Current Progress in Advanced Functional Membranes for Water-Pollutant Removal: A Critical Review
Abstract
1. Introduction
2. General Concept of Advanced Functional Membranes
3. Metal–Organic Framework (MOF)-Based Membranes
3.1. MOF-Based Membranes in Water Purification
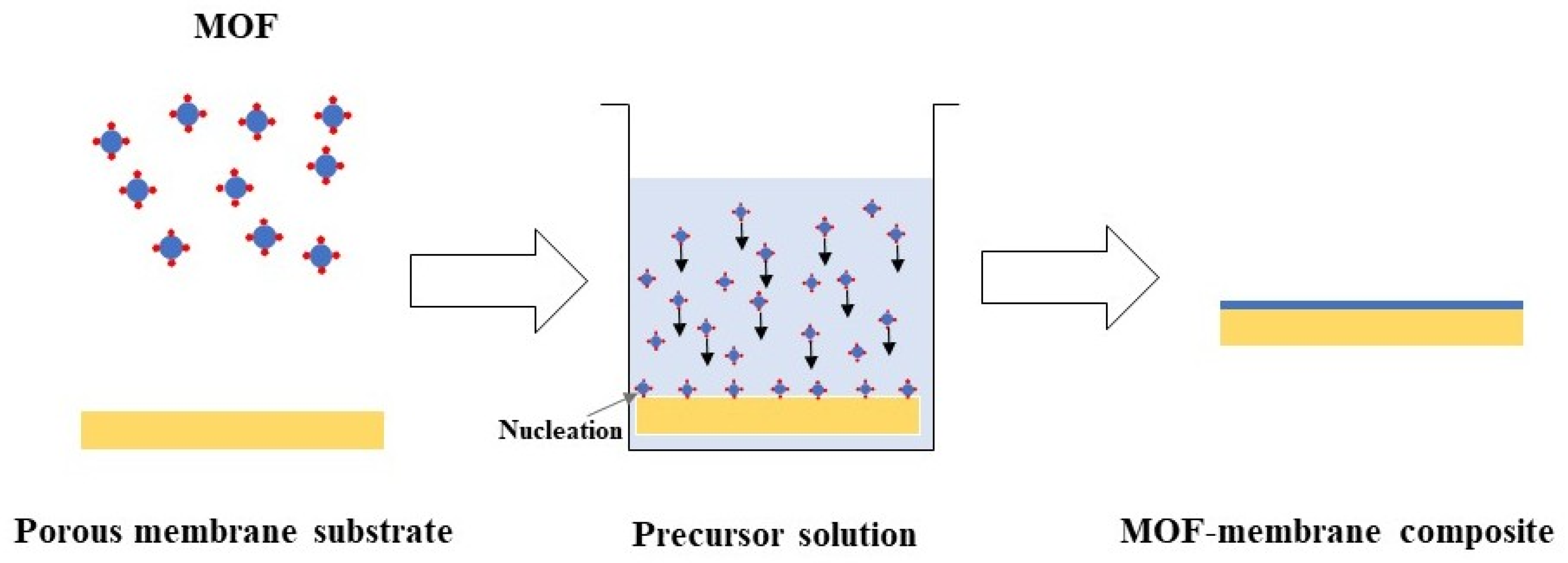
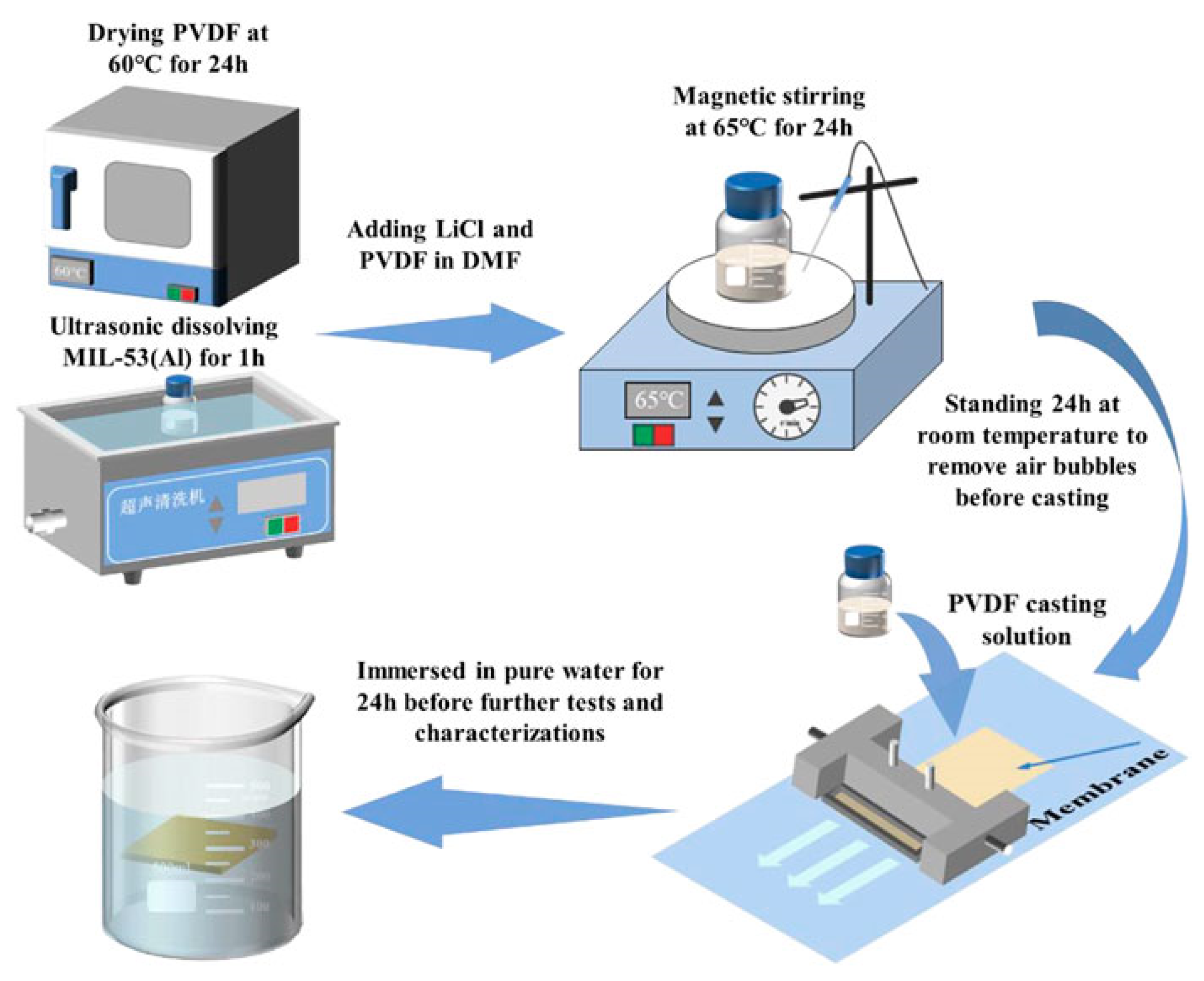
3.2. Synthesis Strategies of MOF-Based Membranes
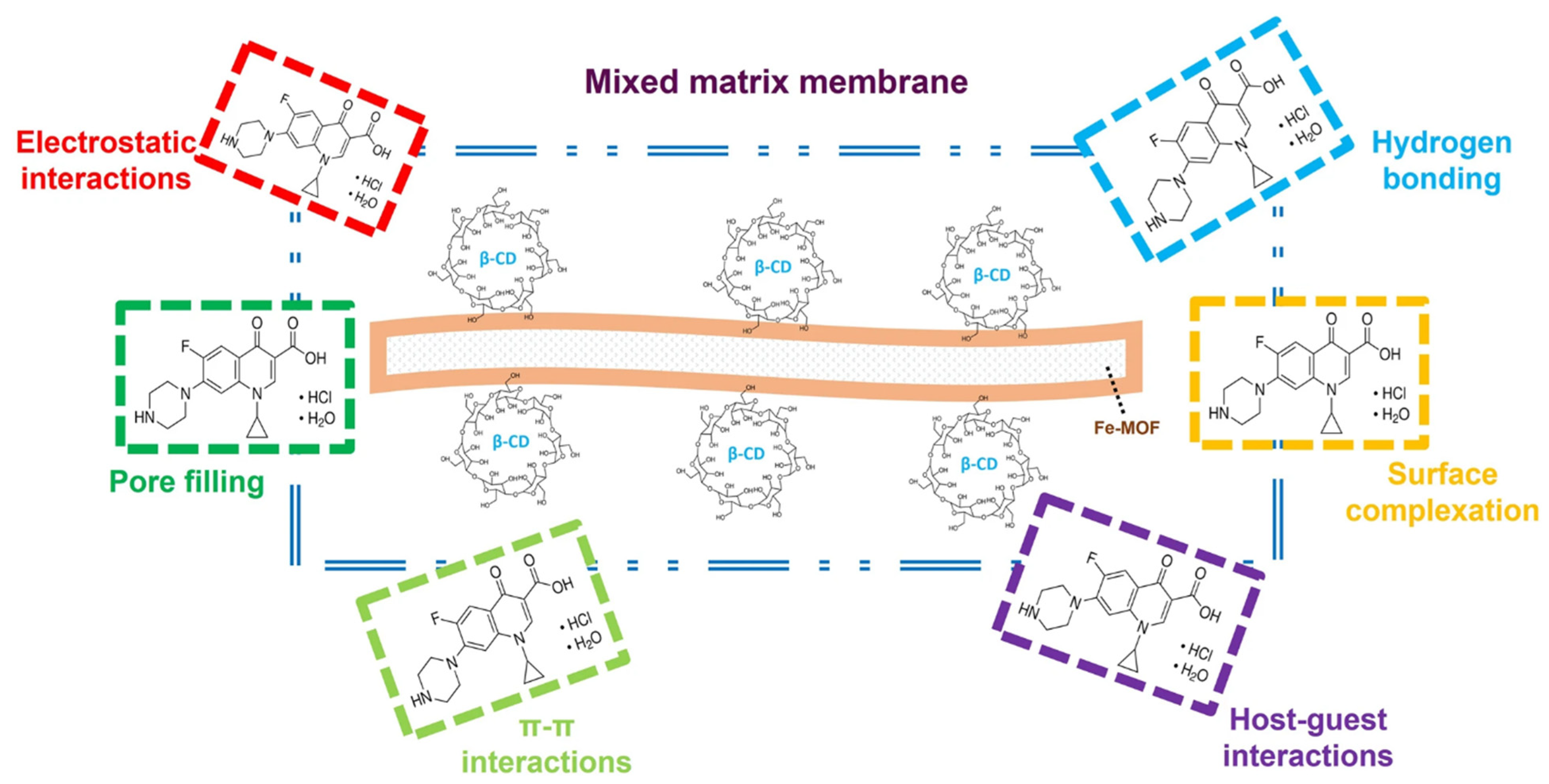
3.3. Pollutant Removal Mechanisms
3.3.1. Heavy Metals
3.3.2. Organic Dye Removal
3.3.3. Antibiotics and Pharmaceuticals
3.3.4. Per- and Polyfluoroalkyl Substances
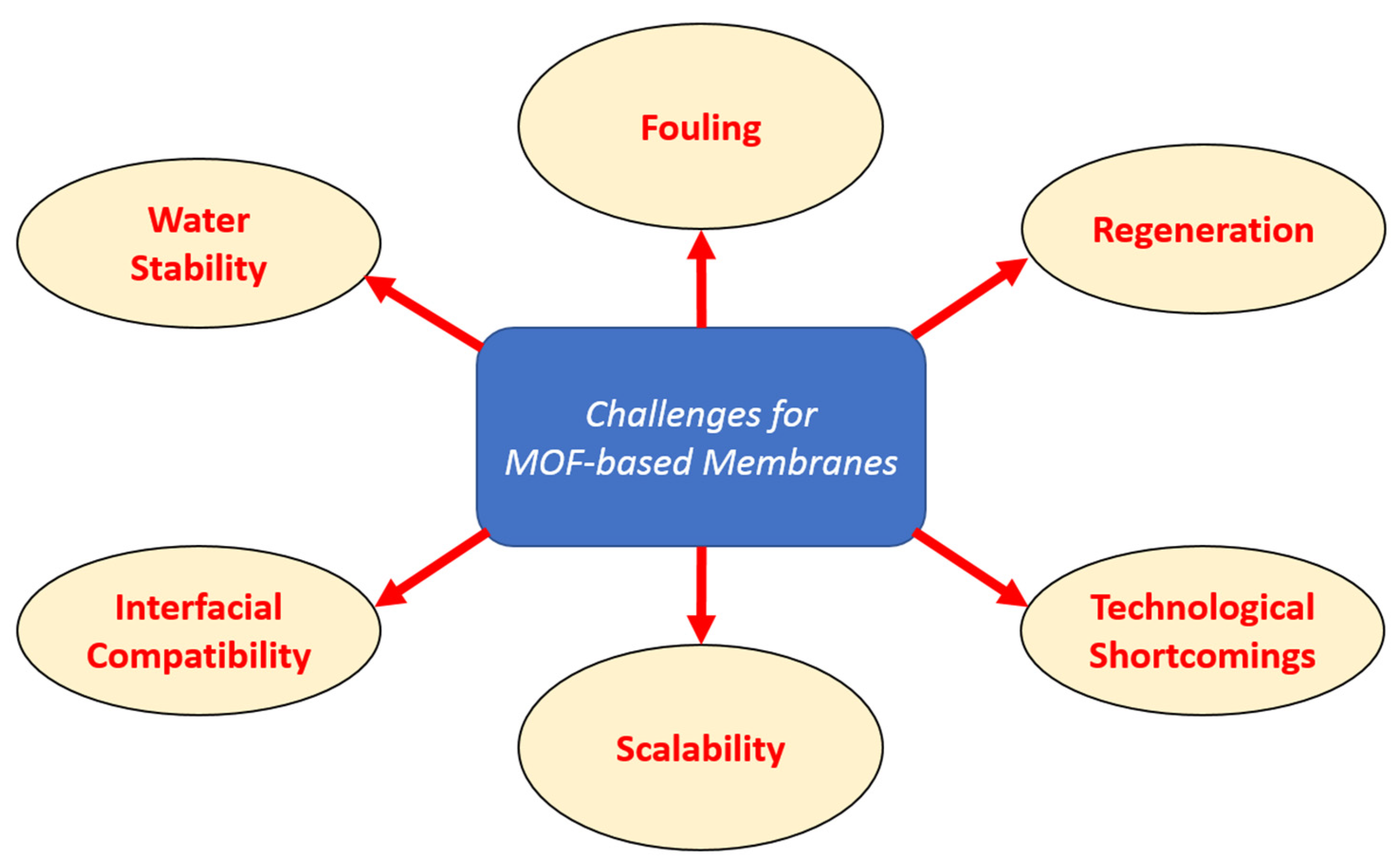
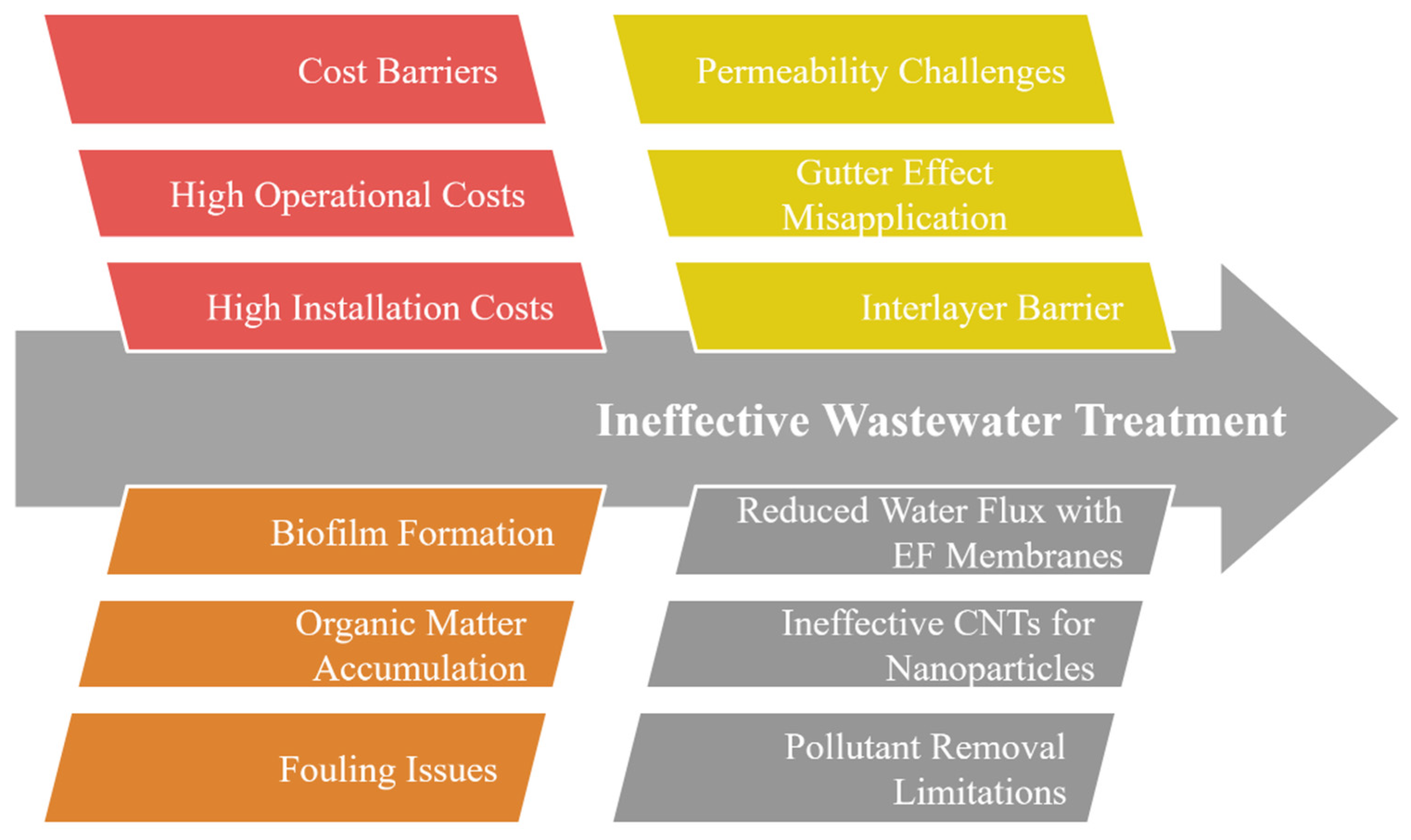
3.4. Current Challenges
4. Carbon Nanotube (CNT)-Based Membranes
4.1. Properties of CNTs for Membrane Application
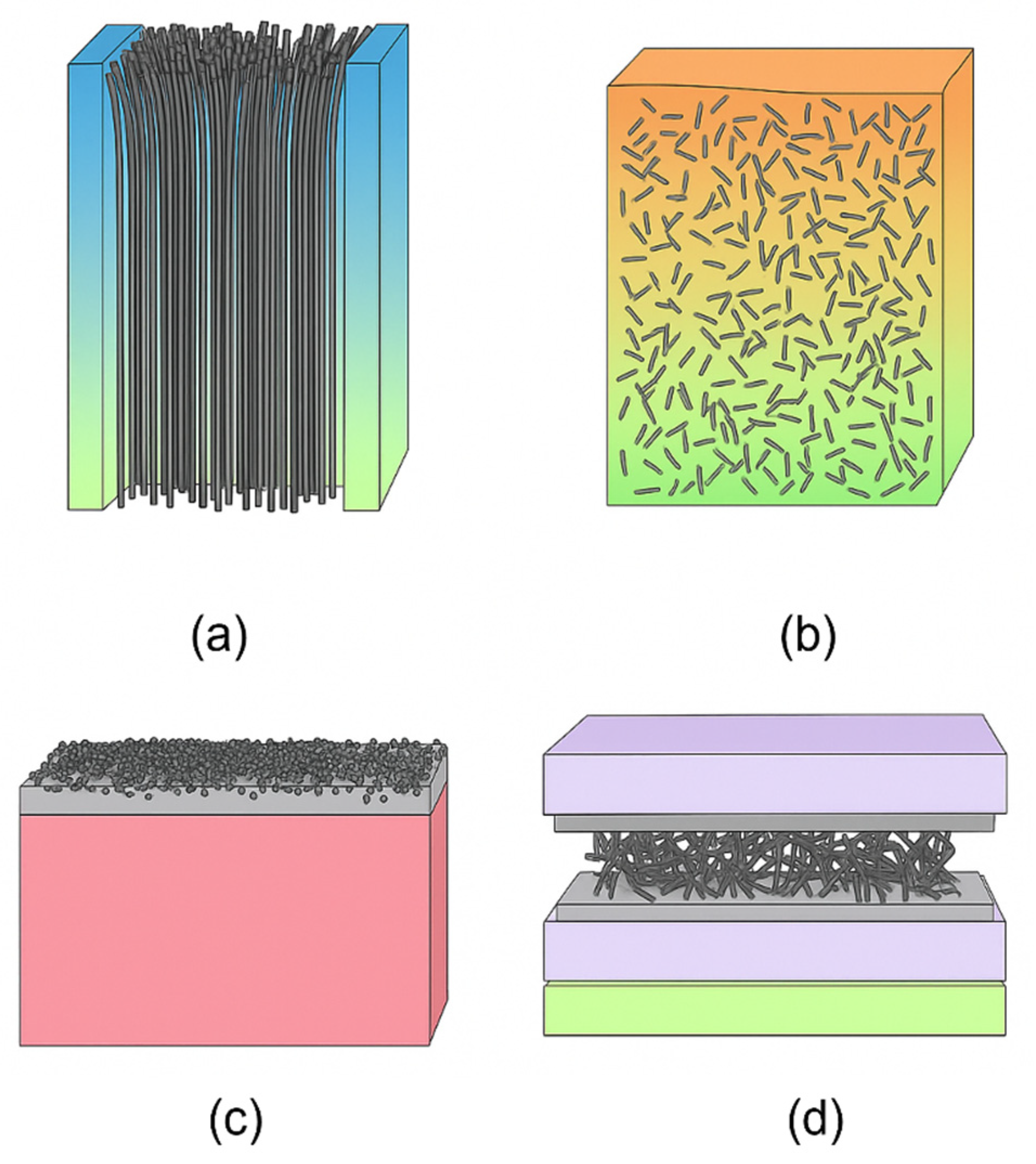
4.2. Fabrication Approaches and Structural Variation
4.3. Applications in Pollutant Removal
4.4. Advantages and Current Challenges
5. Electro-Fenton and Electro–Catalytic Hybrid Membranes
5.1. Concept of Reactive Electrochemical Membranes
5.2. Design of Conductive Membranes
5.3. Different Aspects on EF-Based Pollutant Degradation Processes
5.4. Advantages and Current Challenges
6. Critical Assessments on AFMs-Based Water Treatment
7. Environmental and Economic Considerations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide Cases of Water Pollution by Emerging Contaminants: A Review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty Years of China’s Water Pollution Control: Experiences and Challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef]
- Lin, L.; Yang, H.; Xu, X. Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- Arman, N.Z.; Salmiati, S.; Aris, A.; Salim, M.R.; Nazifa, T.H.; Muhamad, M.S.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- Walker, T.R.; Fequet, L. Current Trends of Unsustainable Plastic Production and Micro(Nano)Plastic Pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, E.; Singh, S.; Pandey, A.; Bhargava, P.C. Micro- and Nano-Plastics (MNPs) as Emerging Pollutant in Ground Water: Environmental Impact, Potential Risks, Limitations and Way Forward towards Sustainable Management. Chem. Eng. J. 2023, 459, 141568. [Google Scholar] [CrossRef]
- Sana, S.S.; Dogiparthi, L.K.; Gangadhar, L.; Chakravorty, A.; Abhishek, N. Effects of Microplastics and Nanoplastics on Marine Environment and Human Health. Environ. Sci. Pollut. Res. 2020, 27, 44743–44756. [Google Scholar] [CrossRef]
- Ugrina, M.; Jurić, A. Current Trends and Future Perspectives in the Remediation of Polluted Water, Soil and Air—A Review. Processes 2023, 11, 3270. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, J.; He, H.; Zhu, Y.; Dionysiou, D.D.; Liu, Z.; Zhao, C. Do Membrane Filtration Systems in Drinking Water Treatment Plants Release Nano/Microplastics? Sci. Total Environ. 2021, 755, 142658. [Google Scholar] [CrossRef]
- Hofman-Caris Roberta and Hofman, J. Limitations of Conventional Drinking Water Technologies in Pollutant Removal. In Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment; Antonio, G., Galeano, L.A., Vincente, M.Á., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 21–51. ISBN 978-3-319-76882-3. [Google Scholar]
- Mandal, M.; Roy, A.; Popek, R.; Sarkar, A. Micro- and Nano- Plastic Degradation by Bacterial Enzymes: A Solution to “White Pollution”. Microbe 2024, 3, 100072. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of Organic Water Pollutants by Clays and Clay Minerals Composites: A Comprehensive Review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiao, X.; Qu, H.; Hu, M.; Au, C.; Nashalian, A.; Xiao, X.; Wang, Y.; Yang, L.; Jia, F.; et al. Ultrafast and Selective Nanofiltration Enabled by Graphene Oxide Membranes with Unzipped Carbon Nanotube Networks. ACS Appl. Mater. Interfaces 2022, 14, 1850–1860. [Google Scholar] [CrossRef]
- Rad, S.M.; Ray, A.K.; Barghi, S. Water Pollution and Agriculture Pesticide. Clean. Technol. 2022, 4, 1088–1102. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.R.; Reshma, B. Effective Water/Wastewater Treatment Methodologies for Toxic Pollutants Removal: Processes and Applications towards Sustainable Development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, C.; Latrach, A.; Rabiei, M.; Venugopal, K. Produced Water Treatment Technologies: A Review. Energies 2025, 18, 63. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge Production from Municipal Wastewater Treatment in Sewage Treatment Plant. Energy Sources Part. A Recovery Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Swartz, C.; Coomans, C.; du Plessis, J.; Kamish, W. Decision-Support Model for the Selection and Costing of Direct Potable Reuse Systems from Municipal Wastewater; Water Research Commission: Lynnwood, Pretoria, 2014. [Google Scholar]
- Bello, M.M.; Abdul Raman, A.A.; Asghar, A. A Review on Approaches for Addressing the Limitations of Fenton Oxidation for Recalcitrant Wastewater Treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Umar, W.; Zia ur Rehman, M.; Umair, M.; Ayub, M.A.; Naeem, A.; Rizwan, M.; Zia, H.; Karri, R.R. Chapter 10-Use of Nanotechnology for Wastewater Treatment: Potential Applications, Advantages, and Limitations. In Sustainable Nanotechnology for Environmental Remediation; Micro and Nano Technologies; Koduru, J.R., Karri, R.R., Mubarak, N.M., Bandala, E.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–272. ISBN 978-0-12-824547-7. [Google Scholar]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Aziz, S.; Mazhar, A.R.; Ubaid, A.; Shah, S.M.H.; Riaz, Y.; Talha, T.; Jung, D.-W. A Comprehensive Review of Membrane-Based Water Filtration Techniques. Appl. Water Sci. 2024, 14, 169. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Fuzil, N.S.; Marpani, F.; Shahruddin, M.Z.; Chew, C.M.; David Ng, K.M.; Lau, W.J.; Ismail, A.F. A Review on the Use of Membrane Technology Systems in Developing Countries. Membranes 2022, 12, 30. [Google Scholar] [CrossRef]
- Rahman, M.M.; Uddin, M.N.; Parvez, M.M.H.; Mohotadi, M.A.A.; Ferdush, J. Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials 2025, 15, 933. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.B.; Zhang, Z. Ceramic Membrane Technology for Water and Wastewater Treatment: A Critical Review of Performance, Full-Scale Applications, Membrane Fouling and Prospects. Chem. Eng. J. 2021, 418, 129481. [Google Scholar] [CrossRef]
- Denny, M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal–Organic Frameworks for Membrane-Based Separations. Nat. Rev. Mater. 2016, 1, 16078. [Google Scholar] [CrossRef]
- Liu, Y.; Ban, Y.; Yang, W. Microstructural engineering and architectural design of metal–organic framework membranes. Adv. Mater. 2017, 29, 1606949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of Water-Stable Metal-Organic Frameworks in the Removal of Water Pollutants: A Review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent Advances in Metal-Organic Framework Membranes for Water Treatment: A Review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.U.; Najam, T.; Islam, M.; Hassan, A.M.; Assiri, M.A.; Rauf, A.; ur Rehman, A.; Shah, S.S.A.; Nazir, M.A. Engineering of Metal Organic Framework (MOF) Membrane for Waste Water Treatment: Synthesis, Applications and Future Challenges. J. Water Process Eng. 2024, 57, 104676. [Google Scholar] [CrossRef]
- Esteras-Saz, J.; Paseta, L.; Echaide-Górriz, C.; Malankowska, M.; Luque-Alled, J.M.; Zornoza, B.; Téllez, C.; Coronas, J. Microfluidic Preparation of Thin Film Composite Hollow Fiber Membrane Modules for Water Nanofiltration: Up-Scaling, Reproducibility and MOF Based Layers. J. Taiwan Inst. Chem. Eng. 2023, 150, 105063. [Google Scholar] [CrossRef]
- Chen, C.; Fei, L.; Wang, B.; Xu, J.; Li, B.; Shen, L.; Lin, H. MOF-Based Photocatalytic Membrane for Water Purification: A Review. Small 2024, 20, 2305066. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.H.; Irizarry, N.; Uddin, M.N. Polymer Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]
- Sianipar, M.; Kim, S.H.; Khoiruddin; Iskandar, F.; Wenten, I.G. Functionalized Carbon Nanotube (CNT) Membrane: Progress and Challenges. RSC Adv. 2017, 7, 51175–51198. [Google Scholar] [CrossRef]
- Yuan, J.; Hung, W.-S.; Zhu, H.; Guan, K.; Ji, Y.; Mao, Y.; Liu, G.; Lee, K.-R.; Jin, W. Fabrication of ZIF-300 Membrane and Its Application for Efficient Removal of Heavy Metal Ions from Wastewater. J. Membr. Sci. 2019, 572, 20–27. [Google Scholar] [CrossRef]
- Humoud, M.S.; Roy, S.; Mitra, S. Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation. Membranes 2020, 10, 325. [Google Scholar] [CrossRef]
- Almusawy, A.M.; Al-Anbari, R.H.; Alsalhy, Q.F.; Al-Najar, A.I. Carbon Nanotubes-Sponge Modified Electro Membrane Bioreactor (EMBR) and Their Prospects for Wastewater Treatment Applications. Membranes 2020, 10, 433. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The Photocatalytic Process in the Treatment of Polluted Water. Chem. Pap. 2023, 77, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Reji Kumar, R.; B, K.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V.V. Utilization of Solar Energy for Wastewater Treatment: Challenges and Progressive Research Trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gu, S.; Jia, X.; Su, X.; Li, Y.; Zhang, Y.; Du, Y.; Ding, Y. The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles. Water 2024, 16, 2906. [Google Scholar] [CrossRef]
- Obotey Ezugbe, E.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Judd, S.J.; Carra, I. Low-Pressure Membrane Technology for Potable Water Filtration: True Costs. Water Res. 2021, 191, 116826. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; O’Connell, M.G.; Xu, H.; Bernstein, R.; Kim, J.-H.; Sankhala, K.; Segal-Peretz, T.; Shevate, R.; Zhang, W.; Zhou, X.; et al. Assessing Advances in Anti-Fouling Membranes to Improve Process Economics and Sustainability of Water Treatment. ACS EST Eng. 2022, 2, 2159–2173. [Google Scholar] [CrossRef]
- Violleau, D.; Essis-Tome, H.; Habarou, H.; Croué, J.P.; Pontié, M. Fouling Studies of a Polyamide Nanofiltration Membrane by Selected Natural Organic Matter: An Analytical Approach. Desalination 2005, 173, 223–238. [Google Scholar] [CrossRef]
- Abdul Ghani, L.; Ali, N.; Nazaran, I.S.; Hanafiah, M.M. Environmental Performance of Small-Scale Seawater Reverse Osmosis Plant for Rural Area Water Supply. Membranes 2021, 11, 40. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling Membranes for Sustainable Water Purification: Strategies and Mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.N.R.; Mohammad, A.W.; Mahmoudi, E.; Ang, W.L.; Leo, C.P.; Teow, Y.H. An Overview of the Modification Strategies in Developing Antifouling Nanofiltration Membranes. Membranes 2022, 12, 1276. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Amin, S.; Mohamed, A.A. Fouling in Reverse Osmosis Membranes: Monitoring, Characterization, Mitigation Strategies and Future Directions. Heliyon 2023, 9, e14908. [Google Scholar] [CrossRef]
- Díez, B.; Rosal, R. A Critical Review of Membrane Modification Techniques for Fouling and Biofouling Control in Pressure-Driven Membrane Processes. Nanotechnol. Environ. Eng. 2020, 5, 15. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, M.; Guo, Y.; Mamrol, N.; Yang, X.; Gao, C.; Van der Bruggen, B. Metal-Organic Framework Based Membranes for Selective Separation of Target Ions. J. Membr. Sci. 2021, 634, 119407. [Google Scholar] [CrossRef]
- Jun, B.-M.; Al-Hamadani, Y.A.J.; Son, A.; Park, C.M.; Jang, M.; Jang, A.; Kim, N.C.; Yoon, Y. Applications of Metal-Organic Framework Based Membranes in Water Purification: A Review. Sep. Purif. Technol. 2020, 247, 116947. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Smith, S.J.D.; Jiang, S.; Wang, H.; Zhang, K.; Ladewig, B.P. Long-Term Stable Metal Organic Framework (MOF) Based Mixed Matrix Membranes for Ultrafiltration. J. Membr. Sci. 2021, 635, 119339. [Google Scholar] [CrossRef]
- Azar, A.N.V.; Velioglu, S.; Keskin, S. Large-Scale Computational Screening of Metal Organic Framework (MOF) Membranes and MOF-Based Polymer Membranes for H2/N2 Separations. ACS Sustain. Chem. Eng. 2019, 7, 9525–9536. [Google Scholar] [CrossRef]
- Guo, J.; Song, G.; Zhang, X.; Zhou, M. Transition Metal Catalysts in the Heterogeneous Electro-Fenton Process for Organic Wastewater Treatment: A Review. Environ. Sci. Water Res. Technol. 2023, 9, 2429–2445. [Google Scholar] [CrossRef]
- Parvez, M.M.H.; Rahman, M.M.; Ferdush, J.; Mohotadi, M.A.A.; Mondal, J.; Uddin, M.N. State-of-the-Art Nanocomposites: Tailoring Material Properties for Next-Generation Applications. Next Res. 2025, 2, 100865. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Tan, Y.H.; Abdullah, E.C.; Rahman, M.E.; Karri, R.R. A Comprehensive Review on Micropollutants Removal Using Carbon Nanotubes-Based Adsorbents and Membranes. J. Environ. Chem. Eng. 2021, 9, 106647. [Google Scholar] [CrossRef]
- Roy, K.; Mukherjee, A.; Maddela, N.R.; Chakraborty, S.; Shen, B.; Li, M.; Du, D.; Peng, Y.; Lu, F.; García Cruzatty, L.C. Outlook on the Bottleneck of Carbon Nanotube in Desalination and Membrane-Based Water Treatment—A Review. J. Environ. Chem. Eng. 2020, 8, 103572. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-Based Membrane Materials and Applications in Water and Wastewater Treatment: A Review. Environ. Chem. Lett. 2021, 19, 1457–1475. [Google Scholar] [CrossRef]
- Paul, S.; Bhoumick, M.C.; Roy, S.; Mitra, S. Carbon Nanotube Enhanced Filtration and Dewatering of Kerosene. Membranes 2022, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, M.M.; Mathaba, M.; Moothi, K. Effect of Carbon Nanotubes Loading and Pressure on the Performance of a Polyethersulfone (PES)/Carbon Nanotubes (CNT) Membrane. Sci. Rep. 2021, 11, 23805. [Google Scholar] [CrossRef]
- Abdul Hamid, M.R.; Qian, Y.; Wei, R.; Li, Z.; Pan, Y.; Lai, Z.; Jeong, H.-K. Polycrystalline Metal-Organic Framework (MOF) Membranes for Molecular Separations: Engineering Prospects and Challenges. J. Membr. Sci. 2021, 640, 119802. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Thornton, A.W.; Hill, A.J.; Wang, H.; Konstas, K. Lithium Extraction by Emerging Metal–Organic Framework-Based Membranes. Adv. Funct. Mater. 2021, 31, 2105991. [Google Scholar] [CrossRef]
- Yu, H.; Hossain, S.M.; Wang, C.; Choo, Y.; Naidu, G.; Han, D.S.; Shon, H.K. Selective Lithium Extraction from Diluted Binary Solutions Using Metal-Organic Frameworks (MOF)-Based Membrane Capacitive Deionization (MCDI). Desalination 2023, 556, 116569. [Google Scholar] [CrossRef]
- Raggam, S.; Mohammad, M.; Choo, Y.; Naidu, G.; Zargar, M.; Shon, H.K.; Razmjou, A. Advances in Metal Organic Framework (MOF)–Based Membranes and Adsorbents for Lithium-Ion Extraction. Sep. Purif. Technol. 2023, 307, 122628. [Google Scholar] [CrossRef]
- Cheng, Y.; Datta, S.J.; Zhou, S.; Jia, J.; Shekhah, O.; Eddaoudi, M. Advances in Metal–Organic Framework-Based Membranes. Chem. Soc. Rev. 2022, 51, 8300–8350. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ara, G.; Susan, M.A.B.H. Green Nanomaterials for Photocatalytic Degradation of Toxic Organic Compounds. Curr. Pharm. Biotechnol. 2023, 24, 118–144. [Google Scholar] [CrossRef] [PubMed]
- Argurio, P.; Fontananova, E.; Molinari, R.; Drioli, E. Photocatalytic Membranes in Photocatalytic Membrane Reactors. Processes 2018, 6, 162. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Conlan, X.A.; Zeng, X.; Kong, L.; O’Dell, L.A.; Sadek, A.; Merenda, A.; Dumée, L.F. Stimuli-Responsive Heterojunctions Based Photo-Electrocatalytic Membrane Reactors for Reactive Filtration of Persistent Organic Pollutants. Chem. Eng. J. 2023, 452, 139374. [Google Scholar] [CrossRef]
- Kumari, P.; Bahadur, N.; Conlan, X.A.; Laleh, M.; Kong, L.; O’Dell, L.A.; Dumée, L.F.; Merenda, A. Atomically-Thin Schottky-like Photo-Electrocatalytic Cross-Flow Membrane Reactors for Ultrafast Remediation of Persistent Organic Pollutants. Water Res. 2022, 218, 118519. [Google Scholar] [CrossRef]
- Johnson, J.K.; Hoffman, C.M., Jr.; Smith, D.A.; Xia, Z. Advanced Filtration Membranes for the Removal of Perfluoroalkyl Species from Water. ACS Omega 2019, 4, 8001–8006. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, G.; Miao, Z.; Dong, X.; Zhang, X. Integration of Membrane Filtration and Peroxymonosulfate Activation on CNT@nitrogen Doped Carbon/Al2O3 Membrane for Enhanced Water Treatment: Insight into the Synergistic Mechanism. Sep. Purif. Technol. 2020, 252, 117479. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Chen, J.; Niu, J. Porous Ti/SnO2-Sb Anode as Reactive Electrochemical Membrane for Removing Trace Antiretroviral Drug Stavudine from Wastewater. Environ. Int. 2019, 133, 105157. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science (1979) 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.B.; Le, V.N.; Zafar, M.N.; Rabani, I.; Do, H.H.; Nguyen, X.C.; Hoang Bui, V.K.; Hur, J. Recent Advancements in Modifications of Metal–Organic Frameworks-Based Materials for Enhanced Water Purification and Contaminant Detection. Chemosphere 2024, 356, 141972. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sivaram, N.; Nath, B.; Khan, N.A.; Singh, J.; Ramamurthy, P.C. Metal Organic Frameworks for Wastewater Treatment, Renewable Energy and Circular Economy Contributions. NPJ Clean. Water 2024, 7, 124. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, S.; Chen, F.; Guo, C. Functional Group Modified 1D Interpenetrated Metal-Organic Frameworks on Perfluorooctanoic Acid Adsorption: Experimental and Theoretical Calculation Study. Environ. Res. 2022, 211, 113083. [Google Scholar] [CrossRef] [PubMed]
- Kavak, S.; Durak, Ö.; Kulak, H.; Polat, H.M.; Keskin, S.; Uzun, A. Enhanced Water Purification Performance of Ionic Liquid Impregnated Metal–Organic Framework: Dye Removal by [BMIM][PF6]/MIL-53(Al) Composite. Front. Chem. 2021, 8, 622567. [Google Scholar] [CrossRef]
- Yang, X.; Ji, Y.; Jian, M.; Meng, N.; Zhu, Y.; Tan, C.; Li, H. Fabrication of Metal-organic Frameworks Macro-Structures for Adsorption Applications in Water Treatment: A Review. Sep. Purif. Technol. 2025, 354, 129376. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Li, Z.; Han, Z.; Fan, J. Review of Synthesis and Separation Application of Metal-Organic Framework-Based Mixed-Matrix Membranes. Polymers 2023, 15, 1950. [Google Scholar] [CrossRef]
- Missaoui, N.; Kahri, H.; Demirci, U.B. Rapid Room-Temperature Synthesis and Characterizations of High-Surface-Area Nanoparticles of Zeolitic Imidazolate Framework-8 (ZIF-8) for CO2 and CH4 Adsorption. J. Mater. Sci. 2022, 57, 16245–16257. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, M.; Lin, Y.S. Stability of ZIF-8 in Water under Ambient Conditions. Microporous Mesoporous Mater. 2019, 279, 201–210. [Google Scholar] [CrossRef]
- Paul, A.; Banga, I.K.; Muthukumar, S.; Prasad, S. Engineering the ZIF-8 Pore for Electrochemical Sensor Applications—A Mini Review. ACS Omega 2022, 7, 26993–27003. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. UiO-66 Metal-Organic Framework Membranes: Structural Engineering for Separation Applications. Membranes 2025, 15, 8. [Google Scholar] [CrossRef]
- Kazemi, A.; Moghadaskhou, F.; Pordsari, M.A.; Manteghi, F.; Tadjarodi, A.; Ghaemi, A. Enhanced CO2 Capture Potential of UiO-66-NH2 Synthesized by Sonochemical Method: Experimental Findings and Performance Evaluation. Sci. Rep. 2023, 13, 19891. [Google Scholar] [CrossRef]
- Chi, H.-Y.; Song, S.; Zhao, K.; Hsu, K.-J.; Liu, Q.; Shen, Y.; Sido Belin, A.F.; Allaire, A.; Goswami, R.; Queen, W.L.; et al. Non-van-Der-Waals Oriented Two-Dimensional UiO-66 Films by Rapid Aqueous Synthesis at Room Temperature. J. Am. Chem. Soc. 2025, 147, 7255–7263. [Google Scholar] [CrossRef]
- Han, G.; Qian, Q.; Mizrahi Rodriguez, K.; Smith, Z.P. Hydrothermal Synthesis of Sub-20 Nm Amine-Functionalized MIL-101(Cr) Nanoparticles with High Surface Area and Enhanced CO2 Uptake. Ind. Eng. Chem. Res. 2020, 59, 7888–7900. [Google Scholar] [CrossRef]
- Zou, M.; Zhu, H.; Dong, M.; Zhao, T. Template Method for Synthesizing Hierarchically Porous MIL-101(Cr) for Efficient Removal of Large Molecular Dye. Materials 2022, 15, 5763. [Google Scholar] [CrossRef] [PubMed]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Zhang, S.; Yao, Y.; Wang, J.; Zhu, X.; Jiang, R.; Zhang, J.; Zhang, W.; Wang, C. MOFs Meet Electrospinning: New Opportunities for Water Treatment. Chem. Eng. J. 2023, 453, 139669. [Google Scholar] [CrossRef]
- Xing, Q.; Xu, X.; Li, H.; Cui, Z.; Chu, B.; Xie, N.; Wang, Z.; Bai, P.; Guo, X.; Lyu, J. Fabrication Methods of Continuous Pure Metal–Organic Framework Membranes and Films: A Review. Molecules 2024, 29, 3885. [Google Scholar] [CrossRef]
- Xu, X.; Hartanto, Y.; Zheng, J.; Luis, P. Recent Advances in Continuous MOF Membranes for Gas Separation and Pervaporation. Membranes 2022, 12, 1205. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Lin, R.; Liu, Y.; Chen, F.; Cui, L.; Meng, X.; Hou, J. Improving the Hydrostability of ZIF-8 Membrane by Biomolecule towards Enhanced Nanofiltration Performance for Dye Removal. J. Membr. Sci. 2021, 618, 118630. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Q.; Wang, Y.; Zhu, Z.; Guo, Z.; Li, J.; Lv, Y.; Chow, Y.T.; Wang, X.; Zhu, L.; et al. Water-Stable Metal–Organic Framework (UiO-66) Supported on Zirconia Nanofibers Membrane for the Dynamic Removal of Tetracycline and Arsenic from Water. Appl. Surf. Sci. 2022, 596, 153559. [Google Scholar] [CrossRef]
- Chen, D.-H.; Gliemann, H.; Wöll, C. Layer-by-Layer Assembly of Metal-Organic Framework Thin Films: Fabrication and Advanced Applications. Chem. Phys. Rev. 2023, 4, 011305. [Google Scholar] [CrossRef]
- Wang, L.; Fang, M.; Liu, J.; He, J.; Li, J.; Lei, J. Layer-by-Layer Fabrication of High-Performance Polyamide/ZIF-8 Nanocomposite Membrane for Nanofiltration Applications. ACS Appl. Mater. Interfaces 2015, 7, 24082–24093. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wei, W.; Yin, Y.; Liu, M.; Zheng, C.; Zhang, Y.; Deng, P. Continuous Ultrathin UiO-66-NH2 Coatings on a Polymeric Substrate Synthesized by a Layer-by-Layer Method: A Kind of Promising Membrane for Oil–Water Separation. Nanoscale 2020, 12, 6658–6663. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhou, Z.; Li, L.; Xiao, P.; Ma, Y.; Liu, F.; Li, J. PVDF/MOFs Mixed Matrix Ultrafiltration Membrane for Efficient Water Treatment. Front. Chem. 2022, 10, 985750. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.V.; Murthy, Z.V.P. Synthesis and Characterization of ZIF-8-Based PVDF Mixed Matrix Membranes and Application to Treat Pulp and Paper Industry Wastewater Using a Membrane Bioreactor. Environ. Sci. Water Res. Technol. 2022, 8, 881–896. [Google Scholar] [CrossRef]
- Kulak, H.; Thür, R.; Vankelecom, I.F.J. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef]
- Ma, L.; Svec, F.; Lv, Y.; Tan, T. Engineering of the Filler/Polymer Interface in Metal–Organic Framework-Based Mixed-Matrix Membranes to Enhance Gas Separation. Chem. Asian J. 2019, 14, 3502–3514. [Google Scholar] [CrossRef]
- Zadehahmadi, F.; Eden, N.T.; Mahdavi, H.; Konstas, K.; Mardel, J.I.; Shaibani, M.; Banerjee, P.C.; Hill, M.R. Removal of Metals from Water Using MOF-Based Composite Adsorbents. Environ. Sci. Water Res. Technol. 2023, 9, 1305–1330. [Google Scholar] [CrossRef]
- Ding, L.; Luo, X.; Shao, P.; Yang, J.; Sun, D. Thiol-Functionalized Zr-Based Metal–Organic Framework for Capture of Hg(II) through a Proton Exchange Reaction. ACS Sustain. Chem. Eng. 2018, 6, 8494–8502. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/Chitosan Nanofibers for Adsorption and Membrane Filtration of Pb(II), Cd(II) and Cr(VI) Ions from Aqueous Solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Li, J.; Li, J.; Fan, M.; Han, M.; Liu, Z.; Li, Z.; Kong, F. Metal Organic Framework UiO-66 Incorporated Ultrafiltration Membranes for Simultaneous Natural Organic Matter and Heavy Metal Ions Removal. Environ. Res. 2022, 208, 112651. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Aly, H.A.; Mohamed, M.; Ahmed, S.; Shaimaa, K.M. Effective Removal of Methyl Blue and Crystal Violet Dyes Using Improved Polysulfone/ZIF-8 Nanocomposite Ultrafiltration Membrane. Biointerface Res. Appl. Chem. 2021, 12, 7942–7956. [Google Scholar] [CrossRef]
- Zhang, J.; Han, J.; Chen, X.; Xu, D.; Wen, X.; Zhao, Y.; Huang, Y.; Ding, X.; Chen, G.; Xu, D.; et al. Recent Advances in ZIF Membrane: Fabrication, Separation Ability and Its Application. Nanomaterials 2025, 15, 239. [Google Scholar] [CrossRef]
- Zhu, K.; Mohammed, S.; Tang, H.; Xie, Z.; Fang, S.; Liu, S. ZIF-67/SA@PVDF Ultrafiltration Membrane with Simultaneous Adsorption and Catalytic Oxidation for Dyes. Sustainability 2023, 15, 2879. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Jiang, Y.; Ni, W.; Li, M.; Wang, J. UiO-66-SO3H@nylon-6 Membrane for Rapid Adsorption and Removal of Rhodamine B (RhB) from Aqueous Solutions. Langmuir 2025, 41, 16022–16032. [Google Scholar] [CrossRef] [PubMed]
- Regmi, S.; Sarker, M.S.; Oglesby, A.K.; Banning, K.N.; Lanier, E.E.; Xia, C.; Sabuj, S.T.; Yang, H.; Khan, N.; Liu, J. Fabrication of NZVI/RGO via an Innovative Thermal Co-Reduction Method for Enhanced PFAS Removal through Sequential Adsorption and Photocatalytic Degradation. J. Hazard. Mater. 2025, 497, 139586. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Palma, M.S.; Bhandari, D.; Tian, F. Electrodeposition of Ag/ZIF-8-Modified Membrane for Water Remediation. Langmuir 2023, 39, 2291–2300. [Google Scholar] [CrossRef]
- Njaramba, L.K.; Yoon, Y.; Park, C.M. Fabrication of Porous Beta-Cyclodextrin Functionalized PVDF/Fe–MOF Mixed Matrix Membrane for Enhanced Ciprofloxacin Removal. NPJ Clean. Water 2024, 7, 14. [Google Scholar] [CrossRef]
- Wang, J.; Teng, Y.; Jia, S.; Li, W.; Yang, T.; Cheng, Y.; Zhang, H.; Li, X.; Li, L.; Wang, C. Highly Efficient Removal of Salicylic Acid from Pharmaceutical Wastewater Using a Flexible Composite Nanofiber Membrane Modified with UiO-66(Hf) MOFs. Appl. Surf. Sci. 2023, 625, 157183. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Q.; Xie, D.; Wang, F.; Zhang, G.; Shan, H. Facile Fabrication of Zeolitic Imidazolate Framework-8@Regenerated Cellulose Nanofibrous Membranes for Effective Adsorption of Tetracycline Hydrochloride. Molecules 2024, 29, 4146. [Google Scholar] [CrossRef]
- Moharrami, E.; Keshipour, S. Photocatalytic Degradation of Tetracycline Antibiotic Using Nitrogen-Doped Reduced Graphene Oxide-Supported Titania/Platinum Nanoparticles. Npj Mater. Degrad. 2025, 9, 57. [Google Scholar] [CrossRef]
- Wang, C.; Sun, H.; Wang, N.; An, Q.-F. Robust ZIF-8 and Its Derivative Composite Membrane for Antibiotic Desalination with High Performance. Sep. Purif. Technol. 2023, 307, 122857. [Google Scholar] [CrossRef]
- Kontogiannis, A.; Evgenidou, E.; Nannou, C.; Bikiaris, D.; Lambropoulou, D. MOF-Based Photocatalytic Degradation of the Antibiotic Lincomycin Enhanced by Hydrogen Peroxide and Persulfate: Kinetics, Elucidation of Transformation Products and Toxicity Assessment. J. Environ. Chem. Eng. 2022, 10, 108112. [Google Scholar] [CrossRef]
- Han, C.-H.; Park, H.-D.; Kim, S.-B.; Yargeau, V.; Choi, J.-W.; Lee, S.-H.; Park, J.-A. Oxidation of Tetracycline and Oxytetracycline for the Photo-Fenton Process: Their Transformation Products and Toxicity Assessment. Water Res. 2020, 172, 115514. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Qu, S.; Bhattacharjee, L.; Lim, X.E.; Yang, H.; Liu, J. Degradation of Perfluoroalkyl Substances Using UV/Fe0 System with and without the Presence of Oxygen. Environ. Technol. 2023, 44, 2725–2736. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, P.; Liu, Y.; Xia, S. Efficient Removal of Per- and Polyfluoroalkyl Substances by a Metal-Organic Framework Membrane with High Selectivity and Stability. Water Res. 2024, 265, 122276. [Google Scholar] [CrossRef]
- Minhas, S.A.; Pandey, R.P.; Abi Jaoude, M.; Hasan, S.W. MOF/Polydopamine-Modified MXene Based Mixed Matrix Membrane for per- and Polyfluoroalkyl Substances Removal from Real Wastewater. Sep. Purif. Technol. 2025, 370, 133139. [Google Scholar] [CrossRef]
- Prajapati, M.; Kalla, S.; Sivaiah, A. A CuBTC@PVDF-Based Super Hydrophobic Membrane for Perfluoroheptanoic Acid (PFHpA) Rejection through Membrane Distillation. Process Saf. Environ. Prot. 2025, 194, 152–163. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Gao, L.; Gray, S.; Xie, Z. Study of MOF Incorporated Dual Layer Membrane with Enhanced Removal of Ammonia and Per-/Poly-Fluoroalkyl Substances (PFAS) in Landfill Leachate Treatment. Sci. Total Environ. 2022, 806, 151207. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Meng, X.; Tan, Z.; Geng, Q.; Peng, J.; Yong, Q.; Sun, X.; Guo, M.; Wang, X. A Novel ZIF-L/PEI Thin Film Nanocomposite Membrane for Removing Perfluoroalkyl Substances (PFASs) from Water: Enhanced Retention and High Flux. Sci. Total Environ. 2024, 925, 171727. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K.; Nehache, S.; Vankelecom, I.; Deratani, A.; Quemener, D. MOF-Mixed Matrix Membranes: Precise Dispersion of MOF Particles with Better Compatibility via a Particle Fusion Approach for Enhanced Gas Separation Properties. J. Membr. Sci. 2015, 492, 21–31. [Google Scholar] [CrossRef]
- Cheng, Y.; Joarder, B.; Datta, S.J.; Alsadun, N.; Poloneeva, D.; Fan, D.; Khairova, R.; Bavykina, A.; Jia, J.; Shekhah, O.; et al. Mixed Matrix Membranes with Surface Functionalized Metal–Organic Framework Sieves for Efficient Propylene/Propane Separation. Adv. Mater. 2023, 35, 2300296. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.S.Q.; Hansen, N.; Motadayen, M.; Lock, N.; Henriksen, M.L.; Quinson, J. A Review of Metal-Organic Frameworks and Polymers in Mixed Matrix Membranes for CO2 Capture. Beilstein J. Nanotechnol. 2025, 16, 155–186. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Fang, W.; Shrestha, B.B.; Huang, M.; Jin, J. Constructing Strong Interfacial Interactions under Mild Conditions in MOF-Incorporated Mixed Matrix Membranes for Gas Separation. ACS Appl. Mater. Interfaces 2021, 13, 3166–3174. [Google Scholar] [CrossRef]
- Yu, S.; Li, C.; Zhao, S.; Chai, M.; Hou, J.; Lin, R. Recent Advances in the Interfacial Engineering of MOF-Based Mixed Matrix Membranes for Gas Separation. Nanoscale 2024, 16, 7716–7733. [Google Scholar] [CrossRef]
- Altaf, F.; Ahmed, S.; Usman, M.; Batool, T.; Shamshad, J.; Bocchetta, P.; Batool, R. Removal of Heavy Metals from Wastewater Using Novel Polydopamine-Modified Cnts-Based Composite Membranes. Processes 2021, 9, 2120. [Google Scholar] [CrossRef]
- Terrón, D.; Sanromán, A.; Pazos, M. Metal–Organic Frameworks: Next-Generation Materials for Environmental Remediation. Catalysts 2025, 15, 244. [Google Scholar] [CrossRef]
- Dong, M.; Guo, J.; Liu, Q.; Zeng, J.; Xiong, X.; Gai, X.; Wang, Y.; Wu, Y. Low-Pressure Carbon Nanotube Membrane with Different Surface Properties for the Removal of Organic Dyes and PPCPs. J. Environ. Chem. Eng. 2023, 11, 110131. [Google Scholar] [CrossRef]
- Farahani, S.K.; Hosseini, S.M. A Highly Promoted Nanofiltration Membrane by Incorporating of Aminated Zr-Based MOF for Efficient Salts and Dyes Removal with Excellent Antifouling Properties. Chem. Eng. Res. Des. 2022, 188, 764–778. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Q.; Lei, L.; Barton, G. Electro-Fenton Method for the Removal of Methyl Red in an Efficient Electrochemical System. Sep. Purif. Technol. 2007, 57, 380–387. [Google Scholar] [CrossRef]
- Ting, H.; Chi, H.Y.; Lam, C.H.; Chan, K.Y.; Kang, D.Y. High-Permeance Metal-Organic Framework-Based Membrane Adsorber for the Removal of Dye Molecules in Aqueous Phase. Environ. Sci. Nano 2017, 4, 2205–2214. [Google Scholar] [CrossRef]
- Dai, R.; Wang, X.; Tang, C.Y.; Wang, Z. Dually Charged MOF-Based Thin-Film Nanocomposite Nanofiltration Membrane for Enhanced Removal of Charged Pharmaceutically Active Compounds. Environ. Sci. Technol. 2020, 54, 7619–7628. [Google Scholar] [CrossRef] [PubMed]
- Lomba-Fernández, B.; Fdez-Sanromán, A.; Pazos, M.; Sanromán, M.A.; Rosales, E. Iron Metal-Organic Framework Nanofiber Membrane for the Integration of Electro-Fenton and Effective Continuous Treatment of Pharmaceuticals in Water. Chemosphere 2024, 366, 143447. [Google Scholar] [CrossRef]
- Wang, Z.; He, M.; Jiang, H.; He, H.; Qi, J.; Ma, J. Photocatalytic MOF Membranes with Two-Dimensional Heterostructure for the Enhanced Removal of Agricultural Pollutants in Water. Chem. Eng. J. 2022, 435, 133870. [Google Scholar] [CrossRef]
- Heo, J.; Joseph, L.; Yoon, Y.; Park, Y.G.; Her, N.; Sohn, J.; Yoon, S.H. Removal of Micropollutants and NOM in Carbon Nanotube-UF Membrane System from Seawater. Water Sci. Technol. 2011, 63, 2737–2744. [Google Scholar] [CrossRef]
- Alterkaoui, A.; Arslan, H.; Saleh, M.; Dizge, N.; Balakrishnan, D.; Naik, N. Integrated Electro-Fenton and Membrane Filtration Technologies for Effective Organic Pollutant Removal and Salt Recovery from Sesame Process Wastewater. Environ. Syst. Res. 2025, 14, 1–12. [Google Scholar] [CrossRef]
- Golgoli, M.; Najafi, M.; Farahbakhsh, J.; Khiadani, M.; Johns, M.L.; Zargar, M. Microplastics-Resistant FO Membranes: Zwitterionic MOF Nanoparticles for Superior Fouling Control. J. Environ. Chem. Eng. 2025, 13, 115668. [Google Scholar] [CrossRef]
- Li, S.; Zhuang, Y.; Wu, H.; Sang, C.; Wang, L.; Pang, S.; Yao, S.; Yang, H.; Guo, Z.; Lu, L.; et al. Self-Cleaning PDMS Membranes via UV-Triggered Integration with Photocatalytic MOF. J. Membr. Sci. 2025, 728, 124154. [Google Scholar] [CrossRef]
- Xia, G.; Zhou, M.; Shao, W.; Adeli, M.; Li, S.; Liao, Y.; Wu, H.; Wang, X.; Wang, M.; Ren, X.; et al. Self-Cleaning Antifouling Membrane Engineered by Oxygen-Activated MOF-Derived Catalysts for Efficient Organic Wastewater Treatment. ACS Appl. Mater. Interfaces 2025, 17, 10637–10649. [Google Scholar] [CrossRef]
- Ghomi, M. Metal-Organic Framework-Based Membranes for Water Decontamination. Mater. Chem. Horiz. 2024, 3, 1–24. [Google Scholar] [CrossRef]
- Kumar, P.; Anand, B.; Tsang, Y.F.; Kim, K.-H.; Khullar, S.; Wang, B. Regeneration, Degradation, and Toxicity Effect of MOFs: Opportunities and Challenges. Environ. Res. 2019, 176, 108488. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Vagena, I.-A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Yang, B.; Xiao, K.; Duan, H.; Zhao, H. The Chemical Stability of Metal-Organic Frameworks in Water Treatments: Fundamentals, Effect of Water Matrix and Judging Methods. Chem. Eng. J. 2022, 450, 138215. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Nadzri, N.; Wang, R. Thin-Film Composite (TFC) Membranes for Sustainable Desalination and Water Reuse: A Perspective. Desalination 2025, 599, 118451. [Google Scholar] [CrossRef]
- Mahlangu, O.T.; Motsa, M.M.; Richards, H.; Mamba, B.B.; George, M.J.; Nthunya, L.N. The Impact of Nanoparticle Leach on Sustainable Performance of the Membranes—A Critical Review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100984. [Google Scholar] [CrossRef]
- Kajau, A.; Motsa, M.; Mamba, B.B.; Mahlangu, O. Leaching of CuO Nanoparticles from PES Ultrafiltration Membranes. ACS Omega 2021, 6, 31797–31809. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Mohammadhosseini, M.; Abadi, A.J.N.; Hasanzadeh, A.; Abasi, E.; Aberoumandi, S.M.; Panahi, Y. Nanomaterials Toxin Contamination in Laboratories and Potential Harmful Effects of Their Products: A Review. Toxin Rev. 2016, 35, 180–186. [Google Scholar] [CrossRef]
- Pattan, G.; Kaul, G. Health Hazards Associated with Nanomaterials. Toxicol. Ind. Health 2014, 30, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Zadehahmadi, F.; Sadiq, M.M.; Sutton, A.L.; Mahdavi, H.; Hill, M.R. Challenges and Solutions to the Scale-up of Porous Materials. Commun. Mater. 2024, 5, 170. [Google Scholar] [CrossRef]
- Selim, S. Metal-Organic Frameworks 2025–2035: Markets, Technologies, and Forecasts; IDTechEx: Cambridge, UK, 2025. [Google Scholar]
- He, L.; Wang, Z.; Wang, H.; Wu, Y. Are MOFs Ready for Environmental Applications: Assessing Stability against Natural Stressors? Coord. Chem. Rev. 2025, 526, 216361. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Baratta, M.; Nezhdanov, A.V.; Mashin, A.I.; Nicoletta, F.P.; De Filpo, G. Carbon Nanotubes Buckypapers: A New Frontier in Wastewater Treatment Technology. Sci. Total Environ. 2024, 924, 171578. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.-S.; Yun, E.-T.; Ham, S.-Y.; Park, J.-H.; Ahn, C.H.; Lee, S.H.; Park, H.-D. Vertically Aligned Carbon Nanotube Membranes: Water Purification and Beyond. Membranes 2020, 10, 273. [Google Scholar] [CrossRef]
- Alfei, S.; Zuccari, G. Carbon-Nanotube-Based Nanocomposites in Environmental Remediation: An Overview of Typologies and Applications and an Analysis of Their Paradoxical Double-Sided Effects. J. Xenobiot. 2025, 15, 76. [Google Scholar] [CrossRef]
- Intrchom, W.; Thakkar, M.; Hamilton, R.F.; Holian, A.; Mitra, S. Effect of Carbon Nanotube-Metal Hybrid Particle Exposure to Freshwater Algae Chlamydomonas Reinhardtii. Sci. Rep. 2018, 8, 15301. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon Nanotubes: A Review on Properties, Synthesis Methods and Applications in Micro and Nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N.; et al. Mechanical Performance and Applications of CNTs Reinforced Polymer Composites—A Review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.Z.; Sagadevan, S.; Bin Johan, R.; Shah, S.T.; Adebesi, A.; Md, S.I.; Rafique, R.F. A Review on Electrochemically Modified Carbon Nanotubes (CNTs) Membrane for Desalination and Purification of Water. Mater. Res. Express 2018, 5, 102001. [Google Scholar] [CrossRef]
- Kallumottakkal, M.; Hussein, M.I.; Haik, Y.; Abdul Latef, T. Bin Functionalized-CNT Polymer Composite for Microwave and Electromagnetic Shielding. Polymers 2021, 13, 3907. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.I.; Jehangir, S.S.; Rajmohan, I.J.; Haik, Y.; Abdulrehman, T.; Clément, Q.; Vukadinovic, N. Microwave Absorbing Properties of Metal Functionalized-CNT-Polymer Composite for Stealth Applications. Sci. Rep. 2020, 10, 16013. [Google Scholar] [CrossRef]
- Pandele, A.M.; Serbanescu, O.S.; Voicu, S.I. Polysulfone Composite Membranes with Carbonaceous Structure. Synthesis and Applications. Coatings 2020, 10, 609. [Google Scholar] [CrossRef]
- Ma, L.; Dong, X.; Chen, M.; Zhu, L.; Wang, C.; Yang, F.; Dong, Y. Fabrication and Water Treatment Application of Carbon Nanotubes (CNTs)-Based Composite Membranes: A Review. Membranes 2017, 7, 16. [Google Scholar] [CrossRef]
- Rashed, A.O.; Huynh, C.; Merenda, A.; Qin, S.; Maghe, M.; Kong, L.; Kondo, T.; Razal, J.M.; Dumée, L.F. Electrocatalytic Ultrafiltration Membrane Reactors Designed from Dry-Spun Self-Standing Carbon Nanotube Sheets. Chem. Eng. J. 2023, 458, 141517. [Google Scholar] [CrossRef]
- Castellano, R.J.; Praino, R.F.; Meshot, E.R.; Chen, C.; Fornasiero, F.; Shan, J.W. Scalable Electric-Field-Assisted Fabrication of Vertically Aligned Carbon Nanotube Membranes with Flow Enhancement. Carbon. N. Y 2020, 157, 208–216. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, H.; Guo, D.; Guo, P.; Wang, J.; Liu, M.; Wu, S.; Bao, C. Multiscale Preparation of Graphene Oxide/Carbon Nanotube-Based Membrane Evaporators by a Spray Method for Efficient Solar Steam Generation. ACS Appl. Nano Mater. 2022, 5, 7198–7207. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Li, S.; Cui, L.; Zhuang, Y.; Xing, W.; Jing, W. Assembly of Multidimensional MXene-Carbon Nanotube Ultrathin Membranes with an Enhanced Anti-Swelling Property for Water Purification. J. Membr. Sci. 2021, 623, 119075. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, N.; Abdelghani-Idrissi, S.; Trégouët, C.; Perez-Carvajal, J.; Colin, A.; Ma, M.; Niguès, A.; Siria, A. Advanced nanoscale functionalities for water and energy technologies. Adv. Phys. Res. 2025, 240, 0195. [Google Scholar] [CrossRef]
- Zhang, W.; Mo, J.; Liang, W.; Du, X. Carbon Nanotube-Adsorptive Dynamic Membrane (CNT-ADM) for Water Purification. J. Water Process Eng. 2023, 51, 103433. [Google Scholar] [CrossRef]
- Ismail, A.F.; Goh, P.S.; Sanip, S.M.; Aziz, M. Transport and separation properties of carbon nanotube-mixed matrix membrane. Sep. Purif. Technol. 2009, 70, 12–26. [Google Scholar] [CrossRef]
- Elmarghany, M.R.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Sabry, M.N.; Matsuyama, H.; Nady, N. Triple-Layer Nanocomposite Membrane Prepared by Electrospinning Based on Modified PES with Carbon Nanotubes for Membrane Distillation Applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef]
- Liao, M.; Zhu, Y.; Gong, G.; Qiao, L. Thin-Film Composite Membranes with a Carbon Nanotube Interlayer for Organic Solvent Nanofiltration. Membranes 2022, 12, 817. [Google Scholar] [CrossRef]
- Gu, Y.; Li, H.; Liu, L.; Li, J.; Zhang, B.; Ma, H. Constructing CNTs-Based Composite Membranes for Oil/Water Emulsion Separation via Radiation-Induced “Grafting to” Strategy. Carbon. N. Y. 2021, 178, 678–687. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q.; Wang, S.; Wang, M.; Chen, X.; Hou, X. Hydrophilic Carbon Nanotube Membrane Enhanced Interfacial Evaporation for Desalination. Chin. Chem. Lett. 2022, 33, 2155–2158. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Zhu, L.; Cui, F.; Xie, C.; Chen, X.; Li, N. High-Performance Nanofiltration Membrane with Structurally Controlled PES Substrate Containing Electrically Aligned CNTs. J. Membr. Sci. 2020, 605, 118104. [Google Scholar] [CrossRef]
- Manorma; Ferreira, I.; Alves, P.; Gil, M.H.; Gando-Ferreira, L.M. Lignin Separation from Black Liquor by Mixed Matrix Polysulfone Nanofiltration Membrane Filled with Multiwalled Carbon Nanotubes. Sep. Purif. Technol. 2021, 260, 118231. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, Z.; Wang, Z.; Liu, Y.; He, Z.; Cao, A.; Zhou, L.; Zhu, L.; Zhao, S. Super-Adsorptive and Photo-Regenerable Carbon Nanotube Based Membrane for Highly Efficient Water Purification. J. Membr. Sci. 2021, 621, 119000. [Google Scholar] [CrossRef]
- Si, Y.; Sun, C.; Li, D.; Yang, F.; Tang, C.Y.; Quan, X.; Dong, Y.; Guiver, M.D. Flexible Superhydrophobic Metal-Based Carbon Nanotube Membrane for Electrochemically Enhanced Water Treatment. Environ. Sci. Technol. 2020, 54, 9074–9082. [Google Scholar] [CrossRef]
- Koo, S.-H.; Jee, M.H.; Baik, D.H. Effects of Multi-Walled Carbon Nanotubes on Structures and Properties of Heat-Resistant Poly(m-Phenylene Isophthalamide)-Based Hollow Fiber Ultrafiltration Membranes. Polym. Eng. Sci. 2024, 64, 554–564. [Google Scholar] [CrossRef]
- Peng, J.; He, Y.; Zhou, C.; Su, S.; Lai, B. The Carbon Nanotubes-Based Materials and Their Applications for Organic Pollutant Removal: A Critical Review. Chin. Chem. Lett. 2021, 32, 1626–1636. [Google Scholar] [CrossRef]
- Yang, D.-C.; Castellano, R.J.; Silvy, R.P.; Lageshetty, S.K.; Praino, R.F.; Fornasiero, F.; Shan, J.W. Fast Water Transport through Subnanometer Diameter Vertically Aligned Carbon Nanotube Membranes. Nano Lett. 2023, 23, 4956–4964. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer Carbon Nanotubes for Contaminant Removal in Water and Wastewater: A Critical Review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, X.; Yue, Y. Determination of pore size and pore size distribution on the surface of hollow-fiber filtration membranes: A review of methods. Desalination 2000, 129, 107–123. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of Heavy Metals and Pollutants by Membrane Adsorption Techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Jain, N.; Jee Kanu, N. The Potential Application of Carbon Nanotubes in Water Treatment: A State-of-the-Art-Review. Mater. Today Proc. 2021, 43, 2998–3005. [Google Scholar] [CrossRef]
- Rashed, A.O.; Merenda, A.; Kondo, T.; Lima, M.; Razal, J.; Kong, L.; Huynh, C.; Dumée, L.F. Carbon Nanotube Membranes–Strategies and Challenges towards Scalable Manufacturing and Practical Separation Applications. Sep. Purif. Technol. 2021, 257, 117929. [Google Scholar] [CrossRef]
- Liu, H.Y.; Sun, Y.; Sun, D.M.; Cheng, H.M. Carbon nanotube 3D integrated circuits: From design to applications. Adv. Funct. Mater. 2025, 242, 4012. [Google Scholar] [CrossRef]
- Goh, K.; Jiang, W.; Karahan, H.E.; Zhai, S.; Wei, L.; Yu, D.; Fane, A.G.; Wang, R.; Chen, Y. All-Carbon Nanoarchitectures as High-Performance Separation Membranes with Superior Stability. Adv. Funct. Mater. 2015, 25, 7348–7359. [Google Scholar] [CrossRef]
- Ren, L.; Ma, J.; Chen, M.; Qiao, Y.; Dai, R.; Li, X.; Wang, Z. Recent Advances in Electrocatalytic Membrane for the Removal of Micropollutants from Water and Wastewater. iScience 2022, 25, 104342. [Google Scholar] [CrossRef]
- Casado, J. Minerals as Catalysts of Heterogeneous Electro-Fenton and Derived Processes for Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2023, 30, 76405–76420. [Google Scholar] [CrossRef]
- Qin, L.; Chen, W.; Fu, Y.; Tang, J.; Yi, H.; Li, L.; Xu, F.; Zhang, M.; Cao, W.; Huang, D.; et al. Hemin Derived Iron and Nitrogen-Doped Carbon as a Novel Heterogeneous Electro-Fenton Catalyst to Efficiently Degrade Ciprofloxacin. Chem. Eng. J. 2022, 449, 137840. [Google Scholar] [CrossRef]
- Chen, J.; Wan, J.; Li, C.; Wei, Y.; Shi, H. Synthesis of Novel Fe0-Fe3O4/CeO2/C Composite Cathode for Efficient Heterogeneous Electro-Fenton Degradation of Ceftriaxone Sodium. J. Hazard. Mater. 2022, 437, 129393. [Google Scholar] [CrossRef]
- Zhong, S.; Zhu, Z.-S.; Duan, X.; Wang, S. Electro-Fenton-Based Membrane System for Organic Micropollutant Removal: New Trend and Prospect. ACS ES&T Eng. 2023, 3, 2147–2160. [Google Scholar] [CrossRef]
- Ramírez-Valencia, L.D.; Bailón-García, E.; Moral-Rodríguez, A.I.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Carbon Gels–Green Graphene Composites as Metal-Free Bifunctional Electro-Fenton Catalysts. Gels 2023, 9, 665. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. Electro-Fenton Process with Emphasis on Its Challenges and Future Prospects for Wastewater Treatment: A Review. 2022. [Google Scholar] [CrossRef]
- Deng, F.; Jiang, J.; Sirés, I. State-of-the-Art Review and Bibliometric Analysis on Electro-Fenton Process. Carbon. Lett. 2023, 33, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, J.; Liu, B.; Hou, H.; Yang, J.; Li, Y.; Zhu, Y.; Liang, S.; Xiao, K. Degradation of Refractory Organics in Dual-Cathode Electro-Fenton Using Air-Cathode for H2O2 Electrogeneration and Microbial Fuel Cell Cathode for Fe2+ Regeneration. J. Hazard. Mater. 2021, 412, 125269. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, S.; Chen, Y.; Wang, Y.; Bai, K.; Mai, Z.; Xiao, Z. Electrocatalytic Composite Membrane with Deep-Permeation Nano Structure Fabricated by Flowing Synthesis for Enhanced Catalysis. J. Membr. Sci. 2021, 636, 119616. [Google Scholar] [CrossRef]
- Yang, L.; Xu, D.; Yang, H.; Luo, X.; Liang, H. Structurally-Controlled FeNi LDH/CNTs Electro-Fenton Membrane for in-Situ Electro-Generation and Activation of Hydroxyl Radicals toward Organic Micropollutant Treatment. Chem. Eng. J. 2022, 432, 134436. [Google Scholar] [CrossRef]
- Liu, C.; Chu, Y.; Wang, R.; Fan, J. Preparation of Lotus-Leaf-like Carbon Cathode for the Electro-Fenton Oxidation Process: Hydrogen Peroxide Production, Various Organics Degradation and Printing Wastewater Treatment. J. Water Process Eng. 2023, 52, 103596. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Li, K.; Guo, J.; Yang, C.; Liu, H.; Wang, J. In Situ Coupling of Electrochemical Oxidation and Membrane Filtration Processes for Simultaneous Decontamination and Membrane Fouling Mitigation. Sep. Purif. Technol. 2022, 290, 120918. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Hou, C.; Chen, W.; Li, S.; Ren, R.; Li, Z. Efficient Degradation of Perfluorooctanoic Acid by Solar Photo-Electro-Fenton like System Fabricated by MOFs/Carbon Nanofibers Composite Membrane. Chem. Eng. J. 2021, 414, 128940. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Li, W.; Ma, J.; He, K.; Yang, K.; Cui, J.; Liu, Q.; Lv, S.; Zhang, M.; et al. FeIII-Driven Self-Cycled Fenton via Contact-Electro-Catalysis for Water Purification. NPJ Clean. Water 2025, 8, 42. [Google Scholar] [CrossRef]
- Luo, X.; Zhu, R.; Zhao, L.; Gong, X. Dual-Functional Electrocatalyst of Defective Cobalt-Nitrogen-Doped Porous Carbon for Enhanced in-Situ Hydrogen Peroxide Generation and Electro-Fenton Tetracycline Degradation. Sep. Purif. Technol. 2024, 346, 127451. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, J.; Xu, D.; Luo, X.; Han, Y.; Tang, X.; Liang, H. Rational Design of a Hydrophilic Nanoarray-Structured Electro-Fenton Membrane for Antibiotics Removal and Fouling Mitigation: An Intensified Catalysis Process in an Oxygen Vacancy-mediated Cathodic Microreactor. J. Hazard. Mater. 2024, 470, 134138. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Wang, K.; Wang, Z.; Liu, Z.; Zhu, S.; Qu, D.; Zhang, Y.; Wang, J. A Novel Electro-Fenton Hybrid System for Enhancing the Interception of Volatile Organic Compounds in Membrane Distillation Desalination. J. Environ. Sci. 2024, 138, 189–199. [Google Scholar] [CrossRef]
- Liu, B.; Liu, P.; Deng, K.; Chen, Y.; Lv, X.; Wang, C.; Tian, W.; Tan, S.; Ji, J. In Situ Transformation of Hierarchical FeOOH/CuO Arrays with Electro-Cleaning Capability for Oil-in-Water Emulsion Separation and Electro-Fenton Degradation of Organic Dye. Sep. Purif. Technol. 2023, 311, 123274. [Google Scholar] [CrossRef]
- Mirzaei, M.; Moazeni, K.; Baghdadi, M.; Aliasghar, A.; Mehrdadi, N. A Hybrid Process of Electrocoagulation and Electro-Fenton for Treatment of Paper Wastewater. Int. J. Environ. Sci. Technol. 2024, 21, 8391–8401. [Google Scholar] [CrossRef]
- Soltani, F.; Navidjouy, N.; Rahimnejad, M. A Review on Bio-Electro-Fenton Systems as Environmentally Friendly Methods for Degradation of Environmental Organic Pollutants in Wastewater. RSC Adv. 2022, 12, 5184–5213. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; He, M.; Duan, X.; Yan, B.; Chen, G.; Wang, S. Catalytic Membrane-Based Oxidation-Filtration Systems for Organic Wastewater Purification: A Review. J. Hazard. Mater. 2021, 414, 125478. [Google Scholar] [CrossRef] [PubMed]
- Bruguera-Casamada, C.; Araujo, R.M.; Brillas, E.; Sirés, I. Advantages of Electro-Fenton over Electrocoagulation for Disinfection of Dairy Wastewater. Chem. Eng. J. 2019, 376, 119975. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Angelidaki, I.; Zhang, Y. Bio-Electro-Fenton Processes for Wastewater Treatment: Advances and Prospects. Chem. Eng. J. 2018, 354, 492–506. [Google Scholar] [CrossRef]
- Chen, R.; Han, M.; Shi, Y.; Guo, W.; Wu, Y.; Zhang, T.; Han, X.; Du, C.; Yu, C.; Feng, J.; et al. Construction of Integrated Oxygen-Rich Carbon-Based Metal-Free Cathode to Simultaneous Boost Wastewater Treatment Performance and Energy Recovery in Bio-Electro-Fenton System. Chem. Eng. J. 2024, 487, 150532. [Google Scholar] [CrossRef]
- Sun, Y.; Tu, S.; Li, Y.; Sui, X.; Geng, S.; Wang, H.; Duan, X.; Chang, L. Degradation Mechanism of Methylene Blue by Heterogenous Electro-Fenton with CeO2/RGO Composite Cathode. Colloids Surf. A Physicochem. Eng. Asp. 2024, 690, 133861. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater Treatment: A Short Assessment on Available Techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Im, S.J.; Fortunato, L.; Jang, A. Real-Time Fouling Monitoring and Membrane Autopsy Analysis in Forward Osmosis for Wastewater Reuse. Water Res. 2021, 197, 117098. [Google Scholar] [CrossRef]
- Ding, J.; Wang, J.; Luo, X.; Xu, D.; Liu, Y.; Li, P.; Li, S.; Wu, R.; Gao, X.; Liang, H. A Passive-Active Combined Strategy for Ultrafiltration Membrane Fouling Control in Continuous Oily Wastewater Purification. Water Res. 2022, 226, 119219. [Google Scholar] [CrossRef]
- Long, L.; Wu, C.; Yang, Z.; Tang, C.Y. Carbon Nanotube Interlayer Enhances Water Permeance and Antifouling Performance of Nanofiltration Membranes: Mechanisms and Experimental Evidence. Environ. Sci. Technol. 2022, 56, 2656–2664. [Google Scholar] [CrossRef]
- Ge, L.; Song, H.; Zhu, J.; Zhang, Y.; Zhou, Z.; der Bruggen, B. Metal/Covalent–Organic Framework Based Thin Film Nanocomposite Membranes for Advanced Separations. J. Mater. Chem. A 2024, 12, 7975–8013. [Google Scholar] [CrossRef]
- Jiang, W.-L.; Haider, M.R.; Han, J.-L.; Ding, Y.-C.; Li, X.-Q.; Wang, H.-C.; Adeel Sharif, H.M.; Wang, A.-J.; Ren, N.-Q. Carbon Nanotubes Intercalated RGO Electro-Fenton Membrane for Coenhanced Permeability, Rejection and Catalytic Oxidation of Organic Micropollutants. J. Membr. Sci. 2021, 623, 119069. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; Arafat, H.A. Recent Developments in the Rational Fabrication of Thin Film Nanocomposite Membranes for Water Purification and Desalination. ACS Omega 2020, 5, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.; Abdelaal, A.B.; Esawi, A.M.K.; Ramadan, A.R. Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance. Membranes 2023, 13, 477. [Google Scholar] [CrossRef]
- Almansouri, H.E.; Edokali, M.; Abu Seman, M.N.; Ndia Ntone, E.P.; Che Ku Yahya, C.K.M.F.; Mohammad, A.W. Antifouling and Desalination Enhancement of Forward Osmosis-Based Thin Film Composite Membranes via Functionalized Multiwalled Carbon Nanotubes Mixed Matrix Polyethersulfone Substrate. Membranes 2025, 15, 240. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Ma, X.; Zhou, Z.; Guo, H.; Yao, Z.; Feng, S.-P.; Tang, C.Y. Fabrication of a Novel and Green Thin-Film Composite Membrane Containing Nanovoids for Water Purification. J. Membr. Sci. 2019, 570–571, 314–321. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.; Wei, Z.; Zhou, S.; Yao, Z.; Fan, F.; Mei, Y. Thin Film Composite Nanofiltration Membrane with Tannic Acid-Fe(III) Complexes Functionalized CNTs Interlayer toward Energy Efficient Remediation of Groundwater. Desalination 2023, 552, 116438. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Ostwal, M.; Zhu, L.; Sekizkardes, A.; Mohamed, M.H.; Gipple, M.; McCutcheon, J.R.; Hopkinson, D. 3D Printed MOF-Based Mixed Matrix Thin-Film Composite Membranes. RSC Adv. 2021, 11, 25658–25663. [Google Scholar] [CrossRef]
- Kamali, K.; Mohammadi, T.; Zarghami, S. Thin Film Composite Nanofiltration Membrane Mediated by MOF Interlayer with Novel MOF Templating Technique for Water Treatment. Ind. Eng. Chem. Res. 2025, 64, 16299–16311. [Google Scholar] [CrossRef]
- Han, G.; Studer, R.M.; Lee, M.; Rodriguez, K.M.; Teesdale, J.J.; Smith, Z.P. Post-Synthetic Modification of MOFs to Enhance Interfacial Compatibility and Selectivity of Thin-Film Nanocomposite (TFN) Membranes for Water Purification. J. Membr. Sci. 2023, 666, 121133. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zong, Z.; Lin, R.; Zhang, X.; Chen, F.; Ding, W.; Zhang, L.; Meng, X.; Hou, J. Thin Film Nanocomposite Membrane Incorporated with 2D-MOF Nanosheets for Highly Efficient Reverse Osmosis Desalination. J. Membr. Sci. 2022, 653, 120520. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and Prospects of Advanced Oxidation Water Treatment Processes Using Catalytic Nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Rivallin, M.; Cerneaux, S.; Lacour, S.; Petit, E.; Cretin, M. Coupling Cathodic Electro-Fenton Reaction to Membrane Filtration for AO7 Dye Degradation: A Successful Feasibility Study. J. Membr. Sci. 2016, 510, 182–190. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon Nanotube Membranes with Ultrahigh Specific Adsorption Capacity for Water Desalination and Purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef] [PubMed]
- Bux, H.; Chmelik, C.; Van Baten, J.M.; Krishna, R.; Caro, J. Novel MOF-Membrane for Molecular Sieving Predicted by IR-Diffusion Studies and Molecular Modeling. Adv. Mater. 2010, 22, 4741–4743. [Google Scholar] [CrossRef] [PubMed]
- Daramola, M.O.; Hlanyane, P.; Sadare, O.O.; Oluwasina, O.O.; Iyuke, S.E. Performance of Carbon Nanotube/Polysulfone (CNT/Psf) Composite Membranes during Oil–Water Mixture Separation: Effect of CNT Dispersion Method. Membranes 2017, 7, 14. [Google Scholar] [CrossRef]
- Solcova, O.; Dlaskova, M.; Kastanek, F. Innovative Sorbents for the Removal of Micropollutants from Water. Molecules 2025, 30, 1444. [Google Scholar] [CrossRef]
- Saththasivam, J.; Yiming, W.; Wang, K.; Jin, J.; Liu, Z. A Novel Architecture for Carbon Nanotube Membranes towards Fast and Efficient Oil/Water Separation. Sci. Rep. 2018, 8, 7418. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Chen, Z.; Khan, N.A.; Ni, B.J. Removing Polyfluoroalkyl Substances (PFAS) from Wastewater with Mixed Matrix Membranes. Sci. Total Environ. 2024, 912, 168881. [Google Scholar] [CrossRef]
- Chung, J.H.; Hasyimah, N.; Hussein, N. Application of Carbon Nanotubes (CNTs) for Remediation of Emerging Pollutants-A Review. Trop. Aquat. Soil. Pollut. 2021, 2, 13–26. [Google Scholar] [CrossRef]
- Kahoush, M.; Behary, N.; Cayla, A.; Nierstrasz, V. Bio-Fenton and Bio-Electro-Fenton as Sustainable Methods for Degrading Organic Pollutants in Wastewater. Process Biochem. 2018, 64, 237–247. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.d.C.B.S.M. Removal of Contaminants from Water by Membrane Filtration: A Review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and Challenges of Carbon Nanotube Membrane in Water Treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, E.; Li, Z.; Xie, D.; Shao, Q.; Sun, Z.; Li, R.; Wang, E.; Liu, K.; Liu, K. Graphene Oxide-Carbon Nanotube Hybrid Membranes for High-Pressure and High-Flux Nanofiltration. Adv. Funct. Mater. 2025, 2503432. [Google Scholar] [CrossRef]
- Xu, H.; Chen, S.; Zhao, Y.-F.; Wang, F.; Guo, F. MOF-Based Membranes for Remediated Application of Water Pollution. Chempluschem 2024, 89, e202400027. [Google Scholar] [CrossRef]
- Sutherland, A.J.; Ruiz-Caldas, M.-X.; de Lannoy, C.-F. Electro-Catalytic Microfiltration Membranes Electrochemically Degrade Azo Dyes in Solution. J. Membr. Sci. 2020, 611, 118335. [Google Scholar] [CrossRef]
- Mattia, D.; Leese, H.; Lee, K.P. Carbon Nanotube Membranes: From Flow Enhancement to Permeability. J. Membr. Sci. 2015, 475, 266–272. [Google Scholar] [CrossRef]
- Wang, X.; Lyu, Q.; Tong, T.; Sun, K.; Lin, L.-C.; Tang, C.Y.; Yang, F.; Guiver, M.D.; Quan, X.; Dong, Y. Robust Ultrathin Nanoporous MOF Membrane with Intra-Crystalline Defects for Fast Water Transport. Nat. Commun. 2022, 13, 266. [Google Scholar] [CrossRef] [PubMed]
- Su, N.C.; Sun, D.T.; Beavers, C.M.; Britt, D.K.; Queen, W.L.; Urban, J.J. Enhanced Permeation Arising from Dual Transport Pathways in Hybrid Polymer-MOF Membranes. Energy Environ. Sci. 2016, 9, 922–931. [Google Scholar] [CrossRef]
- Zhu, X.; Jassby, D. Electroactive Membranes for Water Treatment: Enhanced Treatment Functionalities, Energy Considerations, and Future Challenges. Acc. Chem. Res. 2019, 52, 1177–1186. [Google Scholar] [CrossRef]
- Farid, M.U.; Khanzada, N.K.; An, A.K. Understanding Fouling Dynamics on Functionalized CNT-Based Membranes: Mechanisms and Reversibility. Desalination 2019, 456, 74–84. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, J.; Wang, Z.; Yang, Z.; Yang, W.; Yin, Z. Defective MOFs-Based Electrocatalytic Self-Cleaning Membrane for Wastewater Reclamation: Enhanced Antibiotics Removal, Membrane Fouling Control and Mechanisms. Water Res. 2022, 220, 118635. [Google Scholar] [CrossRef]
- Caro, J. Are MOF Membranes Better in Gas Separation than Those Made of Zeolites? Curr Opin Chem Eng 2011, 1, 77–83. [Google Scholar] [CrossRef]
- Duan, Y.; Li, L.; Shen, Z.; Cheng, J.; He, K. Engineering Metal-Organic-Framework (MOF)-Based Membranes for Gas and Liquid Separation. Membranes 2023, 13, 480. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Luan, H.Y.; Farid, M.U.; Huang, H. Challenges and Opportunities in Functional Carbon Nanotubes for Membrane-Based Water Treatment and Desalination. Sci. Total Environ. 2019, 646, 1126–1139. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Vecitis, C.D. Prospects of an Electroactive Carbon Nanotube Membrane toward Environmental Applications. Acc. Chem. Res. 2020, 53, 2892–2902. [Google Scholar] [CrossRef]
- Lin, R.B.; Zhang, Z.; Chen, B. Achieving High Performance Metal-Organic Framework Materials through Pore Engineering. Acc. Chem. Res. 2021, 54, 3362–3376. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bahadur, N.; Cretin, M.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Electro-Catalytic Membrane Reactors for the Degradation of Organic Pollutants-A Review. React. Chem. Eng. 2021, 6, 1508–1526. [Google Scholar] [CrossRef]
- Hashem, T.; Valadez Sanchez, E.P.; Bogdanova, E.; Ugodchikova, A.; Mohamed, A.; Schwotzer, M.; Alkordi, M.H.; Wöll, C. Stability of Monolithic Mof Thin Films in Acidic and Alkaline Aqueous Media. Membranes 2021, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.O.; Ralph, S.F. Carbon Nanotube Membranes: Synthesis, Properties, and Future Filtration Applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton Technology for Wastewater Treatment: A Bibliometric Analysis of Current Research Trends, Future Perspectives and Energy Consumption Analysis. J. Water Process Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Hani, A.; Haikal, R.R.; El-Mehalmey, W.A.; Safwat, Y.; Alkordi, M.H. Durable and Recyclable MOF@polycaprolactone Mixed-Matrix Membranes with Hierarchical Porosity for Wastewater Treatment. Nanoscale 2023, 15, 19617–19628. [Google Scholar] [CrossRef]
- Ni, L.; Wang, P.; Wang, Y. Sustainable Electro-Fenton Antifouling Strategy Simultaneously Improves Nitrogen-Removal Efficiency of Anammox in Membrane Bioreactors. Environ. Sci. Technol. 2025, 59, 17545–17557. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater Treatment and Reuse: A Review of Its Applications and Health Implications. Water Air Soil. Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.-V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liang, S.; Zhang, Q.; Wang, K.; Liang, P.; Huang, X. Coupling Anodic and Cathodic Reactions Using an Electrocatalytic Dual-Membrane System Actuates Ultra-Efficient Degradation with Regulable Mechanisms. Water Res. 2023, 233, 119741. [Google Scholar] [CrossRef]
- Ma, B.; Ulbricht, M.; Hu, C.; Fan, H.; Wang, X.; Pan, Y.-R.; Hosseini, S.S.; Panglisch, S.; Van der Bruggen, B.; Wang, Z. Membrane Life Cycle Management: An Exciting Opportunity for Advancing the Sustainability Features of Membrane Separations. Environ. Sci. Technol. 2023, 57, 3013–3020. [Google Scholar] [CrossRef]
- Li, S.; Hua, T.; Li, F.; Zhou, Q. Bio-Electro-Fenton Systems for Sustainable Wastewater Treatment: Mechanisms, Novel Configurations, Recent Advances, LCA and Challenges. An Updated Review. J. Chem. Technol. Biotechnol. 2020, 95, 2083–2097. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Salehipour, M.; Nikpour, S.; Rezaei, S.; Mohammadi, S.; Rezaei, M.; Ilbeygi, D.; Hosseini-Chegeni, A.; Mogharabi-Manzari, M. Safety of Metal–Organic Framework Nanoparticles for Biomedical Applications: An in Vitro Toxicity Assessment. Inorg. Chem. Commun. 2023, 152, 110655. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, H.; Wang, X.; Wang, L.; Wu, J. Electro-Fenton Removal of Orange II in a Divided Cell: Reaction Mechanism, Degradation Pathway and Toxicity Evolution. Sep. Purif. Technol. 2014, 122, 533–540. [Google Scholar] [CrossRef]











| MOF | Metal Node | Organic Linker | Surface Area (m2/g) | Pore Size | Water Stability | Targeted Pollutants | Reference |
|---|---|---|---|---|---|---|---|
| ZIF-8 | Zn2+ | 2-methylimidazole | ~1600 | ~3.4 Å | Moderate–poor; hydrolyzes over time at ambient conditions | VOCs, dyes, some metal ions | [90,91,92] |
| UiO-66 | Zr4+ | Terephthalic acid | ~800–1000 | ~6–8 Å | Excellent aqueous stability (acid/base) | Heavy metals (Cr6+), antibiotics, dyes | [93,94,95] |
| MIL-101 (Cr) | Cr3+ | Terephthalic acid | ~2700 | 29–34 Å (cage diameters) | Good hydrothermal stability and durability | Dyes, pharmaceuticals, antibiotics | [96,97] |
| Pollutant Type | MOF-Based Membranes | CNT-Based Membranes | Electro-Fenton/Electro–Catalytic Membranes | References |
|---|---|---|---|---|
| Heavy Metals | NH2-MIL-53(Al)/PAN shows 95% Co2+ removal | Removal up to 90–96% Cr6+, 70–90% Pb depending on CNT loading | Less common; mainly organic degradation | [139,140] |
| Dyes | ZIF-8/PVDF composite membranes remove 83.6% of Reactive Black, 95.8% of Methylene Blue, and 94.2% of Rhodamine B | Promotes removal by 30% when introduced | Electro-Fenton shows 80% dye degradation | [141,142,143,144] |
| Pharmaceuticals | >90% removal for both positively and negatively charged pharmaceutical pollutants | Removal more than 70% pharmaceutical pollutants | >85% removal for the treatment of pharmaceuticals in water | [139,145,146] |
| Organic micropollutants | MOF composites can achieve up to 99% rejection of volatile organic compounds (VOCs) | Can remove up to 79% Bisphenol A (BPA) while also rejecting 80% of DOC in seawater | Electro-Fenton achieves 97.68% COD removal with 82.41% salt recovery | [147,148,149] |
| CNT Type | Structure & Morphology | Key Properties | Advantages in Membranes | Challenges/ Limitations |
|---|---|---|---|---|
| SWCNT (Single-Walled) | Single graphene sheet rolled into a tube (diameter ~0.7–2 nm) | High surface area, high conductivity, excellent selectivity | High adsorption capacity, ideal for sensing and separation at molecular level | Difficult to purify, high cost, dispersibility issues |
| MWCNT (Multi-Walled) | Multiple concentric CNT layers (diameter ~5–50 nm) | Mechanically strong, thermally stable, cost-effective | Good for composite membranes, high chemical stability | Lower surface area than SWCNTs, less flexible for tuning |
| VACNT (Vertically Aligned) | CNTs aligned perpendicular to membrane surface | High flux, low flow resistance, directional transport | Superior water permeability, highly organized pore structure | Complex and costly fabrication |
| Buckypaper | Randomly entangled CNT sheets pressed into thin films | Freestanding membrane, moderate conductivity, porous | Easy to fabricate, scalable, can serve as standalone membrane or layer | Lower selectivity, needs reinforcement for mechanical strength |
| CNT-Polymer Composites | CNTs dispersed in polymer matrix (e.g., PSf, PES, PVDF) | Tunable porosity, mechanical strength, antifouling properties | Versatile, improves polymer membrane properties (flux, fouling, rejection) | Requires functionalization for dispersion, cost scaling |
| Functionalized CNTs | CNTs modified with -OH, -COOH, NH2, etc. | Improved dispersion, hydrophilicity, specific binding sites | Tailored for removal of specific contaminants, better integration with polymers | Functionalization may damage structure or reduce conductivity |
| CNT Properties | CVD | Template- Assisted CVD | Polymer Blending | In Situ Polymerization | Layer-by-Layer (LbL) Assembly | Direct Coating |
|---|---|---|---|---|---|---|
| Compatible Membrane Material | Inorganic | Inorganic | Polymeric | Polymeric | Polymeric | Inorganic or Polymeric |
| Bonding Mechanism | In situ growth | In situ growth | Weak interactions (Van der Waals, H-bonding) | Covalent bonding | Electrostatic and Covalent | Surface adhesion/covalent |
| CNT Arrangement | Aligned or Random | Aligned | Random | Random or Aligned | Mostly Aligned | Random |
| Mechanical Stability | Excellent | Excellent | Good | Poor | Good | Good |
| Common Defects | Impurities | Impurities | Poor dispersion | Low durability | Pinholes | Delamination/peeling |
| Scalability | Limited to substrate size | Limited to template size | Easily scalable | Limited by processing | Scalable with control | Limited to substrate size |
| Pore Size Control | Precise | Precise | Moderate | Precise | Precise | Moderate |
| Ease of Fabrication | Moderate | Complicated | Simple | Complicated | Complex | Simple |
| Practical Use | Industrially promising | Moderate | Suitable for general use | Experimental stage | Lab-scale and advanced studies | Simple but effective |
| Parameter | CNT-Based Membranes | MOF-Based Membranes | Electro-Fenton/Electro–Catalytic Hybrid Membranes | References |
|---|---|---|---|---|
| Main Mechanism | Physical filtration + Adsorption | Molecular sieving + Adsorption | Electrochemical degradation + Filtration | [245,246,247] |
| Typical Applications | Oil-water separation, dye removal, heavy metals | Heavy metals, dyes, PFAS, antibiotics | Pharmaceuticals, endocrine disruptors, pesticides | [248,249,250] |
| Contaminant Types Removed | Moderate range: dyes, oils, some metals | Broad range: organics, metals, emerging pollutants | Very broad: persistent organics, complex molecules | [251,252,253] |
| Rejection Rate | Moderate (60–90% for many contaminants) | High (up to 99% for targeted pollutants) | Very high (>99% for organics) | [149,254,255] |
| Water Flux | Very High (found up to ~1000–5500 L·m−2·h−1·bar−1) | Moderate to high (~30–250 L·m−2·h−1·bar−1) | Variable (depends on electrochemical setup) | [250,256,257,258,259,260] |
| Energy Requirement | Low | Low to moderate | High (due to electrical input) | [255,261,262] |
| Fouling Resistance | Moderate (requires functionalization) | Moderate (susceptible to pore blockage) | High (self-cleaning due to oxidation) | [263,264,265] |
| Material Cost/Scalability | Moderate to High (due to CNT synthesis) | High (MOFs expensive, sensitive to water) | High (complex fabrication, power requirement) | [253,266,267] |
| Strengths | High permeability, tunable surface, light weight | High selectivity, customizable pore structures | Complete breakdown of pollutants, no secondary waste | [268,269,270] |
| Limitations | Costly synthesis, limited removal of micropollutants | Stability in aqueous media, scalability | High energy use, electrode degradation | [271,272,273] |
| Reusability | Good (if fouling is controlled) | Moderate (regeneration needed) | Excellent (can be self-regenerating) | [274,275,276] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mannaf, M.M.; Rahman, M.M.; Sabuj, S.T.; Talukder, N.; Lee, E.S. Current Progress in Advanced Functional Membranes for Water-Pollutant Removal: A Critical Review. Membranes 2025, 15, 300. https://doi.org/10.3390/membranes15100300
Mannaf MM, Rahman MM, Sabuj ST, Talukder N, Lee ES. Current Progress in Advanced Functional Membranes for Water-Pollutant Removal: A Critical Review. Membranes. 2025; 15(10):300. https://doi.org/10.3390/membranes15100300
Chicago/Turabian StyleMannaf, Manseeb M., Md. Mahbubur Rahman, Sonkorson Talukder Sabuj, Niladri Talukder, and Eon Soo Lee. 2025. "Current Progress in Advanced Functional Membranes for Water-Pollutant Removal: A Critical Review" Membranes 15, no. 10: 300. https://doi.org/10.3390/membranes15100300
APA StyleMannaf, M. M., Rahman, M. M., Sabuj, S. T., Talukder, N., & Lee, E. S. (2025). Current Progress in Advanced Functional Membranes for Water-Pollutant Removal: A Critical Review. Membranes, 15(10), 300. https://doi.org/10.3390/membranes15100300







