Mitigation Techniques of Membranes’ Biofouling in Bioelectrochemical Cells (BEC Cells): Recent Advances
Abstract
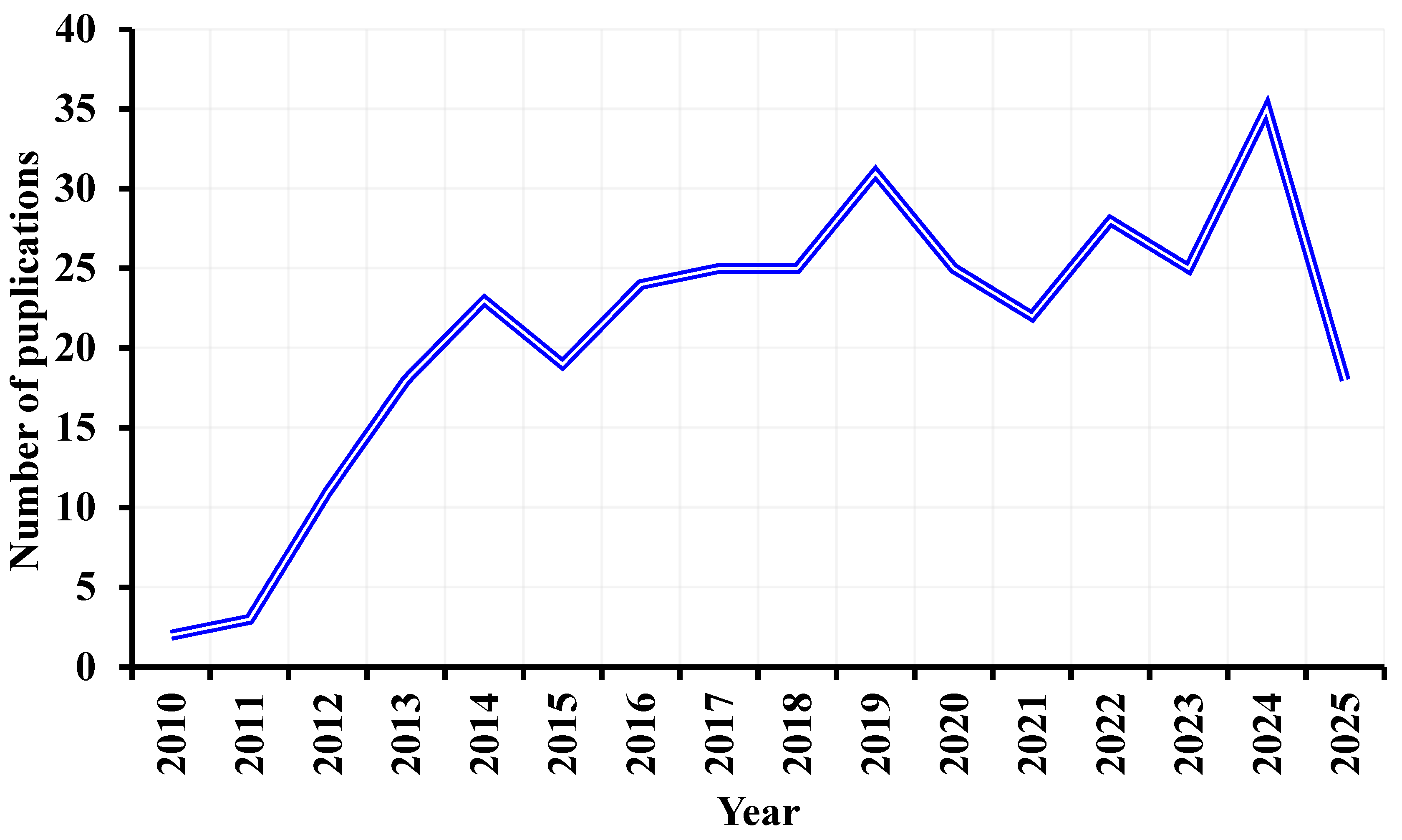
1. Introduction
2. Methodology
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
- ○
- Inclusion Criteria:
- ▪
- Peer-reviewed journal articles focusing on antibiofouling mechanisms in BECs.
- ▪
- Studies published in English between 2019–2025.
- ▪
- Experimental or modeling studies reporting fouling mitigation strategies of membranes or separators in BECs.
- ○
- Exclusion Criteria:
- ▪
- Studies addressing cathodic biofouling exclusively.
- ▪
- Articles focusing solely on novel BEC configurations or electrolyte modifications without membrane fouling analysis.
- ▪
- Review articles, book chapters, conference abstracts, or non-peer-reviewed studies
2.3. Screening and Selection Process
3. Results and Discussion
3.1. Limitations of Conventional Ion-Exchange Membranes
3.2. Alternative Commercial and Low-Cost Membranes
3.3. Biodegradable and Ceramic Membrane Alternatives
3.4. Ionic Liquid and Ionogel Membranes
3.5. Physicochemical Determinants of Antifouling Behavior
3.6. Biopolymer-Based Membranes
3.7. Metal Nanoparticles and Quaternary Ammonium Compounds
3.8. Electro-Assisted Mitigation Methods
| Type of the Cell | Membrane Type | Main Idea | Biofouling Capabilities | Ref. |
|---|---|---|---|---|
| MBR | DC electric field membrane | The use of electric fields to repel the bacteria; hence, there is less biofilm formation, accumulation, and blocking. | Lower zeta potential (−16.9 mV), soluble microbial product (SMP) decline (8.61–4.28 mg/L), and LB-EPS reduction (4.29–2.48 mg/g MLSS). | [115] |
| MFC | AnMBR (conductive membranes) | AnMBR was used as the anode to study the effect of passing a self-generated current on biofouling mitigation. | SMP, LB-EPS, and TB-EPS reductions: R-10 membrane exhibited 82.01%, 66.35%, and 72.57% lower polysaccharide content and 59.47%, 38.97%, and 80.42% lower protein concentrations compared to R-0 membrane. | [116] |
| Cross-flow filtration and electrolysis setup | Nano zeolite/CNS with PVDF as a binder. | Developing an electro-ceramic membrane made from nano-zeolite and evaluating its antifouling performance. | Periodic electrolysis cleaning increased flux by 21% after the first cycle, with subsequent cycles showing additional increases of 23%, 22%, and 17%. | [117] |
| AnOMEBR | Thin film nanocomposite FO membrane with nanocarbons. | Developing an electro-assisted anaerobic forward osmosis membrane bioreactor (AnOMEBR) to treat wastewater and reduce membrane fouling using a conductive FO membrane as both the separation unit and cathode. | SMP reduced by 26%, PN/PS ratio reduced by 15%, and steady flux decline (10.88–2.41 LMH in 134 h) compared to AnOMBR (9.60–1.15 LMH in 72 h). | [118] |
| AnEMBR | Carbon nanotube hollow fiber membranes 16 (CNTs-HFMs) | Electro-assisted Anaerobic Electrochemical Membrane Bioreactor (AnEMBR) using CNTs-HFMs to improve antifouling performance. | Lower transmembrane pressure (TMP) (0.35 bar), reduced EPS adhesion, minimized gel layer formation, and inhibited pollutant penetration. | [119] |
| Single-chamber (AnEMBR) | Nickel-based hollow fiber membrane (Ni-HFM) | Investigating biofouling control by varying the cathode surface-to-cathode area (SCSA). | The 8 m2/m3 AnEMBR effectively reduced biofouling. | [120] |
| Single-chambered Membrane Bio-Electro-Reactor (MBER) | Carbon fiber cloth composite membrane anode (PVDF/PVP-based) | Integrating electrooxidation with a conductive membrane anode to enhance ammonia removal and biofouling resistance. | 1.4 V anode potential generated free chlorine, reducing PVDF composite membrane biofouling. | [121] |
| MFC-MBR | PVDF hollow fiber membrane of the MFC. | Backwashable conductive carbon nanotube (CNT) membrane deposited in PVDF membrane. | TMP rise to 30 kPa extended by 8 days. Reduction in phenol byproducts, cholesterol margarate, and carboxylic acid. | [126] |
| Crossflow membrane filtration system | Polyethersulfone (PES) membranes were CNT-coated with polyvinyl alcohol (PVA) crosslinked by succinic acid or glutaraldehyde. | Enhancing ECM stability in separation applications by examining antifouling properties of CNT/PVA-based ECMs with two crosslinking agents. | ECMs exhibited 21% flux reduction vs. 69% in PES membranes and 91% bacterial retention due to CNT porous structure. | [127] |
| Filtration system | (CNT)-PVDF conductive membrane | CNT-PVDF conductive membrane was tested in multi-cycle lab-scale filtration–backwashing experiments, with a DC voltage of −1.5 V applied across the membrane. | Significantly reduced irreversible biofouling, decreasing flux decline from 68% to 38% for live bacteria, effectively inhibited bacterial adhesion and enhanced backwashing, achieving up to 93% flux recovery. | [128] |
| MFC-AnMBR | PPy/AQDS/PTFE | Conductive anode membranes are used to treat Na+- and Mg2+-containing wastewater. | Reduction in protein and polysaccharide content by up to 44% and 58% for Na+ and up to 40% and 38% for Mg2+, while extending operational times for up to 43 days, with mitigation linked to current for Na+ and voltage for Mg2+. | [129] |
4. Future Directions
- Material Innovation and Surface Engineering: The development of highly hydrophilic, conductive, and antimicrobial membranes is crucial for improving biofouling resistance. A promising approach is the incorporation of nanocomposite materials, such as functionalized graphene derivatives, metal–organic frameworks (MOFs), carbon nanotubes, and bio-inspired coatings, into the polymer matrix. Ceramic membranes have demonstrated superior chemical and mechanical stability, making them promising alternatives for long-term biofouling mitigation. Moreover, electro-assisted ceramic membranes can further enhance antifouling performance.
- Advanced Monitoring and Real-Time Diagnostics: Future studies should move beyond post hoc characterization and develop methods for the real-time, quantitative assessment of membrane health. A key direction is the integration of real-time diagnostic tools, such as polarization loss decomposition, to dynamically estimate the membrane’s health state. This approach can quantitatively separate and track the contribution of biofouling to membrane resistance, enabling proactive fouling management rather than reactive cleaning.
- System Operational Strategies and Thermal Management: Optimizing operational parameters is a vital complement to material-based solutions. Temperature control is a critical operational variable, as optimized thermal management can suppress microbial metabolic rates and EPS production, thereby delaying biofilm growth. Future research should explore thermal management strategies as a systematic approach to complement material-based antifouling approaches.
- Sustainability and Scalable Fabrication: Future research should focus on the development of low-cost, eco-friendly membrane materials and fabrication processes to ensure the long-term viability of BEC systems. This includes investigating green synthesis techniques, biodegradable membrane materials, and energy-efficient fabrication methods. Since even with efforts to develop anti-biofouling membranes that mitigate biofouling growth, membrane performance will still deteriorate over time, eventually requiring the replacement of fouled modules.
- Large-Scale Applications: Membrane modifications that demonstrated excellent performance in lab-scale and pilot-scale studies are essential to be tested under real-world, large-scale conditions to evaluate their long-term efficiency, durability, and practicality.
- Hybrid Systems: One should explore the integration of BECs with other technologies, such as forward osmosis [133], anaerobic membrane bioreactors, and electrochemical systems, to enhance biofouling resistance and overall system efficiency.
- Long-Term Studies: One should conduct extended operational studies to evaluate the durability and antifouling performance of membranes under real-world conditions.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, R.; Leach, M.; Bauen, A. Progress in renewable energy. Environ. Int. 2003, 29, 105–122. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Stoppato, A.; Benato, A. The Importance of Energy Storage. In Energy Storage; World Scientific Series in Current Energy Issues; World Scientific: Hackensack, NJ, USA, 2017; pp. 1–26. [Google Scholar] [CrossRef]
- Alinejad, Z.; Parham, N.; Tawalbeh, M.; Al-Othman, A.; Almomani, F. Progress in green hydrogen production and innovative materials for fuel cells: A pathway towards sustainable energy solutions. Int. J. Hydrogen Energy 2025, 140, 1078–1094. [Google Scholar] [CrossRef]
- Mei, J.; Meng, X.; Tang, X.; Li, H.; Hasanien, H.; Alharbi, M.; Dong, Z.; Shen, J.; Sun, C.; Fan, F.; et al. An Accurate Parameter Estimation Method of the Voltage Model for Proton Exchange Membrane Fuel Cells. Energies 2024, 17, 2917. [Google Scholar] [CrossRef]
- Torrisi, S.; Anastasi, E.; Longhitano, S.; Longo, I.C.; Zerbo, A.; Borzi, G. Circular Economy and the Benefits of Biomass as a Renewable Energy Source. In Proceedings of the 22th International Trade Fair of Material & Energy Recovery and Sustainable Development, ECOMONDO, Rimini, Italy, 6–9 November 2018. [Google Scholar]
- Sonawane, J.M.; Ezugwu, C.I.; Ghosh, P.C. Microbial Fuel Cell-Based Biological Oxygen Demand Sensors for Monitoring Wastewater: State-of-the-Art and Practical Applications. ACS Sens. 2020, 5, 2297–2316. [Google Scholar] [CrossRef]
- Bennetto, H.P. Electricity generation by microorganisms. Biotechnol. Educ. 1990, 1, 163–168. [Google Scholar] [CrossRef]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706–716. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, J.; Miao, H.; Ruan, W.; Wang, X. Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane. Membranes 2020, 10, 122. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Singh, K.; Douba, I.; Kabakebji, D.; Alkasrawi, M. Microbial desalination cells for water purification and power generation: A critical review. Energy 2020, 209, 118493. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and comparison of various hydrogen production methods based on costs and life cycle impact assessment indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Sugumar, M.; Dharmalingam, S. Statistical optimization of process parameters in microbial fuel cell for enhanced power production using Sulphonated Polyhedral Oligomeric Silsesquioxane dispersed Sulphonated Polystyrene Ethylene Butylene Polystyrene nanocomposite membranes. J. Power Sources 2020, 469, 228400. [Google Scholar] [CrossRef]
- Mohan, S.V.; Sravan, J.S.; Butti, S.K.; Krishna, K.V.; Modestra, J.A.; Velvizhi, G.; Kumar, A.N.; Varjani, S.; Pandey, A. Microbial Electrochemical Technology. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 3–18. [Google Scholar]
- Al-Murisi, M.; Al-Muqbel, D.; Al-Othman, A.; Tawalbeh, M. Integrated microbial desalination cell and microbial electrolysis cell for wastewater treatment, bioelectricity generation, and biofuel production: Success, experience, challenges, and future prospects. In Integrated Environmental Technologies for Wastewater Treatment and Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–166. [Google Scholar]
- Benemann, J. Hydrogen biotechnology: Progress and prospects. Nat. Biotechnol. 1996, 14, 1101–1103. [Google Scholar] [CrossRef]
- Ki, D.; Popat, S.C.; Torres, C.I. Reduced overpotentials in microbial electrolysis cells through improved design, operation, and electrochemical characterization. Chem. Eng. J. 2016, 287, 181–188. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef]
- Rasten, E.; Hagen, G.; Tunold, R. Electrocatalysis in water electrolysis with solid polymer electrolyte. Electrochim. Acta 2003, 48, 3945–3952. [Google Scholar] [CrossRef]
- Sharma, A.; Hussain Mehdi, S.E.; Pandit, S.; Eun-Oh, S.; Natarajan, V. Factors affecting hydrogen production in microbial electrolysis cell (MEC): A review. Int. J. Hydrogen Energy 2024, 61, 1473–1484. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Nayak, J.K.; Ress, N.V.; Steinberger-Wilckens, R.; Ghosh, U.K. Bio-hydrogen production through microbial electrolysis cell: Structural components and influencing factors. Chem. Eng. J. 2023, 455, 140535. [Google Scholar] [CrossRef]
- Xu, G.; Dong, X.; Xue, B.; Huang, J.; Wu, J.; Cai, W. Recent Approaches to Achieve High Temperature Operation of Nafion Membranes. Energies 2023, 16, 1565. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Voropaeva, D.Y.; Lysova, A.A.; Korchagin, O.V.; Bogdanovskaya, V.A.; Yaroslavtsev, A.B. On the Properties of Nafion Membranes Recast from Dispersion in N-Methyl-2-Pyrrolidone. Polymers 2022, 14, 5275. [Google Scholar] [CrossRef]
- Shi, S.; Weber, A.Z.; Kusoglu, A. Structure/property relationship of Nafion XL composite membranes. J. Memb. Sci. 2016, 516, 123–134. [Google Scholar] [CrossRef]
- Lufrano, E.; Simari, C.; Di Vona, M.L.; Nicotera, I.; Narducci, R. How the Morphology of Nafion-Based Membranes Affects Proton Transport. Polymers 2021, 13, 359. [Google Scholar] [CrossRef]
- Min, K.; Al Munsur, A.Z.; Paek, S.Y.; Jeon, S.; Lee, S.Y.; Kim, T.-H. Development of High-Performance Polymer Electrolyte Membranes through the Application of Quantum Dot Coatings to Nafion Membranes. ACS Appl. Mater. Interfaces 2023, 15, 15616–15624. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Đelević, L.; Herkendell, K. Next-Generation Proton-Exchange Membranes in Microbial Fuel Cells: Overcoming Nafion’s Limitations. Energy Technol. 2024, 12, 2301346. [Google Scholar] [CrossRef]
- Miskan, M.; Ismail, M.; Ghasemi, M.; Md Jahim, J.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 543–552. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Pandit, S.; Sonawane, J.M.; Gupta, P.K.; Prasad, R.; Chendake, A.D. Effect of membrane biofouling on the performance of microbial electrochemical cells and mitigation strategies. Bioresour. Technol. Rep. 2021, 15, 100822. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Debeer, D.; Caldwell, D.; Korber, D.; James2, G. MINIREVIEW Biofilms, the Customized Microniche. J. Bateriol. 1994, 176, 2137–2142. [Google Scholar]
- Lin, H.; Zhang, M.; Wang, F.; Meng, F.; Liao, B.-Q.; Hong, H.; Chen, J.; Gao, W. A critical review of extracellular polymeric substances (EPSs) in membrane bioreactors: Characteristics, roles in membrane fouling and control strategies. J. Memb. Sci. 2014, 460, 110–125. [Google Scholar] [CrossRef]
- Lin, H.; Wang, F.; Ding, L.; Hong, H.; Chen, J.; Lu, X. Enhanced performance of a submerged membrane bioreactor with powdered activated carbon addition for municipal secondary effluent treatment. J. Hazard. Mater. 2011, 192, 1509–1514. [Google Scholar] [CrossRef]
- Satyawali, Y.; Balakrishnan, M. Effect of PAC addition on sludge properties in an MBR treating high strength wastewater. Water Res. 2009, 43, 1577–1588. [Google Scholar] [CrossRef]
- Jamal Khan, S.; Visvanathan, C.; Jegatheesan, V. Effect of powdered activated carbon (PAC) and cationic polymer on biofouling mitigation in hybrid MBRs. Bioresour. Technol. 2012, 113, 165–168. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Yue, Z. Factors influencing the production of extracellular polymeric substances by Rhodopseudomonas acidophila. Int. Biodeterior. Biodegrad. 2006, 58, 89–93. [Google Scholar] [CrossRef]
- Ye, F.; Peng, G.; Li, Y. Influences of influent carbon source on extracellular polymeric substances (EPS) and physicochemical properties of activated sludge. Chemosphere 2011, 84, 1250–1255. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef]
- Siddiqui, M.F.; Rzechowicz, M.; Harvey, W.; Zularisam, A.W.; Anthony, G.F. Quorum sensing based membrane biofouling control for water treatment: A review. J. Water Process Eng. 2015, 7, 112–122. [Google Scholar] [CrossRef]
- Muras, A.; Parga, A.; Mayer, C.; Otero, A. Use of Quorum Sensing Inhibition Strategies to Control Microfouling. Mar. Drugs 2021, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.-Y.; Kim, H.-S.; Cha, E.; Park, J.-H.; Park, H.-D. Mitigation of membrane biofouling by a quorum quenching bacterium for membrane bioreactors. Bioresour. Technol. 2018, 258, 220–226. [Google Scholar] [CrossRef]
- Bouayed, N.; Dietrich, N.; Lafforgue, C.; Lee, C.-H.; Guigui, C. Process-Oriented Review of Bacterial Quorum Quenching for Membrane Biofouling Mitigation in Membrane Bioreactors (MBRs). Membranes 2016, 6, 52. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.-P.; Luo, H.-W.; Li, W.-W.; Wang, L.-F.; Yu, H.-Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Wan Daud, W.R.; Ismail, M.; Rahimnejad, M.; Ismail, A.F.; Leong, J.X.; Miskan, M.; Ben Liew, K. Effect of pre-treatment and biofouling of proton exchange membrane on microbial fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 5480–5484. [Google Scholar] [CrossRef]
- Xia, L.; Vemuri, B.; Saptoka, S.; Shrestha, N.; Chilkoor, G.; Kilduff, J.; Gadhamshetty, V. Antifouling Membranes for Bioelectrochemistry Applications. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 195–224. [Google Scholar]
- Tawalbeh, M.; Qalyoubi, L.; Al-Othman, A.; Qasim, M.; Shirazi, M. Insights on the development of enhanced antifouling reverse osmosis membranes: Industrial applications and challenges. Desalination 2023, 553, 116460. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The behavior of suspensions and macromolecular solutions in crossflow microfiltration. J. Memb. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Zuo, K.; Chen, M.; Liu, F.; Xiao, K.; Zuo, J.; Cao, X.; Zhang, X.; Liang, P.; Huang, X. Coupling microfiltration membrane with biocathode microbial desalination cell enhances advanced purification and long-term stability for treatment of domestic wastewater. J. Memb. Sci. 2018, 547, 34–42. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Kunteng, D.; Veerman, J.; Saakes, M.; Nijmeijer, K. Periodic Feedwater Reversal and Air Sparging As Antifouling Strategies in Reverse Electrodialysis. Environ. Sci. Technol. 2014, 48, 3065–3073. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, H.; Kilduff, J.E.; Langer, R.; Anderson, D.G.; Belfort, G. High-throughput membrane surface modification to control NOM fouling. Environ. Sci. Technol. 2009, 43, 3865–3871. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Aljaghoub, H.; Qasim, M.; Al-Othman, A. Surface modification techniques of membranes to improve their antifouling characteristics: Recent advancements and developments. Front. Chem. Sci. Eng. 2023, 17, 1837–1865. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Villalobos García, J.; Dow, N.; Milne, N.; Zhang, J.; Naidoo, L.; Gray, S.; Duke, M. Membrane Distillation Trial on Textile Wastewater Containing Surfactants Using Hydrophobic and Hydrophilic-Coated Polytetrafluoroethylene (PTFE) Membranes. Membranes 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; De Rosset, A.; Tyszkiewicz, N.; Widera, B.; Greenman, J.; Ieropoulos, I. Prevention and removal of membrane and separator biofouling in bioelectrochemical systems: A comprehensive review. iScience 2022, 25, 104510. [Google Scholar] [CrossRef]
- Nasruddin, N.I.S.M.; Abu Bakar, M.H. Mitigating membrane biofouling in biofuel cell system-A review. Open Chem. 2021, 19, 1202–1215. [Google Scholar] [CrossRef]
- Koók, L.; Bakonyi, P.; Harnisch, F.; Kretzschmar, J.; Chae, K.-J.; Zhen, G.; Kumar, G.; Rózsenberszki, T.; Tóth, G.; Nemestóthy, N.; et al. Biofouling of membranes in microbial electrochemical technologies: Causes, characterization methods and mitigation strategies. Bioresour. Technol. 2019, 279, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Desmond, P.; Huisman, K.T.; Sanawar, H.; Farhat, N.M.; Traber, J.; Fridjonsson, E.O.; Johns, M.L.; Flemming, H.-C.; Picioreanu, C.; Vrouwenvelder, J.S. Controlling the hydraulic resistance of membrane biofilms by engineering biofilm physical structure. Water Res. 2022, 210, 118031. [Google Scholar] [CrossRef] [PubMed]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef]
- Fan, L.; Shi, J.; Xi, Y. PVDF-Modified Nafion Membrane for Improved Performance of MFC. Membranes 2020, 10, 185. [Google Scholar] [CrossRef]
- Noori, M.T.; Ghangrekar, M.M.; Mukherjee, C.K.; Min, B. Biofouling effects on the performance of microbial fuel cells and recent advances in biotechnological and chemical strategies for mitigation. Biotechnol. Adv. 2019, 37, 107420. [Google Scholar] [CrossRef]
- Flimban, S.G.A.; Hassan, S.H.A.; Rahman, M.M.; Oh, S.E. The effect of Nafion membrane fouling on the power generation of a microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 13643–13651. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Carmona, F.J.; Alonso, R.M.; Prádanos, P.; Morán, A.; Escapa, A. Assessing the ageing process of cation exchange membranes in bioelectrochemical systems. Int. J. Hydrogen Energy 2019, 44, 25287–25296. [Google Scholar] [CrossRef]
- Inamuddin; Shakeel, N.; Imran Ahamed, M.; Kanchi, S.; Abbas Kashmery, H. Green synthesis of ZnO nanoparticles decorated on polyindole functionalized-MCNTs and used as anode material for enzymatic biofuel cell applications. Sci. Rep. 2020, 10, 5052. [Google Scholar] [CrossRef]
- Sigwadi, R.; Mokrani, T.; Dhlamini, M.S.; Nonjola, P.; Msomi, P.F. Nafion®/ sulfated zirconia oxide-nanocomposite membrane: The effects of ammonia sulfate on fuel permeability. J. Polym. Res. 2019, 26, 108. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Sotres, A.; Alonso, R.M.; Díaz-Marcos, J.; Morán, A.; Escapa, A. Assessing anodic microbial populations and membrane ageing in a pilot microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 17304–17315. [Google Scholar] [CrossRef]
- Haupt, D.R.; Landwehr, L.; Schumann, R.; Hahn, L.; Issa, M.; Coskun, C.; Kunz, U.; Sievers, M. A New Reactor Concept for Single-Chamber Microbial Fuel Cells and Possible Anti-Fouling Strategies for Long-Term Operation. Microorganisms 2022, 10, 2421. [Google Scholar] [CrossRef]
- Wang, C.; Yang, F.; Meng, F.; Zhang, H.; Xue, Y.; Fu, G. High flux and antifouling filtration membrane based on non-woven fabric with chitosan coating for membrane bioreactors. Bioresour. Technol. 2010, 101, 5469–5474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shi, Q.; Luan, S.; Song, L.; Yang, H.; Shi, H.; Jin, J.; Li, X.; Yin, J.; Stagnaro, P. Improved biocompatibility and antifouling property of polypropylene non-woven fabric membrane by surface grafting zwitterionic polymer. J. Memb. Sci. 2011, 369, 5–12. [Google Scholar] [CrossRef]
- Pasternak, G.; Ormeno-Cano, N.; Rutkowski, P. Recycled waste polypropylene composite ceramic membranes for extended lifetime of microbial fuel cells. Chem. Eng. J. 2021, 425, 130707. [Google Scholar] [CrossRef]
- Luthfiana, A.; Mulyati, S.; Arahman, N.; Bilad, M.R.; Aulia, M.P. Cigarette butt filter as membrane material with tannic acid and FeCl3 additives for improve antifouling properties. Case Stud. Chem. Environ. Eng. 2025, 11, 101105. [Google Scholar] [CrossRef]
- Sun, B.; Pan, X.; Tian, Y.; Bi, W.; Feng, M.; Liu, F.; Hou, Q. Enhancement of electrochemical performance in PVDF-co-HFP cation exchange membrane with modifications by doping PP13-TFSI ionic liquid and sulfonation. Environ. Technol. Innov. 2025, 37, 103968. [Google Scholar] [CrossRef]
- Solomon, J.; Dharmalingam, S. Phosphomolybdic acid functionalized nano silica for enhanced anti-biofouling effect in polymer electrolyte membranes for microbial fuel cell application. Process Saf. Environ. Prot. 2024, 191, 942–958. [Google Scholar] [CrossRef]
- Colantoni, S.; Pillot, G.; Cvoro, S.; Kerzenmacher, S.; Santiago, Ó. Evaluation of separators for potential use in microbial electrolysis cells under anaerobic digester conditions. J. Memb. Sci. 2025, 722, 123887. [Google Scholar] [CrossRef]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Kurniawan, T.A.; Puteh, M.H.; Jaafar, J.; Rahman, M.A.; Ismail, A.F.; Rajamohan, N.; Abdullah, H.; et al. Recent strategies for enhancing the performance and lifespan of low-cost ceramic membranes in water filtration and treatment processes: A review. J. Water Process Eng. 2024, 62, 105399. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Kwon, Y. Perovskite ceramic membrane separator with improved biofouling resistance for yeast-based microbial fuel cells. J. Memb. Sci. 2020, 599, 117843. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bakonyi, P.; Göllei, A.; Rózsenberszki, T.; Takács, P.; Salekovics, A.; Kumar, G.; Bélafi-Bakó, K. On the efficiency of dual-chamber biocatalytic electrochemical cells applying membrane separators prepared with imidazolium-type ionic liquids containing [NTf2]− and [PF6]− anions. Chem. Eng. J. 2017, 324, 296–302. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Pérez de los Ríos, A.; Mateo-Ramírez, F.; Godínez, C.; Lozano-Blanco, L.J.; Moreno, J.I.; Tomás-Alonso, F. New application of supported ionic liquids membranes as proton exchange membranes in microbial fuel cell for waste water treatment. Chem. Eng. J. 2015, 279, 115–119. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef]
- Grzybek, P.; Dudek, G.; van der Bruggen, B. Cellulose-based films and membranes: A comprehensive review on preparation and applications. Chem. Eng. J. 2024, 495, 153500. [Google Scholar] [CrossRef]
- He, S.; Kamio, E.; Zhang, J.; Matsuoka, A.; Nakagawa, K.; Yoshioka, T.; Matsuyama, H. Development of an ion gel membrane containing a CO2-philic ionic liquid in interpenetrating semi-crystalline and crosslinkable polymer networks. J. Memb. Sci. 2023, 685, 121912. [Google Scholar] [CrossRef]
- Szakács, S.; Martínez, E.O.; Koók, L.; Santos, G.M.; Alarcon, J.T.; Jeison, D.; Pientka, Z.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Biofouling-focused assessment of a novel, cellulose-based ionogel membrane applied in a microbial fuel cell. Bioresour. Technol. Rep. 2024, 26, 101817. [Google Scholar] [CrossRef]
- Tomar, R.; Chandra, S.; Pandit, S.; Shahid, M.; Sharma, K.; Raj, S.; Geetha, S.J.; Joshi, S.J. Novel ionic liquid-infused PVA-based anion exchange membranes boosting bioelectricity yield from microbial fuel cells. Heliyon 2025, 11, e41426. [Google Scholar] [CrossRef]
- Koók, L.; Žitka, J.; Szakács, S.; Rózsenberszki, T.; Otmar, M.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Efficiency, operational stability and biofouling of novel sulfomethylated polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene cation exchange membrane in microbial fuel cells. Bioresour. Technol. 2021, 333, 125153. [Google Scholar] [CrossRef]
- Nagar, H.; Badhrachalam, N.; Rao, V.V.B.; Sridhar, S. A novel microbial fuel cell incorporated with polyvinylchloride/4A zeolite composite membrane for kitchen wastewater reclamation and power generation. Mater. Chem. Phys. 2019, 224, 175–185. [Google Scholar] [CrossRef]
- Ben Liew, K.; Leong, J.X.; Wan Daud, W.R.; Ahmad, A.; Hwang, J.J.; Wu, W. Incorporation of silver graphene oxide and graphene oxide nanoparticles in sulfonated polyether ether ketone membrane for power generation in microbial fuel cell. J. Power Sources 2020, 449, 227490. [Google Scholar] [CrossRef]
- Solomon, J.; Dharmalingam, S. Modified polymer electrolyte membrane for microbial fuel cell: Performance analysis, investigation on anti-biofouling effect, and microbial community analysis on biofouled membrane. J. Power Sources 2023, 580, 233452. [Google Scholar] [CrossRef]
- Shirvani, B.; Rahimi, M.; Zinadini, S. High electricity generation and wastewater treatment enhancement using a microbial fuel cell equipped with conductive and anti-biofouling CuGNSs/SPES proton exchange membrane. Energy Convers. Manag. 2023, 294, 117559. [Google Scholar] [CrossRef]
- Asadollahi, M.; Bastani, D.; Musavi, S.A. Enhancement of surface properties and performance of reverse osmosis membranes after surface modification: A review. Desalination 2017, 420, 330–383. [Google Scholar] [CrossRef]
- Hosseini, Z.; Kargari, A. Fabrication and characterization of hydrophobic/hydrophilic dual-layer polyphenyl sulfone Janus membrane for application in direct contact membrane distillation. Desalination 2024, 571, 117100. [Google Scholar] [CrossRef]
- Pupiales, H.; Soria, R.B.; Arboleda, D.; Cevallos, C.; Alcívar, C.; Francis, L.; Xu, X.; Luis, P. ZIF-8/Chitosan Composite Hydrogel as a High-Performance Separator for Bioelectrochemical Systems. Membranes 2025, 15, 282. [Google Scholar] [CrossRef] [PubMed]
- Alvear Méndez, S.; Soria, R.B.; Arboleda, D.; Cevallos, C.; Alcívar, C.; Jimenez, Y.; Teran, R.; Pupiales, H.; Luis, P. Influence of Er- and Al-Doped ZnO on Mixed-Matrix Membranes of Chitosan Derivatives in Bioelectrochemical Systems. Molecules 2025, 30, 3759. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Q.; Zhu, X.; Wu, Q.; Zheng, T.; Yuan, H.; Zhou, Z. The preparation of anti-fouling dual-layer composite membrane with embedding graphene oxide. Asia-Pac. J. Chem. Eng. 2023, 18, e2949. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Noori, M.T.; Tiwari, B.R.; Mukherjee, C.K.; Ghangrekar, M.M. Enhancing the performance of microbial fuel cell using Ag Pt bimetallic alloy as cathode catalyst and anti-biofouling agent. Int. J. Hydrogen Energy 2018, 43, 19650–19660. [Google Scholar] [CrossRef]
- Park, S.G.; Rajesh, P.P.; Hwang, M.H.; Chu, K.H.; Cho, S.; Chae, K.J. Long-term effects of anti-biofouling proton exchange membrane using silver nanoparticles and polydopamine on the performance of microbial electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 11345–11356. [Google Scholar] [CrossRef]
- Kugarajah, V.; Dharmalingam, S. Effect of silver incorporated sulphonated poly ether ether ketone membranes on microbial fuel cell performance and microbial community analysis. Chem. Eng. J. 2021, 415, 128961. [Google Scholar] [CrossRef]
- Ping, M.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Surface modification of polyvinylidene fluoride membrane by atom-transfer radical-polymerization of quaternary ammonium compound for mitigating biofouling. J. Memb. Sci. 2019, 570–571, 286–293. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, X.; Wang, Z.; Wang, L.; Wu, Z. QAC modified PVDF membranes: Antibiofouling performance, mechanisms, and effects on microbial communities in an MBR treating municipal wastewater. Water Res. 2017, 120, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, P.; Zhang, Q.; Xiao, H.; Zheng, H.; Li, L.; Cao, Z.; Zhang, M.; Ma, C.; Xie, G.; et al. Anti-adhesive and antimicrobial coatings on ultrafiltration membranes via dopamine-assisted co-deposition of zwitterionic-cationic copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2025, 726, 137774. [Google Scholar] [CrossRef]
- Ng, M.K.; Mont, M.A.; Bonutti, P.M. Clinical and Environmental Harms of Quaternary Ammonium Disinfectants and the Promise of Ultraviolet-C (UV-C) Alternatives: A Narrative Review. Cureus 2025, 17, e84022. [Google Scholar] [CrossRef] [PubMed]
- EPA. Drinking Water Regulations and Contaminants. Safe Drinking Water Act. Available online: https://www.epa.gov/sdwa/drinking-water-regulations-and-contaminants (accessed on 1 October 2025).
- Khanzada, N.K.; Al-Juboori, R.A.; Khatri, M.; Ahmed, F.E.; Ibrahim, Y.; Hilal, N. Sustainability in Membrane Technology: Membrane Recycling and Fabrication Using Recycled Waste. Membranes 2024, 14, 52. [Google Scholar] [CrossRef]
- Ranganathan, H.; Vinothkannan, M.; Kim, A.R.; Subramanian, V.; Oh, M.S.; Yoo, D.J. Simultaneous improvement of power density and durability of sulfonated poly(ether ether ketone) membrane by embedding CeO2-ATiO2: A comprehensive study in low humidity proton exchange membrane fuel cells. Int. J. Energy Res. 2022, 46, 9041–9057. [Google Scholar] [CrossRef]
- Zhao, N.; Meng, S.; Li, X.; Liu, H.; Liang, D. Enhancing proton transport in polyvinylidenedifluoride membranes and reducing biofouling for improved hydrogen production in microbial electrolysis cells. Bioresour. Technol. 2024, 402, 130842. [Google Scholar] [CrossRef]
- Koók, L.; Žitka, J.; Bakonyi, P.; Takács, P.; Pavlovec, L.; Otmar, M.; Kurdi, R.; Bélafi-Bakó, K.; Nemestóthy, N. Electrochemical and microbiological insights into the use of 1,4-diazabicyclo[2.2.2]octane-functionalized anion exchange membrane in microbial fuel cell: A benchmarking study with Nafion. Sep. Purif. Technol. 2020, 237, 116478. [Google Scholar] [CrossRef]
- Solomon, J.; Ganesh, N.; Sundaram, C.M.; Ravichandran, S.; Dharmalingam, S. Sulphonated graphene oxide as functionalized filler for polymer electrolyte membrane with enhanced anti-biofouling in microbial fuel cells. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 699, 134675. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Yin, G.; Du, K.; Jia, R.; Yu, X.; Wang, W.; Guo, J. Membrane fouling mitigation in Anoxic-Oxic membrane bioreactor with carbon nanotube/dopamine modification. J. Water Process Eng. 2025, 71, 107231. [Google Scholar] [CrossRef]
- Shen, Y.; Badireddy, A.R. A Critical Review on Electric Field-Assisted Membrane Processes: Implications for Fouling Control, Water Recovery, and Future Prospects. Membranes 2021, 11, 820. [Google Scholar] [CrossRef]
- Li, C.; Guo, X.; Wang, X.; Fan, S.; Zhou, Q.; Shao, H.; Hu, W.; Li, C.; Tong, L.; Kumar, R.R.; et al. Membrane fouling mitigation by coupling applied electric field in membrane system: Configuration, mechanism and performance. Electrochim. Acta 2018, 287, 124–134. [Google Scholar] [CrossRef]
- Dan Grossman, A.; Qi, S.; Aregawi Gebretsadkan, A.; Euni Beyioku, O.; Turkeltaub, T.; Shames, A.I.; Oren, Y.; Ronen, A.; Bernstein, R. Mechanism of mitigating organic fouling on an electro-conductive membrane under anaerobic conditions and cathodic operation. Appl. Surf. Sci. 2024, 654, 159473. [Google Scholar] [CrossRef]
- Qian, L.; Yuan, C.; Wang, X.; Zhang, H.; Du, L.; Wei, G.; Chen, S. Conductive MXene ultrafiltration membrane for improved antifouling ability and water quality under electrochemical assistance. RSC Adv. 2023, 13, 15872–15880. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.; Mukherjee, S.; Chakraborty, S. Electrophoretic motion of a non-uniformly charged particle in a viscoelastic medium in thin electrical double layer limit. J. Fluid Mech. 2021, 924, A41. [Google Scholar] [CrossRef]
- Hou, B.; Liu, X.; Zhang, R.; Li, Y.; Liu, P.; Lu, J. Investigation and evaluation of membrane fouling in a microbial fuel cell-membrane bioreactor systems (MFC-MBR). Sci. Total Environ. 2022, 814, 152569. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Cao, X.; Sakamaki, T.; Zhang, C.; Li, X. Study on the performance and mechanism of bio-electrochemical system to mitigate membrane fouling in bioreactors. Bioresour. Technol. 2022, 365, 128163. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Khair, M.; Hashaikeh, R.; Hilal, N. Electro-ceramic self-cleaning membranes for biofouling control and prevention in water treatment. Chem. Eng. J. 2021, 415, 128395. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Gao, T.; Wang, Y.; Teng, J.; Lu, M. Customized thin and loose cake layer to mitigate membrane fouling in an electro-assisted anaerobic forward osmosis membrane bioreactor (AnOMEBR). Sci. Total Environ. 2020, 729, 138663. [Google Scholar] [CrossRef]
- Yang, Y.; Qiao, S.; Jin, R.; Zhou, J.; Quan, X. Novel Anaerobic Electrochemical Membrane Bioreactor with a CNTs Hollow Fiber Membrane Cathode to Mitigate Membrane Fouling and Enhance Energy Recovery. Environ. Sci. Technol. 2019, 53, 1014–1021. [Google Scholar] [CrossRef]
- Sapireddy, V.; Ragab, A.; Katuri, K.P.; Yu, Y.; Lai, Z.; Li, E.; Thoroddsen, S.T.; Saikaly, P.E. Effect of specific cathode surface area on biofouling in an anaerobic electrochemical membrane bioreactor: Novel insights using high-speed video camera. J. Memb. Sci. 2019, 577, 176–183. [Google Scholar] [CrossRef]
- Song, J.; Yin, Y.; Li, Y.; Gao, Y.; Liu, Y. In-situ membrane fouling control by electrooxidation and microbial community in membrane electro-bioreactor treating aquaculture seawater. Bioresour. Technol. 2020, 314, 123701. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Hawari, A.; Baune, M.; Thöming, J. Dielectrophoretically intensified cross-flow membrane filtration. J. Memb. Sci. 2009, 336, 71–78. [Google Scholar] [CrossRef]
- Huotari, H.M.; Huisman, I.H.; Trägårdh, G. Electrically enhanced crossflow membrane filtration of oily waste water using the membrane as a cathode. J. Memb. Sci. 1999, 156, 49–60. [Google Scholar] [CrossRef]
- Bowen, W.R.; Kingdon, R.S.; Sabuni, H.A.M. Electrically enhanced separation processes: The basis of in situ intermittent electrolytic membrane cleaning (IIEMC) and in situ electrolytic membrane restoration (IEMR). J. Memb. Sci. 1989, 40, 219–229. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: The cost benefit of optimisation. Water Res. 2010, 44, 1389–1398. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, R.; Liu, X.; Li, Y.; Liu, P.; Lu, J. Study of membrane fouling mechanism during the phenol degradation in microbial fuel cell and membrane bioreactor coupling system. Bioresour. Technol. 2021, 338, 125504. [Google Scholar] [CrossRef]
- Halali, M.A.; Larocque, M.; de Lannoy, C.-F. Investigating the stability of electrically conductive membranes. J. Memb. Sci. 2021, 627, 119181. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Hu, W.; Ma, C.; Cui, C.; Wang, L. Mitigation of irreversible membrane biofouling by CNTs-PVDF conductive composite membrane. Environ. Res. 2025, 267, 120703. [Google Scholar] [CrossRef]
- Hu, J.; Cao, X.; Qu, L.; Khodseewong, S.; Zhang, S.; Sakamaki, T.; Li, X. Study on the mechanism of mitigating membrane fouling in MFC-AnMBR coupling system treating sodium and magnesium ion-containing wastewater. Environ. Technol. 2024, 45, 6210–6223. [Google Scholar] [CrossRef]
- Lei, J.; Yu, F.; Xie, H.; Ma, J. Ti3C2Tx MXene/carbon nanofiber multifunctional electrode for electrode ionization with antifouling activity. Chem. Sci. 2023, 14, 3610–3621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Duan, W.; Huang, Z.; Tian, L.; Wu, W.; Dang, Z.; Feng, C. An Anti-Scaling Strategy for Electrochemical Wastewater Treatment: Leveraging Tip-Enhanced Electric Fields. Environ. Sci. Technol. 2024, 58, 13145–13156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, D.; Zhao, Y.; Li, J.; Wang, J.; Wang, Y.; Wang, J.; Zhang, H.; Chen, L.; Zhang, M. Pressure-induced piezoelectric response for mitigating membrane fouling in surface water treatment: Insights from continuous operation and biofouling characterization. Water Res. 2025, 268, 122554. [Google Scholar] [CrossRef] [PubMed]
- Alhajar, A.; Tawalbeh, M.; Arjomand, D.; Rahman, N.A.; Khan, H.; Al-Othman, A. Chapter 14—Integrating forward osmosis into microbial fuel cells for wastewater treatment. In Integrated Environmental Technologies for Wastewater Treatment and Sustainable Development; Kumar, V., Kumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–336. ISBN 978-0-323-91180-1. [Google Scholar]
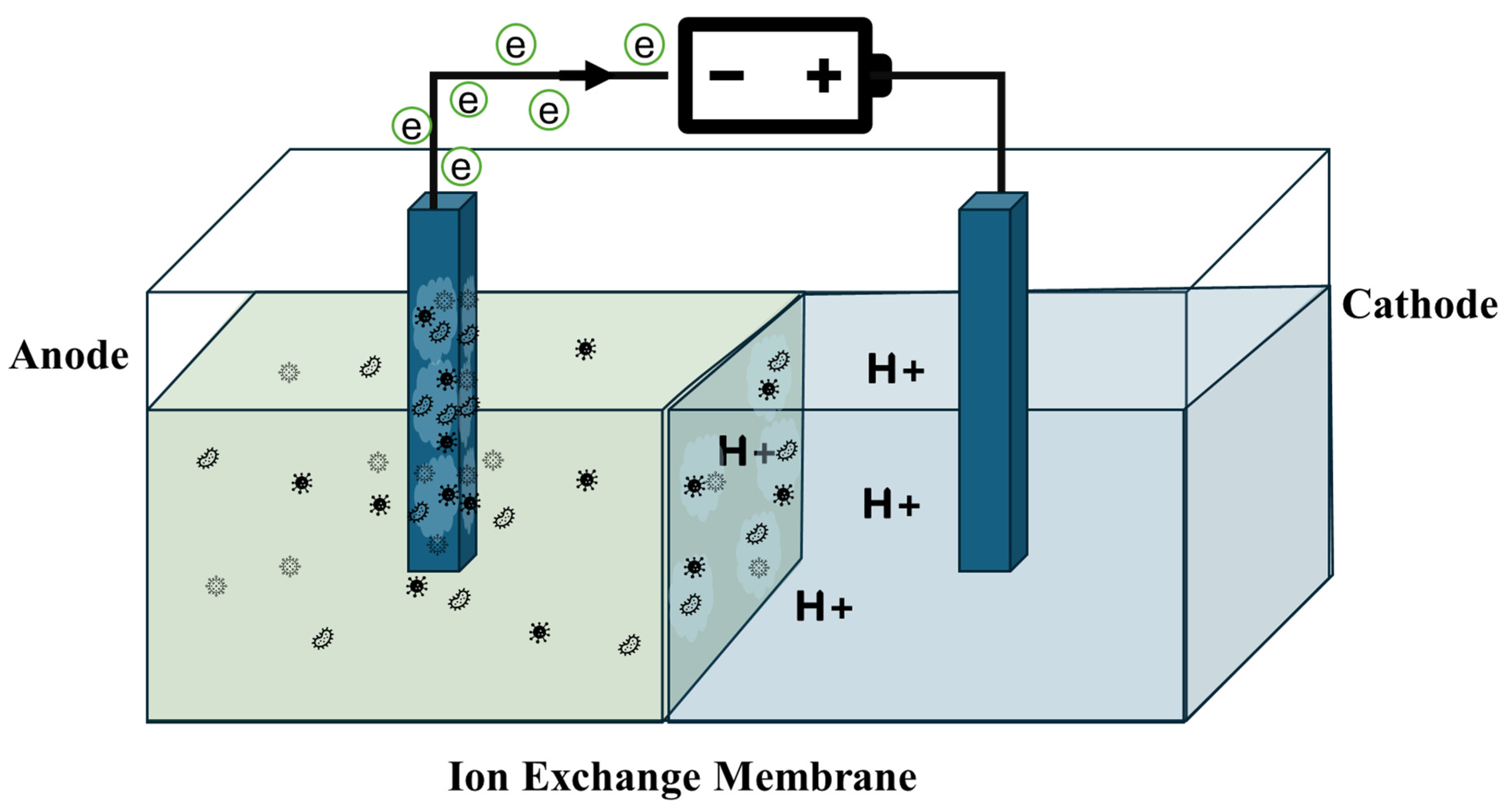
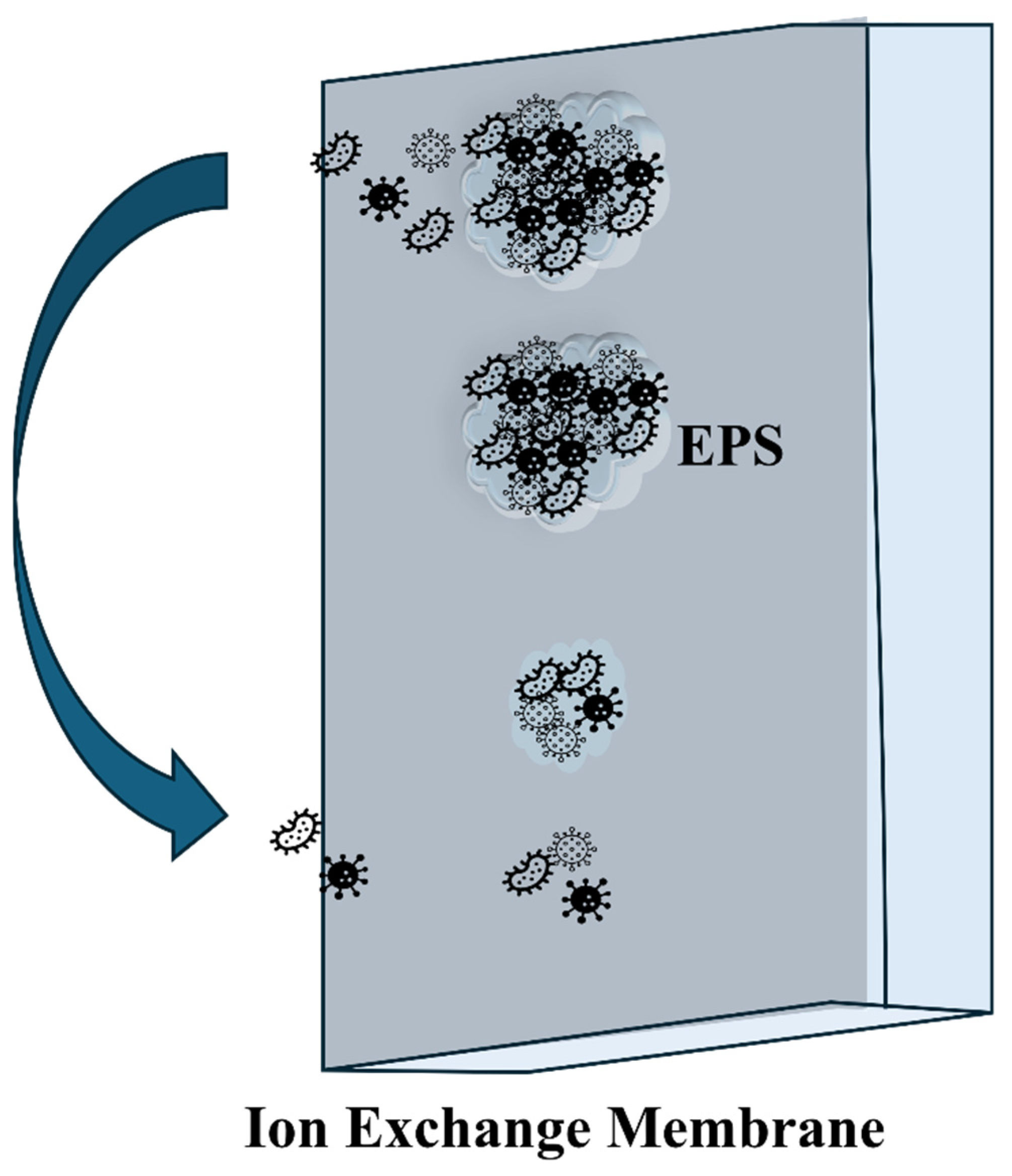
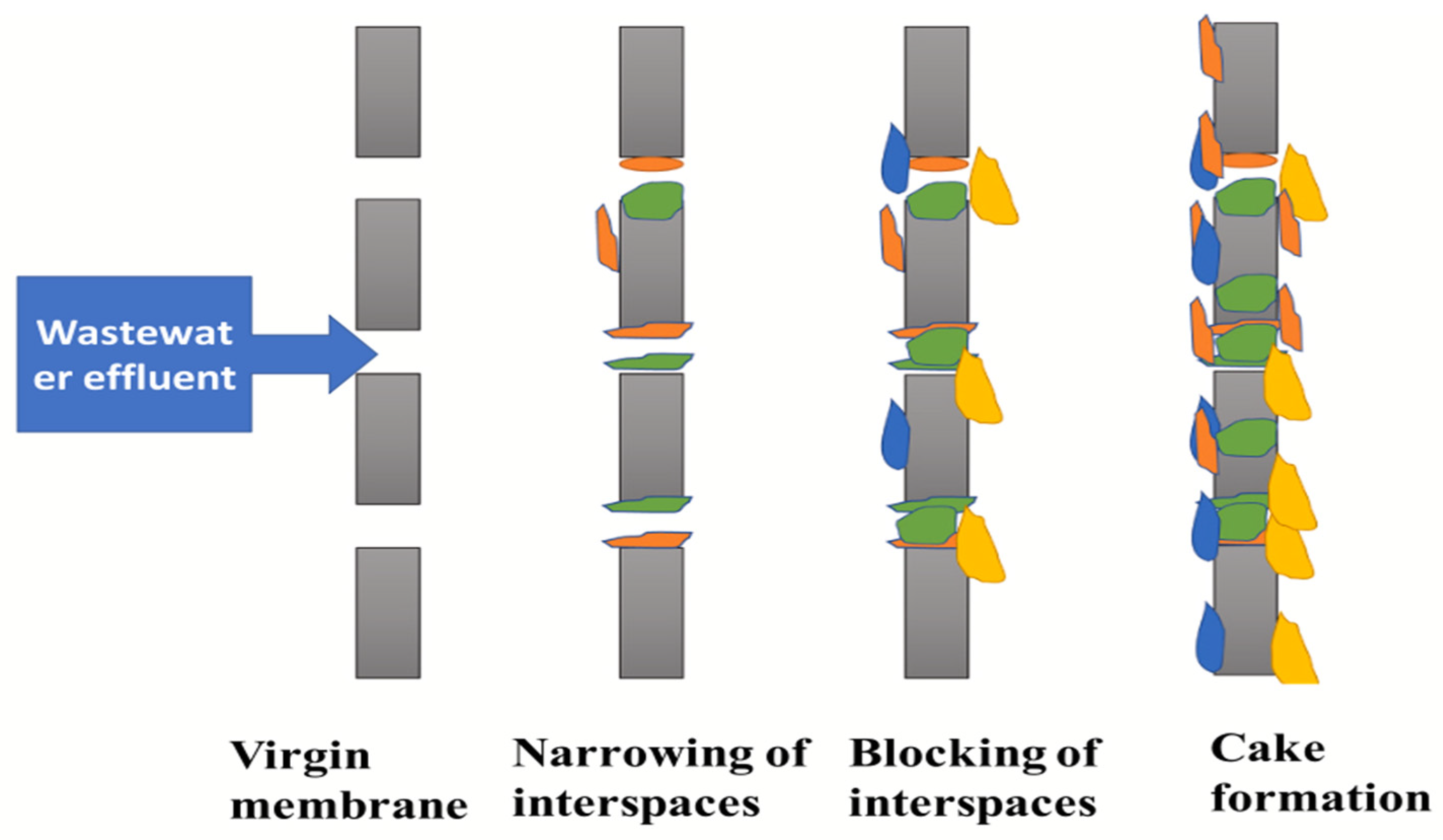
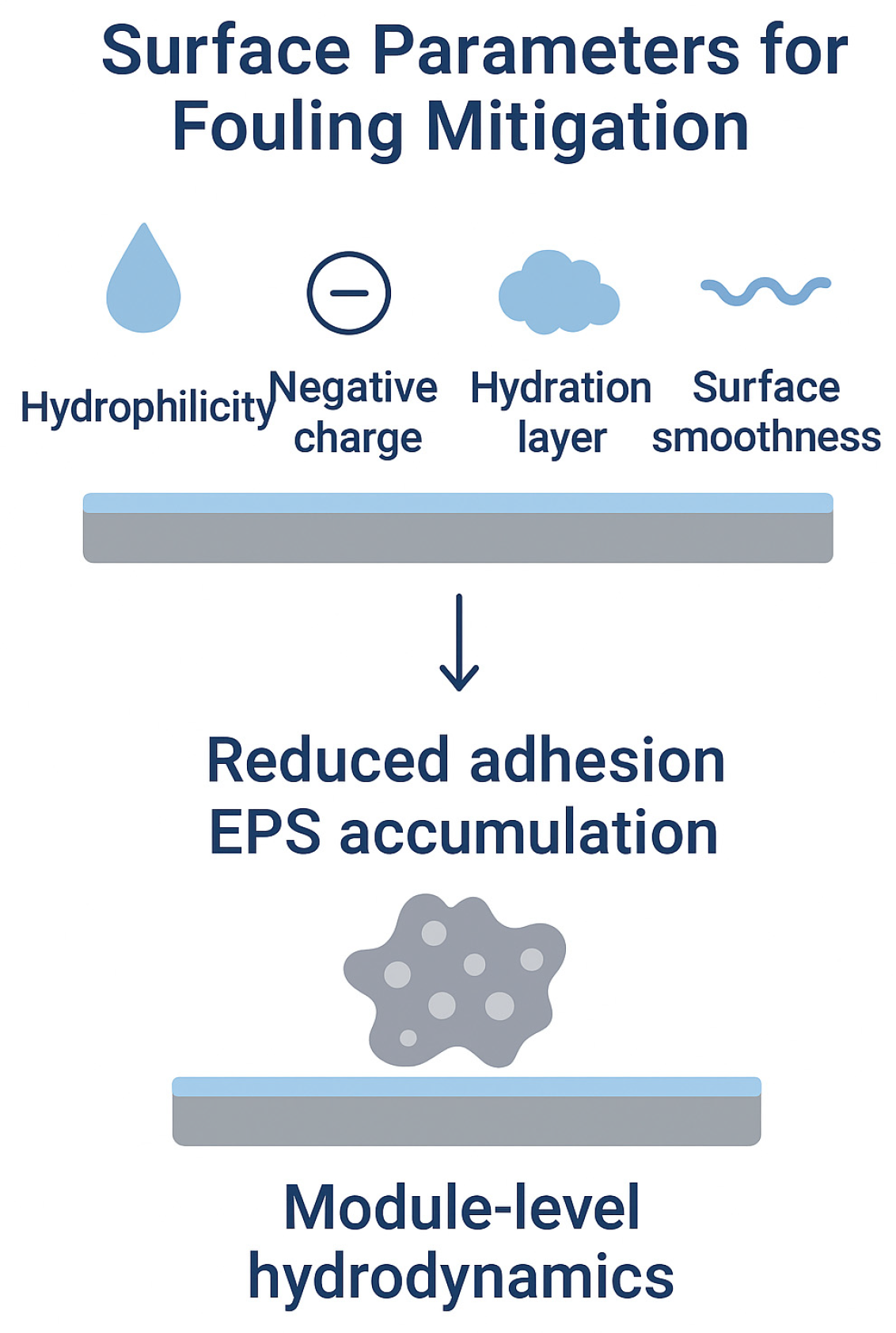

| Property | Structural Origin | Significance in BEC Applications | Refs. |
|---|---|---|---|
| High Proton Conductivity | Nanoscale hydrophilic domains form interconnected ion-conducting pathways upon hydration. | Enables efficient transport of protons (H+) across the membrane, which is essential for cell performance. | [23,24] |
| Excellent Chemical Stability | Highly inert PTFE backbone resists chemical attack. | Provides long-term durability in the harsh electrochemical environments. | [25] |
| Strong Acidity | Sulfonic acid groups stabilized in perfluorinated matrix | Enhances proton dissociation and conduction. | [26] |
| Mechanical Strength | PTFE backbone and phase-separated morphology | Maintains membrane integrity under stress | [25] |
| Selectivity | Phase-separated structure limits crossover of gases and ions | Reduces fuel/oxidant crossover, improving efficiency | [27] |
| Membrane | Cost (€ m−2) | Ion Exchange Capacity (IEC) (meq g−1) | Selectivity | Durability (Test Duration/Conditions) |
|---|---|---|---|---|
| Nafion 117 | 400 | 0.95–1.01 | - | 6 months at 30 °C, pH 7; 37% power loss due to fouling |
| CMI-7000 | 170 | 1.6 | >0.97% | 120 days; roughness increased, indicating fouling |
| Zirfon® | 45 | - | - | 120 days; minimal fouling and stable conductivity |
| FKB | 320 | 0.9–1.0 | >0.98% | 120 days; slow fouling progression |
| FKE | 195 | >1 | >0.98% | 120 days; stable mechanical and chemical resistance |
| Type of the Cell | Membrane Type | Maximum Power Density (mW/m2) | H2 Production | Main Idea | Biofouling Capabilities | Ref. |
|---|---|---|---|---|---|---|
| Tabular MFC | SPSEBS +6% SPOSS | 126 | Studying the biofilm formation on SPOSS-incorporated SPSEBS nanocomposites at different weight percentages of nano fillers (2–8%) | Biofouling results explain that the addition of nanofiller reduces the biofilm formation and increases the proton conductivity. | [13] | |
| Two-Chamber MEC | Nafion-117, CMI-7000, Zirfon UTP 500, FKE, and FKB | Constant power density of around 1000 | - | Studying mechanical, chemical, and electrochemical properties of five commercially available CEMs developed over four months in MEC. | Over four months, fouling increased membrane roughness, with CMI and Nafion rising from 7 nm to 26 nm and 23 nm, respectively, while Zirfon and FKB/FKE showed slower increases. | [64] |
| Direct Methanol Fuel Cell (DMFC) | Nafion®/S-ZrO2 (NH3SO4) and Nafion®/S-ZrO2 membranes | Maximum power density Nafion®/S-ZrO2 (NH3SO4): 1.83 × 106 and Nafion®/S-ZrO2: 1.58 × 106 | Modifying Nafion® membranes for fuel cells by incorporating sulfated zirconium compared to commercial Nafion® 117. | he Nafion®/S-ZrO2 (NH3SO4) membranes showed improved proton conductivity (7.891 S/cm), better hydrophilicity (contact angles reduced to 68°). | [66] | |
| Two-Chamber MEC | CMI7000 | 0.2 LH2/Lreactor·day with a purity always higher than 98% | Investigating the durability of cation exchange membrane (CEM) in MECs operated in fed-batch mode for a period of 78 days and used for hydrogen production and ammonia recovery from pig slurry. | The membrane’s Ion Exchange Capacity (IEC) dropped by up to 22% at the inlet and outlet, while biofouling caused increased surface roughness, especially in samples M1, M2, and M6. | [67] | |
| Single-Chamber MFC | (fumasep® FFFA-3-30, FUMATECH BWT GmbH, Bietigheim-Bissingen, Germany) | 200 | A membrane-electrode assembly was tested using an antibacterial membrane affixed to a gas diffusion electrode. | A thin biofilm developed on the electrode surface but was not cross-linked to the membrane, allowing for easier removal. | [68] | |
| Single-Chamber AC-MFC | PP-373 and PP-468 | 81 | - | Developing a ceramic membrane using recycled polypropylene on ceramic materials. | PP-373 maintained the lowest contact angle over time and neglectable weight gain after operation. | [71] |
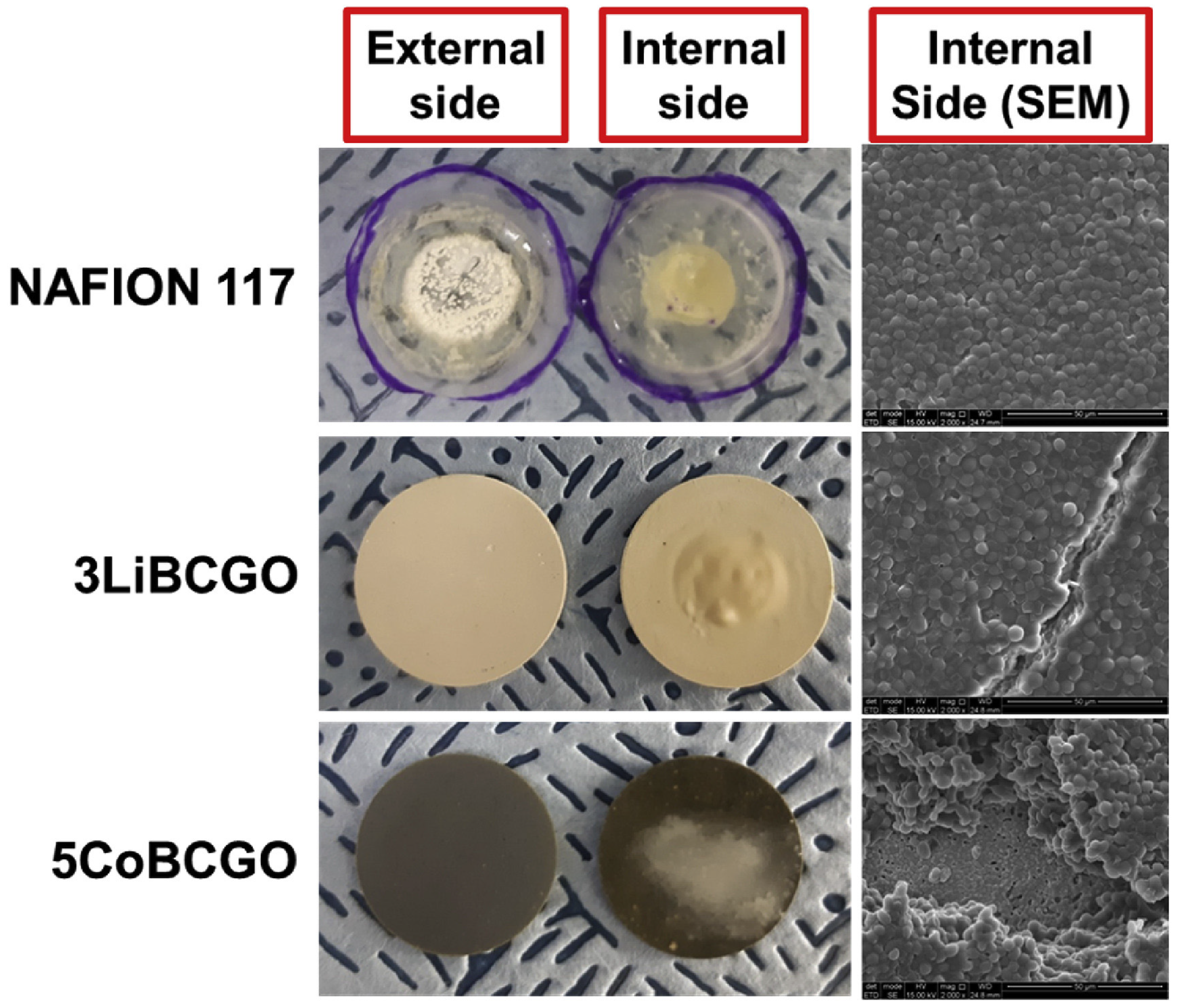
| MFC | BCGO, 3LiBCGO and 5CoBCGO | - | - | To dope BCGO (ceramic membrane) with two different materials (Co and Li). | Yeast cells are prevented from crossing the membranes with ions. 5CoBCGO had better biofouling mitigation capabilities with the thinnest thickness of biofilm. | [77] |
| Two-Chamber MFC | Bio-cellulose-[BMIM][Cl] | ~173 | Comparing ionogel membrane efficiency and biofilm behavior against a Nafion membrane, both equipped in an acetate-fed MFC. | The ionogel membrane boosted microbial growth but had higher biofouling and resistance, lowering MFC performance compared to Nafion. | [83] | |
| Two-Chamber MFC | (GO-SPEEK) and (AgGO-GO-SPEEK). | 1049 for AgGO-GO-SPEEK | - | GO enhances the mechanical structure of the polymeric membrane as well as the proton conductivity. Ag-Go is an antibacterial material. | AgGO-GO-SPEEK exhibits the lowest internal resistance with 23.83%. GO hydrophilicity and Ag antibacterial activity. | [87] |
| MFC | SPEEK/SSA + 1.5 wt.% CuO@rGO as membrane filler | 186 ± 5 | - | The SPEEK/SSA hydrophilicity and CuO@rGO antibacterial properties improved the biofouling mitigation. | The membrane had a biofilm thickness of 77 ± 0.6 micrometers and a contact angle of 34.6°. | [88] |
| Two-Chamber MFC | CuGNSs (2.0)/SPES | 84.1 | - | The use of CuGNSs as modifier agents in PEM, which were biosynthesized from plants. | The membrane obtained a contact angle of 59.7°, and a high negative zeta potential was obtained for PH higher than 5 (almost −70 mV for pH = 7.5) | [89] |
| Two-Chamber MFC | Zeolite 4A to the PVC matrix (PVC-Zeolite membranes) | 250 ± 5 at 340 mA/m2 current density. | - | The use of zeolite 4A hydrophilicity with the hydrophobicity of PVC matrix | Biofilm attachment was minimized due to antibacterial properties and high hydrophilicity of the composite membrane. | [86] |
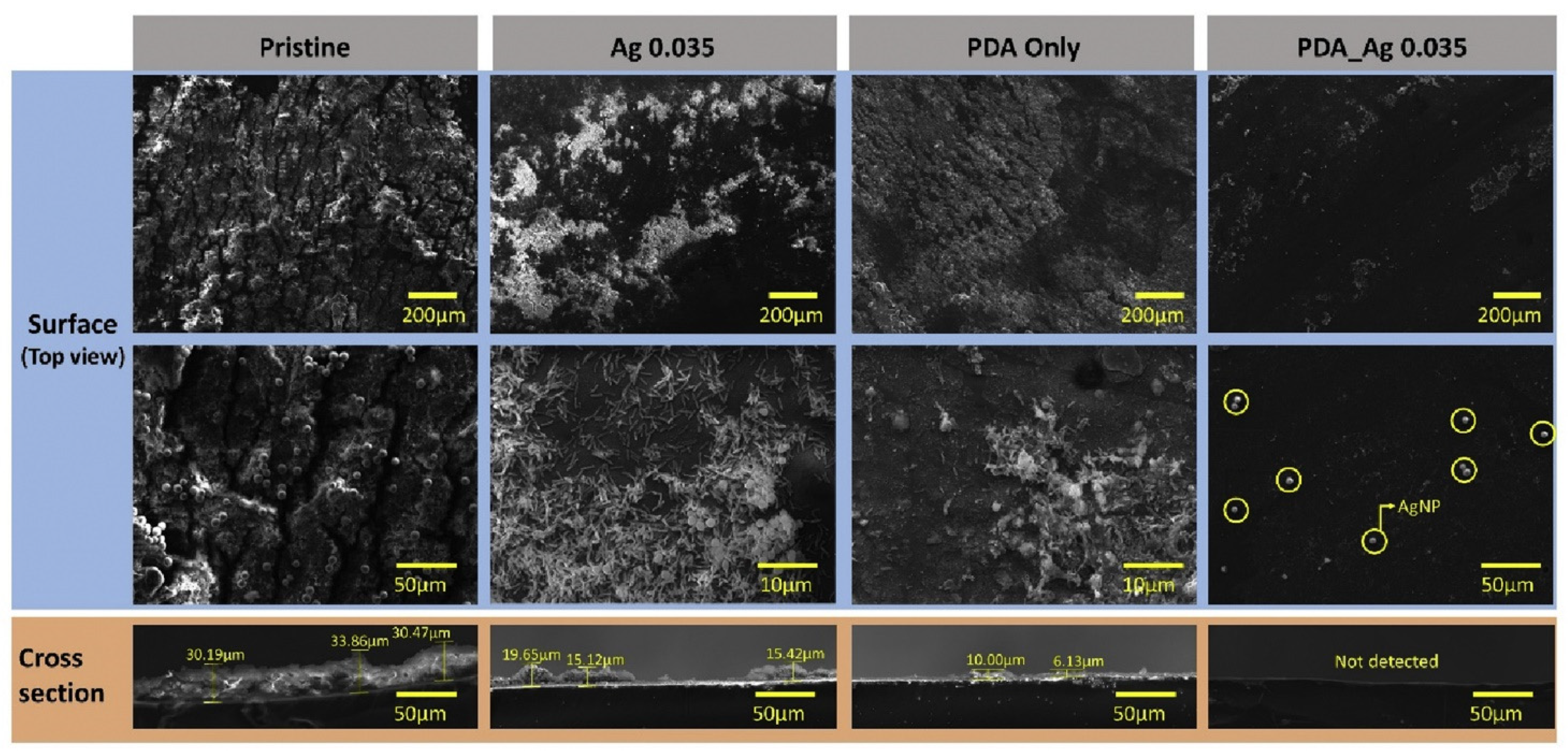
| Two-Chamber MEC | AgNP-PDA/SPAES/PIN | - | Hydrogen production maintained by a reduction of 31.88% | SPAES/PIN-PEM modified by surface functionalization with AgNP and PDA, multiple coating types and orders were investigated. | The biofilm formation was reduced by 80.74% relative to the pristine membrane. The contact angle of PDA-Ag 0.036 was 37.41°. | [97] |
| MFC | SPEEK + 7.5 wt.% Ag | 156 ± 0.5 | - | SPEEK with (2.5, 5, 7.5 or 10 wt.%) Ag additives was investigated. | The presence of biofilm formation was reduced. The addition of silver increased power density by mitigating biofouling over time. | [98] |
| MFC | SPEEK/CeO2-AtiO2 (2%) | Maximum power density: 1.17 × 106 at 100% Relative Humidity (RH) | - | The incorporation of (CeO2-ATiO2) as a bifunctional nanofiller i00n SPEEK to reduce cost and enhance performance. | Durability of the composite membrane was enhanced, OCV decay of 0.925 mV/h at T = 60 °C and RH = 30%. | [105] |
| Two-Chamber H-type MFC | Nafion 117 | Maximum power density of rice straw: 119.35 mW/m2 potato peels: 152.55 mW/m2 | - | The biofouling effect was investigated using SEM. | A reduction in power generation was observed after biofouling by 37%. Biofouling was highly detectable. | [63] |
| Osmotic Microbial Fuel Cell (OsMFC) | AgNP modified thin film composite (TFC) polyamide FO membrane | 3.67 W/m2 | - | Advanced hydrophilicity, more negative zeta potential and better antibacterial properties led to biofouling mitigation. | A decrease of 25% in the number of E. coli bacteria colonies was observed | [10] |
| Two-Chamber MFC | PSEBS SU22 PSEBS-based, sulfomethylated, CEM membrane | 149 mW/m2 at 595 mA/m−2 | - | Biofouling was mechanically removed and followed by chemical conditioning. | Minor differences could be observed between the fouling layers of Nafion 115 and PSEBS SU22 | [85] |
| Two-Chamber MEC | PDA-PEI-Ag@PVDF | 2.65 ± 0.02 m3/m3/d. An overall hydrogen recovery of 86–94%. | Improving hydrogen production in MEC by enhancing PVDF membranes with a PDA, PEI, and AgNP coating. | An improved hydrophilicity with a contact angle of 8.0 ± 1°. The membrane exhibited high antibacterial activity due to the formation of AgNPs in comparison to a pristine membrane. | [106] | |
| Two-Chamber MFC | PSEBS DABCO | Current Density: AEM: 400 mA/m2 at Acetate concentration = 5 mM. PEM: 285 mA/m2 at Acetate concentration = 5 mM. | - | Comparing the performance of PEM (Nafion) and AEM (PSEBS-DABCO), combining the physical properties of PSEBS and the chemical properties | AEM showed a lower internal resistance of 145 Ω, which was lower than PEM by 194 Ω. PEM thickness increased by 16%, whereas AEM thickness changes were negligible. | [107] |
| Two-Chamber MFC | SPEEK/SSA + 5% SGO | 203 mW/m2 | Investigates the development of SGO functionalized PEM for MFCs to enhance power generation and mitigate biofouling | 31.52% biofilm inhibition, enhanced hydrophilicity (33.5° water contact angle) | [108] | |
| Filtration cell/system | PDA/p(DM)-3-PVDF | - | - | Ultrafiltration PVDF membranes were coated with zwitterionic (MPC, CBMA) and cationic (DMC) copolymers for dual antifouling and antibacterial performance. | (PDA/p(DCD)-1-PVDF) achieved BSA rejection of more than 97%, flux recovery 84.78%, FDR = 9.57%, and 100% antibacterial efficiency. | [101] |
| - | W-CBF 2 | - | - | Recycled cigarette filter waste modified with tannic acid and FeCl3 coatings was used to develop a sustainable antifouling ultrafiltration membrane | Flux recovery ratios of 85% and 76%, with reduced irreversible fouling and increased reversible fouling. | [72] |
| Anaerobic/Oxic MBR | M-CNTs/PDA | - | - | CNTs/PDA- membrane was fabricated and systematically evaluated its performance in a continuous-flow A/O-MBR. | M-CNTs/PDA achieved high nitrogen removal efficiency (~85%), maintained a 30–40% higher permeability, and demonstrated stronger antifouling performance with over 70% flux recovery. | [109] |
| Four-Chamber Test Cell | ZIF-8/CS | Chitosan (CS), a cheap, biodegradable biopolymer, was combined with ZIF-8 to create a composite material. | High ionic conductivity of 0.099 S/cm, competing with Nafion-117 (0.13 S/cm), and only a 25.88% increase in surface roughness compared to Nafion’s 179.48% | [92] | ||
| Four-Chamber Test Cell | Chitosan-based membranes doped with Er/Al/ZnO | Fabricated and tested photocatalyst-enhanced chitosan membranes with Er/Al/ZnO improve ionic conductivity and resistance to fouling and biofouling | Surface resistance dropped f to 6.84 Ω·cm2 (MQ), 5.15 Ω·cm2 (MQ1), and 5.06 Ω·cm2 (MQ2), biofouling resistance improved from 13.09 v to 8.06 Ω·cm2, and Nafion fouled 10.21 more | [93] | ||
| MFC | Sulfonation + PP13-TFSI on PVDF-co-HFP | 325 mW/m2 | PVDF-co-HFP was modified via sulfonation and ionic liquid (PP13-TFSI) incorporation | Achieved 22.8% water uptake, 1.25 meq/g IEC, 10.83 mS/cm proton conductivity, 54.1° contact angle, and minimal performance loss over 5 cycles and a cost saving of $188–1388 per m2 | [73] | |
| MEC | PDMS-modified Cellophane | (PDMS)-modified Cellophane separator was tested for its low cost, effectively maintains a stable pH environment, and exhibits manageable energy loss | Costs only 10% of a commercial membrane, doubles the lifespan of plain Cellophane from 1 to 2 months, and effectively prevents pH imbalance like an AEM | [75] | ||
| Membrane Cathode Assembly (MCA) single Chamber MFC | PVA–NH4I–EMMIB | Investigated the development of cheap ionic liquid-doped PVA anion exchange membrane. | High ionic conductivity of 2.4 × 10−2 S/cm at the optimal loading, which is a 26-fold increase over the plain PVA membrane, and reducing cell biomass by over 60% compared to the control. | [84] | ||
| MFC | SPEEK/SSA + SPMA | 196.6 mW/m2 | Studied novel nanocomposite membrane (SPEEK/SSA with phosphomolybdic acid-functionalized silica. | Achieved proton conductivity of 3.75 × 10−2 S cm and a significant 67.52% biofilm inhibition. | [74] | |
| GO-PSF | Created a thin, top layer of GO mixed with polysulfone (PSF) on a standard PSF support. | Improved hydrophilicity (contact angle 47.29° vs. 75.9°), demonstrated superior antifouling performance (83% vs. 52% flux recovery), and offered excellent antibacterial properties. | [94] |
| Risk Factor | Description and Impact | Mitigation Strategies | Ref. |
|---|---|---|---|
| Electrode Corrosion | Electrochemical oxidation and reverse currents degrade electrodes, reducing lifespan. | Advanced electrode materials (like MXene/carbon nanofiber, corrosion-resistant coatings), periodic polarity reversal, and real-time monitoring. | [130] |
| By-Product Formation | Generation of free chlorine, mineral scales, or other undesirable compounds. | Physical barrier layers, optimized voltage/current control, selective catalysts, and electrolyte additives. | [131] |
| pH Drift | Water electrolysis causes local pH changes, affecting microbial inactivation and scaling. | Buffered electrolytes, careful voltage/current control, and system design to manage local pH. | [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyazouri, S.; Tawalbeh, M.; Al-Othman, A. Mitigation Techniques of Membranes’ Biofouling in Bioelectrochemical Cells (BEC Cells): Recent Advances. Membranes 2025, 15, 332. https://doi.org/10.3390/membranes15110332
Alyazouri S, Tawalbeh M, Al-Othman A. Mitigation Techniques of Membranes’ Biofouling in Bioelectrochemical Cells (BEC Cells): Recent Advances. Membranes. 2025; 15(11):332. https://doi.org/10.3390/membranes15110332
Chicago/Turabian StyleAlyazouri, Shatha, Muhammad Tawalbeh, and Amani Al-Othman. 2025. "Mitigation Techniques of Membranes’ Biofouling in Bioelectrochemical Cells (BEC Cells): Recent Advances" Membranes 15, no. 11: 332. https://doi.org/10.3390/membranes15110332
APA StyleAlyazouri, S., Tawalbeh, M., & Al-Othman, A. (2025). Mitigation Techniques of Membranes’ Biofouling in Bioelectrochemical Cells (BEC Cells): Recent Advances. Membranes, 15(11), 332. https://doi.org/10.3390/membranes15110332








