Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrochemical Cell
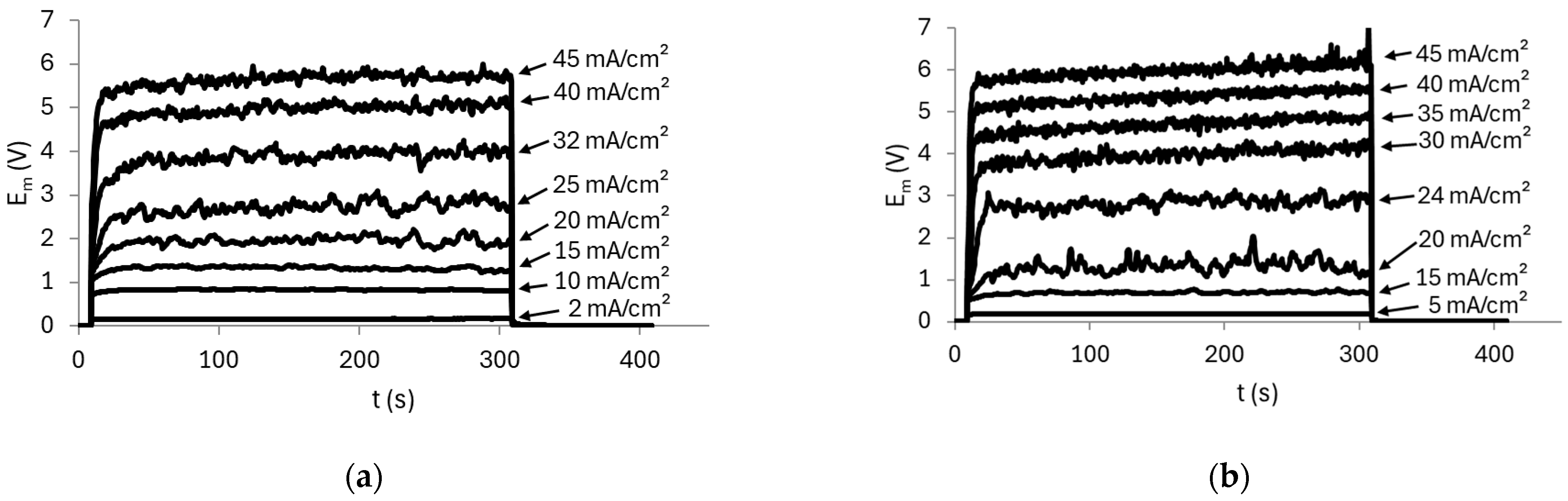
2.2. Obtention of Chronopotentiometric and Polarization Curves
2.3. Ion-Exchange Membranes
2.4. Working Solutions
3. Results
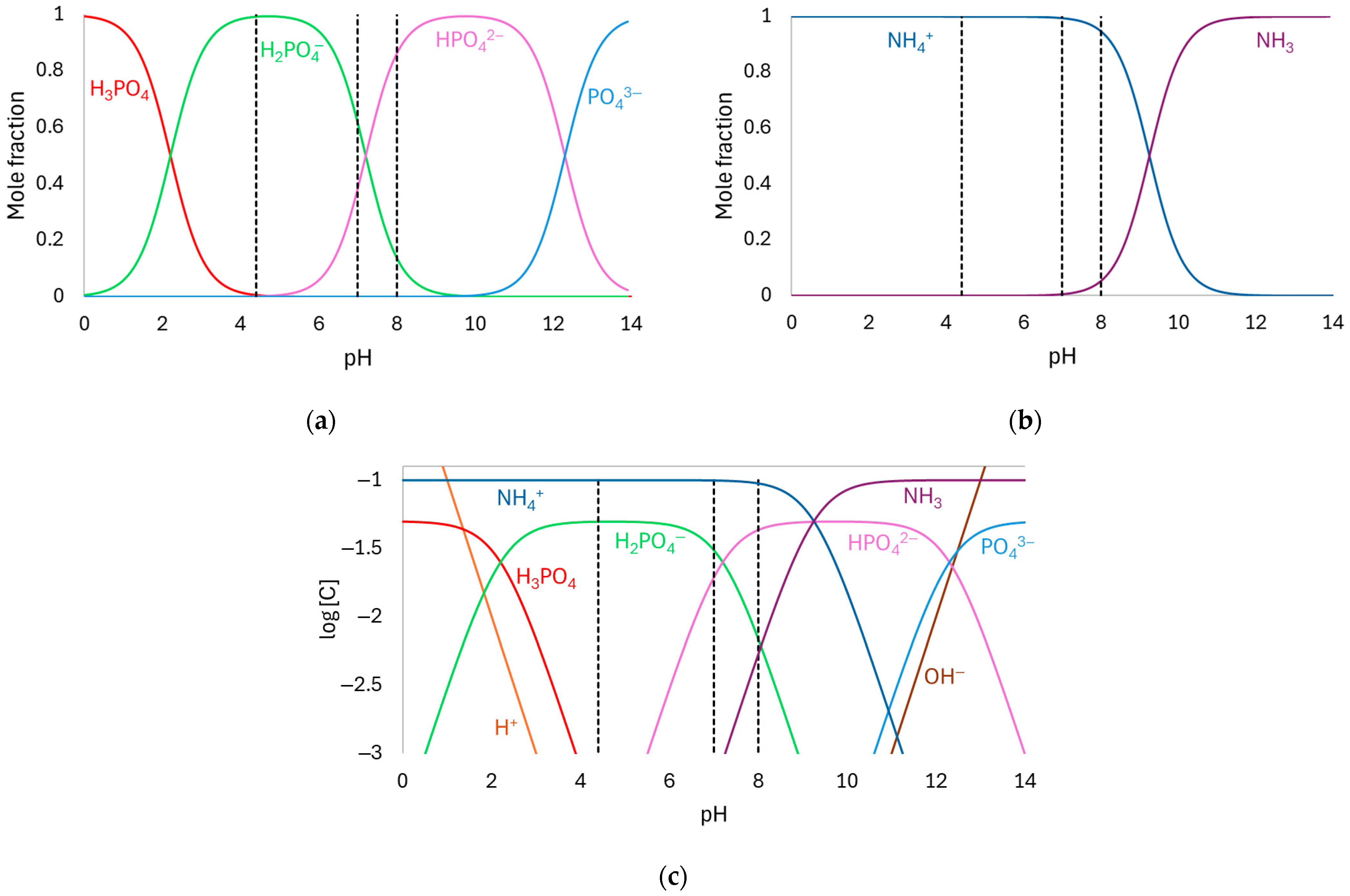
3.1. Speciation Diagram
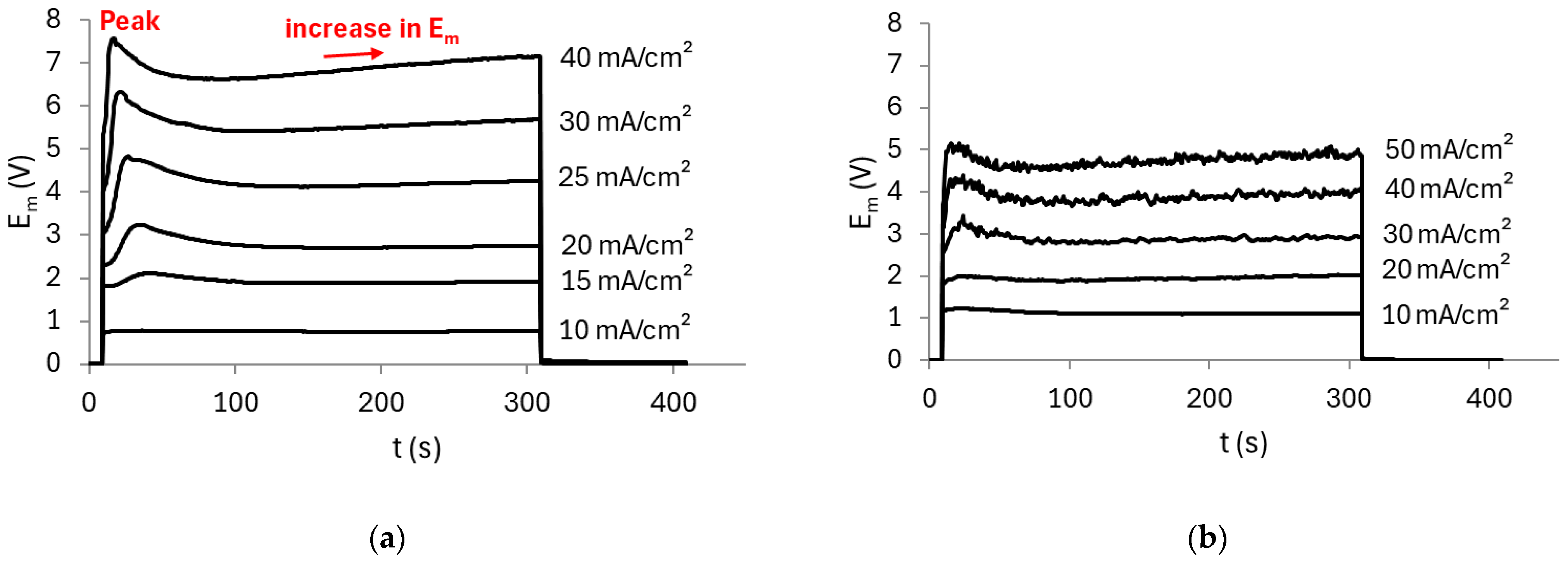
3.1.1. Stability of the Electrochemical Response of the Membrane/Solution System
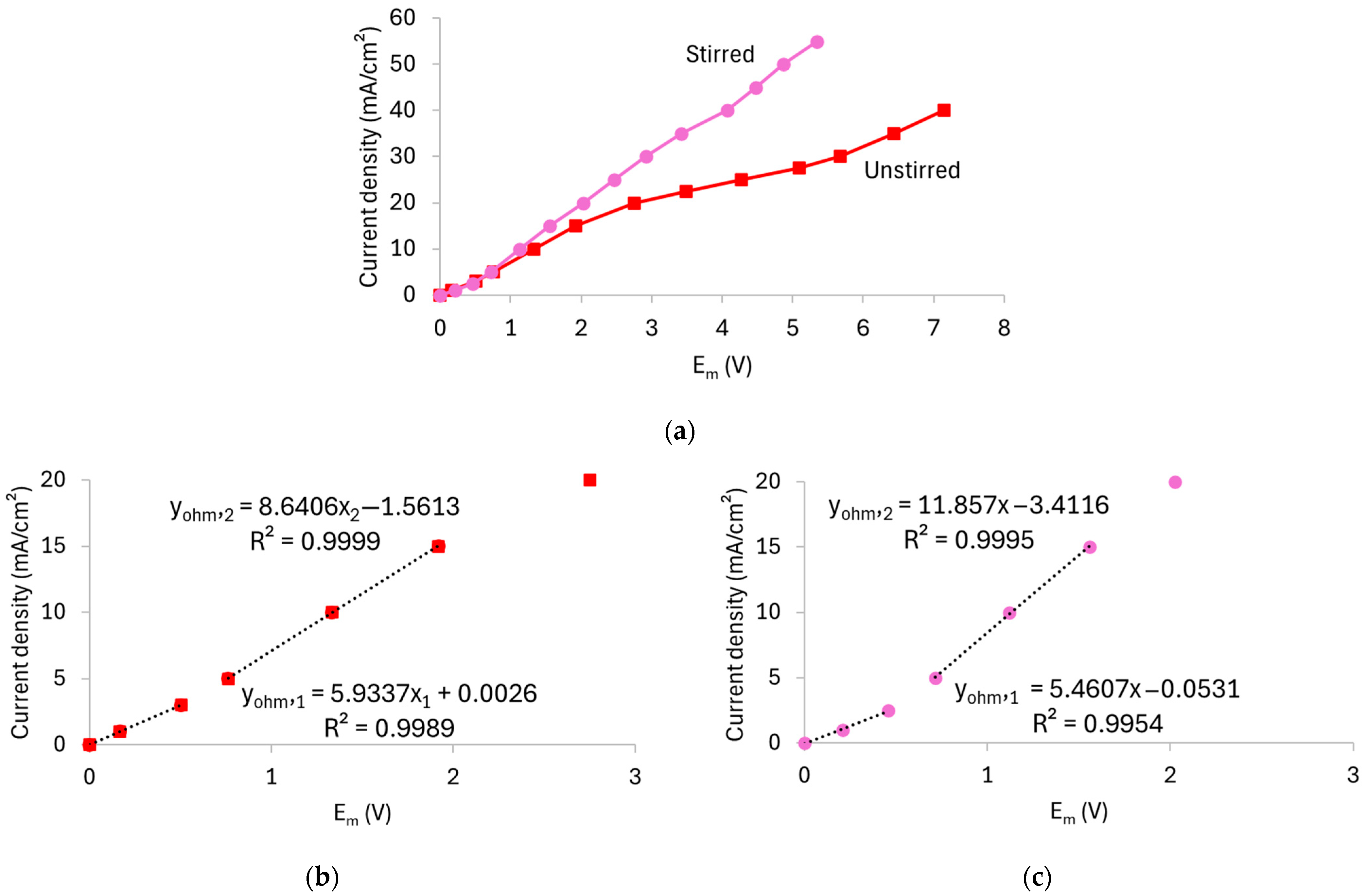
3.1.2. Effect of Stirring on the Current–Voltage Response
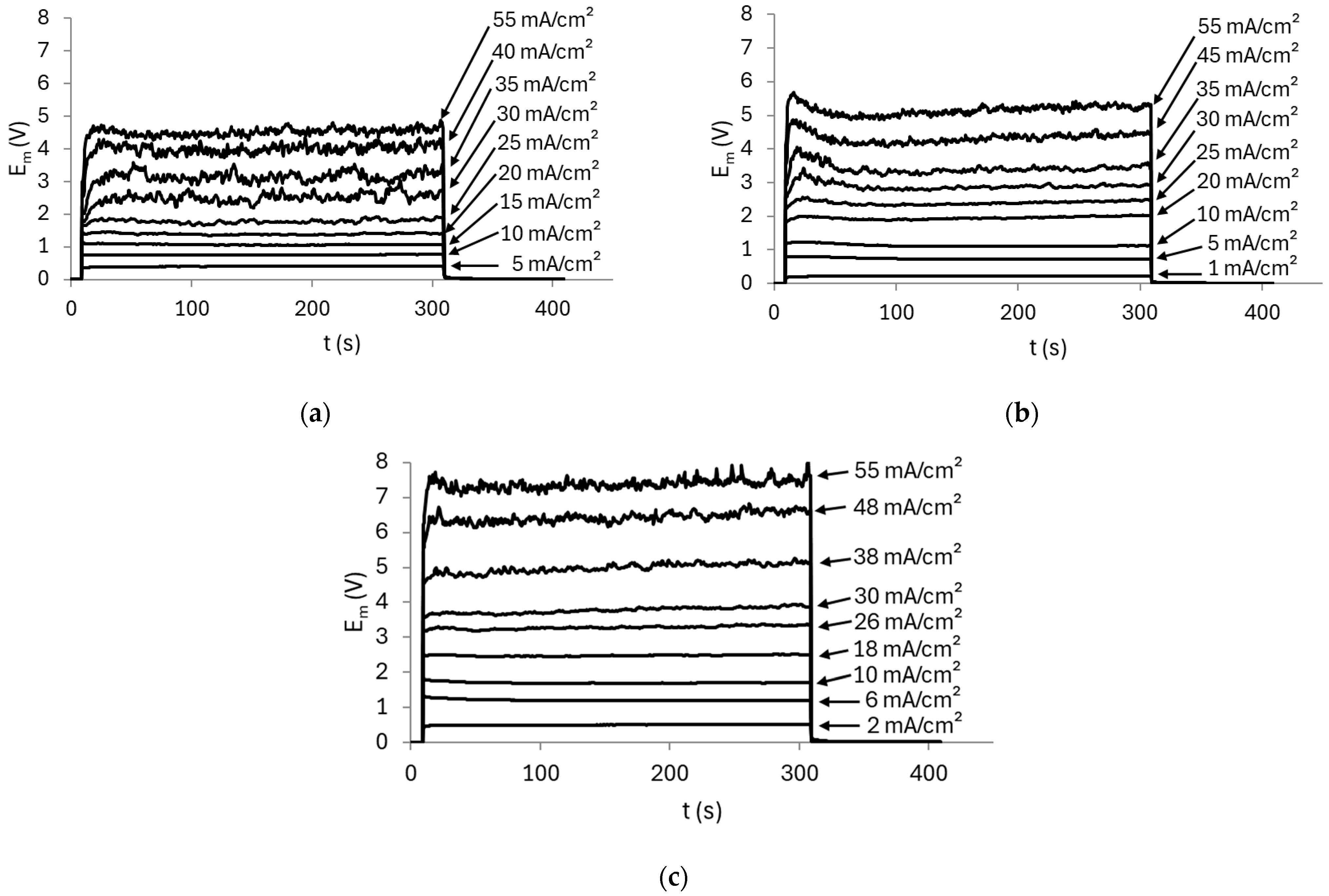
3.2. Effect of pH Under Stirring Conditions
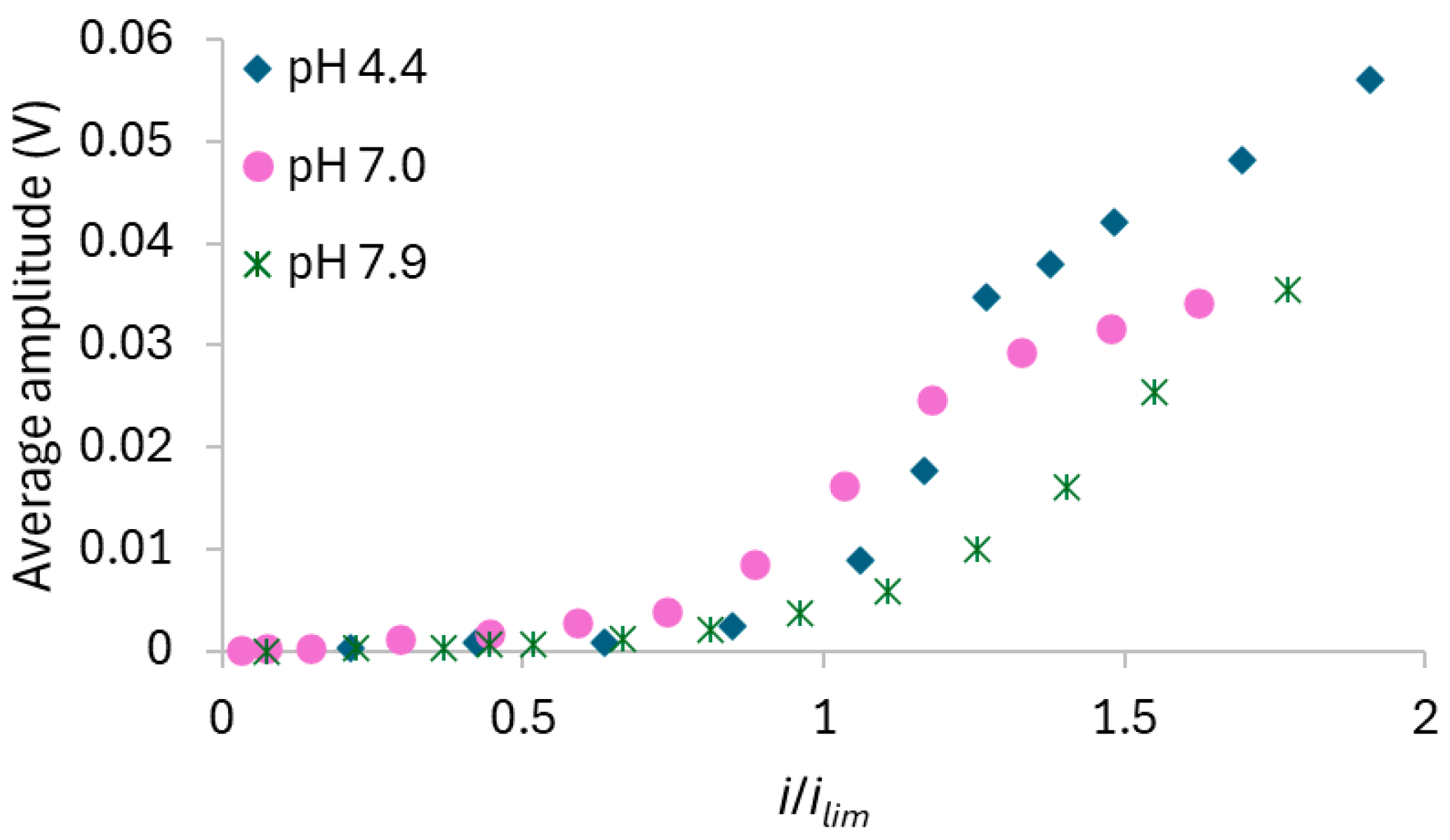
3.2.1. AMV Membrane
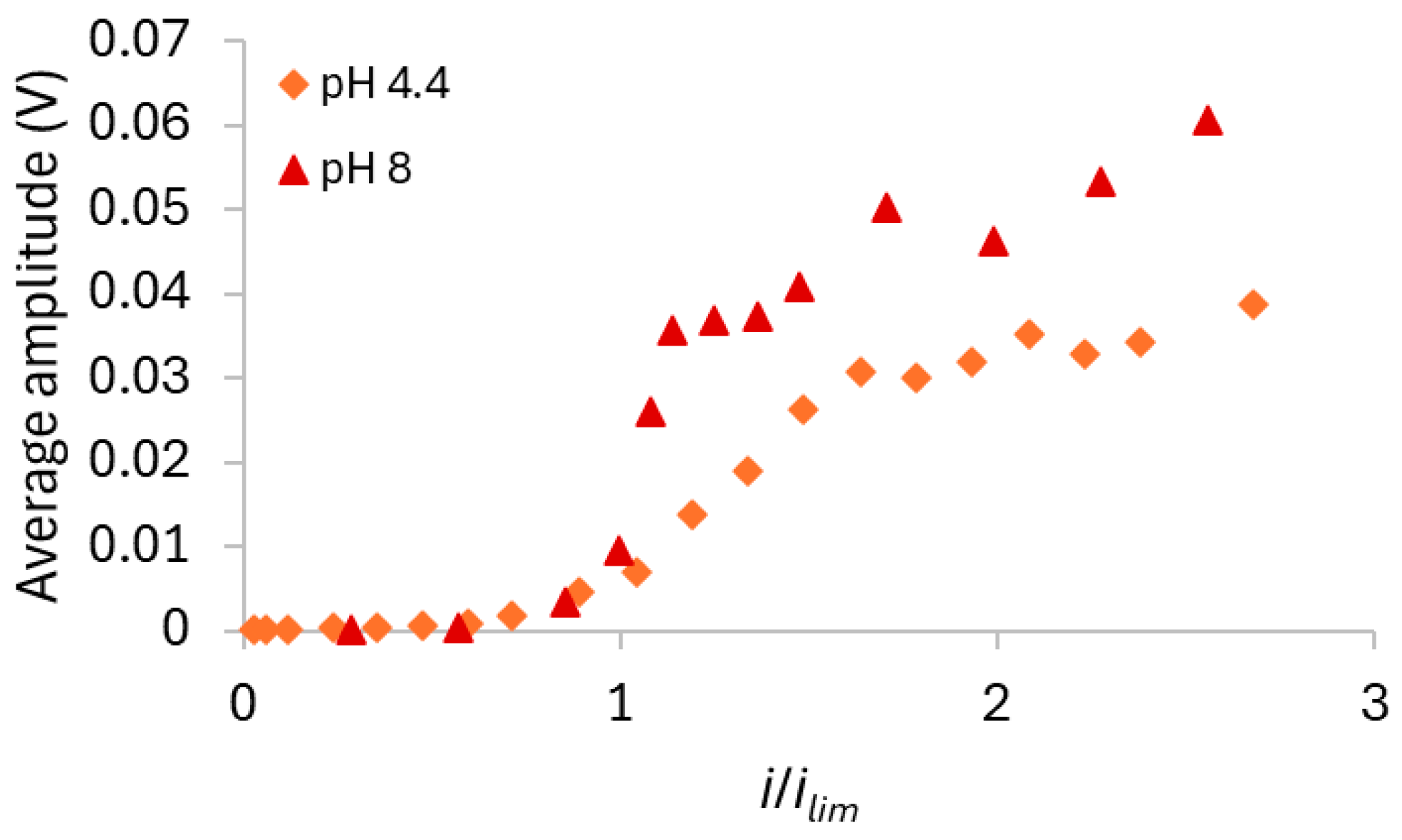
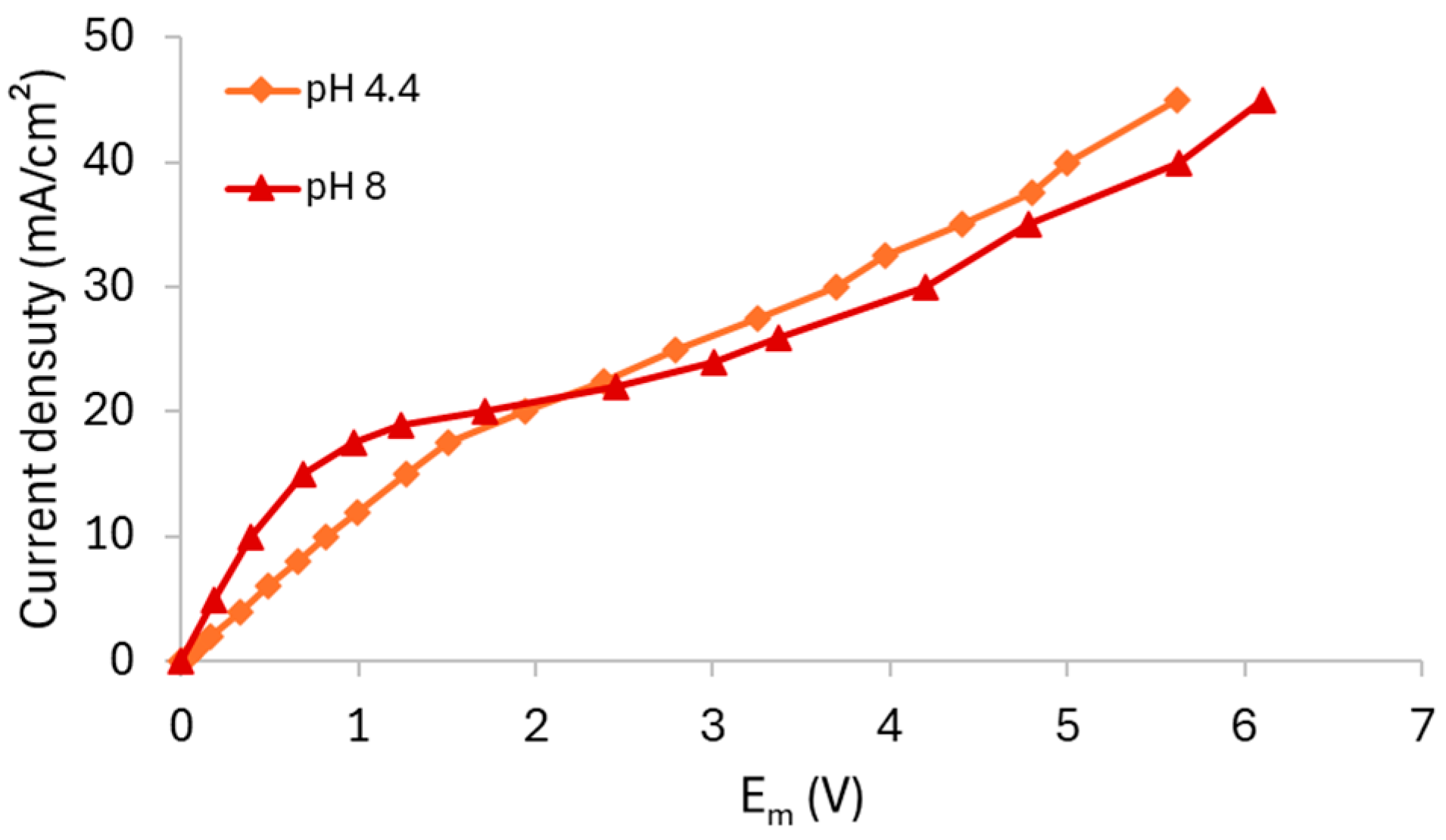
3.2.2. HC-A Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brownlie, W.J.; Sutton, M.A.; Cordell, D.; Reay, D.S.; Heal, K.V.; Withers, P.J.A.; Vanderbeck, I.; Spears, B.M. Phosphorus price spikes: A wake-up call for phosphorus resilience. Front. Sustain. Food Syst. 2023, 7, 1088776. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials. 2023. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en (accessed on 7 June 2025).
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Amador, P.; Soria, J.; Moratalla-López, J.; Rico, A. Looking beyond the surface: Understanding the role of multiple stressors on the eutrophication status of the Albufera Lake (Valencia, Spain). Sci. Total. Environ. 2024, 956, 177247. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Hernández-Crespo, C.; Andrés-Doménech, I.; Benedito-Durá, V. Fifty years of eutrophication in the Albufera lake (Valencia, Spain): Causes, evolution and remediation strategies. Ecol. Eng. 2020, 155, 105932. [Google Scholar] [CrossRef]
- Meng, J.; Shi, X.; Wang, S.; Hu, Z.; Koseoglu-Imer, D.Y.; Lens, P.N.; Zhan, X. Application of electrodialysis technology in nutrient recovery from wastewater: A review. J. Water Process Eng. 2024, 65, 105855. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Membr. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Yurchenko, O.; Mareev, S.; Zhang, C.; Pismenskaya, N.; Nikonenko, V. Study of non-stationary phosphorus transport with phosphoric acid anions through an anion-exchange membrane by chronopotentiometry: Experiments and modeling. J. Water Process Eng. 2024, 64, 105711. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Solonchenko, K.; Pasechnaya, E.; Sarapulova, V.; Wang, Y.; Jiang, C.; Xu, T.; Nikonenko, V. How Chemical Nature of Fixed Groups of Anion-Exchange Membranes Affects the Performance of Electrodialysis of Phosphate-Containing Solutions? Polymers 2023, 15, 2288. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Solonchenko, K.V.; Nikonenko, V.V.; Pismenskaya, N.D. Investigation of Causes of Low Current Efficiency in Electrodialysis of Phosphate-Containing Solutions. Membr. Membr. Technol. 2021, 3, 220–230. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Solonchenko, K.; Butylskii, D.; Nikonenko, V. Phosphates Transfer in Pristine and Modified CJMA-2 Membrane during Electrodialysis Processing of NaxH(3−x)PO4 Solutions with pH from 4.5 to 9.9. Membranes 2023, 13, 647. [Google Scholar] [CrossRef]
- Rotta, E.H.; Martí-Calatayud, M.C.; Pérez-Herranz, V.; Bernardes, A.M. Evaluation by Means of Electrochemical Impedance Spectroscopy of the Transport of Phosphate Ions through a Heterogeneous Anion-Exchange Membrane at Different pH and Electrolyte Concentration. Water 2023, 15, 9. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Mareev, S.; Rybalkina, O.; Tsygurina, K.; Nikonenko, V.; Pismenskaya, N. How do proton-transfer reactions affect current-voltage characteristics of anion-exchange membranes in salt solutions of a polybasic acid? Modeling and experiment. J. Membr. Sci. 2023, 683, 121786. [Google Scholar] [CrossRef]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Martí-Calatayud, M.C.; Barros, K.S. Concentration polarization in ion-exchange membranes. In Current Trends and Future Developments on (Bio-) Membranes: Recent Achievements for Ion-Exchange Membranes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 321–345. [Google Scholar] [CrossRef]
- Al-Amshawee, S.K.A.; Yunus, M.Y.B.M. Impact of membrane spacers on concentration polarization, flow profile, and fouling at ion exchange membranes of electrodialysis desalination: Diagonal net spacer vs. ladder-type configuration. Chem. Eng. Res. Des. 2023, 191, 197–213. [Google Scholar] [CrossRef]
- Al-Amshawee, S.K.A.; Yunus, M.Y.B.M. Electrodialysis membrane desalination with diagonal membrane spacers: A review. Environ. Sci. Pollut. Res. 2023, 32, 13064–13088. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Bittencourt, S.D.; Marder, L.; Benvenuti, T.; Ferreira, J.Z.; Bernardes, A.M. Analysis of different current density conditions in the electrodialysis of zinc electroplating process solution. Sep. Sci. Technol. 2017, 52, 2079–2089. [Google Scholar] [CrossRef]
- Gangrade, A.S.; Cassegrain, S.; Ghosh, P.C.; Holdcroft, S. Permselectivity of ionene-based, Aemion® anion exchange membranes. J. Membr. Sci. 2022, 641, 119917. [Google Scholar] [CrossRef]
- Prakash, P.; Hoskins, D.; SenGupta, A.K. Application of homogeneous and heterogeneous cation-exchange membranes in coagulant recovery from water treatment plant residuals using Donnan membrane process. J. Membr. Sci. 2004, 237, 131–144. [Google Scholar] [CrossRef]
- Dimitrova, P.; Friedrich, K.; Stimming, U.; Vogt, B. Modified Nafion®-based membranes for use in direct methanol fuel cells. Solid State Ion. 2002, 150, 115–122. [Google Scholar] [CrossRef]
- Beck, A.; Ernst, M. Kinetic modeling and selectivity of anion exchange in Donnan dialysis. J. Membr. Sci. 2015, 479, 132–140. [Google Scholar] [CrossRef]
- Dinda, M.; Chatterjee, U.; Kulshrestha, V.; Sharma, S.; Ghosh, S.; Desale, G.R.; Shahi, V.K.; Makwana, B.S.; Maru, P.; Bhadja, V.; et al. Sustainable synthesis of a high performance inter-polymer anion exchange membrane employing concentrated solar radiation in a crucial functionalization step. J. Membr. Sci. 2015, 493, 373–381. [Google Scholar] [CrossRef]
- Nafion 117. 2016. Available online: https://fuelcellearth.com/pdf/nafion-N115-N117-N1110.pdf?srsltid=AfmBOoqjPTVu5HJlzS16kVNggmnNXj0ji3VsljdJAb4fUojp7UQbUQHY (accessed on 13 March 2025).
- Izquierdo-Gil, M.; Barragán, V.; Villaluenga, J.; Godino, M. Water uptake and salt transport through Nafion cation-exchange membranes with different thicknesses. Chem. Eng. Sci. 2012, 72, 1–9. [Google Scholar] [CrossRef]
- Breytus, A.; Hasson, D.; Semiat, R.; Shemer, H. Ion exchange membrane adsorption in Donnan dialysis. Sep. Purif. Technol. 2019, 226, 252–258. [Google Scholar] [CrossRef]
- Barros, K.S.; Carvalheira, M.; Marreiros, B.C.; Reis, M.A.M.; Crespo, J.G.; Pérez-Herranz, V.; Velizarov, S. Donnan Dialysis for Recovering Ammonium from Fermentation Solutions Rich in Volatile Fatty Acids. Membranes 2023, 13, 347. [Google Scholar] [CrossRef]
- Barros, K.S.; Giacobbo, A.; Agnol, G.D.; Velizarov, S.; Pérez–Herranz, V.; Bernardes, A.M. Evaluation of mass transfer behaviour of sulfamethoxazole species at ion–exchange membranes by chronopotentiometry for electrodialytic processes. J. Electroanal. Chem. 2023, 931, 117214. [Google Scholar] [CrossRef]
- Barros, K.S.; Marreiros, B.C.; Reis, M.A.; Crespo, J.G.; Pérez-Herranz, V.; Velizarov, S. Recovery and fractionation of volatile fatty acids from fermented solutions by electrodialysis: Electrochemical characterization of anion-exchange membranes. J. Environ. Chem. Eng. 2024, 12, 114457. [Google Scholar] [CrossRef]
- Uzdenova, A. 2D mathematical modelling of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells in galvanodynamic mode. Membranes 2019, 9, 39. [Google Scholar] [CrossRef]
- Belashova, E.D.; Kharchenko, O.A.; Sarapulova, V.V.; Nikonenko, V.V.; Pismenskaya, N.D. Effect of Protolysis Reactions on the Shape of Chronopotentiograms of a Homogeneous Anion-Exchange Membrane in NaH2PO4 Solution. Pet. Chem. 2017, 57, 1207–1218. [Google Scholar] [CrossRef]
- Melnikova, E.; Pismenskaya, N.; Bazinet, L.; Mikhaylin, S.; Nikonenko, V. Effect of ampholyte nature on current-voltage characteristic of anion-exchange membrane. Electrochimica Acta 2018, 285, 185–191. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Belashova, E.; Pismenskaya, N.; Nikonenko, V.; Sistat, P.; Pourcelly, G. Current-voltage characteristic of anion-exchange membrane in monosodium phosphate solution. Modelling and experiment. J. Membr. Sci. 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Mareev, S.; Moroz, I.; Nikonenko, V.; Pismenskaya, N. Partial fluxes of phosphoric acid anions through anion-exchange membranes in the course of NaH2PO4 solution electrodialysis. Int. J. Mol. Sci. 2019, 20, 3593. [Google Scholar] [CrossRef]
- Hernández-Pérez, L.; Martí-Calatayud, M.C.; Montañés, M.T.; Pérez-Herranz, V. Interplay between Forced Convection and Electroconvection during the Overlimiting Ion Transport through Anion-Exchange Membranes: A Fourier Transform Analysis of Membrane Voltage Drops. Membranes 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Urtenov, M.; Uzdenova, A.; Kovalenko, A.; Nikonenko, V.; Pismenskaya, N.; Vasil’Eva, V.; Sistat, P.; Pourcelly, G. Basic mathematical model of overlimiting transfer enhanced by electroconvection in flow-through electrodialysis membrane cells. J. Membr. Sci. 2013, 447, 190–202. [Google Scholar] [CrossRef]
- Gil, V.V.; Andreeva, M.A.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Dammak, L. Effect of counterion hydration numbers on the development of Electroconvection at the surface of heterogeneous cation-exchange membrane modified with an MF-4SK film. Pet. Chem. 2016, 56, 440–449. [Google Scholar] [CrossRef]
- Chiang, Y.; Kresge, A.J.; VAN Do, S.; Weeks, D.P. ChemInform Abstract: Catalysis by Undissociated H3PO4 in Aqueous H2PO4-/HPO42- Buffer Solutions: Dependence on the Magnitude of the Brnsted Exponent. J. Org. Chem. 1987, 51, 4035–4037. [Google Scholar] [CrossRef]
- Rybalkina, O.; Tsygurina, K.; Melnikova, E.; Pourcelly, G.; Nikonenko, V.; Pismenskaya, N. Catalytic effect of ammonia-containing species on water splitting during electrodialysis with ion-exchange membranes. Electrochim. Acta 2019, 299, 946–962. [Google Scholar] [CrossRef]
- Barros, K.S.; Martí-Calatayud, M.C.; Ortega, E.M.; Pérez-Herranz, V.; Espinosa, D.C.R. Chronopotentiometric study on the simultaneous transport of EDTA ionic species and hydroxyl ions through an anion-exchange membrane for electrodialysis applications. J. Electroanal. Chem. 2020, 879, 114782. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Moroz, I.; Mareev, S.; Nikonenko, V. Influence of electroconvection on chronopotentiograms of an anion-exchange membrane in solutions of weak polybasic acid salts. Int. J. Mol. Sci. 2021, 22, 13518. [Google Scholar] [CrossRef]
- Eiberweiser, A.; Nazet, A.; Hefter, G.; Buchner, R. Ion hydration and association in aqueous potassium phosphate solutions. J. Phys. Chem. B 2015, 119, 5270–5281. [Google Scholar] [CrossRef]
- Gil, V.; Andreeva, M.; Jansezian, L.; Han, J.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Dammak, L. Impact of heterogeneous cation-exchange membrane surface modification on chronopotentiometric and current–voltage characteristics in NaCl, CaCl2 and MgCl2 solutions. Electrochim. Acta 2018, 281, 472–485. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Mareev, S.A.; Pis’Menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [Google Scholar] [CrossRef]
- Nikonenko, V.; Nebavsky, A.; Mareev, S.; Kovalenko, A.; Urtenov, M.; Pourcelly, G. Modelling of ion transport in electromembrane systems: Impacts of membrane bulk and surface heterogeneity. Appl. Sci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]


| Selemion AMV | Ionsep-HC-A [19] | Nafion 117 | |
|---|---|---|---|
| Ion group attached | |||
| Heterogeneity | Homogeneous [20] | Heterogeneous | Homogeneous [21,22] |
| Thickness (µm) | 110–120 [23] | 420 [24] | 183 [25] |
| Ion-exchange capacity (Eq/kg) | 1.68 eq/kg [20] | >1.8 (eq/kgdry) | 1.86 eq/kg [26] |
| Density (g/cm3) | 1.1 [27] | Not reported | 1.98 [26] |
| Water uptake (%) | 19 [23] | 30–45 | 38 [25] |
| Resistance (Ω·cm2) | 1.5–3.0 [23] | <20 | Not reported |
| Transport number | >0.96 [20] | >0.89 | Not reported |
| ilim (mA/cm2) | Rohm,1 (Ω·cm2) | Rohm,2 (Ω·cm2) | Roverlim (Ω·cm2) | |
|---|---|---|---|---|
| Unstirred | 15.9 | 168 | 116 | 148 |
| Stirred | 33.9 | 183 | 84 | 84 |
| (meq/L) | ilim (mA/cm2) | Rohm,1 (Ω·cm2) | Rohm,2 (Ω·cm2) | Roverlim (Ω·cm2) | |
|---|---|---|---|---|---|
| pH 4.4 | 49.8 | 23.6 | Not present | 70 | 48 |
| pH 7 | 69.3 | 33.9 | 183 | 84 | 95 |
| pH 8 | 93.2 | 27.1 | 255 | 99 | 145 |
| (meq/L) | ilim (mA/cm2) | Rohm (Ω·cm2) | Roverlim (Ω·cm2) | |
|---|---|---|---|---|
| pH 4.4 | 49.8 | 16.8 | 82 | 131 |
| pH 8 | 93.2 | 17.6 | 39 | 132 |
| Category | AMV Membrane (Homogeneous) | HC-A Membrane (Heterogeneous) |
|---|---|---|
| Ion transport (ohmic conditions) | Simultaneous transport of H2PO4− and HPO42− at pH 7 and 8. Only H2PO4− at pH 4. | No evidence of simultaneous transport of different phosphate species at any pH. |
| Behavior under overlimiting current | Electroconvection at low pH. Significant water dissociation at high pH. Local pH shifts and chemical equilibrium displacement. | Strong electroconvection at all pH values. No water dissociation. No chemical equilibrium displacement. |
| Effect of solution stirring | Intense oscillations in chronopotentiograms under overlimiting current densities. Increase in limiting current density. Reduced electrical resistance. Compression of transition (plateau) region. | Same effects observed as in AMV membrane: changes in ChPs and CVCs due to stirring. |
| NH3 formation (pH 7–8) | Detected at the membrane–solution interface. Associated with increased membrane resistance. | Also detected at the membrane–solution interface. Also associated with increased membrane resistance. |
| pH sensitivity | High sensitivity: transport mechanisms and electrochemical behavior strongly influenced by pH. | Low sensitivity: consistent electrochemical behavior across different pH values. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Barros, K.; Martí-Calatayud, M.C.; Velizarov, S.; Pérez-Herranz, V. Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications. Membranes 2025, 15, 230. https://doi.org/10.3390/membranes15080230
Santana-Barros K, Martí-Calatayud MC, Velizarov S, Pérez-Herranz V. Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications. Membranes. 2025; 15(8):230. https://doi.org/10.3390/membranes15080230
Chicago/Turabian StyleSantana-Barros, Kayo, Manuel César Martí-Calatayud, Svetlozar Velizarov, and Valentín Pérez-Herranz. 2025. "Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications" Membranes 15, no. 8: 230. https://doi.org/10.3390/membranes15080230
APA StyleSantana-Barros, K., Martí-Calatayud, M. C., Velizarov, S., & Pérez-Herranz, V. (2025). Phosphate Transport Through Homogeneous and Heterogeneous Anion-Exchange Membranes: A Chronopotentiometric Study for Electrodialytic Applications. Membranes, 15(8), 230. https://doi.org/10.3390/membranes15080230









