Optimizing Ammonia Separation from Thermophilic Digestate: The Combined Effect of pH and Thermal Gradients in Direct Contact Membrane Distillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic Thermophilic Anaerobic Sludge
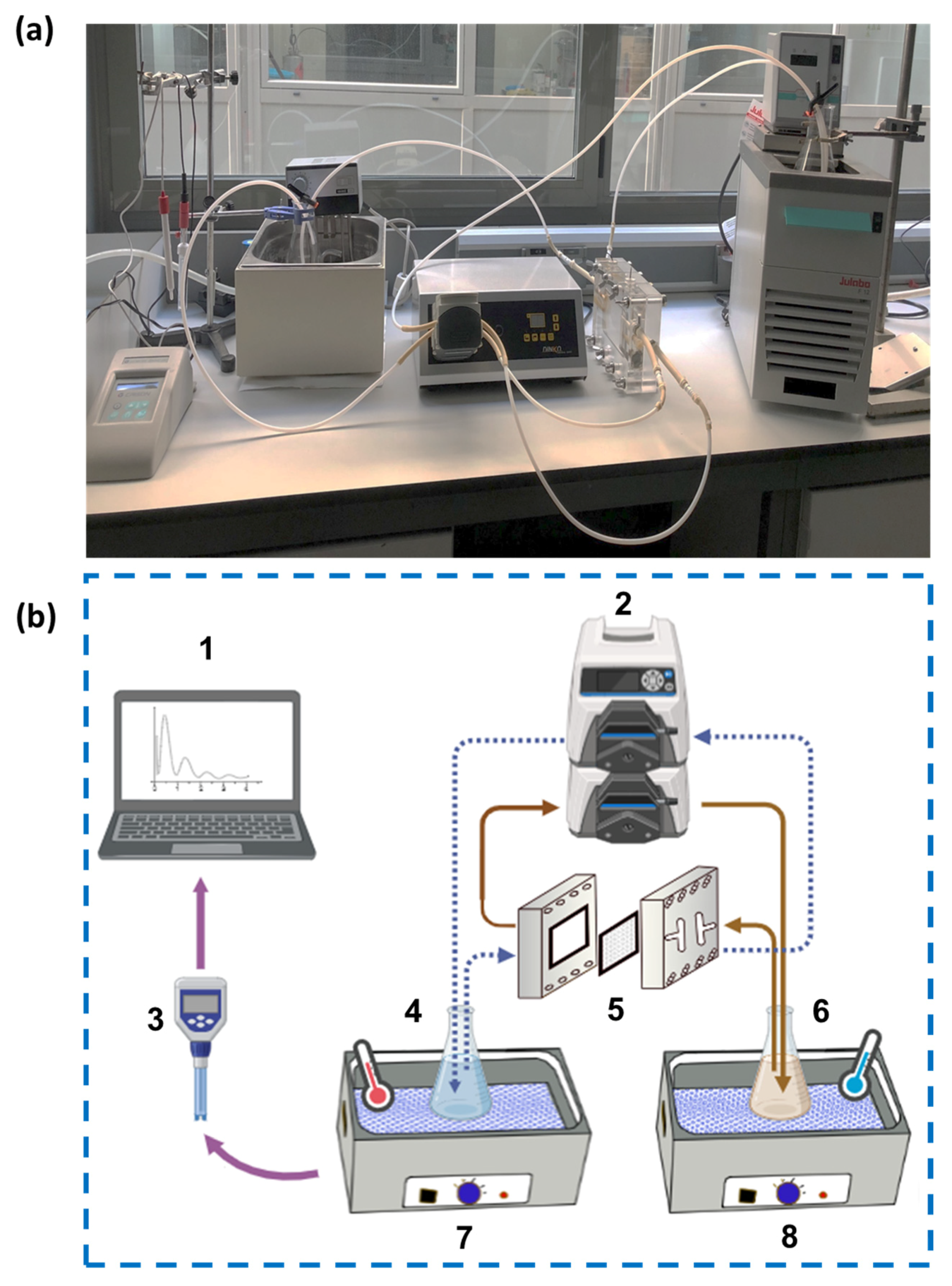
2.2. Experimental Setup
2.3. Operational Conditions and Process Evaluation
2.4. Analytical Methods and Data Analysis
2.5. Membrane Characterization Techniques
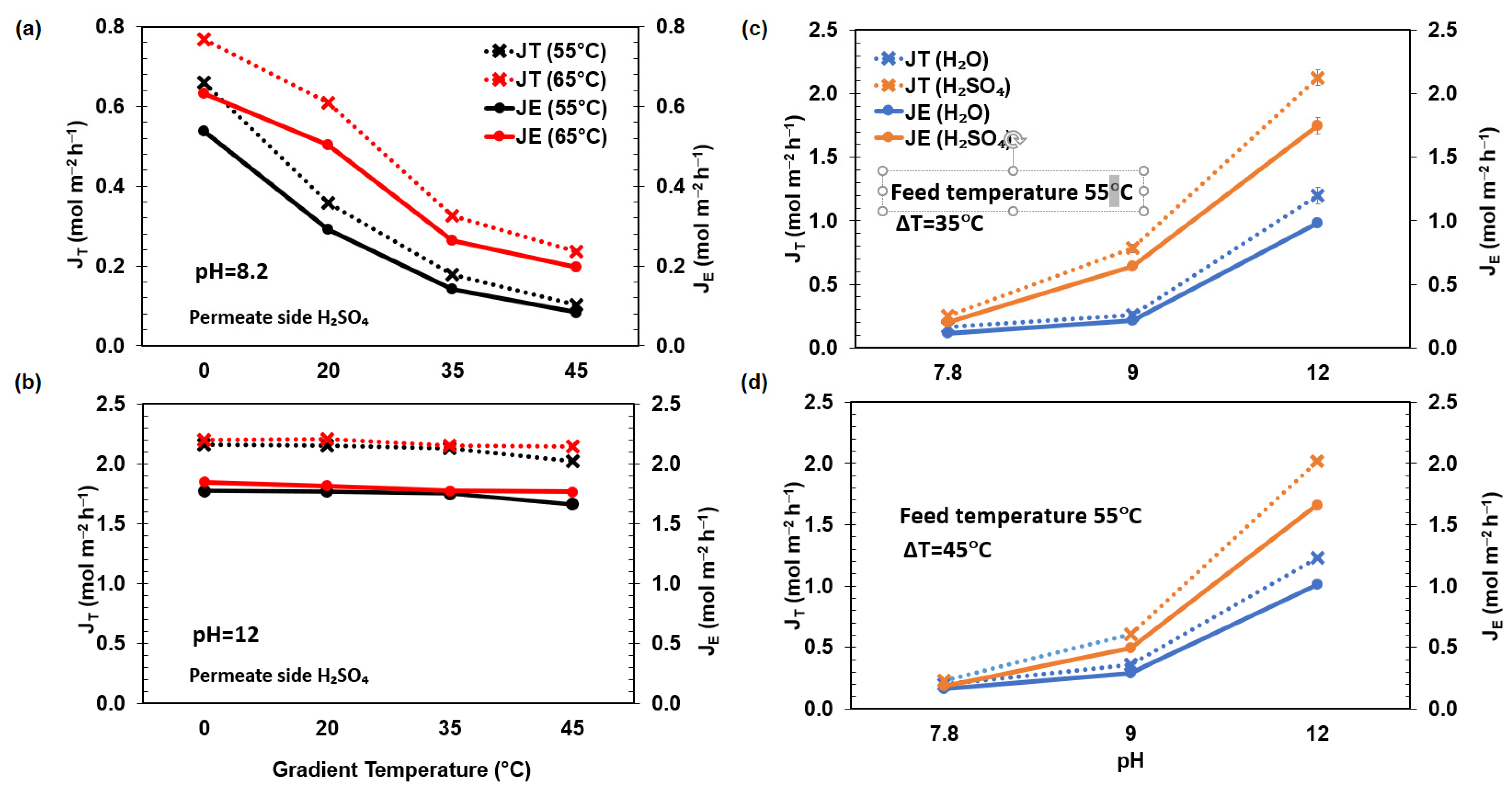
3. Results and Discussion
3.1. Influence of the Capture and Dissolution of NH3
3.2. Effect of System Conditions Using H2SO4 as a Receiving Solution Recovery
3.3. Statistical Analysis
3.4. Validation of Theoretical Flux Predictions with Experimental Data
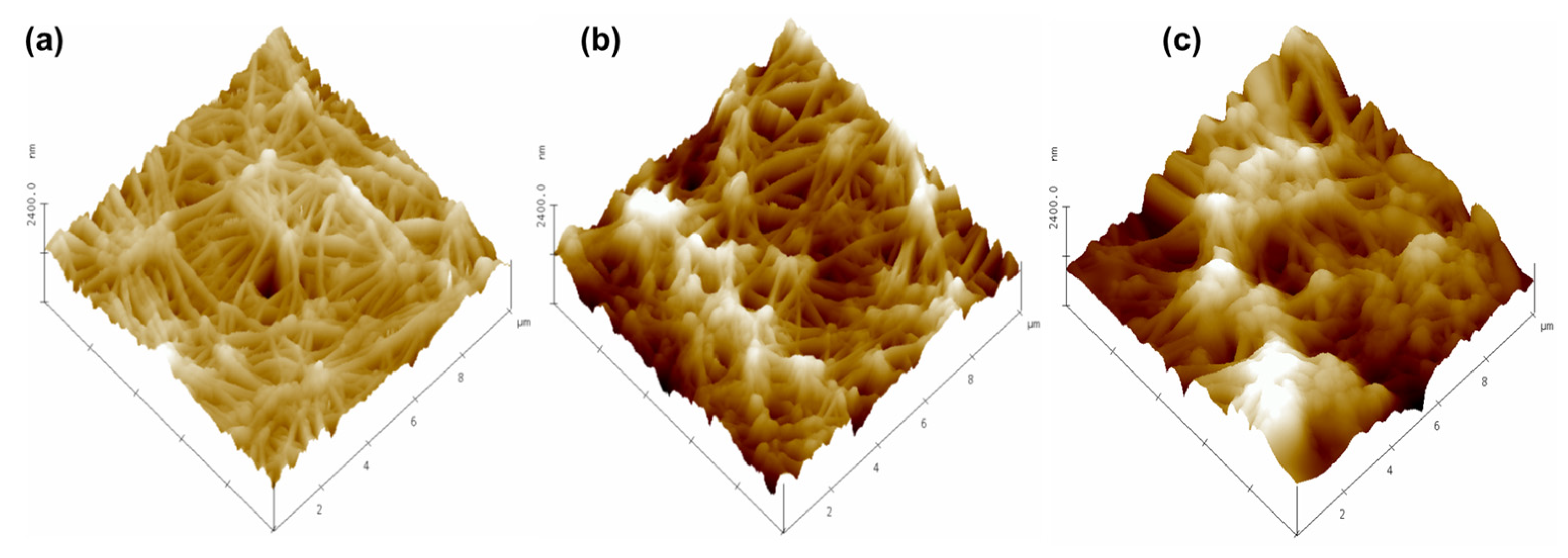
3.5. Membrane Morphology Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erisman, J.W.; Bleeker, A.; Galloway, J.; Sutton, M.S. Reduced Nitrogen in Ecology and the Environment. Environ. Pollut. 2007, 150, 140–149. [Google Scholar] [CrossRef]
- Kavanagh, I.; Burchill, W.; Healy, M.G.; Fenton, O.; Krol, D.J.; Lanigan, G.J. Mitigation of Ammonia and Greenhouse Gas Emissions from Stored Cattle Slurry Using Acidifiers and Chemical Amendments. J. Clean. Prod. 2019, 237, 117822. [Google Scholar] [CrossRef]
- Ma, R.; Li, K.; Guo, Y.; Zhang, B.; Zhao, X.; Linder, S.; Guan, C.H.; Chen, G.; Gan, Y.; Meng, J. Mitigation Potential of Global Ammonia Emissions and Related Health Impacts in the Trade Network. Nat. Commun. 2021, 12, 6308. [Google Scholar] [CrossRef]
- Asman, W.A.H.; Sutton, M.A.; Schjørring, J.K. Ammonia: Emission, Atmospheric Transport and Deposition. New Phytol. 1998, 139, 27–48. [Google Scholar] [CrossRef]
- Temkin, A.; Evans, S.; Manidis, T.; Campbell, C.; Naidenko, O.V. Exposure-Based Assessment and Economic Valuation of Adverse Birth Outcomes and Cancer Risk Due to Nitrate in United States Drinking Water. Environ. Res. 2019, 176, 108442. [Google Scholar] [CrossRef]
- Chen, T.L.; Chen, L.H.; Lin, Y.J.; Yu, C.P.; Ma, H.W.; Chiang, P.C. Advanced Ammonia Nitrogen Removal and Recovery Technology Using Electrokinetic and Stripping Process towards a Sustainable Nitrogen Cycle: A Review. J. Clean. Prod. 2021, 309, 127369. [Google Scholar] [CrossRef]
- European Parliament and Council. Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and Repealing Directive 2001/81/EC. In Official Journal of the European Union; European Union: Brussels, Belgium, 2016; Volume L 344, pp. 1–31. [Google Scholar]
- Burton, F.L.; Stensel, H.D.; Tchobanoglous, G. Wastewater Engineering Treatment, Disposal and Reuse; McGraw-Hill Inc.: New York, NY, USA, 2013. [Google Scholar]
- US EPA Biosolids. Available online: https://www.epa.gov/biosolids (accessed on 30 October 2025).
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane Bioreactor for Wastewater Treatment: A Review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Sońta, M.; Łozicki, A.; Szymańska, M.; Sosulski, T.; Szara, E.; Wąs, A.; van Pruissen, G.W.P.; Cornelissen, R.L. Duckweed from a Biorefinery System: Nutrient Recovery Efficiency and Forage Value. Energies 2020, 13, 5261. [Google Scholar] [CrossRef]
- Laureni, M.; Palatsi, J.; Llovera, M.; Bonmatí, A. Influence of Pig Slurry Characteristics on Ammonia Stripping Efficiencies and Quality of the Recovered Ammonium-Sulfate Solution. J. Chem. Technol. Biotechnol. 2013, 88, 1654–1662. [Google Scholar] [CrossRef]
- Winkler, M.K.; Straka, L. New Directions in Biological Nitrogen Removal and Recovery from Wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Reig, M.; Vecino, X.; Lopez, J.; Rezakazemi, M.; Valderrama, C.A.; Cortina, J.L. Liquid–Liquid Membrane Contactors Incorporating Surface Skin Asymmetric Hollow Fibres of Poly(4-Methyl-1-Pentene) for Ammonium Recovery as Liquid Fertilisers. Sep. Purif. Technol. 2022, 283, 120212. [Google Scholar] [CrossRef]
- Vecino, X.; Reig, M.; Gibert, O.; Valderrama, C.; Cortina, J.L. Integration of Liquid-Liquid Membrane Contactors and Electrodialysis for Ammonium Recovery and Concentration as a Liquid Fertilizer. Chemosphere 2020, 245, 125606. [Google Scholar] [CrossRef]
- Wakimoto, K.; Yan, W.W.; Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Ammonia Permeation of Fluorinated Sulfonic Acid Polymer/Ceramic Composite Membranes. J. Memb. Sci. 2022, 658, 120718. [Google Scholar] [CrossRef]
- Kuntke, P.; Sleutels, T.H.J.A.; Rodríguez Arredondo, M.; Georg, S.; Barbosa, S.G.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. (Bio)Electrochemical Ammonia Recovery: Progress and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y. Solar-Powered Forward Osmosis as a Sustainable Water Treatment Solution: A Review. J. Environ. Chem. Eng. 2025, 13, 116332. [Google Scholar] [CrossRef]
- Rivera, F.; Akpan, J.; Prádanos, P.; Hernández, A.; Palacio, L.; Muñoz, R. Side-Stream Membrane-Based NH3 Extraction to Improve the Anaerobic Digestion of Poultry Manure. J. Water Process Eng. 2023, 54, 103990. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Guan, K.; Gonzales, R.R.; Ishigami, T.; Xue, M.; Yoshioka, T.; Matsuyama, H. An Experimental Study on Recovering and Concentrating Ammonia by Sweep Gas Membrane Distillation. Process Saf. Environ. Prot. 2023, 171, 555–560. [Google Scholar] [CrossRef]
- Criscuoli, A. Membrane Distillation Process. Membranes 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Horseman, T.; Yin, Y.; Christie, K.S.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, Scaling, and Fouling in Membrane Distillation: State-of-the-Art Insights on Fundamental Mechanisms and Mitigation Strategies. ACS EST Eng. 2021, 1, 117–140. [Google Scholar] [CrossRef]
- Mariah, L.; Buckley, C.A.; Brouckaert, C.J.; Curcio, E.; Drioli, E.; Jaganyi, D.; Ramjugernath, D. Membrane Distillation of Concentrated Brines-Role of Water Activities in the Evaluation of Driving Force. J. Memb. Sci. 2006, 280, 937–947. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Han Seo, D.; Van Der Laan, T.; Lee, M.; Sung Yun, J.; Jun Park, M.; Bendavid, A.; Kyong Shon, H.; Halil Avci, A.; et al. Dimensionally Controlled Graphene-Based Surfaces for Photothermal Membrane Crystallization. J. Colloid. Interface Sci. 2022, 623, 607–616. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Moritz, E.; Hobisch, M.; Muster-Slawitsch, B. Recovery of Ammonia from Centrate Water in Urban Waste Water Treatment Plants via Direct Contact Membrane Distillation: Process Performance in Long-Term Pilot-Scale Operation. J. Memb. Sci. 2023, 667, 121161. [Google Scholar] [CrossRef]
- Hu, Y.; Loh, C.Y.; Xie, M.; Chen, G.; Huang, M.; Qiao, J. Ammonia Recovery via Direct Contact Membrane Distillation: Modeling and Performance Optimization. J. Environ. Manag. 2024, 365, 121683. [Google Scholar] [CrossRef]
- Kywe, P.P.; Ratanatamskul, C. Influences of Permeate Solution and Feed PH on Enhancement of Ammonia Recovery from Wastewater by Negatively Charged PTFE Membranes in Direct Contact Membrane Distillation Operation. ACS Omega 2022, 7, 27722–27733. [Google Scholar] [CrossRef]
- Müller, T.; Walter, B.; Wirtz, A.; Burkovski, A. Ammonium Toxicity in Bacteria. Curr. Microbiol. 2006, 52, 400–406. [Google Scholar] [CrossRef]
- Rivera, F.; Villareal, L.; Prádanos, P.; Hernández, A.; Palacio, L.; Muñoz, R. Enhancement of Swine Manure Anaerobic Digestion Using Membrane-Based NH3 Extraction. Bioresour. Technol. 2022, 362, 127829. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Torres-Franco, A.F.; Zuluaga, M.; Hernández-Roldán, D.; Leroy-Freitas, D.; Sepúlveda-Muñoz, C.A.; Blanco, S.; Mota, C.R.; Muñoz, R. Assessment of the Performance of an Anoxic-Aerobic Microalgal-Bacterial System Treating Digestate. Chemosphere 2021, 270, 129437. [Google Scholar] [CrossRef] [PubMed]
- Montalvillo, M.; Silva, V.; Palacio, L.; Calvo, J.I.; Carmona, F.J.; Hernández, A.; Prádanos, P. Charge and Dielectric Characterization of Nanofiltration Membranes by Impedance Spectroscopy. J. Memb. Sci. 2014, 454, 163–173. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Q.; Shu, X.; Yu, H.; Rong, H.; Qu, F.; Liang, H. Simultaneous Ammonium and Water Recovery from Landfill Leachate Using an Integrated Two-Stage Membrane Distillation. Water Res. 2023, 240, 120080. [Google Scholar] [CrossRef] [PubMed]
- McCartney, S.N.; Williams, N.A.; Boo, C.; Chen, X.; Yip, N.Y. Novel Isothermal Membrane Distillation with Acidic Collector for Selective and Energy-Efficient Recovery of Ammonia from Urine. ACS Sustain. Chem. Eng. 2020, 8, 7324–7334. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology NIST Chemistry WebBook. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C7732185 (accessed on 27 May 2025).
- Bottino, A.; Capannelli, G.; Grosso, A.; Monticelli, O.; Cavalleri, O.; Rolandi, R.; Soria, R. Surface Characterization of Ceramic Membranes by Atomic Force Microscopy. J. Memb. Sci. 1994, 95, 289–296. [Google Scholar] [CrossRef]
- Rivera, F.; Muñoz, R.; Prádanos, P.; Hernández, A.; Palacio, L. A Systematic Study of Ammonia Recovery from Anaerobic Digestate Using Membrane-Based Separation. Membranes 2022, 12, 19. [Google Scholar] [CrossRef]
- Rivera, F.; Sepúlveda-Muñoz, C.A.; Prádanos, P.; Hernández, A.; Palacio, L.; Muñoz, R. Influence of PH on the Performance of Anaerobic Piggery Wastewater Treatment Coupled with Membrane-Based NH3 Extraction. J. Water Process Eng. 2023, 55, 104226. [Google Scholar] [CrossRef]
- He, Q.; Tu, T.; Yan, S.; Yang, X.; Duke, M.; Zhang, Y.; Zhao, S. Relating Water Vapor Transfer to Ammonia Recovery from Biogas Slurry by Vacuum Membrane Distillation. Sep. Purif. Technol. 2018, 191, 182–191. [Google Scholar] [CrossRef]
- Yang, K.; Qin, M. Understanding Ammonia and Water Transport in Direct Contact Membrane Distillation toward Selective Ammonia Recovery. ACS ES&T Eng. 2024, 4, 1321–1330. [Google Scholar] [CrossRef]
- Tun, L.L.; Jeong, D.; Jeong, S.; Cho, K.; Lee, S.; Bae, H. Dewatering of Source-Separated Human Urine for Nitrogen Recovery by Membrane Distillation. J. Memb. Sci. 2016, 512, 13–20. [Google Scholar] [CrossRef]
- Resch, C.; Wörl, A.; Waltenberger, R.; Braun, R.; Kirchmayr, R. Enhancement Options for the Utilisation of Nitrogen Rich Animal By-Products in Anaerobic Digestion. Bioresour. Technol. 2011, 102, 2503–2510. [Google Scholar] [CrossRef]
- Panichnumsin, P.; Nopharatana, A.; Ahring, B.; Chaiprasert, P. Production of Methane by Co-Digestion of Cassava Pulp with Various Concentrations of Pig Manure. Biomass Bioenergy 2010, 34, 1117–1124. [Google Scholar] [CrossRef]
- Kister, Z.H. Dsitillation Design, 1st ed.; McGraw-Hill Education: New York, NY, USA, 1992; ISBN 9780070349094. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane Distillation: Recent Developments and Perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Fan, S.; Feng, Z.; Lu, R.; Lv, L.; Dong, L. Enhanced Extractive Distillation Processes for Separating N-Hexane and 1,2-Dichloroethane via Bottom Flash Heat Pump. Chem. Eng. Process.-Process Intensif. 2022, 179, 109069. [Google Scholar] [CrossRef]
- Wang, S.J.; Wong, D.S.H.; Lee, E.K. Control of a Reactive Distillation Column in the Kinetic Regime for the Synthesis of N-Butyl Acetate. Ind. Eng. Chem. Res. 2003, 42, 5182–5194. [Google Scholar] [CrossRef]
- Kiss, A.A. Advanced Distillation Technologies: Design, Control and Applications; Wiley: Hoboken, NJ, USA, 2013; ISBN 9781119993612. [Google Scholar]
- Tan, X.; Tan, S.P.; Teo, W.K.; Li, K. Polyvinylidene Fluoride (PVDF) Hollow Fibre Membranes for Ammonia Removal from Water. J. Memb. Sci. 2006, 271, 59–68. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, W.; Yu, S.; Zhu, Y.; Zhang, R.; Li, L. Optimization of Cleaning Conditions on a Polytetrafluoroethylene (PTFE) Microfiltration Membrane Used in Treatment of Oil-Field Wastewater. RSC Adv. 2015, 5, 104960–104971. [Google Scholar] [CrossRef]
- Xu, P.; Bellona, C.; Drewes, J.E. Fouling of Nanofiltration and Reverse Osmosis Membranes during Municipal Wastewater Reclamation: Membrane Autopsy Results from Pilot-Scale Investigations. J. Memb. Sci. 2010, 353, 111–121. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Norddahl, B. Ammonia Recovery from Agricultural Wastes by Membrane Distillation: Fouling Characterization and Mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow Fibre Membrane Contactors for Ammonia Recovery: Current Status and Future Developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Chang, I.-S.; Le Clech, P.; Jefferson, B.; Judd, S. Membrane Fouling in Membrane Bioreactors for Wastewater Treatment. J. Environ. Eng. 2002, 128, 1018–1029. [Google Scholar] [CrossRef]


| Series | Assay | Feed pH | Feed Temp. (°C) | Receiving Solution | Receiving Solution Temp. (°C) | Temp. Gradient (°C) |
|---|---|---|---|---|---|---|
| I | 1 | 7.8 | 55 | DI H2O | 20 | 35 |
| 2 | 7.8 | 55 | H2SO4 | 20 | 35 | |
| 3 | 7.8 | 55 | DI H2O | 10 | 45 | |
| 4 | 7.8 | 55 | H2SO4 | 10 | 45 | |
| 5 | 9 | 55 | DI H2O | 20 | 35 | |
| 6 | 9 | 55 | H2SO4 | 20 | 35 | |
| 7 | 9 | 55 | DI H2O | 10 | 45 | |
| 8 | 9 | 55 | H2SO4 | 10 | 45 | |
| 9 | 12 | 55 | DI H2O | 20 | 35 | |
| 10 | 12 | 55 | H2SO4 | 20 | 35 | |
| 11 | 12 | 55 | DI H2O | 10 | 45 | |
| 12 | 12 | 55 | H2SO4 | 10 | 45 | |
| II | 1 | 7.8 | 55 | H2SO4 | 55 | 0 |
| 2 | 7.8 | 55 | 35 | 20 | ||
| 3 | 9 | 55 | 55 | 0 | ||
| 4 | 9 | 55 | 35 | 20 | ||
| 5 | 12 | 55 | 55 | 0 | ||
| 6 | 12 | 55 | 35 | 20 | ||
| III | 1 | 8.2 | 55 | H2SO4 | 55 | 0 |
| 2 | 8.2 | 55 | 35 | 20 | ||
| 3 | 8.2 | 55 | 20 | 35 | ||
| 4 | 8.2 | 55 | 10 | 45 | ||
| IV | 1 | 8.2 | 65 | H2SO4 | 65 | 0 |
| 2 | 8.2 | 65 | 45 | 20 | ||
| 3 | 8.2 | 65 | 30 | 35 | ||
| 4 | 8.2 | 65 | 20 | 45 | ||
| 5 | 12 | 65 | 65 | 0 | ||
| 6 | 12 | 65 | 45 | 20 | ||
| 7 | 12 | 65 | 30 | 35 | ||
| 8 | 12 | 65 | 20 | 45 |
| Membrane | (nm) | Bearing Volume (μ3) |
|---|---|---|
| New membrane | 200 ± 8 | 9400 ± 90 |
| Used membrane (Active layer) | 246 ± 25 | 11,800 ± 130 |
| Used membrane (Support layer) | 147 ± 6 | 5000 ± 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, F.; Villarreal, L.; Prádanos, P.; Muñoz, R.; Palacio, L.; Hernández, A. Optimizing Ammonia Separation from Thermophilic Digestate: The Combined Effect of pH and Thermal Gradients in Direct Contact Membrane Distillation. Membranes 2025, 15, 348. https://doi.org/10.3390/membranes15120348
Rivera F, Villarreal L, Prádanos P, Muñoz R, Palacio L, Hernández A. Optimizing Ammonia Separation from Thermophilic Digestate: The Combined Effect of pH and Thermal Gradients in Direct Contact Membrane Distillation. Membranes. 2025; 15(12):348. https://doi.org/10.3390/membranes15120348
Chicago/Turabian StyleRivera, Fanny, Luis Villarreal, Pedro Prádanos, Raúl Muñoz, Laura Palacio, and Antonio Hernández. 2025. "Optimizing Ammonia Separation from Thermophilic Digestate: The Combined Effect of pH and Thermal Gradients in Direct Contact Membrane Distillation" Membranes 15, no. 12: 348. https://doi.org/10.3390/membranes15120348
APA StyleRivera, F., Villarreal, L., Prádanos, P., Muñoz, R., Palacio, L., & Hernández, A. (2025). Optimizing Ammonia Separation from Thermophilic Digestate: The Combined Effect of pH and Thermal Gradients in Direct Contact Membrane Distillation. Membranes, 15(12), 348. https://doi.org/10.3390/membranes15120348








