Ultrapure Water Production by a Saline Industrial Effluent Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Food Effluent Valorization to Treatment and Water Recovery
2.3. Desalination Technology for Industrial Effluent Treatment
2.4. Water Recovery from Food Effluent Desalination Process
2.5. Ultrapure Water Production from RO Stream
3. Results
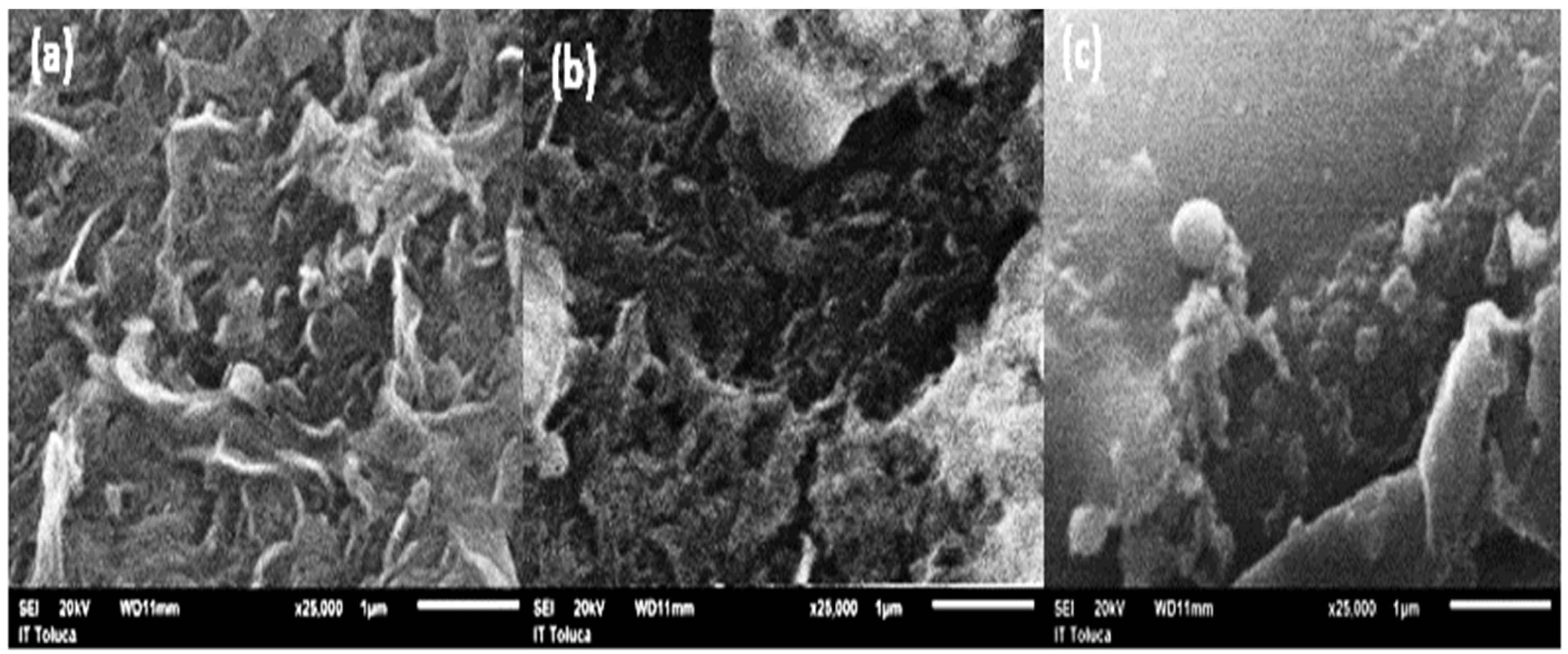
3.1. Physicochemical Characteristics of Industrial Effluent
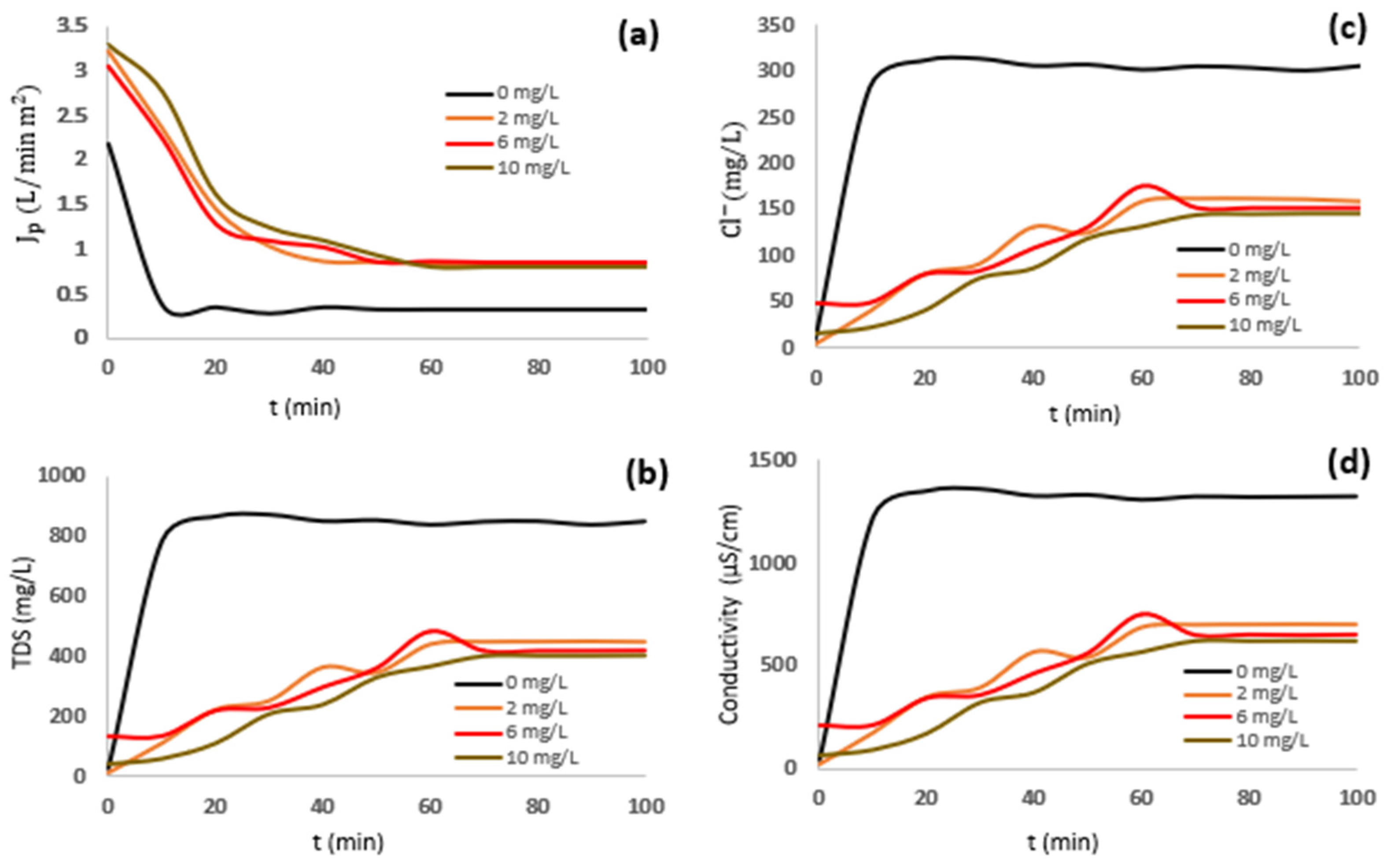
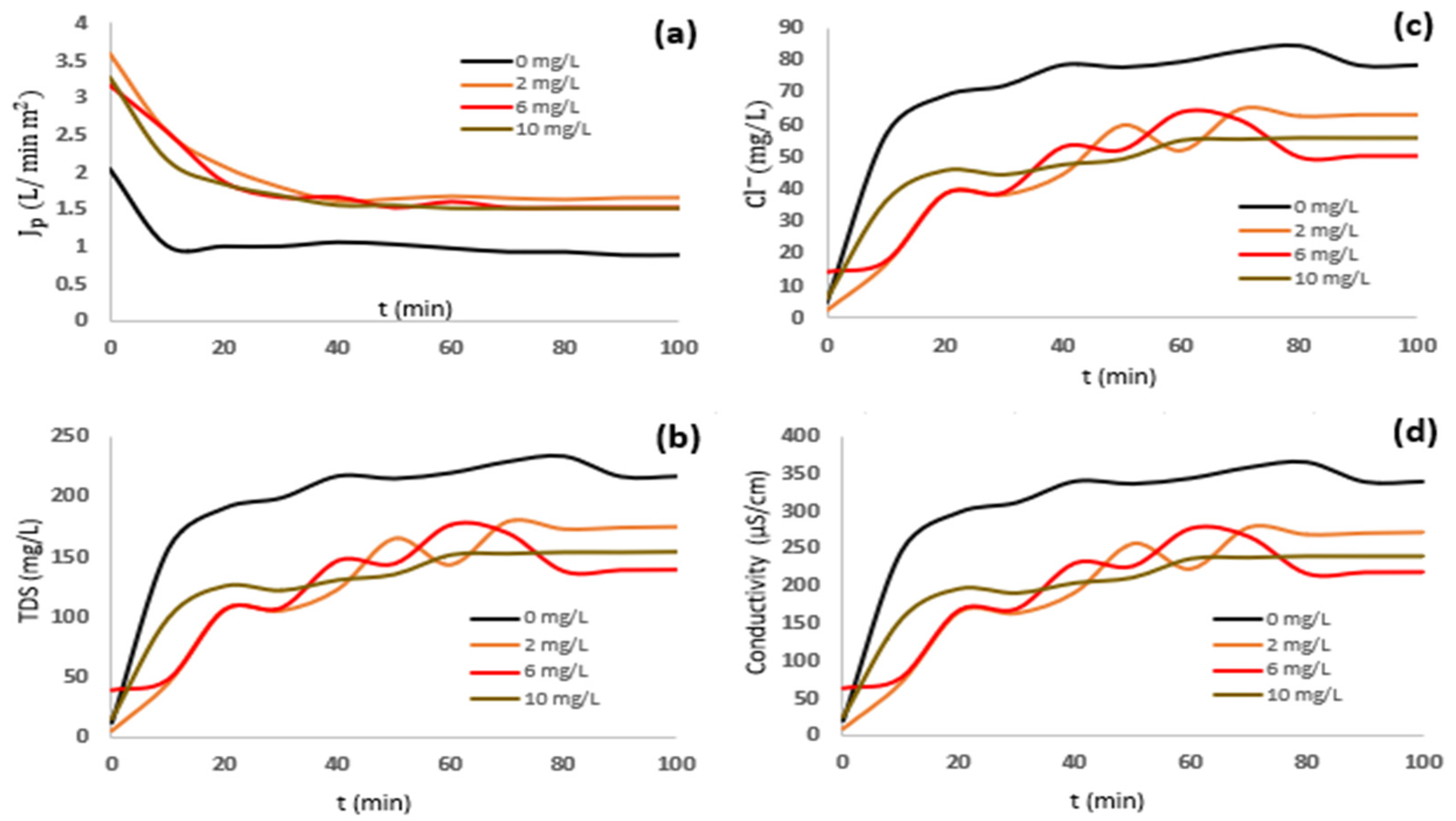
3.2. Water Recovery Efficiency from Effluent Desalination Process
3.3. Production of Ultrapure Water
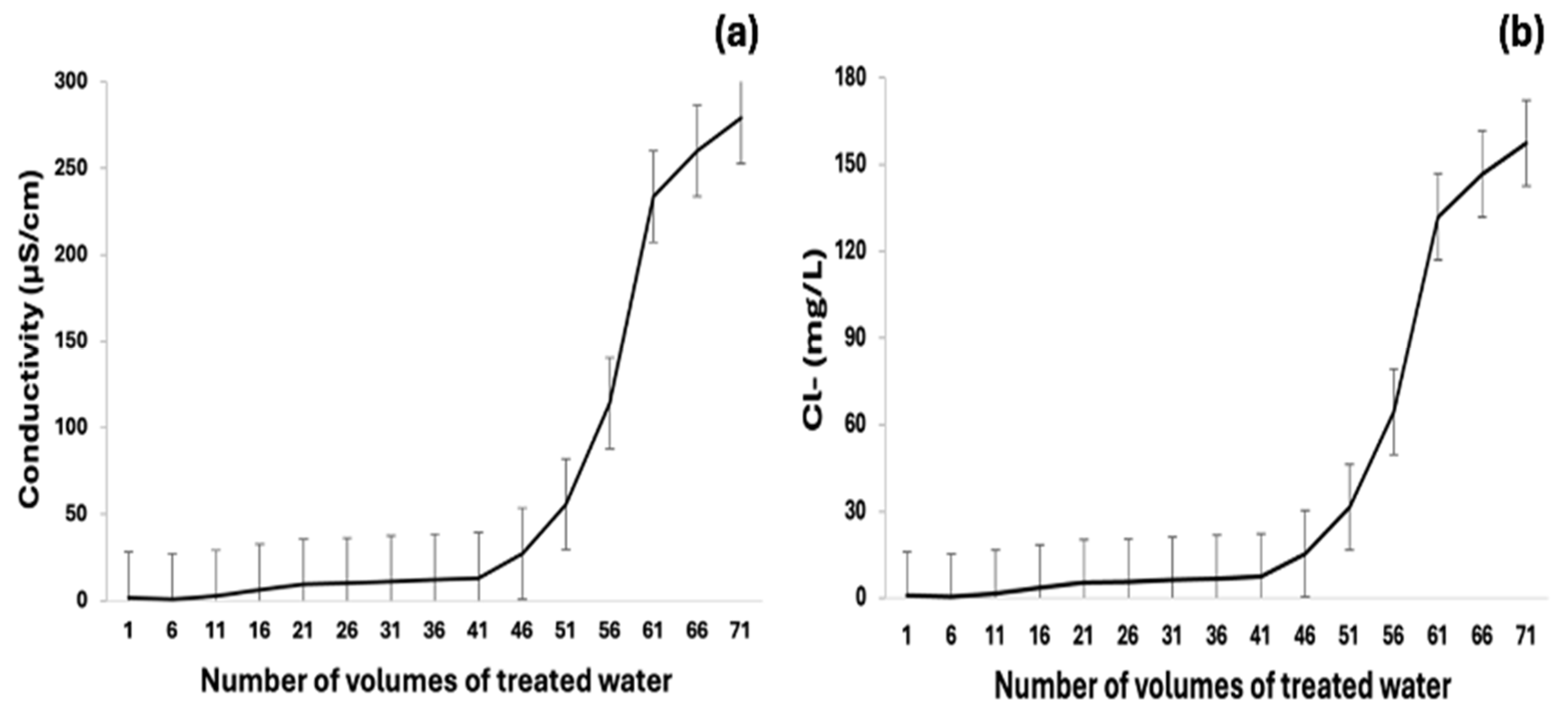
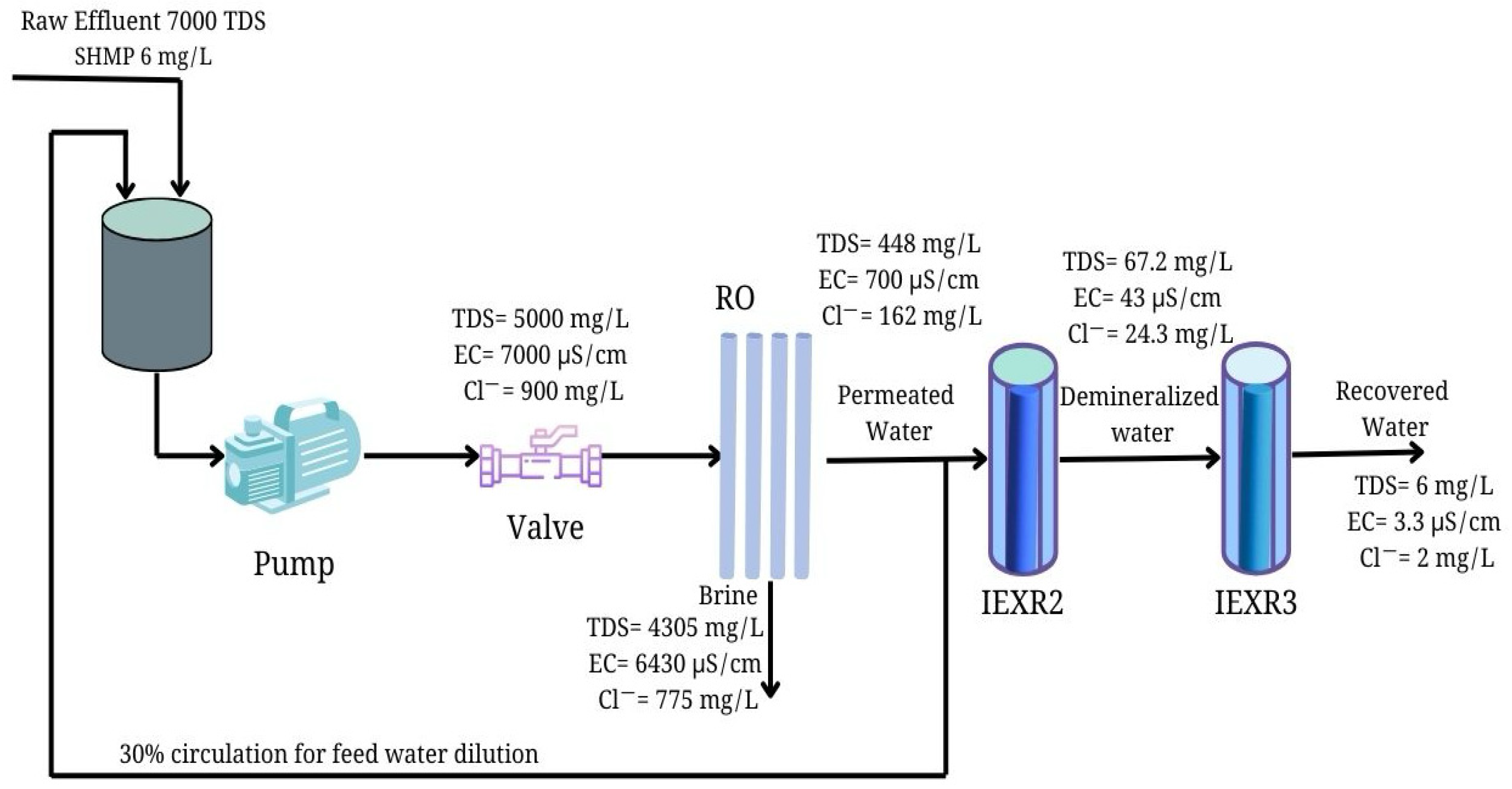
3.4. Integrated System of RO for Ultrapure Water Production from Saline Industrial Effluent
3.5. Operational Costs of Ultrapure Water Production from Saline Industrial Effluent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Cross-sectional area |
| Cp | Concentration of TDS in the permeate |
| EC | Electric conductivity |
| EDI | Electrodialysis |
| EIEX | Electrodesionization |
| F% | The percentage of fouling |
| HRM | The hydraulic resistance of the membra |
| IEX | Ion exchange |
| IEXR | Ion exchange resins |
| IP% | Ion’s permeation percentage |
| IR% | Ion’s rejection percentage |
| Jp | Permeate flux density |
| Lp | Hydraulic permeability coefficient |
| LDH | Layered double hydroxides |
| ∆P | Pressure difference |
| PTM | Transmembrane pressure |
| Qa | Feed flow |
| Qp | Permeate flow |
| R% | Percentage of water recovery |
| RO | Inverse osmosis |
| SDI | Fouling index of the membrane |
| SEM | Scanning electron microscope |
| SHMP | Sodium hexametaphosphate |
| SP% | Salts permeation percentage |
| SR% | Salts rejection percentage |
| SS | Suspended solids |
| TDS | Total dissolved solids |
| TS | Total solids |
| TSS | Total suspended solids |
| t15 min | Operation time to fill 25 mL |
| ti | Initial operation time |
| T | Time elapsed between initial and second measurement. |
References
- Hernández, K.; Muro, C.; Monroy, O.; Diaz-Blancas, V.; Alvarado, Y.; Diaz, M.C. Membrane Water Treatment for Drinking Water Production from an Industrial Effluent Used in the Manufacturing of Food Additives. Membranes 2022, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Hernández, K.; Muro, C.; Ortega, R.E.; Velazquez, S.; Riera, F. Water recovery by treatment of food industry wastewater using membrane processes. Environ. Technol. 2019, 42, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Eke, J.; Yusuf, A.; Giwa, A.; Sodiq, A. The global status of desalination: An assessment of current desalination technologies, plants and capacity. Desalination 2020, 495, 114633. [Google Scholar] [CrossRef]
- Shabib, A.; Tatan, B.; Elbaz, Y.; Hassan, A.A.; Hamouda, M.A.; Maraqa, M.A. Advancements in reverse osmosis desalination: Technology, environment, economy, and bibliometric insights. Desalination 2025, 598, 118413. [Google Scholar] [CrossRef]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Mohamed, A.A. Evaluation and optimization of antiscalant substances for enhanced reverse osmosis performance. J. Saudi Chem. Soc. 2024, 28, 101923. [Google Scholar] [CrossRef]
- Vázquez, E.; Muro, C.; Illescas, J.; Burillo, G.; Hernández, O.; Rivera, E. Obtainment and Characterization of Hydrophilic Polysulfone Membranes by N-Vinylimidazole Grafting Induced by Gamma Irradiation. Polymers 2020, 12, 1284. [Google Scholar] [CrossRef]
- Mangal, M.; Yangali-Quintanilla, V.A.; Salinas-Rodriguez, S.G.; Dusseldorp, J.; Blankert, B.; Kemperman, A.J.; Schippers, J.C.; Kennedy, M.D.; van der Meer, W.G. Application of a smart dosing pump algorithm in identifying real-time optimum dose of antiscalant in reverse osmosis systems. J. Membr. Sci. 2022, 658, 120717. [Google Scholar] [CrossRef]
- Khalek, D.E.; Abd-El-Nabey, B.A. Evaluation of sodium hexametaphosphate as scale and corrosion inhibitor in cooling water using electrochemical techniques. Desalination 2013, 311, 227–233. [Google Scholar] [CrossRef]
- de Morais, S.C.; de Lima, D.F.; Ferreira, T.M.; Domingos, J.B.; de Souza, M.A.F.; Castro, B.B.; Balaban, R.d.C. Effect of pH on the efficiency of sodium hexametaphosphate as calcium carbonate scale inhibitor at high temperature and high pressure. Desalination 2020, 491, 114548. [Google Scholar] [CrossRef]
- Hasson, D.; Cornel, A. Effect of residence time on the degree of CaCO3 precipitation in the presence of an anti-scalant. Desalination 2017, 401, 64–67. [Google Scholar] [CrossRef]
- Yerzhan, A.; Galina, P.; Nina, P.; Yerengaip, M.; Hervé, M.; Colombeau, P.; Frochot, C. Synthesis of unexplored aminophosphonic acid and evaluation as scale inhibitor for industrial water applications. Water Process Eng. 2018, 22, 192–202. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Gao, Y.; Fan, L.; Roddick, F.A.; Liu, Z. Antiscaling effect of polyaspartic acid and its derivative for RO membranes used for saline wastewater and brackish water desalination. Desalination 2017, 404, 224–229. [Google Scholar] [CrossRef]
- Shen, C.; Xu, X.; Hou, X.; Wu, D.; Yin, J. Molecular weight effect on PAA antiscale performance in LT-MED desalination system: Static experiment and MD simulation. Desalination 2018, 445, 1–5. [Google Scholar] [CrossRef]
- Ramírez-García, P.; Durán-Olivencia, M.A.; Kellermeier, M.; Van Driessche, A.E.S. Determining the operational window of green antiscalants: A case study for calcium sulfate. Desalination 2022, 544, 116128. [Google Scholar] [CrossRef]
- Alvarado, L.; Chen, A. Electrodeionization: Principles, Strategies and Applications. Electrochim. Acta 2014, 132, 583–597. [Google Scholar] [CrossRef]
- Song, J.H.; Yeon, K.H.; Cho, J. Effects of the operating parameters on the reverse osmosis-electrodeionization performance in the production of high purity water. Korean J. Chem. Eng. 2005, 22, 108–114. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; Al-Shamaileh, E.M.; Al-Whoosh, K.; Al-Ma’abrah, A.; Al-Zorqan, R.; Zanoon, R.; Rawajfeh, K.; Al-Jufout, S. Adsorption desalination of chloride ions on composite natural–synthetic materials: An approach for the reduction of chlorine corrosion in electrodeionization units. Ind. Eng. Chem. 2013, 19, 1895–1902. [Google Scholar] [CrossRef]
- Carmona, M.; Pérez, A.; de Lucas, A.; Rodríguez, L.; Rodriguez, J.F. Removal of chloride ions from an industrial polyethylenimine flocculant shifting it into an adhesive promoter using the anion exchange resin Amberlite IRA-420. React. Funct. Polym. 2008, 68, 1218–1224. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Long, J.; Jiang, D.; Liu, J.; Li, S.; Qi, J.; Zhang, P.; Wang, J.; Gong, J.; et al. Simultaneous removal of thallium and chloride from a highly saline industrial wastewater using modified anion exchange resins. Hazard Mater. 2017, 333, 179–185. [Google Scholar] [CrossRef]
- Okyere Abayie, S.; Leiviskä, T. Removal of nitrate from underground mine waters using selective ion exchange resins. J. Environ. Chem. Eng. 2022, 10, 108642. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Lipps, W.C.B.T., Braun-Howland, E.B., Eds.; American Public Health Association: Washington, DC, USA, 2023. [Google Scholar]
- Popov, K.; Oshchepkov, M.; Pervov, A.; Golovesov, V.; Ryabova, A.; Trukhina, M.; Tkachenko, S. A Case Study of Calcium Carbonate Crystallization during Reverse Osmosis Water Desalination in Presence of Novel Fluorescent-Tagged Antiscalants. Membranes 2022, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Devora-Isiordia, G.E.; Villegas-Peralta, Y.; Piña-Martínez, H.A.; Sánchez-Duarte, R.G.; Álvarez-Sánchez, J. Determination of the concentration polarization in a reverse osmosis plant to desalinate sea water. Rev. Mex. Ing. Chim. 2023, 22, 2349. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Testa, F.; Lawler, D.F.; Freeman, B.D.; Moulin, P. The effect of antiscalant addition on calcium carbonate precipitation for a simplified synthetic brackish water reverse osmosis concentrate. Water Res. 2010, 44, 2957–2969. [Google Scholar] [CrossRef]
- Chen, X.; Bi, Y.; Zhang, H.; Wang, J. Chlorides Removal and Control through water-washing process on MSWI Fly Ash. Procedia Environ. Sci. 2016, 31, 560–566. [Google Scholar] [CrossRef]
- Boonpanaid, C.; Piyamongkala, K. Using commercial resin for ion exchange to remove hardness from domestic water supply. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Li, J.; Koner, S.; German, M.; SenGupta, A.K. Aluminum-Cycle Ion Exchange Process for Hardness Removal: A New Approach for Sustainable Softening. J. Environ. Sci. Technol. 2016, 50, 11943–11950. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Hakim, A.N.; Alkhadra, M.A.; Bazant, M.Z.; Wenten, I.G. Development and long-term field test of electrodeionization for decentralized desalination facility. Chem. Eng. Process-Process Intensif. 2023, 192, 109502. [Google Scholar] [CrossRef]
- Otero, C.; Urbina, A.; Rivero, E.P.; Rodríguez, F.A. Desalination of brackish water by electrodeionization: Experimental study and mathematical modeling. Desalination 2021, 504, 114803. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E. Enhancement of hardness and chloride removal and reduction of Cl2 release and corrosion in electrodeionization units. Water Process Eng. 2015, 5, 160–165. [Google Scholar] [CrossRef]
- Santos, A.B.; Giacobbo, A.; Rodrigues, M.A.; Bernardes, A.M. Integrated membrane process (UF/RO/EDI) for treating a petrochemical wastewater to obtain ultrapure water for industrial reuse. Process Saf. Environ. Prot. 2023, 177, 223–231. [Google Scholar] [CrossRef]
- Thu, N.T.; Patra, S.; Pranudta, A.; Nguyen, T.T.; El-Moselhy, M.M.; Padungthon, S. Desalination of brackish groundwater using self-regeneration hybrid ion exchange and reverse osmosis system (HSIX-RO). Desalination 2023, 550, 116378. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, Z.; Zhang, D.; Zhang, Y.; Zhang, B.; Tang, Z. Optimization of design and operational parameters of hybrid MED-RO desalination system via modelling, simulation and engineering application. Results Eng. 2024, 24, 103253. [Google Scholar] [CrossRef]
- Mangosing, O.R.P.; Ang, H.J.T.; Galarrita, K.A.L.; Sable, S.R.J.; Ucab, P.M.L.; Cascon, H.R.; Cabaraban, M.T.I.; Tan, N.P.B. Techno-economic analysis on the production of domestic water using solar-driven membrane seawater desalination device in the Philippines. Case Stud. Therm. Eng. 2023, 41, 102575. [Google Scholar] [CrossRef]
- Triki, Z.; Bouaziz, M.N.; Boumaza, M. Techno-economic and environmental analysis of an integrated solar vacuum membrane distillation system for the treatment of reverse osmosis desalination brine. Desalination Water Treat. 2017, 83, 193–2023. [Google Scholar] [CrossRef]

| Characteristics | Units | Raw Effluent | Effluent Dilution 35% |
|---|---|---|---|
| pH | - | 8.5 ± 0.1 | 7.8 ± 0.1 |
| EC | mS/cm | 10 ± 0.1 | 6.5 ± 0.1 |
| TDS | mg/L | 7000 ± 50 | 5000 ± 50 |
| TS | mg/L | 7075 ± 35 | 5500 ± 15 |
| Cl− | mg/L | 1530 ± 5 | 810 ± 10 |
| Ca2+ | mg/L | 516 ± 0.7 | 450 ± 0.8 |
| Mg2+ | mg/L | 349 ± 0.5 | 262 ± 0.5 |
| SO42− | mg/L | 301 ± 0.5 | 250 ± 0.3 |
| Salts Removal Efficiency by RO | Raw Effluent (Initial TDS = 7000 mg/L) | Diluted Effluent (Initial TDS = 5000 mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| SHMP Concentration (mg/L) | ||||||||
| 0 | 2 | 6 | 10 | 0 | 2 | 6 | 10 | |
| TDS | 50 ± 2 | 63 ± 5 | 66 ± 1 | 77 ± 5 | 70 ± 1 | 71 ± 2 | 76 ± 1 | 78 ± 3 |
| Ca2+ | 60 ± 1 | 68 ± 1 | 70 ± 0.5 | 71 ± 0.1 | 65 ± 5 | 85 ± 0.1 | 90 ± 5 | 91 ± 2 |
| Mg2+ | 70 ± 0.5 | 79 ± 0.3 | 80 ± 0.5 | 80 ± 0.1 | 80 ± 4 | 90 ± 0.1 | 90 ± 0.7 | 90 ± 0.5 |
| SO42− | 70 ± 0.4 | 72 ± 0.1 | 75 ± 0.1 | 78 ± 0.5 | 75 ± 2 | 90 ± 0.5 | 90 ± 0.5 | 90 ± 0.3 |
| Cl− | 60 ± 0.5 | 65 ± 0.5 | 69 ± 0.3 | 70 ± 0.3 | 50 ± 0.1 | 78 ± 0.5 | 79 ± 0.4 | 79 ± 0.5 |
| Functional Parameters in RO | Raw Effluent (Initial TDS = 7000 mg/L) | Diluted Effluent (Initial TDS = 5000 mg/L) | ||||||
|---|---|---|---|---|---|---|---|---|
| SHMP Concentration (mg/L) | ||||||||
| 0 | 2 | 6 | 10 | 0 | 2 | 6 | 10 | |
| Jp% | 50 ± 2 | 50 ± 1 | 55 ± 1 | 55 ± 2 | 60 ± 4 | 62 ± 1 | 65 ± 1 | 65 ± 2 |
| SR% | 50 ± 2 | 70 ± 5 | 75 ± 3 | 75 ± 5 | 60 ± 1 | 90 ± 2 | 93 ± 0.5 | 93 ± 5 |
| F% | 94 ± 1 | 87 ± 0.5 | 85 ± 0.5 | 90 ± 0.2 | 84 ± 0.5 | 50 ± 1 | 35 ± 3 | 44 ± 1 |
| SDI | 6 ± 0.1 | 4 ± 0.01 | 4 ± 0.2 | 5 ± 0.1 | 4 ± 0.1 | 2 ± 0.01 | 3 ± 0.05 | 3 ± 0.1 |
| Ionic Resin Comercial Identification/Matrix | Identification/Functional Group | Resins Operation | TDS (mg/L) | EC (µS/cm) | Cl− (mg/L) | |||
|---|---|---|---|---|---|---|---|---|
| Input | Output | Input | Output | Input | Output | |||
| HPA25 (Strong base anion)/Styrene-DVB, Porous) | EXR1/trimethyl ammonium | First step Dichlorination and anions | 448 ± 1 | 91 ± 7 | 287 ± 1 | 58 ± 9 | 162 ± 1 | 35 ± 2 |
| WA21J (Weak base anion/Styrene-DVB, Porous) | IEXR2/polyamine | First step Dichlorination and anions | 448 ± 2 | 67 ± 5 | 287 ± 1 | 40 ± 5 | 162 ± 2 | 24 ± 3 |
| MB3710 (strongly acidic cationic gel and strongly anionic base gel Type I) | IEXR3/Sulfonic Acid and Quaternary Ammonium | Second step Remanant ions (cations and anions) | 448 ± 2 | 6 ± 1 | 287 ± 1 | 3.34 ± 1 | 162 ± 3 | 2 ± 1 |
| TDS (mg/L) | EC (µS/cm) | Cl− (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Input RO | Output RO | Output IEX2 | Output IEX3 | Input RO | Output RO | Output IEX2 | Output IEX3 | Input RO | Output RO | Output IEX2 | Output IEX3 |
| 5000 ± 50 | 448 ± 2 | 67.2 ± 5 | 6 ± 1 | 6500 ± 50 | 700 ± 5 | 40 ± 5 | 3.3 ± 1 | 810 ± 10 | 162 ± 3 | 24.3 ± 3 | 2 ± 1 |
| Operational Parameters | Description | Ultrapure Water Production m3/year | Annual Cost of Ultrapure Water Production USD/year | Cost of Water Recovery USD/m3 |
|---|---|---|---|---|
| Electric energy | 15 kW/h 0.7 USD/kW | 87,600 | 13,140 | 0.15 |
| Raw Materials | Membranes | 87,600 | 92,820 | 1.07 |
| Resins | 875 | |||
| Chemicals | SHMP | 87,600 | 200 | 0.2 |
| Cleaners | 300 | |||
| Labor operation | Plant manager | 87,600 | 10,800 8400 | 0.8 |
| Process engineer | ||||
| Technician | 6600 | |||
| Maintenance | Salary | 87,600 | 30,500 | 0.4 |
| Total cost | 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miraflores, A.H.; Gómez, K.H.; Muro, C.; Hernández, M.C.D.; Blancas, V.D.; Álvarez Sánchez, J.; Isordia, G.E.D. Ultrapure Water Production by a Saline Industrial Effluent Treatment. Membranes 2025, 15, 116. https://doi.org/10.3390/membranes15040116
Miraflores AH, Gómez KH, Muro C, Hernández MCD, Blancas VD, Álvarez Sánchez J, Isordia GED. Ultrapure Water Production by a Saline Industrial Effluent Treatment. Membranes. 2025; 15(4):116. https://doi.org/10.3390/membranes15040116
Chicago/Turabian StyleMiraflores, Adriana Hernández, Karina Hernández Gómez, Claudia Muro, María Claudia Delgado Hernández, Vianney Díaz Blancas, Jesús Álvarez Sánchez, and German Eduardo Devora Isordia. 2025. "Ultrapure Water Production by a Saline Industrial Effluent Treatment" Membranes 15, no. 4: 116. https://doi.org/10.3390/membranes15040116
APA StyleMiraflores, A. H., Gómez, K. H., Muro, C., Hernández, M. C. D., Blancas, V. D., Álvarez Sánchez, J., & Isordia, G. E. D. (2025). Ultrapure Water Production by a Saline Industrial Effluent Treatment. Membranes, 15(4), 116. https://doi.org/10.3390/membranes15040116








