Electrodialysis Metathesis for the Production of Potassium Phosphate
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Materials
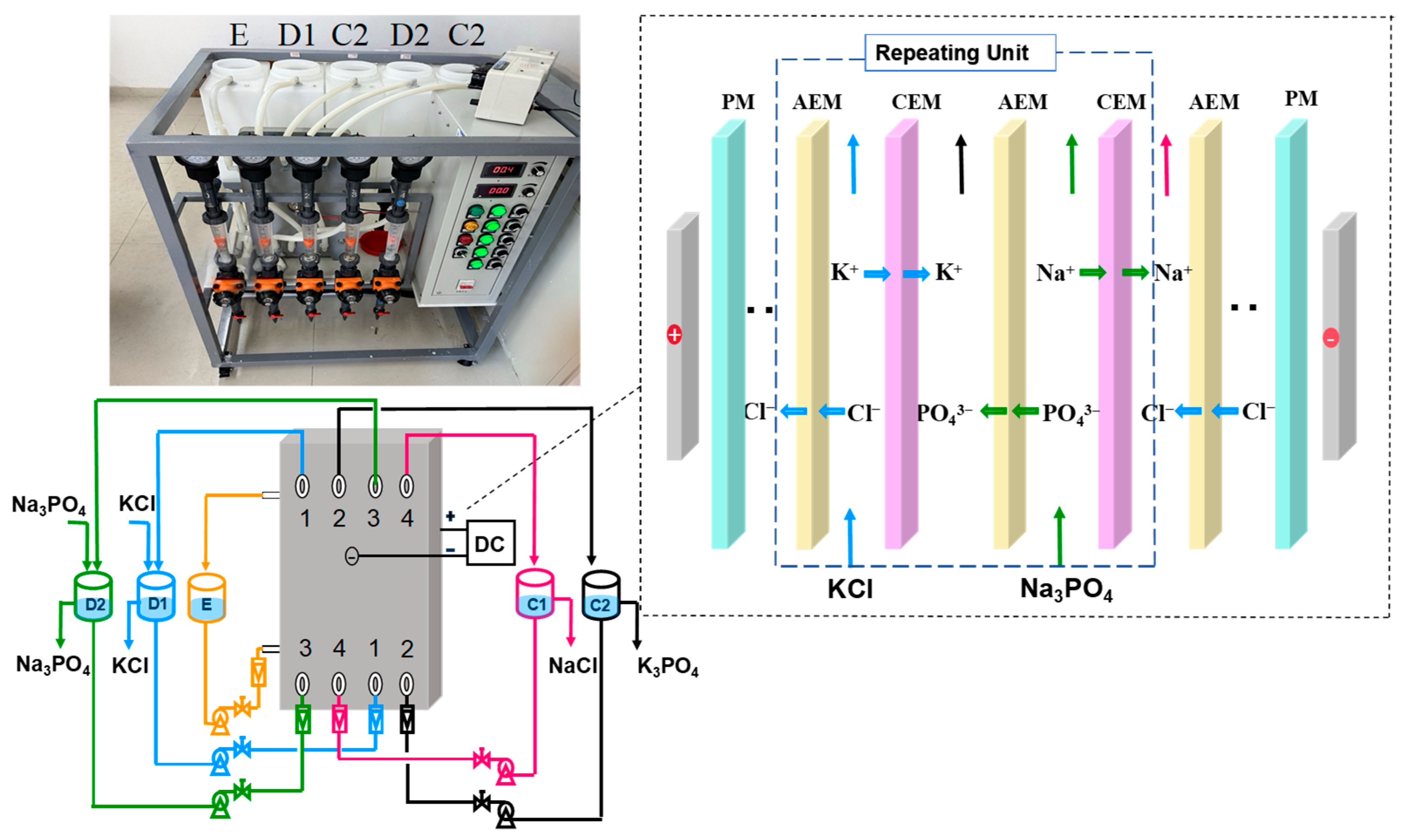
2.2. EDM Equipment
2.3. EDM Experimental Procedure
2.4. Analysis and Calculation
2.4.1. Concentration Analysis Methods
2.4.2. Parameter Calculation Methods
- 1.
- Ion flux;
- 2.
- Apparent water flux;
- 3.
- Energy Consumption;
- 4.
- Current efficiency;
- 5.
- Purity.
3. Results and Discussion
3.1. Effect of Voltage
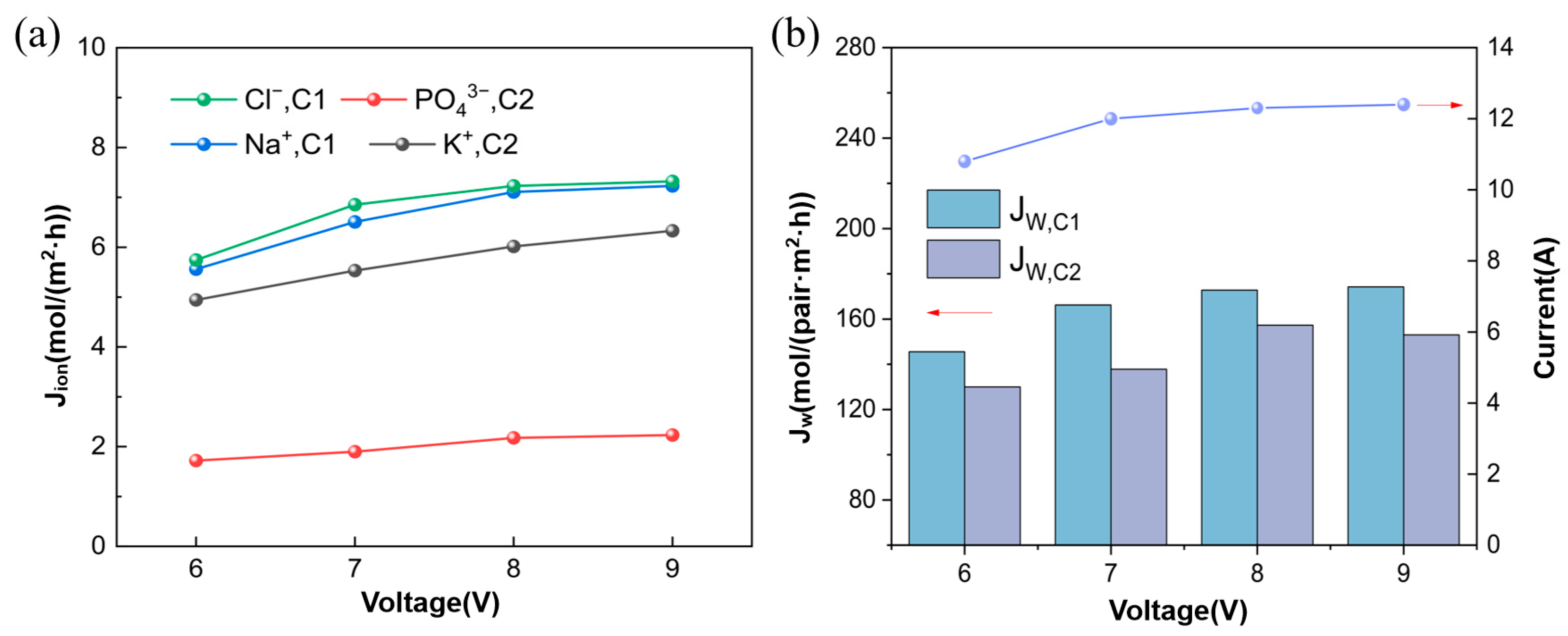
3.1.1. Ion Flux and Apparent Water Flux
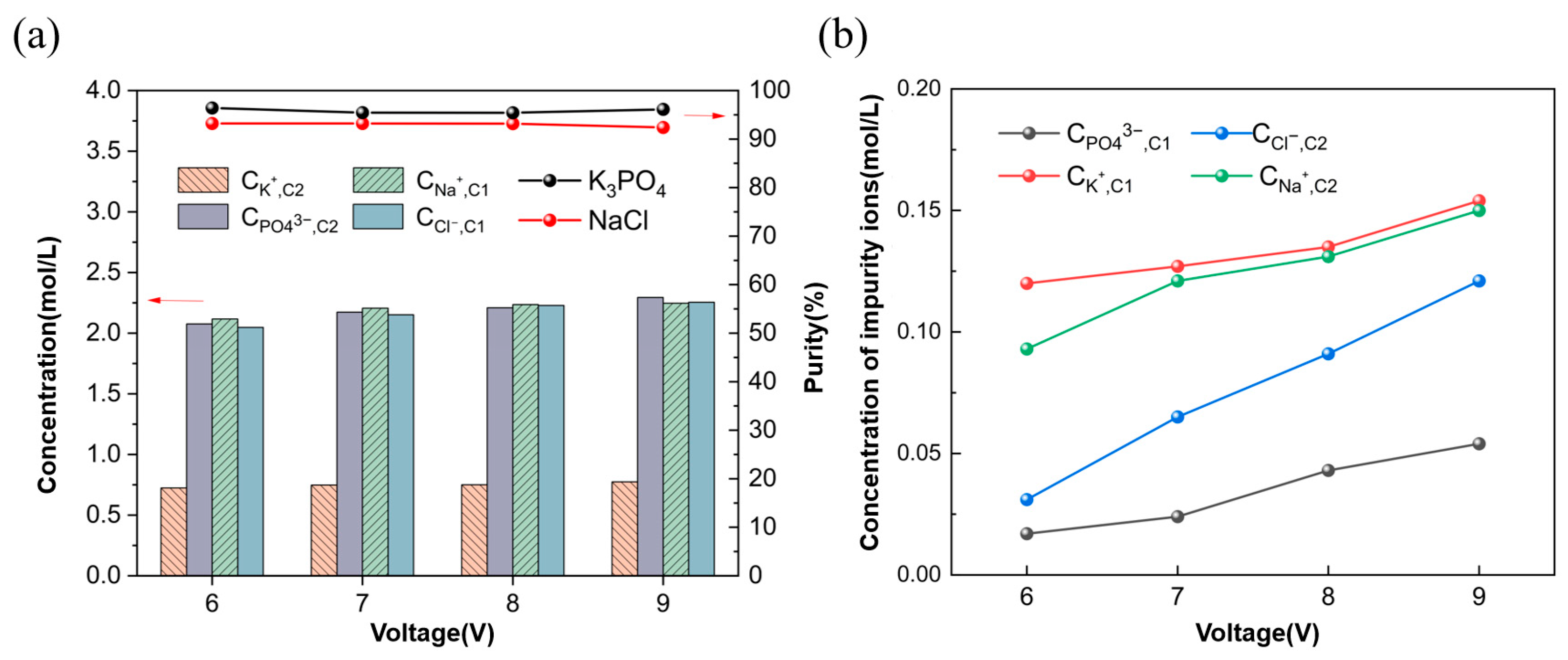
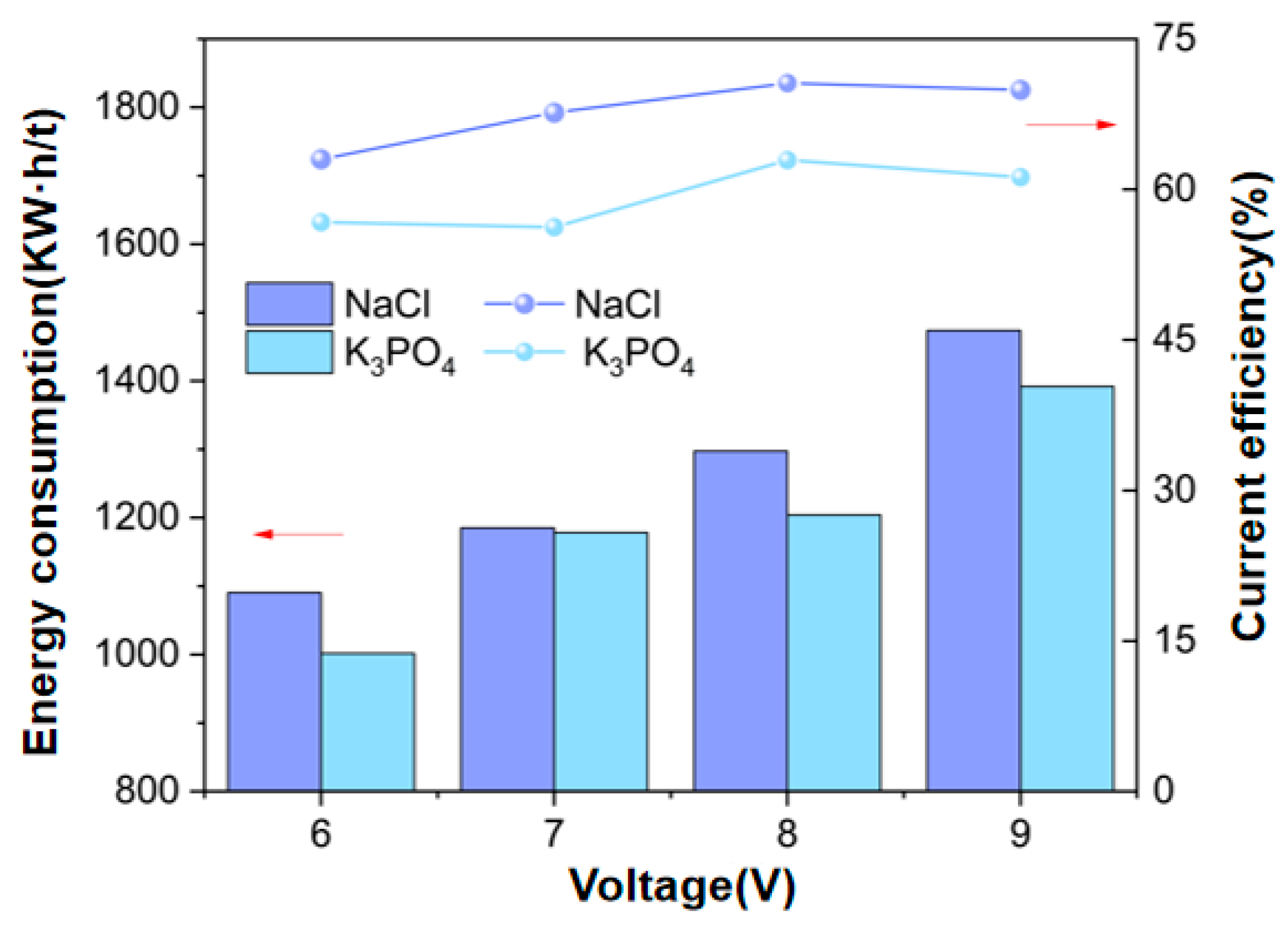
3.1.2. Ion Equilibrium Concentration and Energy Consumption
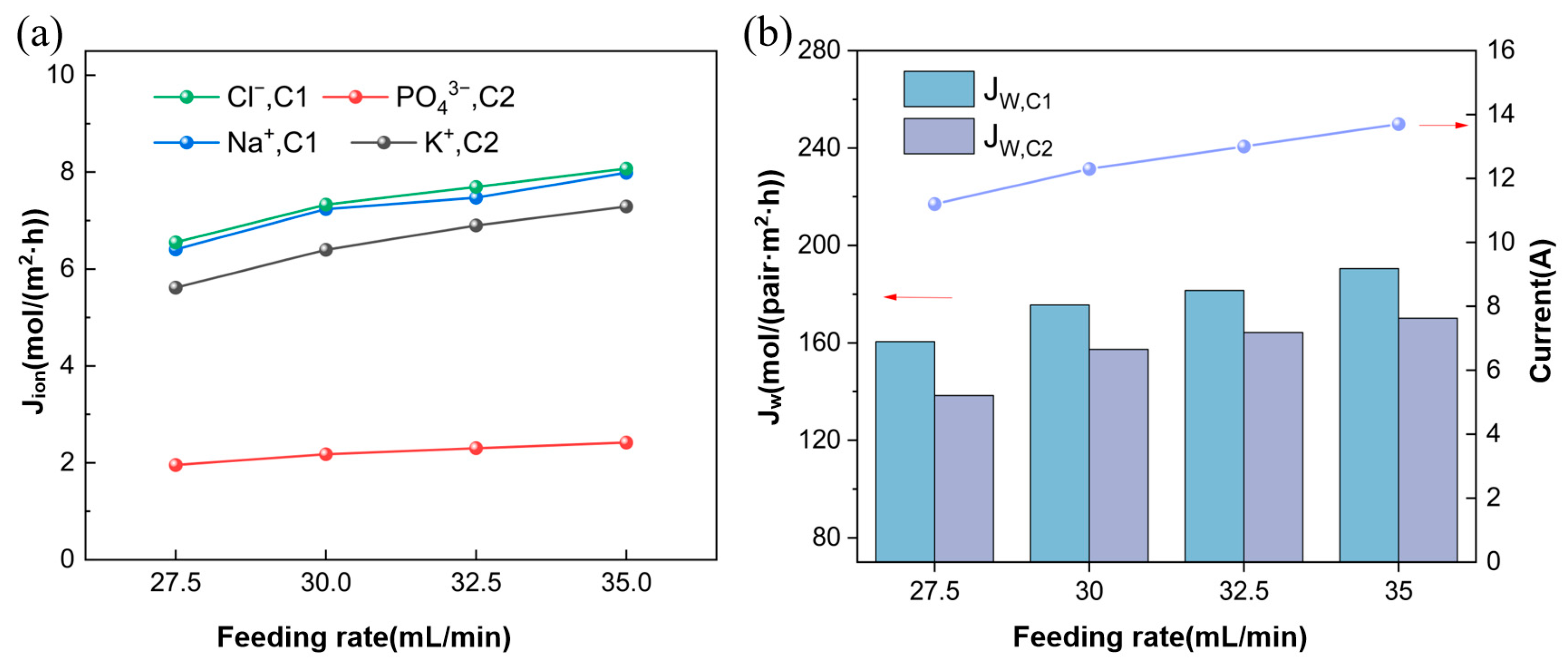
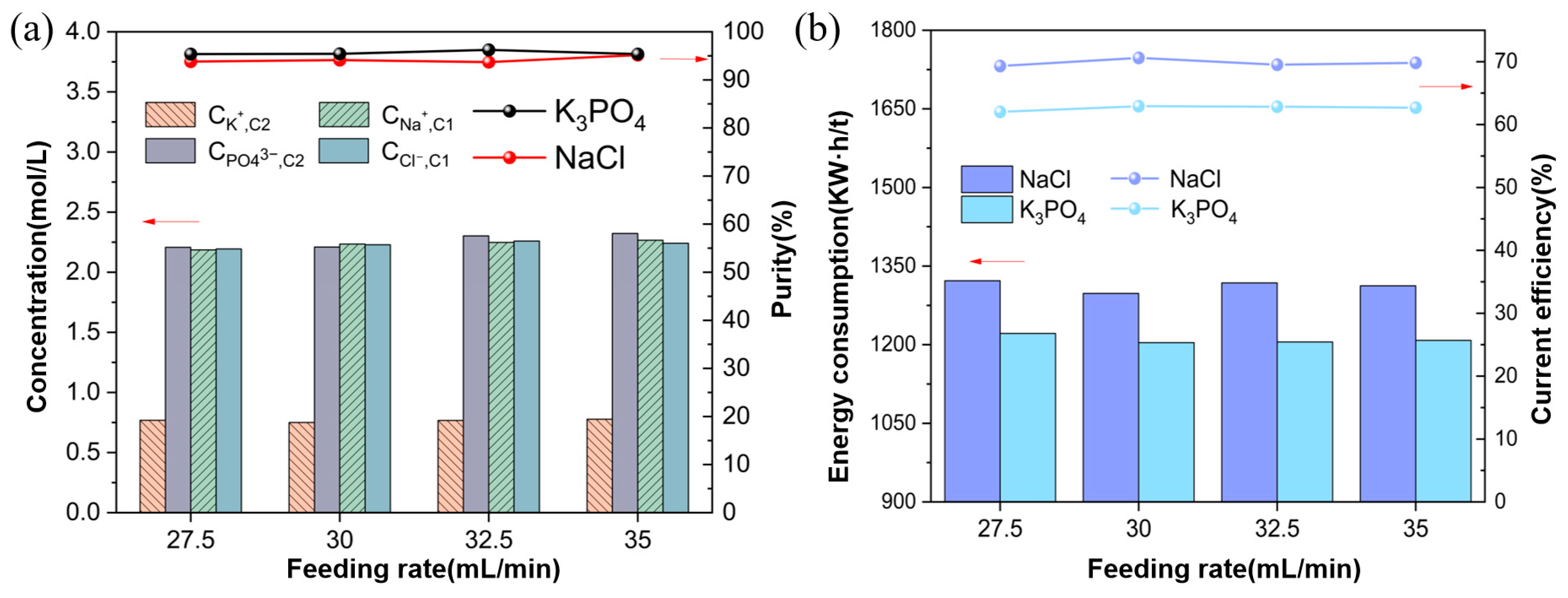
3.2. Effect of Feed Flow Rate
3.2.1. Ion Flux and Apparent Water Flux
3.2.2. Ion Equilibrium Concentration and Energy Consumption
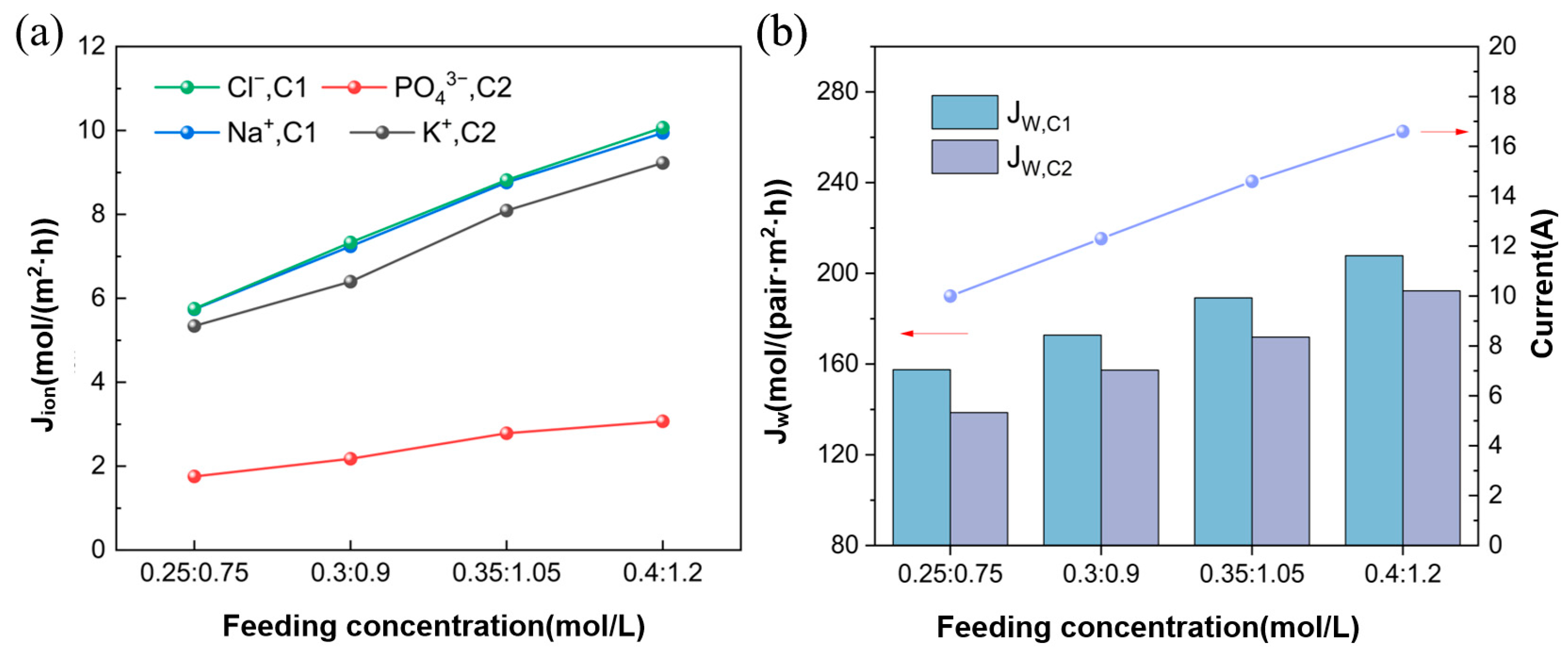
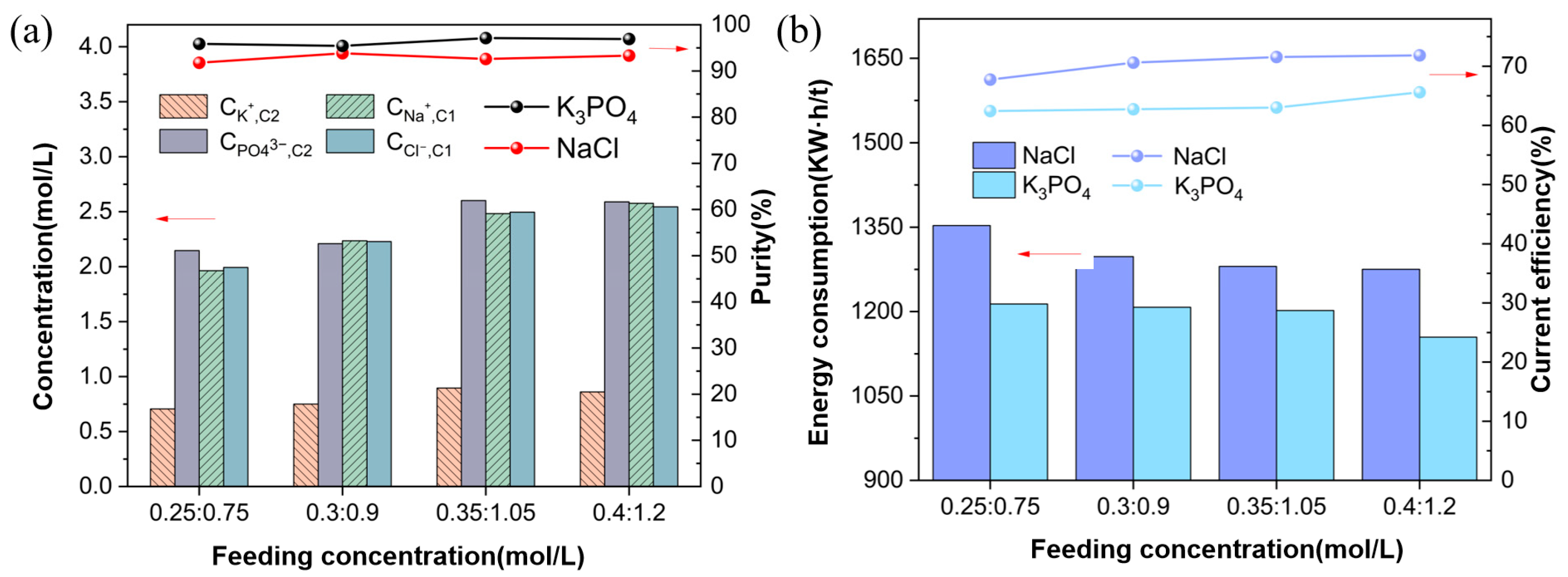
3.3. Effect of Concentration of the Feed Solution
3.3.1. Ion Flux and Apparent Water Flux
3.3.2. Ion Equilibrium Concentration and Energy Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, D.-Z.; Zhang, Q.-Y.; Chen, T.-T.; Fan, W.-J.; Li, Y.-N. Preparing Potassium Dihydrogen Phosphate Using Waste Phosphoric Acid: A Green Chemistry Experiment. J. Chem. Educ. 2025, 102, 783–786. [Google Scholar] [CrossRef]
- Kirish, K.; Vlasov, P.; Dmitrevskii, B. Production of Potassium Phosphate by a Conversion Method. Fibre Chem. 2020, 52, 148–153. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Xie, L.; Wang, K.; Zhang, W. Study on the Treatment of Refined Sugar Wastewater by Electrodialysis Coupled with Upflow Anaerobic Sludge Blanket and Membrane Bioreactor. Membranes 2023, 13, 527. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.S.; Bhadja, V.; Makwana, B.S.; Jewrajka, S.K.; Chatterjee, U. Sustainable process for the preparation of potassium sulfate by electrodialysis and its concentration and purification by a nanofiltration process. RSC Adv. 2016, 6, 71807–71817. [Google Scholar] [CrossRef]
- Chen, T.; Bi, J.; Sun, M.; Liu, J.; Yuan, J.; Zhao, Y.; Ji, Z. Electrodialysis metathesis for high-value resource conversion and recovery: From sustainable applications to future prospects. Chem. Eng. J. 2023, 473. [Google Scholar] [CrossRef]
- Meng, H.; Li, H.; Li, C.; Li, L. Synthesis of ionic liquid using a four-compartment configuration electrodialyzer. J. Membr. Sci. 2008, 318, 1–4. [Google Scholar] [CrossRef]
- Rottiers, T.; Bruggen, B.V.d.; Pinoy, L. Synthesis and transport of impurities in electrodialysis metathesis: Production of choline dihydrogen phosphate. J. Membr. Sci. 2017, 541, 550–557. [Google Scholar] [CrossRef]
- Gao, W.; Zhao, H.; Wei, X.; Meng, X.; Wu, K.; Liu, Y. A Green and Economical Method for Preparing Potassium Glutamate through Electrodialysis Metathesis. Ind. Eng. Chem. Res. 2022, 61, 1486–1493. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, Y.; Ge, S.; Liang, G.; Afsar, N.U. Electrodialysis Metathesis (EDM) Desalination for the Effective Removal of Chloride and Nitrate from Tobacco Extract: The Effect of Membrane Type. Membranes 2023, 13, 214. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Liu, J.; Xu, F.; Ji, Z.; Guo, X.; Li, F.; Yuan, J. Modeling and simulation of continuous electrodialysis metathesis process for conversion of Na2SO4 to K2SO4. Desalination 2022, 528, 115605. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Song, W.; Jiang, C.; Wang, Y.; Xu, T. Ion exchange membrane related processes towards carbon capture, utilization and storage: Current trends and perspectives. Sep. Purif. Technol. 2022, 296, 121390. [Google Scholar] [CrossRef]
- Han, X.; Yan, X.; Wang, X.; Ran, J.; Wu, C.; Zhang, X. Preparation of chloride-free potash fertilizers by electrodialysis metathesis. Sep. Purif. Technol. 2018, 191, 144–152. [Google Scholar] [CrossRef]
- Jaroszek, H.; Dydo, P. Potassium nitrate synthesis by electrodialysis-metathesis: The effect of membrane type. J. Membr. Sci. 2018, 549, 28–37. [Google Scholar] [CrossRef]
- Chen, Q.B.; Li, P.F.; Wang, J.; Xu, Y.; Zhao, J. Reclamation of sulfate-laden wastewater via a hybrid selective nanofiltration and electrodialysis metathesis process. J. Environ. Chem. Eng. 2022, 10, 108712. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Yuan, J.; Ji, Z.; Liu, J.; Wang, S.; Guo, X.; Li, F.; Wang, J.; Bi, J. An efficient electrodialysis metathesis route to recover concentrated NaOH-NH4Cl products from simulated ammonia and saline wastewater in coal chemical industry. Sep. Purif. Technol. 2022, 301, 122042. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Haerens, K.; De Vreese, P.; Matthijs, E.; Pinoy, L.; Binnemans, K.; Van der Bruggen, B. Production of ionic liquids by electrodialysis. Sep. Purif. Technol. 2012, 97, 90–95. [Google Scholar] [CrossRef]
- Chai, P.; Wang, J.; Lu, H. The cleaner production of monosodium L-glutamate by resin-filled electro-membrane reactor. J. Membr. Sci. 2015, 493, 549–556. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Li, N.; Sun, Y.; Ma, Z.; Xu, D.; Gao, J.; Wang, J.; Wang, L.; Gao, X. Techno-economic evaluation of preparing high-valued TPAOH from its low-cost bromide via electrodialysis metathesis (EDM). Sep. Purif. Technol. 2020, 237, 116371. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X.; Yan, X.; Chen, X.; Jin, Z.; Hu, X. Continuous synthesis of high purity KNO3 through electrodialysis metathesis. Sep. Purif. Technol. 2019, 222, 85–91. [Google Scholar] [CrossRef]
- Liu, J.; Li, D.; Xu, F.; Yuan, J.; Ji, Z.; Zhao, Y.; Li, F.; Guo, X.; Wang, S. The efficient conversion of K2SO4 from Na2SO4 by continuous electrodialysis metathesis process. Desalination Water Treat. 2021, 238, 267–276. [Google Scholar] [CrossRef]
- Sharma, P.P.; Yadav, V.; Rajput, A.; Kulshrestha, V. Synthesis of Chloride-Free Potash Fertilized by Ionic Metathesis Using Four-Compartment Electrodialysis Salt Engineering. ACS Omega 2018, 3, 6895–6902. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.F.; Wang, J.; Zhong, C.Y.; Chen, Q.; Zhang, W.M. Direct production of lithium nitrate from the primary lithium salt by electrodialysis metathesis. J. Membr. Sci. 2022, 654, 120555. [Google Scholar] [CrossRef]
- Zhong, C.-Y.; Lv, Y.-P.; Wen, W.-F.; Chen, Q.; Zhang, W.-M. Sustainable Production of Lithium Acetate by Bipolar Membrane Electrodialysis Metathesis. ACS Sustain. Chem. Eng. 2022, 10, 6045–6056. [Google Scholar] [CrossRef]
- Liu, J.; Xu, F.; Yuan, J.; Ji, Z.; Zhao, Y.; Li, F.; Guo, X. High-value conversion of Na2SO4 wastewater by a continuous electrodialytic metathesis process: Effects of coexisting ions. J. Membr. Sci. 2020, 615, 118584. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Yang, B.; Zou, D.; Li, W. Solid-Liquid Equilibria in the Quaternary System K+//H2PO42−, SO42−, Cl−-H2O at 298.2 K and 323.2 K. J. Chem. Eng. Data ACS J. Data 2016, 61, 330–335. [Google Scholar] [CrossRef]
- Available online: https://ndls.org.cn/standard/detail/c27e8ed457ab094ec6e941b8f2121a62 (accessed on 15 March 2025).
- Tiezheng, T.; Menachem, E. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar]
- Available online: https://www.100ppi.com/mprice/plist-1-4876-1.html (accessed on 15 March 2025).
| Membrane Type | Membrane Size/mm2 | Thickness/mm | Resistance /Ω·cm2 | Burst Strength /MPa | Recommended Operating Temperature/°C |
|---|---|---|---|---|---|
| CEM (YRJCM-I) | 400 × 200 | 0.26 | 4.0 | ≥0.25 | 15–40 |
| AEM (YRJAM-I) | 400 × 200 | 0.17 | 3.5 | ≥0.25 | 15–40 |
| Items | K3PO4 | Remarks |
|---|---|---|
| Membrane area, m2 | 0.08 | |
| Repeating units | 4 | |
| Process capacity, kg K3PO4/year | 737 | Annual operation Days = 300 |
| Membrane life and amortization of the peripheral equipment, years | 5 | |
| Cation exchange membrane price, CNY/m2 | 500 | |
| Anion exchange membrane price, CNY/m2 | 1000 | |
| Membrane cost, CNY | 480 | |
| Stack cost, CNY | 720 | 1.5 times membrane cost |
| Peripheral equipment cost, CNY | 1080 | 1.5 times stack cost |
| Total investment cost, CNY | 2280 | |
| Amortization, CNY/year | 456 | |
| Total process cost, CNY/t K3PO4 | 619 |
| Type | Name | Consumption | Specific Value (CNY/ton) | Value (CNY) |
|---|---|---|---|---|
| Material consumption | KCl | 1.054 ton | 3200 | 3162 |
| Na3PO4 | 0.773 ton | 3200 | 2474 | |
| Electric consumption | EDM(K3PO4) | 1191 kW·h/t | 0.7 | 834 |
| EDM(NaCl) | 1285 kW·h/t | 0.7 | 896 | |
| MVR(K3PO4) | 184 kW·h | 0.7 | 129 | |
| MVR(NaCl) | 236 kW·h | 0.7 | 165 | |
| Equipment consumption | EDM process cost | 1 ton | 619 | 619 |
| Total | 8314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Zhang, Z.; Zhang, L.; Xie, L.; Zhang, W. Electrodialysis Metathesis for the Production of Potassium Phosphate. Membranes 2025, 15, 136. https://doi.org/10.3390/membranes15050136
Xu S, Zhang Z, Zhang L, Xie L, Zhang W. Electrodialysis Metathesis for the Production of Potassium Phosphate. Membranes. 2025; 15(5):136. https://doi.org/10.3390/membranes15050136
Chicago/Turabian StyleXu, Shichang, Zhen Zhang, Long Zhang, Lixin Xie, and Wen Zhang. 2025. "Electrodialysis Metathesis for the Production of Potassium Phosphate" Membranes 15, no. 5: 136. https://doi.org/10.3390/membranes15050136
APA StyleXu, S., Zhang, Z., Zhang, L., Xie, L., & Zhang, W. (2025). Electrodialysis Metathesis for the Production of Potassium Phosphate. Membranes, 15(5), 136. https://doi.org/10.3390/membranes15050136







