Effect of Folded Structures on Interfacial Solar-Driven Seawater Desalination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Photothermal CNTs@rPET Membranes
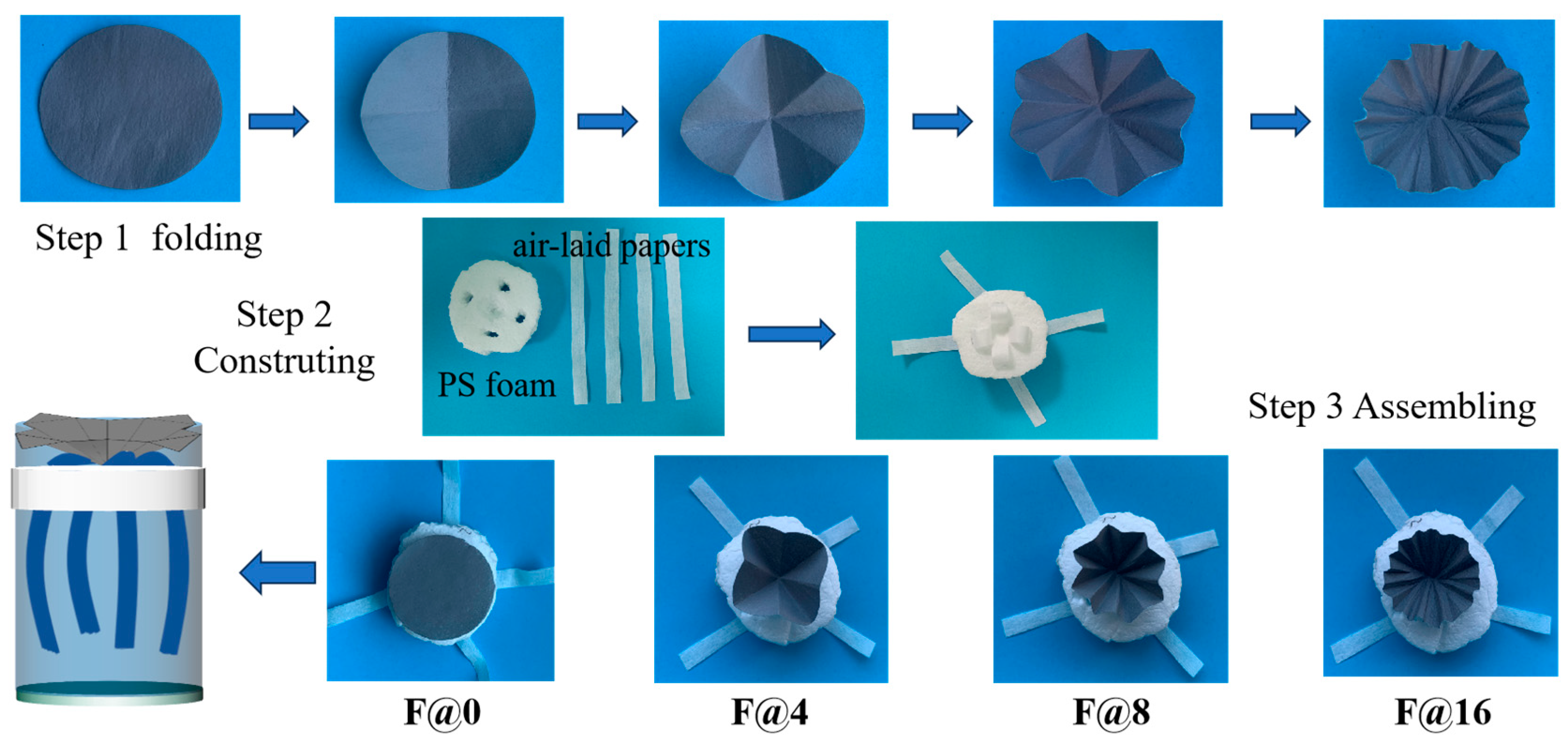
2.3. Preparation of Folded Structure 3D Evaporator
2.4. Characterization
2.5. Photothermal Evaporation Experiments
3. Results and Discussion
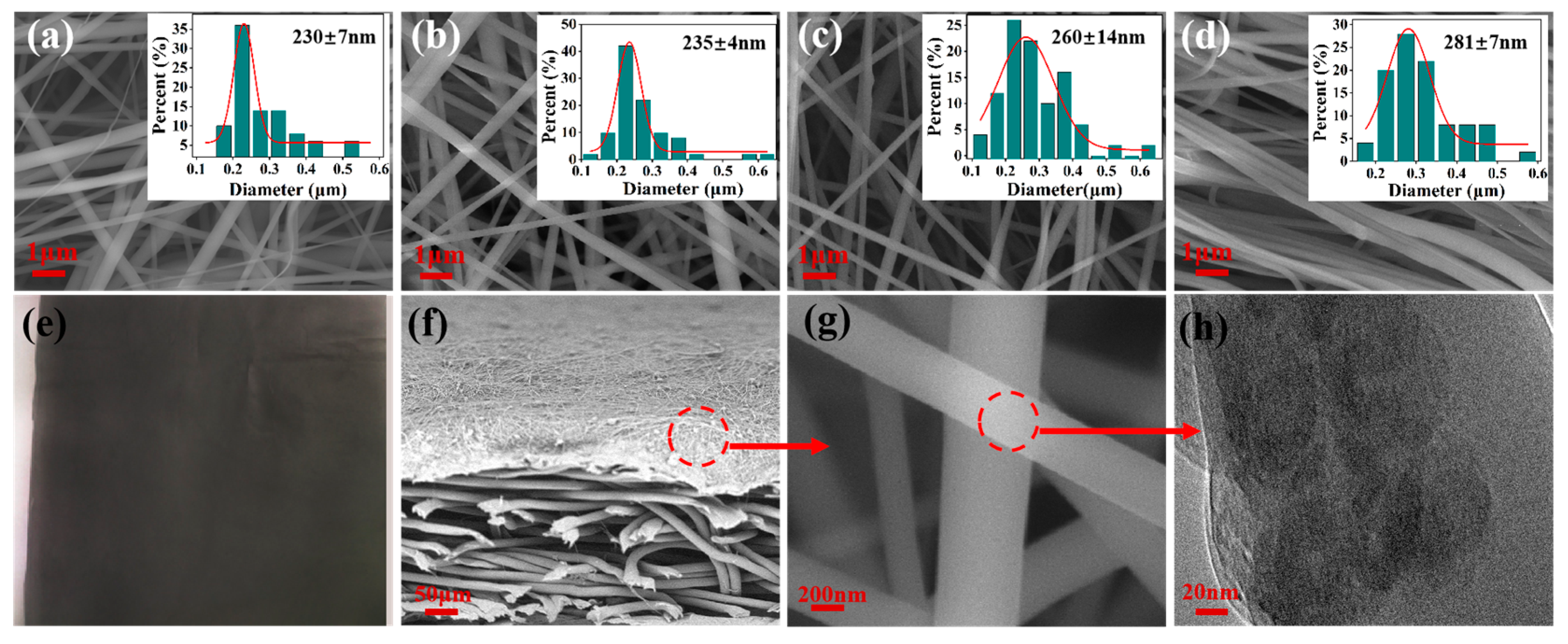
3.1. Morphology of the As-Spun CNTs@rPET Membranes
3.2. Wettability and Stability of CNTs@rPET-P Membrane
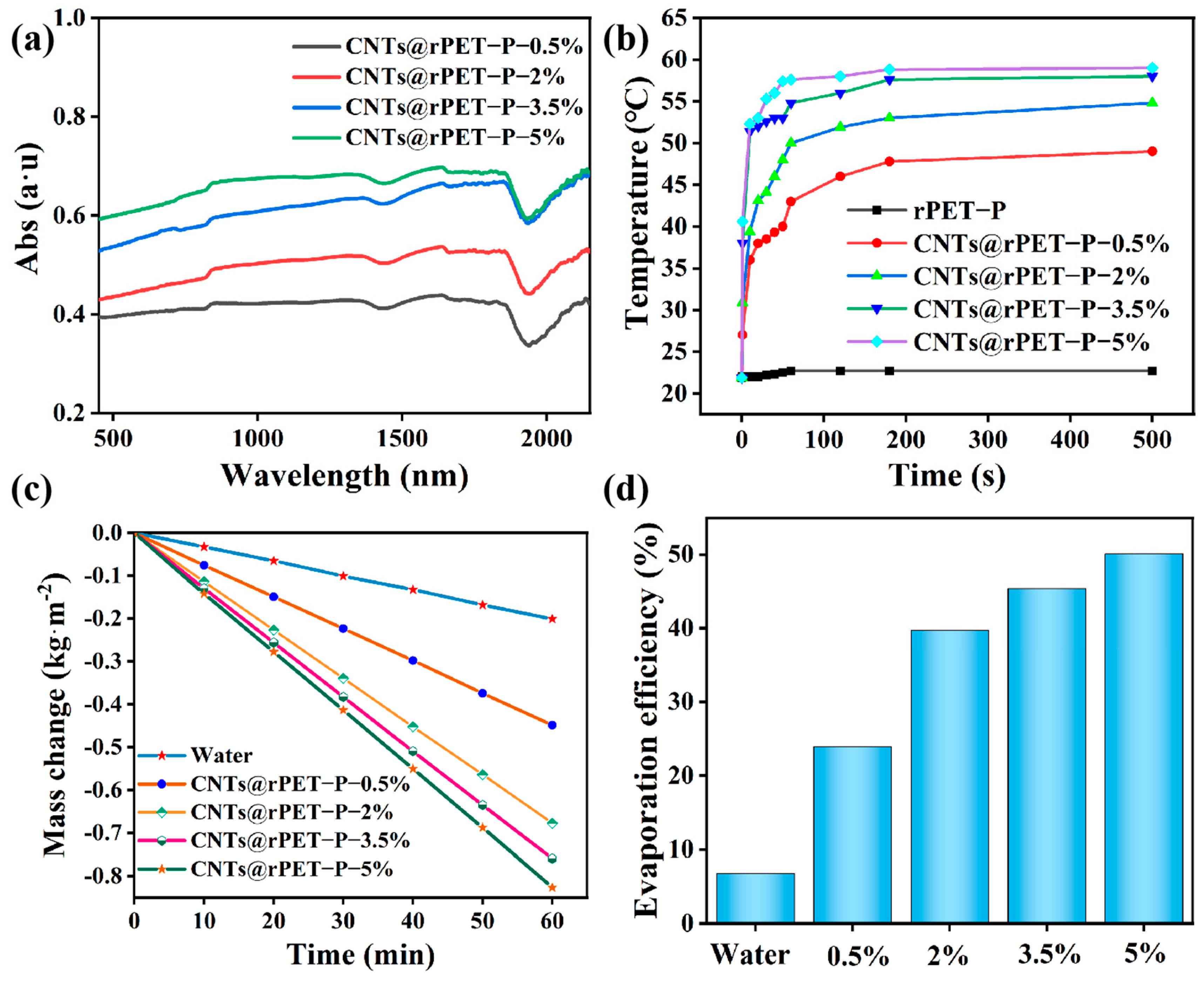
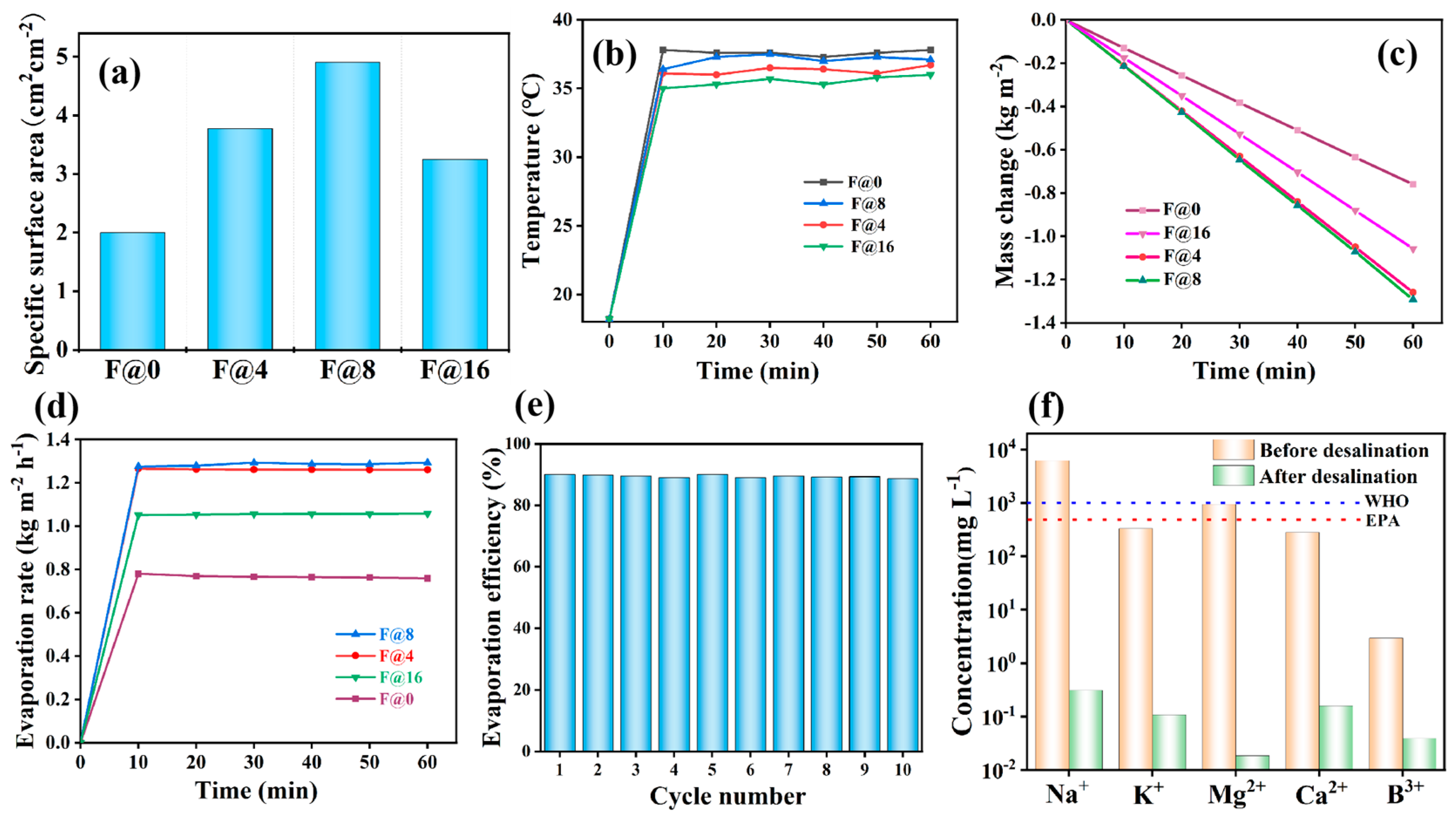
3.3. Evaporation Performance of CNTs@rPET-P Membranes
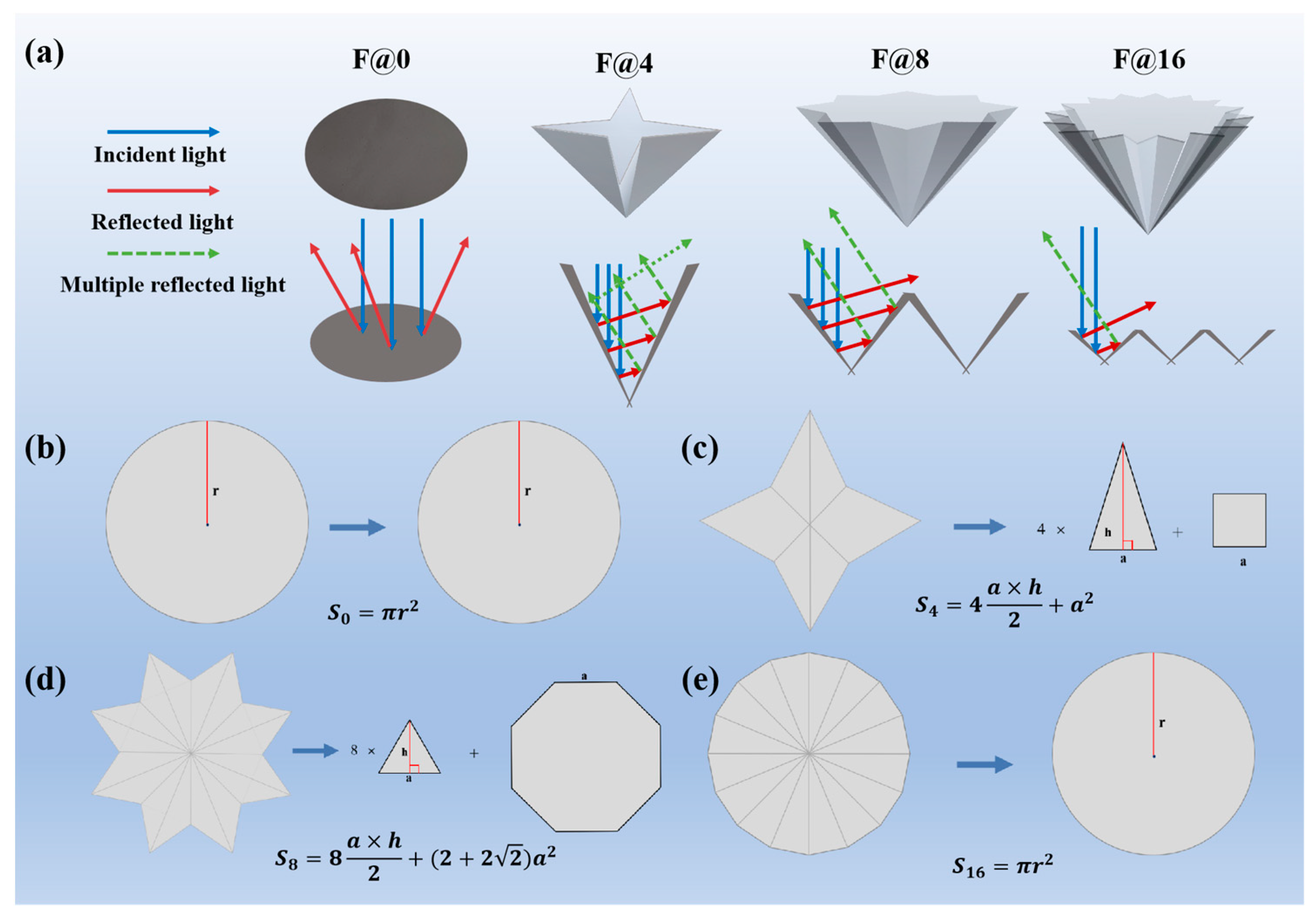
3.4. Optical Property of F@N
3.5. Photothermal Performances of F@N
3.6. Cost Benefit Analysis of F@N
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prajapati, M.; Shah, M.; Soni, B. A review of geothermal integrated desalination: A sustainable solution to overcome potential freshwater shortages. J. Clean. Prod. 2021, 326, 129412. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of water: A review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Alawad, S.M.; Khalifa, A.E. Analysis of water gap membrane distillation process for water desalination. Desalination 2019, 470, 114088. [Google Scholar] [CrossRef]
- Lee, T.; Rahardianto, A.; Cohen, Y. Flexible reverse osmosis (FLERO) desalination. Desalination 2019, 452, 123–131. [Google Scholar] [CrossRef]
- Stuart-Dahl, S.; Martinez-Guerra, E.; Kokabian, B.; Gude, V.G.; Smith, R.; Brooks, J. Resource recovery from low strength wastewater in a bioelectrochemical desalination process. Eng. Life Sci. 2020, 20, 54–66. [Google Scholar] [CrossRef]
- Alkhadra, M.A.; Gao, T.; Conforti, K.M.; Tian, H.; Bazant, M.Z. Small-scale desalination of seawater by shock electrodialysis. Desalination 2020, 476, 114219. [Google Scholar] [CrossRef]
- Saavedra, A.; Valdés, H.; Mahn, A.; Acosta, O. Comparative analysis of conventional and emerging technologies for seawater desalination: Northern Chile as a case study. Membranes 2021, 11, 180. [Google Scholar] [CrossRef]
- Luo, X.; Shi, J.; Zhao, C.; Luo, Z.; Gu, X.; Bao, H. The energy efficiency of interfacial solar desalination. Appl. Energy 2021, 302, 117581. [Google Scholar] [CrossRef]
- Liu, X.; Mishra, D.D.; Wang, X.; Peng, H.; Hu, C. Towards highly efficient solar-driven interfacial evaporation for desalination. J. Mater. Chem. A 2020, 8, 17907–17937. [Google Scholar] [CrossRef]
- Jia, X.; Niu, Y.; Zhu, S.; He, H.; Yan, X. Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review. C 2024, 10, 86. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, J.; Tao, Y.; Kong, F.; Li, P. The emerging development of solar evaporators in materials and structures. Chemosphere 2022, 289, 133210. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.K.; Gong, J.Z.; Dai, M.; Zhang, Y.F.; Wang, S.G.; Lin, Z.D.; Du, F.P.; Fu, P. Reduced graphene oxide/Ag nanoparticle aerogel for efficient solar water evaporation. J. Alloys Compd. 2023, 930, 167404. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, H.; Wang, H.; Wang, D.; van Aken, P.A.; Schaaf, P. Plasmon-Enhanced Light Absorption Below the Bandgap of Semiconducting SnS2 Microcubes for Highly Efficient Solar Water Evaporation. Small 2024, 20, 2400588. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, X.; Li, Y.; Li, H.; Cai, Y.; Wang, W.; Min, X.; Xiong, J.; Li, M. Fully waste-based solar evaporator in interfacial solar-driven seawater desalination. J. Environ. Chem. Eng. 2023, 11, 110879. [Google Scholar] [CrossRef]
- Saleque, A.M.; Ma, S.; Thakur, A.K.; Saidur, R.; Han, T.K.; Hossain, M.I.; Qarony, W.; Sathyamurthy, R.; Tsang, Y.H. MXene/MnO2 nanocomposite coated superior salt-rejecting biodegradable luffa sponge for efficient solar steam generation. Desalination 2023, 554, 116488. [Google Scholar] [CrossRef]
- Xiao, B.; Zhou, W.; Yu, F.; Wang, J.; Xiong, X.; Liu, G.; Xia, Y.; Wang, X. Materials design and system structures of solar steam evaporators. Environ. Prog. Sustain. Energy 2023, 42, e13944. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Gao, T.; Lu, Y.; Yang, X.; Chen, G.Y.; Owens, G.; Xu, H. Same materials, bigger output: A reversibly transformable 2D-3D photothermal evaporator for highly efficient solar steam generation. Nano Energy 2021, 79, 105477. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, D.; Yu, H.; Wu, X.; Lu, Y.; Yang, X.; Owens, G.; Xu, H. Recent innovations in 3D solar evaporators and their functionalities. Sci. Bull. 2024, 69, 3590–3617. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xi, Z.; Yu, L.; Yan, H.; Chen, M. Thermal management of the solar evaporation process. Langmuir 2023, 39, 8900–8907. [Google Scholar] [CrossRef]
- Liang, C.G.; Yasin, A.; Zhang, L.; Zhang, P.; Zhou, K.; Hao, B.; Li, H.; Ma, P.C. Solar interfacial evaporator with three-dimensional architecture for seawater desalination. Colloids Surf. A 2024, 702, 135035. [Google Scholar] [CrossRef]
- Li, B.; Lin, M.; Cheng, C.; Li, X. Three-dimensional freeze-casting solar evaporator for highly efficient water evaporation and high salinity desalination. Desalination 2024, 580, 117542. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, C.; Liu, J.; Han, M.Y.; Pan, R.J.; Yu, Z.Z.; Li, X. Three-Dimensional Spiral Evaporator with Side Channels for Efficient Solar-Driven Water Purification. ACS Appl. Mater. Interfaces 2023, 15, 48196–48206. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Kim, S.K. An easily scalable, durable, and highly efficient three-dimensional solar evaporator inspired by a rice paddy field. Desalination 2023, 548, 116251. [Google Scholar] [CrossRef]
- Jia, L.; Feng, M.; Zhang, F.; Lin, H.; Guo, W.; Yu, K.; Yang, C.; Qu, F. Three-dimensional water evaporator based on carbonized silkworm cocoon for highly effective solar-driven water evaporation and wastewater purification. Mater. Lett. 2022, 312, 131661. [Google Scholar] [CrossRef]
- Sun, M.H.; Li, C.; Liu, J.; Min, P.; Yu, Z.Z.; Li, X. Three-dimensional mirror-assisted and concave pyramid-shaped solar-thermal steam generator for highly efficient and stable water evaporation and brine desalination. ACS Appl. Mater. Interfaces 2023, 15, 27120–27129. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Guo, S.; Tan, S.C. Towards highly salt-rejecting solar interfacial evaporation: Photothermal materials selection, structural designs, and energy management. Nano Res. Energy 2022, 1, 9120014. [Google Scholar] [CrossRef]
- Sun, Y.; Tan, X.; Yuan, X.; Li, J. Solar-driven interfacial evaporation: Research advances in structural design. Chem. Eng. J. 2024, 495, 153316. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Mishra, D.D.; Chen, Z.; Zhao, J.; Hu, C. Evaporation rate far beyond the input solar energy limit enabled by introducing convective flow. Chem. Eng. J. 2022, 429, 132335. [Google Scholar] [CrossRef]
- Hong, S.; Shi, Y.; Li, R.; Zhang, C.; Jin, Y.; Wang, P. Nature-inspired 3D origami solar steam generator toward near full utilization of solar energy. ACS Appl. Mater. Interfaces 2018, 10, 28517–28524. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Wang, L.; Qin, X.; Yu, J. Nanofiber based origami evaporator for multifunctional and omnidirectional solar steam generation. Carbon 2021, 177, 199–206. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Chen, F.; Mu, Y.; Caratenuto, A.; Minus, M.L.; Zheng, Y. A waterbomb origami tower for convertible photothermal evaporation J. Mater. Chem. A 2022, 10, 18657–18670. [Google Scholar] [CrossRef]
- Zhu, S.; Jia, X.; Ni, Y.; Pan, B.; Long, Y.; Miao, D.; Yan, X. A 3D solar-driven evaporator based on electrospun recycled PET film for efficient seawater desalination. J. Cleaner Prod. 2023, 408, 137113. [Google Scholar] [CrossRef]
- Hussain, N.; Mehdi, M.; Yousif, M.; Ali, A.; Ullah, S.; Hussain Siyal, S.; Hussain, T.; Kim, I.S. Synthesis of highly conductive electrospun recycled polyethylene terephthalate nanofibers using the electroless deposition method. Nanomaterials 2021, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Hong, J.L. Effect of folding on 3D photothermal cones with efficient solar-driven water evaporation. Appl. Therm. Eng. 2020, 178, 115636. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Falinski, M.M.; Wang, Y.; Li, T.; Zheng, S.; Sun, D.; Dai, J.; Bian, Y.; Zhu, X.; et al. Sustainable off-grid desalination of hypersaline waters using Janus wood evaporators. Energy Environ. Sci. 2021, 14, 5347–5357. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, Y.; Li, N.; Yang, P.; Yang, Y.; Duan, G.; Wang, X.; Xu, Y.; Li, Y. A robust and 3D-printed solar evaporator based on naturally occurring molecules. Sci. Bull. 2023, 68, 203–213. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Meng, L.; Wang, B.; Bai, J.; Wang, G.; Ma, S. Three dimensional hydrogel evaporator made of nano level antireflection particles for high-efficiency solar steam generation. Sustain. Energy Technol. Assess. 2022, 52, 102074. [Google Scholar] [CrossRef]
- Fan, D.; Min, H.; Zhang, H.; Tang, Y.; Yang, X.; Lu, Y. Architecting a bifunctional solar evaporator of perovskite La0.5Sr0.5CoO3 for solar evaporation and degradation. J. Mater. Sci. 2021, 56, 18625–18635. [Google Scholar] [CrossRef]
- Huang, Q.; Du, C.; Huang, C. Nature-inspired pyramid-shaped 3-dimensional structure for cost-effective heat-localized solar evaporation with high efficiency and salt localization. Appl. Therm. Eng. 2022, 215, 118950. [Google Scholar] [CrossRef]


| Literature | Structure | Material | Evaporation Efficiency (%) | Cost (USD/m2) | Quality Factor (m2/$) |
|---|---|---|---|---|---|
| F@8 | Eight folded petals | CNTs, rPET, air-laid paper | 90.59 | 0.26 | 17.33 |
| [37] | Cone-shaped array | Tannic acid, ferric chloride, 3D printing ink | 94.4 | ≈11 | 0.41 |
| [38] | Three-dimensional porous cubic shape | Silver nitrate, polyvinylpyrrolidone, silica, squid powder | 94 | ≈2 | 2.27 |
| [39] | Porous cylinder | Lanthanum nitrate hexahydrate, cobalt nitrate hexahydrate, strontium nitrate, polyvinyl alcohol solution, chitosan solution | 93 | ≈3 | 1.51 |
| [40] | Durian shell shaped | Pyrrole, iron oride hexahydrate | 91 | ≈1 | 4.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Niu, Y.; Yan, X. Effect of Folded Structures on Interfacial Solar-Driven Seawater Desalination. Membranes 2025, 15, 134. https://doi.org/10.3390/membranes15050134
Zhu S, Niu Y, Yan X. Effect of Folded Structures on Interfacial Solar-Driven Seawater Desalination. Membranes. 2025; 15(5):134. https://doi.org/10.3390/membranes15050134
Chicago/Turabian StyleZhu, Shufang, Yuke Niu, and Xu Yan. 2025. "Effect of Folded Structures on Interfacial Solar-Driven Seawater Desalination" Membranes 15, no. 5: 134. https://doi.org/10.3390/membranes15050134
APA StyleZhu, S., Niu, Y., & Yan, X. (2025). Effect of Folded Structures on Interfacial Solar-Driven Seawater Desalination. Membranes, 15(5), 134. https://doi.org/10.3390/membranes15050134






