Comparison of Different Polymeric Membranes in Direct Contact Membrane Distillation and Air Gap Membrane Distillation Configurations
Abstract
1. Introduction
2. Materials and Methods
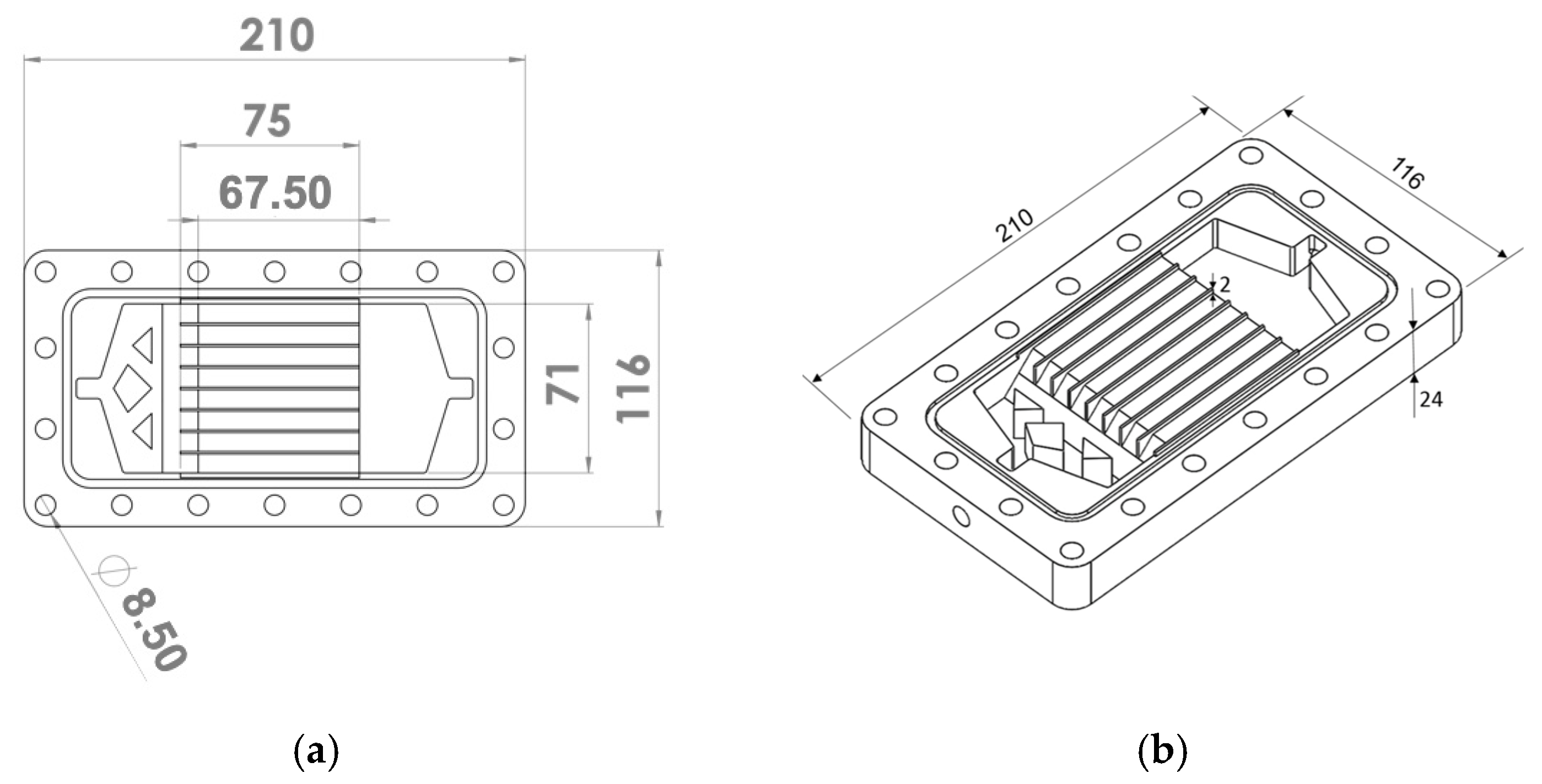
2.1. Membranes and Module Configuration
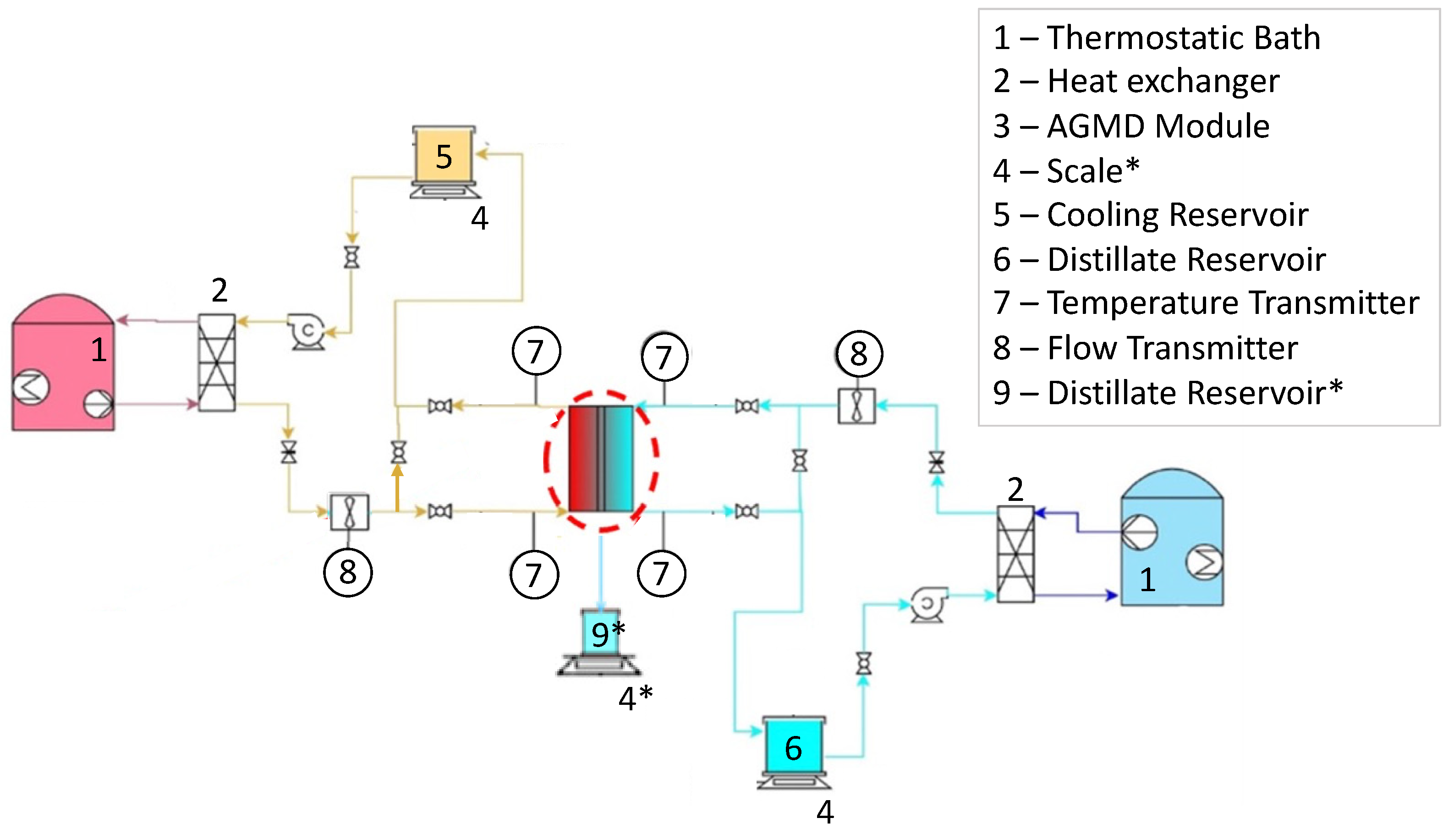
2.2. Experimental Setup
3. Theoretical Model
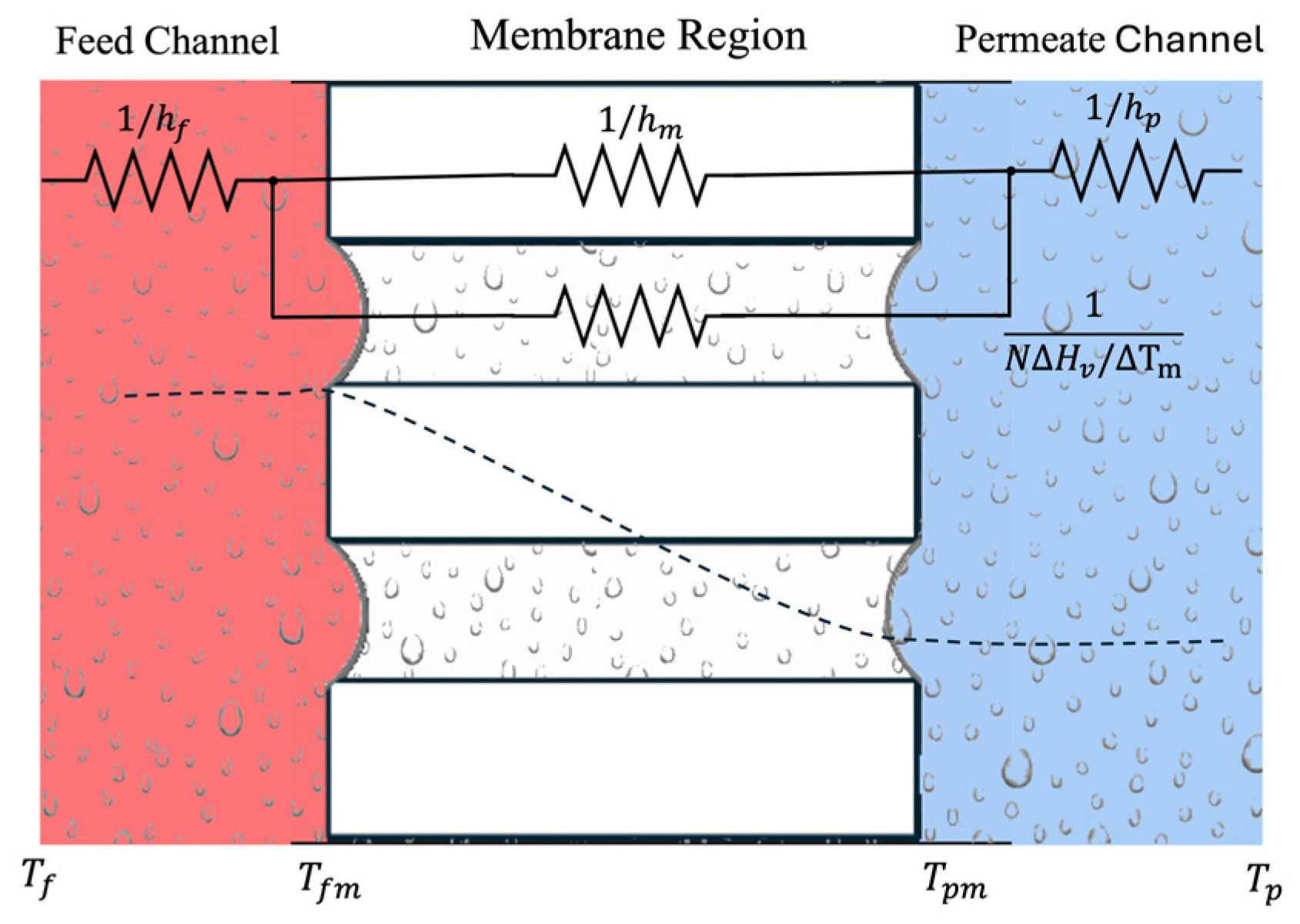
3.1. Heat Transfer
3.2. Mass Transfer
3.3. Implementation of the Computational Model
4. Results and Discussion
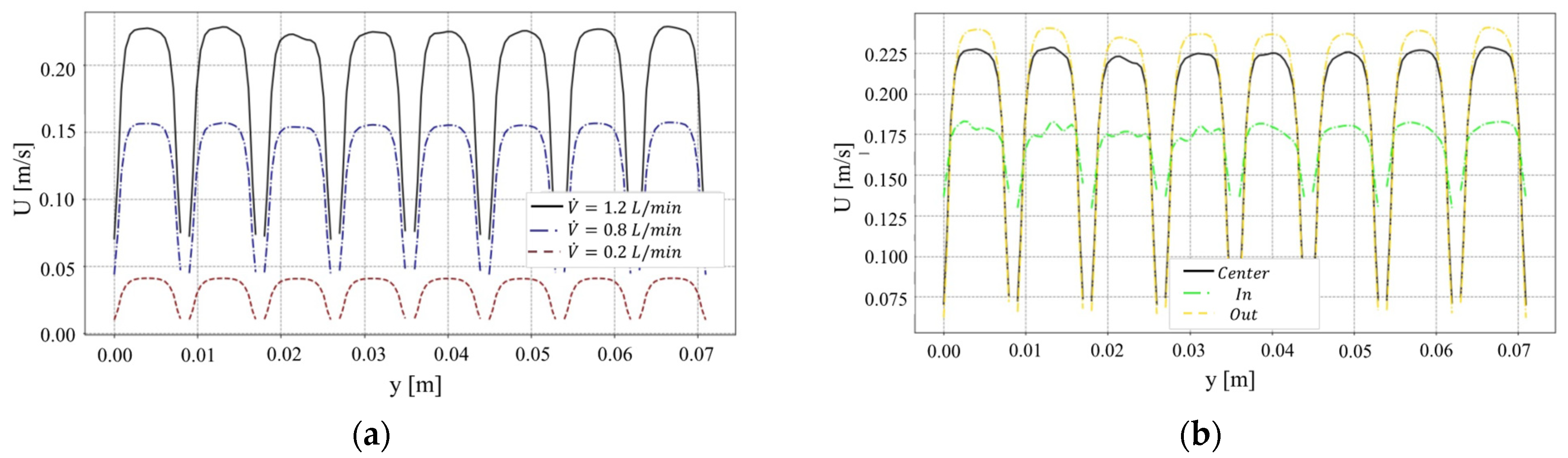
4.1. Analysis of Membrane Parameters in the MD Performance
4.2. Comparison of Energy Efficiency
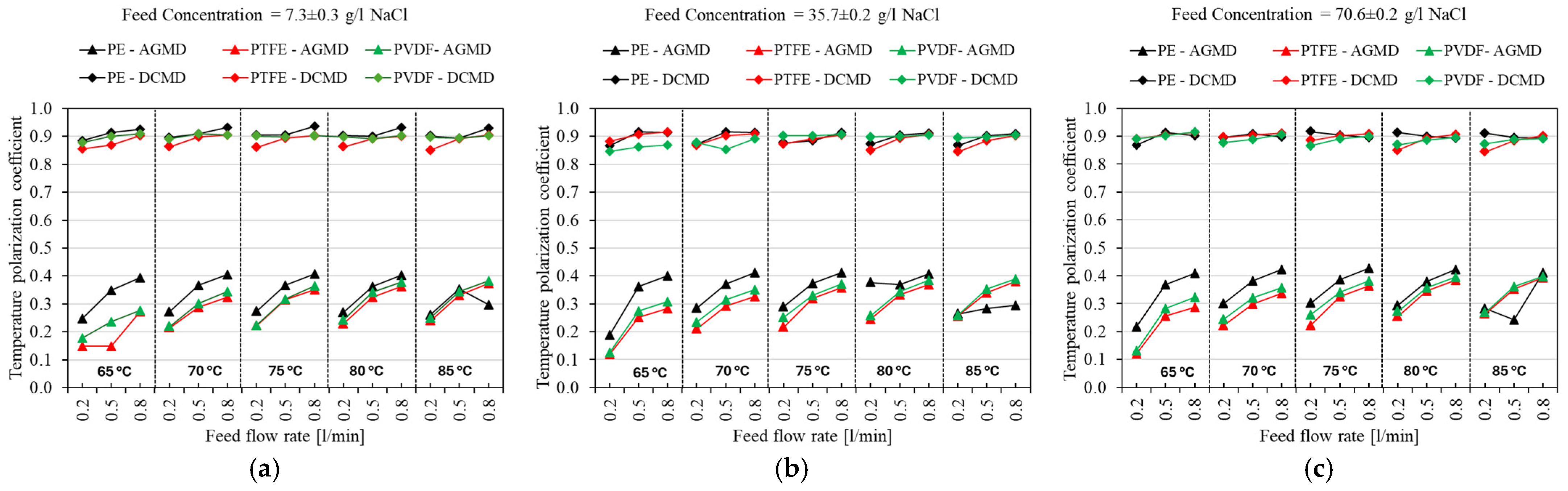
4.3. Temperature Polarization
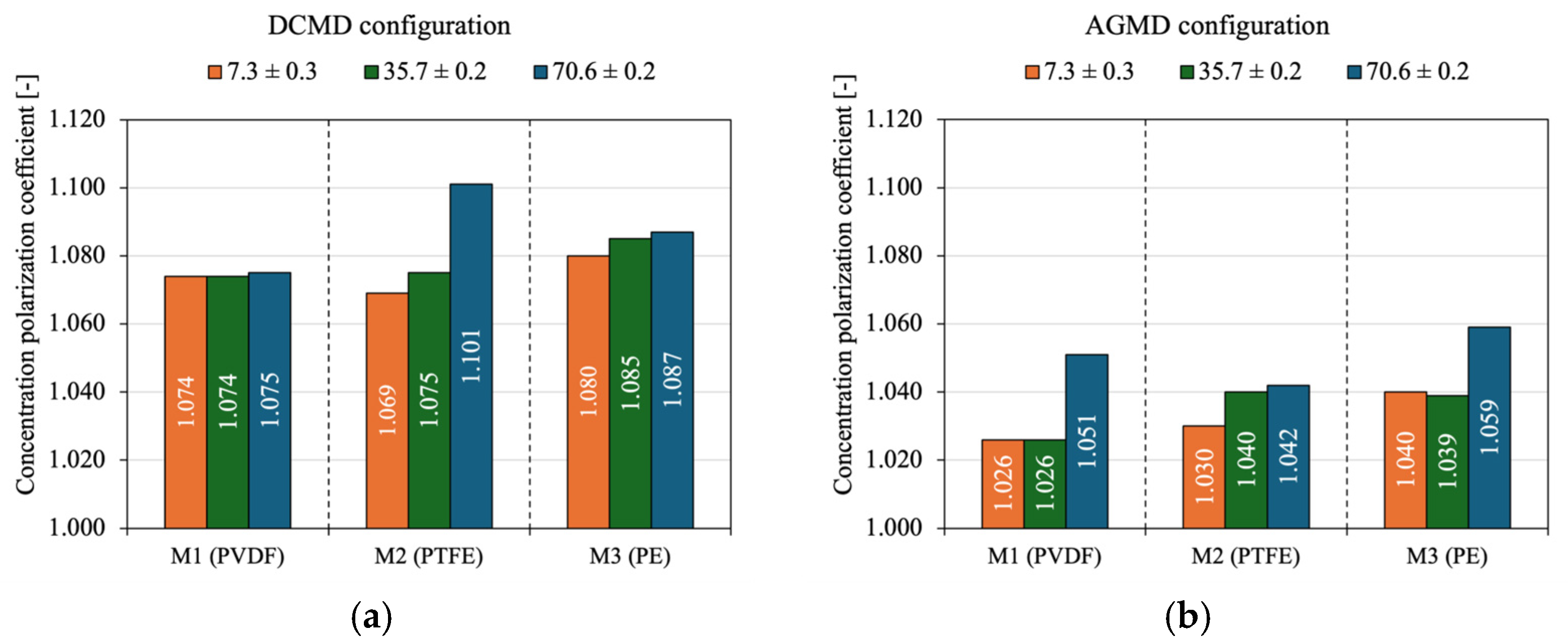
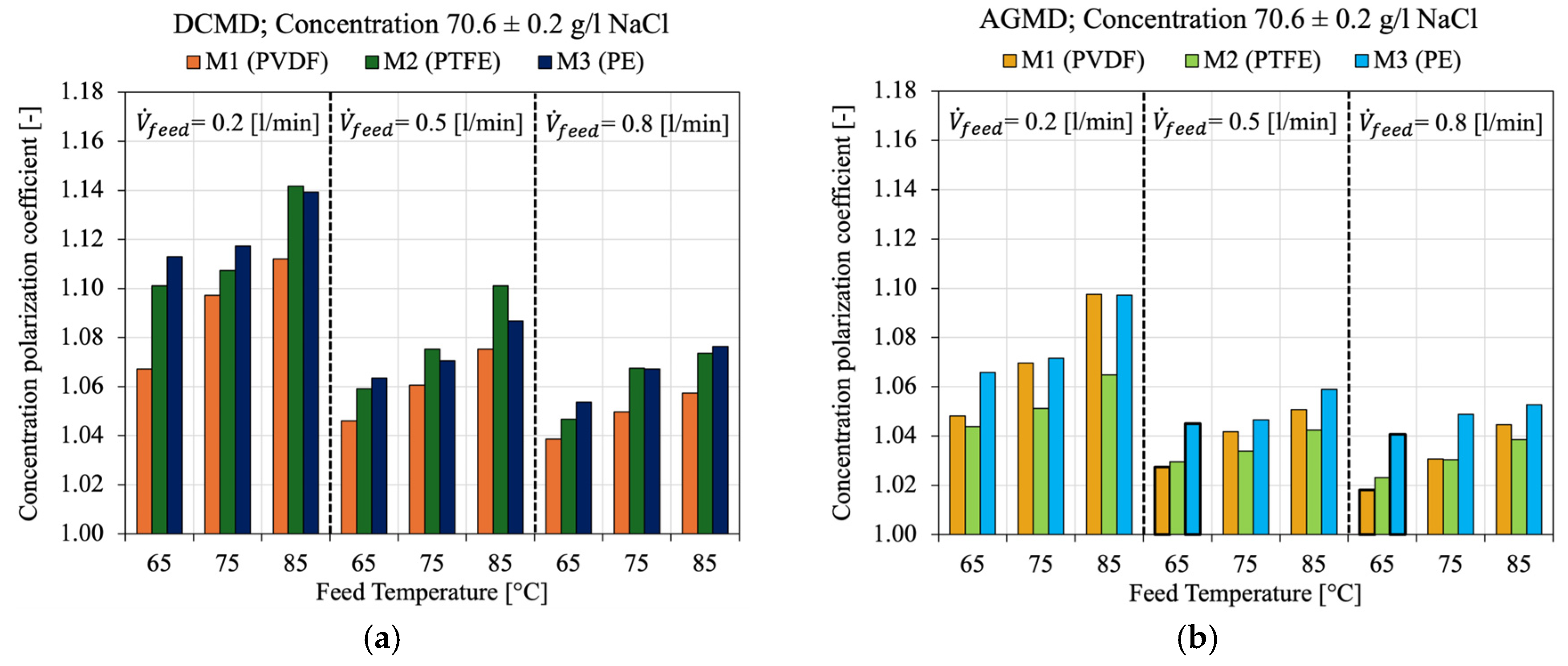
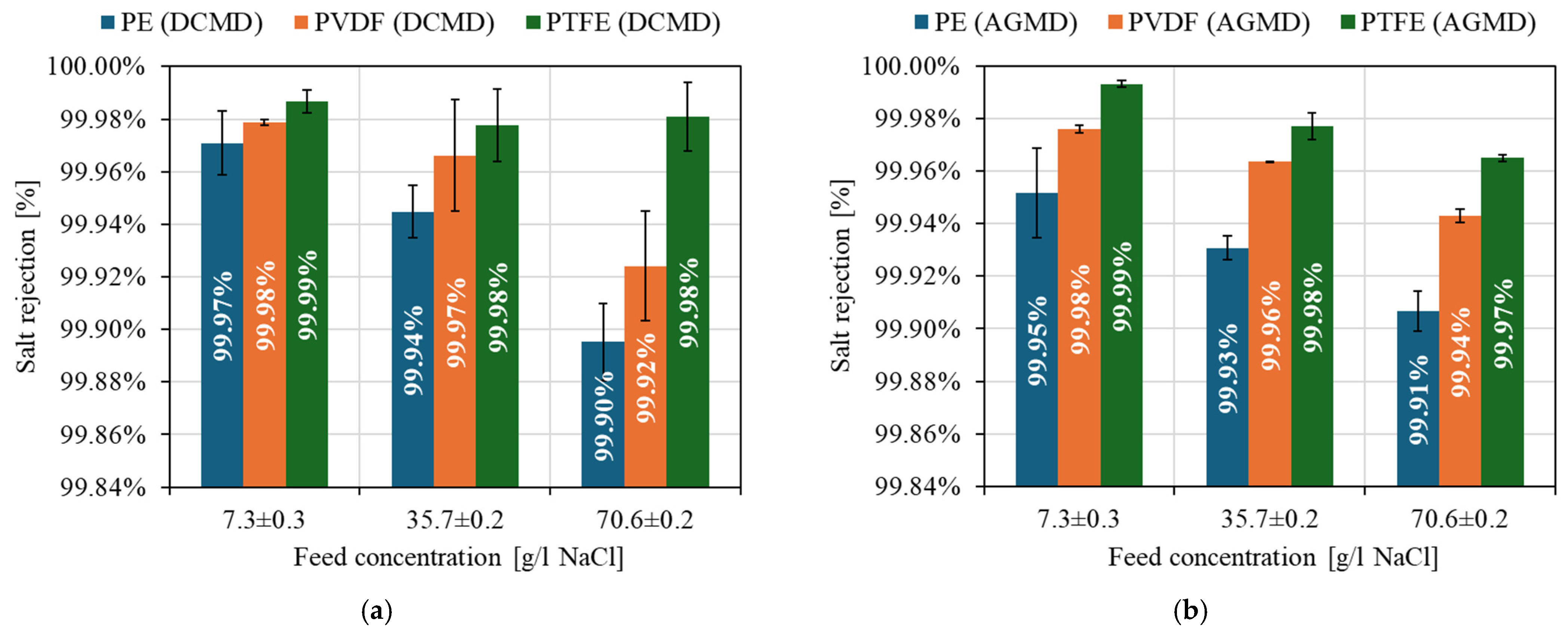
4.4. Concentration Polarization and Salt Rejection
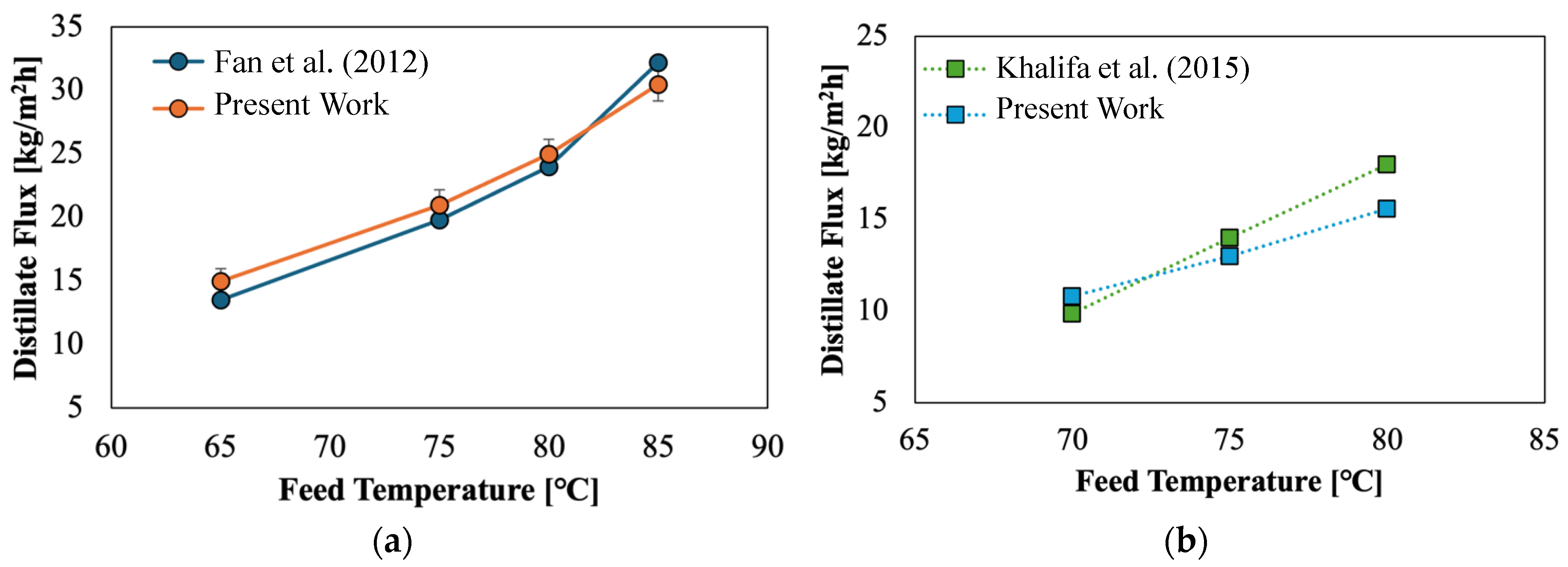
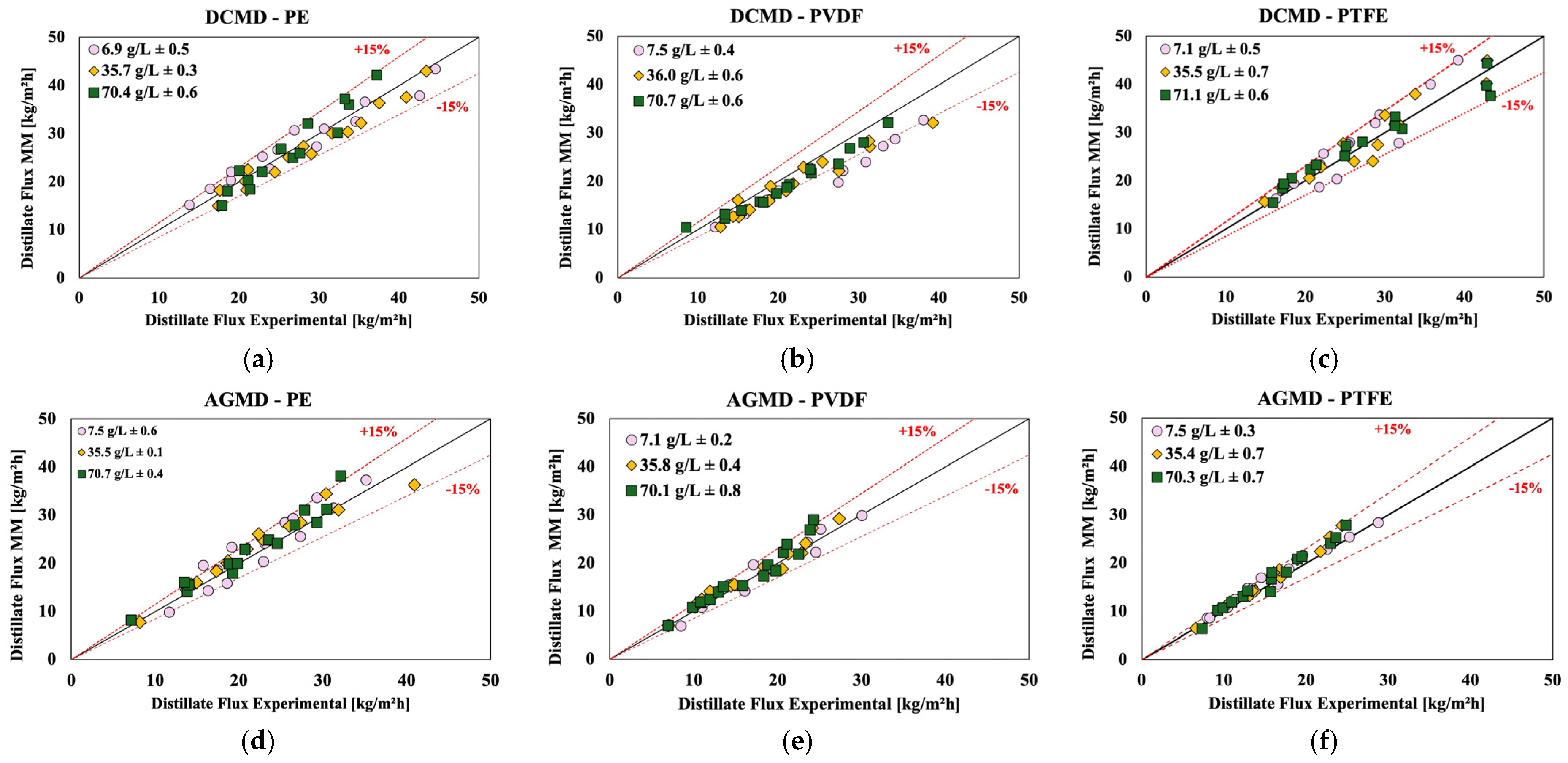
4.5. Comparison of Experimental Results and the Mathematical Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baghbanzadeh, M.; Lan, C.Q.; Rana, D.; Matsuura, T. Membrane Distillation. Nanostructured Polym. Membr. 2016, 1, 419–455. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-Distillation Desalination: Status and Potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane Distillation: Recent Developments and Perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and Applications of Direct Contact Membrane Distillation (DCMD): A Comprehensive Review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Kebria, M.R.S.; Rahimpour, A. Membrane Distillation: Basics, Advances, and Applications. Advances in Membrane Technologies; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar] [CrossRef]
- Hou, Y.; Shah, P.; Constantoudis, V.; Gogolides, E.; Kappl, M.; Butt, H.J. A Super Liquid-Repellent Hierarchical Porous Membrane for Enhanced Membrane Distillation. Nat. Commun. 2023, 14, 6886. [Google Scholar] [CrossRef]
- Giraldo-Mejía, H.; Quintero, Y.M.; Mery, F.; Rodriguez, F.; Curcio, E.; Estay, H.; Garcia, A. Plasma-Grafting Surface Modifications to Enhance Membrane Hydrophobicity for Brine Membrane Distillation. Desalination 2023, 567, 116942. [Google Scholar] [CrossRef]
- Lobregas, M.O.S.; Rangkupan, R.; Riassetto, D.; Klaysom, C. Advancing Surface-Enhanced Electrospun Nanofiber Membranes: Customizing Properties for Enhanced Performance in Membrane Distillation. Macromol. Mater. Eng. 2024, 309, 2300461. [Google Scholar] [CrossRef]
- Yu, L.; Xie, Y.; Li, L.; Zhang, M.; Yu, Y. Membrane Hydrophobicity Enhanced by Non-Solvent Induced Separation Strategy for Improving Membrane Distillation Performance. Colloids Surf. C Environ. Asp. 2024, 2, 100032. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hakami, M.; Zacharof, M.P.; Homod, H.A.; Alsadun, A. Mercury, Arsenic and Lead Removal by Air Gap Membrane Distillation: Experimental Study. Water 2020, 12, 1574. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Dumée, L.F. Membrane Distillation for Sustainable Wastewater Treatment. J. Water Process Eng. 2022, 47, 102670. [Google Scholar] [CrossRef]
- Elcik, H.; Fortunato, L.; Alpatova, A.; Soukane, S.; Orfi, J.; Ali, E.; AlAnsary, H.; Leiknes, T.O.; Ghaffour, N. Multi-Effect Distillation Brine Treatment by Membrane Distillation: Effect of Antiscalant and Antifoaming Agents on Membrane Performance and Scaling Control. Desalination 2020, 493, 114653. [Google Scholar] [CrossRef]
- Zhang, Z.; Lokoare, O.R.; Gusa, A.V.; Vidic, R.D. Pretreatment of Brackish Water Reverse Osmosis (BWRO) Concentrate to Enhance Water Recovery in Inland Desalination Plants by Direct Contact Membrane Distillation (DCMD). Desalination 2021, 508, 115050. [Google Scholar] [CrossRef]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane Distillation Using Low-Grade Energy for Desalination: A Review. J. Environ. Chem. Eng. 2021, 9, 105818. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Gorgojo, P. The Use of Carbon Nanomaterials in Membrane Distillation Membranes: A Review. Front. Chem. Sci. Eng. 2021, 15, 755–774. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Cath, T.Y.; Nghiem, L.D. Rejection and Fate of Trace Organic Compounds (TrOCs) during Membrane Distillation. J. Memb. Sci. 2014, 453, 636–642. [Google Scholar] [CrossRef]
- Salls, K.A.; Won, D.; Kolodziej, E.P.; Childress, A.E.; Hiibel, S.R. Evaluation of Semi-Volatile Contaminant Transport in a Novel, Gas-Tight Direct Contact Membrane Distillation System. Desalination 2018, 427, 35–41. [Google Scholar] [CrossRef]
- Duong, H.C.; Cooper, P.; Nelemans, B.; Cath, T.Y.; Nghiem, L.D. Evaluating Energy Consumption of Air Gap Membrane Distillation for Seawater Desalination at Pilot Scale Level. Sep. Purif. Technol. 2016, 166, 55–62. [Google Scholar] [CrossRef]
- Liu, C.; Rainwater, K.; Song, L. Calculation of Energy Consumption for Crossflow RO Desalination Processes. Desalination Water Treat. 2012, 42, 295–303. [Google Scholar] [CrossRef]
- Moudjeber, D.E.; Ruiz-Aguirre, A.; Ugarte-Judge, D.; Mahmoudi, H.; Zaragoza, G. Solar Desalination by Air-Gap Membrane Distillation: A Case Study from Algeria. Desalination Water Treat. 2016, 57, 22718–22725. [Google Scholar] [CrossRef]
- Curcino, I.V.; Reis, B.S.; Gomez, A.O.C.; Chenche, L.E.P.; Lima, J.A.; Naveira-Cotta, C.P.; Cotta, R.M. Cotta Pilot-Scale Sustainable Solar-Driven Vacuum-Enhanced Air Gap Membrane Distillation Plant for Water Desalination. In Advances in Sustainable Energy and Renewable Fuels; Gupta, A.K., Ed.; Begell House: Redding, CT, USA, 2025; Chapter 24; pp. 1–28. [Google Scholar]
- Naveira-Cotta, C.P.; Curcino, I.V.; Reis, B.S.; Gómez, A.O.C.; Costa, P.R.S.; Carvalho, J.D.C.G.; Chenche, L.E.P.; Lima, J.A.; Cotta, R.M. A Sustainable Polycogeneration Prototype for Decentralized Production of Electricity, Distilled Water and Biodiesel. In Proceedings of the 8th Thermal and Fluids Engineering Conference (TFEC), College Park, MD, USA, 26–29 March 2023; pp. 703–707. [Google Scholar] [CrossRef]
- Eykens, L.; Reyns, T.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to Select a Membrane Distillation Configuration? Process Conditions and Membrane Influence Unraveled. Desalination 2016, 399, 105–115. [Google Scholar] [CrossRef]
- González, D.; Amigo, J.; Suárez, F. Membrane Distillation: Perspectives for Sustainable and Improved Desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Janajreh, I.; El Kadi, K.; Hashaikeh, R.; Ahmed, R. Numerical Investigation of Air Gap Membrane Distillation (AGMD): Seeking Optimal Performance. Desalination 2017, 424, 122–130. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Santos, L.V.d.S.; Amaral, M.C.S. Membrane Distillation and Ion Exchange Combined Process for Mining Wastewater Treatment, Water Reuse, and Byproducts Recovery. Chem. Eng. J. 2023, 466, 143181. [Google Scholar] [CrossRef]
- Moreira, V.R.; Castro, L.M.C.; Balarini, J.C.; Santos, T.L.M.; Amaral, M.C.S. Recovering and Reusing Water, H2SO4, Nickel and Cobalt from Gold Mining Wastewater Using Air-Gap Membrane Distillation and Solvent Extraction. Desalination 2024, 580, 117525. [Google Scholar] [CrossRef]
- Moreira, V.R.; Castro, L.M.C.; Amaral, M.C.S. Comparative Analysis of Direct Contact and Air–Gap Membrane Distillation Techniques for Water Recovery from Gold Mining Wastewater. Sep. Purif. Technol. 2024, 344, 127300. [Google Scholar] [CrossRef]
- Ahmed, N. Title: Bioethanol Production from Lignocellulosic and Macroalgae Feedstock and Its Separation by Membrane Distillation: Effect of Pretreatments on Reducing Sugar and Bioethanol Yield. Ph.D. Dissertation, University of Wollongong, Wollongong, Australia, 2022. [Google Scholar]
- Kumar, R.; Ghosh, A.K.; Pal, P. Fermentative Ethanol Production from Madhuca Indica Flowers Using Immobilized Yeast Cells Coupled with Solar Driven Direct Contact Membrane Distillation with Commercial Hydrophobic Membranes. Energy Convers. Manag. 2019, 181, 593–607. [Google Scholar] [CrossRef]
- Loulergue, P.; Balannec, B.; Fouchard-Le Graët, L.; Cabrol, A.; Sayed, W.; Djelal, H.; Amrane, A.; Szymczyk, A. Air-Gap Membrane Distillation for the Separation of Bioethanol from Algal-Based Fermentation Broth. Sep. Purif. Technol. 2019, 213, 255–263. [Google Scholar] [CrossRef]
- Silva, A.F.R.; Ricci, B.C.; Koch, K.; Weißbach, M.; Amaral, M.C.S. Dissolved Hydrogen Sulfide Removal from Anaerobic Bioreactor Permeate by Modified Direct Contact Membrane Distillation. Sep. Purif. Technol. 2020, 233, 116036. [Google Scholar] [CrossRef]
- Sim, L.N.; Jayaraman, P.; Lau, Y.H.; Chong, T.H.; Wang, R. Pharmaceutical Wastewater Treatment Using Direct Contact Membrane Distillation. J. Water Process Eng. 2024, 68, 106563. [Google Scholar] [CrossRef]
- Khayet, M. Treatment of Radioactive Wastewater Solutions by Direct Contact Membrane Distillation Using Surface Modified Membranes. Desalination 2013, 321, 60–66. [Google Scholar] [CrossRef]
- Essalhi, M.; Afsar, N.U.; Bouyer, D.; Sundman, O.; Holmboe, M.; Khayet, M.; Jonsson, M.; Tavajohi, N. Gamma-Irradiated Janus Electrospun Nanofiber Membranes for Desalination and Nuclear Wastewater Treatment. J. Memb. Sci. 2024, 700, 122726. [Google Scholar] [CrossRef]
- Korolkov, I.V.; Yeszhanov, A.B.; Zdorovets, M.V.; Gorin, Y.G.; Güven, O.; Dosmagambetova, S.S.; Khlebnikov, N.A.; Serkov, K.V.; Krasnopyorova, M.V.; Milts, O.S.; et al. Modification of PET Ion Track Membranes for Membrane Distillation of Low-Level Liquid Radioactive Wastes and Salt Solutions. Sep. Purif. Technol. 2019, 227, 115694. [Google Scholar] [CrossRef]
- Al-Salmi, M.; Laqbaqbi, M.; Al-Obaidani, S.; Al-Maamari, R.S.; Khayet, M.; Al-Abri, M. Application of Membrane Distillation for the Treatment of Oil Field Produced Water. Desalination 2020, 494, 114678. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of Technologies for Oil and Gas Produced Water Treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Laqbaqbi, M.; García-Payo, M.C.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of Direct Contact Membrane Distillation for Textile Wastewater Treatment and Fouling Study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Ge, J.; Peng, Y.; Li, Z.; Chen, P.; Wang, S. Membrane Fouling and Wetting in a DCMD Process for RO Brine Concentration. Desalination 2014, 344, 97–107. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, L.; Ji, R. Direct Contact Membrane Distillation (DCMD) Process for Simulated Brackish Water Treatment: An Especial Emphasis on Impacts of Antiscalants. J. Memb. Sci. 2022, 643, 120017. [Google Scholar] [CrossRef]
- Muhamad, N.A.S.; Hanoin, M.A.H.M.; Mokhtar, N.M.; Lau, W.J.; Jaafar, J. Industrial Application of Membrane Distillation Technology Using Palm Oil Mill Effluent in Malaysia. Mater. Today Proc. 2022, 57, 1282–1287. [Google Scholar] [CrossRef]
- Shi, M.; He, Q.; Xu, R.; Sheng, Z.; Luo, H.; Meers, E.; Yan, S. Ammonia Recovery from Anaerobic Digestion Effluent Using Membrane Distillation: Membrane Fouling at Different Water Vapor Transfer Scenarios. Chem. Eng. J. 2025, 505, 159098. [Google Scholar] [CrossRef]
- Li, L.; Sirkar, K.K. Studies in Vacuum Membrane Distillation with Flat Membranes. J. Memb. Sci. 2017, 523, 225–234. [Google Scholar] [CrossRef]
- Khalifa, A.; Lawal, D.; Antar, M.; Khayet, M. Experimental and Theoretical Investigation on Water Desalination Using Air Gap Membrane Distillation. Desalination 2015, 376, 94–108. [Google Scholar] [CrossRef]
- Khalifa, A.E.; Lawal, D.U. Application of Response Surface and Taguchi Optimization Techniques to Air Gap Membrane Distillation for Water Desalination—A Comparative Study. Desalination Water Treat. 2016, 57, 28513–28530. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S. Membrane Fouling and Wetting in Membrane Distillation and Their Mitigation by Novel Membranes with Special Wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.R.; Anwar, N.; Jassby, D.; Rahaman, M.S. Fouling and Wetting in the Membrane Distillation Driven Wastewater Reclamation Process—A Review. Adv. Colloid. Interface Sci. 2019, 269, 370–399. [Google Scholar] [CrossRef]
- Medaer, B.; Bindels, M. Numerical Modelling and Optimization of Air Gap Membrane Distillation. Master’s Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2019. [Google Scholar]
- Okati, V.; Moghadam, A.J.; Farzaneh-Gord, M.; Moein-Jahromi, M. Thermo-Economical and Environmental Analyses of a Direct Contact Membrane Distillation (DCMD) Performance. J. Clean. Prod. 2022, 340, 130613. [Google Scholar] [CrossRef]
- Abdelrazeq, H.; Khraisheh, M. Porosity Effect of Polystyrene Membranes on Desalination Performance: A Combined Experimental and Numerical Heat and Mass Transfer Study in Direct Contact Membrane Distillation. Polymers 2023, 15, 1821. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Carlson, K.H.; Lee, J.; Tong, T. Membrane Fouling and Reusability in Membrane Distillation of Shale Oil and Gas Produced Water: Effects of Membrane Surface Wettability. J. Memb. Sci. 2018, 567, 199–208. [Google Scholar] [CrossRef]
- Samadi, A.; Ni, T.; Fontananova, E.; Tang, G.; Shon, H.; Zhao, S. Engineering Antiwetting Hydrophobic Surfaces for Membrane Distillation: A Review. Desalination 2023, 563, 116722. [Google Scholar] [CrossRef]
- Chang, H.; Liu, B.; Zhang, Z.; Pawar, R.; Yan, Z.; Crittenden, J.C.; Vidic, R.D. A Critical Review of Membrane Wettability in Membrane Distillation from the Perspective of Interfacial Interactions. Environ. Sci. Technol. 2021, 55, 1395–1418. [Google Scholar] [CrossRef]
- Ye, Y.; Zhao, L.; Liu, Z.; Wang, Z.; Niu, Q.J. Recent Advances in Novel Membranes for Improved Anti-Wettability in Membrane Distillation: A Mini Review. Desalination 2024, 571, 117066. [Google Scholar] [CrossRef]
- Sun, Q.; Kong, S.; Wang, L.; Luo, H.; Zhou, X.; Zhang, W.; Wu, L. Facile Preparation of Superhydrophobic PVDF/MWCNTs Distillation Membranes: Synthesis, Characteristics and Separation Performance. Sep. Purif. Technol. 2024, 347, 127567. [Google Scholar] [CrossRef]
- Park, Y.; Lee, S. Analysis of Thermal Energy Efficiency for Hollow Fiber Membranes in Direct Contact Membrane Distillation. Environ. Eng. Res. 2019, 24, 347–353. [Google Scholar] [CrossRef]
- Woldemariam, D.; Kullab, A.; Fortkamp, U.; Magner, J.; Royen, H.; Martin, A. Membrane Distillation Pilot Plant Trials with Pharmaceutical Residues and Energy Demand Analysis. Chem. Eng. J. 2016, 306, 471–483. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–477. [Google Scholar] [CrossRef]
- Jabed Perves Bappy, M.; Bahar, R.; Firdaus Ariff, T. Effect of air gap in the performance of AGMD system. ARPN J. Eng. Appl. Sci. 2016, 11, 4087–4093. [Google Scholar]
- Liu, Z.; Gao, Q.; Lu, X.; Zhao, L.; Wu, S.; Ma, Z.; Zhang, H. Study on the Performance of Double-Pipe Air Gap Membrane Distillation Module. Desalination 2016, 396, 48–56. [Google Scholar] [CrossRef]
- Sabzekar, M.; Pourafshari Chenar, M.; Maghsoud, Z.; Mostaghisi, O.; García-Payo, M.C.; Khayet, M. Cyclic Olefin Polymer as a Novel Membrane Material for Membrane Distillation Applications. J. Memb. Sci. 2021, 621, 118845. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Heat and Mass Transfer Resistance Analysis of Membrane Distillation. J. Memb. Sci. 2006, 282, 362–369. [Google Scholar] [CrossRef]
- Ali, A.; Macedonio, F.; Drioli, E.; Aljlil, S.; Alharbi, O.A. Experimental and Theoretical Evaluation of Temperature Polarization Phenomenon in Direct Contact Membrane Distillation. Chem. Eng. Res. Des. 2013, 91, 1966–1977. [Google Scholar] [CrossRef]
- Eleiwi, F.; Ghaffour, N.; Alsaadi, A.S.; Francis, L.; Laleg-Kirati, T.M. Dynamic Modeling and Experimental Validation for Direct Contact Membrane Distillation (DCMD) Process. Desalination 2016, 384, 1–11. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and Mass Transfer Analysis in Direct Contact Membrane Distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat Transport and Membrane Distillation Coefficients in Direct Contact Membrane Distillation. J. Memb. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Hitsov, I.; Maere, T.; de Sitter, K.; Dotremont, C.; Nopens, I. Modelling Approaches in Membrane Distillation: A Critical Review. Sep. Purif. Technol. 2015, 142, 48–64. [Google Scholar] [CrossRef]
- Huang, F.Y.C.; Reprogle, R. Thermal Conductivity of Polyvinylidene Fluoride Membranes for Direct Contact Membrane Distillation. Environ. Eng. Sci. 2019, 36, 420–430. [Google Scholar] [CrossRef]
- García-Payo, M.C.; Izquierdo-Gil, M.A. Thermal Resistance Technique for Measuring the Thermal Conductivity of Thin Microporous Membranes. J. Phys. D Appl. Phys. 2004, 37, 3008. [Google Scholar] [CrossRef]
- Andrjesdóttir, Ó.; Ong, C.L.; Nabavi, M.; Paredes, S.; Khalil, A.S.G.; Michel, B.; Poulikakos, D. An Experimentally Optimized Model for Heat and Mass Transfer in Direct Contact Membrane Distillation. Int. J. Heat. Mass. Transf. 2013, 66, 855–867. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane Distillation. J. Memb. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Lisboa, K.M.; Busson, D.M.; Moraes, D.; Naveira-Cotta, C.P.; Cotta, R.M. Analysis of the Membrane Effects on the Energy Efficiency of Water Desalination in a Direct Contact Membrane Distillation (DCMD) System with Heat Recovery. Appl. Therm. Eng. 2021, 182, 116063. [Google Scholar] [CrossRef]
- Deshmukh, A.; Elimelech, M. Understanding the Impact of Membrane Properties and Transport Phenomena on the Energetic Performance of Membrane Distillation Desalination. J. Memb. Sci. 2017, 539, 458–474. [Google Scholar] [CrossRef]
- Lisboa, K.M.; Curcino, I.V.; Gómez, A.O.C.; Chenche, L.E.P.; Cotta, R.M.; Naveira-Cotta, C.P. A Reduced Model for Pilot-Scale Vacuum-Enhanced Air Gap Membrane Distillation (V-AGMD) Modules: Experimental Validation and Paths for Process Improvement. Sep. Purif. Technol. 2024, 350, 127891. [Google Scholar] [CrossRef]
- Curcino, I.V.; Costa Júnior, P.R.S.; Cárdenas Gómez, A.O.; Peñaranda Chenche, L.E.; Lima, J.A.; Naveira-Cotta, C.P.; Cotta, R.M. Analysis of Effective Thermal Conductivity and Tortuosity Modeling in Membrane Distillation Simulation. Micro Nano Eng. 2022, 17, 100165. [Google Scholar] [CrossRef]
- Curcino, I.V.; Costa Júnior, P.R.S.; Cárdenas Gómez, A.O.; Peñaranda Chenche, L.E.; Lima, J.A.; Naveira-Cotta, C.P.; Cotta, R.M. Influence of Membrane Effective Thermal Conductivity and Tortuosity Modelis on the Performance of DCMD Systems for Desalination. In Proceedings of the CONEN2022—XI Congresso Nacional de Engenharia Mecânica, Teresina, Brasil, 7 August 2022. [Google Scholar]
- Liu, J.; Wang, Q.; Shan, H.; Guo, H.; Li, B. Surface Hydrophobicity Based Heat and Mass Transfer Mechanism in Membrane Distillation. J. Memb. Sci. 2019, 580, 275–288. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane Distillation at the Water-Energy Nexus: Limits, Opportunities, and Challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Delgado, J.M.P.Q. A Simple Experimental Technique to Measure Tortuosity in Packed Beds. Can. J. Chem. Eng. 2006, 84, 651–655. [Google Scholar] [CrossRef]
- Iversen, N.; Jørgensen, B.B. Diffusion Coefficients of Sulfate and Methane in Marine Sediments: Influence of Porosity. Geochim. Cosmochim. Acta 1993, 57, 571–578. [Google Scholar] [CrossRef]
- Beeckman, J.W. Mathematical Description of Heterogeneous Materials. Chem. Eng. Sci. 1990, 45, 2603–2610. [Google Scholar] [CrossRef]
- Tsai, D.S.; Strieder, W. Effective conductivities of random fiber beds. Chem. Eng. Commun. 2007, 40, 207–218. [Google Scholar] [CrossRef]
- Weissberg, H.L. Effective Diffusion Coefficient in Porous Media. J. Appl. Phys. 2004, 34, 2636. [Google Scholar] [CrossRef]
- Ghanbarian, B.; Hunt, A.G.; Sahimi, M.; Ewing, R.P.; Skinner, T.E. Percolation Theory Generates a Physically Based Description of Tortuosity in Saturated and Unsaturated Porous Media. Soil Sci. Soc. Am. J. 2013, 77, 1920–1929. [Google Scholar] [CrossRef]
- Kim, W.J.; Campanella, O.; Heldman, D.R. Predicting the Performance of Direct Contact Membrane Distillation (DCMD): Mathematical Determination of Appropriate Tortuosity Based on Porosity. J. Food Eng. 2021, 294, 110400. [Google Scholar] [CrossRef]
- Mackie, J.S.; Meares Katchalsky, P.; Shavit, A.; Eisenberg, N.; Kressman, H.; Kitchener, R.E.; Kressman, J.A.; Lifson, J.A.; Katchalsky, S.; Oosawa, A.; et al. The Diffusion of Electrolytes in a Cation-Exchange Resin Membrane I. Theoretical. Proc. R. Soc. Lond. A Math. Phys. Sci. 1955, 232, 498–509. [Google Scholar] [CrossRef]
- Jonsson, G. The Influence of the Porous Sublayer on the Salt Rejection and Reflection Coefficient of Asymmetric CA Membranes. Desalination 1980, 34, 141–157. [Google Scholar] [CrossRef]
- Iversen, S.B.; Bhatia, V.K.; Dam-Johansen, K.; Jonsson, G. Characterization of Microporous Membranes for Use in Membrane Contactors. J. Memb. Sci. 1997, 130, 205–217. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Alfadul, S.M.; Al-Romaih, H.S.; Al Shabouna, F.S.; Al-Harbi, O.A.; Drioli, E. Effect of Selected Spinning Parameters on PVDF Hollow Fiber Morphology for Potential Application in Desalination by VMD. Desalination 2014, 344, 28–35. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2002; ISBN 9780080532127. [Google Scholar]
- Wang, J.; Liu, Y.; Rao, U.; Dudley, M.; Ebrahimi, N.D.; Lou, J.; Han, F.; Hoek, E.M.V.; Tilton, N.; Cath, T.Y.; et al. Conducting Thermal Energy to the Membrane/Water Interface for the Enhanced Desalination of Hypersaline Brines Using Membrane Distillation. J. Memb. Sci. 2021, 626, 119188. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y. Application of PVDF Membranes in Desalination and Comparison of the VMD and DCMD Processes. Chem. Eng. Sci. 2012, 79, 94–102. [Google Scholar] [CrossRef]
- Khalifa, A.E. Water and Air Gap Membrane Distillation for Water Desalination—An Experimental Comparative Study. Sep. Purif. Technol. 2015, 141, 276–284. [Google Scholar] [CrossRef]
- Yao, M.; Ren, J.; Akther, N.; Woo, Y.C.; Tijing, L.D.; Kim, S.-H.; Shon, H.K. Improving Membrane Distillation Performance: Morphology Optimization of Hollow Fiber Membranes with Selected Non-Solvent in Dope Solution. Chemosphere 2019, 230, 117–126. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How To Optimize the Membrane Properties for Membrane Distillation: A Review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Summers, E.K.; Arafat, H.A.; Lienhard, J.H. Energy Efficiency Comparison of Single-Stage Membrane Distillation (MD) Desalination Cycles in Different Configurations. Desalination 2012, 290, 54–66. [Google Scholar] [CrossRef]
- Banat, F.A.; Simandl, J. Theoretical and Experimental Study in Membrane Distillation. Desalination 1994, 95, 39–52. [Google Scholar] [CrossRef]
- Kimura, S.; Nakao, S.-I.; Shimatani, S.-I. Transport Phenomena in Membrane Distillation. J. Memb. Sci. 1987, 33, 285–298. [Google Scholar] [CrossRef]
- Luo, A.; Lior, N. Study of Advancement to Higher Temperature Membrane Distillation. Desalination 2017, 419, 88–100. [Google Scholar] [CrossRef]
- Soukane, S.; Naceur, M.W.; Francis, L.; Alsaadi, A.; Ghaffour, N. Effect of Feed Flow Pattern on the Distribution of Permeate Fluxes in Desalination by Direct Contact Membrane Distillation. Desalination 2017, 418, 43–59. [Google Scholar] [CrossRef]
- Alsaadi, A.S.; Francis, L.; Amy, G.L.; Ghaffour, N. Experimental and Theoretical Analyses of Temperature Polarization Effect in Vacuum Membrane Distillation. J. Memb. Sci. 2014, 471, 138–148. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Khraisheh, M.A.M.M.; Fard, A.K.; Benyahia, F.; Adham, S. A Predictive Model for the Assessment of the Temperature Polarization Effect in Direct Contact Membrane Distillation Desalination of High Salinity Feed. Desalination 2014, 341, 38–49. [Google Scholar] [CrossRef]
- Kuang, Z.; Long, R.; Liu, Z.; Liu, W. Analysis of Temperature and Concentration Polarizations for Performance Improvement in Direct Contact Membrane Distillation. Int. J. Heat. Mass. Transf. 2019, 145, 118724. [Google Scholar] [CrossRef]
- Lokare, O.R.; Vidic, R.D. Impact of Operating Conditions on Measured and Predicted Concentration Polarization in Membrane Distillation. Environ. Sci. Technol. 2019, 53, 11869–11876. [Google Scholar] [CrossRef]
- Jang, E.; Nam, S.H.; Hwang, T.M.; Lee, S.; Choi, Y. Effect of Operating Parameters on Temperature and Concentration Polarization in Vacuum Membrane Distillation Process. Desalination Water Treat. 2015, 54, 871–880. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and Fouling in Membrane Distillation for Desalination Applications: A Review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Suleman, M.; Asif, M.; Jamal, S.A. Temperature and Concentration Polarization in Membrane Distillation: A Technical Review. Desalination Water Treat. 2021, 229, 52–68. [Google Scholar] [CrossRef]



| Units | M1 (Millipore) | M2 (Millipore) | M3 (Aquastill) | |
|---|---|---|---|---|
| Membrane Material | - | Polyvinylidene fluoride | Polytetrafluoroethylene | Polyethylene |
| Support layer material | - | - | High-Density polyethylene (HDPE) | - |
| Membrane Thickness | μm | 125 | 150 | 110 |
| Average Pore Size | μm | 0.22 | 0.22 | 0.32 |
| Porosity () | % | 75 | 85 | 85 |
| Water Contact Angle () | 128.2° (±2%) | 143.4 (±2%) | 99.5° (±2%) | |
| Water Entry Pressure | bar | 8.09 | 3.68 | 2.38 |
| Thermal conductivity 1 | W/m·K | 0.19 to 0.25 | 0.23 to 0.25 | 0.33 to 0.35 |
| Component | Unit | Values |
|---|---|---|
| Feed Tank | L | 5 |
| Permeate Tank | L | 5 |
| Feed Temperature | 65; 70; 75; 80; 85 | |
| Permeate Temperature | 32.5 | |
| Feed flow rate | L/min | 0.2; 0.5; 0.8 |
| Permeate flow rate | L/min | 0.5 |
| Feed Concentrate | g/L | 7; 35; 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesquita, C.R.S.; Gómez, A.O.C.; Cotta, C.P.N.; Cotta, R.M. Comparison of Different Polymeric Membranes in Direct Contact Membrane Distillation and Air Gap Membrane Distillation Configurations. Membranes 2025, 15, 91. https://doi.org/10.3390/membranes15030091
Mesquita CRS, Gómez AOC, Cotta CPN, Cotta RM. Comparison of Different Polymeric Membranes in Direct Contact Membrane Distillation and Air Gap Membrane Distillation Configurations. Membranes. 2025; 15(3):91. https://doi.org/10.3390/membranes15030091
Chicago/Turabian StyleMesquita, Cristiane Raquel Sousa, Abdul Orlando Cárdenas Gómez, Carolina Palma Naveira Cotta, and Renato Machado Cotta. 2025. "Comparison of Different Polymeric Membranes in Direct Contact Membrane Distillation and Air Gap Membrane Distillation Configurations" Membranes 15, no. 3: 91. https://doi.org/10.3390/membranes15030091
APA StyleMesquita, C. R. S., Gómez, A. O. C., Cotta, C. P. N., & Cotta, R. M. (2025). Comparison of Different Polymeric Membranes in Direct Contact Membrane Distillation and Air Gap Membrane Distillation Configurations. Membranes, 15(3), 91. https://doi.org/10.3390/membranes15030091







