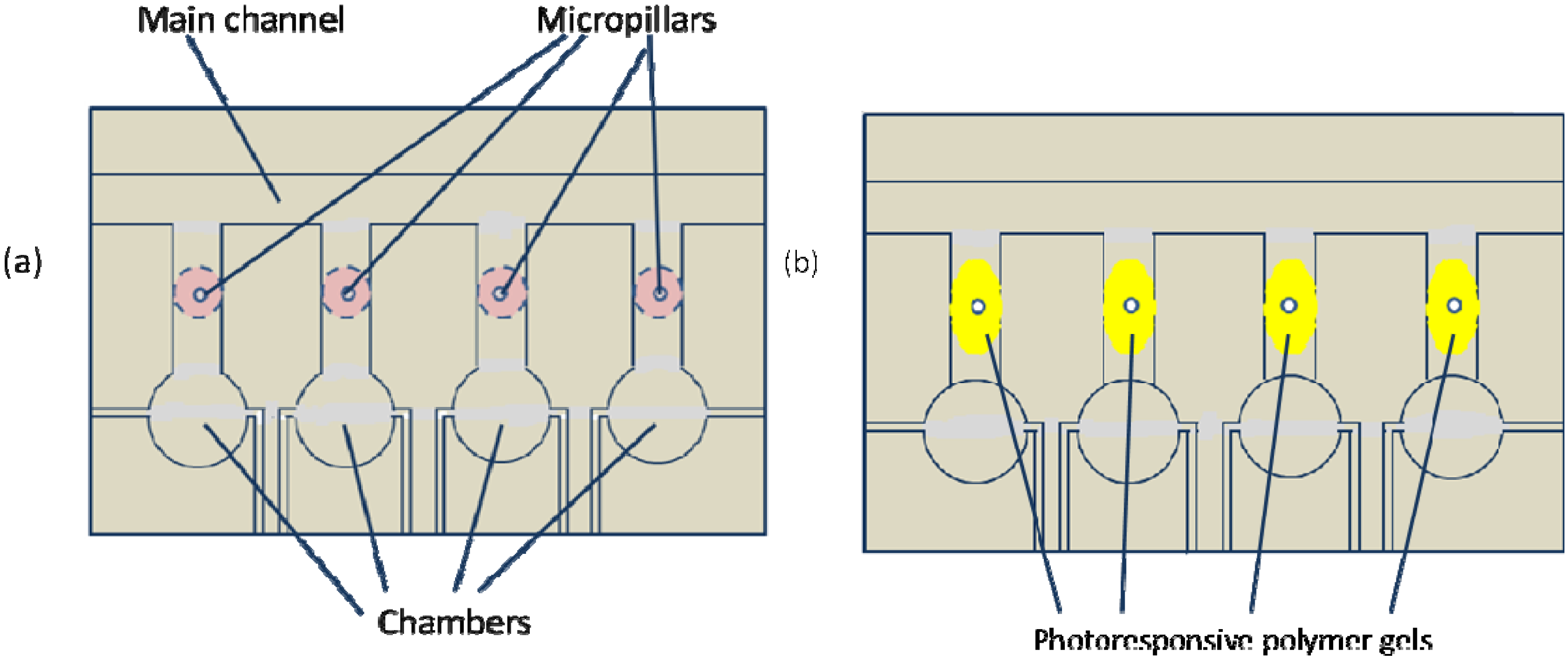
5.1. Light-Valved Microfluidics
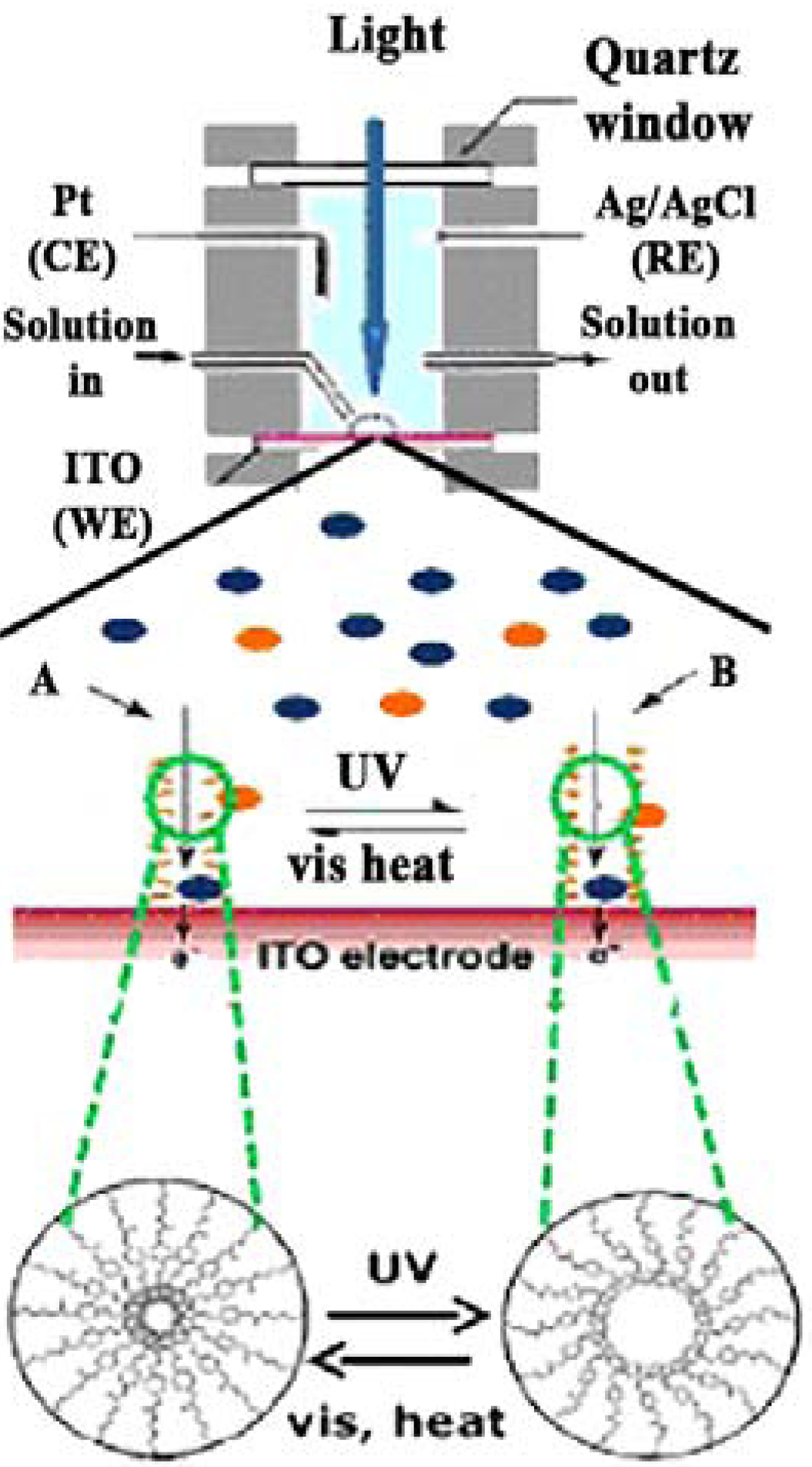
5.2. Photo-Catalytic Membranes
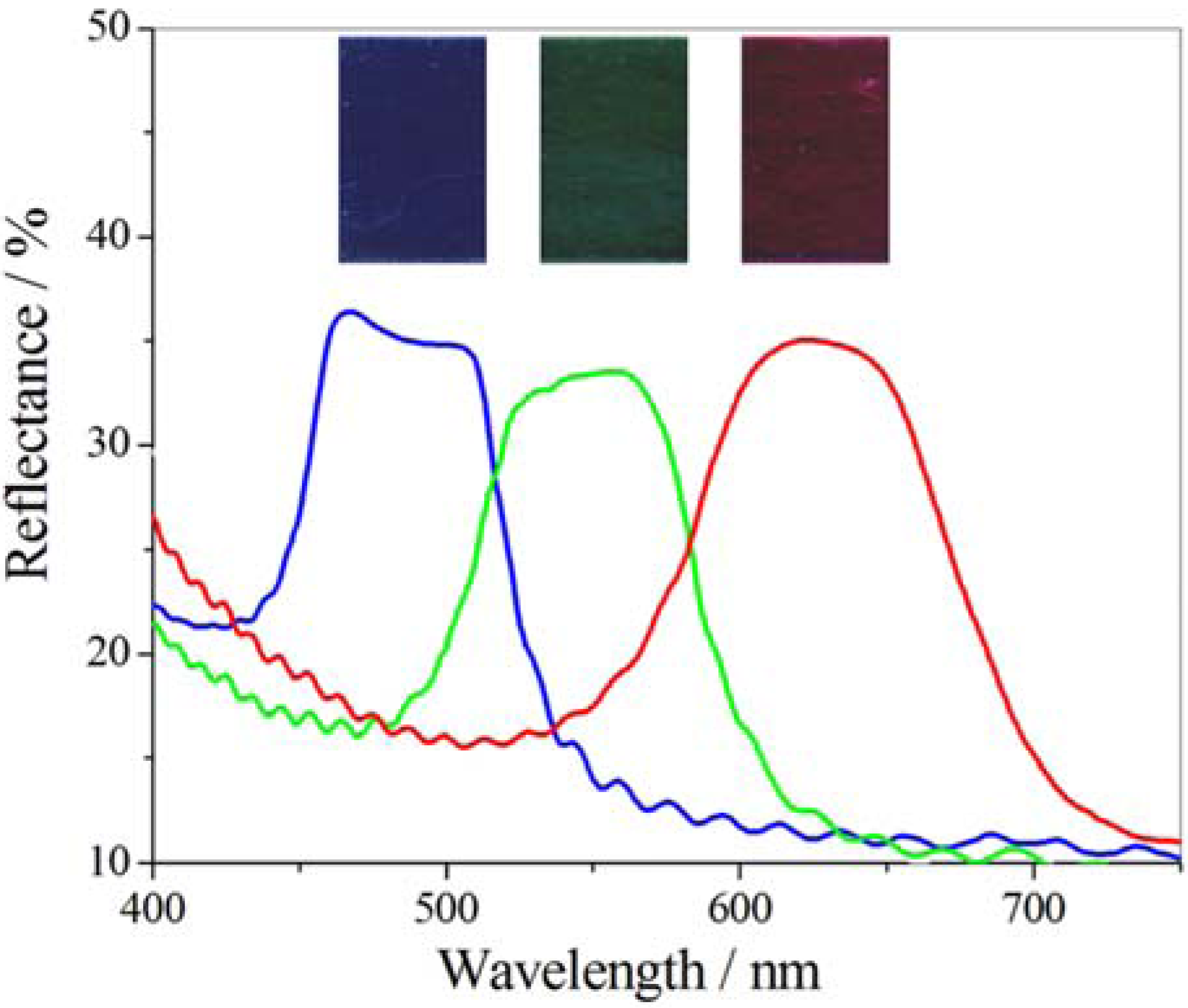
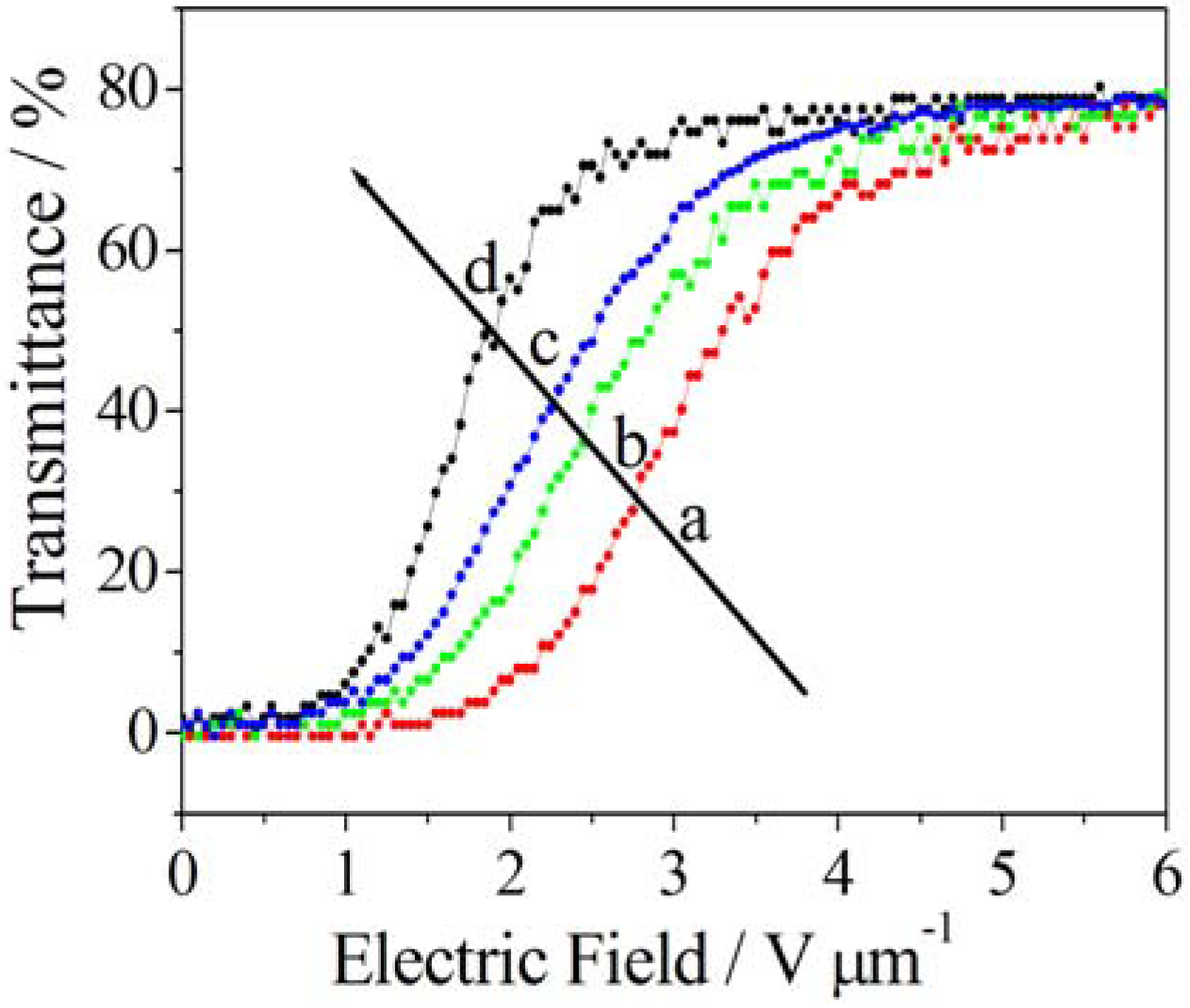
5.3. Photo-Responsive PDLCs and Membranes
| Transparency tuning → | ||||
|---|---|---|---|---|
| ↓ Color tuning | Electric fieldUV intensity | 0 V μm−1 | → | 3.5 V μm−1 |
| 0% | Transparent and uncolored | → | Opaque and uncolored | |
| ↓ | ↓ | ↓ | ||
| 100% | Transparent and colored | → | Opaque and colored | |

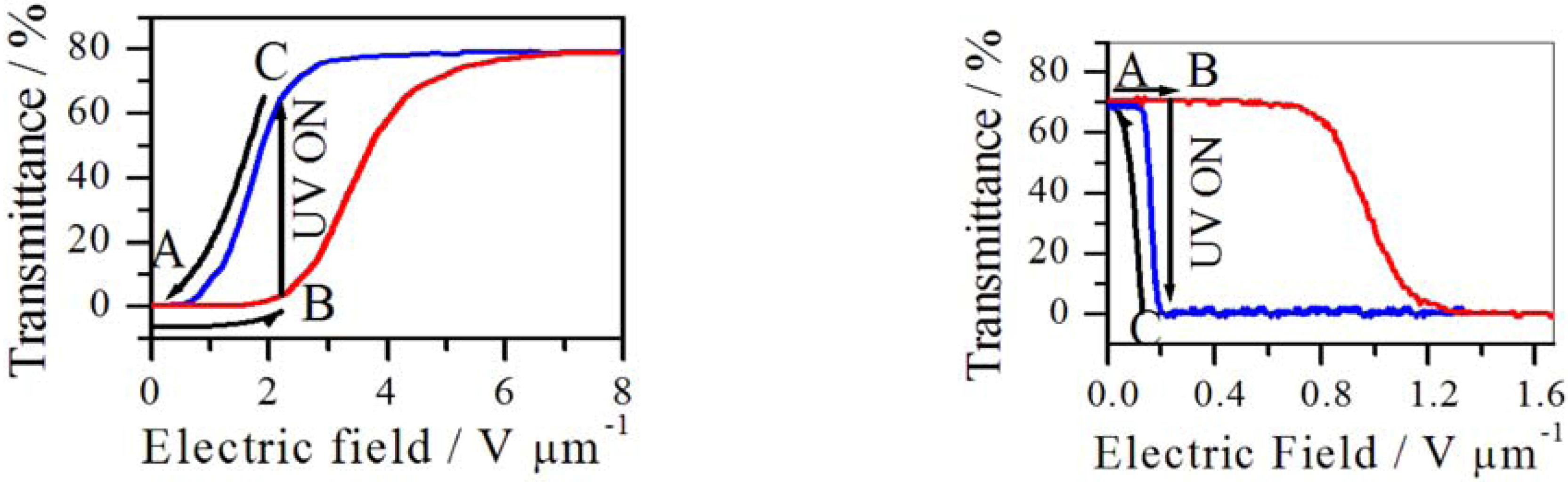
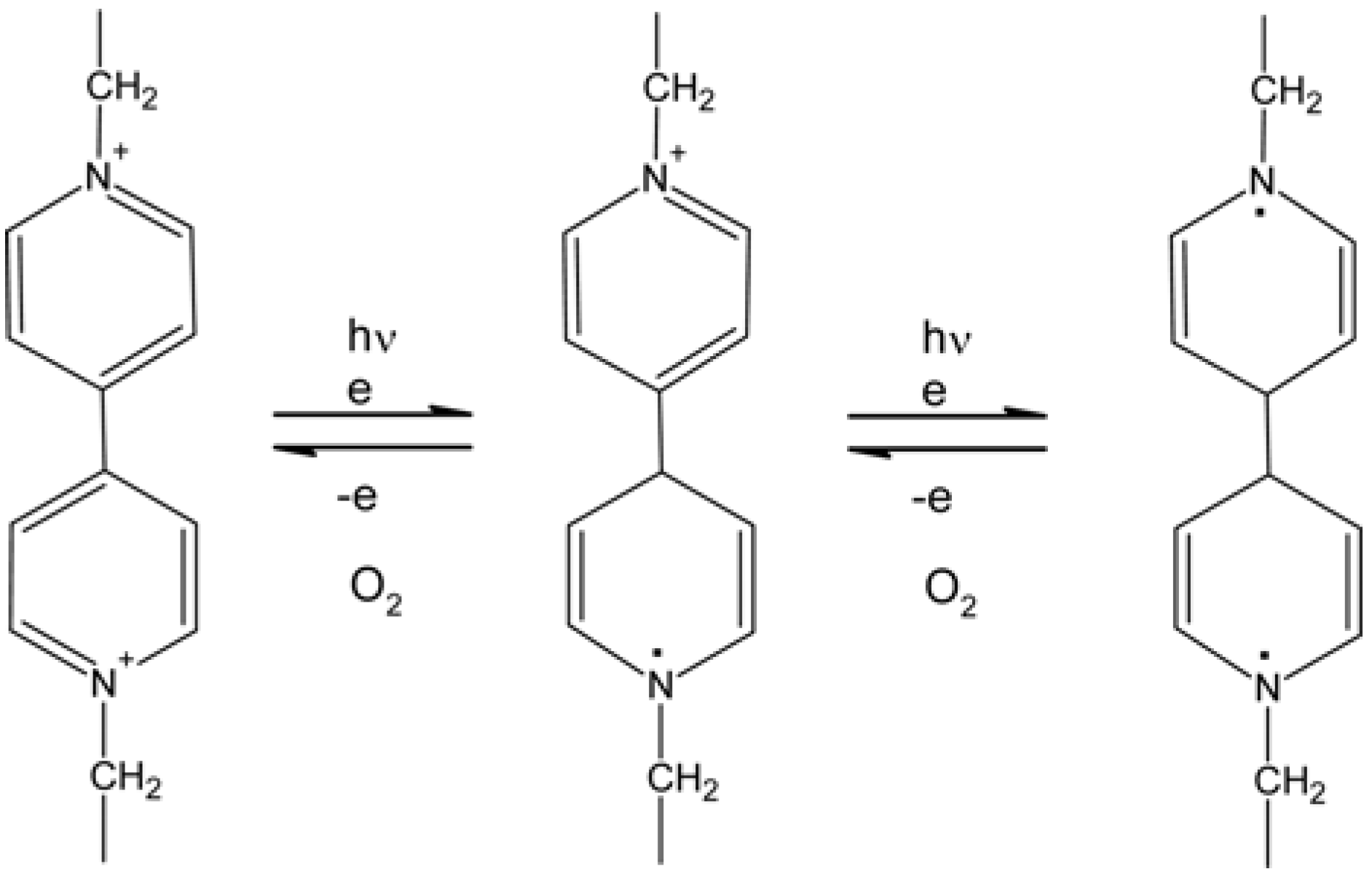
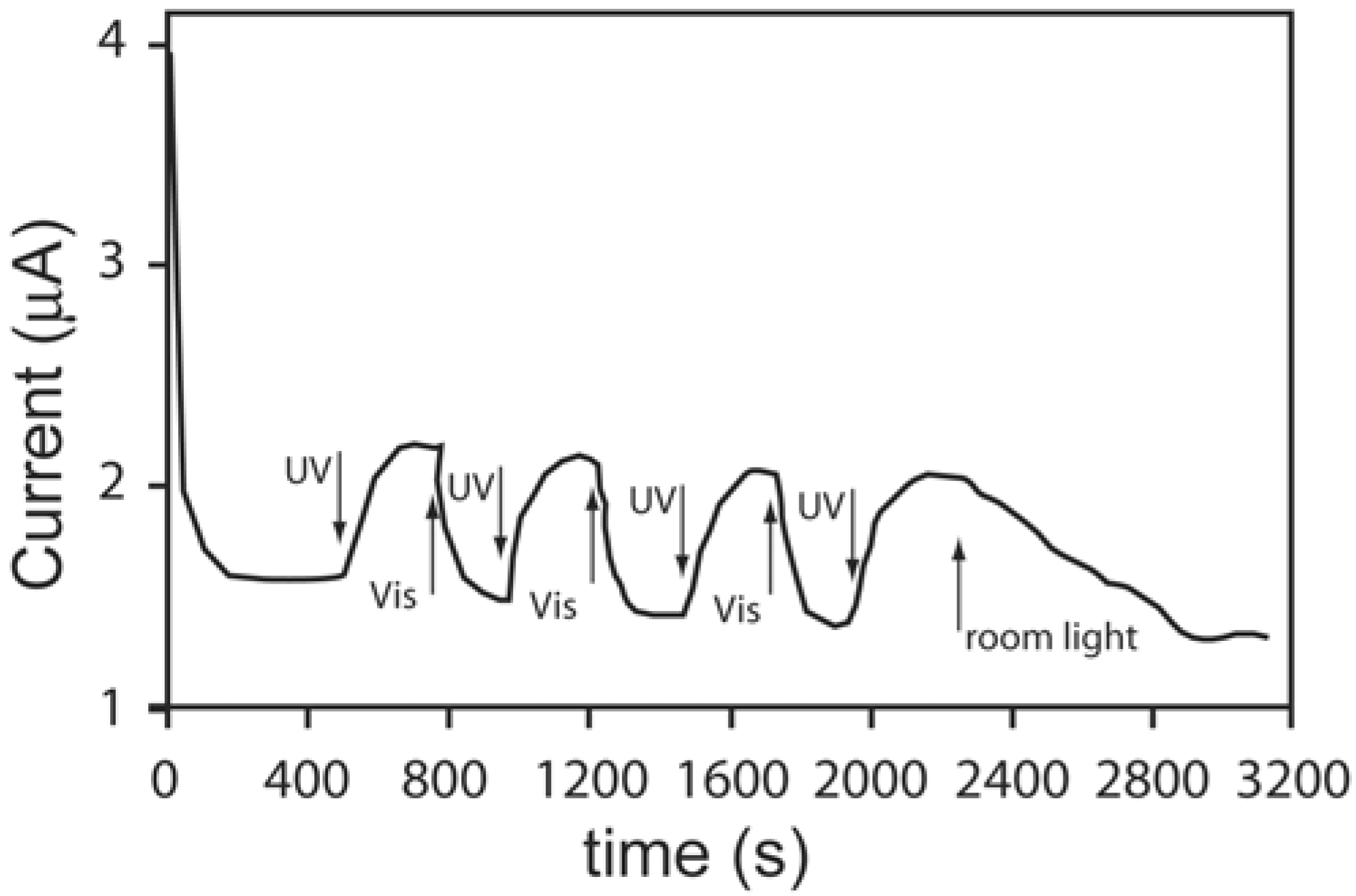
5.4. Photo-Electrochromic Systems
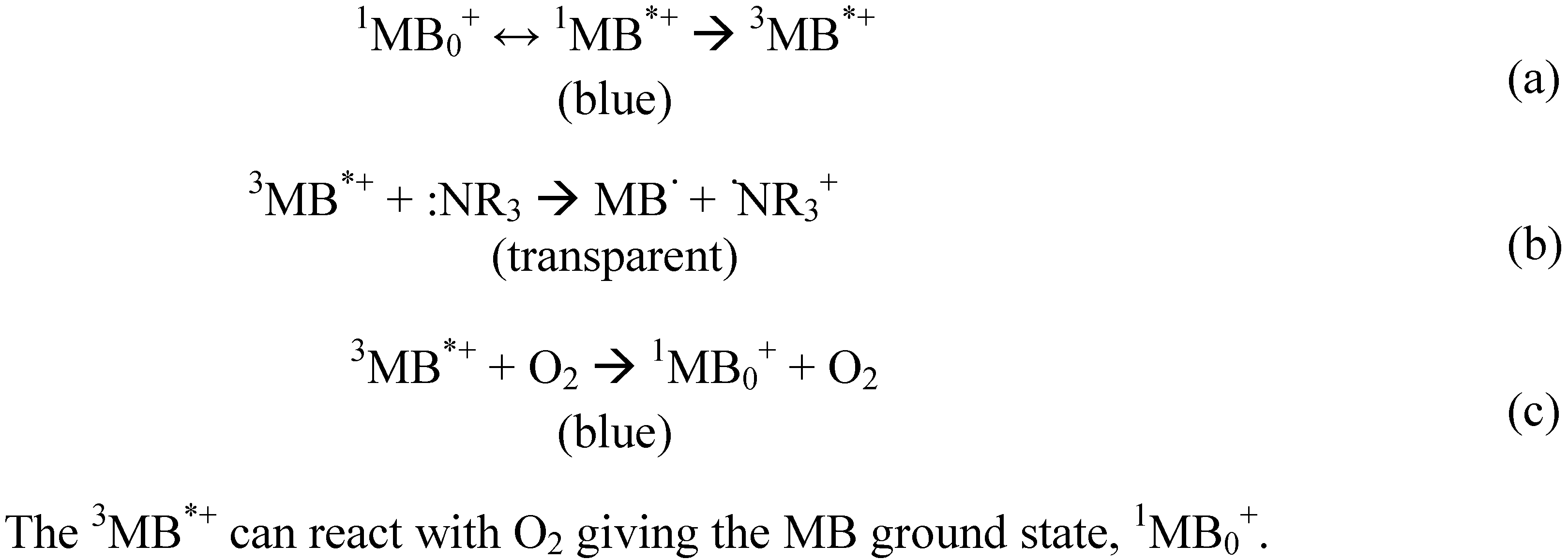
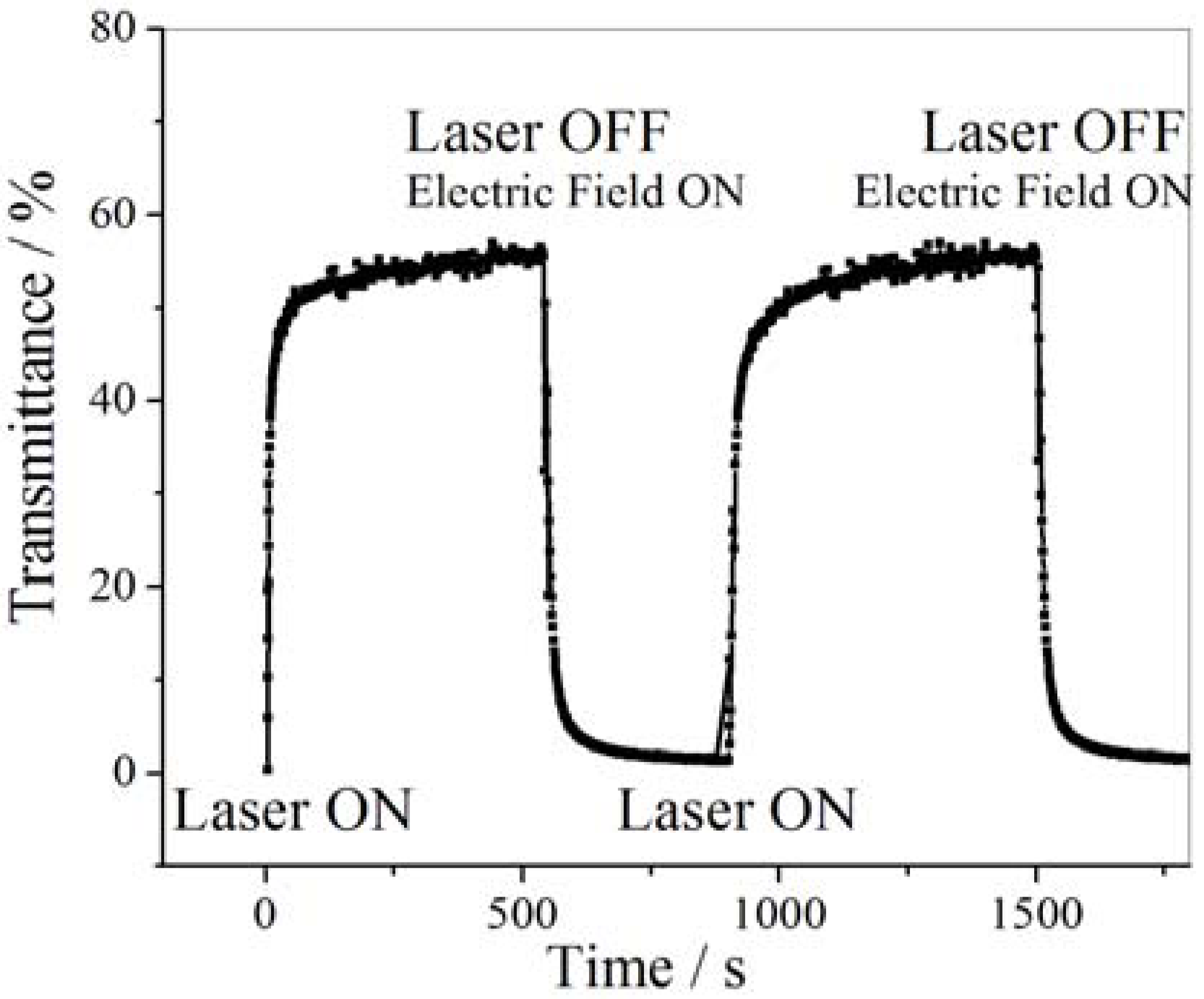
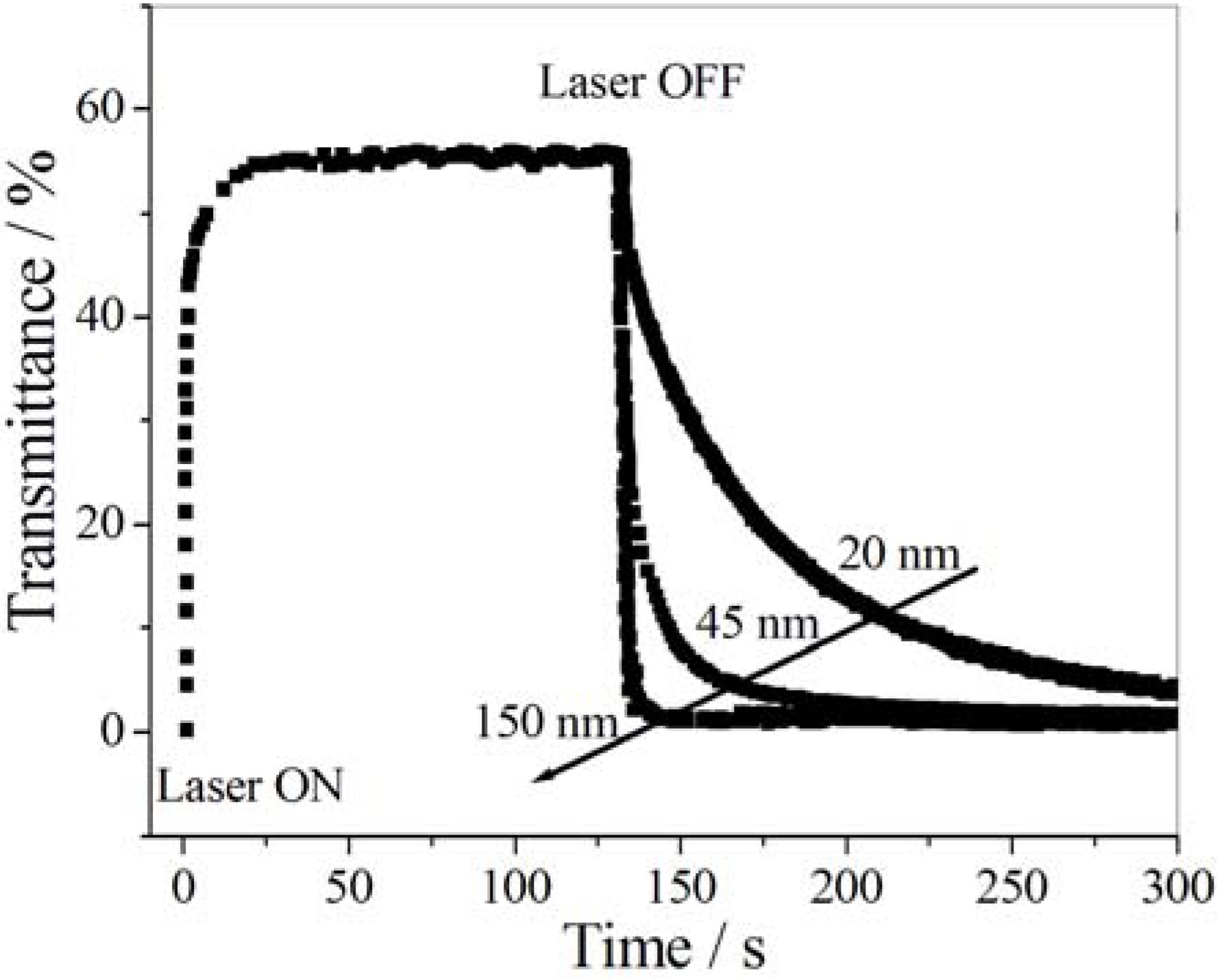
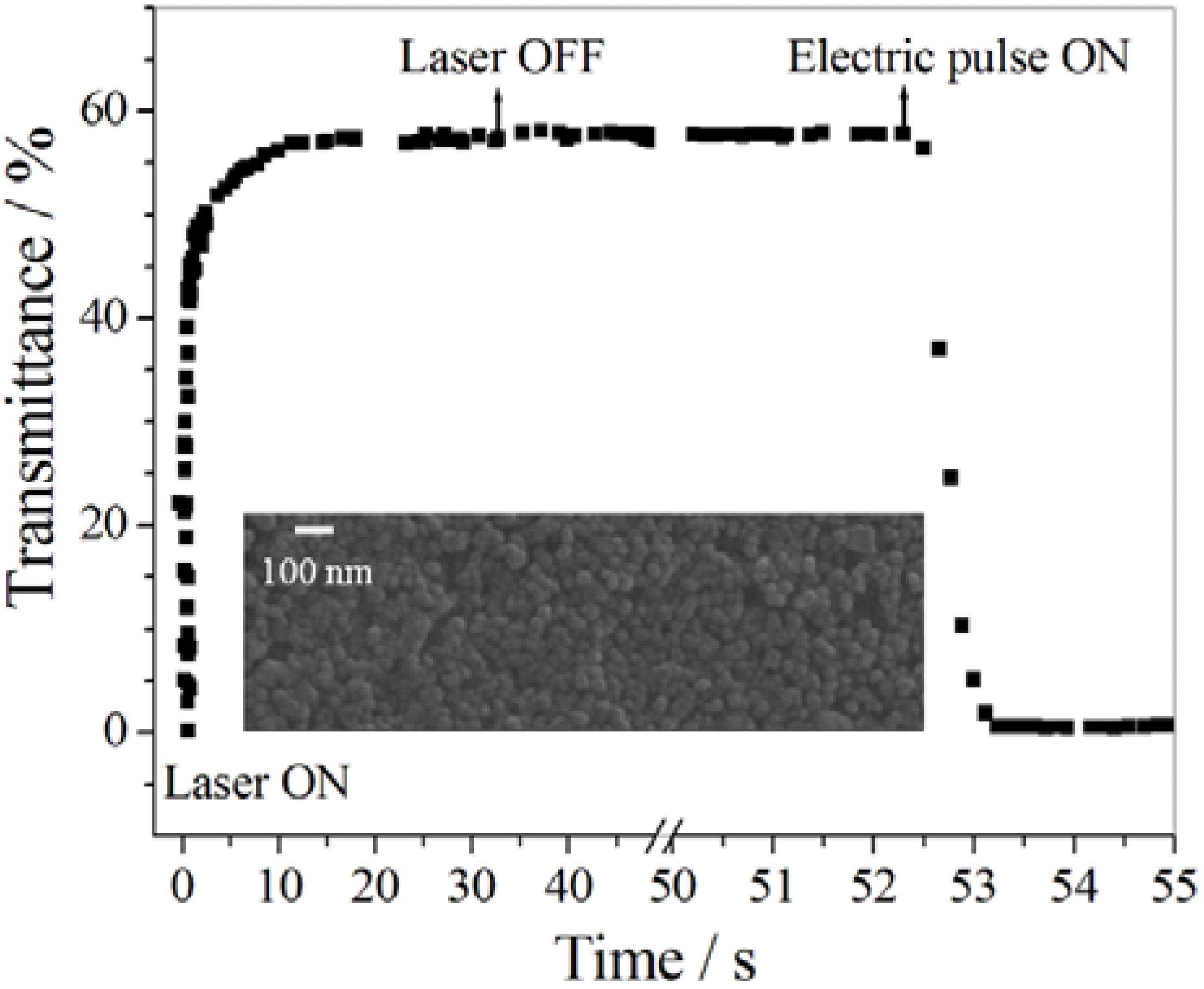
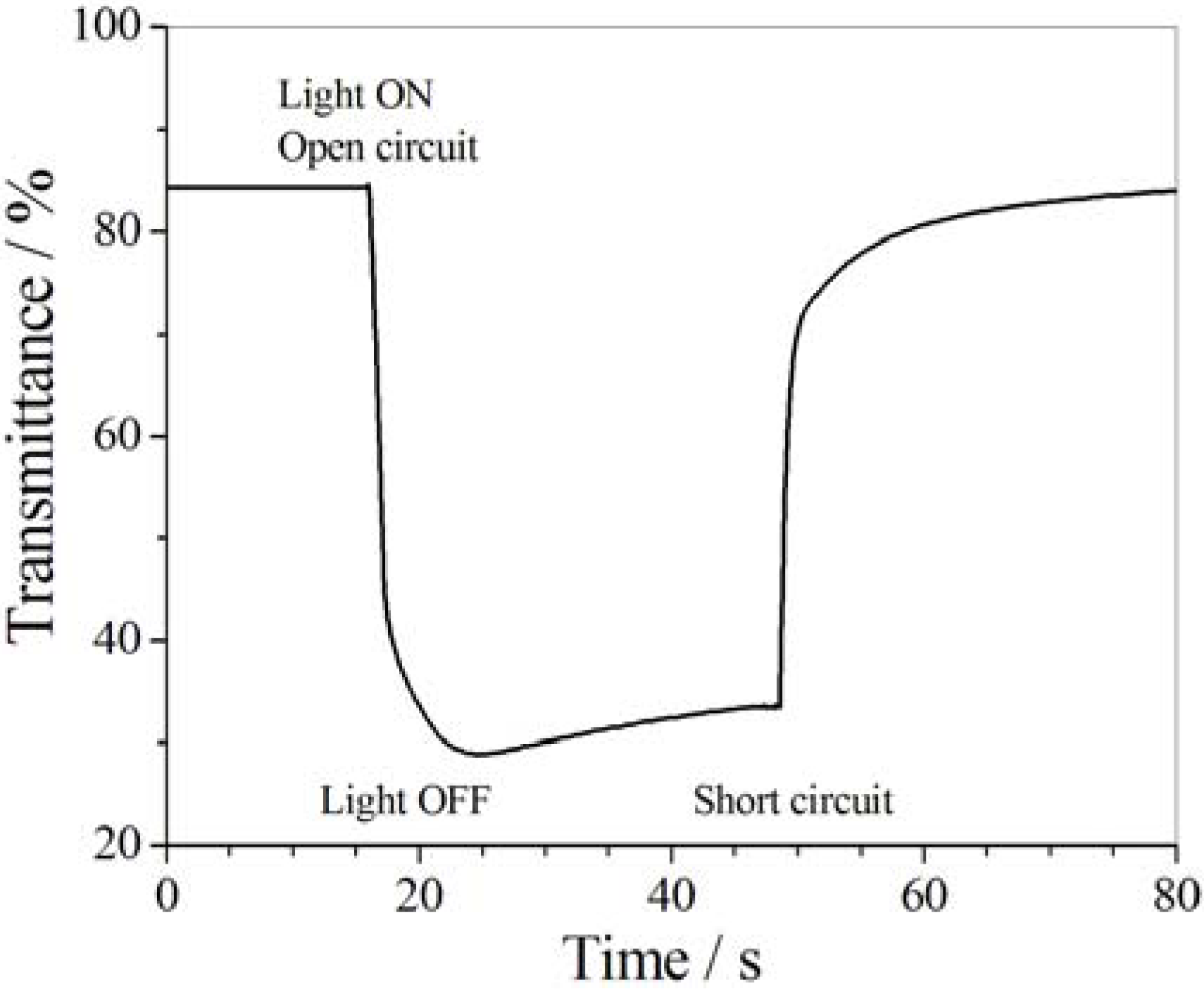
6. Biological Applications
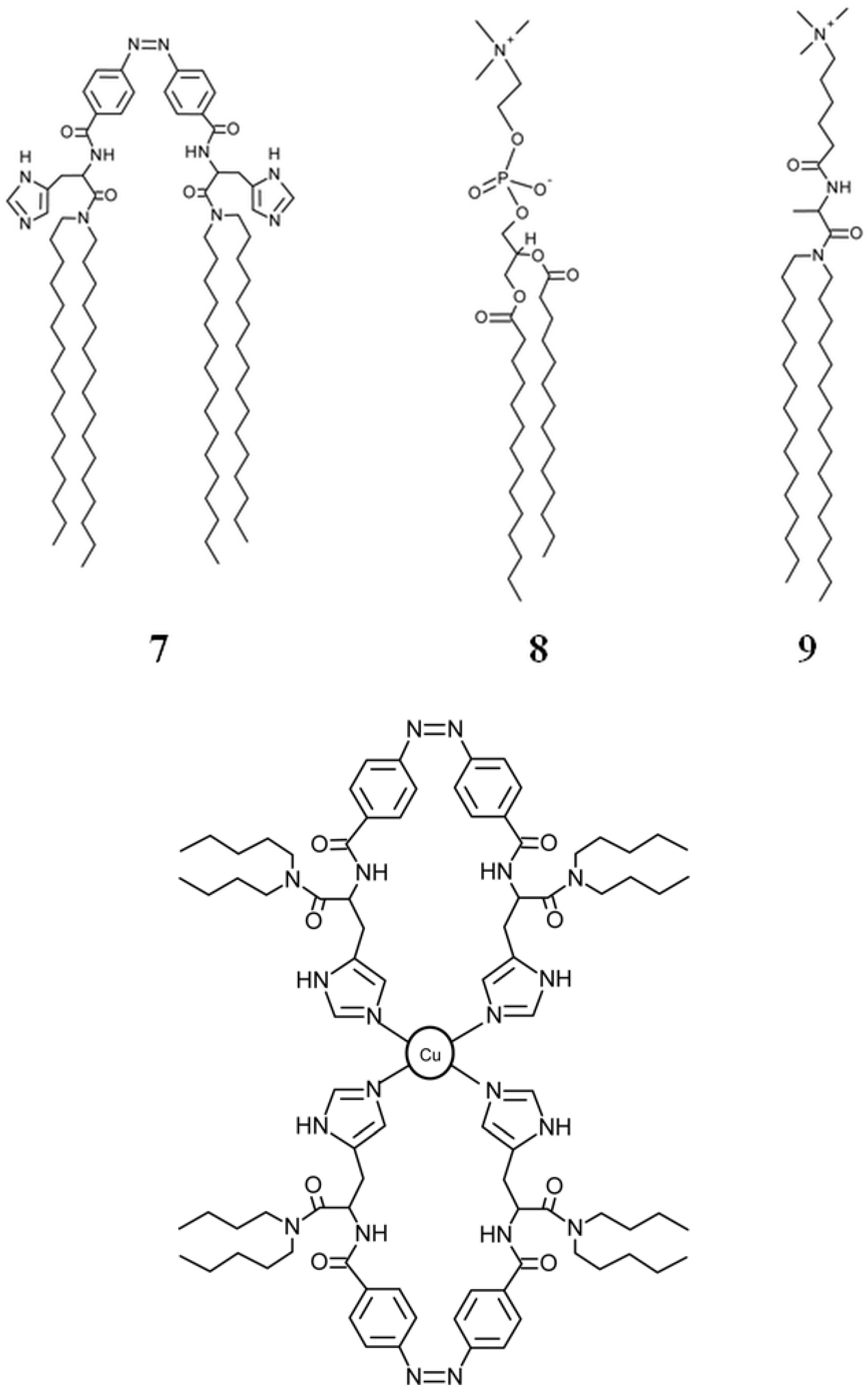
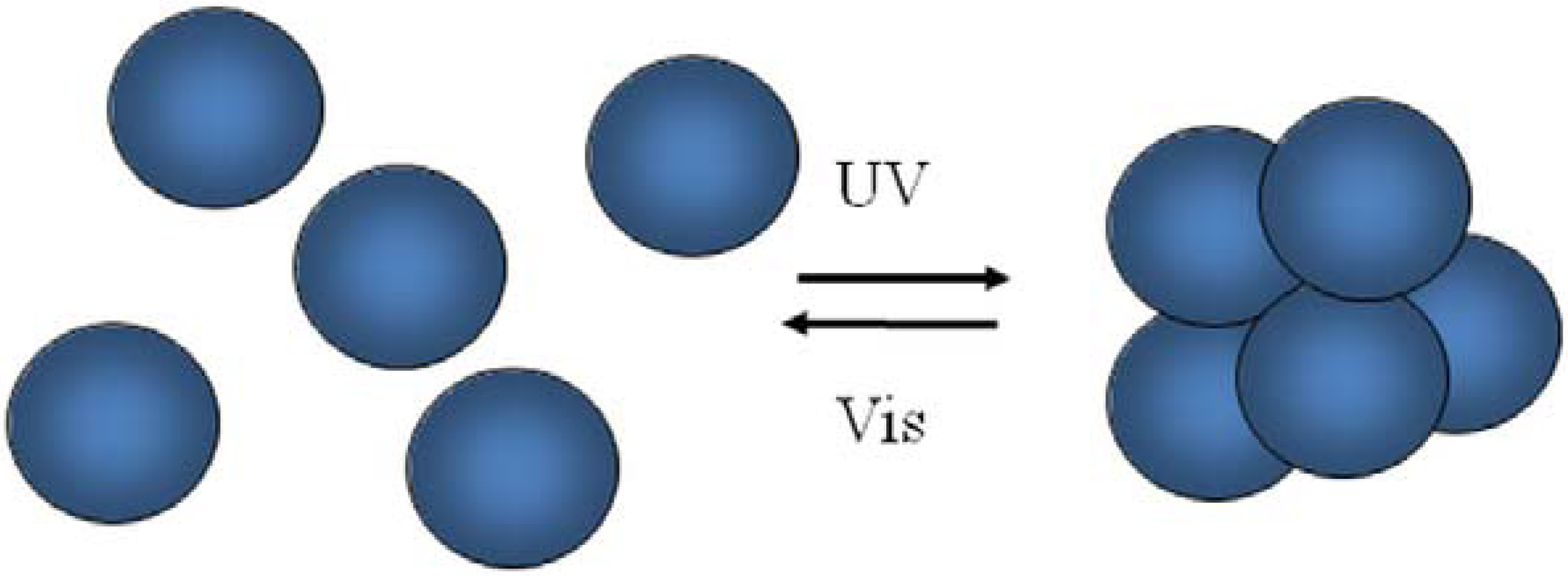
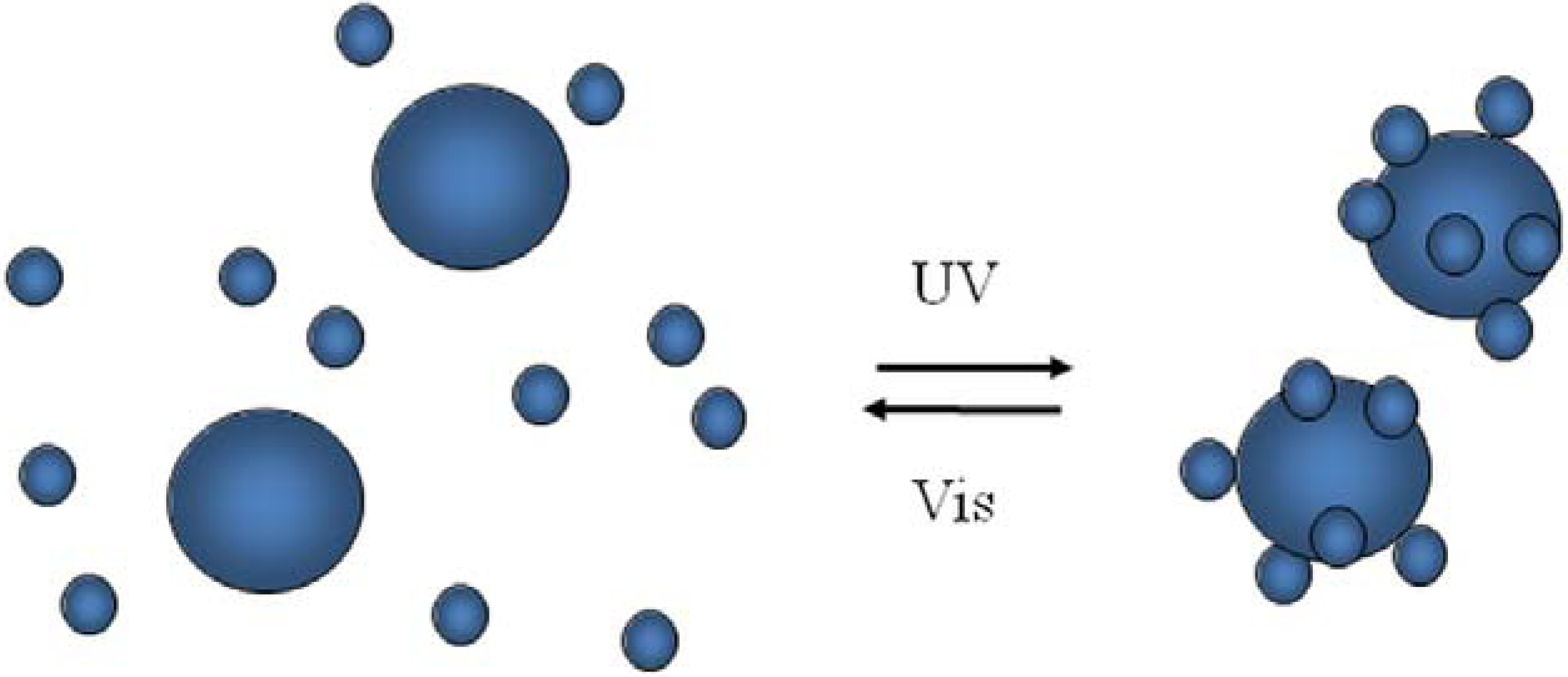
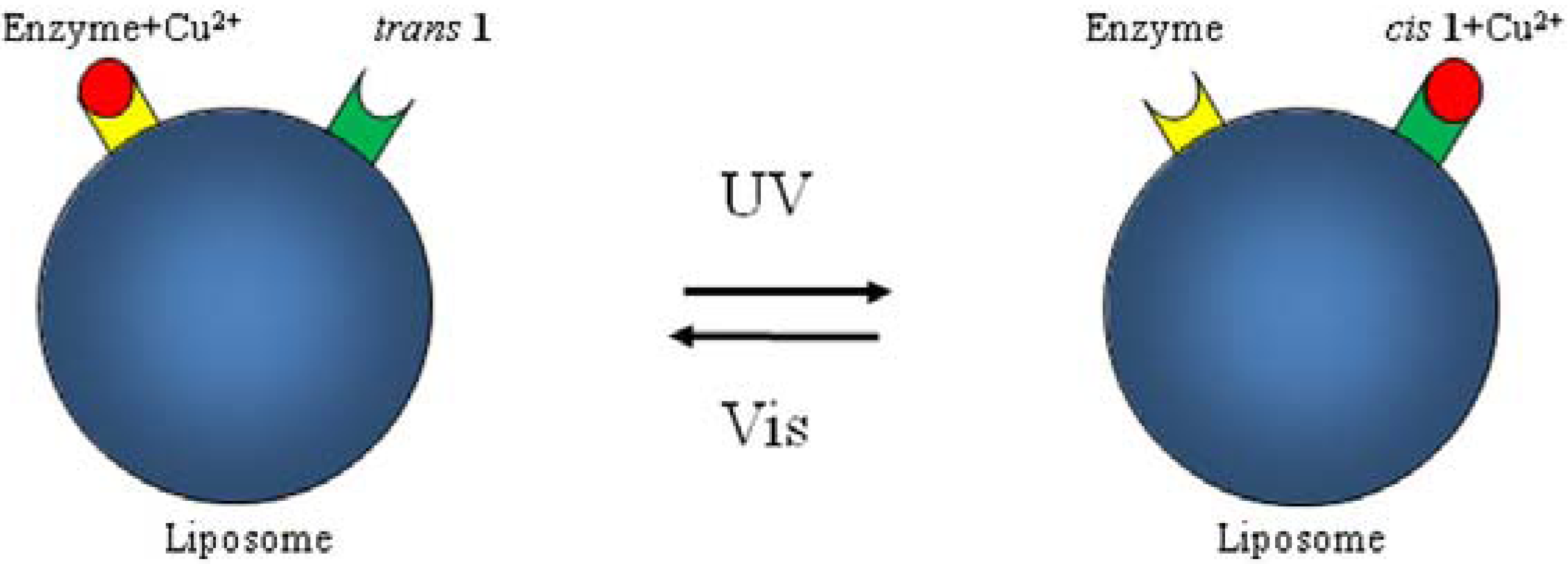
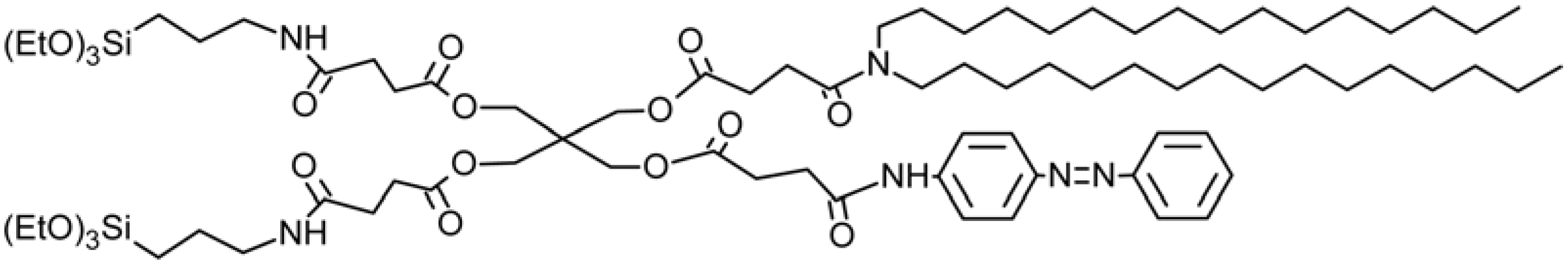
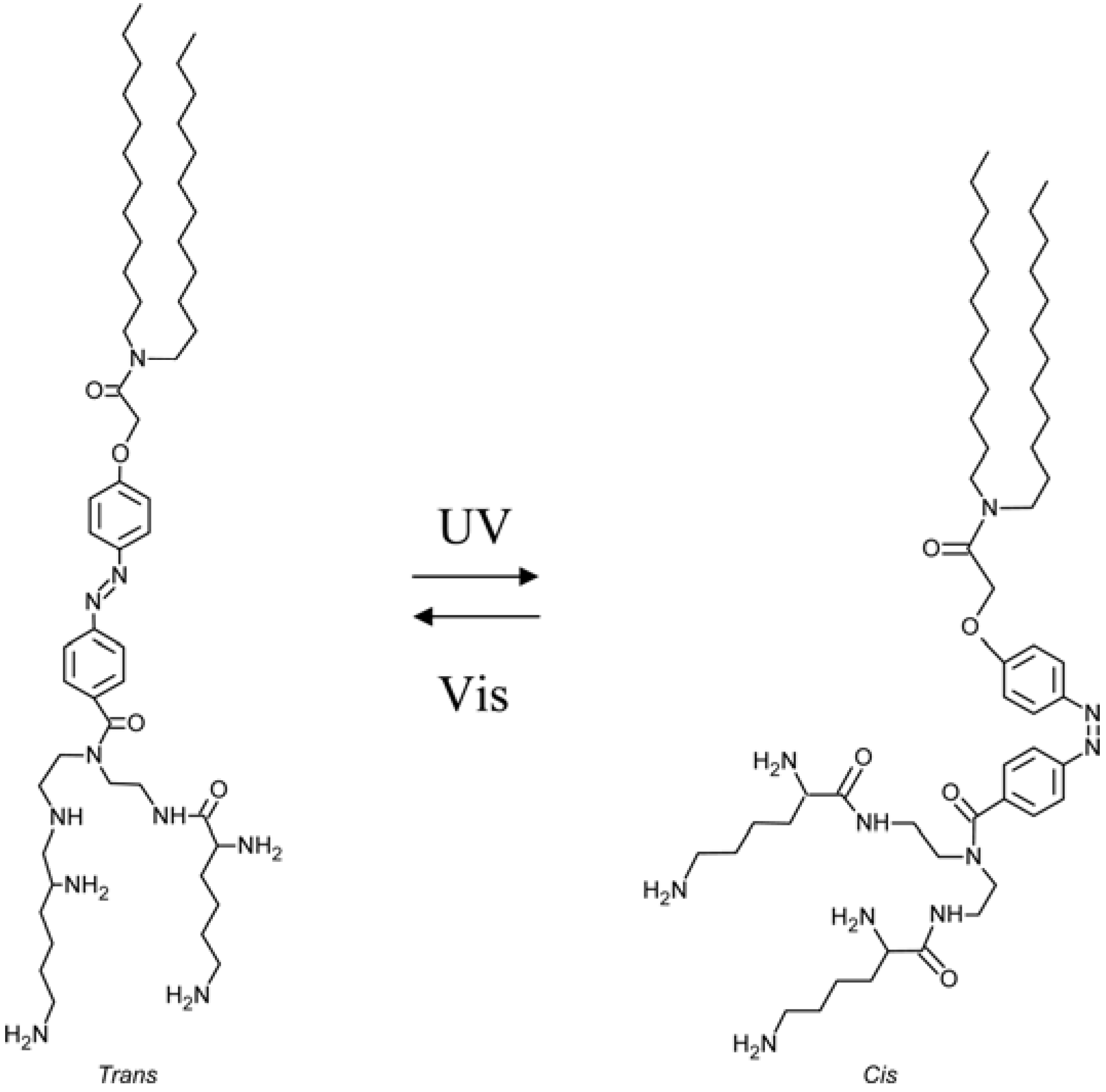
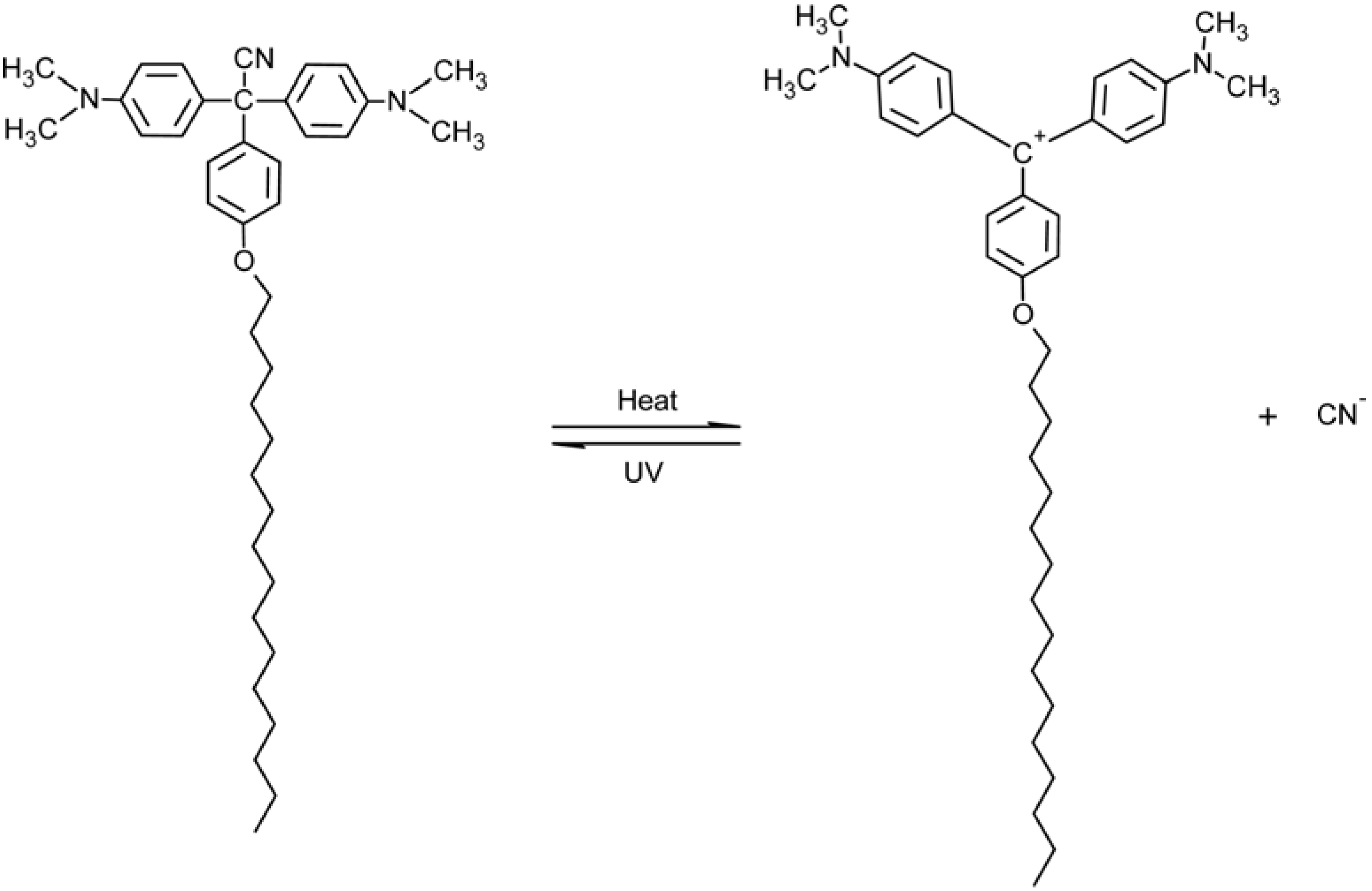
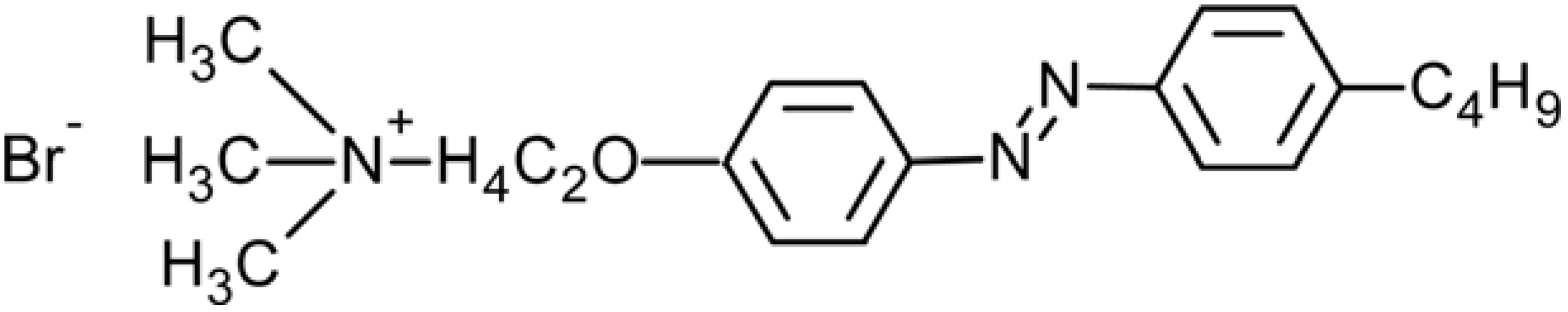
7. Conclusions
8. List of Abbreviations
| AZB | azobenzene |
| AZTMA | 4-butylazobenzene-4’-(oxyethyl)trimethylammoniumbromide |
| BSP | benzospiropyran |
| CA | contact angle |
| CD | circular dichroism |
| CPP | critical packing parameter |
| CTAC | cetyltrimethylammonium chloride |
| DMF | N,N-dimethylformamide |
| E, Eappl | electric field across liquid crystal droplet, applied external field |
| FM | 4-{4-[2,6-bis(n-butylamino)pyridine-4-yl]-phenylazo}-phenyl methacrylate |
| Fy | net force on the drop |
| G0, G1, G2 | first, second, third generation dendrimers |
| h | Planck’s constant |
| MB | methylene blue |
| MC | merocyanine |
| MGL | malachite green leuconitrile |
| MIP | molecularly imprinted polymer |
| MMA, PMMA | methylmethacrylate, polymethylmethacrylate |
| PA | polyacrylamide |
| PDLC | polymer dispersed liquid crystals |
| PEG | poly(ethylene glycol) |
| PEG-CA | cinnamylidene acetate modified PEG |
| PET | polyethylene terephthalate |
| PGA | poly(L-glutamic acid) |
| PMO | periodic mesoporous organosilica |
| PM6AzCOOH | carboxylic azopolymer |
| PNA | 2-nitro-4’-methoxyazobenzene |
| poly(NSP-co-MMA) | nitrobenzospiropyran-methyl methacrylate copolymer |
| PVHQ | poly(2-vinyl-8-hydroxyquinoline-r-8-vinyl-1-naphthoic acid) |
| SAM | self-assembled monolayer |
| SP | spiropyran |
| SPMMA/MMA | spirobenzopyran/methylmethacrylate copolymer |
| TPMLH | bis-[4-{dimethylamino}phenyl] {4-vinyl-phenyl} methyl leucohydroxide |
| SDBS | sodium dodecylbenzenesulfonate |
| SOS | sodium octyl sulfate |
| Strans/cis | trans/cis selectivity |
| V | volume of the hydrophobic group |
| w | drop width |
| a0 | surface area of the hydrophilic group |
| lc | length of the hydrophobic group |
| UV | ultraviolet light |
| Vis | visible light |
| α(a/b) | mixture separation factor |
| Δ | heat or thermal relaxation |
| ΔE | energy band-gap |
| ν, ν’ | frequency |
| θ | contact angle value |
| γLV | surface free energy of the liquid-vapor interface |
| γSL | surface free energy of the solid-liquid interface |
| γSV | surface free energy of the solid-vapor interface |
| σmatrix , σLC | polymer matrix electric conductivity, liquid crystal electric conductivity |
References
- Fendler, J.H. Membrane Mimetic Chemistry; John Wiley Press: New York, NY, USA, 1982. [Google Scholar]
- Kotyk, A.; Janacek, K.; Koryta, J. Biophysical Chemistry of Membrane Functions; John Wiley Press: New York, NY, USA, 1988. [Google Scholar]
- Kinoshita, T. Biomembrane mimetic systems. Prog.Polym. Sci. 1995, 20, 527–583. [Google Scholar] [CrossRef]
- Balzani, V. Supramolecular Photo-Chemistry; Reidel Publishing: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Irie, M. Photo-responsive polymers. Adv. Polym. Sci. 1990, 94, 27–67. [Google Scholar] [CrossRef]
- Kumano, A.; Niwa, O.; Kajiyama, T.; Takayanagi, M.; Kano, K.; Shinkai, S. Photo-induced ion permeation through ternary composite membrane composed of polymer/liquid crystal/azobenzene bridged crown ether. Chem. Lett. 1983, 12, 1327–1330. [Google Scholar]
- Anzai, J.; Hasebe, Y.; Ueno, A.; Osa, T. Photo-excitable membranes. Photo-induced potential changes across poly(vinyl chloride) membranes doped with a photo-sensitive crown ether having lipophilic side chain. J. Polym. Sci. A-Polym. Chem. 1988, 26, 1519–1529. [Google Scholar] [CrossRef]
- Anzai, J.; Sasaki, A.; Ueno, A.; Osa, T. Photo-chemically-induced asymmetric membrane-potential across poly(vinyl-chloride) spirobenzopyran membranes. Chem. Lett. 1985, 14, 1443–1446. [Google Scholar]
- Smetz, G.; Braeken, J.; Irie, M. Photo-mechanical effects in photo-chromic systems. Pure Appl. Chem. 1978, 50, 845–856. [Google Scholar] [CrossRef]
- Shinkai, S.; Kinda, H.; Manabe, O. Photo-responsive complexation of metal cations with an azobenzene-crown-azobenzene bridge immobilized in polymer supports. J. Am. Chem. Soc. 1982, 104, 2933–2934. [Google Scholar] [CrossRef]
- Irie, M.; Tanaka, H. Photo-responsive polymers. 5. Reversible solubility change of polystyrene having azobenzene pendant groups. Macromolecules 1983, 16, 210–214. [Google Scholar] [CrossRef]
- Ishihara, K.; Negishi, N.; Shinohara, I. Adsorption of photo-chromic azo dye onto styrene-divinylbenzene copolymer. J. Polym. Sci. Polym. Lett. Ed. 1981, 19, 593–587. [Google Scholar] [CrossRef]
- Negishi, N.; Iida, K.; Ishihara, K.; Shinohara, I. Photo-regulated binding ability of a polymeric adsorbent containing a spiro[2H-chromen-2,2’-indoline] moiety. Makromol. Chem.-Rapid 1981, 2, 617–620. [Google Scholar] [CrossRef]
- Malcolm, B.R.; Pieroni, O. The photo-response of an azobenzene-containing poly(L-lysine) in the monolayer state. Biopolymers 1990, 29, 1121–1123. [Google Scholar] [CrossRef]
- Morishima, Y.; Tsuji, M.; Kamachi, M.; Hatada, K. Photo-chromic isomerization of azobenzene moieties compartmentalized in hydrophobic microdomains in a microphase structure of amphiphilic polyelectrolytes. Macromolecules 1992, 25, 4406–4410. [Google Scholar] [CrossRef]
- Crano, J.C.; Flood, T.; Knowles, D.; Kumar, A.; van Gemert, B. Photocromic compounds: Chemistry and application in ophthalmic lenses. Pure Appl. Chem. 1996, 68, 1395–1398. [Google Scholar] [CrossRef]
- Minkin, V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem. Rev. 2004, 104, 2751–2776. [Google Scholar] [CrossRef]
- Radu, A.; Byrne, R.; Alhashimy, N.; Fusaro, M.; Scarmagnani, S.; Diamond, D. N,S co-doped and N-doped Degussa P-25 powders with visible light response prepared by mechanical mixing of thiourea and urea. Reactivity towards E. coli inactivation and phenol oxidation. J. Photoc. Photobio A 2009, 206, 109–115. [Google Scholar] [CrossRef]
- Gorner, H.; Chibisov, A.K. Complexes of spiropyran-derived merocyanines with metal ions thermally activated and light-induced processes. J. Chem. Soc. Faraday T. 1998, 94, 2557–2564. [Google Scholar] [CrossRef]
- Andersson, J.; Li, S.; Lincoln, P.; Andreasson, J. Photoswitched DNA-binding of a photochromic spiropyran. J. Am. Chem. Soc. 2008, 130, 11836–11837. [Google Scholar]
- Ipe, B.I.; Mahima, S.; Thomas, K.G. Light-induced modulation of self-assembly on spiropyran-capped gold nanoparticles: A potential system for the controlled release of amino acid derivatives. J. Am. Chem. Soc. 2003, 125, 7174–7175. [Google Scholar]
- Scarmagnani, S.; Slater, C.; Benito-Lopez, F.; Diamond, D.; Walsh, Z.; Paull, B.; Macka, M. Photoreversible ion-binding using spiropyran modified silica microbeads. Int. J. Nanomanufacturing 2010, 5, 38–52. [Google Scholar] [CrossRef]
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed; Clarendon Press: Oxford, UK, 1993. [Google Scholar]
- Seki, T.; Tamaki, T.; Suzuki, Y.; Kawanishi, K.; Ichimura, K.; Aoki, K. Photo-chemical alignment regulation of a nematic liquid crystal by Langmuir-Blodgett layers of azobenzene polymers as “command surfaces”. Macromolecules 1989, 22, 3505–3506. [Google Scholar] [CrossRef]
- Seki, T.; Fukuda, R.; Tamakia, T.; Ichimura, K. Alignment photo-regulation of liquid crystals on precisely area controlled azobenzene Langmuir-Blodgett monolayers. Thin Solid Films 1994, 243, 675–678. [Google Scholar] [CrossRef]
- Seki, T.; Sakuragi, M.; Kawanishi, K.; Suzuki, Y.; Tamaki, T.; Fukuda, R.; Ichimura, K. “Command surfaces” of Langmuir-Blodgett films. Photo-regulation of liquid crystal alignment by molecularly tailored surface azobenzene layers. Langmuir 1993, 9, 211–218. [Google Scholar] [CrossRef]
- Zolot’ko, A.S.; Kitaeva, V.F.; Kroo, N.; Sobolev, N.N.; Chillag, L. The effect of an optical field on the nematic phase of the liquid crystal OCBP. JETP Lett. 1980, 32, 158–162. [Google Scholar]
- Durbin, S.D.; Arakelian, S.M.; Shen, Y.H. Optical-field-induced birefringence and Freedericksz transition in a nematic liquid crystal. Phys. Rev. Lett. 1981, 47, 1411–1414. [Google Scholar] [CrossRef]
- Khoo, I.C. Optically induced molecular reorientation and third-order nonlinear optical processes in nematic liquid crystals. Phys. Rev. A 1981, 28, 2077–2081. [Google Scholar] [CrossRef]
- Janossy, I.; Csillag, L.; Lloyd, A.D. Temperature dependence of the optical Freedericksz transition in dyed nematic liquid crystals. Phys. Rev. A 1991, 44, 8410–8413. [Google Scholar] [CrossRef]
- Zebger, I.; Rutloh, M.; Hoffmann, U.; Stumpe, J.; Siesler, H.W.; Hvilsted, S. Photoorientation of a liquid crystalline polyester with azobenzene side groups.1. Effects of irradiation with linearly polarized blue light. J. Phys. Chem. A 2002, 106, 3454–3462. [Google Scholar]
- Folks, W.R.; Keast, S.; Krentzel, T.A.; Zalar, B.; Zeng, H.; Reznikov, Yu.A.; Neubert, M.; Kumar, S.; Finotello, D.; Lavrentovich, O.D. Photocontrol of smectic spacing. Mol. Cryst. Liq. Cryst. 1998, 320, 77–88. [Google Scholar] [CrossRef]
- Lansac, Y.; Glaser, M.A.; Clark, N.A.; Lavrentovich, O.D. Photocontrolled nanophase segregation in a liquid-crystal solvent. Nature 1999, 398, 54–57. [Google Scholar]
- Voloschenko, D.; Lavrentovich, O.D. Light-induced director-controlled microassembly of dye molecules from a liquid crystal matrix. J. Appl. Phys. 1999, 86, 4843–4846. [Google Scholar] [CrossRef]
- Chandran, S.P.; Mondiot, F.; Mondain-Monval, O.; Loudet, J.C. Photonic control of surface anchoring on solid colloids dispersed in liquid crystals. Langmuir 2011, 27, 15185–15198. [Google Scholar] [CrossRef]
- De Filpo, G.; Nicoletta, F.P.; Chidichimo, G. Cholesteric emulsions for colored displays. Adv. Mater. 2005, 17, 1150–1152. [Google Scholar] [CrossRef]
- Kinoshita, T.; Sato, M.; Takizawa, A.; Tsujita, Y. Photo-control of polypeptide membrane permeabilities by cis-trans isomerism in side chain azobenzene groups. J. Chem. Soc. Chem. Comm. 1984, 14, 929–930. [Google Scholar]
- Kinoshita, T.; Sato, M.; Takizawa, A.; Tsujita, Y. Photo-control of polypeptide membrane functions by cis-trans isomerization in side-chain azobenzene groups. Macromolecules 1986, 19, 51–55. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y. Photo-induced conformational transition of polypeptides containing azobenzenesulfonate in the side chains. Macromolecules 1988, 21, 1612–1616. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y.; Osada, T. Photo-control of polypeptide membrane structure and functions by cis-trans isomerization in side-chain azobenzenesulfonate groups. Polym. J. 1989, 21, 533–541. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y.; Ito, R. Permeability changes of a porous membrane by an adsorbed photo-responsive polypeptide. Polym.J. 1989, 21, 761–769. [Google Scholar]
- Kinoshita, T.; Sato, M.; Takizawa, A.; Tsujita, Y. Photo-induced reversible conformational transition of polypeptide solid membranes. J. Am. Chem. Soc. 1986, 108, 6399–6401. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y. Photo-induced conformational transition of polypeptide membrane composed of poly(L-glutamic acid) containing pararosaniline groups in the side chains. Macromolecules 1988, 21, 3419–3424. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y.; Osada, T. Photo-control of polypeptide membrane functions by photo-dissociation of pararosaniline side-chain groups. Polym. J. 1988, 20, 729–738. [Google Scholar] [CrossRef]
- Sato, M.; Kinoshita, T.; Takizawa, A.; Tsujita, Y.; Osada, T. Photo-control of the structure and functions of a polypeptide membrane composed of poly(L-glutamic acid) containing pararosaniline leucocyanide groups in the side-chains. Polym. J. 1989, 21, 369–376. [Google Scholar] [CrossRef]
- Aoyama, M.; Youda, A.; Watanabe, J.; Inoue, S. Synthesis and circular dichroic and photo-responsive properties of a graft copolymer containing an azoaromatic polypeptide branch and its membrane. Macromolecules 1990, 23, 1458–1463. [Google Scholar] [CrossRef]
- Aoyama, M.; Watanabe, J.; Inoue, S. Photo-regulation of permeability across a membrane from graft copolymer containing a photo-responsive polypeptide branch. J. Am. Chem. Soc. 1990, 112, 5542–5545. [Google Scholar] [CrossRef]
- Higuchi, M.; Kinoshita, T. Photo-responsive behavior of self-assembling systems by amphiphilic α-helix with azobenzene unit. J. Photochem. Photobiol. B Biol. 1998, 42, 143–150. [Google Scholar] [CrossRef]
- Higuchi, M.; Minoura, N.; Kinoshita, T. Photo-control of molecular orientation of a photo-responsive amphiphilic α-helix in a lipid monolayer. Langmuir 1997, 13, 1616–1622. [Google Scholar] [CrossRef]
- Klajn, R. Immobilized azobenzenes for the construction of photoresponsive materials. Pure Appl. Chem. 2010, 82, 2247–2279. [Google Scholar] [CrossRef]
- Han, M.; Ishikawa, D.; Honda, T.; Ito, E.; Hara, M. Light-driven molecular switches in azobenzene self-assembled monolayers: effect of molecular structure on reversible photoisomerization and stable cis state. Chem. Commun. 2010, 46, 3598–3600. [Google Scholar]
- Takamatsu, D.; Fukui, K.; Aroua, S.; Yamakoshi, Y. Photoswitching tripodal single molecular tip for noncontact AFM measurements: synthesis, immobilization, and reversible configurational change on gold surface. Org. Biomol. Chem. 2010, 8, 3655–3664. [Google Scholar] [CrossRef]
- Tamada, K.; Akiyama, H.; Wei, T.X.; Kim, S.A. Photoisomerization reaction of unsymmetrical azobenzene disulfide self-assembled monolayers: modification of azobenzene dyes to improve thermal endurance for photoreaction. Langmuir 2003, 19, 2306–2312. [Google Scholar] [CrossRef]
- Nagahiro, T.; Akiyama, H.; Hara, M.; Tamada, K. Photoisomerization of azobenzene containing self-assembled monolayers investigated by Kelvin probe work function measurements. J. Electron Spectrosc. Relat. Phenom. 2009, 172, 128–133. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Schouten, A.; Kroon, J. Structural studies of the system trans-azobenzene /trans-stilbene. I. A reinvestigation of the disorder in the crystal structure of trans-azobenzene, C12H10N2. ACTA Crystallogra. A 1983, 39, 1121–1123. [Google Scholar]
- Jung, U.; Muller, M.; Fujimoto, N.; Ikeda, K.; Uosaki, K.; Cornelissen, U.; Tuczek, F.; Bornholdt, C.; Zargarani, D.; Herges, R.; Magnussen, O. Gap-mode SERS studies of azobenzene-containing self-assembled monolayers on Au(111). J. Colloid Interf. Sci. 2010, 341, 366–375. [Google Scholar] [CrossRef]
- Good, R.J.; van Oss, C.J. The modern theory of contact angles and the hydrogen bond components of surface energies. In Modern Approaches to Wettability; Schrader, M.E., Loeb, G.L., Eds.; Plenum Press: New York, NY, USA, 1992. [Google Scholar]
- Siewierski, L.M.; Brittain, W.J.; Pdtrash, S.; Foster, M.D. Photoresponsive monolayers containing in-chain azobenzene. Langmuir 1996, 12, 5838–5844. [Google Scholar] [CrossRef]
- Tylkowski, B.; Peris, S.; Giamberini, M.; Garcia-Valls, R.; Reina, J.A.; Ronda, J.C. Light-induced switching of the wettability of novel asymmetrical poly(vinyl alcohol)-co-ethylene membranes blended with azobenzene polymers. Langmuir 2010, 26, 14821–14829. [Google Scholar] [CrossRef]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Nayak, A.; Liu, H.; Belfort, G. An optically reversible switching membrane surface. Angew. Chem. Int. Ed. 2006, 45, 4094–4098. [Google Scholar] [CrossRef]
- Vlassiouk, I.; Park, C.D.; Vail, S.A.; Gust, D.; Smirnov, S. Control of nanopore wetting by a photocromic spiropyran—A light-controlled valve and electrical switch. Nano Lett. 2006, 6, 1013–1017. [Google Scholar] [CrossRef]
- Uchida, K.; Izumi, N.; Sukata, S.; Kojima, Y.; Nakamura, S.; Irie, M. Photoinduced reversible formation of microfibrils on a photochromic diarylethene microcrystalline surface. Angew. Chem. Int. Ed. 2006, 45, 6470–6473. [Google Scholar] [CrossRef]
- Lim, H.S.; Han, J.T.; Kwak, D.; Jin, M.; Cho, K. Photoreversibly switchable superhydrophobic surface with erasable and rewritable pattern. J. Am. Chem. Soc. 2006, 128, 14458–14459. [Google Scholar] [CrossRef]
- Berna, J.; Leigh, D.; Lubomska, M.; Mendoza, S.; Perez, E.; Rudolf, P.; Teobaldi, G.; Zerbetto, F. Macroscopic transport by synthetic molecular machines. Nat. Mater. 2005, 4, 704–710. [Google Scholar] [CrossRef]
- Delorme, N.; Bardeau, J.; Bulou, A.; Poncin-Epaillard, F. Azobenzene-containing monolayer with photoswitchable wettability. Langmuir 2005, 21, 12278–12282. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, G.; He, Y.; Wang, X.; An, Y.; Song, Y.; Jiang, L. Photo-switched wettability on an electrostatic self-assembly azobenzene monolayer. Chem. Commun. 2005, 28, 3550–3552. [Google Scholar]
- Ichimura, K.; Oh, S.K.; Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef]
- Yang, D.; Piech, M.; Bell, N.S.; Gust, D.; Vail, S.; Garcia, A.A.; Schneider, J.; Park, C.D.; Hayes, M.A.; Picraux, S.T. Photon control of liquid motion on reversibly photoresponsive surfaces. Langmuir 2007, 23, 10864–10872. [Google Scholar]
- Kumar, S.K.; Hong, J.-D. Photoresponsive ion gating function of an azobenzene polyelectrolyte multilayer spin-self-assembled on a nanoporous support. Langmuir 2008, 24, 4190–4193. [Google Scholar] [CrossRef]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive properties of poly(N-isopropylacrylamide) hydrogel partly modified with spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef]
- Zhou, X.F.; Yu, C.Z.; Tang, J.W.; Yan, X.X.; Zhao, D.Y. The effect of water content on the preparation of mesoporous monoliths and films. Micropo. Mesopor. Mat. 2005, 79, 283–289. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Maeda, K.; Nishiyama, T.; Yamazaki, T.; Suzuki, T.; Seki, T. Reversible photoswitching liquid-phase adsorption on azobenzene derivative-grafted mesoporous silica. Chem. Lett. 2006, 35, 736–737. [Google Scholar] [CrossRef]
- Ogawa, M.; Ishii, T.; Miyamoto, N.; Kuroda, K. Photocontrol of the basal spacing of azobenzene-magadiite intercalation compound. Adv. Mater. 2001, 13, 1107–1109. [Google Scholar] [CrossRef]
- Alvaro, M.; Ferrer, B.; Garcia, H.; Rey, F. Photochemical modification of the surface area and tortuosity of a trans-1,2-bis(4-pyridyl)ethylene periodic mesoporous MCM organosilica. Chem. Commun. 2002, 18, 2012–2013. [Google Scholar]
- Liu, N.; Chen, Z.; Dunphy, D.R.; Jiang, Y.B.; Assink, R.A.; Brinker, C.J. Photoresponsive nanocomposite formed by self-assembly of an azobenzene-modified silane. Angew. Chem. Int. 2003, 42, 1731–1734. [Google Scholar] [CrossRef]
- Liu, N.; Dunphy, D.R.; Atanassov, P.; Bunge, S.D.; Chen, Z.; Lopez, G.P.; Boyle, T.J.; Brinker, J. Photoregulation of mass transport through a photoresponsive azobenzene-modified nanoporous membrane. Nano Lett. 2004, 4, 551–554. [Google Scholar] [CrossRef]
- Banghart, M.R.; Volgraf, M.; Trauner, D. Engeneering light-gated ion channels. Biochemistry 2006, 45, 15129–15141. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membrane Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Sata, T.; Matsusaki, K. Generation of light-induced electrical potential from ion exchange membranes containing 4,4’-bipyridine moiety II. Effect of species of anion exchange membranes on photovoltage. J. Polym. Sci. Polym. Chem. Ed. 1996, 34, 2123–2134. [Google Scholar] [CrossRef]
- Sata, T.; Matsuo, Y.; Yamaguchi, T.; Matsusaki, K. Preparation and transport properties of anion-exchange membranes containing viologen moieties as anion exchange groups in the presence or absence of photoirradiation. J. Chem. Soc. Faraday Trans. 1997, 93, 2553–2560. [Google Scholar] [CrossRef]
- Sata, T.; Shimokawa, Y.; Matsusaki, K. Preparation of ion-permeable membranes having an azobenzene moiety and their transport properties in electrodialysis. J. Membrane Sci. 2000, 171, 31–43. [Google Scholar] [CrossRef]
- Byrne, R.; Ventura, C.; Lopez, F.B.; Walther, A.; Heise, A.; Diamond, D. Characterisation and analytical potential of a photo-responsive polymeric material based on spiropyran. Biosens. Bioelectron. 2010, 26, 1392–1398. [Google Scholar] [CrossRef]
- Byrne, R.; Diamond, D. Chemo/bio-sensor networks. Nat. Mater. 2006, 5, 421–424. [Google Scholar] [CrossRef]
- Zakharova, M.I.; Coudret, C.; Pimienta, V.; Micheau, J.C.; Delbaere, S.; Vermeersch, G.; Metelitsa, A.V.; Voloshin, N.; Minkin, V.I. Quantitative investigations of cation complexation of photochromic 8-benzothiazole-substituted benzopyran: Towards metal-ion sensors. Photochem. Photobiol. Sci. 2010, 9, 199–207. [Google Scholar] [CrossRef]
- Fries, K.H.; Driskell, J.D.; Samanta, S.; Locklin, J. Spectroscopic analysis of metal ion binding in spiropyran containing copolymer thin films. Anal. Chem. 2010, 82, 3306–3314. [Google Scholar] [CrossRef]
- Ito, Y.; Park, Y.S. Signal-responsive gating of porous membranes by polymer brushes. Polym. Adv. Technol. 2000, 11, 136–144. [Google Scholar] [CrossRef]
- Irie, M.; Menju, A.; Hayashi, K. Photoresponsive polymers: Reversible solution viscosity change of poly(methyl methacrylate) having spirobenzopyran side groups. Macromolecules 1979, 12, 1176–1180. [Google Scholar] [CrossRef]
- Irie, M.; Iwanaga, T.; Taniguchi, Y. Photoresponsive polymers. 7. Reversible solubility change of polystyrene having pendant spirobenzopyran groups and its application to photoresists. Macromolecules 1985, 18, 2418–2422. [Google Scholar] [CrossRef]
- Balazs, A.C.; Kuksenok, O.; Alexeev, A. Modeling the interactions between membranes and inclusions: Designing self-cleaning films and resealing pores. Macromol. Theor. Simul. 2009, 18, 11–24. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M.; Ruhmann, R.; Hoffmann, K.; Toussaint, P.; Caro, J. Modification of the transport properties of a polymethacrylate-azobenzene membrane by photochemical switching. Chem. Eng. Technol. 1998, 21, 408–412. [Google Scholar] [CrossRef]
- Okahata, Y.; Lim, H.; Hachiya, S. Bilayer coated capsule membranes. Part 2. Photoresponsive permeability control of sodium chloride across a capsule membrane. J. Chem. Soc. Perkin Trans. 2 1984, 6, 989–994. [Google Scholar]
- Balasubramanian, D.; Subramani, S.; Kumar, C. Dopamine inhibits adenylate cyclase in human prolactin-secreting pituitary adenomas. Nature 1975, 254, 252–254. [Google Scholar] [CrossRef]
- Tachibana, H.; Nakamura, T.; Matsumoto, M.; Komizu, H.; Manda, E.; Niino, H.; Yabe, A.; Kawabata, Y. Photochemical switching in conductive Langmuir-Blodgett films. J. Am. Chem. Soc. 1989, 111, 3080–3081. [Google Scholar] [CrossRef]
- Anzai, J.; Osa, T. Photosensitive artificial membranes based on azobenzene and spirobenzopyran derivatives. Tetrahedron 1994, 50, 4039–4070. [Google Scholar] [CrossRef]
- Pyrasch, M.; Toutianoush, A.; Jin, W.; Schnepf, J.; Tieke, B. Self-assembled films of Prussian blue and analogues: Optical and electrochemical properties and application as ion-sieving membranes. Chem. Mater. 2003, 15, 245–254. [Google Scholar] [CrossRef]
- Balachandra, A.M.; Dai, J.; Bruening, M.L. Enhancing the anion-transport selectivity of multilayer polyelectrolyte membranes by templating with Cu2+. Macromolecules 2002, 35, 3171–3178. [Google Scholar] [CrossRef]
- Hong, S.U.; Malaisamy, R.; Bruening, M.L. Separation of fluoride from other monovalent anions using multilayer polyelectrolyte nanofiltration membranes. Langmuir 2007, 23, 1716–1722. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Bruening, M.L. Ultrathin, gas-selective polyimide membranes prepared from multilayer polyelectrolyte films. Chem. Mater. 2003, 15, 281–287. [Google Scholar] [CrossRef]
- Krasemann, L.; Tieke, B. Ultrathin self-assembled polyelectrolyte membranes for pervaporation. J. Membr. Sci. 1998, 150, 23–30. [Google Scholar] [CrossRef]
- Sun, L.; Baker, G.L.; Bruening, M.L. Polymer brush membranes for pervaporation of organic solvents from water. Macromolecules 2005, 38, 2307–2314. [Google Scholar] [CrossRef]
- Jin, W.; Toutianoush, A.; Tieke, B. Use of polyelectrolyte layer-by-layer assemblies as nanofiltration and reverse osmosis membranes. Langmuir 2003, 19, 2550–2553. [Google Scholar] [CrossRef]
- Hiller, J.; Rubner, M.F. Reversible molecular memory and pH-switchable swelling transitions in polyelectrolyte multilayers. Macromolecules 2003, 36, 4078–4083. [Google Scholar] [CrossRef]
- Park, M.-K.; Deng, S.; Advincula, R.C. pH-sensitive bipolar ion-permselective ultrathin films. J. Am. Chem. Soc. 2004, 126, 13723–13731. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B.; Mohwald, H. Influence of the ionic strength on the polyelectrolyte multilayers permeability. Langmuir 2003, 19, 2444–2448. [Google Scholar]
- Ito, Y.; Inaba, M.; Chung, D.J.; Imanishi, Y. Control of water permeation by pH and ionic strength through a porous membrane having poly(carboxylic acid) surface-grafted. Macromolecules 1992, 25, 7313–7316. [Google Scholar] [CrossRef]
- Jaber, J.A.; Schlenoff, J.B. Polyelectrolyte multilayers with reversible thermal responsivity. Macromolecules 2005, 38, 1300–1306. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Tyutina, J.; Sukhishvili, S.A. Hydrogen-bonded multilayers of thermoresponsive polymers. Macromolecules 2005, 38, 10523–10531. [Google Scholar] [CrossRef]
- Kocer, A.; Walko, M.; Meijberg, W.; Feringa, B.L. A light-actuated nanovalve derived from a channel protein. Science 2005, 309, 755–758. [Google Scholar] [CrossRef]
- Hamada, T.; Sugimoto, R.; Vestergaard, M.C.; Nagasaki, T.; Takagi, M. Membrane disk and sphere: Controllable mesoscopic structure for the capture and release of a targeted object. J. Am. Chem. 2010, 132, 10528–10532. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Fernando, G.F.; Sabey, C.; Winter, D.; Badcock, R.A.; Akhavan, J.; Krofli, E. Synthesis and characterization of a novel class of photo-actuating and photo-rheological polymers. Proc. SPIE-Int. Soc. Opt. Eng. 2004, 5464, 209–220. [Google Scholar]
- Ueda, M.; Kudo, K.; Ichimura, K. Photo-chromic behavior of a spirobenzopyran chemisorbed on a colloidal silica surface. J. Mater. Chem. 1995, 5, 1007–1011. [Google Scholar] [CrossRef]
- Ueda, M.; Kim, H.-B.; Ichimura, K. Photo-controlled aggregation of colloidal silica. J. Mater. Chem. 1994, 4, 883–889. [Google Scholar] [CrossRef]
- Bell, N.S.; Piech, M. Photo-physical effects between spirobenzopyran-methyl methacrylate-functionalized colloidal particles. Langmuir 2006, 22, 1420–1427. [Google Scholar] [CrossRef]
- Rosario, R.; Gust, D.; Garcia, A.A.; Hayes, M.; Taraci, J.L.; Clement, T.; Dailey, J.W.; Picraux, S.T. Lotus effect amplifies light-induced contact angle switching. J. Phys. Chem. B 2004, 108, 12640–12642. [Google Scholar]
- And, E.; Miyazaki, J.; Moriomoto, K.J. Aggregation of photo-chromic spiropyran in Langmuir-Blodgett-films. Thin Solid Films 1985, 133, 21–28. [Google Scholar] [CrossRef]
- Park, Y.S.; Ito, Y.; Imanishi, Y. Photo-controlled gating by polymer brushes grafted on porous glass filter. Macromolecules 1998, 31, 2606–2610. [Google Scholar] [CrossRef]
- Chung, D.J.; Ito, Y.; Imanishi, Y. Preparation of porous membranes grafted with poly(spiropyran-containing methacrylate) and photo-control of permeability. J. Appl. Polym. Sci. 1994, 51, 2027–2033. [Google Scholar] [CrossRef]
- Kalisky, Y.; Williams, D.J. Laser photo-lysis studies of photo-induced aggregation in polymers containing spiropyran units. Macromolecules 1984, 17, 292–296. [Google Scholar] [CrossRef]
- Kimura, K.; Sumida, M.; Yokoyama, M. Drastic metal-ion enhancement in photo-induced aggregation of copolymers carrying crown ether and spirobenzopyran moieties. Chem. Commun. 1997, 15, 1417–1418. [Google Scholar]
- Kimura, K.; Sakamoto, H.; Nakamura, T. Application of photo-responsive polymers carrying crown ether and spirobenzopyran side chains to photo-chemical valve. J. Nanosci. Nanotechnol. 2006, 6, 1741–1749. [Google Scholar] [CrossRef]
- Krongauz, V.A.; Goldburt, E.S. Crystallization of poly(spiropyran methacrylate) with cooperative spiropyran-merocyanine conversion. Macromolecules 1981, 14, 1382–1386. [Google Scholar] [CrossRef]
- Fyles, T.M. Electrostatic ion binding by synthetic receptors. In Cation Binding by Macrocycles: Complexation of Cationic Species by Crown Ethers; Inoue, Y., Gokel, G.W., Eds.; Marcel-Dekker: New York, NY, USA, 1990; pp. 203–251. [Google Scholar]
- Shinkai, S. Dynamic control of cation binding. In Cation Binding by Macrocycles: Complexation of Cationic Species by Crown Ethers; Inoue, Y., Gokel, G.W., Eds.; Marcel-Dekker: New York, NY, USA, 1990; pp. 397–428. [Google Scholar]
- Sakamoto, H.; Takagaki, H.; Nakamura, M. Liquid-liquid extraction of alkali metal ions with photo-chromic crowned spirobenzopyrans. Anal. Chem. 2005, 77, 1999–2006. [Google Scholar] [CrossRef]
- Sakamoto, H.; Yokohata, T.; Yamakura, T.; Kimura, K. Liquid-liquid extraction of alkali metal ions with photo-chromic crowned spirobenzopyrans. Anal. Chem. 2002, 74, 2522–2528. [Google Scholar] [CrossRef]
- Nakamura, M.; Takahashi, K.; Fujioka, T.; Kado, S.; Sakamoto, H.; Kimura, K. Evaluation of photo-induced changes in stability constants for metal-ion complexes of crowned spirobenzopyran derivatives by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1110–1115. [Google Scholar] [CrossRef]
- Haupt, K.; Mosbach, K. Molecularly imprinted polymers and their use in biomimetic sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Wulff, G. Enzyme-like catalysis by molecularly imprinted polymers. Chem. Rev. 2002, 102, 1–27. [Google Scholar] [CrossRef]
- Hilt, J.Z.; Byrne, M.E. Configurational biomimesis in drug delivery: Molecular imprinting of biologically significant molecules. Adv. Drug Deliv. Rev. 2004, 56, 1599–1620. [Google Scholar] [CrossRef]
- Alexander, C.; Andersson, H.S.; Andersson, L.I.; Ansell, R.J.; Kirsch, N.; Nicholls, I.A.; O’Mahony, J.; Whitcombe, M.J. Molecular imprinting science and technology: A survey of the literature for the years up to and including 2003. J. Mol. Recognit. 2006, 19, 106–180. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, L.; Mosbach, K. Non-covalent molecular imprinting with emphasis on its application in separation and drug development. J. Mol. Recognit. 2006, 19, 248–259. [Google Scholar] [CrossRef]
- Ye, L.; Mosbach, K. Molecular imprinting: Synthetic materials as substitutes for biological antibodies and receptors. Chem. Mater. 2008, 20, 859–868. [Google Scholar] [CrossRef]
- Sellergren, B. Molecularly imprinted polymers: Shaping enzyme inhibitors. Nat. Chem. 2010, 2, 7–8. [Google Scholar] [CrossRef]
- Andersson, L.I. Molecular imprinting: Developments and applications in the analytical chemistry field. J. Chromatogr. B 2000, 745, 3–13. [Google Scholar] [CrossRef]
- Sellergren, B. Imprinted polymers with memory for small molecules, proteins, or crystals. Angew. Chem. Int. Ed. Engl. 2000, 39, 1031–1037. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Sellergren, B.; Hall, A.J. In Molecularly Imprinted Polymers: Man-Made Mimics of Antibodies and Their Applications in Analytical Chemistry; Sellergren, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2001; Chapter 4. [Google Scholar]
- Zhang, H.; Piacham, T.; Drew, M.; Patek, M.; Mosbach, K.; Ye, L. Molecularly imprinted nanoreactors for regioselective huisgen 1,3-dipolar cycloaddition reaction. J. Am. Chem. Soc. 2006, 128, 4178–4179. [Google Scholar]
- Liu, J.Q.; Wulff, G. Functional mimicry of carboxypeptidase A by a combination of transition state stabilization and a defined orientation of catalytic moieties in molecularly imprinted polymers. J. Am. Chem. Soc. 2008, 130, 8044–8054. [Google Scholar] [CrossRef]
- Cutivet, A.; Schembri, C.; Kovensky, J.; Haupt, K. Molecularly imprinted microgels as enzyme inhibitors. J. Am. Chem. Soc. 2009, 131, 14699–14702. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Picci, N. Stimuli-responsive molecularly imprinted polymers for drug delivery: A review. Curr. Drug Deliv. 2008, 5, 85–96. [Google Scholar] [CrossRef]
- El Khoury, J.M.; Zhou, X.L.; Qu, L.T.; Dai, L.M.; Urbas, A.; Li, Q. Organo-soluble photoresponsive azo thiol monolayer-protected gold nanorods. Chem. Commun. 2009, 16, 2109–2111. [Google Scholar]
- Gong, C.B.; Wong, K.L.; Lam, M.H.-W. Photoresponsive molecularly imprinted hydrogels for the photoregulated release and uptake of pharmaceuticals in the aqueous media. Chem. Mater. 2008, 20, 1353–1358. [Google Scholar] [CrossRef]
- Gomy, C.; Schmitzer, A.R. Synthesis and photoresponsive properties of a molecularly imprinted polymer. Org. Lett. 2007, 9, 3865–3868. [Google Scholar] [CrossRef]
- Kempe, H.; Kempe, M. Development and evaluation of spherical molecularly imprinted polymer beads. Anal. Chem. 2006, 78, 3659–3666. [Google Scholar] [CrossRef]
- Takeuchi, T.; Akeda, K.; Murakami, S.; Shinmori, H.; Inoue, S.; Lee, W.S.; Hishiya, T. Photoresponsive porphyrin-imprinted polymers prepared using a novel functional monomer having diaminopyridine and azobenzene moieties. Org. Biomol. Chem. 2007, 5, 2368–2374. [Google Scholar] [CrossRef]
- Yagai, S.; Karatsu, T.; Kitamura, A. Photocontrollable self-assembly. Chem. Eur. J. 2005, 11, 4054–4063. [Google Scholar] [CrossRef]
- Minoura, N.; Idei, K.; Rachkov, A.; Uzawa, H.; Matsuda, K. Molecularly imprinted polymer membranes with photoregulated template binding. Chem. Mater. 2003, 15, 4703–4704. [Google Scholar] [CrossRef]
- Minoura, N.; Idei, K.; Rachkov, A.; Choi, Y.; Ogiso, M.; Matsuda, K. Binding efficiency and transport properties of molecularly imprinted polymer thin films. Macromolecules 2004, 37, 9571–9576. [Google Scholar] [CrossRef]
- Marx-Tibbon, S.; Willner, I. Photostimulated imprinted polymers: A light-regulated medium for transport of amino acids. J. Chem. Soc., Chem. Commun. 1994, 10, 1261–1262. [Google Scholar] [CrossRef]
- Gong, C.; Lam, M.H.; Yu, H. The fabrication of a photoresponsive molecularly imprinted polymer for the photoregulated uptake and release of caffeine. Adv. Funct. Mater. 2006, 16, 1759–1767. [Google Scholar] [CrossRef]
- Stephens, D.A.; Bohn, P.W. Absorption spectrometry of bound monolayers on integrated optical structures. Anal. Chem. 1989, 61, 386–390. [Google Scholar] [CrossRef]
- Matsuda, N.; Takatsu, A.; Kato, K. Absorption spectra of rhodamine 6G by slab optical waveguide spectroscopy. Chem. Lett. 1996, 2, 105–106. [Google Scholar]
- Mendes, S.B.; Li, L.; Burke, J.J. Broad-band attenuated total reflection spectroscopy of a hydrated protein film on a single mode planar waveguide. Langmuir 1996, 12, 3374–3376. [Google Scholar] [CrossRef]
- Mitsuishi, M.; Tanuma, T.; Matsui, J.; Chen, J.; Miyashita, T. In situ monitoring of photo-cross-linking reaction of anthracene chromophores in polymer Langmuir-Blodgett films by an integrated optical waveguide technique. Langmuir 2001, 17, 7449–7451. [Google Scholar] [CrossRef]
- Bradshaw, J.T.; Mendes, S.B.; Armstrong, N.R.; Saavedra, S.S. Broadband coupling into a single-mode, electroactive integrated optical waveguide for spectroelectrochemical analysis of surface-confined redox couples. Anal. Chem. 2003, 75, 1080–1088. [Google Scholar] [CrossRef]
- Ogawa, K.; Harada, J.; Fujiwara, T.; Takahashi, H. UV–vis absorption spectra of powdered materials: Direct measurements by optical waveguide spectroscopy. Chem. Lett. 2004, 33, 1446–1447. [Google Scholar] [CrossRef]
- Fujita, K.; Taniguchi, K.; Ohno, H. Dynamic analysis of aggregation of methylene blue with polarized optical waveguide spectroscopy. Talanta 2005, 65, 1066–1070. [Google Scholar] [CrossRef]
- Fang, L.; Chen, S.; Zhang, Y.; Zhang, H. Azobenzene-containing molecularly imprinted polymer microspheres with photoresponsive template binding properties. J. Mater. Chem. 2011, 21, 2320–2329. [Google Scholar] [CrossRef]
- Jiang, G.S.; Zhomg, S.A.; Chen, L.; Blakey, I.; Whitaker, A. Synthesis of molecularly imprinted organic–inorganic hybrid azobenzene materials by sol–gel for radiation induced selective recognition of 2,4-dichlorophenoxyacetic acid. Radiat. Phys. and Chem. 2011, 80, 130–135. [Google Scholar]
- Gehrke, S.H. Mass transfer in pH-sensitive hydrogels. Chem. Eng. Sci. 1989, 44, 559–566. [Google Scholar] [CrossRef]
- Bell, C.L.; Peppas, N.A. Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-gethylene glycol). Biomaterials 1996, 17, 1203–1218. [Google Scholar] [CrossRef]
- Gudeman, L.F.; Peppas, N.A. pH-sensitive membranes from poly(vinyl alcohol)/poly(acrylic acid) interpenetrating networks. J. Membrane Sci. 1995, 107, 239–248. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv.Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hariharan, D.; Khare, A.R. Bioresponsive hydrogels for controlled release of solutes. Polym. Mater. Sci. Eng. 1992, 66, 83–84. [Google Scholar]
- Brazel, C.S.; Peppas, N.A. Temperature and pH-sensitive hydrogels for controlled release of antithrombogenic agents. Mater. Res. Soc. Symp. Proc. 1994, 331, 211–216. [Google Scholar]
- Hoffman, A.S. Intelligent’ polymers in medicine and biotechnology. Artif. Organs 1995, 19, 458–467. [Google Scholar] [CrossRef]
- Galaev, I.Y.; Mattiasson, B. Thermoreactive water-soluble polymers, nonionic surfactants, and hydrogels as reagents in biotechnology. Enzym. Microb. Tech. 1993, 15, 354–366. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Thermal cycling effects on the bioreactor performances of immobilized b-galactosidase in temperature-sensitive hydrogel beads. Enzym. Microb. Tech. 1993, 15, 476–482. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Chemical and physical structure of polymers as carriers for controlled release of bioactive agents: a review. JMS-Rev. Macromol. Chem. Phys. 1983, 23, 61–126. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Beckman, E.; Russel, A.J. Photoscissable hydrogel synthesis via rapid photopolymerization of novel PEG-based polymers in the absence of photoinitiators. J. Am. Chem. Soc. 1996, 118, 6235–6240. [Google Scholar] [CrossRef]
- Andreopoulos, F.M.; Beckman, E.; Russel, A.J. Light-induced tailoring of PEG-hydrogel properties. Biomaterials 1998, 19, 1343–1352. [Google Scholar] [CrossRef]
- Kodzwa, M.G.; Staben, M.E.; Rethwisch, D.G. Photoresponsive control of ion-exchange in leucohydroxide containing hydrogel membranes. J. Membrane Sci. 1999, 158, 85–92. [Google Scholar] [CrossRef]
- Irie, M.; Kunwatchakun, D. Photoresponsive polymer 8. Reversible photostimulated dilation of polyacrylamide gels having triphenilmethane leuco-derivate. Macromolecules 1986, 19, 2476–2480. [Google Scholar] [CrossRef]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Biomacromolecules 1990, 19, 1517–1519. [Google Scholar]
- Ishikawa, M.; Kitamura, N.; Masuhara, H.; Irie, M. Size effect on photoinduced volume change of polyacrylamide microgels containg triphenylmathane leucoderivates. Makromol. Chem. Rapid Commun. 1991, 12, 887. [Google Scholar]
- Tanaka, T.; Fillmore, D.; Sun, S.T.; Nishio, I.; Swislow, G.; Shah, A. Phase transitions in ionic gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Sasaki, H.; Ueno, A.; Osa, T. Photoinduced extraction and active transport of anions by a triphenylmethane derivative. Bull. Chem. Soc. 1988, 61, 2325–2326. [Google Scholar]
- Willner, I.; Sussan, S.; Rubin, S. Photostimulated transport of carboxylate and phenolate anions across a liquid-liquid membrane using a photochromic cationic copolymer as carrier. J. Chem. Soc. Chem. Commun. 1992, 2, 100–101. [Google Scholar]
- Willner, I.; Rubin, S.; Shatzmiller, R.; Zor, T. Reversible light stimulated activation and deactivation of α-chymotrypsin by its immobilization in photoisomerizable copolymers. J. Am. Chem. Soc. 1993, 115, 8690–8694. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komori, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and light control of the sol-gel phase transition in cholesterol-based organic gels. Novel helical aggregation modes as detected by circular dichroism and electron microscopic observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Mamiya, J.; Kanie, K.; Hiyama, T.; Ikeda, T.; Kato, T. A rodlike organogelator: Fibrous aggregation of azobenzene derivatives with a syn-chiral carbonate moiety. Chem. Commun. 2002, 17, 1870–1871. [Google Scholar]
- Moriyama, M.; Mizoshita, M.; Yokota, T.; Kishimoto, K.; Kato, T. Photoresponsive anisotropic soft solids: Liquid-crystalline physical gels based on a chiral photochromic gelator. Adv. Mater. 2003, 15, 1335–1338. [Google Scholar]
- Yagai, S.; Nakajima, T.; Kishikawa, K.; Kohmoto, S.; Karatsu, T.; Kitamura, A. Hierarchical organization of photoresponsive hydrogen-bonded rosettes. J. Am. Chem. Soc. 2005, 127, 11134–11139. [Google Scholar] [CrossRef]
- Ishi-I, T.; Shinkai, S. Dye-based organogels: Stimuli-responsive soft materials based on one-dimensional self-assembling aromatic dyes. Top. Curr. Chem. 2005, 258, 119–160. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Xu, M.; Yi, T.; Xiao, S.Z.; Zhou, Z.G.; Li, F.Y.; Huang, C.H. Morphology-tunable and photoresponsive properties in a self-assembled two-component gel system. Lagmuir 2007, 23, 202–208. [Google Scholar] [CrossRef]
- Deindorfen, P.; Davis, R.; Zentel, R. Photoresponsive anisotropic and isotropic gels of semicarbazide–azobenzene organogelators: The use of magnetic polymer colloids to detect gel–sol transformation. Soft Matter 2007, 3, 1308–1311. [Google Scholar] [CrossRef]
- Kitahara, T.; Fujita, N.; Shinkai, S. Photoresponsive fluorescence color change derived from TICT in an organogel system. Chem. Lett. 2008, 37, 912–920. [Google Scholar] [CrossRef]
- Palui, G.; Banerjee, A. Fluorescent gel from a self-assembling new chromophoric moiety containing azobenzene based tetraamide. J. Phys. Chem. B 2008, 112, 10107–10115. [Google Scholar] [CrossRef]
- Suzuki, T.; Shinkai, S.; Sada, K. Supramolecular crosslinked linear poly(trimethylene iminium trifluorosulfonimide) polymer gels sensitive to light and thermal stimuli. Adv. Mater. 2006, 18, 1043–1046. [Google Scholar] [CrossRef]
- Pouliquen, G.; Tribet, C. Light-triggered association of bovine serum albumin and azobenzene-modified poly(acrylic acid) in dilute and semidilute solutions. Macromolecules 2006, 39, 373–383. [Google Scholar] [CrossRef]
- Ruchmann, J.; Fouilloux, S.; Tribret, C. Light-responsive hydrophobic association of surfactants with azobenzene-modified polymers. Soft Matter 2008, 4, 2098–2108. [Google Scholar] [CrossRef]
- Pouliquen, G.; Amiel, C.; Tribret, C. Photoresponsive viscosity and host−guest association in aqueous mixtures of poly-cyclodextrin with azobenzene-modified poly(acrylic) acid. J. Phys. Chem. B 2007, 111, 5587–5595. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bromberg, L.; Smith, K.A.; Hatton, T.A. Photoresponsive behavior of amphiphilic copolymers of azobenzene and N,N-dimethylacrylamide in aqueous solutions. Langmuir 2009, 25, 3459–3466. [Google Scholar]
- Kasha, M.; Rawls, H.R.; Ashraf El-Bayoumi, M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Pedrosa, J.M.; Romero, M.T.M.; Camacho, L.; Mobius, D. Organization of an amphiphilic azobenzene derivative in monolayers at the air−water interface. J. Phys. Chem. B 2002, 106, 2583–2591. [Google Scholar]
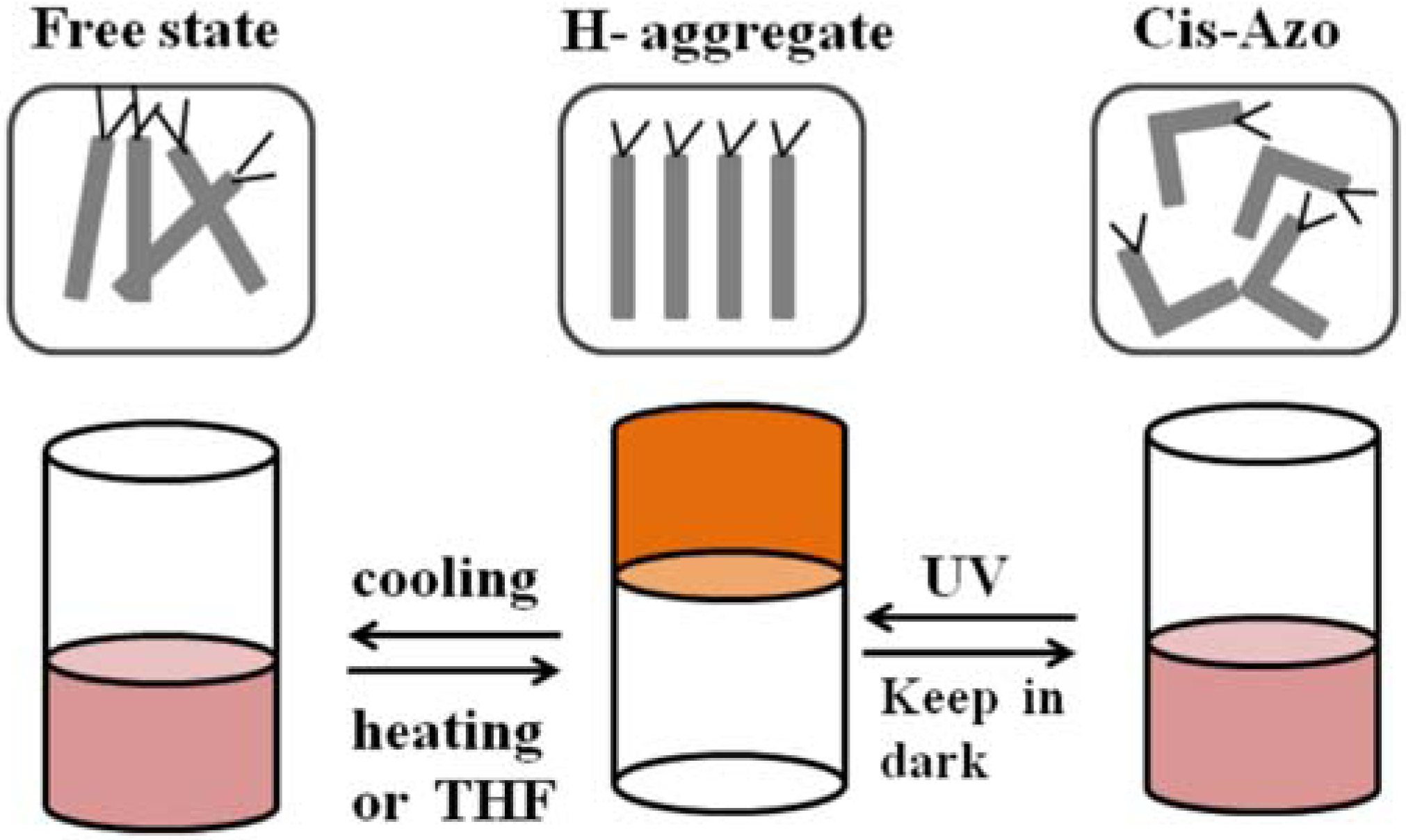
- Deng, Y.; Li, Y.; Wang, X. Colloidal sphere formation, H-aggregation, and photoresponsive properties of an amphiphilic random copolymer bearing branched azo side chain. Macromolecules 2006, 39, 6590–6598. [Google Scholar]
- Whitten, D.G.; Chen, L.H.; Geiger, H.C.; Perlstein, J.; Song, X.D. Self-assembly of aromatic-functionalized amphiphiles: The role and consequences of aromatic-aromatic noncovalent interactions in building supramolecular aggregates and novel assemblies. J. Phys. Chem. B 1998, 102, 10098–10111. [Google Scholar]
- Chen, D.; Liu, H.; Kobayashi, T.; Yu, H. Multiresponsive reversible gels based on a carboxylic azopolymer. J. Mat. Chem. 2010, 20, 3610–3614. [Google Scholar] [CrossRef]
- Sumaru, K.; Kameda, M.; Kanamori, T.; Shinbo, T. Reversible and efficient proton dissociation of spirobenzopyran-functionalized poly(N-isopropylacrylamide) in aqueous solution triggered by light irradiation and temporary temperature rise. Macromolecules 2004, 37, 7854–7856. [Google Scholar] [CrossRef]
- Benito-Lopez, F.; Byrne, R.; Raduta, A.M.; Vrana, N.E.; McGuinness, G.; Diamond, D. Ionogel-based light-actuated valves for controlling liquid flow in microfluidic manifolds. Lab Chip 2010, 10, 195–201. [Google Scholar] [CrossRef]
- Deloncle, R.; Caminade, A.-M. Stimuli-responsive dendritic structure: the case of light-driven azobenzene-containing dendrimers and dendrons. J. Photochem. Photobiol. C photo. 2010, 11, 25–45. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, J.; Yang, Z.; Shi, W. Aggregation behaviors and photo-responsive properties of azobenzene constructed phosphate dendrimers. Polymer 2007, 48, 4466–4481. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Lu, Y.; Ganguli, R.; Drewien, C.A.; Anderson, M.T.; Brinker, C.J.; Gong, W.L.; Guo, Y.X.; Soyez, H.; Dunn, B.; Huang, M.H.; Zink, J.I. Continuous formation of supported cubic and hexagonal mesoporous films by sol gel dip-coating. Nature 1997, 389, 364–368. [Google Scholar] [CrossRef]
- Wirnsberger, G.; Scott, B.J.; Stucky, G.D. pH Sensing with mesoporous thin films. Chem. Commun. 2001, 1, 119–120. [Google Scholar]
- Melosh, N.A.; Lipic, P.; Bates, F.S.; Wudl, F.; Stucky, G.D.; Fredrickson, H.G.; Chmelka, B.F. Molecular and mesoscopic structures of transparent block copolymer-silica monoliths. Macromolecules 1999, 32, 4332–4342. [Google Scholar] [CrossRef]
- Grosso, D.; Balkenende, A.R.; Albouy, P.A.; Ayral, A.; Amenitsch, H.; Babonneau, F. Two-dimensional hexagonal mesoporous silica thin films prepared from block copolymers: Detailed characterization and formation mechanism. Chem. Mater. 2001, 13, 1848–1856. [Google Scholar] [CrossRef]
- Feng, X.; Fryxell, G.E.; Wang, L.-Q.; Kim, A.Y.; Liu, J.; Kemner, K.M. Functionalized monolayers on ordered mesoporous supports. Science 1997, 276, 923–926. [Google Scholar]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Oshuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Grondey, H.; Coombs, N.; Ozin, G.A. Metamorphic channels in periodic mesoporous methylene silica. Angew. Chem. Int. Ed. 2000, 39, 1808–1811. [Google Scholar] [CrossRef]
- MacLachlan, M.J.; Asefa, T.; Ozin, G.A. Writing on the wall with a new synthetic quill. Chem. Eur. J. 2000, 6, 2507–2511. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Nair, S.; Vogt, T.; Dickinson, L.C.; Tsapatsis, M.A. Highly crystalline layered silicate with three-dimensionally microporous layers. Nat. Mater. 2003, 2, 53–58. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M.; Sieber, I.; Caro, J. Permeation of single gases and gas mixtures through faujasite-type molecular sieve membranes. Micropor. Mesopor. Mat. 2002, 54, 27–36. [Google Scholar] [CrossRef]
- Lassinantti, M.; Jareman, F.; Hedlund, J.; Creaser, D.; Sterte, J. Preparation and evaluation of thin ZSM-5 membranes synthesized in the absence of organic template molecules. Catal. Today 2001, 67, 109–119. [Google Scholar]
- Wan, Y.S.S.; Chau, J.L.H.; Gavriilidis, A.; Yeung, K.L. Design and fabrication of zeolite-based microreactors and membrane microseparators. Micropor Mesopor. Mat. 2001, 42, 157–175. [Google Scholar] [CrossRef]
- Hedlund, J.; Noack, M.; Kolsch, P.; Creaser, D.; Caro, J.; Sterte, J. ZSM-5 membranes synthesized without organic templates using a seeding technique. J. Membrane Sci. 1999, 159, 263–273. [Google Scholar] [CrossRef]
- Noack, M.; Kolsch, P.; Venzke, D.; Toussaint, P.; Caro, J. A new one-dimensional-membilkne: aligned AlPO4–5 molecular sieve crystals in a nickel foil. Stud. Surf. Sci. Catal. 1995, 98, 276–277. [Google Scholar] [CrossRef]
- Kolsch, P.; Venzke, D.; Noack, M.; Toussaint, P.; Caro, J. Zeolite-in-metal membranes reparation and testing. J. Chem. Soc. Chem. Commun. 1994, 21, 2491–2492. [Google Scholar]
- Kolsch, P.; Venzke, D.; Noack, M.; Lieske, E.; Toussaint, P.; Caro, J. Preparation and testing of silicalite-in-metal-membranes. Stud. Surf. Sci. Catal. 1994, 84, 1075–1082. [Google Scholar] [CrossRef]
- Noack, M.; Kolsch, P.; Venzke, D.; Toussaint, P.; Caro, J. New one dimensional membrane: aligned AlPO4 5 molecular sieve crystals in a nickel foil. Microporous Mater. 1994, 3, 201–206. [Google Scholar] [CrossRef]
- Matsukata, M.; Nishiyama, N.; Ueyama, K. Zeolitic membrane synthesized on a porous alumina support. J. Chem. Soc. Chem. Commun. 1994, 3, 339–340. [Google Scholar]
- Ozin, G.A.; Kuperman, A.; Stein, A. Advanced zeolite materials science. Angew. Chem. 1989, 101, 373–390. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M. Modification of gas permeation by optical switching of molecular sieve-azobezene membranes. In Host-guest-Systems Based on Nanoporous Crystals; Laeri, F., Schüth, F., Simon, U., Wark, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 484–500. [Google Scholar]
- Kim, Y.; Dutta, P.K. Photo-chemical studies with a zeolite Y membrane formed via secondary growth. Res. Chem. Intermediat. 2004, 30, 147–161. [Google Scholar] [CrossRef]
- Weh, K.; Noack, M.; Hoffmann, K.; Schroder, K.P.; Caro, J. Change of gas permeation by photo-induced switching of zeolite-azobenzene membranes of type MFI and FAU. Micropor. Mesopor. Mat. 2002, 54, 15–26. [Google Scholar] [CrossRef]
- Noack, M.; Koelsch, P.; Caro, J.; Weh, K. Gas permeable membrane and use thereof for controlling gas passage. Eur. Patent 1,095,694, 2 May 2001. [Google Scholar]
- Liu, N.; Yu, K.; Smarsly, B.; Dunphy, D.R.; Jiang, Y.-B.; Brinker, C.J. Self-directed assembly of photo-active hybrid silicates derived from an azobenzene-bridged silsesquioxane. J. Am. Chem. Soc. 2002, 124, 14540–14541. [Google Scholar] [CrossRef]
- Schomburg, C.; Wohrle, G.; Schultz-Ekloff, G. In situ synthesis of azo dyes in mesoporous Y zeolites. Zeolites 1996, 17, 232–236. [Google Scholar] [CrossRef]
- Alvaro, M.; Benitez, M.; Das, D.; Garcia, H.; Peris, E. Reversible porosity changes in photo-responsive azobenzene-containing periodic mesoporous silicas. Chem. Mater. 2005, 17, 4958–4964. [Google Scholar] [CrossRef]
- Sugiura, S.; Sumaru, K.; Ohi, K.; Hiroki, K.; Takagi, T.; Kanamori, T. Photoresponsive polymer gel microvalves controlled by local light irradiation. Sensor. Actuator. A Phys. 2007, 140, 176–184. [Google Scholar] [CrossRef]
- Arana, J.; Melian, J.A.H.; Rodriguez, J.M.D.; Diaz, O.G.; Viera, A.; Pena, J.P.; Sosa, P.M.M.; Jimenez, V.E. TiO2-photocatalysis as a tertiary treatment of naturally treated wastewater. Catal. Today 2002, 76, 279–289. [Google Scholar]
- Shon, H.K.; Vigneswaran, S.; Ngo, H.H.; Kim, J.H. Chemical coupling of photocatalysis with flocculation and adsorption in the removal of organic matter. Wat. Res. 2005, 39, 2549–2558. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes in Water and Wastewater Treatment; International Water Association Press: London, UK, 2004. [Google Scholar]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Mozia, S.; Morawski, A.W. Hybridization of photocatalysis and membrane distillation for purification of wastewater. Catal. Today 2006, 118, 181–188. [Google Scholar]
- Ho, D.P.; Vigneswaran, S.; Ngo, H.H. Integration of photocatalysis and microfiltration in removing effluent organic matter from treated sewage effluent. Sep. Sci. Technol. 2010, 45, 155–162. [Google Scholar] [CrossRef]
- Drzaic, P.S. Liquid Crystal Dispersions; World Scientific Press: Singapore, 1995. [Google Scholar]
- Glowacki, E.; Horovitz, K.; Tang, C.W.; Maeshall, K.L. Photoswitchable gas permeation membranes based on liquid crystals. Adv. Funct. Mater. 2010, 20, 2778–2785. [Google Scholar] [CrossRef]
- Macchione, M.; De Filpo, G.; Nicoletta, F.P.; Chidichimo, G. Photo-chromic reverse mode polymer dispersed liquid crystals. Liq. Cryst. 2000, 27, 917–920. [Google Scholar] [CrossRef]
- Kwak, S.; Hurditch, R.J. Photochromic compound and articles containing the same. U.S. Patent 4,637,698, 20 January 1987. [Google Scholar]
- Nicoletta, F.P.; Cupelli, D.; De Filpo, G.; Chidichimo, G. Electrochromism in switchable nematic emulsions. Appl. Phys. Lett. 2004, 84, 4260–4262. [Google Scholar]
- Cupelli, D.; Nicoletta, F.P.; De Filpo, G.; Chidichimo, G.; Fazio, A.; Gabriele, B.; Salerno, G. Fine adjustment of conductivity in polymer-dispersed liquid crystals. Appl. Phys. Lett. 2004, 85, 3292–3294. [Google Scholar] [CrossRef]
- Cupelli, D.; De Filpo, G.; Chidichimo, G.; Nicoletta, F.P. Photo-switching in polymer dispersed liquid crystals. J. Appl. Phys. 2006, 100, 024508. [Google Scholar] [CrossRef]
- Cupelli, D.; Nicoletta, F.P.; Manfredi, S.; Vivacqua, M.; Formoso, P.; De Filpo, G.; Chidichimo, G. Self-adjusting smart windows based on polymer-dispersed liquid crystals. Sol. Energy Mater. Sol. Cells 2009, 93, 2008–2012. [Google Scholar] [CrossRef]
- Macchione, M.; De Filpo, G.; Mashin, A.; Nicoletta, F.P.; Chidichimo, G. Laser-writable, electrically erasable photo-electrochromic organic film. Adv. Mater. 2003, 15, 327–329. [Google Scholar] [CrossRef]
- Macchione, M.; De Filpo, G.; Nicoletta, F.P.; Chidichimo, G. Improvement of response times in photo-electrochromic organic film. Chem. Mater. 2004, 16, 1400–1401. [Google Scholar] [CrossRef]
- De Filpo, G.; Nicoletta, F.P.; Chidichimo, G. Flexible nanophoto-electrochromic film. Chem. Mater. 2005, 18, 4662–4666. [Google Scholar]
- Argun, A.A.; Cirpan, A.; Reynolds, J.R. The first truly all-polymer electrochromic devices. Adv. Mater. 2003, 15, 1338–1341. [Google Scholar] [CrossRef]
- De Filpo, G.; Mormile, S.; Nicoletta, F.P.; Chidichimo, G. Fast, self-supplied, all-solid photo-electrochromic film. J. Power Sources 2010, 195, 4365–4369. [Google Scholar] [CrossRef]
- Higuchi, A.; Hamamura, A.; Shindo, Y.; Kitamura, H.; Yoon, B.O.; Mori, T.; Uyama, T.; Umezawa, A. Photo-n-modulated changes of cell attachments on poly(spiropyran-co-methyl methacrylate) membranes. Biomacromolecules 2004, 5, 1770–1774. [Google Scholar] [CrossRef]
- Menon, S.; Thekkayil, R.; Varghese, S.; Das, S. Photoresponsive soft materials: synthesis and photophysical studies of a stilbene-based diblock copolymer. J. Polym. Sci. Pol. Chem. 2011, 49, 5063–5073. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwamoto, S.; Mukai, M.; Kikuchi, J. Photo- and thermo-responsive assembly of liposomal membranes triggered by a gemini peptide lipid as a molecular switch. J. Photochem. Photobiol. A Chem. 2006, 183, 309–314. [Google Scholar] [CrossRef]
- Mukai, M.; Maruo, K.; Kikuchi, J.; Sasaki, Y.; Hiyama, S.; Moritani, Y.; Suda, T. Propagation and amplification of molecular information using a photo-responsive molecular switch. Supramol. Chem. 2009, 21, 284–291. [Google Scholar] [CrossRef]
- Liang, X.; Yue, X.; Dai, Z.; Kikuchi, J. Photo-responsive liposomal nanohybrid cerasomes. Chem. Commun. 2011, 47, 4751–4753. [Google Scholar]
- Kikuchi, J.; Ariga, K.; Miyazaki, T.; Ikeda, K. An artificial signal transduction system. control of lactate dehydrogenase activity performed by an artificial cell-surface receptor. Chem. Lett. 1999, 28, 253–254. [Google Scholar]
- Kikuchi, J.; Kamijyo, Y.; Etoh, H.; Murakami, Y. Catalytic performance of a supramolecular bienzyme complex formed with artificial aminotransferase and natural lactate dehydrogenase. Chem. Lett. 1996, 25, 427–428. [Google Scholar]
- Yasuhara, K.; Sasaki, Y.; Kikuchi, J. A photo-responsive cholesterol capable of inducing a morphological transformation of the liquid-ordered microdomain in lipid bilayers. Colloid Polym. Sci. 2008, 286, 1675–1680. [Google Scholar] [CrossRef]
- Benkoski, J.J.; Jesorka, A.; Edvardsson, M.; Hook, F. Light-regulated release of liposomes from phospholipid membranes via photo-responsive polymer–DNA conjugates. Soft Matter 2006, 2, 710–715. [Google Scholar] [CrossRef]
- Hamada, T.; Sato, Y.T.; Yoshikawa, K.; Nagasaki, T. Reversible photo-switching in a cell-sized vesicle. Langmuir 2005, 21, 7626–7628. [Google Scholar] [CrossRef]
- Ishii, K.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible control of exo-and endo-budding transitions in a photo-sensitive lipid membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef]
- Riske, K.A.; Sudbrack, T.P.; Archilha, N.L.; Uchoa, A.F.; Schröder, A.P.; Marques, C.M.; Baptista, M.S.; Itri, R. Giant vesicles under oxidative stress induced by a membrane-anchored photo-sensitizer. Biophys. J. 2009, 97, 1362–1370. [Google Scholar] [CrossRef]
- Uda, R.M.; Yamashita, D.; Sakurai, Y.; Kimura, K. Photo-induced increase in vesicle size and role of photo-responsive malachite green leuconitrile derivative in vesicle fusion. Langmuir 2007, 23, 7936–7941. [Google Scholar] [CrossRef]
- Uda, R.M.; Hiraishi, E.; Ohnishi, R.; Nakahara, Y.; Kimura, K. Morphological changes in vesicles and release of an encapsulated compound triggered by a photo-responsive Malachite Green leuconitrile derivative. Langmuir 2010, 26, 5444–5450. [Google Scholar]
- Matsumura, A.; Tsuchiya, K.; Torigoe, K.; Sakai, K.; Sakai, H.; Abe, M. Photo-chemical control of molecular assembly formation in a catanionic surfactant system. Langmuir 2011, 27, 1610–1617. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Orihara, H.; Matsumura, A.; Saito, Y.; Ogawa, N.; Saji, T.; Yamaguchi, A.; Sakai, H.; Abe, M. Reversible release control of an oily substance using photo-responsive micelles. Langmuir 2001, 17, 6072–6076. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nicoletta, F.P.; Cupelli, D.; Formoso, P.; De Filpo, G.; Colella, V.; Gugliuzza, A. Light Responsive Polymer Membranes: A Review. Membranes 2012, 2, 134-197. https://doi.org/10.3390/membranes2010134
Nicoletta FP, Cupelli D, Formoso P, De Filpo G, Colella V, Gugliuzza A. Light Responsive Polymer Membranes: A Review. Membranes. 2012; 2(1):134-197. https://doi.org/10.3390/membranes2010134
Chicago/Turabian StyleNicoletta, Fiore Pasquale, Daniela Cupelli, Patrizia Formoso, Giovanni De Filpo, Valentina Colella, and Annarosa Gugliuzza. 2012. "Light Responsive Polymer Membranes: A Review" Membranes 2, no. 1: 134-197. https://doi.org/10.3390/membranes2010134
APA StyleNicoletta, F. P., Cupelli, D., Formoso, P., De Filpo, G., Colella, V., & Gugliuzza, A. (2012). Light Responsive Polymer Membranes: A Review. Membranes, 2(1), 134-197. https://doi.org/10.3390/membranes2010134






