Recent Developments in Polymer Inclusion Membranes: Advances in Selectivity, Structural Integrity, Environmental Applications and Sustainable Fabrication
Abstract
1. Introduction
1.1. Overview of Polymer Inclusion Membranes (PIMs)
1.2. Historical Development and Current Research Trends
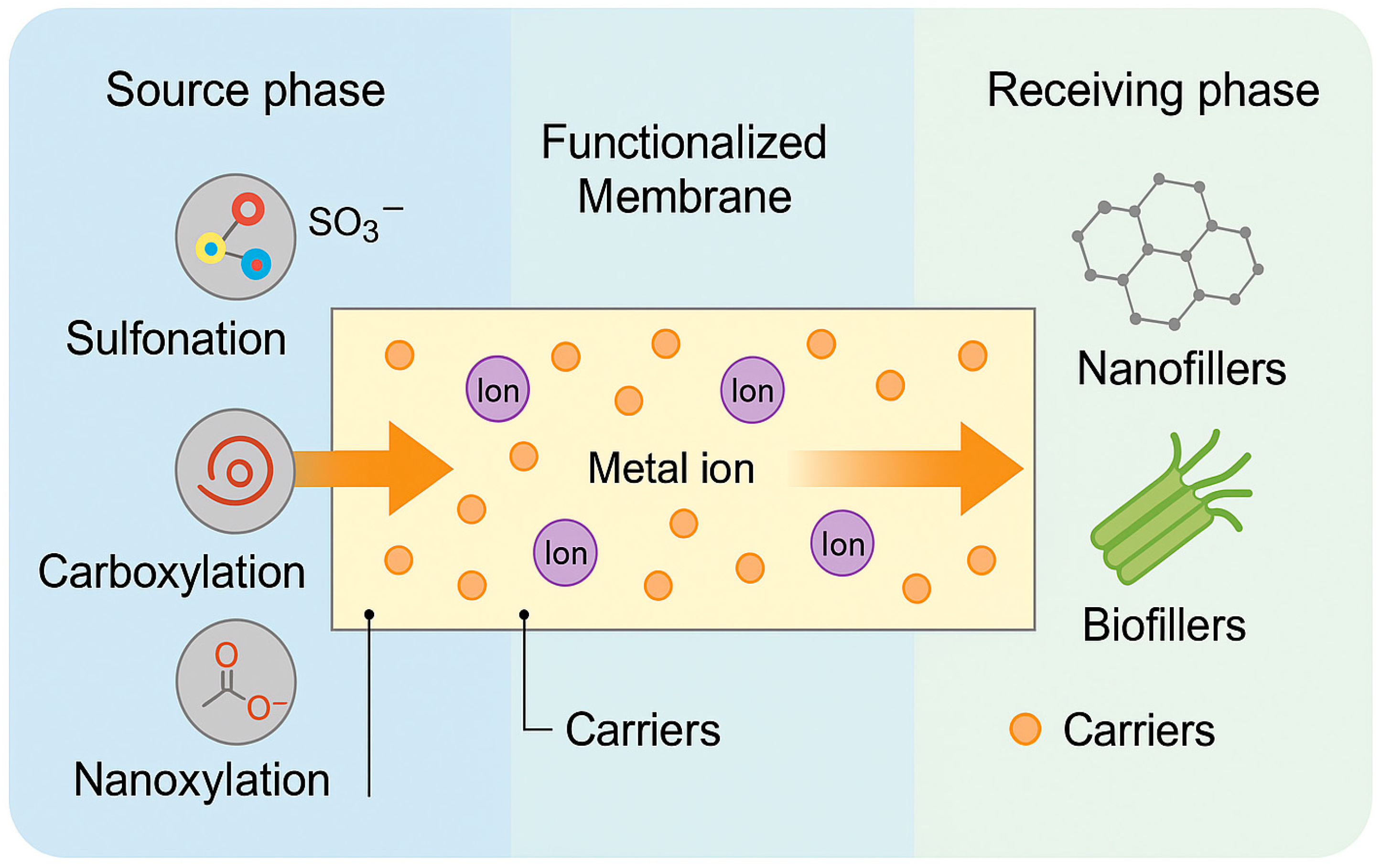
2. Advances in the Selectivity and Functionalization of PIMs
2.1. Novel Carriers and Extractants: Ionic Liquids, Task-Specific Molecules
2.2. Polymer Matrix Functionalization and Its Impact on Selectivity
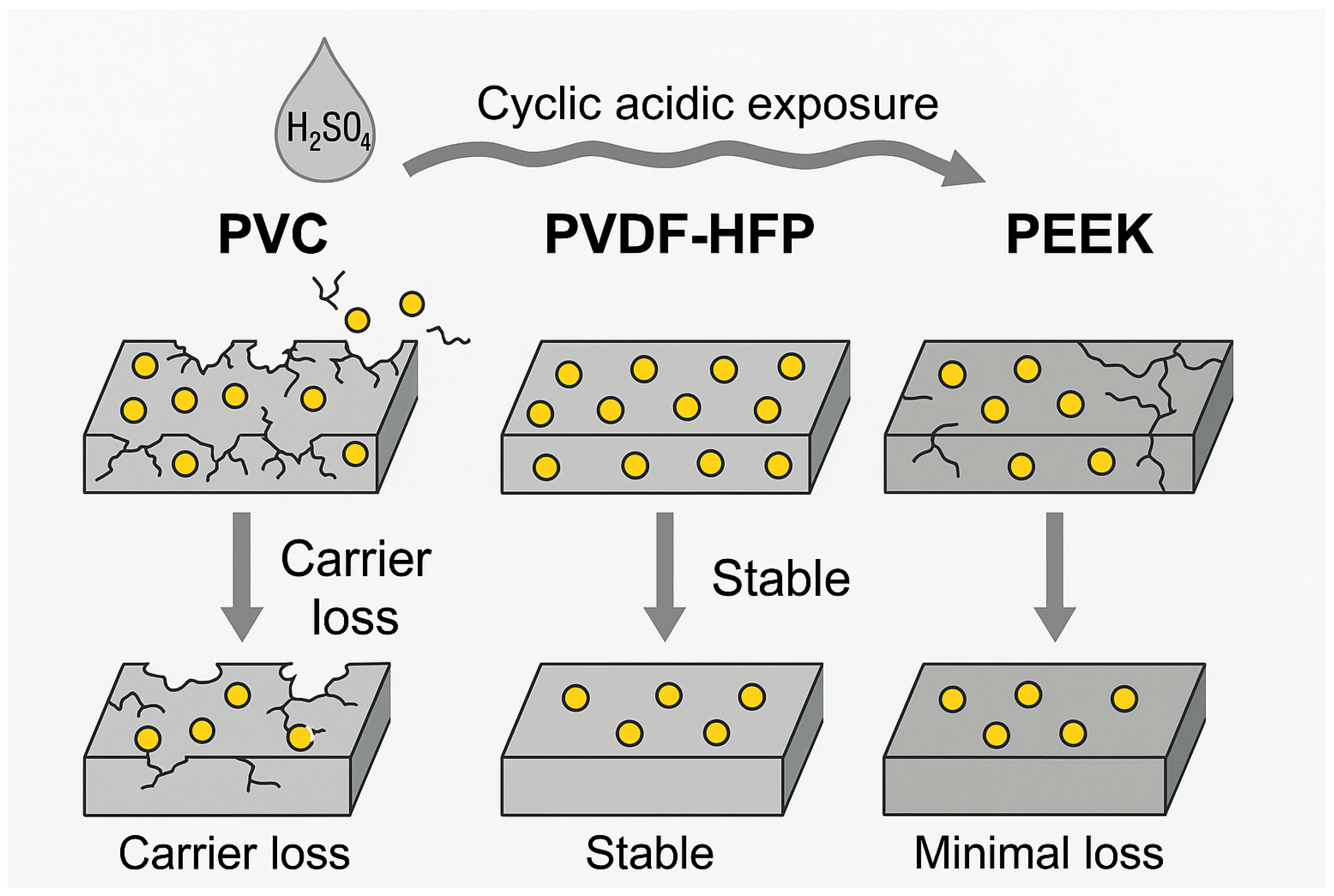
3. Enhancement of Mechanical and Chemical Stability
3.1. Utilization of Advanced Polymers
3.1.1. PVDF- and PVDF-HFP-Based PIMs
3.1.2. PEEK-Based PIMs
3.1.3. Comparative Properties and Potential Applications
- Wider pH Stability: PVDF-HFP and PEEK maintain stability across a broad pH range of approximately 1 to 10, making them suitable for use in both acidic and alkaline environments.
- Enhanced Thermal Resistance: PVDF-HFP can withstand temperatures up to around 120 °C, while PEEK remains stable at temperatures exceeding 250 °C. This capability allows for applications in thermally intensive processes.
- Improved Carrier Retention: Due to their lower surface energy and dense polymeric structure, these matrices reduce carrier volatilization and leaching, thereby extending membrane lifetime and selectivity.
- Antifouling Behavior: Their hydrophobic and chemically inert surfaces minimize organic and microbial adhesion, resulting in lower fouling rates and reduced cleaning frequency.
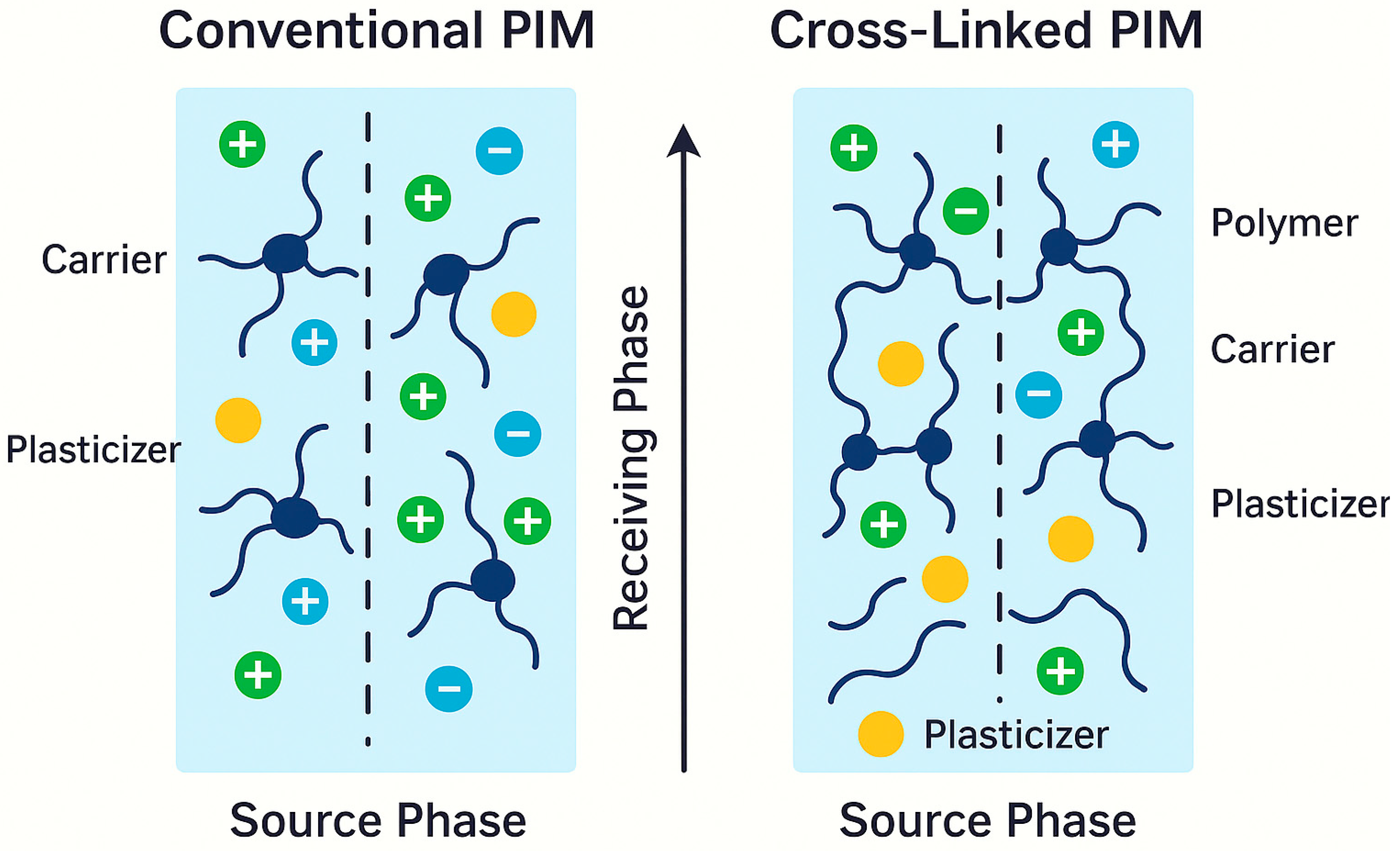
3.2. Cross-Linking Strategies and Stability Under Harsh Conditions
4. Environmental Applications of PIMs
4.1. Heavy Metal and Organic Pollutant Removal from Water
4.1.1. Mechanism of Removal
4.1.2. Heavy Metal Removal
4.1.3. Organic Micropollutant Removal
- High ion selectivity due to the molecular specificity of the carriers;
- Low energy demands, operating under ambient pressure and temperature;
- Minimal leaching and long-term reusability, especially when incorporating ILs or bio-based matrices;
- Tailorability for specific targets, including non-ionic pharmaceuticals, nutrients, or charged dyes;
- Greater chemical resistance, particularly with CTA, PVDF-HFP, or IL-based systems.
4.1.4. Practical Benefits and Benchmarks
- Exceptional selectivity toward specific target analytes, enabled by the molecular recognition capabilities of tailored carriers;
- Energy efficiency, as separation processes are typically conducted under ambient- temperature and- pressure conditions;
- Operational simplicity and renewability, with facile carrier reactivation and membrane reuse achievable through controlled pH modulation between phases;
- Enhanced sustainability, particularly when incorporating biodegradable or bio-derived fillers that reduce the environmental impact of membrane fabrication and disposal.
4.2. Gas Separation and Carbon Capture
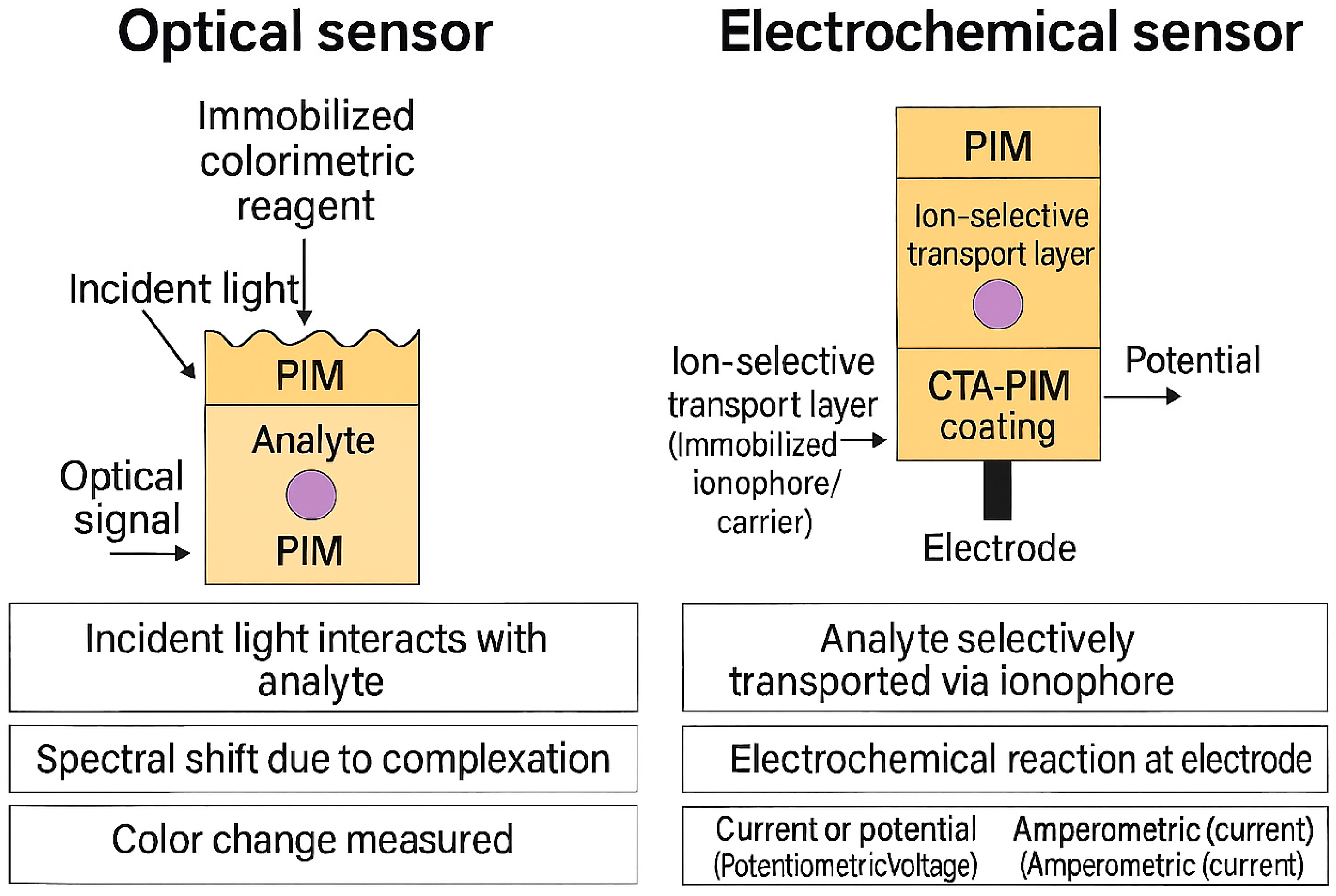
5. Integration of PIMs in Sensing Technologies
5.1. Optical and Electrochemical Sensors Based on PIMs
5.2. Performance Matrix: Sensitivity, Selectivity, and Response Time
6. Sustainable and Green Approaches in PIM Fabrication
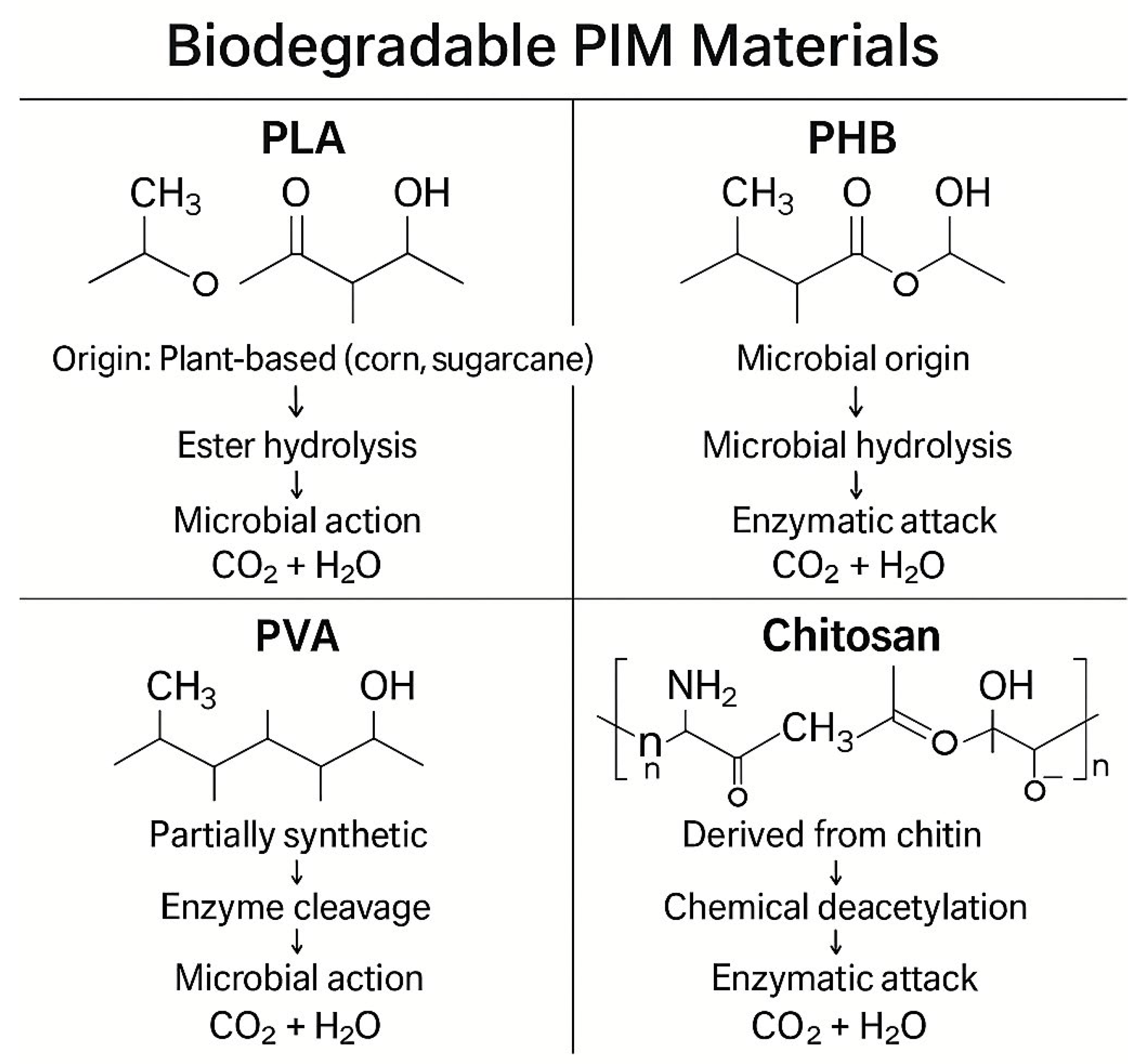
6.1. Biodegradable and Renewable Polymers
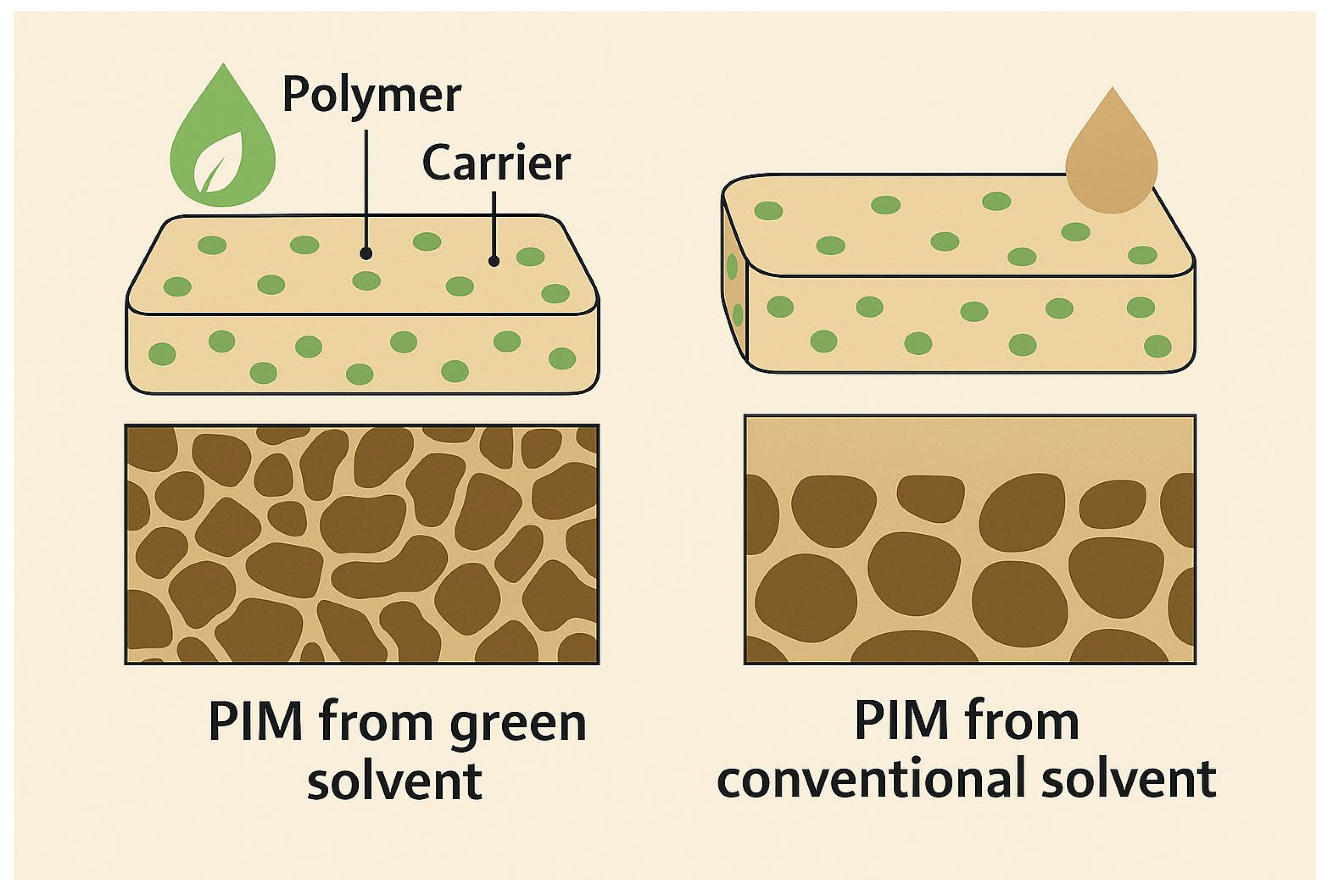
6.2. Green Solvents and Plasticizers
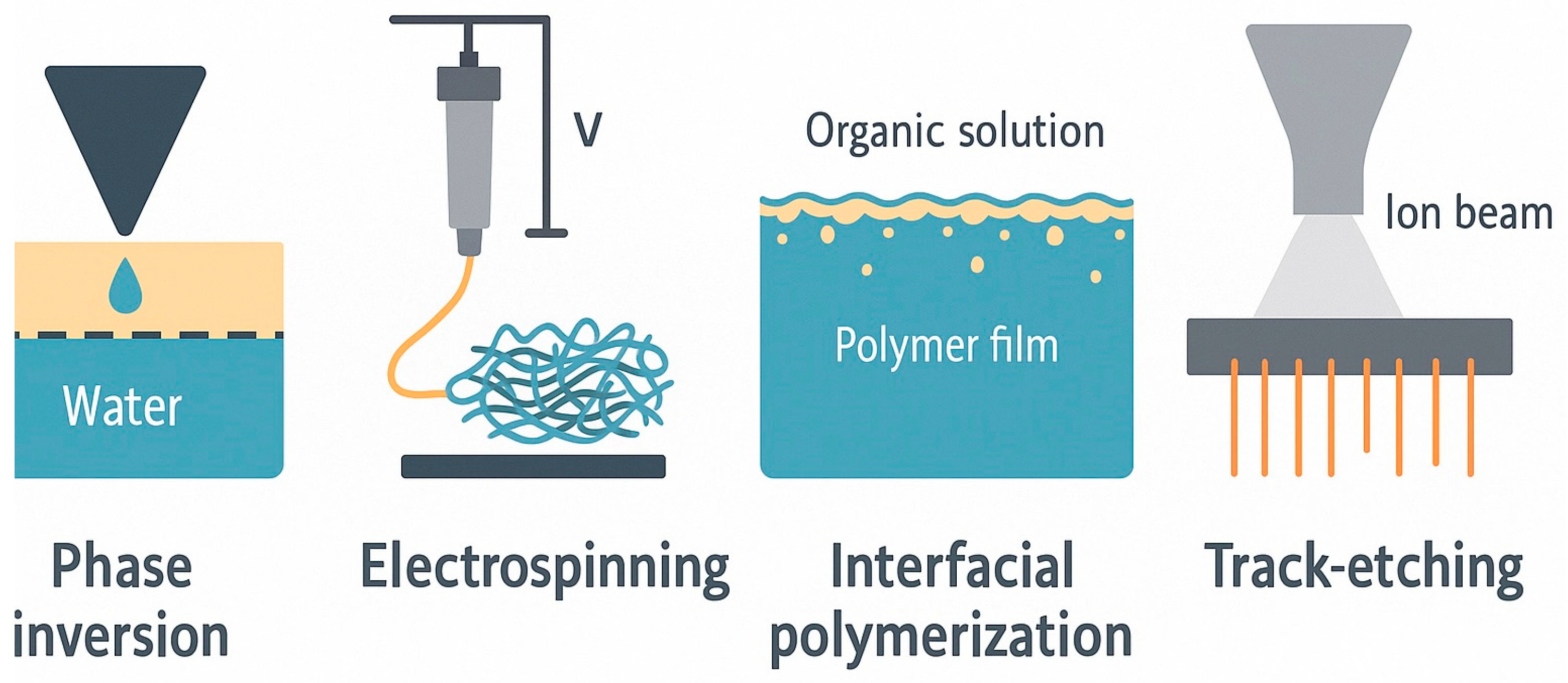
7. Scalable Fabrication and Process Optimization
7.1. Techniques: Phase Inversion, Electrospinning, and Others
7.2. Industrial Feasibility and Cost Considerations
| Material | Approx. Cost ($/kg) | Reusability Cycles | Carrier Leaching | Environmental Impact |
|---|---|---|---|---|
| CTA | 20–40 | 30–50 | Moderate | Low |
| PVDF | 60–90 | 50–80 | Low | Moderate |
| PVA | 10–25 | 15–30 | High | Biodegradable |
| PLA | 5–15 | 1020 | Moderate | Biodegradable |
| PEEK | 100–150 | >100 | Low | Low |
8. Challenges and Future Perspectives
8.1. Remaining Limitations in Current PIMs
8.2. Future Reasearch Directions for PIMs
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kagaya, S.; Maeno, T.; Ito, K.; Gemmei-Ide, M.; Catrrrall, R.W.; Kolev, S.D. Improvement of Chromium(VI) extraction from acidic solutions using a poly(vinyl chloride)-based polimer inclusion membranę with Aliquat336 as the carrier. Anal. Sci. 2017, 33, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Kagaya, S.; Ryokan, Y.; Tohda, T.; Cattrall, R.W.; Kolev, S.D. Stability Studies of Poly(vinyl chloride)-Based Polymer Inclusion Membranes Containing Aliquat 336 as a Carrier. Sep. Purif. Technol. 2012, 101, 69–75. [Google Scholar] [CrossRef]
- Uğur, A.; Sener, I.; Hol, A.; Alpoguz, H.K.; Elci, L. Facilitated transport of Zn(II) and Cd(II) ions through polymer inclusion membranes immobilized with calix[4]resorcinarene derivative. J. Macromol. Sci. A 2014, 51, 611–618. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Suksaroj, C.; Héran, M.; Allègre, C.; Persin, F. Treatment of textile plant effluent by nanofiltration and/or reverse osomosis for water reuse. Desalination 2005, 178, 333–341. [Google Scholar] [CrossRef]
- Vera, R.; Anticó, E.; Fontàs, C. The Use of a Polymer Inclusion Membrane for Arsenate Determination in Groundwater. Water 2018, 10, 1093. [Google Scholar] [CrossRef]
- Fontàs, C.; Vera, R.; Anticó, E. New Insights on the effects of water on polimer inclusion membranes containing Aliquat 336 Derivatives as Carriers. Membranes 2022, 12, 192. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Cristea, M.; Grigoras, V.C.; Bourceanu, G. New polymer inclusion membrane. Preparation and characteryzation. Dig. J. Nanomater. Biostructures 2011, 6, 1499–1508. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- AI-Haddabi, M.; Vuthaluru, H.; Ahmed, M.; Znad, H. Use of Ceramic Membrane Technology for Sustainable Management of Oil Production Water: A Review. In Recent Progress in Desalination, Environmental and Marine Outfall Systems; Springer Nature: Berlin/Heidelberg, Germany, 2015; pp. 11–23. [Google Scholar]
- Hakami, M.W.; Alkhudhri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Zielińska, M.; Galik, M. Use of Ceramic Membranes in a Membrane Filtration Supported by Coagulation for the Treatment of Dairy wastewater. Water Air Soil Pollut. 2017, 228, 173. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic membranę technology for water and wastewater treatment: A critical review of performance, fullscale applications, membranę fouling and prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Almeida, M.I.; Cattrall, R.W.; Kolev, S. Polymer Inclusion Membranes: Concept and applications. Procedia Eng. 2012, 44, 681–682. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Namiesnik, J. Green Analytical Chemistry Past, Present and Perspectives; Springer Nature: Berlin/Heidelberg, Germany, 2019; ISBN 978-981-13-9104-0. [Google Scholar]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D.; Yang, Y.; Bartsch, R.A. Heavy metal separation with polimer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Ouchn, R.; Chaouqi, Y.; Oukkass, S.; Santoro, S.; Avci, A.H.; Curcio, E.; Hlaibi, M. Advancing Sustainable Direct Contact Membrane Distillation: Performance and Stability of Novel Polymer Inclusion membranes. Sep. Purif. Technol. 2025, 354, 129056. [Google Scholar] [CrossRef]
- Musa, A.A.; Bello, A.; Adams, S.M.; Onwualu, A.P.; Anye, V.C.; Bello, K.O.; Obianyo, I.I. Nano-Enhanced Polymer Composite Materials: A Review of Current Advancements and Challenges. Polymers 2025, 17, 893. [Google Scholar] [CrossRef]
- Nitti, F.; Selan, O.T.E.; Hoque, B.; Tambaru, D.; Djunaidi, M.C. Improving the Performance Inclusion Membranes in Separation Process Using Alternative Base Polymers: A review. Indones. J. Chem. 2022, 22, 284–302. [Google Scholar] [CrossRef]
- Zante, G.; Boltoeva, M.; Masmoudi, A.; Barillon, R.; Trébouet, D. Supported ionic liquid and polimer inclusion membranes for metal separation. Sep. Purif. Rev. 2022, 51, 100–116. [Google Scholar] [CrossRef]
- Xiao, T.; Zhu, Z.; Li, L.; Shi, J.; Li, Z.; Zuo, X. Mebrane fouling and cleaning strategies in microfiltration/ultrafiltration and dynamic membrane. Sep. Purif. Technol. 2023, 318, 123977. [Google Scholar] [CrossRef]
- Zulkefli, N.F.; Alias, N.H.; Jamaluddin, N.S.; Abdullah, N.; Manaf, S.F.A.; Othman, N.H.; Marpani, F.; Mat-Shayuti, M.S.; Kusworo, T.D. Recent Mitigation Strategies on Membrane Fouling for Oily Wastewater Treatment. Membranes 2021, 12, 26. [Google Scholar] [CrossRef]
- Shukla, B.K.; Sharma, P.K.; Yadav, H.; Singh, S.; Tyagi, K.; Yadav, Y.; Rajpoot, N.K.; Rawat, S.; Verma, S. Advanced membranę technologies for water treatment: Utilization of nanomaterials and nanoparticles in membranes fabrication. J. Nanoparticle Res. 2024, 26, 222. [Google Scholar] [CrossRef]
- Sellami, F.; Senhadji, O.K.; Marais, S.; Fatyeyeva, K. PVC/EVA-based polymer inclusion membranes with improved stability and Cr(VI) extraction capacity: Water plasticization effect. J. Hazard. Mater. 2022, 436, 129069. [Google Scholar] [CrossRef] [PubMed]
- Ovejero-Pérez, A.; Nakasu, P.Y.S.; Hopson, C.; Costa, J.M.; Hallett, J.P. Challenges and opportunities on the utilisation of ionic liquid for biomass pretreatment and valorisation. Mater. Sustain. 2024, 2, 7. [Google Scholar] [CrossRef]
- Wong, K.C.; Gog, P.S.; Ismail, A.F.; Kang, H.S.; Guo, Q.; Jiang, X.; Ma, J. The State-of-the-Art Functionalized Nanomaterials for Carbon Dioxide Separation Membrane. Membranes 2022, 12, 186. [Google Scholar] [CrossRef]
- Hernández-Fernández, A.; Iniesta-López, E.; Ginestá-Anzola, A.; Garrido, Y.; Pérez de los Ríos, A.; Quesada-Medina, J.; Hernádez-Fernádez, F. Polymeric Inclusion Membranes Based on Ionic Liquids for Selective Separation of Metal Ions. Membranes 2023, 13, 795. [Google Scholar] [CrossRef]
- Adigun, B.; Thapaliya, B.P.; Luo, H.; Dai, S. Ionic Liquid-Based Extraction of Metal Ions via Polymer Inclusion Membranes: A Critical Review. RSC Sustain. 2024, 2, 2768–2780. [Google Scholar] [CrossRef]
- Kazemi, D.; Yaftian, M.R. PVDF-HFP-Based Polymer Inclusion Membrane Functionalized with D2EHPA for the Selective Extraction of Bismuth(III) from sulfate media. Sci. Rep. 2024, 14, 11622. [Google Scholar] [CrossRef]
- Senila, M. Polymer Inclusion Membranes (PIMs) for Metal Separation-Toward Environmentally Friendly Production and Applications. Polymers 2025, 17, 725. [Google Scholar] [CrossRef] [PubMed]
- Keskin, B.; Zeytuncu-Gökoğlu, B.; Koyuncu, I. Polymer inclusion membrane applications for transport of metal ions: A critical review. Chemosphere 2021, 279, 130604. [Google Scholar] [CrossRef] [PubMed]
- Yaacob, S.F.F.; Olasupo, A.; Suah, F.B.M. Polymer Inclusion Membranes Based Optode: Recent Advances and Perspectives. TrAC—Trends Anal. Chem. 2024, 171, 117498. [Google Scholar] [CrossRef]
- Bloch, R.; Kedem, O.; Vofsi, D. Ion Specific Mambrane. Nature 1963, 199, 802–803. [Google Scholar] [CrossRef]
- Kolev, S.D.; Ines, M.I.G.; Cattrall, R.W. Polymer Inclusion Membranes. In Handbook of Membrane Separations; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Kaczorowska, M. The use of polymer inclusion membranes for the removal of metal ions from aqueous solutions—The Latest Achievements and potential industrial applications: A review. Membranes 2022, 12, 1135. [Google Scholar] [CrossRef]
- Latos-Brozios, M.; Rułka, K.; Masek, A. Review of Bio-fillers Dedicated of Polymer Compositions. Chem. Biodivers. 2025, 22, e202500406. [Google Scholar] [CrossRef]
- Maiphetlho, K.; Shumbula, N.; Motsoane, N.; Chimuka, L.; Richards, H. Evaluation of Silver-Nanocomposite PIMs for Trace Metal Transports. Selectivity and stability studies. J. Water Process Eng. 2020, 37, 101527. [Google Scholar] [CrossRef]
- Negul, E.A.; Fontas, C.; McKelvie, I.D.; Cattrall, R.W.; Kolev, S.D. The use of a polymer inclusion membrane for separation and preconcentration of orthophosphate in flow analysis. Anal. Chim. Acta 2013, 803, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- O’Rourke, M.; Cattrall, R.W.; Kolev, S.D.; Potter, I.D. Electrochemical Impedance Spectroscopy-A Simple Method for the Characterization of Polymer Inclusion Membranes Containing Aliquat 336. Membranes 2011, 1, 132–148. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Bourceanu, G.; Timpu, D. Experimental and modeling studies of lead(II) sorption onto a polyvinyl-chloride inclusion membrane. Chem. Eng. J. 2011, 172, 817–827. [Google Scholar] [CrossRef]
- Kolev, S.D.; St John, A.M.; Cattrall, R.W. Mathematical modelling of the extraction of uranium(VI) into a polymer inclusion membrane composed of PVC and di-(2-ethylhexyl) phosphoric acid. J. Membr. Sci. 2013, 425–426, 169–175. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Matamoros, V.; Kolev, S.D.; Fontàs, C. Development of a polimer inclusion membranę (PIM) for the preconcentration of antibiotics in environmental water samples. J. Membr. Sci. 2015, 492, 32–39. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takemori, S.; Tahara, Y. Lactic acid permeation through deep eutectic solvents-based polymer inclusion membranes. Membranes 2020, 10, 244. [Google Scholar] [CrossRef]
- Kończyk, J.; Ciesielski, W. Calixresorcin[4]arene-Mediated Transport of Pb)II) Ions through Polymer Inclusion Membrane. Membranes 2021, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ponce, L.; Casanueva-Marenco, M.J.; Diaz-de-Alba, M.; Galindo-Riano, M.D.; Granada-Castro, M.D. A novel polymer inclusion membrane-based green optical sensor for selective determination of Iron: Design, characterization, and analytical applications. Polymers 2023, 15, 4082. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Vatanpour, V.; Ganjali, M.R.; Saeb, M.R. Deep Eutectic Solvents in Membrane science and technology: Fundamental, preparation, application and future perspective. Sep. Purif. Technol. 2020, 258, 118015. [Google Scholar] [CrossRef]
- Kandel, D.R.; Kwak, D.; Lee, S.; Lim, Y.J.; Subedi, S.; Lee, J. Harnessing natural antifouling agents for enhancing water and wastewater treatment membranes. Sep. Purif. Technol. 2025, 359, 130254. [Google Scholar] [CrossRef]
- Fang, H.; Han, Y.; Wang, J.; Ding, Y.; He, M.; Lan, J.; Ding, X.; Shi, W.; Liu, X. Calix[4]arene—Decorated polymers of intrinsic microporosity for lithium isotopes separation. Chem. Eng. J. 2024, 500, 156916. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Burgard, M.; Dieleman, C.B.; Matt, D. Rare-earth metal-ion separation using a supported liquid membrane mediated by a narrow rim phosphorylated calix[4]arene. J. Membr. Sci. 1998, 144, 57–64. [Google Scholar] [CrossRef]
- Syeda, S.E.Z.; Nowacka, D.N.; Khan, M.S.; Skwierawska, A. Recent Advancements in Cyclodextrin-Based Adsorbants for the Removal of Hazardous pollutants from Waters. Polymers 2022, 14, 2341. [Google Scholar] [CrossRef]
- Kozlowski, C.A.; Walkowaik, W.; Girek, T. Modified cyclodextrin polymers as selectiveion carriers for Pb(II) separation across plasticized membranes. J. Membr. Sci. 2008, 310, 312–320. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Z.; Li, M.; Sun, P.; Yu, T.; Zhou, L. Novel porous magnetic nanophores funcionalized by ß-cyclodextrin polimer and its application in organic pollutants from aqueous solution. Environ. Pollut. 2019, 250, 639–649. [Google Scholar] [CrossRef]
- Miyah, Y.; El Messaoudi, N.; Benjelloun, M.; Georgin, J.; Franco, D.S.P.; El-Habacha, M.; Ali, O.A.; Acikbas, Y. A comprehensive review of ß-cyclodextrinpolymer nanocomposities exploration for heavy metal removal from wastewater. Carbohydr. Polym. 2025, 350, 122981. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Zhao, X.; Zhang, Y. Fabrication of three-dimensional β-cyclodextrin/chitosan functionalized Graphene oxide hydrogel for methylene blue removal from aqueous solution. Colloids Surf. A 2019, 539, 1–10. [Google Scholar] [CrossRef]
- Moulahcene, L.; Skiba, M.; Bounoure, F.; Benamor, M.; Milon, N.; Hallouard, F.; Lahiani-Skiba, M. New Polymer Inclusion membranę Containing β-cyclodextrin Polymer: Application for Pharmaceutical Pollutant Removal from Wastewater. Int. J. Environ. Res. Public Health. 2019, 16, 414. [Google Scholar] [CrossRef] [PubMed]
- Edward, K.; Yuvaraj, K.M.; Kapoor, A. Chitosan-blende membranes for heavy metal removal from aqueous systems: A review of synthesis, separation, mechanism, and performance. Int. J. Biol. Macromol. 2024, 279, 134996. [Google Scholar] [CrossRef] [PubMed]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(I) using polymer inclusion membranes containing calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Kozlowski, C. Removal of Pb(II) ions using polymer inclusion membranes containing calix[4]resorcinarene derivative as ion carrier. Polymers 2019, 11, 2111. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Influence of the ionic liquid cation on the solvent extraction of trivalent rare-earth ions by mixtures of Cyanex 923 and ionic liquids. Dalton Trans. 2015, 44, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Lee, M.-S. Separation of Co(II) and Ni(II) from chloride leach solution of nickel laterite ore by solvent extraction with Cyanex 301. Int. J. Miner. Process. 2017, 166, 45–52. [Google Scholar] [CrossRef]
- Skoronski, E.; Fernandes, M.; Malaret, F.J.; Hallett, J.P. Use of phosphonium ionic liquids for highly efficient extraction of phenolic compounds from water. Sep. Purif. Technol. 2020, 248, 117069. [Google Scholar] [CrossRef]
- Fraser, K.J.; MacFarlane, D.R. Phosphonium-based Ionic Liquids: An Overview. Aust. J. Chem. 2009, 62, 309–321. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Liang, X.; Luo, J.; Wan, Y. Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent. Polymers 2021, 13, 1170. [Google Scholar] [CrossRef]
- Kozłowski, C.; Zawierucha, I. Polymer Inclusion Membranes Based on Sulfonic Acid Derivatives as Ion Carriers for Selective Separation of Pb(II) Ions. Membranes 2025, 15, 146. [Google Scholar] [CrossRef]
- Bashiri, A.; Nikzad, A.; Maleki, R.; Asadnia, M.; Razmjou, A. Rare earth elements recovery using selective membranes via extraction and rejection. Membranes 2022, 12, 80. [Google Scholar] [CrossRef]
- Croft, C.F.; Nagul, E.A.; Almeida, M.I.G.S.; Kolev, S.D. Polymer-based extracting materials in the green recycling of rare earth elements: A review. ACS Omega 2024, 9, 40315–40328. [Google Scholar] [CrossRef]
- Husna, S.M.; Yusoff, A.H.; Mohan, M.; Azmi, N.A.; Ter, T.P.; Shoparwe, N.F.; Sulaiman, A.Z. Graphene Oxide (GO) Membrane in Removing Heavy Metals from Wastewater: Effect of Graphene Oxide on the Properties of Polymer Inclusion Membranes for Gold Extraction from Acidic Solution. Membranes 2022, 12, 996. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.; Tran, H.M.; Kandel, D.R.; Lee, J. Synergistic effects of MOF catalysts and a 3D junction enable thin bipolar membranes to reach the thermodynamic minimum potential for water dissociation. Chem. Eng. J. 2025, 507, 160573. [Google Scholar] [CrossRef]
- Ncib, S.; Barhoumi, A.; Bouguerra, W.; Larchet, C.; Dammak, L.; Hamrouni, B.; Elaloui, E. Preparation and Characterization of Cellulose Triacetate Polymer Inclusion Membrane Blended with Acetylated Kraft Lignin: Effect of AKL and Application to the Extraction of Copper(II) from Acidic Media. Desalination Water Treat. 2018, 104, 263–272. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. Electrospun polystyrene/Aliquat 336 for preconcentration and determination of thiocyanate in flow analysis. Electrospinning 2017, 1, 100–110. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.; Lu, Y.; Fan, W.; Huo, M.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Membr. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Bi, W.; Jiang, Z.; Zhang, M.; Pang, J. High-strength corrosion resistant membranes for the separation of oil/water mixtures and immiscible oil mixtures based on PEEK. J. Membr. Sci. 2020, 616, 118418. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A New Generation of Highly Stable and Permeable Polymer Inclusion Membranes (PIMs) with Their Carrier Immobilized in a Cross-Linked Semi-Interpenetrating Polymer Network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Aristizábal, S.L.; Chisca, S.; Pulido, B.A.; Nunes, S.P. Preparation of PEEK Membranes with Excellent Stability Using Common Organic Solvents. Ind. Eng. Chem. Res. 2020, 59, 5218–5226. [Google Scholar] [CrossRef]
- Ncib, S.; Brahmi, K.; Othmen, K.; Lasaad, D.; Elaloui, E.; Bouguerra, W. Enhanced Ni(II) recovery via D2EHPA-cellulose triacetate-based polymer inclusion membranes: Optimized extraction and stability analysis. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2245–2263. [Google Scholar] [CrossRef]
- Chang, S.; Kandal, D.R.; Lee, U.; Tran, H.M.; Lee, J. Improving separation efficiency of various micropollutants from water by polymer-enhanced ultrafiltration using oxidized alginate featuring less viscous and low filtration resistance. J. Environ. Chem. Eng. 2024, 12, 114753. [Google Scholar] [CrossRef]
- Wang, H.; He, S.; Qin, X.; Li, C.; Li, T. Interfacialo Engineering in metal organic framework-based mixed matrix membranes using covalently grafted polyimide Brushes. J. Am. Chem. Sci. 2018, 140, 17203–17210. [Google Scholar] [CrossRef]
- Astorino, C.; De Nardo, E.; Lattieri, S.; Ferraro, G.; Pirri, C.F.; Bocchini, S. Advancements in gas separation for energy applications: Exploring the potential of polymer membranes with intrinsic microporosity (PIM). Membranes 2023, 13, 903. [Google Scholar] [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.; Escobar, I.C. Polymers and solvents used in membrane fabrication: A review focusing on sustainable membrane development. Membranes 2021, 11, 309. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, W.; Wu, A.; Shu, W.; Zhang, Y. Recent progress on CO2 separation membranes. RSC Adv. 2024, 14, 20714. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wang, D.-M.; Hsien, T.-Y.; Chan, K.-Y.; Lai, J.-Y. Polymer Inclusion Membranes with Strip Dispersion. Water 2017, 9, 399. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I. New polymer inclusion membrane in the separation of nonferrous metal ion from aqueous solutions. Membranes 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Choi, M.; Chang, J.; Ji, D.; Kang, S.W.; Hong, J. Highly permeable graphene oxide/polyelectrolytes hybrid thin films for enhanced CO2/N2 separation performance. Sci. Rep. 2017, 7, 456. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.W. CO2 Separation with polymer/aniline composite membranes. Polymers 2020, 12, 1363. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Waheed, H.; Raza, A.; Mukhtar, A.; Ahmad, F. A Critical Review on membrane Technology and Its application in CO2 Capturing. Chem. Methodol. 2024, 8, 662–698. [Google Scholar]
- Tong, Z.; Sekizkardes, A.K. Recent developments in high-performance membranes for CO2 separation. Membranes 2021, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Yahia, M.; Refaat, D.; Coronas, J.; Tellez, C. Enhancing CO2/CH4 separation performance in PIM-1 based MXene nanosheets mixed matrix membranes. Sep. Purif. Technol. 2025, 356, 129825. [Google Scholar] [CrossRef]
- Ferraro, G.; Astorino, C.; Bartoli, M.; Martis, A.; Lattieri, S.; Pirri, C.F.; Bocchini, S. Ionic Liquid-Polymer of Intrinsic Microporosity (PIMs) Blend Membranes for CO2 Separation. Membranes 2022, 12, 1262. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, X.; Xu, D.; Luo, Y. Porous adsorption materials for carbon dioxide capture in industrial flue gas. Front. Chem. 2022, 10, 939701. [Google Scholar] [CrossRef]
- Sánchez-Ponce, L.; Galindo-Riano, M.D.; Casanueva-Marenco, M.J.; Granada-Castro, M.D.; Diaz-de-Alba, M. Sensing Cd(II) using a disposable optical sensor based on a Schiff base immobilization on a polymer-inclusion membrane. Applications in water and art paint samples. Polymers 2021, 13, 4414. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nitti, F.; Almeida, M.I.G.S.; Kolev, S.D. Water monitoring using polymer inclusion membranes: A review. Environ. Chem. Lett. 2019, 18, 129–150. [Google Scholar] [CrossRef]
- Alshehri, R.F.; Amin, A.S.; Aish, M. PVC-DOS membrane immobilized with 2-amino-4-(3-chloro-phenylozo) pyridine-3-ol for environmentally friendly detection of Cd(II) ions. Chem. Data Collect. 2023, 48, 101098. [Google Scholar] [CrossRef]
- Suah, F.B.M.; Ahmad, M. Preparation and characterization of polimer inclusion membranę based optode for determination of Al3+ ion. Anal. Chim. Acta 2017, 951, 133–139. [Google Scholar] [CrossRef]
- Alharbi, K.H. A Review on Organic Colorimetric and Fluoroscent Chemosensors for the Detection of Zn(II) Ions. Crit. Rev. Anal. Chem. 2023, 53, 1472–1488. [Google Scholar] [CrossRef]
- Alizadeh, N.; Moemeni, A.; Shamsipur, M. Poly(vinyl chloride)-membrane ion-selective bulk optode based on 1, 10-dibenzyl-1, 10-diaza-18-crown-6 and 1-(2-pyridylazo)-2-naphthol for Cu2+ and Pb2+ ions. Anal. Chim. Acta 2002, 464, 187–196. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, X.; Long, Y.; Chen, J.; Wang, L.; Zhang, Y. Optical sensing membrane for determination of trace cadmium(II), zinc(II) and cooper(II) based on immobilization of 1-(2-pyridylazo)-2-naphthol on polymer inclusion membrane. Microchem. J. 2021, 162, 105767. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. A new planar potentiometric sensor for in situ measurements. Sensors 2024, 24, 2492. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.A.; Bühlmann, P. Next-generation potentiometric sensors: A review of flexible and wearable technologies. Biosensors 2025, 15, 51. [Google Scholar] [CrossRef]
- Apostu, M.; Vieriu, M.; Bibire, N.; Panainte, A.; Tantaru, G. Design and Study of Electrochemical Sensors Based on Polymer Inclusion Membranes Containing Polyoxometalate. Mater. Plast. 2019, 56, 429–433. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, W.; Yang, G.; Niu, L. Solid-contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Chinthaginjala, R.; Chittathuru, D. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef]
- Mancilla-Rico, A.J. Simultaneous determination of Cu(II), Zn(II), and Pb(II) from aqueous solutions using a polymer inclusion membrane (PIM) based-sensor with 1-(2-pyridylazo)-2-naphthol (PAN) as chromophore and chemometric methods. Front. Anal. Sci. 2022, 2, 971352. [Google Scholar] [CrossRef]
- Suah, F.B.M. Preparation and Characterisation of a Novel Co(II) Optode Based on Polymer Inclusion Membrane. Anal. Chem. Res. 2017, 12, 40–46. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, C.; Kolev, S.; Almeida, M.I. Green Solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Maiphetlho, K.; Chimuka, L.; Tutu, H.; Richards, H. Technical design and optimization of polymer inclusion membranes (PIMs) for sample pretreatment and passive sampling—A review. Sci. Total Environ. 2021, 799, 149483. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, T.; Tiraferri, A.; Drioli, E.; Figoli, A.; Crittenden, J.C.; Liu, B. Toward the Next Generation of Sustainable Membranes from Green Chemistry Principles. ACS Sustain. Chem. Eng. 2021, 9, 50–75. [Google Scholar] [CrossRef]
- Zhao, S.; Samadi, A.; Wang, Z.; Pringle, J.M.; Zhang, Y.; Kolev, S.D. Ionic liquid-based polymer inclusion membranes for metal ions extraction and recovery: Fundamentals, considerations, and prospects. Chem. Eng. J. 2024, 481, 148792. [Google Scholar] [CrossRef]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Prządka, D.; Borysiak, S.; Regel-Rosocka, M. Characteryzation of polymer inclusion membranes (PIMs) containing phosphonium ionic liquids as Zn(II) carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Cindradewi, A.W.; Bandi, R.; Park, C.-W.; Park, J.-S.; Lee, E.-A.; Kim, J.-K.; Kwon, G.-J.; Han, S.-Y.; Lee, S.-H. Preparation and characterization of cellulose acetate film reinforced with cellulose nanofibril. Polymers 2021, 13, 2990. [Google Scholar] [CrossRef]
- Mena-Prado, I.; Fernández-Garcia, M.; Blázquez-Blázquez, E.; Muñoz-Bonilla, A.; Del Campo, A. Plasticizing PLA with Biobased fatty esters: Comprehensive study on film properties. J. Polym. Environ. 2025, 33, 1337–1352. [Google Scholar] [CrossRef]
- Cocco, S.; Resende Assis Silva, R.; Veloso De Oliveira, T.; Stringheta, P.; Moacir Ribeiro Pinto, M. Glycerol and triethyl citrate plasticizer effects om molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar]
- Rachid, O.; Youssf, C.; Louafy, R.; Avci, A.H.; Santoro, S.; Cherkaoui, O.; Hlaibi, M. Polymer inclusion membranes with long term-stability in desalination via membrane distillation. Chem. Eng. Process. 2023, 191, 109442. [Google Scholar]
- Cheng, X.; Bae, J. Recent advancements in fabrication, separation, and purification of hierarchically porous polymer membranes and their applications in next-generation electrochemical energy storage devices. Polymers 2024, 16, 3269. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Tucker, N.; Kny, E.; Thomas, S. Chapter 1: Electrical Spinning to Electrospinning: A Brief History. In Electrospinning: From Basic Research to Commercialization; Royal Society of Chemistry: London, UK, 2025; pp. 1–23. ISBN 978-1-78801-491-5. [Google Scholar]
- Gkika, D.A.; Filiz, V.; Rongou, S.; Kyzas, G.K.; Mitropoulos, A.C. Cost profile of membranes that use polymers of intrinsic microporosity (PIMs). Membranes 2022, 12, 433. [Google Scholar] [CrossRef]
- Caliskan, E.; Shishatskiy, S.; Abetz, V.; Filiz, V. Pioneering the preparation of porous PIM-1 membranes for enhanced water vapor flow. RSC Adv. 2024, 14, 9631–9645. [Google Scholar] [CrossRef]
- Goh, L.M.; Thong, Z.; Li, W.P.; Ooi, T.S.; Esa, F.; Ng, K.S.; Dhalla, A.; Gudipati, C. development and Industrial-scale Fabrication of Next Generation Low-Energy membranes for Desalination. Membranes 2022, 12, 540. [Google Scholar] [CrossRef]
- Dischinger, S.M.; Miller, D.J.; Vermaas, D.A.; Kingsbury, R.S. Unifying the Conversation: Mebrane Separation Performance in Energy, Water, and Industrial Applications. ACS EST Engg. 2024, 4, 277–289. [Google Scholar] [CrossRef]
- Jiang, L.; Yan, C.; Zhai, Y.-M.; Luo, F.; Meng, H.; Yin, B.; Zhang, k.; Yang, M.-B. Highly-toughened PLLA/PVA biodegradable blends: Graft copolymer tailored crystallization and phase morphology. Polymer 2024, 312, 127606. [Google Scholar] [CrossRef]
- Zawierucha, I.; Nowik-Zajac, A.; Malina, G. Selective Removal of As(V) Ions from Acid Mine Drainage Using Polymer Inclusion Membranes. Minerals 2020, 10, 909. [Google Scholar] [CrossRef]
- Ihueze, C.C.; Ekhibise, N.; Onwurah, U.O.; Okafor, C.E. Process Parameters Optimization in Plastic manufacturing Industry using machine Learning. J. Innov. Res. Eng. Sci. 2024, 5, 572–589. [Google Scholar]
- Kian, L.K.; Saba, N.; Jawaid, M.; Sultan, M.T.H. A Review on Processing Techniques of Bast Fibers Nanocellulose and Its Polylactic Acid (PLA) Nanocomposites. Int. J. Biol. Macromol. 2019, 121, 1314–1328. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowska, M.; Bożejewicz, D.; Witt, K. The Application of Polymer inclusion membranes for the Removal of Emerging Contaminants and Synthetic Dyes from Aqueous Solutions—A Mini Review. Membranes 2023, 13, 132. [Google Scholar] [CrossRef]
- Nitti, F.; Boliona, A.A.; Nitbani, F.O.; Naat, J.N.; Lapailaka, T.; Kadang, L.; Pingak, R.K.; Abadi, A.; Kiswandono, A.A. Fabrication of Polymer Inclusion Membrane Using Recycled Polyvinyl Chloride as Sustainable Alternative Support Polymer for the Extraction of Zn(II) from Water. J. Appl. Polym. Sci. 2025, 142, e57365. [Google Scholar] [CrossRef]
- Kunene, P.; Akinbami, O.; Motsoane, N.; Tutu, H.; Chimuka, L.; Richards, H. Feasibility of Polysulfone as Base Polymer in a Polymer Inclusion Membrane: Synthesis and Characrerisation. J. Membr. Sci. Res. 2020, 6, 203–210. [Google Scholar]
- Coscarello, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Alternative Assisted Extraction Methods of Phenolic Compounds Using NaDESs. Antioxidants 2023, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.; Fatyeyeva, K.; Marais, S. Ionic liquid-based polymer inclusion membranes for heavy metal ions removal from water: Achievements and challenges. Chem. Eng. J. 2025, 505, 158916. [Google Scholar] [CrossRef]
- Kerkel, F.; Markiewicz, M.; Stolte, S.; Müller, E.; Kunz, W. The green platform molecule gamma-valerolactone—Ecotoxicity, biodegradability, solvent properties, and potential applications. Green Chem. 2021, 23, 2962–2976. [Google Scholar] [CrossRef]
- Rasool, M.A.; Vankelecom, I.F.J. γ-Valerolactone as Bio-Based Solvent for Nanofiltration Membrane Preparation. Membranes 2021, 11, 418. [Google Scholar] [CrossRef]
- Jose, E.S.; Rosa de la Viuda, M.; Carmona, J.; Soto, C.M.; Palacio, L.; Pradanos, P.; Hernandez, A.; Tena, A. Green Dipolar aprotic Solvents for the Dynamic Polycondensation of High-Performance Polyimide Membranes. Green Chem. 2024, 26, 11984–12007. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Wang, K.; Zhang, M. A PEGylated PVDF antifouling membrane prepared by grafting of mPEGA. Membranes 2022, 11, 16. [Google Scholar]
- Zhang, X.; Zhang, Y.; Gao, J.; Li, Y. Investigation of anti-fouling and UV-cleaning properties of PVDF/TiO2 mixed-matrix membranes. Membranes 2021, 11, 16. [Google Scholar]
- Nikonenko, V.; Nebavsky, A.; Mareev, S.; Kovalenko, A.; Urtenov, M.; Pourcelly, G. Modeling of Ion Transport in Electromembrane Systems: Impacts of membrane Bulk and Surface Heterogeneity. Appl. Sci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Di Salvo, J.L.; de Luca, G.; Cipollina, A.; Micale, G. Effect of ion exchange capacity and water uptake on hydroxide transport in PSU-TMA membranes: A DAFT and molecular dynamics study. J. Membr. Sci. 2020, 599, 117837. [Google Scholar] [CrossRef]
- Witzleben Von, M.; Stoppe, T.; Strauwitz ne Ahlfeld Von, T.; Bernhardt, A.; Polk, M.-L.; Bornitz, M.; Neudert, M.; Gelinsky, M. Biomimetic Tympanic Membrane Replacement Made by Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, 2002089. [Google Scholar] [CrossRef]
- Bonakdar, M.A.; Rodrigue, D. Electrospinning: Processes, Structures and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Vazquez, M.I.; Romero, V.; Fontas, C.; Antico, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical-chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Sellami, F.; Marais, S.; Ounissa, S.-K.; Kobzar, Y.; Fatyeyeva, K. Poly(vinyl-chloride)-based advanced polymer inclusion membranes with Aliquat 336 and inorganic filler for efficient Cr(VI) removal. Chem. Eng. J. 2024, 493, 152056. [Google Scholar] [CrossRef]
- Ismail, N. Sustainable Membrane Fabrication Using Greener Solvents; Cityprint I Norr AB: Umea, Sweden, 2023; ISBN 978-91-8070-134-1. [Google Scholar]
- O’Neill, K.; Dalton, P.D. A decade of melt electrowriting. Small Methods 2023, 7, 2201589. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, M.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Preparation and application of green sustainable solvent Cyrene. Chemistry 2023, 5, 2322–2346. [Google Scholar] [CrossRef]
- Uredat, S.; Gujare, A.; Runge, J.; Truzzolillo, D.; Oberdisse, J.; Hellweg, T. A review of stimuli-responsive polymer-based gating membranes. Phys. Chem. Chem. Phys. 2024, 26, 2732–2744. [Google Scholar] [CrossRef] [PubMed]
- Jurak, I.; Ramos, S.; Caldeira, B.; Domingues, C.; Veiga, F.; Figueiras, A. The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements. Int. J. Mol. Sci. 2024, 25, 9516. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, Y.; Ding, Y.-T.; Jiang, H.-Y.; Zhang, Z.-B.; Li, W.-X.; Liu, M.-L.; Sun, S.-P. Synergistic Construction of Sub-Nanometer Channel Membranes through MOF-Polymer Composites: Strategies and Nanofiltration Applications. Polymers 2024, 16, 1653. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Z.; Chen, J.; Ning, X.; Chen, P.; Jiang, H.; Qiu, H. Enhanced adsorption and synergistic photocatalytic degradation of tetracycline by MOF-801/Go composite via solvothermal synthesis. Environ. Sci. Nano 2022, 9, 4609–4618. [Google Scholar] [CrossRef]
- Chabalala, M.B.; Gumbi, N.N.; Mamba, B.B.; Al-Abri, M.Z.; Nxumalo, E.N. photocatalytic Nanofiber membranes for Degradation of Micropollutants and their antimicrobial Activity: Recent Advances and Future Prospects. Membranes 2021, 11, 678. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2020, 10, 2001232. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Sun, Y.; Ye, X.; Du, L.; Xu, H. Fabrication of janus Composite Membranes with a Gradient Pore Structure based on Melt Electrowriting and Solution Electrospinning for Directional water transport. Langmuir 2023, 39, 7891–7900. [Google Scholar] [CrossRef]
- Polychronopoulos, N.D.; Brouzgou, A. Direct Ink Writing for electrochemical Device fabrication: A review of 3D-Printed Electrodes and Ink Rheology. Catalysts 2024, 14, 110. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Z.; Wang, Y.; Tian, E.; Mo, J. Hierarchical diffusion pathways into VOC adsorption films by direct ink writing and ammonium carbonate treatment. Chem. Eng. J. 2023, 471, 144560. [Google Scholar] [CrossRef]
- Liang, Z.; Yun, Y.; Wang, M.; Liu, G.; Lu, P.; Yang, W.; Li, C. Performance evaluation of interfacial polymerization-fabricated aquaporin-based biomimetic membranes in forward osmosis. RSC. Adv. 2019, 9, 19163. [Google Scholar] [CrossRef]
- Ahuja, R.; Shivhare, V.; Konar, A.D. Recent Advances in Smart Self-Assembled Bioinspired Hydrogels: A Bridging Weapon for Emerging Health Care applications from Bench to Bedside. Macromol. Rapid Commun. 2024, 45, 2400255. [Google Scholar] [CrossRef]
- Osman, A.I.; Nasr, M.; Farghali, M.; Bakr, S.S.; Eltaweil, A.S.; Rashwan, A.K.; Abd El-Monaem, E.M. Machine learning for membrane design in energy production, gas separation, and water treatment: A review. Environ. Chem. Lett. 2024, 22, 505–560. [Google Scholar] [CrossRef]
- Ren, E.; Guilbaud, P.; Coudert, F.-X. High-throughput computational screening of nanoporous materials in targeted applications. Digit. Discov. 2022, 1, 355–374. [Google Scholar] [CrossRef]
- Khanzada, N.K.; AI-Juboori, R.A.; Khatri, M.; Ahmed, F.E.; Ibrahim, Y.; Hilal, N. Sustainability in membrane Technology: Membrane Recycling and fabrication Using Recycled waste. Membranes 2024, 14, 52. [Google Scholar] [CrossRef]
- Goh, W.H.D.; Lau, H.S.; Yong, W.F. An integrated life cycle assessment and techno-economic analysis: Evaluation on the production of polymers of intrinsic microporosity (PIM-1) and UiO-66-NH2 as membrane materials. Sci. Total Environ. 2023, 892, 164582. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, M.; Luo, Z.; Bi, X.; Li, J.; Zhang, S.; Wang, X. Machine learning for membrane design and discovery. Green Energy Environ. 2024, 9, 54–70. [Google Scholar] [CrossRef]
- Sharma, B.; Moghimianavval, H.; Hwang, S.W.; Liu, A.P. Synthetic Cell as a Platform for Understanding Membrane-Membrane Interactions. Membranes 2021, 11, 912. [Google Scholar] [CrossRef] [PubMed]
- Ignacz, G.; Bader, L.; Beke, A.; Ghunaim, Y.; Shastry, T.; Vovusha, H.; Carbone, M.R.; Ghanem, B.; Szekely, G. Machine learning for the advancement of membrane science and technology: A critical review. J. Membr. Sci. 2024, 713, 123256. [Google Scholar] [CrossRef]
- Maleki, R.; Shams, S.M.; Chellehbari, Y.M.; Rezvantalab, S.; Jahromi, A.M.; Asadnia, M.; Abbassi, R.; Aminabhavi, T.; Razmjou, A. Materials discovery of ion-selective membranes usinf artificial intelligence. Commun. Chem. 2022, 5, 132. [Google Scholar] [CrossRef]
- Coronado, S.; Herrera, J.; Pino, M.G.; Martin, S.; Ballesteros-Rueda, L.; Cea, P. Advancements in Engineering Planar Model Cell Membranes: Current Techniques, Applications, and Future Perspectives. Nanomaterials 2024, 14, 1489. [Google Scholar] [CrossRef]






| Period | Key Developments | Representative Publications |
|---|---|---|
| 1960s–1980s | Conceptual foundation; immobilized extractants; early SLMs | Bloch et al. [33] |
| 1990s | Formalization of PIMs; use of CTA/PVC; Aliquat 336 and D2EHPA | Almeida et al. [9], Hakami et al. [11], Zielińska et al. [12] |
| 2000s | Mechanistic studies; polymer-carrier optimization; initial modeling | Nghiem et al. [39], Cattrall et al. [40] |
| 2010–2015 | Broader applications in analytical/environmental science | Gherasim et al. [41], Kolev et al. [42,43] |
| 2016–2020 | ILs and DESs introduced as carriers; modeling transport of complex ions | Hernández-Fernández et al. [27], Matsumoto et al. [44], Kończyk et al. [45] |
| 2021–2022 | Green solvents and biodegradable polymers; sensor integration | Senila et al. [30], Sánchez-Ponce et al. [46] |
| 2023–2025 | Scalable production; biofillers; gas separation and industrial wastewater use | Adigun et al. [28], Kazemi and Yaftian [29], Kaczorowska et al. [35] |
| Carrier Type | Example | Target Ion(s) | Observed Selectivity | Ref. |
|---|---|---|---|---|
| Imidazolium Ionic Liquid | [C8mim][NTf2] + Cyanex 301 | Co2+, Ni2+ | α(Co2+/Li+) > 30 | [27,28,60,61,62,63] |
| Phosphonium Ionic Liquid | [P66614][Cl] | Ag+, Hg2+ | High selectivity for soft metals | [28] |
| Deep Eutectic Solvent (DES) | Choline chloride + glycerol | Cd2+, Zn2+, Pb2+ | Comparable to ILs; high for Cd2+, Pb2+ | [47,48,64] |
| Task-Specific Macrocycle | Calix[4]arene-phosphine oxide | La3+, Eu3+ | α(La3+/Eu3+) > 100 | [49,50] |
| Crown Ether System | 18-crown-6 + Aliquat 336 | Na+, K+ | Size-specific selectivity | [50] |
| Bio-based Carrier (Cyclodextrin) | Carboxymethyl-β-cyclodextrin | BPA, Phenols, Pb2+, Cd2+ | Strong host–guest interaction | [51,52,53,54,55,56,57] |
| Task-Specific Macrocycle | Calix[4]pyrrole derivative | Ag+ | High affinity via anion coordination | [58] |
| Task-Specific Macrocycle | Calixresorcinarene derivative | Pb2+ | >90% removal, reusable | [59] |
| Polymer Matrix | Tensile Strength (MPa) | Max Operating Temp (°C) | Acid/Base Resistance | Carrier Leaching Tendency | Reusability | Ref. |
|---|---|---|---|---|---|---|
| PVC | 40–50 | <80 | Moderate (pH 3–9) | High | Low | [65,71,72] |
| CTA | 20–40 | <90 | Moderate | Moderate | Medium | [65,71,72] |
| PVDF-HFP | 50–60 | 120 | Excellent | Low | High | [28,29,72,73] |
| PEEK | >100 | >250 | Excellent | Very Low | Very High | [30,74] |
| Cross-Linking Type | Polymer Base | Method | Key Benefits | Ref. |
|---|---|---|---|---|
| UV (PEG-DMA) | PVDF-HFP | UV + PEG-DMA | +25% modulus; −50% swelling; stable Pb(II) flux over 10 cycles | [27,75,78,79,80] |
| Thermal Cure | PVDF-HFP | Heat-curing at ~80 °C | >90% flux retention after 0.1 M HCl exposure over 72 h | [9,76] |
| Chemical (Glutaraldehyde) | CTA, PVC | Aqueous/vapor cross-linking | 20–40% extended durability in solvent/surfactant conditions | [77,81] |
| Dual-stress reinforcement | PVDF-HFP, hybrid | Epoxy/acrylate reinforcement; flow and temp. cycling | Functional flux and tensile retention after >50 h of flow at 2 bar; stable under 20–80 °C thermal cycling | [78,79,80] |
| Application | Membrane Composition | Target Contaminants | Removal Efficiency | Operational Stability | Ref. |
|---|---|---|---|---|---|
| Heavy metal removal | CTA + [Bmim][PF6] | Pb2+, Cd2+ | >90% in 2 h | 5 reuse cycles | [27] |
| Biofiller-enhanced CTA | CTA + lignin + D2EHPA | Ni2+, Co2+ | ~88% | Maintained at pH 3–6 | [28] |
| Pharmaceutical removal | PVC + Aliquat 336 | Sulfamethoxazole, DCF | 85–95% | 3 reuse cycles | [30,77] |
| Dye removal | CNC-modified CTA | Methylene Blue | ~90% | Stable after 4 cycles | [78] |
| Membrane System | CO2 Permeability (Barrer) | CO2 Selectivity | Notes | Ref. |
|---|---|---|---|---|
| IL-PVA/aniline on PSf support | ≈300 GPU | CO2/N2 ≈ 30 | Facilitated via IL-aniline chemistry | [82,83,86] |
| IL-PIM-1 + GO | ~6.169 | CO2/N2 ≈ 123.5 | Mixed-matrix, free-volume enhanced | [82,87] |
| PIM-1 + PEG (2.5 wt%) | 1.952 | CO2/CH4 ≈ 39 | Blend tailored for CH4 separation | [82,88,89] |
| Sensor Type | Target Ion(s) | LOD | Response Time | Selectivity/Reusability | Ref. |
|---|---|---|---|---|---|
| Optical (PVC-PIM) | Cd2+ | 0.02 mg/L | 20 min | High/~10 uses | [104] |
| Optical (CTA-PIM) | Fe2+ | ~0.05 mg/L | 15–25 min | Moderate/9+ days | [105] |
| Optical (Multianalyte) | Cu2+, Zn2+, Pb2+ | <0.05 mg/L | 15 min | With chemometric modeling | [30,32] |
| Electrochemical (PIM-SPCE) | Cu2+, Zn2+ | ppb range | <0 s | High/>50 cycles | [32] |
| Plasticizer | Chemical Origin | Target Polymer(s) | Biodegradability | Key Benefit in PIMs | Ref. |
|---|---|---|---|---|---|
| Triethyl citrate | Citrate ester (natural) | PLA, PHB | High | Improves flexibility, non-toxic | [8,30] |
| Glycerol | Biobased polyol | PVA, Chitosan | High | Enhances water retention, compatible with hydrophilic carriers | [28] |
| Acetyl tributyl citrate | Citrate ester (natural) | PLA, PHB | Moderate–High | Reduces brittleness, low leaching | [30,32] |
| Polyethylene glycol (PEG) | Synthetic, degradable | PLA, PVA | Moderate | Enhances elasticity and carrier dispersion | [28,30] |
| Sorbitol derivatives | Sugar alcohols | Chitosan, PVA | High | Maintains mechanical integrity and bio-compatibility | [9] |
| Polymer | Biodegradability | Mechanical Strength | Thermal Stability | Carrier Compatibility | Notable Applications | Limitations | Ref. |
|---|---|---|---|---|---|---|---|
| PLA | High (industrial composting) | Moderate | ~150 °C | Moderate | Heavy metal extraction, ion-selective PIMs | Brittle, low elongation | [8,30,108] |
| PHB | Excellent (natural degradation) | High | ~180 °C | Good | Acid/base-stable PIMs, wastewater recovery | Rigid, high crystallinity | [8,109,110] |
| PVA | Partial (aqueous biodegradation) | High | ~200 °C | Excellent | Optical sensors, hybrid PIMs | Water-sensitive, soluble in humid air | [18] |
| Chitosan | Excellent (enzymatic degradation) | Low-Moderate | ~120 °C | High | Metal-binding, biocompatible membranes | Acid solubility, lower durability | [112,113] |
| Technique | Fiber Size/Porosity | Throughput | Energy Demand | Solvent Required | Industrial Potential | Ref. |
|---|---|---|---|---|---|---|
| NIPS | 5–50 µm/up to 70% | High | Moderate | Yes | Commercially established | [30,35,114,115] |
| Electrospinning | 100–500 nm | Medium | High | Yes | Pilot to industrial | [30,116] |
| MEW | 1–10 µm | Low–Medium | High | No | Emerging/R&D | [29] |
| Research Direction | Objective | Key Challenges |
|---|---|---|
| Green Polymer Matrices | Develop fully biodegradable and non-toxic matrices | Limited mechanical stability and low compatibility with conventional carriers |
| Advanced Carrier Anchoring | Enhance carrier stability through covalent attachment or encapsulation | Synthesis complexity and limited scalability |
| Stimuli-Responsive PIMs | Enable selective transport triggered by pH, light, or temperature | Slow response time and material fatigue under cycling |
| Multiscale Modeling and Simulation | Predict transport performance under complex conditions | High computational cost and lack of experimental validation |
| Hybrid Membrane Structures | Combine PIMs with MOFs, COFs, or electrospun layers | Interfacial incompatibility and manufacturing difficulties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowik-Zając, A.; Sabadash, V. Recent Developments in Polymer Inclusion Membranes: Advances in Selectivity, Structural Integrity, Environmental Applications and Sustainable Fabrication. Membranes 2025, 15, 249. https://doi.org/10.3390/membranes15080249
Nowik-Zając A, Sabadash V. Recent Developments in Polymer Inclusion Membranes: Advances in Selectivity, Structural Integrity, Environmental Applications and Sustainable Fabrication. Membranes. 2025; 15(8):249. https://doi.org/10.3390/membranes15080249
Chicago/Turabian StyleNowik-Zając, Anna, and Vira Sabadash. 2025. "Recent Developments in Polymer Inclusion Membranes: Advances in Selectivity, Structural Integrity, Environmental Applications and Sustainable Fabrication" Membranes 15, no. 8: 249. https://doi.org/10.3390/membranes15080249
APA StyleNowik-Zając, A., & Sabadash, V. (2025). Recent Developments in Polymer Inclusion Membranes: Advances in Selectivity, Structural Integrity, Environmental Applications and Sustainable Fabrication. Membranes, 15(8), 249. https://doi.org/10.3390/membranes15080249







