Changes in the Separation Properties of Aged PVDF Ultrafiltration Membranes During Long-Term Treatment of Car Wash Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes
- (i)
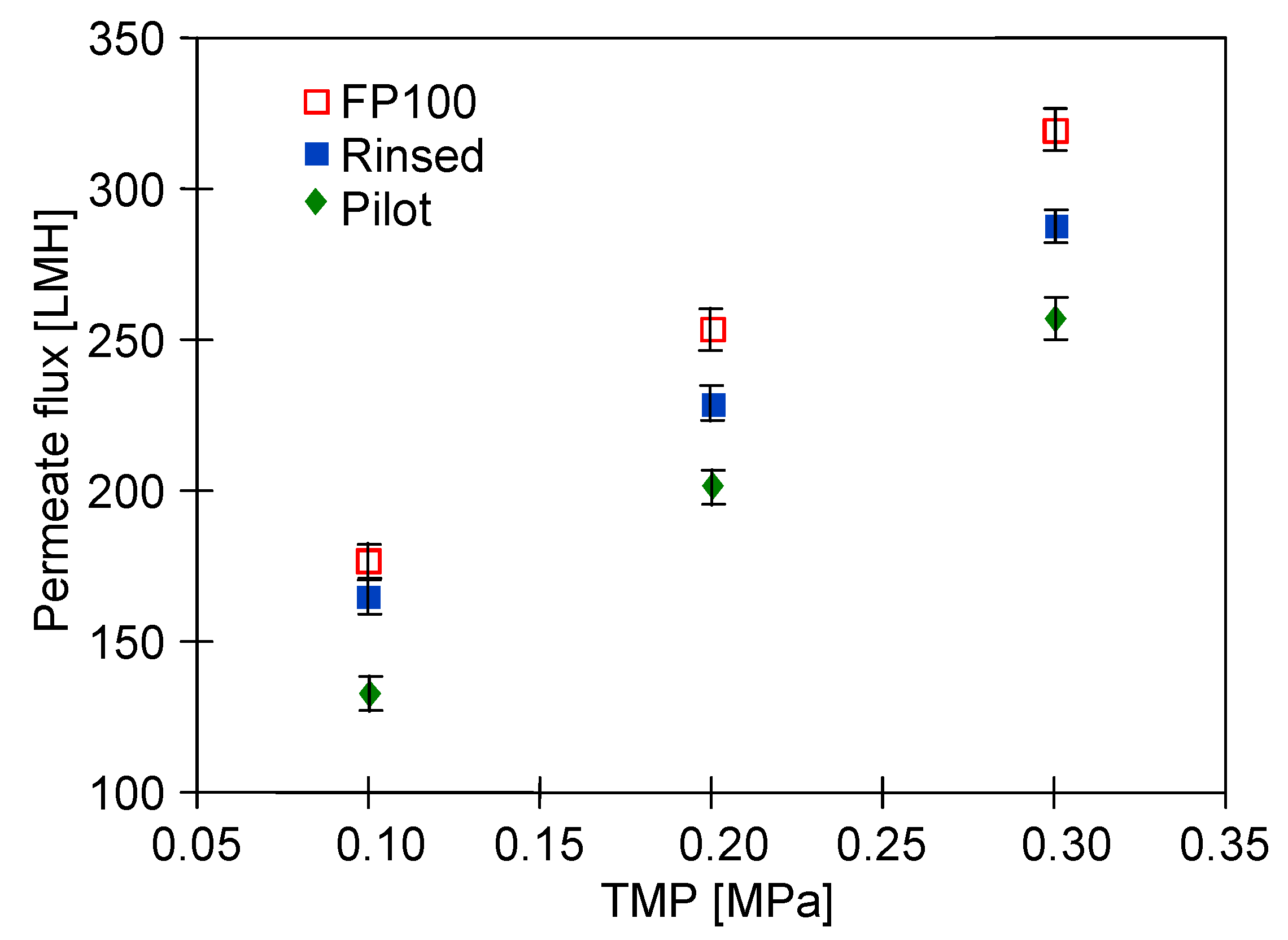
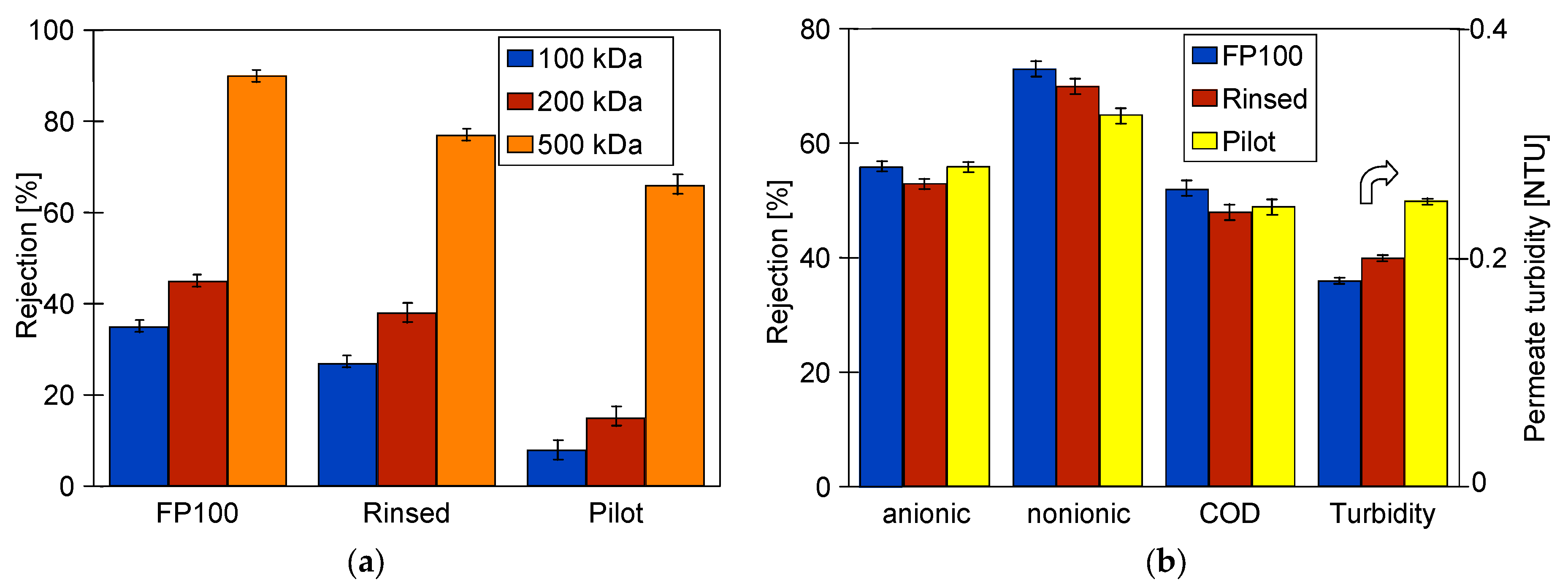
- “FP100”—the new membranes are rinsed several times with DI water (5 h),
- (ii)
- “Rinsed”—chemical cleaning changes the membrane morphology [20,58]; thus, the new membranes which are subjected to preliminary degradation under the influence of aggressive cleaning agents. For this purpose, after dextran separation, membrane “FP100” was rinsed with DI water (5 h), alkaline P3 Ultrasil 11 solution (4 × 0.5 h), 0.5% H3PO4 (0.5 h), DI water (1 h) and then conditioned for 20 h with an alkaline (pH = 11.5) cleaning agent (“Insect”) and filtered (5 h/day, TMP = 0.1 MPa). The above-mentioned procedure corresponded to the impact of cleaning agents that would occur during more than 3 months of operation of the UF installation at a car wash [20].
- (iii)
- “Pilot”—the FP100 membranes operated periodically for over 5 years in a UF pilot installation. The pilot installation was described in the previous work [33]. This installation was equipped with the PCI B1 tubular module equipped with 18 membrane tubes FP100 (total membrane area: 0.9 m2). The installation was used to filter water contaminated with machine oil (50–200 ppm), and for the last 2 months, synthetic car wash wastewater—which was a mixture of cleaning agents (active foam) and waxing used for car paint protection (“Hydrowax”)—was filtered. The composition of these cleaning agents was presented in the previously published study [21]. Due to fouling, the membranes in the pilot installation were systematically (every 5–7 days) washed using alkaline cleaning agents (P3 Ultrasil 11 or “Insect”), and during downtime, the installation was rinsed with the 0.25% sodium disulfite (Na2S2O5) solution for 30 min. As a result, the permeate flux was maintained at the level of 70–80% of the initial flux. However, as it was found, chemical washing also caused minor damage of the polyethersulfone (PES) membranes [21,58]. For this reason, the study investigated whether long-term use of FP100 membranes would deteriorate separation.
2.2. Experimental Set-Up
2.3. Feed Solutions
- (i)
- Solutions of dextran (Polfa, Łódź, Poland) with a molecular weight within the range of 100 and 500 kDa. The solution concentration was equal to 0.5 g/L.
- (ii)
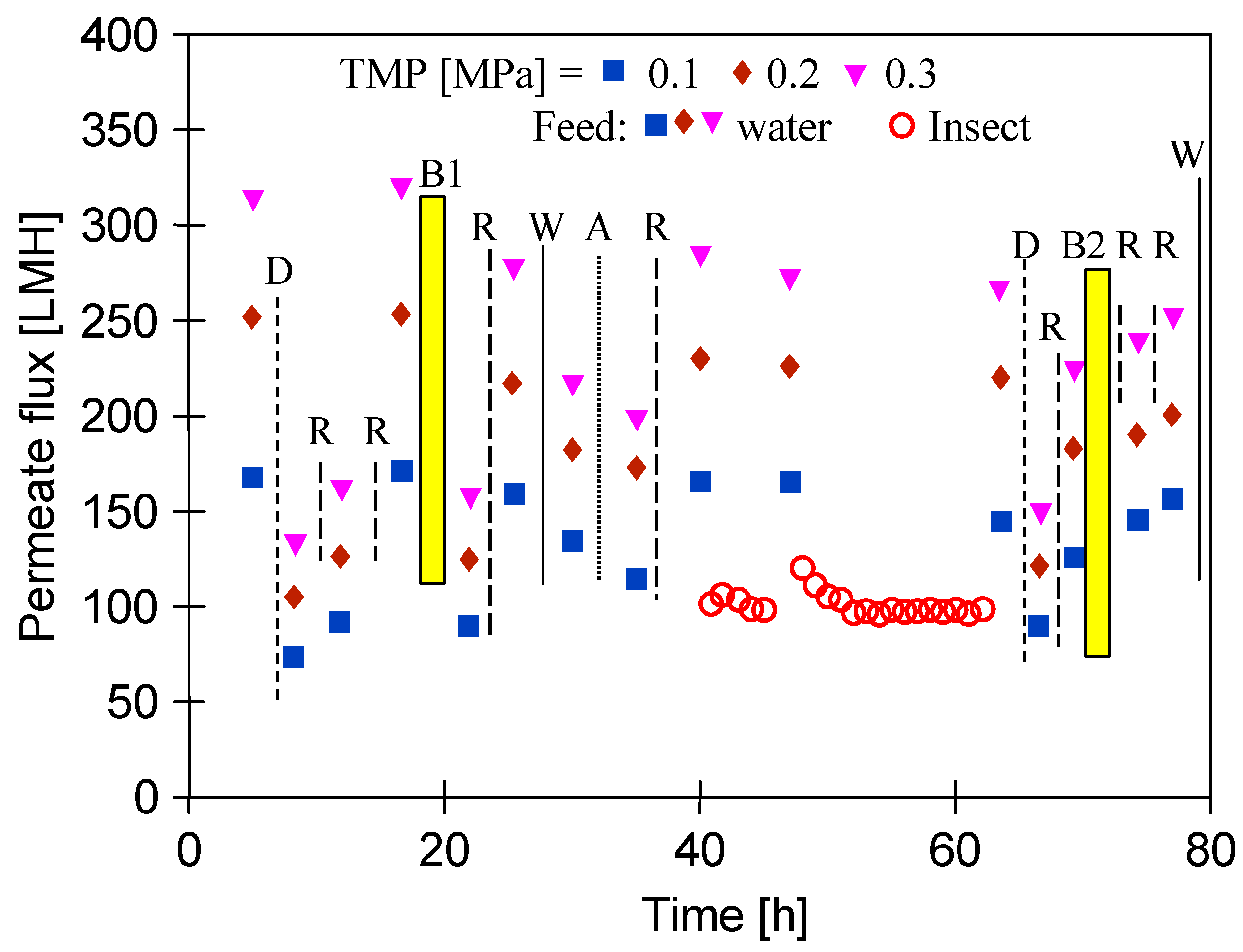
- Solutions of model organism Escherichia coli K12 (ATCC 29425). The feed volume was 2 dm3. The initial bacterial concentration in the feed was approximately 2.1–3.5·106 CFU/mL. Similar bacterial content was found in real car wash wastewater analysed in [59].
- (iii)
- Synthetic CWW consisting of a mixture of 0.5 vol.% foaming agent solution Turbo Active Foam Green (EuroEcol, Łódź, Poland) and 0.2 vol.% Hydrowax (EuroEcol, Łódź, Poland), characteristics of which were presented in a previously published work [60]. The composition of CWW was the same as the composition of effluents generated during car washing and contained surfactants (789 mg/L anionic and 33 mg/L nonionic) and polymeric waxes. It was characterized by the COD = 2680 mg/L and tortuosity equal to 23 NTU.
2.4. Membranes Cleaning
2.5. Analytical Methods
3. Results and Discussion
3.1. Membranes Performance
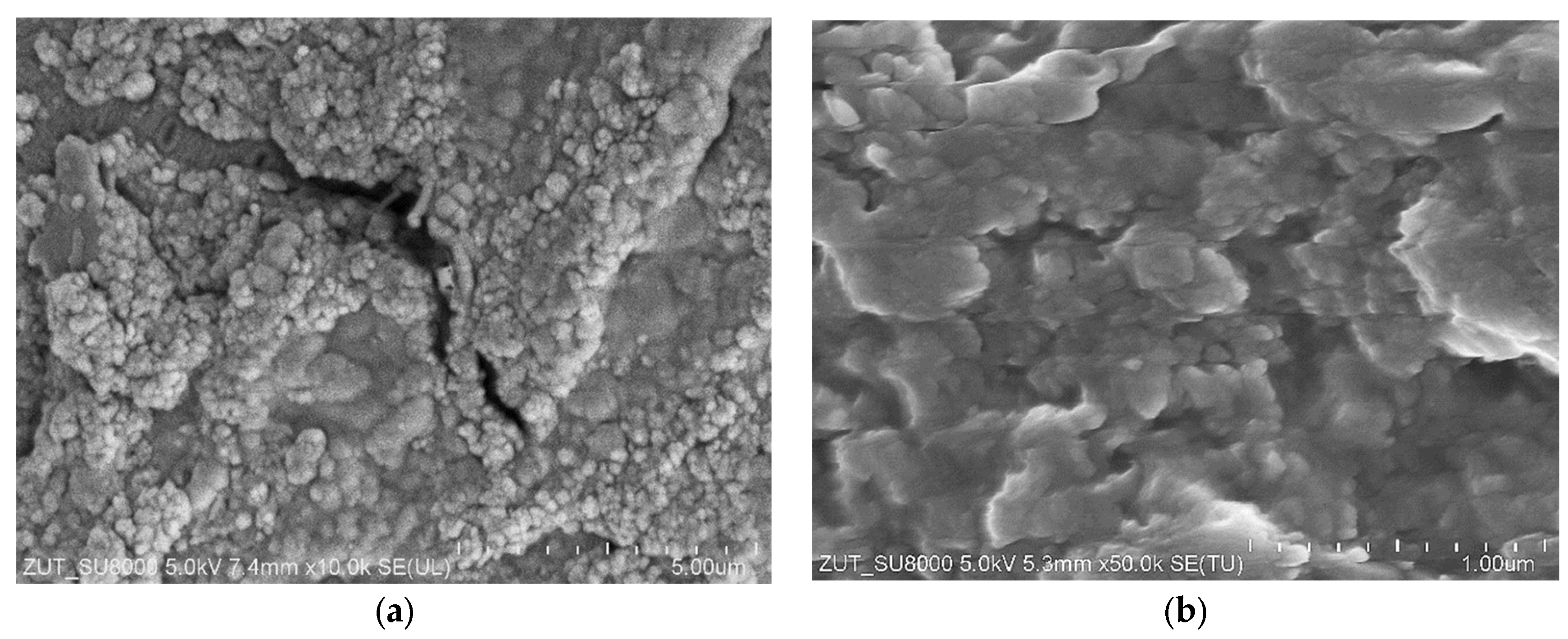
3.2. Membranes Exploitation
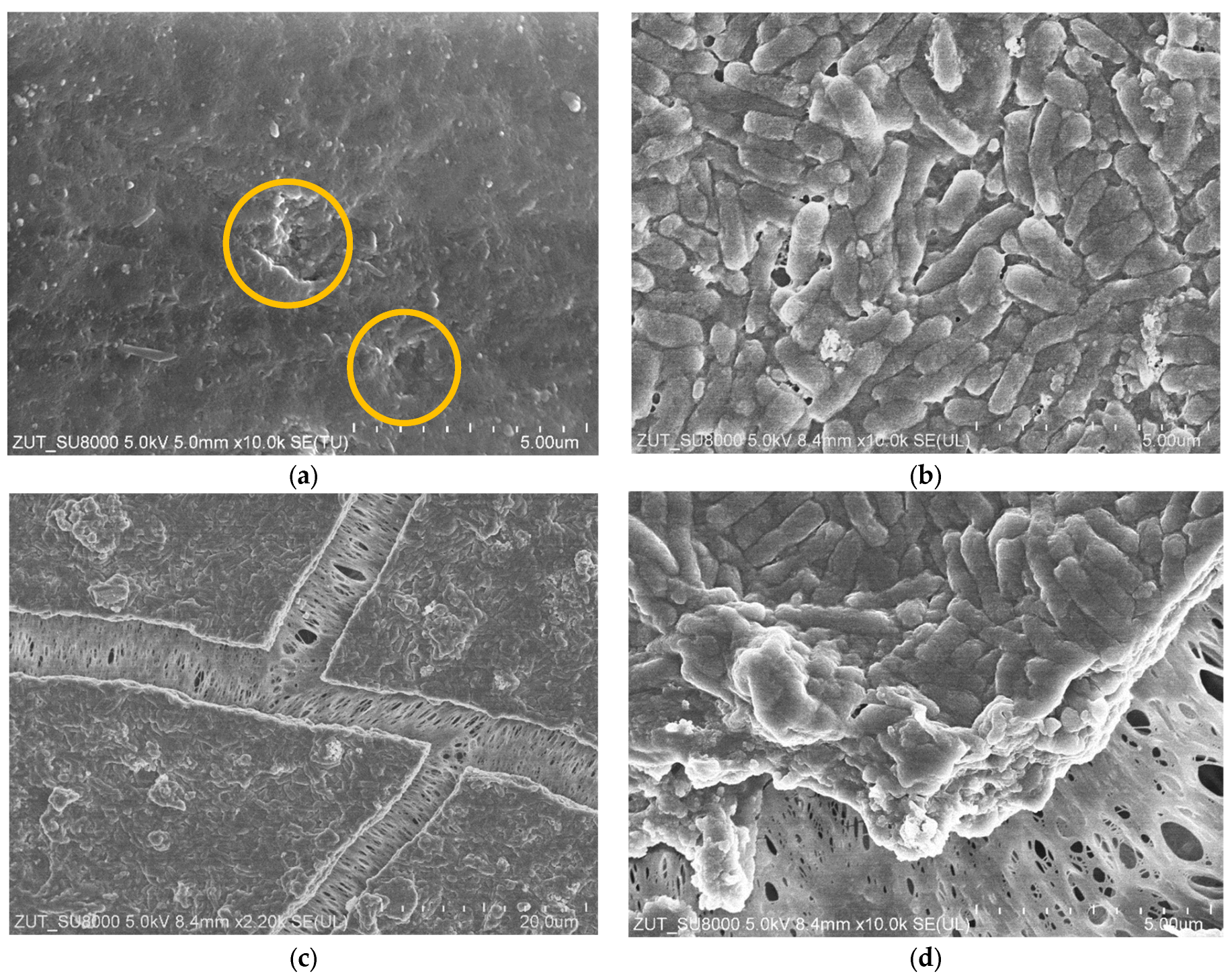
3.3. Membranes Fouling
4. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fayed, M.; Shewitah, M.A.; Dupont, R.R.; Fayed, M.; Badr, M.M. Treatability Study of Car Wash Wastewater Using Upgraded Physical Technique with Sustainable Flocculant. Sustainability 2023, 15, 8581. [Google Scholar] [CrossRef]
- Kuan, W.-H.; Hu, C.-Y.; Ke, L.-W.; Wu, J.-M. A Review of On-Site Carwash Wastewater Treatment. Sustainability 2022, 14, 5764. [Google Scholar] [CrossRef]
- Sarmadi, M.; Foroughi, M.; Najafi Saleh, H.; Sanaei, D.; Zarei, A.A.; Ghahrchi, M.; Bazrafshan, E. Efficient Technologies for Carwash Wastewater Treatment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 34823–34839. [Google Scholar] [CrossRef]
- Zaneti, R.N.; Etchepare, R.; Rubio, J. Car Wash Wastewater Treatment and Water Reuse—A Case Study. Water Sci. Technol. 2013, 67, 82–88. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Bonten, M.; Johnson, J.R.; Van Den Biggelaar, A.H.J.; Georgalis, L.; Geurtsen, J.; De Palacios, P.I.; Gravenstein, S.; Verstraeten, T.; Hermans, P.; Poolman, J.T. Epidemiology of Escherichia coli Bacteremia: A Systematic Literature Review. Clin. Infect. Dis. 2021, 72, 1211–1219. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Kuang, W.; Huang, Q.; Xiao, G. Modeling Escherichia coli Concentrations for Health Risk Analysis in the Three Gorges Reservoir Region, Chongqing, China. J. Hydrol. 2024, 639, 131511. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and Public Health Implications—A Review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Van Elsas, J.D.; Semenov, A.V.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the Environment: Fundamental and Public Health Aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef]
- Vila, J.; Sáez-López, E.; Johnson, J.R.; Römling, U.; Dobrindt, U.; Cantón, R.; Giske, C.G.; Naas, T.; Carattoli, A.; Martínez-Medina, M.; et al. Escherichia coli: An Old Friend with New Tidings. FEMS Microbiol. Rev. 2016, 40, 437–463. [Google Scholar] [CrossRef] [PubMed]
- Ruslan; Wulandari, E.; Sagita, F.; Hung, W.-S.; Kadja, G.T.M. Recent Advances in MXenes and Their Functionalization for Highly Effective Ultrafiltration, Nanofiltration, and Reverse Osmosis. Inorg. Chem. Commun. 2024, 170, 113152. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, Z.; Li, L.; Xiao, P.; Ma, Y.; Liu, F.; Li, J. PVDF/MOFs Mixed Matrix Ultrafiltration Membrane for Efficient Water Treatment. Front. Chem. 2022, 10, 985750. [Google Scholar] [CrossRef]
- Baig, N.; Salhi, B.; Sajid, M.; Aljundi, I.H. Recent Progress in Microfiltration/Ultrafiltration Membranes for Separation of Oil and Water Emulsions. Chem. Rec. 2022, 22, e202100320. [Google Scholar] [CrossRef]
- Ding, J.; Wang, J.; Luo, X.; Xu, D.; Liu, Y.; Li, P.; Li, S.; Wu, R.; Gao, X.; Liang, H. A Passive-Active Combined Strategy for Ultrafiltration Membrane Fouling Control in Continuous Oily Wastewater Purification. Water Res. 2022, 226, 119219. [Google Scholar] [CrossRef]
- Awad, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering 2021, 5, 34. [Google Scholar] [CrossRef]
- Kammakakam, I.; Lai, Z. Next-Generation Ultrafiltration Membranes: A Review of Material Design, Properties, Recent Progress, and Challenges. Chemosphere 2023, 316, 137669. [Google Scholar] [CrossRef]
- Moazzem, S.; Wills, J.; Fan, L.; Roddick, F.; Jegatheesan, V. Performance of Ceramic Ultrafiltration and Reverse Osmosis Membranes in Treating Car Wash Wastewater for Reuse. Environ. Sci. Pollut. Res. 2018, 25, 8654–8668. [Google Scholar] [CrossRef]
- Agostini, S.; Moriconi, F.; Zampirolli, M.; Padoan, D.; Treu, L.; Campanaro, S.; Favaro, L. Monitoring the Microbiomes of Agricultural and Food Waste Treating Biogas Plants over a One-Year Period. Appl. Sci. 2023, 13, 9959. [Google Scholar] [CrossRef]
- Woźniak, P.; Gryta, M. Application of Polymeric Tubular Ultrafiltration Membranes for Separation of Car Wash Wastewater. Membranes 2024, 14, 210. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P. Polyethersulfone Membrane Fouling Mitigation during Ultrafiltration of Wastewaters from Car Washes. Desalination 2024, 574, 117254. [Google Scholar] [CrossRef]
- Wills, J.; Moazzem, S.; Jegatheesan, V. Treating Car Wash Wastewater by Ceramic Ultrafiltration Membranes for Reuse Purposes. In Water Scarcity and Ways to Reduce the Impact; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 63–73. ISBN 978-3-319-75198-6. [Google Scholar]
- Uçar, D. Membrane Processes for the Reuse of Car Washing Wastewater. J. Water Reuse Desalination 2018, 8, 169–175. [Google Scholar] [CrossRef]
- Li, K.; Xu, W.; Wen, G.; Zhou, Z.; Han, M.; Zhang, S.; Huang, T. Aging of Polyvinylidene Fluoride (PVDF) Ultrafiltration Membrane Due to Ozone Exposure in Water Treatment: Evolution of Membrane Properties and Performance. Chemosphere 2022, 308, 136520. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the Production and Modification of PVDF Membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Concha, V.O.C.; Timóteo, L.; Duarte, L.A.N.; Bahú, J.O.; Munoz, F.L.; Silva, A.P.; Lodi, L.; Severino, P.; León-Pulido, J.; Souto, E.B. Properties, Characterization and Biomedical Applications of Polyvinylidene Fluoride (PVDF): A Review. J. Mater. Sci. 2024, 59, 14185–14204. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Sobola, D.; Orudzhev, F.; Ramazanov, S.; Trčka, T. Brief Review of PVDF Properties and Applications Potential. Polymers 2022, 14, 4793. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A Comprehensive Review on Fundamental Properties and Applications of Poly(Vinylidene Fluoride) (PVDF). Adv. Compos. Hybrid Mater. 2021, 4, 8–26. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P. A Comparative Analysis of the Basic Properties and Applications of Poly (Vinylidene Fluoride) (PVDF) and Poly (Methyl Methacrylate) (PMMA). Polym. Bull. 2022, 79, 5635–5665. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and Modification of Poly(Vinylidene Fluoride) (PVDF) Membranes—A Review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Veved, A.; Ejuh, G.W.; Djongyang, N. Review of Emerging Materials for PVDF-Based Energy Harvesting. Energy Rep. 2022, 8, 12853–12870. [Google Scholar] [CrossRef]
- Nivedhitha, D.M.; Jeyanthi, S. Polyvinylidene Fluoride, an Advanced Futuristic Smart Polymer Material: A Comprehensive Review. Polym. Adv. Technol. 2023, 34, 474–505. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Long-Term Performance of Ultrafiltration Membranes: Corrosion Fouling Aspect. Materials 2023, 16, 1673. [Google Scholar] [CrossRef] [PubMed]
- Nyström, M. Ultrafiltration of O/W Emulsions Stabilized by Limiting Amounts of Tall Oil. Colloids Surf. 1991, 57, 99–114. [Google Scholar] [CrossRef]
- Hilal, N.; Busca, G.; Hankins, N.; Mohammad, A.W. The Use of Ultrafiltration and Nanofiltration Membranes in the Treatment of Metal-Working Fluids. Desalination 2004, 167, 227–238. [Google Scholar] [CrossRef]
- Hilal, N.; Busca, G.; Waller, M.D. Treatment of Metalworking Fluids: Development of a Bioconsortium for the Treatment of Nanofiltration Permeate. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 641–648. [Google Scholar] [CrossRef]
- Maddah, H.A.; Alzhrani, A.S.; Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.; Almalki, A.M. Evaluation of Various Membrane Filtration Modules for the Treatment of Seawater. Appl. Water Sci. 2018, 8, 150. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Orecki, A.; Karakulski, K. Treatment of Bilge Water Using a Combination of Ultrafiltration and Reverse Osmosis. Desalination 2005, 185, 203–212. [Google Scholar] [CrossRef]
- Karakulski, K.; Morawski, W.A.; Grzechulska, J. Purification of Bilge Water by Hybrid Ultrafiltration and Photocatalytic Processes. Sep. Purif. Technol. 1998, 14, 163–173. [Google Scholar] [CrossRef]
- Niu, C.; Li, X.; Dai, R.; Wang, Z. Artificial Intelligence-Incorporated Membrane Fouling Prediction for Membrane-Based Processes in the Past 20 Years: A Critical Review. Water Res. 2022, 216, 118299. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane Fouling Control in Ultrafiltration Technology for Drinking Water Production: A Review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Ullah, A.; Tanudjaja, H.J.; Ouda, M.; Hasan, S.W.; Chew, J.W. Membrane Fouling Mitigation Techniques for Oily Wastewater: A Short Review. J. Water Process Eng. 2021, 43, 102293. [Google Scholar] [CrossRef]
- Rajendran, D.S.; Devi, E.G.; Subikshaa, V.S.; Sethi, P.; Patil, A.; Chakraborty, A.; Venkataraman, S.; Kumar, V.V. Recent Advances in Various Cleaning Strategies to Control Membrane Fouling: A Comprehensive Review. Clean Technol. Environ. Policy 2024, 1–16. [Google Scholar] [CrossRef]
- Regula, C.; Carretier, E.; Wyart, Y.; Gésan-Guiziou, G.; Vincent, A.; Boudot, D.; Moulin, P. Chemical Cleaning/Disinfection and Ageing of Organic UF Membranes: A Review. Water Res. 2014, 56, 325–365. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Zhang, H.; Jia, H.; Cui, Z.; Yang, G. Aged PVDF and PSF Ultrafiltration Membranes Restored by Functional Polydopamine for Adjustable Pore Sizes and Fouling Control. J. Membr. Sci. 2019, 570, 156–167. [Google Scholar] [CrossRef]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, Full-Scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A Review on Inorganic Membranes for Desalination and Wastewater Treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-Based Membranes for Water and Wastewater Treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Ranil Wickramasinghe, S.; Banat, F. Recent Developments in Porous Ceramic Membranes for Wastewater Treatment and Desalination: A Review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Karami, P.; Khorshidi, B.; McGregor, M.; Peichel, J.T.; Soares, J.B.P.; Sadrzadeh, M. Thermally Stable Thin Film Composite Polymeric Membranes for Water Treatment: A Review. J. Clean. Prod. 2020, 250, 119447. [Google Scholar] [CrossRef]
- Romero, A.S.; Innocentini, M.D.M.; Vladimir Oliveira, J.; Lider, A.; Fey, T.; Travitzky, N.; Hotza, D. Unveiling the Potential of Silicon Carbide as a Support Material and Membranes for Oily Wastewater Remediation. Sep. Purif. Technol. 2025, 354, 129044. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Gao, F.; Chen, Y.; Zhang, H. A Comparison Study: The Different Impacts of Sodium Hypochlorite on PVDF and PSF Ultrafiltration (UF) Membranes. Water Res. 2017, 109, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, V.; Granville, A.; Le-Clech, P.; Chen, V. Cleaning and Ageing Effect of Sodium Hypochlorite on Polyvinylidene Fluoride (PVDF) Membrane. Sep. Purif. Technol. 2010, 72, 301–308. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, H.; Wu, Z.; Cao, J. Impact of Sodium Hypochlorite Cleaning on the Surface Properties and Performance of PVDF Membranes. Appl. Surf. Sci. 2018, 428, 289–295. [Google Scholar] [CrossRef]
- Abdullah, S.Z.; Bérubé, P.R. Assessing the Effects of Sodium Hypochlorite Exposure on the Characteristics of PVDF Based Membranes. Water Res. 2013, 47, 5392–5399. [Google Scholar] [CrossRef]
- Lye, C.J.M.; Xu, H.; Wang, R. Impact of NaOCl Ageing on Reinforced PVDF Hollow Fiber Membranes Used in Membrane Bioreactor. J. Water Process Eng. 2021, 44, 102408. [Google Scholar] [CrossRef]
- Oliveira Filho, M.; Mailler, R.; Rocher, V.; Fayolle, Y.; Causserand, C. Comprehensive Study of Supported PVDF Membrane Ageing in MBR: A Direct Comparison between Changes at Bench Scale and Full Scale. Sep. Purif. Technol. 2021, 279, 119695. [Google Scholar] [CrossRef]
- Gryta, M.; Woźniak, P.; Mozia, S. Effects of Alkaline Cleaning Agents on the Long-Term Performance and Aging of Polyethersulfone Ultrafiltration Membranes Applied for Treatment of Car Wash Wastewater. Membranes 2024, 14, 122. [Google Scholar] [CrossRef]
- Woźniak, P.; Dubicki, M.; Gryta, M. Microbiological Hazard Analysis of Car WashWastewater. Pol. J. Environ. Stud. 2023, 32, 3871–3882. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef]
- Ji, M.; Luo, J.; Wei, J.; Woodley, J.; Daugaard, A.E.; Pinelo, M. Commercial Polysulfone Membranes Pretreated with Ethanol and NaOH: Effects on Permeability, Selectivity and Antifouling Properties. Sep. Purif. Technol. 2019, 219, 82–89. [Google Scholar] [CrossRef]
- Tomczak, W.; Gryta, M. Clarification of 1,3-Propanediol Fermentation Broths by Using a Ceramic Fine UF Membrane. Membranes 2020, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, C. Study of Critical Flux in Ultrafiltration of Seawater: New Measurement and Sub- and Super-Critical Flux Operations. Chem. Eng. J. 2010, 165, 102–110. [Google Scholar] [CrossRef]
- Crowley, S.V.; O’Callaghan, T.F.; Kelly, A.L.; Fenelon, M.A.; O’Mahony, J.A. Use of Ultrafiltration to Prepare a Novel Permeate for Application in the Functionality Testing of Infant Formula Ingredients. Sep. Purif. Technol. 2015, 141, 294–300. [Google Scholar] [CrossRef]
- Le, T.T.; Vu, L.T.; Le, N.L. Effects of Membrane Pore Size and Transmembrane Pressure on Ultrafiltration of Red-fleshed Dragon Fruit (Hylocereus polyrhizus) Juice. J. Chem. Technol. Biotechnol. 2021, 96, 1561–1572. [Google Scholar] [CrossRef]
- Basavaraju, M.; Gunashree, B.S. Escherichia coli: An Overview of Main Characteristics. In Escherichia Coli—Old and New Insights; Starčič Erjavec, M., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-83969-869-9. [Google Scholar]
- Blount, Z.D. The Unexhausted Potential of E. Coli. eLife 2015, 4, e05826. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, W.; Gryta, M.; Woźniak, P.; Daniluk, M. Changes in the Separation Properties of Aged PVDF Ultrafiltration Membranes During Long-Term Treatment of Car Wash Wastewater. Membranes 2025, 15, 66. https://doi.org/10.3390/membranes15030066
Tomczak W, Gryta M, Woźniak P, Daniluk M. Changes in the Separation Properties of Aged PVDF Ultrafiltration Membranes During Long-Term Treatment of Car Wash Wastewater. Membranes. 2025; 15(3):66. https://doi.org/10.3390/membranes15030066
Chicago/Turabian StyleTomczak, Wirginia, Marek Gryta, Piotr Woźniak, and Monika Daniluk. 2025. "Changes in the Separation Properties of Aged PVDF Ultrafiltration Membranes During Long-Term Treatment of Car Wash Wastewater" Membranes 15, no. 3: 66. https://doi.org/10.3390/membranes15030066
APA StyleTomczak, W., Gryta, M., Woźniak, P., & Daniluk, M. (2025). Changes in the Separation Properties of Aged PVDF Ultrafiltration Membranes During Long-Term Treatment of Car Wash Wastewater. Membranes, 15(3), 66. https://doi.org/10.3390/membranes15030066





