Changes in Tubular PVDF Membrane Performance During Initial Period of Pilot Plant Operation
Abstract
:1. Introduction
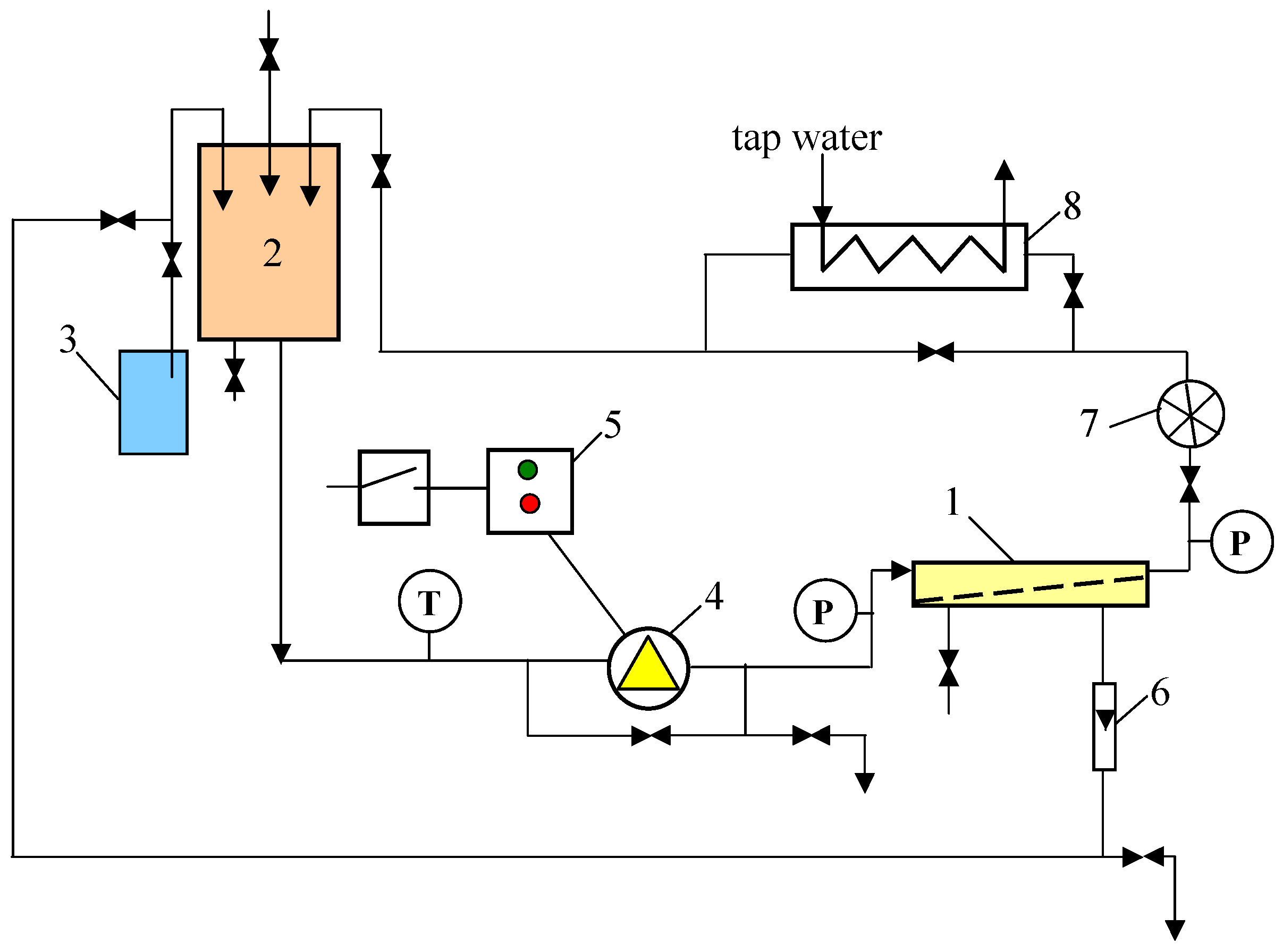
2. Materials and Methods
2.1. UF Studies
2.2. Analytical Procedures
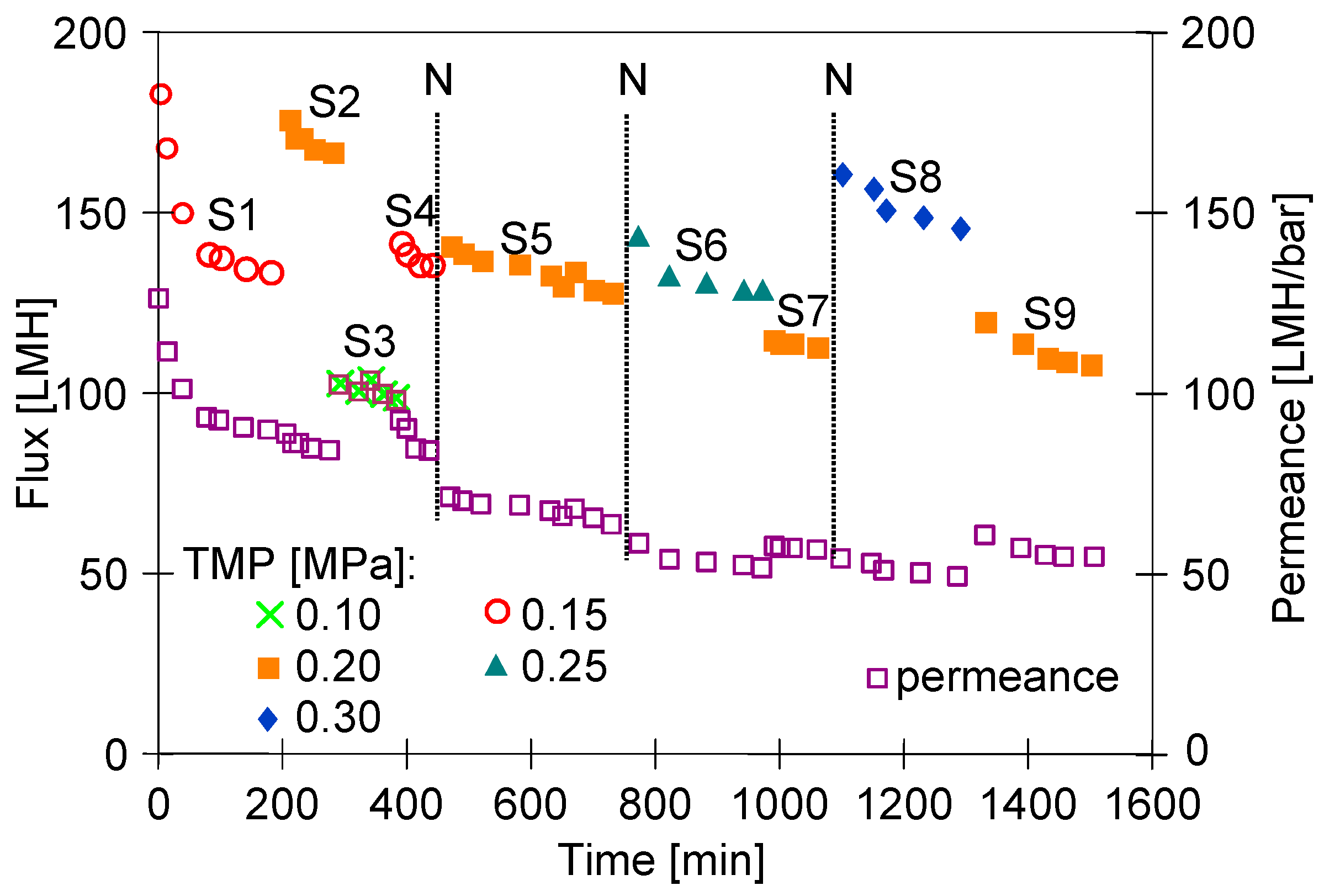
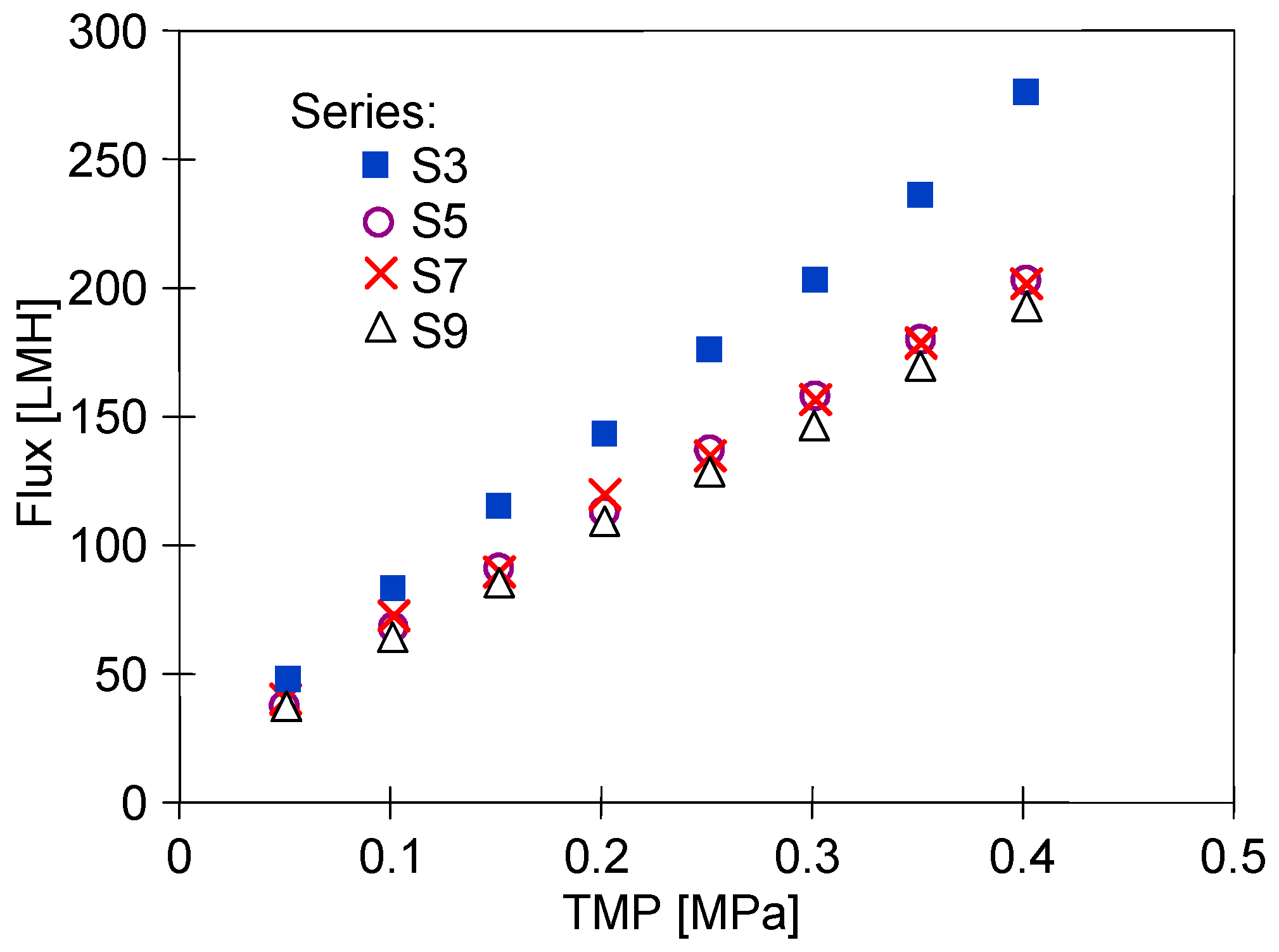
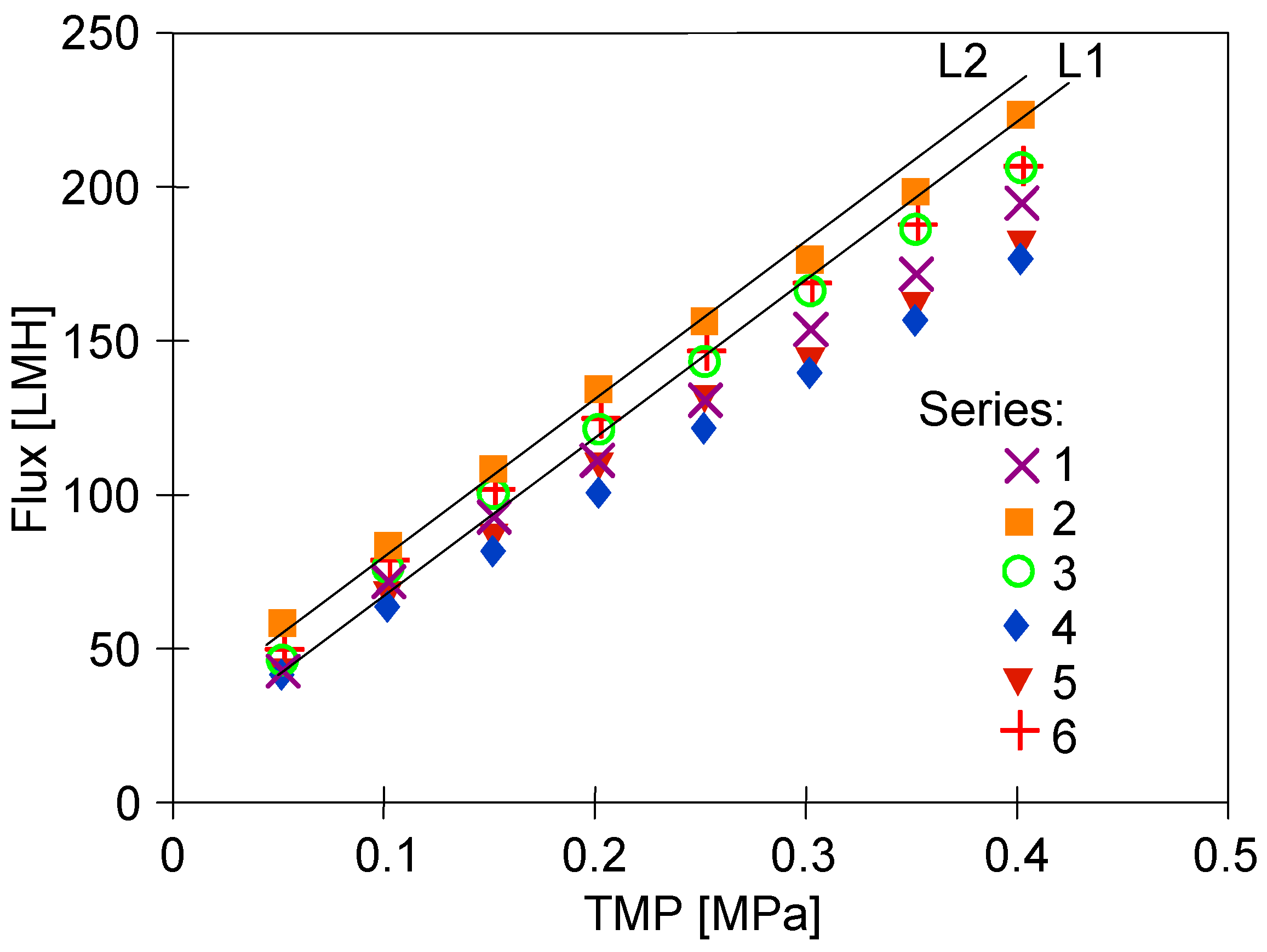
3. Results and Discussion
3.1. Active Layer Porosity
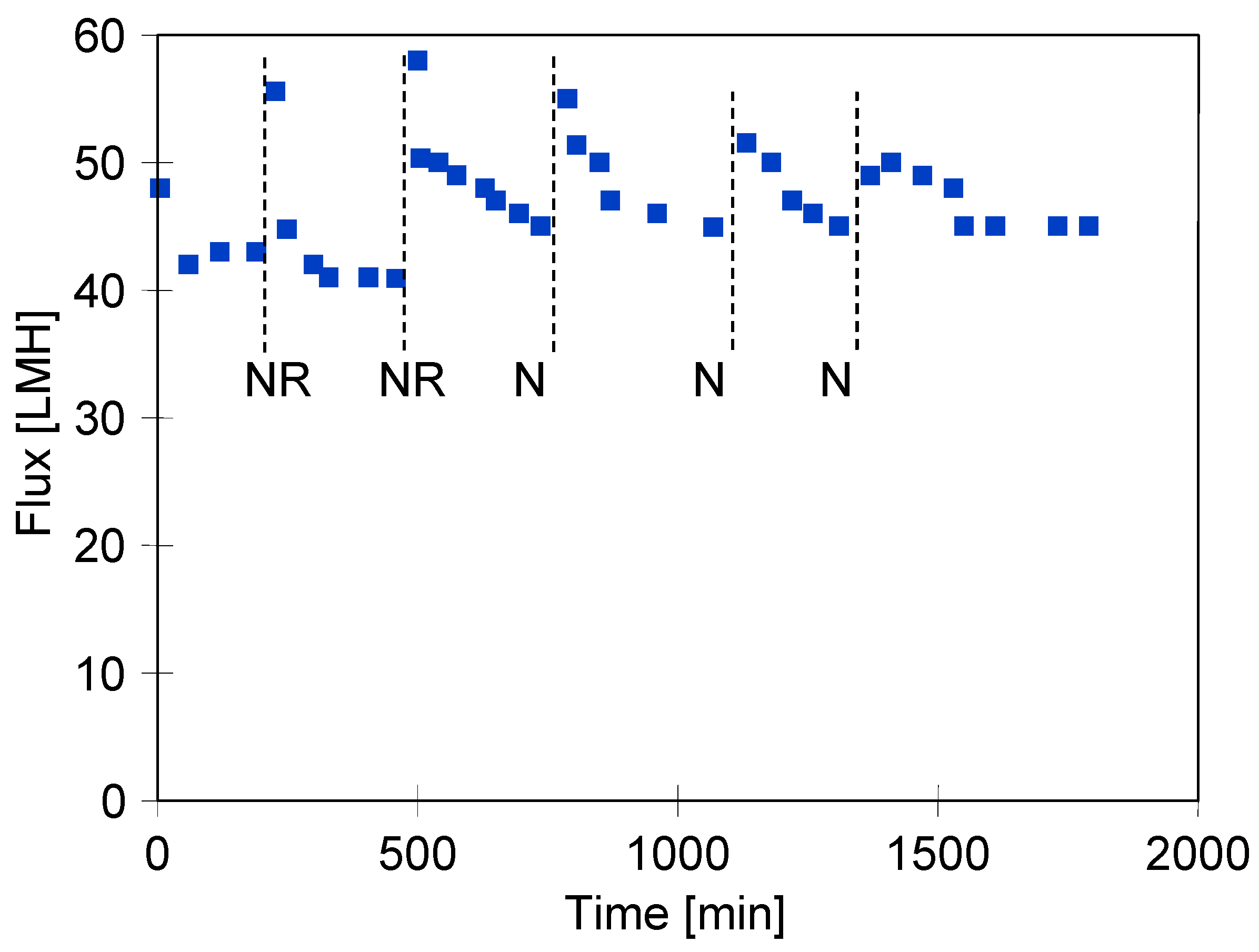
3.2. Membrane Compression
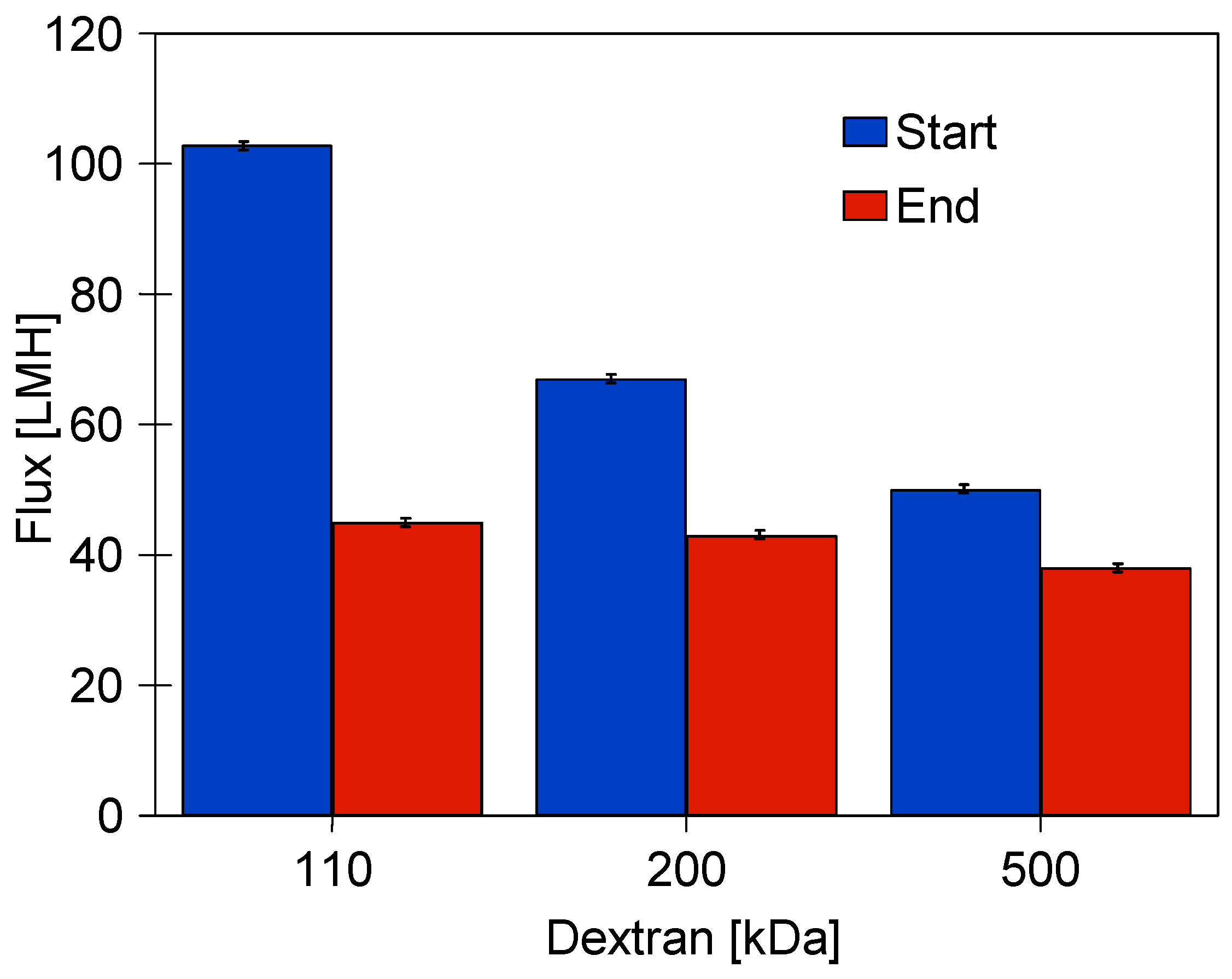
3.3. Dextran Separation
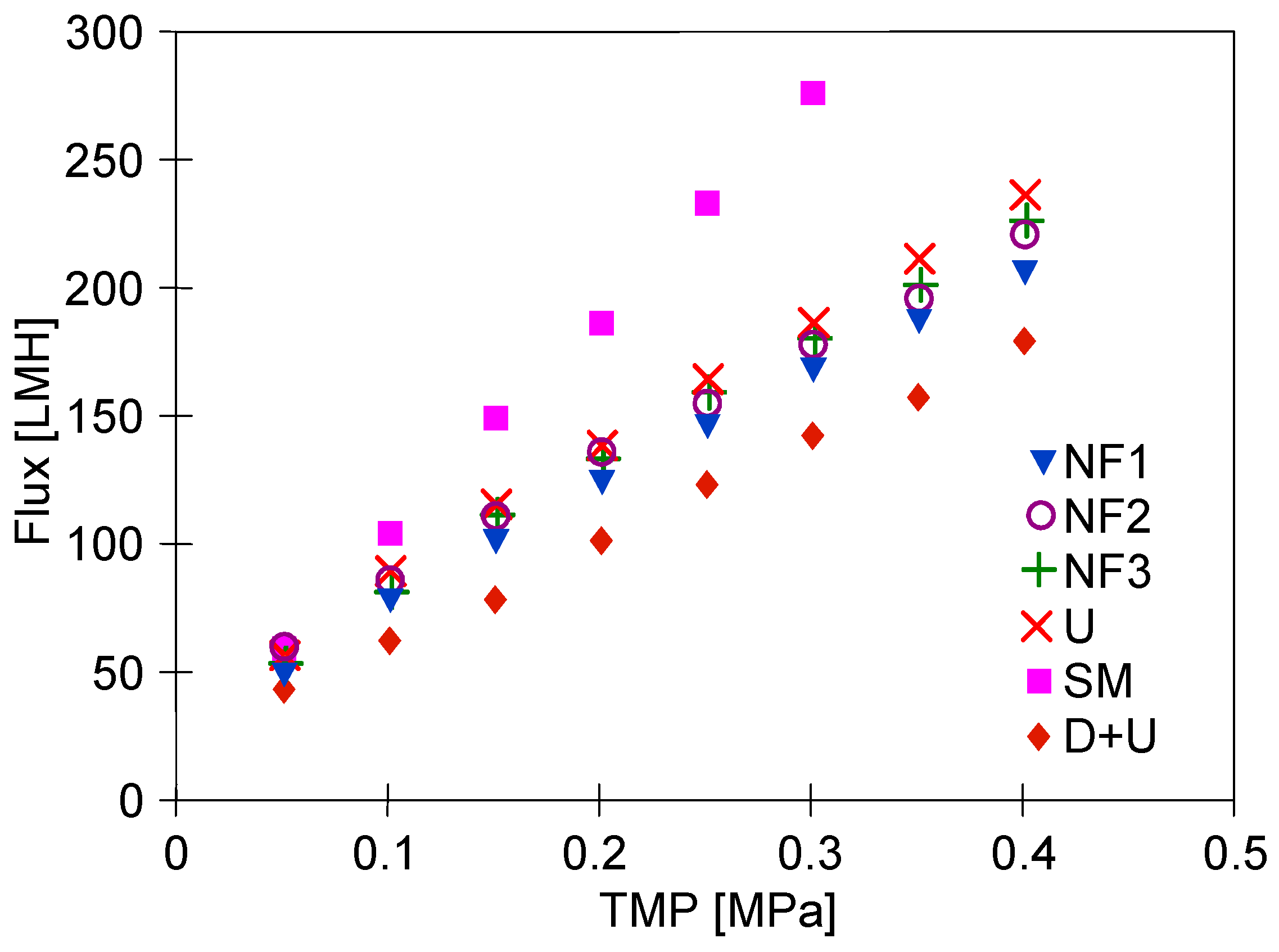
3.4. Wastewater Separation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Botes, J.P.; Jacobs, E.P.; Bradshaw, S.M. Long-term evaluation of a UF pilot plant for potable water production. Desalination 1998, 115, 229–238. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Ozgun, H.; Kaya, R.; Kose Mutlu, B.K.; Kinaci, C.; Koyuncu, I. Treatment of produced water originated from oil and gas production wells: A pilot study and cost analysis. Environ. Sci. Pollut. Res. 2018, 25, 6398–6406. [Google Scholar] [CrossRef] [PubMed]
- Falsanisi, D.; Liberti, L.; Notarnicola, M. Ultrafiltration (UF) Pilot Plant for Municipal Wastewater Reuse in Agriculture: Impact of the Operation Mode on Process Performance. Water 2009, 1, 872–885. [Google Scholar] [CrossRef]
- Farahbakhsh, K.; Svrcek, C.; Guest, R.K.; Smith, D.W. A review of the impact of chemical pretreatment on low-pressure water treatment membranes. J. Environ. Eng. Sci. 2004, 3, 237–253. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Al-Hamdani, H.; Alnaemi, A.; Getan, A. Possibility of Concentrating Milk Enriched with Lemongrass (Cymbopogon) as a Preservative using Ultrafiltration Technology. Asian J. Dairy Food Res. 2024, 43, 404–410. [Google Scholar] [CrossRef]
- Ćurić, I.; Dolar, D.; Horvat, J.; Grgić, K. Effect of Textile Wastewater Secondary Effluent on UF Membrane Characteristics. Polymers 2022, 14, 2035. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Huang, T.; Dong, C.; Li, J.; Zhao, B.; Zhang, S. Chemical Cleaning of Ultrafiltration Membrane Fouled by Humic Substances: Comparison between Hydrogen Peroxide and Sodium Hypochlorite. Int. J. Environ. Res. Public Health 2019, 16, 2568. [Google Scholar] [CrossRef]
- Maddah, H.A.; Alzhrani, A.S.; Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.; Almalki, A.M. Evaluation of various membrane filtration modules for the treatment of seawater. Appl. Water Sci. 2018, 8, 150. [Google Scholar] [CrossRef]
- Ali, B.T.I.; Romadiansyah, T.Q.; Lestari, W.C.; Kusumawati, Y.; Ermavitalini, D.; Widiastuti, N. Modification of PVDF ultrafiltration membrane for high concentration of nannochloropsis as a raw material for bioethanol: Computations and experiments. S. Afr. J. Chem. Eng. 2023, 46, 42–55. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, Y.J.; Kang, D.R.; Lee, S.Y.; Kim, J.H. Fluorine-Containing, Self-Assembled Graft Copolymer for Tuning the Hydrophilicity and Antifouling Properties of PVDF Ultrafiltration Membranes. Polymers 2023, 15, 3623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, Y.; Liu, Q.; Ye, J.; Hu, Q.; Zhang, X. Harvesting of Isochrysis zhanjiangensis using ultrafiltration: Changes in the contribution ratios of cells and algogenic organic matter to membrane fouling under different cross-flow velocities. Algal Res. 2019, 41, 101567. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhao, F.; Yu, Z.; Zhou, X.; Chu, H. Impact of transmembrane pressure (TMP) on membrane fouling in microalgae harvesting with a uniform shearing vibration membrane system. Algal Res. 2018, 35, 613–623. [Google Scholar] [CrossRef]
- Wenten, I.G.; Khoiruddin, K.; Hakim, A.N.; Aryanti, P.T.P.; Rova, N. Long-Term Performance of a Pilot Scale Combined Chemical Precipitation-Ultrafiltration Technique for Waste Brine Regeneration at Chevron Steam Flooding Plant. J. Eng. Technol. Sci. 2020, 52, 501–513. [Google Scholar] [CrossRef]
- Das, S.; Vila, S.; Jové, E. Alternative cleaning method for ultrafiltration membrane system. Desalin. Water Treat. 2017, 61, 222–229. [Google Scholar] [CrossRef]
- Khodadadi, F.; Mansourianfar, M.; Bozorg, A. Application of dextran to manipulate formation mechanism, morphology, and performance of ultrafiltration membranes. Chem. Eng. Res. Des. 2022, 183, 452–465. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Bazarbayeva, A.; Kamal, A.; Kim, J.; Inglezakis, V.; Gitis, V. Tangential streaming potential, transmembrane flux, and chemical cleaning of ultrafiltration membranes. Sep. Purif. Technol. 2021, 258, 118045. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Verardo, V.; Segura-Carretero, A.; Martinez-Ferez, A. Technical optimization of an integrated UF/NF pilot plant for conjoint batch treatment of two-phase olives and olive oil washing wastewaters. Desalination 2015, 364, 82–89. [Google Scholar] [CrossRef]
- Piepiórka-Stepuk, J.; Kufel, A.; Czerwińska, E.; Kalak, T.; Prochaska, K. Impact of storage at room temperature on the properties of CIP solutions. J. Inst. Brew. 2019, 125, 374–382. [Google Scholar] [CrossRef]
- Li, K.; Su, Q.; Li, S.; Wen, G.; Huang, T. Aging of PVDF and PES Ultrafiltration Membranes by Sodium Hypochlorite: Effect of Solution pH. J. Environ. Sci. 2021, 104, 444–455. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Application of Ultrafiltration Ceramic Membrane for Separation of Oily Wastewater Generated by Maritime Transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Abd-Razak, N.H.; Chew, Y.M.J.; Bird, M.R. Orange Juice Ultrafiltration: Characterisation of Deposit Layers and Membrane Surfaces after Fouling and Cleaning. Int. J. Food Eng. 2021, 17, 837–850. [Google Scholar] [CrossRef]
- Woźniak, P.; Gryta, M. Application of Polymeric Tubular Ultrafiltration Membranes for Separation of Car Wash Wastewater. Membranes 2024, 14, 210. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Woźniak, P. The Resistance of Polyethersulfone Membranes on the Alkaline Cleaning Solutions. Membranes 2024, 14, 27. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Polyethersulfone membrane fouling mitigation during ultrafiltration of wastewaters from car washes. Desalination 2024, 574, 117254. [Google Scholar] [CrossRef]
- Forstmeier, M.; Goers, B.; Wozny, G. UF/NF treatment of rinsing waters in a liquid detergent production plant. Desalination 2002, 149, 175–177. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. [Google Scholar] [CrossRef]
- De Souza, N.P.; Basu, O.D. Comparative Analysis of Physical Cleaning Operations for Fouling Control of Hollow Fiber Membranes in Drinking Water Treatment. J. Membr. Sci. 2013, 436, 28–35. [Google Scholar] [CrossRef]
- Van Trana, T.T.; Kumara, S.R.; Lue, S.J. Separation mechanisms of binary dye mixtures using a PVDF ultrafiltration membrane: Donnan effect and intermolecular interaction. J. Membr. Sci. 2019, 575, 38–49. [Google Scholar] [CrossRef]
- Kowalska, I.; Kabsch-Korbutowicz, M.; Majewska-Nowak, K.; Winnicki, T. Separation of anionic surfactants on ultrafiltration membranes. Desalination 2004, 162, 33–40. [Google Scholar] [CrossRef]
- Ji, M.; Luo, J.; Wei, J.; Woodley, J.; Daugaard, A.E.; Pinelo, M. Commercial Polysulfone Membranes Pretreated with Ethanol and NaOH: Effects on Permeability, Selectivity and Antifouling Properties. Sep. Purif. Technol. 2019, 219, 82–89. [Google Scholar] [CrossRef]
- Xiarchos, I.; Doulia, D.; Gekas, V.; Trägårdh, G. Polymeric Ultrafiltration Membranes and Surfactants. Sep. Purif. Rev. 2003, 32, 215–278. [Google Scholar] [CrossRef]



| Dextran | IC [mg/L] | TOC [mg/L] | RTOC | ||
|---|---|---|---|---|---|
| [kDa] | F | P | F | P | [%] |
| Series D1 | |||||
| water | 2.41 | 1.73 | 0.58 | 0.48 | - |
| 40 | 2.65 | 2.11 | 19.02 | 6.11 | 67.9 |
| 70 | 2.73 | 2.15 | 20.20 | 6.69 | 66.9 |
| 110 | 2.70 | 2.19 | 19.57 | 5.34 | 72.7 |
| 200 | 2.72 | 2.18 | 20.10 | 4.88 | 75.7 |
| Series D2 | |||||
| water | 3.40 | - | 0.65 | - | - |
| 110 | 3.29 | 2.75 | 32.41 | 9.95 | 69.3 |
| water | 3.04 | - | 0.38 | - | - |
| 200 | 2.98 | 2.43 | 17.56 | 5.37 | 69.4 |
| water | 2.97 | - | 0.64 | - | - |
| 500 | 3.36 | 2.86 | 16.90 | 3.96 | 76.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryta, M.; Tomczak, W. Changes in Tubular PVDF Membrane Performance During Initial Period of Pilot Plant Operation. Membranes 2025, 15, 119. https://doi.org/10.3390/membranes15040119
Gryta M, Tomczak W. Changes in Tubular PVDF Membrane Performance During Initial Period of Pilot Plant Operation. Membranes. 2025; 15(4):119. https://doi.org/10.3390/membranes15040119
Chicago/Turabian StyleGryta, Marek, and Wirginia Tomczak. 2025. "Changes in Tubular PVDF Membrane Performance During Initial Period of Pilot Plant Operation" Membranes 15, no. 4: 119. https://doi.org/10.3390/membranes15040119
APA StyleGryta, M., & Tomczak, W. (2025). Changes in Tubular PVDF Membrane Performance During Initial Period of Pilot Plant Operation. Membranes, 15(4), 119. https://doi.org/10.3390/membranes15040119








