Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications
Abstract
1. Introduction
| Membrane | Preparation Method | Characterization | Application | Relevant Properties | Ref. |
|---|---|---|---|---|---|
| Na-CMC/PEO | Dip coating on PEO supports | Thermal stability (N2), FESEM, EIS, Na transference number, interfacial properties, LSV and galvanostatic charge (Na+ deinsertion)/discharge (Na+ insertion) | Na-ion batteries | Mechanical resistance as dimensional stability | [23] |
| CMC chitosan membrane | Solution casting | ATR-FTIR, 1H NMR, EA, XRD, EIS and SEM | Development of alternative membrane materials not petroleum-related | Ionic conductivity (0.37 mS at 60 °C) Interaction polymer solvent (acetic acid) by ATR-FTIR | [13] |
| PVA-CS membrane | Blend and solution casting | EIS, LSV and TNM | Li-ion batteries | Ionic conductivity (0.84 mS/cm) vs. ion transport | [19] |
| PVA-KOH hydrogels | Solution casting | OCV (using 6 M KOH), WU, XRD, TGA, XPS, ATR-FTIR, | Zn–air batteries | Functionalization to avoid ZnO precipitation—stability | [27] |
| CS-PVA-Fe2O3 | Solution casting and soaked in iron sand leachate | XRD, FTIR, SEM WU, tensile testing, IEC, methanol permeability and proton conductivity | DMFC | relationship among IEC, ionic conductivity and alcohol crossover | [25] |
| CS-PVA-based mixed-matrix membranes | Blending | SEM, XPS, XRD, TGA, WU, IEC, specific conductivity, alcohol permeability and PEM reactor performance | Organic electrosynthesis in alkaline media | Correlation among IEC, anion conductivity and crossover | [11] |
| MA-chitosan membrane | Blending | TGA, WU, SD, IEC and EIS (H+) | PEMFC | IEC and proton conductivity correlation | [15] |
| PVA-CS AEM | Solution casting, quaternized or not | TGA, FTIR, EIS and alkaline stability | AEMFC | Alkaline stability reduced ionic conductivity loss with time | [14] |
| PVA/PVP AEM | Solution casting | FTIR, TGA, SEM, EIS, KOH uptake, SEM | AEMFC | KOH uptake increased membrane stability | [24] |
2. Materials and Methods
2.1. Membrane Preparation
2.2. Membrane Characterization
2.2.1. Membrane Thickness and Morphology
2.2.2. Water and KOH Uptake
2.2.3. Ion-Exchange Capacity (IEC)
2.2.4. Streaming Potential
2.2.5. NaCl and KOH Permeability
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl alcohol |
| CS | Chitosan |
| ZO | Zinc oxide |
| POP | Porous organic polymer |
| MMM | Mixed-matrix membrane |
| AEM | Anion exchange membrane |
| PEG | Polyethylene glycol |
| PEO | Polyethylene oxide |
| CMC | Carboxymethyl cellulose |
| PVP | Polyvinylpyrrolidone |
| LSV | Linear Sweep Voltammetry |
| ATR-FTIR | Attenuated Total Reflectance–Fourier Transform Infrared |
| FESEM | Field Emission Scanning Electron Microscopy |
| EA | Elemental analysis |
| XRD | X-ray Diffraction |
| EIS | Electrochemical Impedance Spectroscopy |
| SEM | Scanning Electron Microscopy |
| TNM | Transference number analysis |
| OCV | Open Circuit Voltage |
| WU | Water uptake |
| XPS | X-ray Photoelectron Spectroscopy |
| IEC | Ionexchange capacity |
| DMFC | Direct methanol fuel cell |
| KHP | Potassium hydrogen phatalate |
| MA | Maleic anhydride |
References
- Qiu, Z.; Yun, Y.; He, M.; Wang, L. Recent Developments in Ion Conductive Membranes for CO2 Electrochemical Reduction. Chem. Eng. J. 2023, 456, 140942. [Google Scholar] [CrossRef]
- Aili, D.; Kraglund, M.R.; Rajappan, S.C.; Serhiichuk, D.; Xia, Y.; Deimede, V.; Kallitsis, J.; Bae, C.; Jannasch, P.; Henkensmeier, D.; et al. Electrode Separators for the Next-Generation Alkaline Water Electrolyzers. ACS Energy Lett. 2023, 8, 1900–1910. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for Zinc-Air Batteries: Recent Progress, Challenges and Perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Su, F.C.; Yu, H.H.; Yang, H. Anion-Exchange Membranes’ Characteristics and Catalysts for Alkaline Anion-Exchange Membrane Fuel Cells. Membranes 2024, 14, 246. [Google Scholar] [CrossRef]
- Bose, I.; Nousheen; Roy, S.; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Matei (Brazdis), R.I.; Manaila-Maximean, D. Natural and Natural-Based Polymers: Recent Developments in Management of Emerging Pollutants. Polymers 2023, 15, 2063. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2021, 3, 32–58. [Google Scholar] [CrossRef]
- Santos, F.; Urbina, A.; Abad, J.; López, R.; Toledo, C.; Fernández Romero, A.J. Environmental and Economical Assessment for a Sustainable Zn/Air Battery. Chemosphere 2020, 250, 126273. [Google Scholar] [CrossRef]
- Santos, F.; Tafur, J.P.; Abad, J.; Fernández Romero, A.J. Structural Modifications and Ionic Transport of PVA-KOH Hydrogels Applied in Zn/Air Batteries. J. Electroanal. Chem. 2019, 850, 113380. [Google Scholar] [CrossRef]
- Maiti, J.; Kakati, N.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Where Do Poly(Vinyl Alcohol) Based Membranes Stand in Relation to Nafion® for Direct Methanol Fuel Cell Applications? J. Power Sources 2012, 216, 48–66. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Iniesta, J.; Montiel, V.; Irabien, Á. Chitosan: Poly (Vinyl) Alcohol Composite Alkaline Membrane Incorporating Organic Ionomers and Layered Silicate Materials into a PEM Electrochemical Reactor. J. Memb. Sci. 2016, 498, 395–407. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan Biopolymer for Fuel Cell Applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ahmad, A.; Abdullah, M.P.; Ramli, N.; Rahman, M.Y.A. Conductivity Enhancement via Chemical Modification of Chitosan Based Green Polymer Electrolyte. Electrochim. Acta 2013, 92, 161–167. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Dhavale, V.M.; Bhat, S.D. Development of Polyvinyl Alcohol/Chitosan Blend Anion Exchange Membrane with Mono and Di Quaternizing Agents for Application in Alkaline Polymer Electrolyte Fuel Cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar] [CrossRef]
- Septiawan, M.R.; Permana, D.; Sabarwati, S.H.; Ahmad, L.O.; Ramadhan, L.O.A.N. Functionalization of Chitosan with Maleic Anhydride for Proton Exchange Membrane. Indones. J. Chem. 2018, 18, 313–320. [Google Scholar] [CrossRef]
- Song, F.; Fu, Y.; Gao, Y.; Li, J.; Qiao, J.; Zhou, X.D.; Liu, Y. Novel Alkaline Anion-Exchange Membranes Based on Chitosan/Ethenylmethylimidazoliumchloride Polymer with Ethenylpyrrolidone Composites for Low Temperature Polymer Electrolyte Fuel Cells. Electrochim. Acta 2015, 177, 137–144. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Q.; Ma, W.; Dai, Y.; Zhang, S.; Wang, F.; Zhu, H. Hydrophilic Chitosan-Doped Composite Diaphragm Reducing Gas Permeation for Alkaline Water Electrolysis Producing Hydrogen. ACS Appl. Mater. Interfaces 2024, 16, 1394–1403. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; Iniesta, J.; Irabien, A. Use of Chitosan as Copper Binder in the Continuous Electrochemical Reduction of CO2 to Ethylene in Alkaline Medium. Membranes 2022, 12, 783. [Google Scholar] [CrossRef]
- Brza, M.; Aziz, S.B.; Saeed, S.R.; Hamsan, M.H.; Majid, S.R.; Abdulwahid, R.T.; Kadir, M.F.Z.; Abdullah, R.M. Energy Storage Behavior of Lithium-Ion Conducting Poly(Vinyl Alcohol) (Pva): Chitosan(Cs)-Based Polymer Blend Electrolyte Membranes: Preparation, Equivalent Circuit Modeling, Ion Transport Parameters, and Dielectric Properties. Membranes 2020, 10, 381. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M.; Popa, M.; Calderon Moreno, J.M. Chitosan Hydrogels for Water Purification Applications. Gels 2023, 9, 664. [Google Scholar] [CrossRef]
- Sapna; Sharma, R.; Kumar, D. Chitosan-Based Membranes for Wastewater Desalination and Heavy Metal Detoxification; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 154–196. [Google Scholar]
- Wang, J.; Zheng, X.; Wu, H.; Zheng, B.; Jiang, Z.; Hao, X.; Wang, B. Effect of Zeolites on Chitosan/Zeolite Hybrid Membranes for Direct Methanol Fuel Cell. J. Power Sources 2008, 178, 9–19. [Google Scholar] [CrossRef]
- Colò, F.; Bella, F.; Nair, J.R.; Destro, M.; Gerbaldi, C. Cellulose-Based Novel Hybrid Polymer Electrolytes for Green and Efficient Na-Ion Batteries. Electrochim. Acta 2015, 174, 185–190. [Google Scholar] [CrossRef]
- Qiao, J.; Fu, J.; Lin, R.; Ma, J.; Liu, J. Alkaline Solid Polymer Electrolyte Membranes Based on Structurally Modified PVA/PVP with Improved Alkali Stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Permana, D.; Ilimu, E.; Kadidae, L.O. Synthesis and Characterization of Chitosan-Polyvinyl Alcohol-Fe2O3 Composite Membrane for DMFC Application. Makara J. Sci. 2020, 24, 1–9. [Google Scholar] [CrossRef]
- García-Cruz, L.; Casado-Coterillo, C.; Iniesta, J.; Montiel, V.; Irabien, Á. Preparation and Characterization of Novel Chitosan-Based Mixed Matrix Membranes Resistant in Alkaline Media. J. Appl. Polym. Sci. 2015, 132, 42240. [Google Scholar] [CrossRef]
- Santos, F.; Abad, J.; Vila, M.; Castro, G.R.; Urbina, A.; Fernández Romero, A.J. In Situ Synchrotron X-Ray Diffraction Study of Zn/Bi2O3 Electrodes Prior to and during Discharge of Zn-Air Batteries: Influence on ZnO Deposition. Electrochim. Acta 2018, 281, 133–141. [Google Scholar] [CrossRef]
- Matesanz-Niño, L.; Moranchel-Pérez, J.; Álvarez, C.; Lozano, Á.E.; Casado-Coterillo, C. Mixed Matrix Membranes Using Porous Organic Polymers (POPs)—Influence of Textural Properties on CO2/CH4 Separation. Polymers 2023, 15, 4135. [Google Scholar] [CrossRef]
- Wang, M.; Xu, N.; Fu, J.; Liu, Y.; Qiao, J. High-Performance Binary Cross-Linked Alkaline Anion Polymer Electrolyte Membranes for All-Solid-State Supercapacitors and Flexible Rechargeable Zinc-Air Batteries. J. Mater. Chem. A 2019, 7, 11257–11264. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Kutz, R.; Masel, R.I. CO2 elettrolysis to CO and O2 at high selectivity, stability and efficiency using Sustainion membranes. J. Electrochem. Soc. 2018, 165, 3371–3377. [Google Scholar] [CrossRef]
- Imaan, D.U.; Mir, F.Q.; Ahmad, B. Synthesis and Characterization of a Novel Poly (Vinyl Alcohol)-Based Zinc Oxide (PVA-ZnO) Composite Proton Exchange Membrane for DMFC. Int. J. Hydrogen Energy 2021, 46, 12230–12241. [Google Scholar] [CrossRef]
- Rico-Martínez, S.; Álvarez, C.; Hernández, A.; Miguel, J.A.; Lozano, Á.E. Mixed Matrix Membranes Loaded with a Porous Organic Polymer Having Bipyridine Moieties. Membranes 2022, 12, 547. [Google Scholar] [CrossRef]
- Wang, J.; Willson, T.R.; Brückner, S.; Whelligan, D.K.; Sun, C.; Liang, L.; Wang, X.; Strasser, P.; Varcoe, J.; Ju, W. Design of NiNC Single Atom Catalyst Layers and AEM Electrolyzers for Stable and Efficient CO2-to-CO Electrolysis: Correlating Ionomer and Cell Performance. Electrochim. Acta 2023, 461, 142613. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, X.; Wu, H.; Pan, F. Proton Conducting Membranes Prepared by Incorporation of Organophosphorus Acids into Alcohol Barrier Polymers for Direct Methanol Fuel Cells. J. Power Sources 2008, 185, 85–94. [Google Scholar] [CrossRef]
- Permana, D.; Purwanto, M.; Ramadhan, L.O.A.N.; Atmaja, L. Synthesis and Characterization of Chitosan/Phosphotungstic Acid-Montmorillonite Modified by Silane for DMFC Membrane. Indones. J. Chem. 2015, 15, 218–225. [Google Scholar] [CrossRef]
- Franck-Lacaze, L.; Sistat, P.; Huguet, P. Determination of the PKa of Poly (4-Vinylpyridine)-Based Weak Anion Exchange Membranes for the Investigation of the Side Proton Leakage. J. Memb. Sci. 2009, 326, 650–658. [Google Scholar] [CrossRef]
- Zawadzki, J.; Kaczmarek, H. Thermal Treatment of Chitosan in Various Conditions. Carbohydr. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. A Thermal Degradation Mechanism of Polyvinyl Alcohol/Silica Nanocomposites. Polym. Degrad. Stab. 2007, 92, 1061–1071. [Google Scholar] [CrossRef]
- Koyano, T.; Koshizaki, N.; Umehara, H.; Nagura, M.; Minoura, N. Surface States of PVA/Chitosan Blended Hydrogels. Polymer 2000, 41, 4461–4465. [Google Scholar] [CrossRef]
- Shchipunov, Y.; Sarin, S.; Kim, I.; Ha, C.S. Hydrogels Formed through Regulated Self-Organization of Gradually Charging Chitosan in Solution of Xanthan. Green Chem. 2010, 12, 1187–1195. [Google Scholar] [CrossRef]
- Venu Rajendran, M.; Ganesan, S.; Sudhakaran Menon, V.; Raman, R.K.; Alagumalai, A.; Ashok Kumar, S.; Krishnamoorthy, A. Manganese Dopant-Induced Isoelectric Point Tuning of ZnO Electron Selective Layer Enable Improved Interface Stability in Cesium-Formamidinium-Based Planar Perovskite Solar Cells. ACS Appl. Energy Mater. 2022, 5, 6671–6686. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, X.; Zhang, Q.; Cai, J. Rapid dissolution of chitin and chitosan with degree of deacetylation less than 80% in KOH/urea aqueous solution. Green Chem. 2023, 25, 8593–8605. [Google Scholar] [CrossRef]
- Casado-Coterillo, C. Mixed Matrix Membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental Water and Salt Transport Properties of Polymeric Materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Torre-celeizabal, A.; Garea, A.; Casado-Coterillo, C. Chitosan: Polyvinyl alcohol based mixed matrix sustainable coatings for resuing membrens in water treatment: Fouling characterization. Chem. Eng. J. Adv. 2022, 9, 100236. [Google Scholar] [CrossRef]


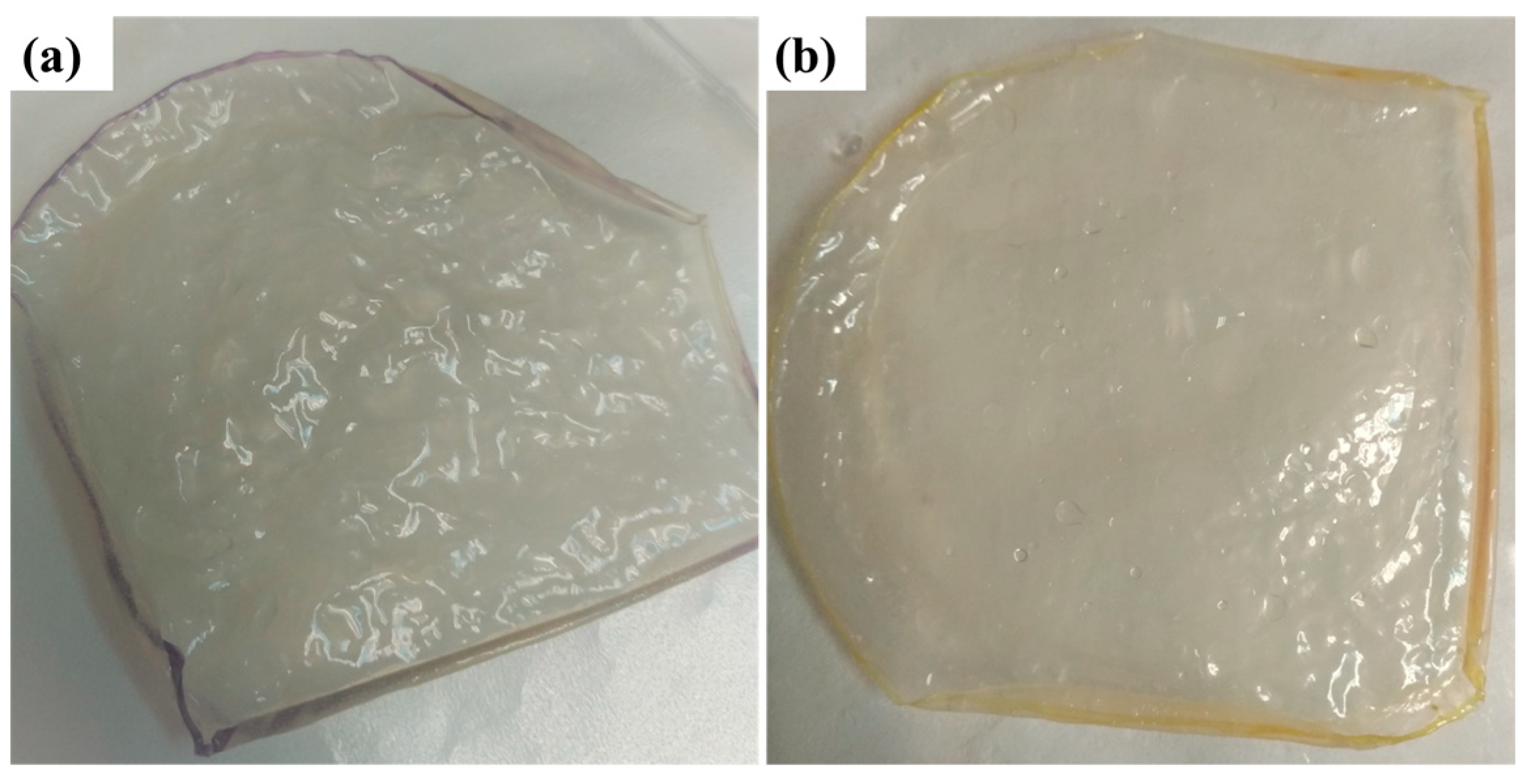

 PVA,
PVA,  PVA-CS-5,
PVA-CS-5,  PVA-CS-6,
PVA-CS-6,  PVA-CS-7,
PVA-CS-7,  P15,
P15,  Sustainion and
Sustainion and  PE (Celgard 3601)).
PE (Celgard 3601)).
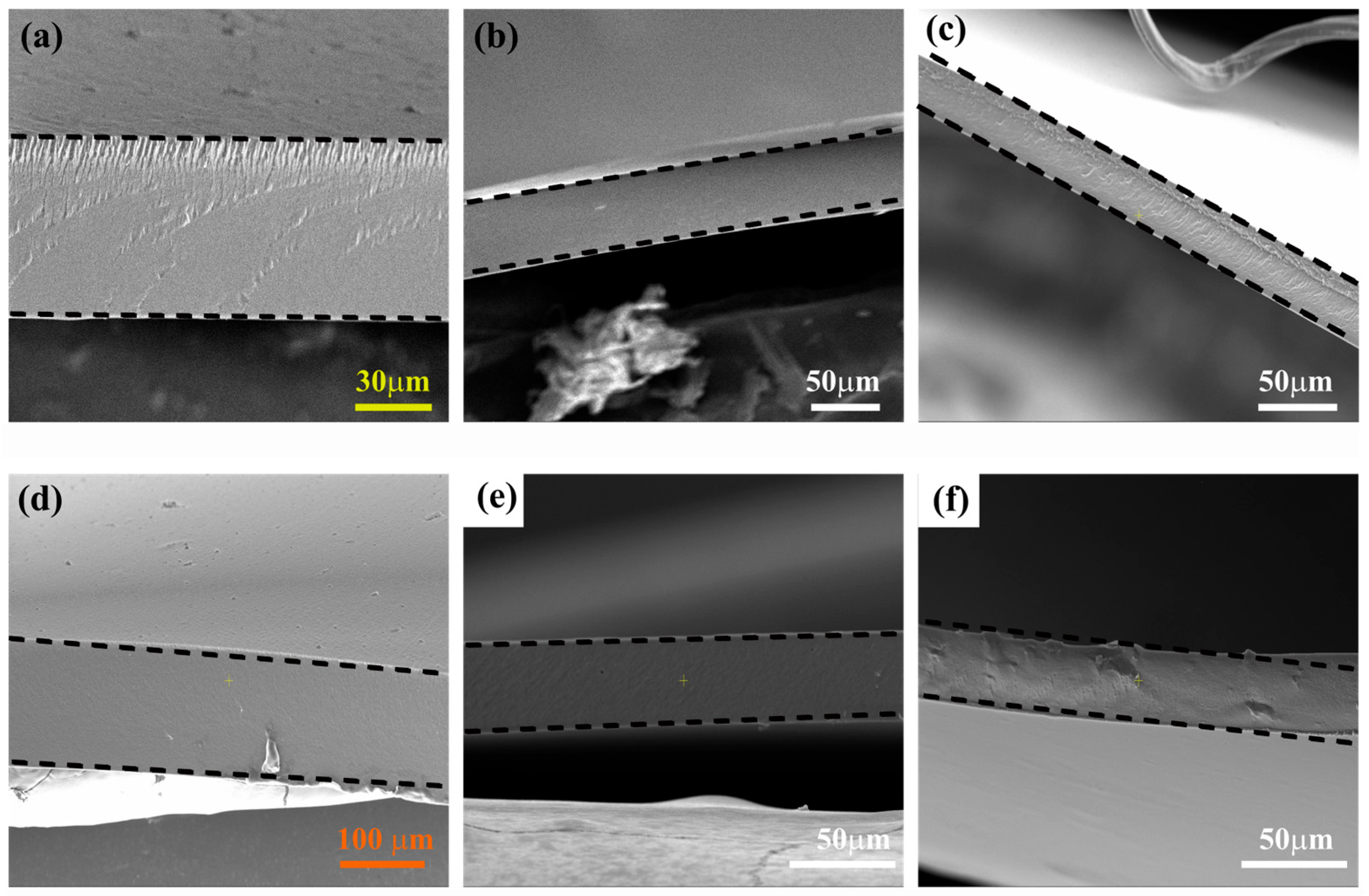
 PVA,
PVA,  PVA-CS-5,
PVA-CS-5,  PVA-CS-6,
PVA-CS-6,  PVA-CS-7,
PVA-CS-7,  P15,
P15,  Sustainion and
Sustainion and  PE (Celgard 3601)).
PE (Celgard 3601)).
 PVA,
PVA,  PVA-CS-5,
PVA-CS-5,  PVA-CS-6,
PVA-CS-6,  PVA-CS-7,
PVA-CS-7,  Z10 and
Z10 and  P10).
P10).
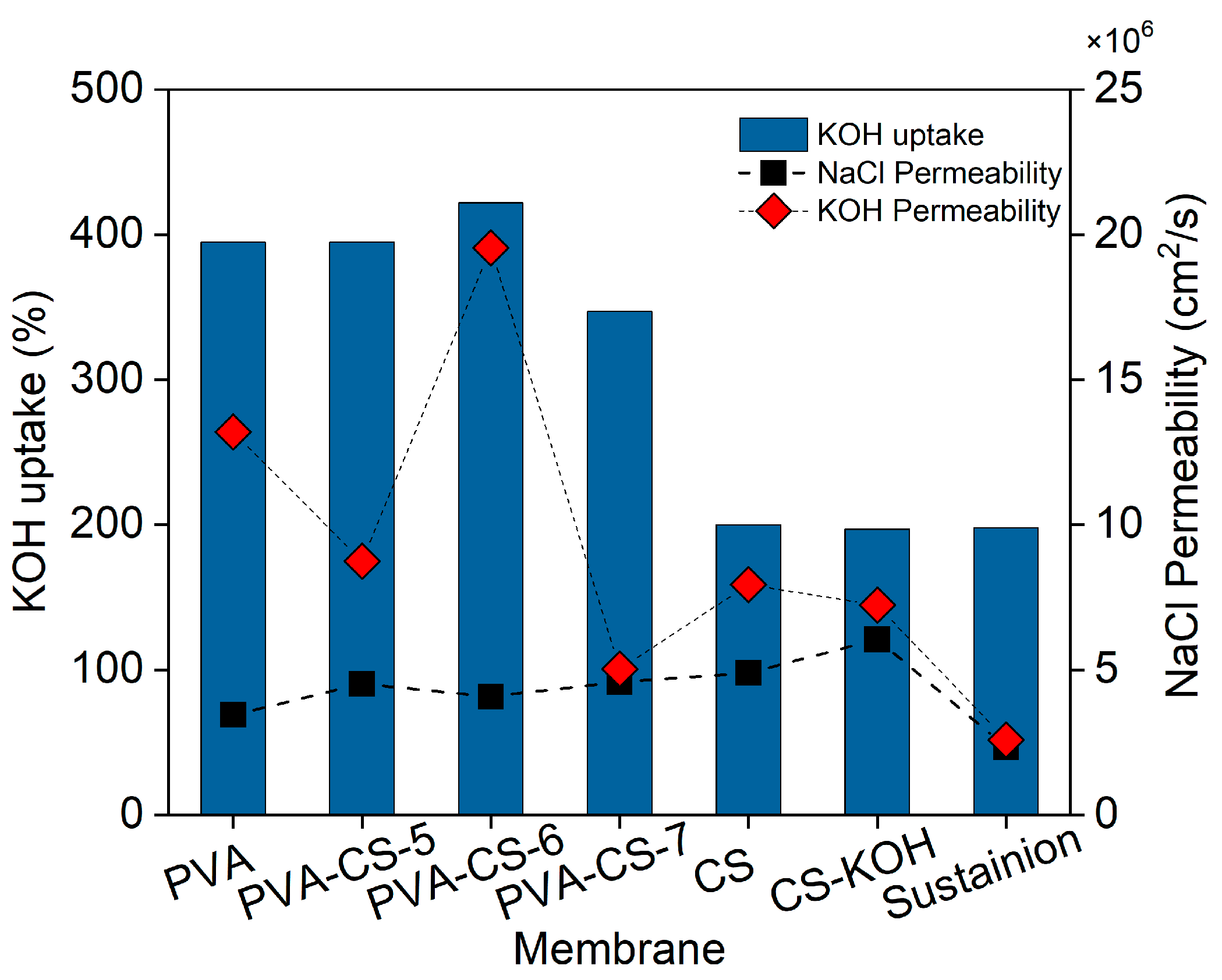
 PVA,
PVA,  PVA-CS-5,
PVA-CS-5,  PVA-CS-6,
PVA-CS-6,  PVA-CS-7,
PVA-CS-7,  Z10 and
Z10 and  P10).
P10).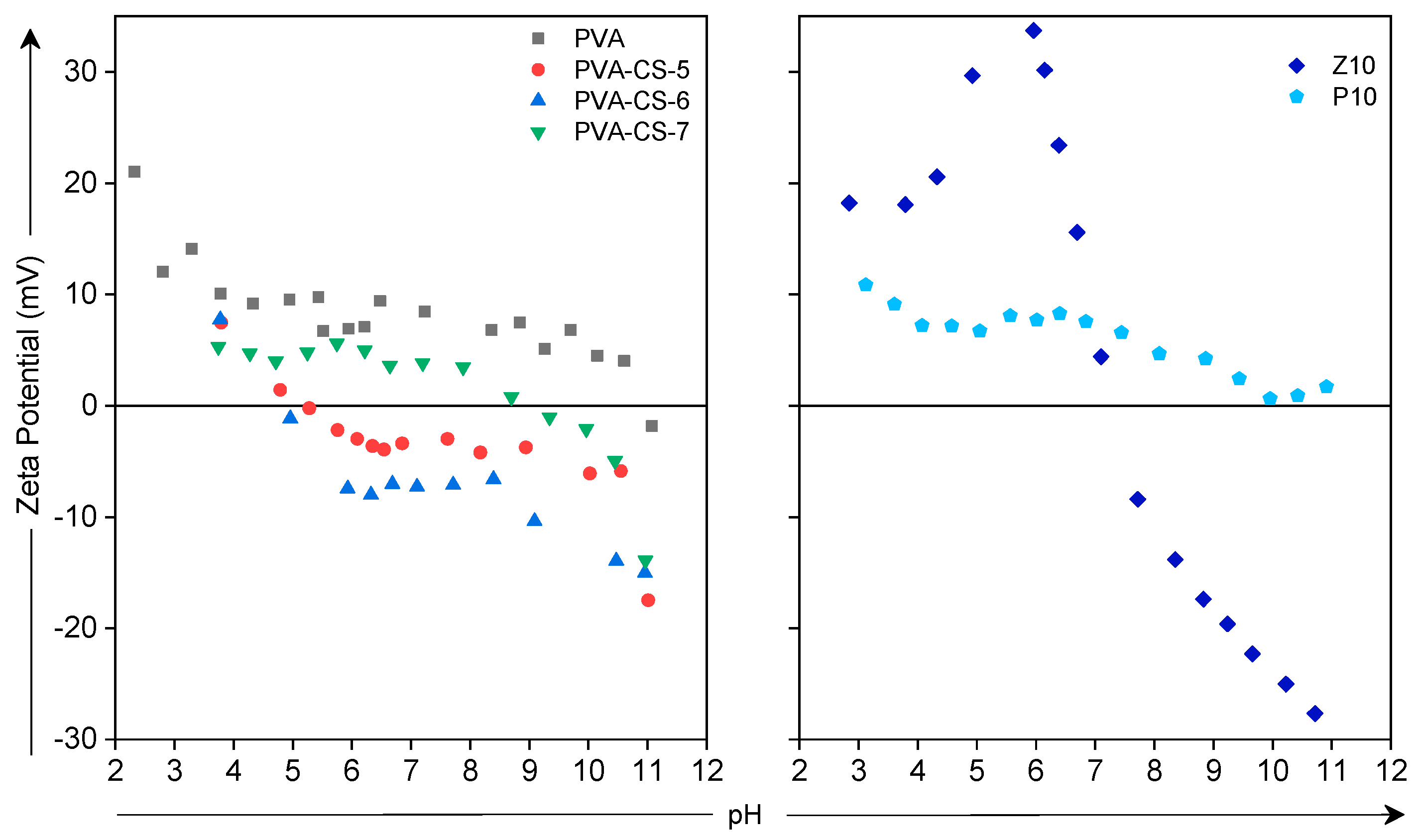
| Membrane Code | PVA Solution Concentration | CS Solution Concentration | CS | Nanomaterial | |
|---|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | Type | (wt.%) | |
| PVA | 4 | - | 0 | - | - |
| CS | - | 4 | 100 | - | - |
| CS-KOH | - | 4 | 100 | - | - |
| PVA-CS-5 | 4 | 2 | 50 | - | - |
| PVA-CS-6 | 4 | 2 | 60 | - | - |
| PVA-CS-7 | 4 | 2 | 70 | - | - |
| Z5 | 4 | 2 | 60 | ZnO | 5 |
| Z10 | 4 | 2 | 60 | ZnO | 10 |
| Z15 | 4 | 2 | 60 | ZnO | 15 |
| P5 | 4 | 2 | 60 | POP-1 | 5 |
| P10 | 4 | 2 | 60 | POP-1 | 10 |
| P15 | 4 | 2 | 60 | POP-1 | 15 |
| Membrane | KOH Uptake (%) | Water Uptake (%) |
|---|---|---|
| CS | 200 | 162 |
| CS-KOH | 197 | 221 |
| PVA | 394 ± 7 | 83.3 ± 0.6 |
| PVA-CS-5 | 405 ± 10 | 82.3 ± 1.5 |
| PVA-CS-6 | 380 ± 61 | 82.3 ± 3.1 |
| PVA-CS-7 | 325 ± 22 | 81.0 ± 1.0 |
| Z5 | 491 | 90 |
| Z10 | 542 | 91 |
| Z15 | 458 (M20) | 70 (M27) |
| P5 | 298 | 88 |
| P10 | 261 | 81 |
| P15 | 288 | 85 |
| Sustainion | 198 | 169 80 1 |
| Celgard | 85 | 140 |
| Membrane | IEC (mmol/g Dry Membrane) | Reference |
|---|---|---|
| CS-KOH | 0.25 1 | This work |
| PVA | 0.13 | This work |
| PVA-CS-5 | 0.29 | This work |
| PVA-CS-6 | 0.26 | This work |
| Z15 | 0.69 | This work |
| P10 | 0.16 | This work |
| Sustainion | 2.52 0.95 | [1] [34] |
| Celgard | 0.05 | This work |
| CS-PVA | 0.27 | [11] |
| PVAPVA/CS | 0.03 2 0.04 2 | [14] |
| PVA-CS-HDT | 0.28 | [35] |
| PCS-PVA-H 5050 Fe2O3 | 1.36 | [10] |
| PCS-PVA-H 6040 Fe2O3 | 1.145 | [25] |
| PVA-PGG-GP | 1.52 | [29] |
| Membrane | Water Content (%) | Td (°C) | Weight Loss (%) |
|---|---|---|---|
| Celgard 3601 | 10.7 | 193 | 9.84 |
| Sustainion | 7.45 | 211 | 12.2 |
| PVA | 24.2 | 190 | 14.0 |
| PVA-CS-6 | 14.8 | 222 | 10.0 |
| PVA-CS-5 | 25.8 | 252 | 12.5 |
| PVA-CS-7 | 27.5 | 252 | 13.9 |
| P15 | 9.62 | 220 | 15.2 |
| Membrane | PNaCl (cm2/s) · 106 | PKOH (cm2/s) · 106 |
|---|---|---|
| CS | 4.89 | 7.94 |
| CS-KOH | 6.05 | 7.23 |
| PVA | 3.45 | 13.20 |
| PVA-CS-5 | 4.52 | 8.75 |
| PVA-CS-6 | 4.08 | 19.54 |
| PVA-CS-7 | 4.59 | 5.03 |
| Z5 | 7.61 | 8.29 |
| Z10 | 8.88 | 19.40 |
| Z15 | 1.06 | NA |
| P5 | 5.20 | 9.01 |
| P10 | 4.94 | 8.38 |
| P15 | 11.60 | 16.40 |
| Sustainion | 2.33 | 2.59 |
| Celgard | 1.23 | 1.39 |
| Membrane | PNaCl (cm2/s) · 106 |
|---|---|
| PVA | 1.48 |
| PVA-CS-5 | 4.98 |
| PVA-CS-6 | 3.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurklu-Kocaoglu, S.; Ramírez-Espinosa, D.; Casado-Coterillo, C. Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications. Membranes 2025, 15, 229. https://doi.org/10.3390/membranes15080229
Kurklu-Kocaoglu S, Ramírez-Espinosa D, Casado-Coterillo C. Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications. Membranes. 2025; 15(8):229. https://doi.org/10.3390/membranes15080229
Chicago/Turabian StyleKurklu-Kocaoglu, Suer, Daniela Ramírez-Espinosa, and Clara Casado-Coterillo. 2025. "Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications" Membranes 15, no. 8: 229. https://doi.org/10.3390/membranes15080229
APA StyleKurklu-Kocaoglu, S., Ramírez-Espinosa, D., & Casado-Coterillo, C. (2025). Enhanced OH− Transport Properties of Bio-Based Anion-Exchange Membranes for Different Applications. Membranes, 15(8), 229. https://doi.org/10.3390/membranes15080229










