Polyphenols from Inula oculus-christi L.-Induced Cell-Specific Membrane and Cytoskeleton Reorganization
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Plant Extracts
2.2. Cell Cultures
2.3. Evaluation of the Cytotoxicity of FFGs and FCQAs
2.4. Di-4-ANEPPDHQ Microscopy Measurements
2.5. Fluorescent Staining of F-Actin
2.6. Statistical Analysis
3. Results
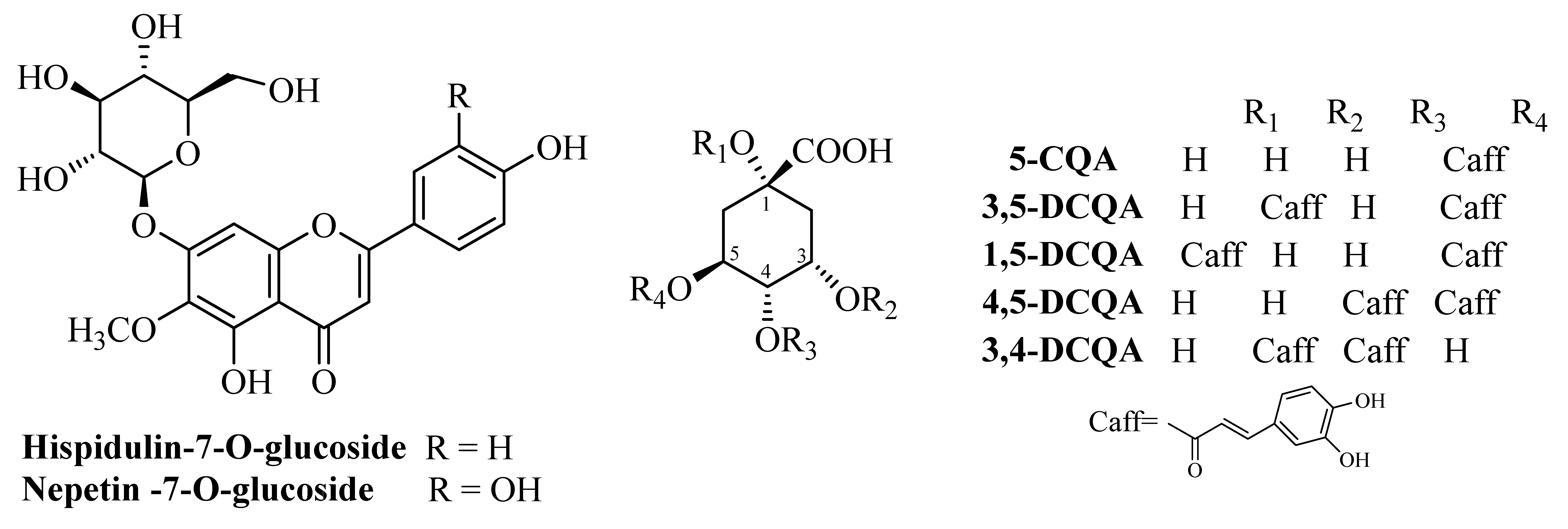
3.1. Dominant Compounds in the Fractions Enriched in Phenolic Acids (FCQAs) and Flavonoid Glycosydes (FFGs) from Inula oculus-christi L.
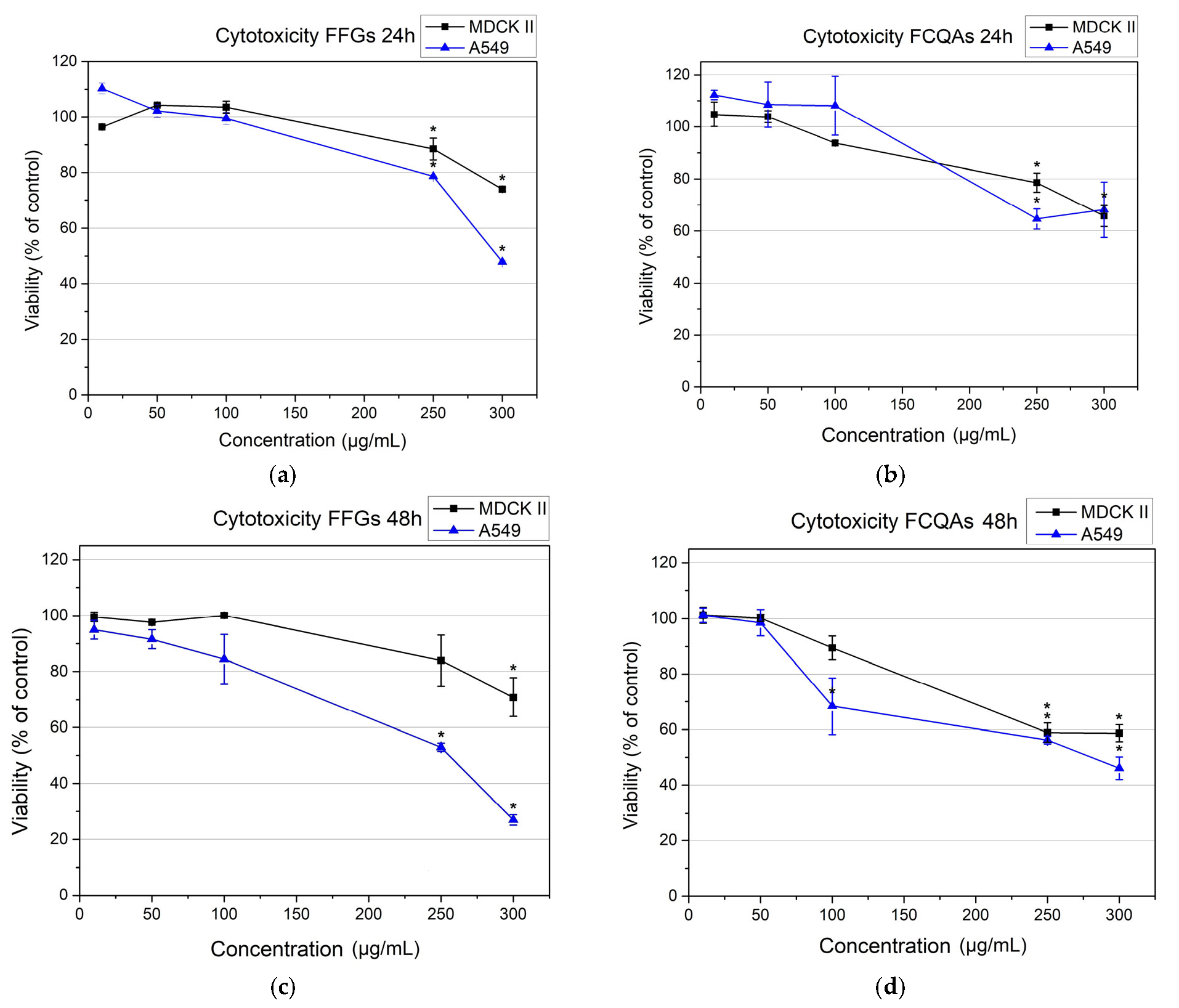
3.2. Cytotoxicity of the Fractions Enriched in Caffeoylquinic Acids (FCQAs) and Flavonoid Glycosydes (FFGs) from Inula oculus-christi L.
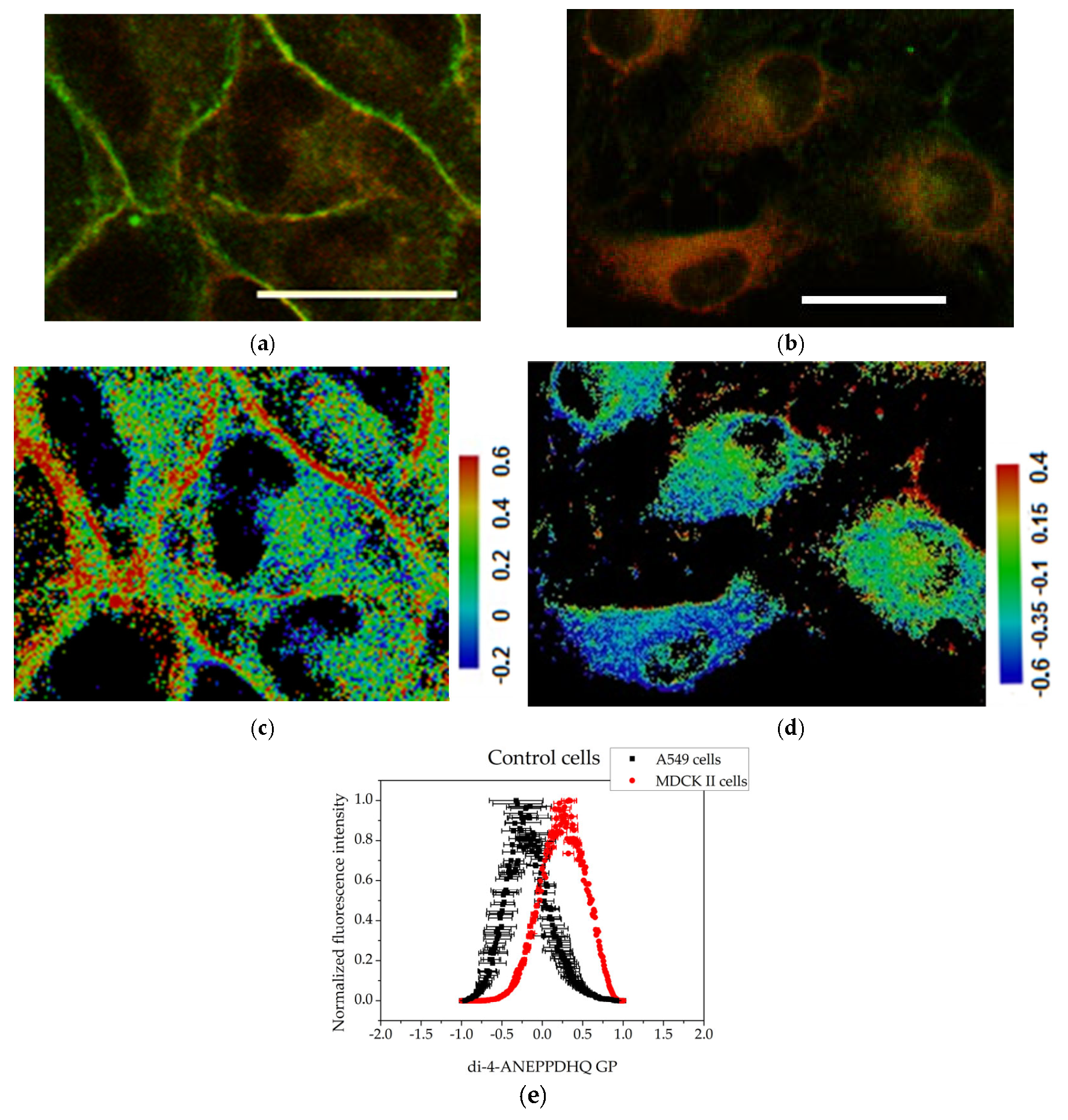
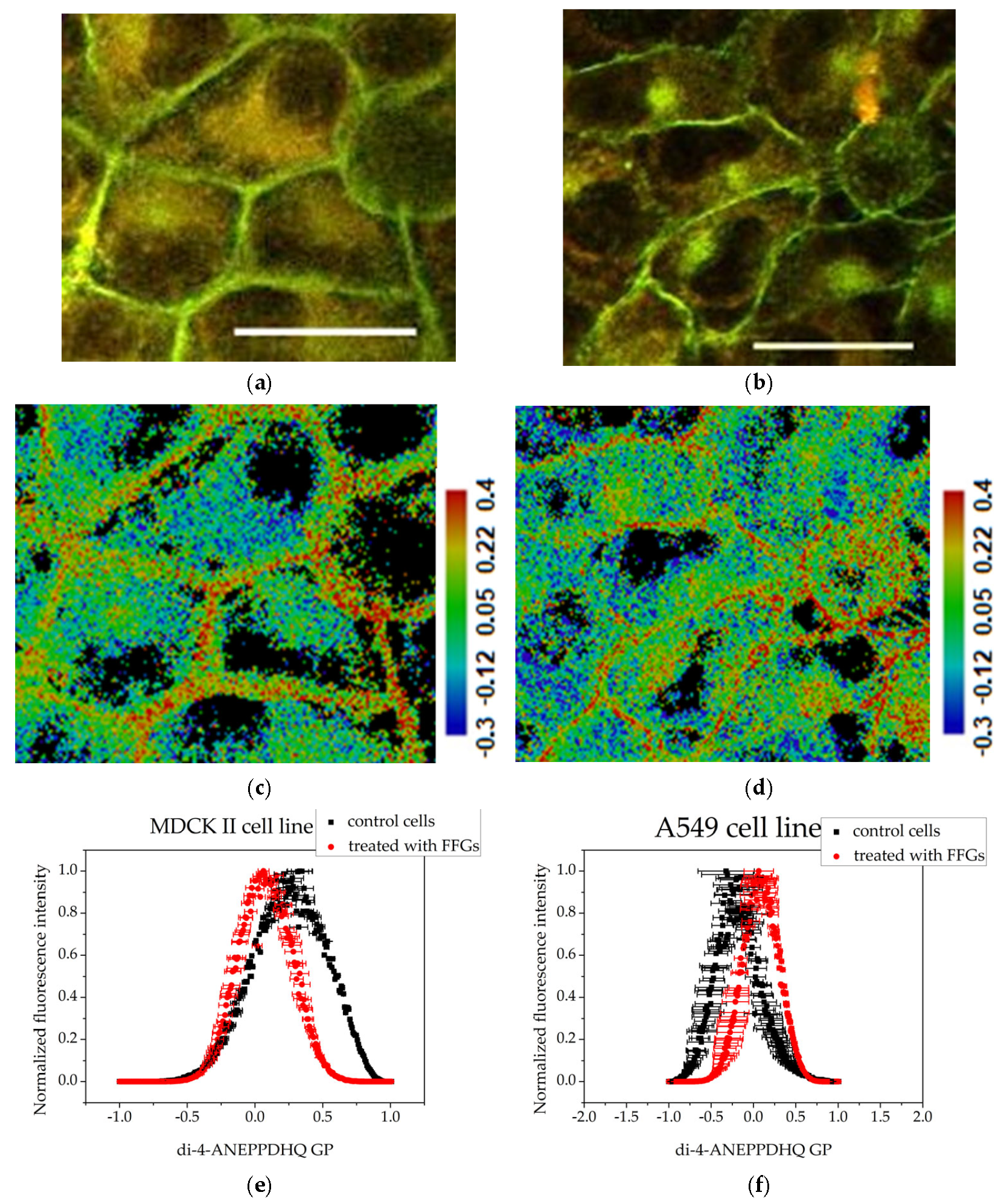
3.3. Membrane Order Imaging of Cells with the Fluorescent Probe Di-4-ANEPPDHQ Through Confocal Microscopy
3.4. GP Image Acquisition
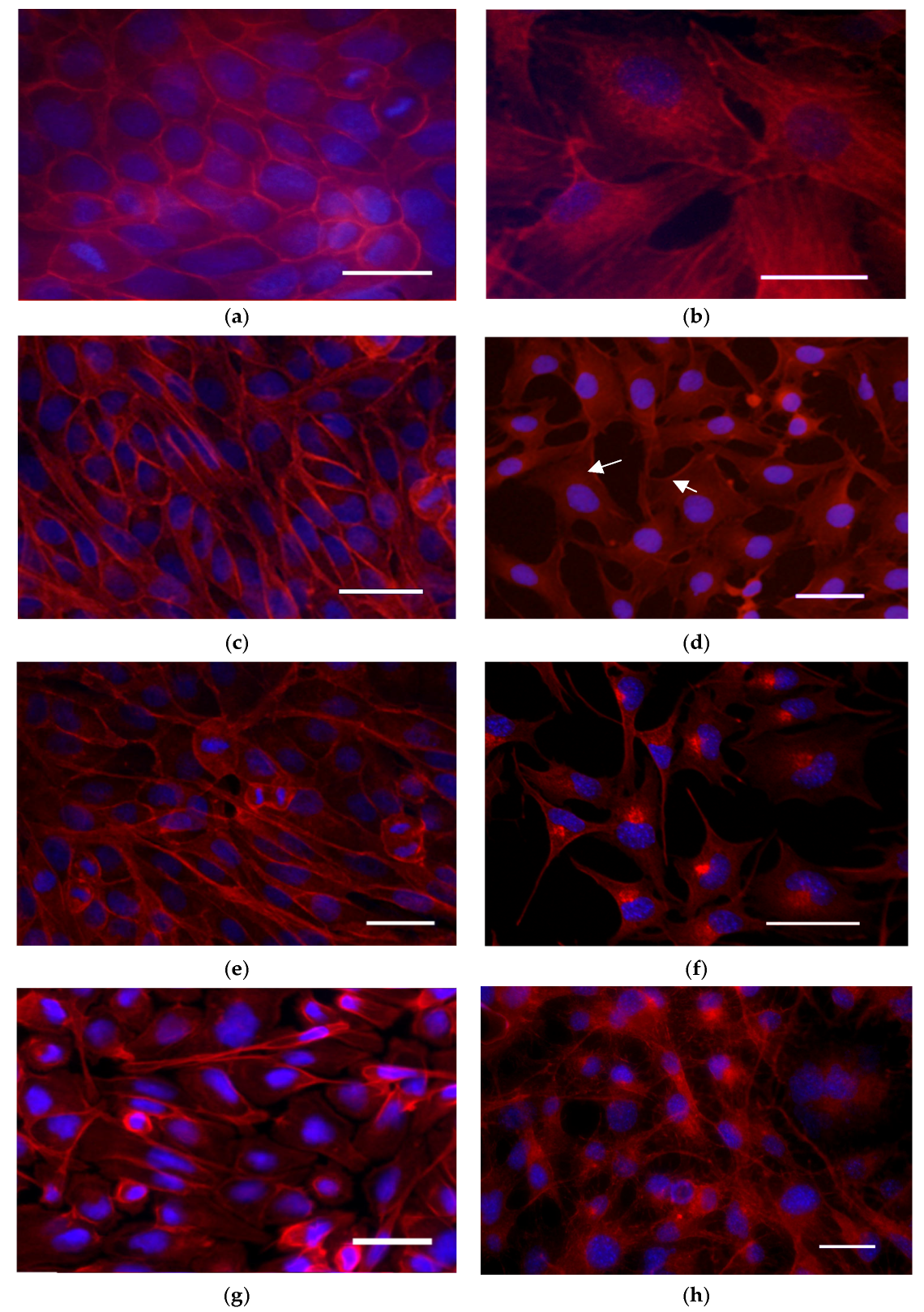
3.5. Actin Cytoskeleton Reorganization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| diCQA | Dicaffeoylquinic acid isomers |
| PA | Phenolic acids |
| FCQAs | Fraction of caffeoylquinic acids |
| CGAs | Chlorogenic acids |
| FFGs | Fraction enriched in flavonoid glycosides |
| GP | Generalized polarization |
References
- Seca, A.M.; Grigore, A.; Pinto, D.C.; Silva, A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Yu, H.; Zheng, S.; Wang, Q.; Jiang, H.; et al. A review of the botany, traditional uses, phytochemistry, and pharmacology of the Flos Inulae. J. Ethnopharmacol. 2021, 276, 114125. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids—Ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Rogozinska, M.; Lisiecki, K.; Czarnocki, Z.; Biesaga, M. Antioxidant activity of sulfate metabolites of chlorogenic acid. Appl. Sci. 2023, 13, 2192. [Google Scholar] [CrossRef]
- Belew, A.A.; Hanan, G.G.M.W.; Meshesha, D.S.; Akele, M.L. Evaluation of total phenolic, flavonoid contents, antioxidant and antibacterial activity of leaf extracts from Rhus vulgaris. Discov. Plants 2025, 2, 141. [Google Scholar] [CrossRef]
- Xie, L.; Deng, Z.; Zhang, J.; Dong, H.; Wang, W.; Xing, B.; Liu, X. Comparison of flavonoid O-glycoside, C-glycoside and their aglycones on antioxidant capacity and metabolism during in vitro digestion and in vivo. Foods 2022, 11, 882. [Google Scholar] [CrossRef]
- Gumisiriza, H.; Olet, E.A.; Mwikali, L.; Akatuhebwa, R.; Omara, T.; Lejju, J.B.; Sesaazi, D.C. Antibacterial and antioxidant activities of flavonoids, phenolic and flavonoid glycosides from Gouania longispicata leaves. Microbiol. Res. 2024, 15, 2085–2101. [Google Scholar] [CrossRef]
- Ghozzi, I.; Fontaine, J.-X.; Molinié, R.; Elboutachfaiti, R.; Akkouche, L.; Sebei, K.; Mathiron, D.; Hano, C.; Garros, L.; Choque, E.; et al. Relationship between the structure of the flavone C-glycosides of linseed (Linum usitatissimum L.) and their antioxidant activity. Molecules 2024, 29, 5829. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, S.; Li, Y.; Xia, B.; Li, M.; Zhang, Z.; Shi, Z.; Peng, Q.; Li, C.; Lin, L.; et al. Phenolic acids from medicinal and edible homologous plants: A potential anti-inflammatory agent for inflammatory diseases. Front. Immunol. 2024, 15, 1345002. [Google Scholar] [CrossRef]
- Ekowati, J.; Tejo, B.A.; Maulana, S.; Kusuma, W.A.; Fatriani, R.; Ramadhanti, N.S.; Norhayati, N.; Nofianti, K.A.; Sulistyowaty, M.I.; Zubair, M.S.; et al. Potential utilization of phenolic acid compounds as anti-inflammatory agents through TNF-α convertase inhibition mechanisms: A network pharmacology, docking, and molecular dynamics approach. ACS Omega 2023, 8, 43243–43254. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Wu, J.; Huang, H.; Gong, L.; Tian, X.; Peng, Z.; Zhu, Y.; Wang, W. A flavonoid glycoside compound from Siraitia grosvenorii with anti-inflammatory and hepatoprotective effects in vitro. Biomolecules 2024, 14, 450. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Zhukov, A.; Vereshchagin, M. Polar glycerolipids and membrane lipid rafts. Int. J. Mol. Sci. 2024, 25, 8325. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jin, J.; Li, S. Role of polyunsaturated phospholipids in liquid-ordered and liquid-disordered phases. RSC Adv. 2021, 11, 27115–27120. [Google Scholar] [CrossRef] [PubMed]
- Pike, L.J. Rafts defined: A report on the Keystone Symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Tsunoyama, T.A.; Tang, B.; Hirosawa, K.M.; Morone, N.; Fujiwara, T.K.; Suzuki, K.G.N. Cholesterol- and actin-centered view of the plasma membrane: Updating the Singer–Nicolson fluid mosaic model to commemorate its 50th anniversary. Mol. Biol. Cell 2023, 34, pl1. [Google Scholar] [CrossRef]
- Brown, D.A.; London, E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 2000, 275, 17221–17224. [Google Scholar] [CrossRef]
- Sharma, P.; Varma, R.; Sarasij, R.C.; Ira; Gousset, K.; Krishnamoorthy, G.; Rao, M.; Mayor, S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 2004, 116, 577–589. [Google Scholar] [CrossRef]
- Trybus, M.; Hryniewicz-Jankowska, A.; Wójtowicz, K.; Trombik, T.; Czogalla, A.; Sikorski, A.F. EFR3A: A new raft domain organizing protein? Cell. Mol. Biol. Lett. 2023, 28, 86. [Google Scholar] [CrossRef]
- Castello-Serrano, I.; Heberle, F.A.; Diaz-Rohrer, B.; Ippolito, R.; Shurer, C.R.; Lujan, P.; Campelo, F.; Levental, K.R.; Levental, I. Partitioning to ordered membrane domains regulates the kinetics of secretory traffic. eLife 2024, 12, RP89306. [Google Scholar] [CrossRef] [PubMed]
- Lietha, D.; Izard, T. Roles of membrane domains in integrin-mediated cell adhesion. Int. J. Mol. Sci. 2020, 21, 5531. [Google Scholar] [CrossRef] [PubMed]
- Daumann, I.M.; Hiesinger, P.R. Lipid rafts, Rab GTPases, and a late endosomal checkpoint for plasma membrane recycling. Proc. Natl. Acad. Sci. USA 2023, 120, e2302320120. [Google Scholar] [CrossRef]
- Chichili, G.R.; Rodgers, W. Cytoskeleton-membrane interactions in membrane raft structure. Cell. Mol. Life Sci. 2009, 66, 2319–2328. [Google Scholar] [CrossRef]
- Liu, A.P.; Fletcher, D.A. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys. J. 2006, 91, 4064–4070. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of membrane/lipid rafts with the cytoskeleton: Impact on signaling and function: Membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim. Biophys. Acta 2014, 1838, 532–545. [Google Scholar] [CrossRef]
- Warda, M.; Tekin, S.; Gamal, M.; Khafaga, N.; Çelebi, F.; Tarantino, G. Lipid rafts: Novel therapeutic targets for metabolic, neurodegenerative, oncological, and cardiovascular diseases. Lipids Health Dis. 2025, 24, 147. [Google Scholar] [CrossRef]
- Kalappurakkal, J.M.; Anilkumar, A.A.; Patra, C.; van Zanten, T.S.; Sheetz, M.P.; Mayor, S. Integrin mechano-chemical signaling generates plasma membrane nanodomains that promote cell spreading. Cell 2019, 177, 1738–1756.e23. [Google Scholar] [CrossRef]
- Veiko, A.G.; Sekowski, S.; Lapshina, E.A.; Wilczewska, A.Z.; Markiewicz, K.H.; Zamaraeva, M.; Zhao, H.; Zavodnik, I.B. Flavonoids modulate liposomal membrane structure, regulate mitochondrial membrane permeability and prevent erythrocyte oxidative damage. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183442. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid–Membrane Interactions: Involvement of Flavonoid–Metal Complexes in Raft Signaling. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1235–1246. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, W.; Dang, M.; Li, X.; Zhang, Y.; Chen, J. Targeting lipid rafts as a rapid screening strategy for potential antiadipogenic polyphenols along with the structure-activity relationship and mechanism elucidation. J. Agric. Food Chem. 2022, 70, 3872–3885. [Google Scholar] [CrossRef] [PubMed]
- Kanga, Y.; Kimb, B.; Kima, S.; Leec, Y.; Yoona, Y. Inhibitory potential of flavonoids on PtdIns(3,4,5)P3 binding with the phosphoinositide-dependent kinase 1 pleckstrin homology domain. Bioorganic Med. Chem. Lett. 2017, 27, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, X.; Li, C. Insights into the surface binding and structural interference of polyphenols with the membrane raft domains in relation to their distinctive ability to inhibit preadipocyte differentiation in 3T3-L1 cells. J. Agric. Food Chem. 2023, 71, 19845–19855. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zeng, F.; Wang, M.; Guo, S.; Tang, Z.; Itagaki, K.; Lin, Y.; Shen, X.; Cao, Y.; Duan, J.; et al. Antimicrobial activities of lavandulylated flavonoids in Sophora flavences against methicillin-resistant Staphylococcus aureus via membrane disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef]
- Veleva, R.; Topouzova-Hristova, T.; Kostadinova, A.; Benkova, D.; Trendafilova, A.; Ivanova, V.; Moskova-Doumanova, V.; Mladenova, K.; Doumanov, J.; Yordanova, V.; et al. Flavonoid Glycosides and Phenolic Acids from Inula oculus-christi Modulate Membrane Organization and Provide Antioxidant Protection. Molecules 2025, 30, 2740. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Yur, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase and tyrosinase enzyme inhibitory activities of six Inula species from Bulgaria. Chem Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Aneva, I. Phenolic constituents and antioxidant capacity of Inula oculus-christi from Bulgaria. Bulg. Chem. Commun. 2017, 49, 176–180. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_49_Special_D_2017/BCC2017-49-SE-D-176-180.pdf (accessed on 23 November 2025).
- Kostadinova, A.; Staneva, G.; Topouzova-Hristova, T.; Moyankova, D.; Yordanova, V.; Veleva, R.; Nikolova, B.; Momchilova, A.; Djilianov, D.; Hazarosova, R. Myconoside Affects the Viability of Polarized Epithelial MDCKII Cell Line by Interacting with the Plasma Membrane and the Apical Junctional Complexes. Separations 2022, 9, 239. [Google Scholar] [CrossRef]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative imaging of membrane lipid order in cells and organisms. Nat Protoc. 2011, 7, 24–35. [Google Scholar] [CrossRef]
- Garrido, J.; Gaspar, A.; Garrido, E.M.; Miri, R.; Tavakkoli, M.; Pourali, S.; Saso, L.; Borges, F.; Firuzi, O. Alkyl esters of hydroxycinnamic acids with improved antioxidant activity and lipophilicity protect PC12 cells against oxidative stress. Biochimie 2012, 94, 961–967. [Google Scholar] [CrossRef]
- Delmondes, P.; Stefani, R. Computational study of natural phenolic acid solubility and their interactions with chitosan. MOL2NET 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Jiali, Y.; Gupta, V.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure–activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Świsłocka, R.; Wołejko, E.; Jabłońska-Trypuć, A.; Wydro, U.; Kozłowski, M.; Koronkiewicz, K.; Piekut, J.; Lewandowski, W. Structural characterization and evaluation of antimicrobial and cytotoxic activity of six plant phenolic acids. PLoS ONE 2024, 19, e0299372. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Hung, Y.-L.; Chen, T.-C.; Li, H.-J.; Lo, Y.-H.; Wu, N.-L.; Chang, D.-C.; Hung, C.-F. Anti-proliferative and anti-migratory activities of hispidulin on human melanoma A2058 cells. Biomolecules 2021, 11, 1039. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Yeager, A.N.; Weber, P.K.; Kraft, M.L. Three-dimensional imaging of cholesterol and sphingolipids within a Madin–Darby canine kidney cell. Biointerphases 2016, 11, 02A309. [Google Scholar] [CrossRef]
- Nicolson, G.L. Cell membrane fluid–mosaic structure and cancer metastasis. Cancer Res. 2015, 75, 1169–1176. [Google Scholar] [CrossRef]
- Xu, X.; Xu, S.; Wan, J.; Wang, D.; Pang, X.; Gao, Y.; Ni, N.; Chen, D.; Sun, X. Disturbing cytoskeleton by engineered nanomaterials for enhanced cancer therapeutics. Bioact. Mater. 2023, 29, 50–71. [Google Scholar] [CrossRef]
- Nurmagambetova, A.; Mustyatsa, V.; Saidova, A.; Vorobjev, I. Morphological and cytoskeleton changes in cells after EMT. Sci. Rep. 2023, 13, 22164. [Google Scholar] [CrossRef]
- Deusser, H.; Groh, I.; Bakuradze, T.; Simson, N.; Kaiser, E.; Barth, H.; Richling, E. Are compounds membrane-associated or present in the cytosol? A study using polyphenols in a colon carcinoma cell line model. Curr. Pharmacol. Rep. 2020, 6, 213–224. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Influence of membrane lipid composition on flavonoid–membrane interactions: Implications on their biological activity. Prog. Lipid Res. 2015, 58, 1–13. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid–membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Filipe, H.A.L.; Sousa, C.; Marquês, J.T.; Vila-Viçosa, D.; de Granada-Flor, A.; Viana, A.S.; Santos, M.S.C.S.; Machuqueiro, M.; de Almeida, R.F.M. Differential targeting of membrane lipid domains by caffeic acid and its ester derivatives. Free Radic. Biol. Med. 2018, 115, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Carrano, R.; Grande, M.; Leti Maggio, E.; Zucca, C.; Bei, R.; Palumbo, C.; Focaccetti, C.; Nardozi, D.; Lucarini, V.; Angiolini, V.; et al. Dietary polyphenols effects on focal adhesion plaques and metalloproteinases in cancer invasiveness. Biomedicines 2024, 12, 482. [Google Scholar] [CrossRef]
- Wang, R.; Peng, J.; Shi, X.; Cao, S.; Xu, Y.; Xiao, G.; Li, C. Change in membrane fluidity induced by polyphenols is highly dependent on the position and number of galloyl groups. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184015. [Google Scholar] [CrossRef]
- Zhou, A.; Xue, B.; Zhong, J.; Liu, J.; Peng, R.; Wang, F.; Zhou, Y.; Tang, J.; Yang, Q.; Chen, X. Cholesterol-rich lipid rafts mediate endocytosis as a common pathway for respiratory syncytial virus entry into different host cells. Microbiol. Spectr. 2025, 13, e0119225. [Google Scholar] [CrossRef]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Hanna, S.J.; McCoy-Simandle, K.; Miskolci, V.; Guo, P.; Cammaer, M.; Hodgson, L.; Cox, D. The role of Rho-GTPases and actin polymerization during macrophage tunneling nanotube biogenesis. Sci. Rep. 2017, 7, 8547. [Google Scholar] [CrossRef]
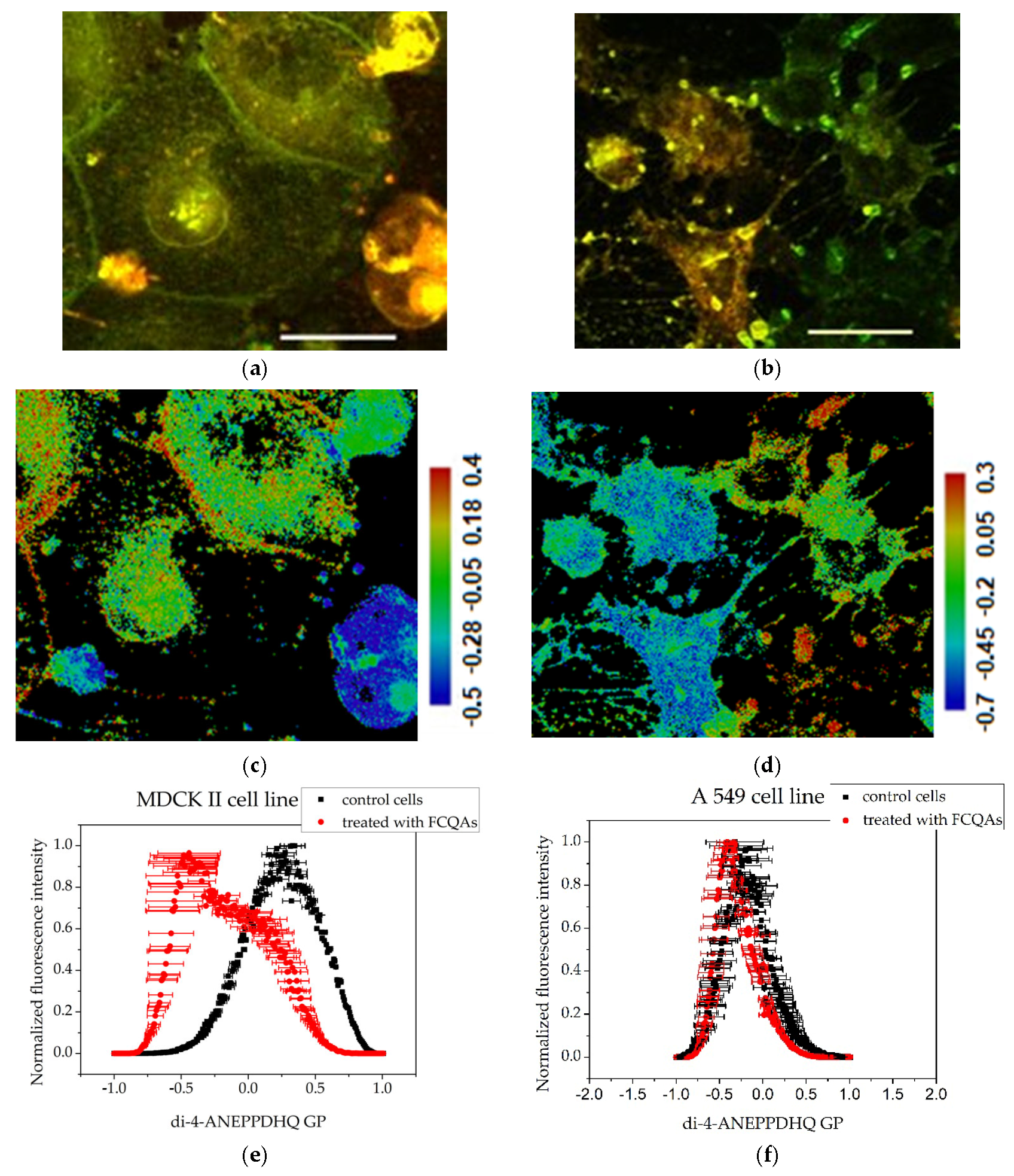
| Cells | MDCK II—GP Peak at | A549—GP Peak at |
|---|---|---|
| Control | 0.331 (SE 0.09026) | −0.323 (SE 0.33225) |
| Treated with FFGs | 0.063 (SE 0.04053) | 0.047 (SE 0.16069) |
| Treated with FCQAs | −0.441 (SE 0.23289) | −0.338 (SE 0.07277) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veleva, R.; Kostadinova, A.; Trendafilova, A.; Ivanova, V.; Moskova-Doumanova, V.; Mladenova, K.; Doumanov, J.; Benkova, D.; Staneva, G.; Topouzova-Hristova, T. Polyphenols from Inula oculus-christi L.-Induced Cell-Specific Membrane and Cytoskeleton Reorganization. Membranes 2025, 15, 357. https://doi.org/10.3390/membranes15120357
Veleva R, Kostadinova A, Trendafilova A, Ivanova V, Moskova-Doumanova V, Mladenova K, Doumanov J, Benkova D, Staneva G, Topouzova-Hristova T. Polyphenols from Inula oculus-christi L.-Induced Cell-Specific Membrane and Cytoskeleton Reorganization. Membranes. 2025; 15(12):357. https://doi.org/10.3390/membranes15120357
Chicago/Turabian StyleVeleva, Ralitsa, Aneliya Kostadinova, Antoaneta Trendafilova, Viktoria Ivanova, Veselina Moskova-Doumanova, Kirilka Mladenova, Jordan Doumanov, Dayana Benkova, Galya Staneva, and Tanya Topouzova-Hristova. 2025. "Polyphenols from Inula oculus-christi L.-Induced Cell-Specific Membrane and Cytoskeleton Reorganization" Membranes 15, no. 12: 357. https://doi.org/10.3390/membranes15120357
APA StyleVeleva, R., Kostadinova, A., Trendafilova, A., Ivanova, V., Moskova-Doumanova, V., Mladenova, K., Doumanov, J., Benkova, D., Staneva, G., & Topouzova-Hristova, T. (2025). Polyphenols from Inula oculus-christi L.-Induced Cell-Specific Membrane and Cytoskeleton Reorganization. Membranes, 15(12), 357. https://doi.org/10.3390/membranes15120357








