Ceramic Nanofiltration Membranes: Creating Nanopores by Calcination of Atmospheric-Pressure Molecular Layer Deposition Grown Titanicone Layers
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Methodology: Atmospheric-Pressure MLD (AP-MLD)
2.3. Preparation of Titanicone Layers Inside Tubular α-Al2O3
2.4. Characterization
2.4.1. In-Line Gas Permeance Study of Tubular α-Al2O3 Membrane
2.4.2. Titanicone Layer Thickness Study on Planar Silicon Substrates
2.4.3. Degree of Porosity and Elemental Distribution of Calcined Titanicone Hybrid Layers on Tubular α-Al2O3
2.4.4. Layer Composition on Tubular α-Al2O3
2.4.5. Pore Size and Distribution, and Size-Selective Separation for Calcined Titanicone Hybrid Layers on Tubular α-Al2O3
3. Results and Discussion
3.1. Degree of Porosity, Elemental Distribution, and Composition of Calcined Titanicone Hybrid Layers on Tubular α-Al2O3 Analysis
3.2. In-Line Gas Permeance Analysis
3.3. Permporometry Analysis Using Water as a Condensable Vapor
3.4. Molecular Weight Cut-Off Analysis Using PEG as Solute Molecules and Water Permeability
3.5. Highlights of Experimental Results and Outlook
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Zhuang, Y.; Liu, L.; Zhang, L.; Du, J.; Zhang, Z. Design and control of a novel side-stream extractive distillation column for separating methanol-toluene binary azeotrope with intermediate boiling entrainer. Sep. Purif. Technol. 2020, 239, 116581. [Google Scholar] [CrossRef]
- El Fadil, A.; Rezaei Hosseinabadi, S.; de Oliveira Silva, R.; Sakellariou, D.; Nijmeijer, K.; Vankelecom, I.F.J. Strong impact of exposure to water/solvent mixtures on permeance of nanofiltration membranes. J. Membr. Sci. 2024, 699, 122651. [Google Scholar] [CrossRef]
- Gu, S.; Li, S.; Xu, Z. Organic solvent nanofiltration membranes for separation in non-polar solvent system. Green Energy Environ. 2024, 10, 244–267. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Lu, Z.; Ao, X.; Li, S. Ceramic nanocomposite membranes and membrane fouling: A review. Water Res. 2020, 175, 115674. [Google Scholar] [CrossRef] [PubMed]
- Merlet, R.; Winnubst, L.; Nijmeijer, A.; Amirilargani, M.; Sudhölter, E.J.R.; de Smet, L.C.P.M.; Cob, S.S.; Vandezande, P.; Dorbec, M.; Sluijter, S.; et al. Comparing the Performance of Organic Solvent Nanofiltration Membranes in Non-Polar Solvents. Chem. Ing. Tech. 2021, 93, 1389–1395. [Google Scholar] [CrossRef]
- Jarvis, P.; Carra, I.; Jafari, M.; Judd, S.J. Ceramic vs polymeric membrane implementation for potable water treatment. Water Res. 2022, 215, 118269. [Google Scholar] [CrossRef]
- Mertens, M.; Van Goethem, C.; Thijs, M.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinked PVDF-membranes for solvent resistant nanofiltration. J. Membr. Sci. 2018, 566, 223–230. [Google Scholar] [CrossRef]
- Merlet, R.B.; Pizzoccaro-Zilamy, M.A.; Nijmeijer, A.; Winnubst, L. Hybrid ceramic membranes for organic solvent nanofiltration: State-of-the-art and challenges. J. Memb. Sci. 2020, 599, 117839. [Google Scholar] [CrossRef]
- Mondal, S.; Wickramasinghe, S.R. Produced water treatment by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2008, 322, 162–170. [Google Scholar] [CrossRef]
- Vartak, S.; Myerson, A.S. Continuous Crystallization with Impurity Complexation and Nanofiltration Recycle. Org. Process Res. Dev. 2017, 21, 253–261. [Google Scholar] [CrossRef]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent surface modification of oxide surfaces. Angew. Chem. Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Sekulić, J.; Ten Elshof, J.E.; Blank, D.H.A. A microporous titania membrane for nanofiltration and pervaporation. Adv. Mater. 2004, 16, 1546–1550. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Fathizadeh, M.; Huang, Y.; Chu, K.H.; Yoon, Y.; Wang, L.; Xu, W.L.; Yu, M. TiO2 nanofiltration membranes prepared by molecular layer deposition for water purification. J. Membr. Sci. 2016, 510, 72–78. [Google Scholar] [CrossRef]
- Erdem, I. Sol-gel applications for ceramic membrane preparation. AIP Conf. Proc. 2017, 1809, 020011. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, X.; Fan, Y.; Xing, W. 1.11 Ceramic Membranes. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 1, pp. 270–297. [Google Scholar] [CrossRef]
- Hsieh, H.P.; Liu, P.K.T.; Dillman, T.R. Microporous Ceramic Membranes. Polym. J. 1991, 23, 407–415. [Google Scholar] [CrossRef][Green Version]
- Bortot Coelho, F.E.; Magnacca, G.; Boffa, V.; Candelario, V.M.; Luiten-Olieman, M.; Zhang, W. From ultra to nanofiltration: A review on the fabrication of ZrO2 membranes. Ceram. Int. 2023, 49, 8683–8708. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, C.; Tang, C.Y. Making waves: Why do we need ultra-permeable nanofiltration membranes for water treatment? Water Res. X 2023, 19, 100172. [Google Scholar] [CrossRef]
- Tsuru, T. Nano/subnano-tuning of porous ceramic membranes for molecular separation. J. Sol-Gel Sci. Technol. 2008, 46, 349–361. [Google Scholar] [CrossRef]
- Kramer, F.C.; Shang, R.; Scherrenberg, S.M.; Rietveld, L.C.; Heijman, S.J.G. Quantifying defects in ceramic tight ultra- and nanofiltration membranes and investigating their robustness. Sep. Purif. Technol. 2019, 219, 159–168. [Google Scholar] [CrossRef]
- Meng, X. An overview of molecular layer deposition for organic and organic-inorganic hybrid materials: Mechanisms, growth characteristics, and promising applications. J. Mater. Chem. A Mater. 2017, 5, 18326–18378. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Xu, R.; Xu, L.; Ding, Y.; Xiao, H.; Li, X.; Li, A.; Fang, G. Atomic/molecular layer deposition mechanism of alucone organic–inorganic hybrid materials. Mater. Today Commun. 2023, 34, 105061. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Van Bui, H.; Grillo, F.; Van Ommen, J.R. Atomic and molecular layer deposition: Off the beaten track. Chem. Commun. 2017, 53, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Weckman, T.; Laasonen, K. First principles study of the atomic layer deposition of alumina by TMA-H2O-process. Phys. Chem. Chem. Phys. 2015, 17, 17322–17334. [Google Scholar] [CrossRef]
- George, S.M.; Dameron, A.A.; Yoon, B. Surface chemistry for molecular layer deposition of organic and hybrid organic-inorganic polymers. Acc. Chem. Res. 2009, 42, 498–508. [Google Scholar] [CrossRef]
- Sundberg, P.; Karppinen, M. Organic and inorganic-organic thin film structures by molecular layer deposition: A review. Beilstein J. Nanotechnol. 2014, 5, 1104–1136. [Google Scholar] [CrossRef]
- Tanskanen, A.; Sundberg, P.; Nolan, M.; Karppinen, M. Atomic/molecular layer deposition of Ti-organic thin films from different aromatic alcohol and amine precursors. Thin Solid Film. 2021, 736, 138896. [Google Scholar] [CrossRef]
- Multia, J.; Karppinen, M. Atomic/Molecular Layer Deposition for Designer’s Functional Metal–Organic Materials. Adv. Mater. Interfaces 2022, 9, 2200210. [Google Scholar] [CrossRef]
- Sengupta, B.; Dong, Q.; Khadka, R.; Behera, D.K.; Yang, R.; Liu, J.; Jiang, J.; Keblinski, P.; Belfort, G.; Yu, M. Carbon-doped metal oxide interfacial nanofilms for ultrafast and precise separation of molecules. Science 2023, 381, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Madadi, M.; Heiska, J.; Multia, J.; Karppinen, M. Atomic and Molecular Layer Deposition of Alkali Metal Based Thin Films. ACS Appl. Mater. Interfaces 2021, 13, 56793–56811. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, H.; Nijboer, M.; Makhoul, E.; Nijmeijer, A.; Roozeboom, F.; Bechelany, M.; Kovalgin, A.; Luiten-Olieman, M. Increasing hydrophobicity of ceramic membranes by post-deposition nitrogen annealing of molecular layer deposition grown hybrid layers. Appl. Surf. Sci. 2025, 683, 161790. [Google Scholar] [CrossRef]
- CoorsTek, The Netherlands. Available online: https://www.coorstek.com/en/materials/alumina/ (accessed on 27 February 2025).
- Nijboer, M.; Jan, A.; Chen, M.; Batenburg, K.; Peper, J.; Aarnink, T.; Roozeboom, F.; Kovalgin, A.; Nijmeijer, A.; Luiten-Olieman, M. Tuning Nanopores in Tubular Ceramic Nanofiltration Membranes with Atmospheric-Pressure Atomic Layer Deposition: Prospects for Pressure-Based In-Line Monitoring of Pore Narrowing. Separations 2024, 11, 24. [Google Scholar] [CrossRef]
- Muñoz-Rojas, D.; Nguyen, V.H.; Masse de la Huerta, C.; Aghazadehchors, S.; Jiménez, C.; Bellet, D. Spatial Atomic Layer Deposition (SALD), an emerging tool for energy materials. Application to new-generation photovoltaic devices and transparent conductive materials. Comptes Rendus. Phys. 2017, 18, 391–400. [Google Scholar] [CrossRef]
- Abdulagatov, A.I.; Hall, R.A.; Sutherland, J.L.; Lee, B.H.; Cavanagh, A.S.; George, S.M. Molecular layer deposition of titanicone films using TiCl4 and ethylene glycol or glycerol: Growth and properties. Chem. Mater. 2012, 24, 2854–2863. [Google Scholar] [CrossRef]
- Dameron, A.A.; Seghete, D.; Burton, B.B.; Davidson, S.D.; Cavanagh, A.S.; Bertrand, J.A.; George, S.M. Molecular layer deposition of alucone polymer films using trimethylaluminum and ethylene glycol. Chem. Mater. 2008, 20, 3315–3326. [Google Scholar] [CrossRef]
- Johs, B.; Woollam, J.A.; Herzinger, C.M.; Hilfiker, J.N.; Synowicki, R.A.; Bungay, C.L. Overview of variable-angle spectroscopic ellipsometry (VASE): II. Advanced applications. In Optical Metrology: A Critical Review; SPIE: Bellingham, WA, USA, 1999; Volume 10294, p. 1029404. [Google Scholar] [CrossRef]
- Agilent. Available online: https://www.agilent.com/en/product/liquid-chromatography/hplc-systems/gpc-sec-solutions/1260-infinity-ii-gpc-sec-system (accessed on 27 February 2025).
- Kaya, S.; Soykan, U.; Sunkar, M.; Karaboğa, S.; Doğan, M.U.; Terzioğlu, R.; Yildirim, G. Annealing-induced modifications on structural, surface chemical bonding, and electrical characteristics of p-NiO/n-TiO2 heterostructure. J. Mater. Sci. Mater. Electron. 2023, 34, 1725. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Gautam, L.; Razeghi, M. Highly Conductive Co-Doped Ga2O3:Si-In Grown by MOCVD. Coatings 2021, 11, 287. [Google Scholar] [CrossRef]
- Tsuru, T.; Hino, T.; Yoshioka, T.; Asaeda, M. Permporometry characterization of microporous ceramic membranes. J. Membr. Sci. 2001, 186, 257–265. [Google Scholar] [CrossRef]
- Cuperus, F.P.; Bargeman, D.; Smolders, C.A. Permporometry. The determination of the size distribution of active pores in UF membranes. J. Membr. Sci. 1992, 71, 57–67. [Google Scholar] [CrossRef]
- Marchetti, P.; Butté, A.; Livingston, A.G. An improved phenomenological model for prediction of solvent permeation through ceramic NF and UF membranes. J. Membr. Sci. 2012, 415–416, 444–458. [Google Scholar] [CrossRef]
- Cao, G.Z.; Meijerink, J.; Brinkman, H.W.; Burggraaf, A.J. Permporometry study on the size distribution of active pores in porous ceramic membranes. J. Membr. Sci. 1993, 83, 221–235. [Google Scholar] [CrossRef]
- Henninger, M.; Engelpracht, M.; Tuchlinski, D.; Ismail, M.; Bardow, A.; Seiler, J. Water, ethanol, or methanol for adsorption chillers? Model-based performance prediction from Infrared–Large-Temperature-Jump experiments. Appl. Therm. Eng. 2024, 236, 121816. [Google Scholar] [CrossRef]
- Cooper, A.R.; Van Derveer, D.S. Characterization of Ultrafiltration Membranes by Polymer Transport Measurements. Sep. Sci. Technol. 1979, 14, 551–556. [Google Scholar] [CrossRef]
- Merdaw, A.A.; Sharif, A.O.; Derwish, G.A.W. Water permeability in polymeric membranes, Part I. Desalination 2010, 260, 180–192. [Google Scholar] [CrossRef]
- Verbeke, R.; Nulens, I.; Thijs, M.; Lenaerts, M.; Bastin, M.; Van Goethem, C.; Koeckelberghs, G.; Vankelecom, I.F.V. Solutes in solvent resistant and solvent tolerant nanofiltration: How molecular interactions impact membrane rejection. J. Membr. Sci. 2023, 677, 121595. [Google Scholar] [CrossRef]
- Yadav, A.R.; Sriram, R.; Carter, J.A.; Miller, B.L. Comparative study of solution-phase and vapor-phase deposition of aminosilanes on silicon dioxide surfaces. Mater. Sci. Eng. C 2014, 35, 283–290. [Google Scholar] [CrossRef]
- Nayeri, D.; Mousavi, S.A. A critical review on the effect of silanization on the ceramic membrane distillation (CMD): Performance, operational factors, and characterization. Water Sci. 2024, 14, 114. [Google Scholar] [CrossRef]
 ), and α-Al2O3 coated with titanicone layers deposited at 125 °C (
), and α-Al2O3 coated with titanicone layers deposited at 125 °C ( ) and after calcination at 350 °C (
) and after calcination at 350 °C ( ). Vertical scale bars (A–F) 10 µm.
). Vertical scale bars (A–F) 10 µm.
 ), and α-Al2O3 coated with titanicone layers deposited at 125 °C (
), and α-Al2O3 coated with titanicone layers deposited at 125 °C ( ) and after calcination at 350 °C (
) and after calcination at 350 °C ( ). Vertical scale bars (A–F) 10 µm.
). Vertical scale bars (A–F) 10 µm.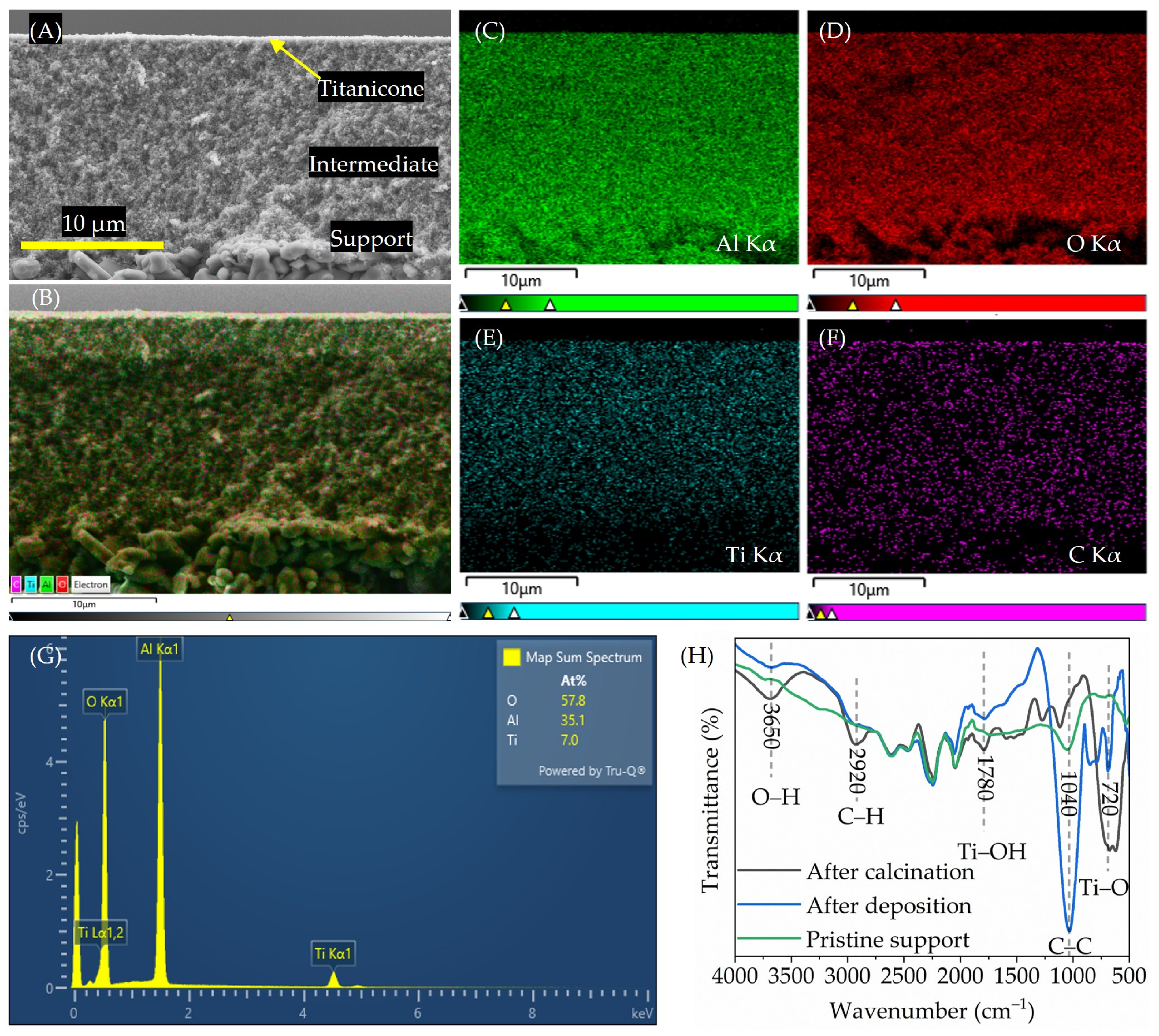
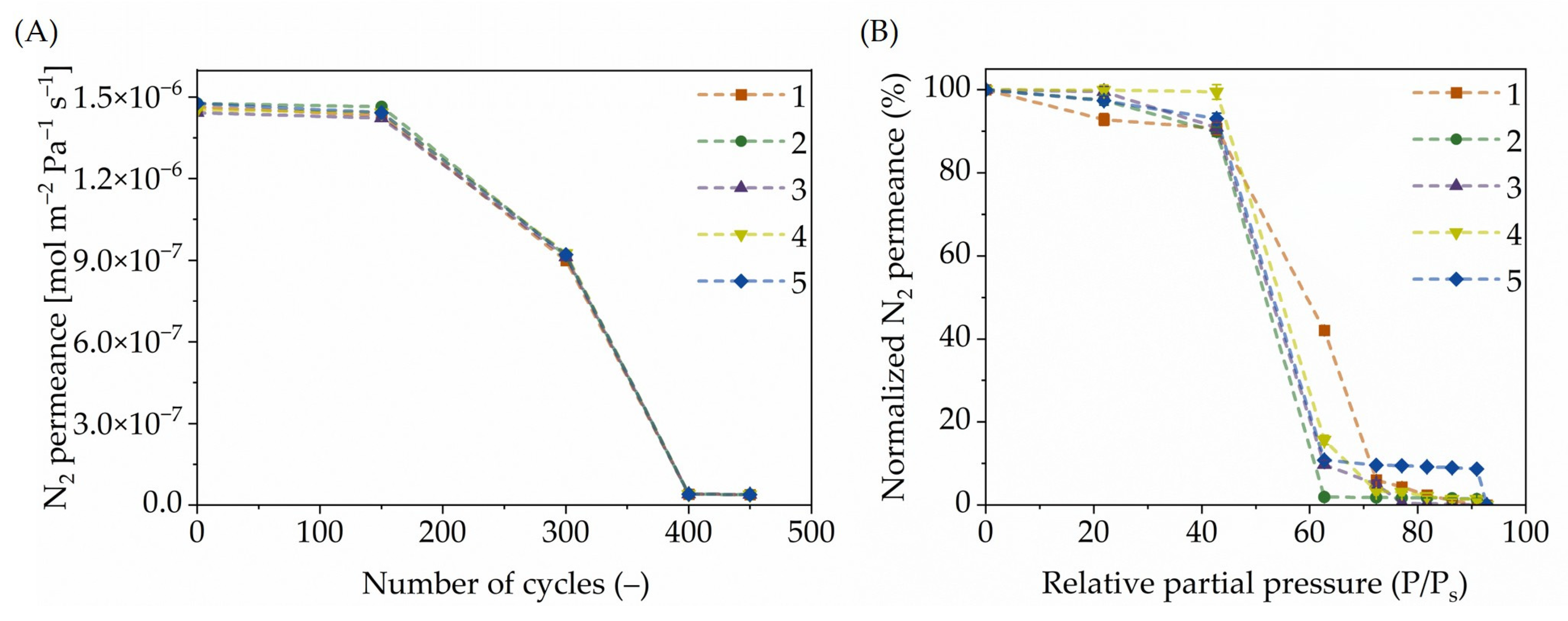
 ), 2 (
), 2 ( ), 3 (
), 3 ( ), 4 (
), 4 ( ), and 5 (
), and 5 ( ).
).
 ), 2 (
), 2 ( ), 3 (
), 3 ( ), 4 (
), 4 ( ), and 5 (
), and 5 ( ).
).
 ) with respect to (A) pore radius (
) with respect to (A) pore radius ( ) and (B) water flux (
) and (B) water flux ( ) of calcined titanicone hybrid layer membranes, calcined at 350 °C.
) of calcined titanicone hybrid layer membranes, calcined at 350 °C.
 ) with respect to (A) pore radius (
) with respect to (A) pore radius ( ) and (B) water flux (
) and (B) water flux ( ) of calcined titanicone hybrid layer membranes, calcined at 350 °C.
) of calcined titanicone hybrid layer membranes, calcined at 350 °C.
 ), 25 °C (
), 25 °C ( ), and 30 °C (
), and 30 °C ( ), for calcined titanicone hybrid layer membranes.
), for calcined titanicone hybrid layer membranes.
 ), 25 °C (
), 25 °C ( ), and 30 °C (
), and 30 °C ( ), for calcined titanicone hybrid layer membranes.
), for calcined titanicone hybrid layer membranes.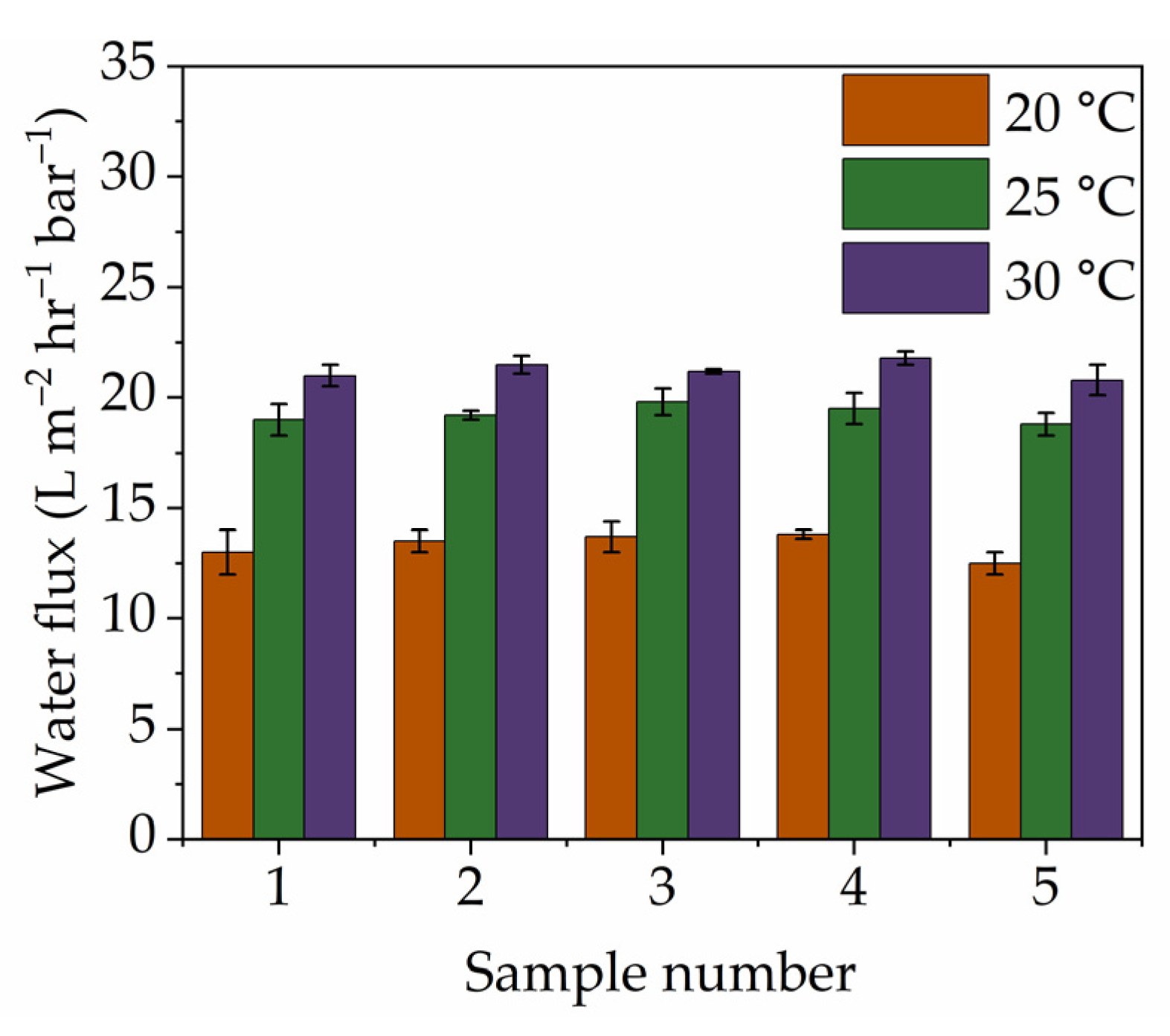
| Sample | Pore Radius (nm) | MWCO (Dalton) | Water Flux ( L·m−2·h−1·bar−1) |
|---|---|---|---|
| (Standard dev.~0.1) | (Standard dev.~6) | (Standard dev.~1) | |
| 1 | 0.8 | 375 | 13 |
| 2 | 0.9 | 383 | 13.5 |
| 3 | 0.9 | 385 | 13.7 |
| 4 | 0.9 | 386 | 13.8 |
| 5 | 0.7 | 370 | 12.5 |
| Membrane Type | Temperature (°C) | Pressure (bar) | MWCO (Da) | Water flux (L·m−2·h−1·bar−1) |
|---|---|---|---|---|
| NF200 [50] | 20 | 20 | 300–360 | 7.7 |
| 25 | 20 | - | 8.65 | |
| 30 | 20 | - | 9.71 | |
| Calcined titanicone hybrid layer | 20 25 30 | 9 9 9 | 380 ± 6 - - | 13 ± 1 19 ± 0.7 21 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sondhi, H.; Chen, M.; Nijboer, M.P.; Nijmeijer, A.; Roozeboom, F.; Bechelany, M.; Kovalgin, A.; Luiten-Olieman, M. Ceramic Nanofiltration Membranes: Creating Nanopores by Calcination of Atmospheric-Pressure Molecular Layer Deposition Grown Titanicone Layers. Membranes 2025, 15, 86. https://doi.org/10.3390/membranes15030086
Sondhi H, Chen M, Nijboer MP, Nijmeijer A, Roozeboom F, Bechelany M, Kovalgin A, Luiten-Olieman M. Ceramic Nanofiltration Membranes: Creating Nanopores by Calcination of Atmospheric-Pressure Molecular Layer Deposition Grown Titanicone Layers. Membranes. 2025; 15(3):86. https://doi.org/10.3390/membranes15030086
Chicago/Turabian StyleSondhi, Harpreet, Mingliang Chen, Michiel Pieter Nijboer, Arian Nijmeijer, Fred Roozeboom, Mikhael Bechelany, Alexey Kovalgin, and Mieke Luiten-Olieman. 2025. "Ceramic Nanofiltration Membranes: Creating Nanopores by Calcination of Atmospheric-Pressure Molecular Layer Deposition Grown Titanicone Layers" Membranes 15, no. 3: 86. https://doi.org/10.3390/membranes15030086
APA StyleSondhi, H., Chen, M., Nijboer, M. P., Nijmeijer, A., Roozeboom, F., Bechelany, M., Kovalgin, A., & Luiten-Olieman, M. (2025). Ceramic Nanofiltration Membranes: Creating Nanopores by Calcination of Atmospheric-Pressure Molecular Layer Deposition Grown Titanicone Layers. Membranes, 15(3), 86. https://doi.org/10.3390/membranes15030086








