Optimized Sulfonated Poly(Ether Ether Ketone) Membranes for In-House Produced Small-Sized Vanadium Redox Flow Battery Set-Up
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of SPEEK Powders
2.3. Preparation of Membranes
2.4. Chemical–Physical Characterization of the Membranes
2.5. Preparation of the Chloride-Based VRFB Electrolyte
2.6. Single-Cell VRFB Tests (Charge/Discharge Measurements and EIS)
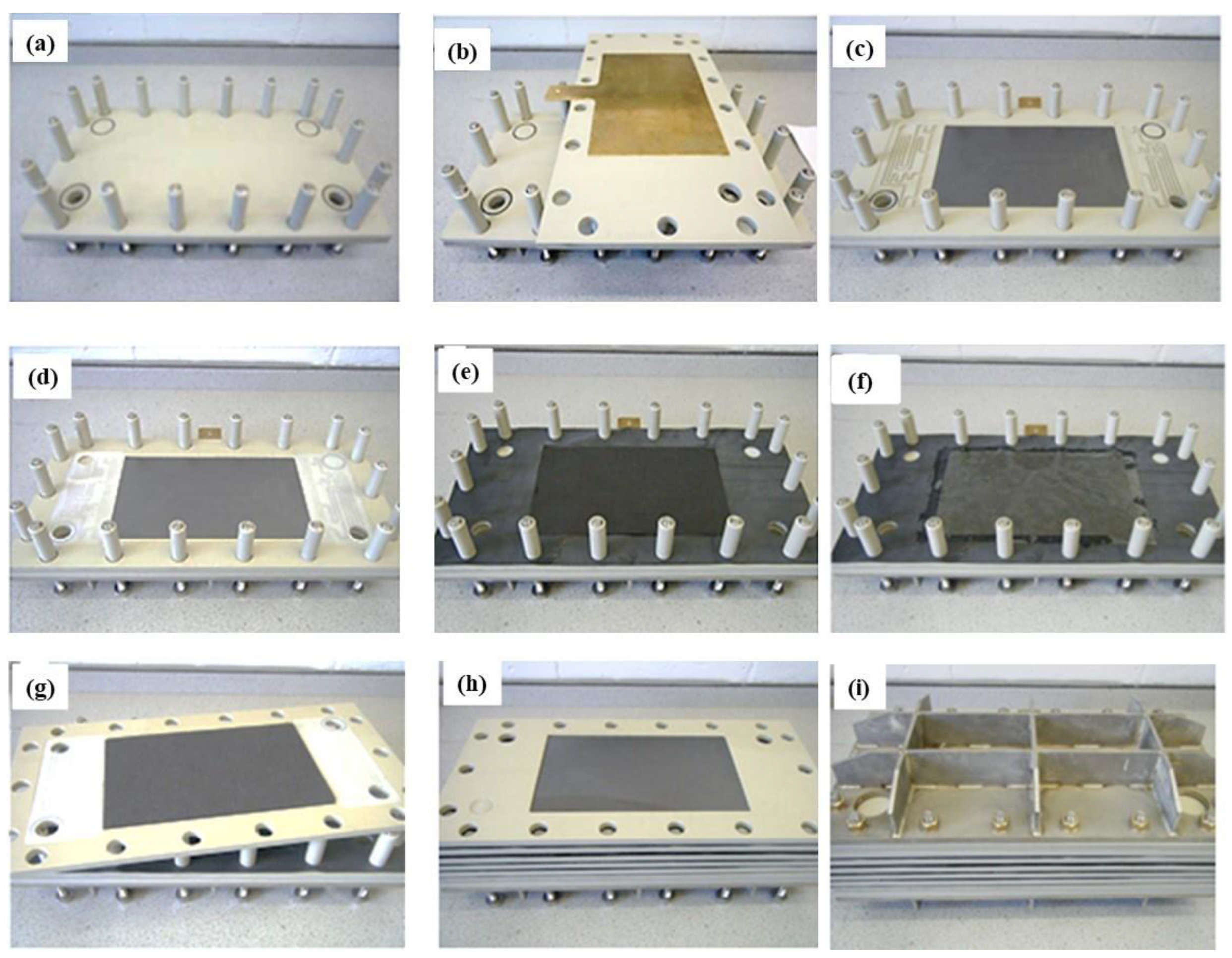
2.7. In-House Five-Cell VRFB Stack System
3. Results and Discussion
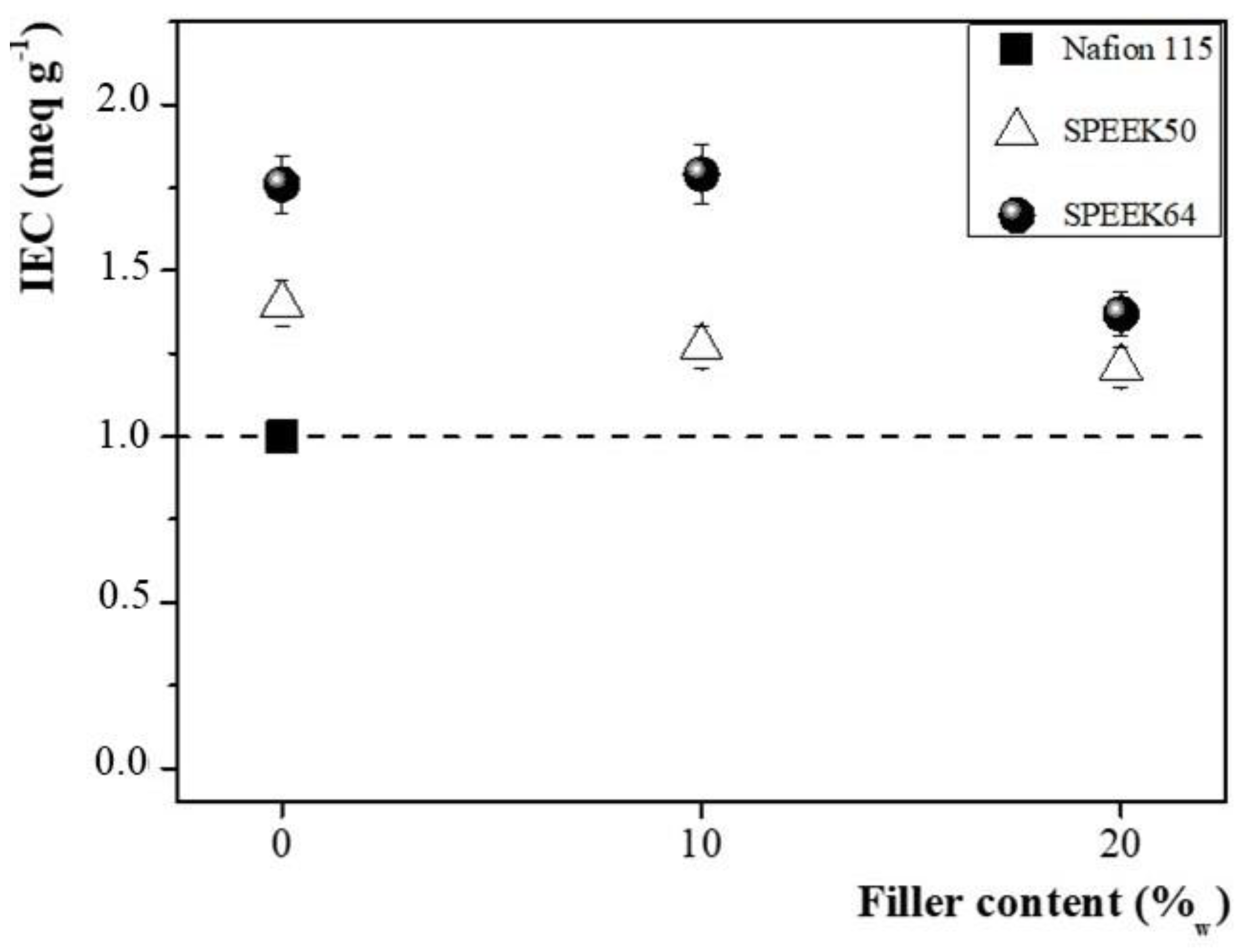
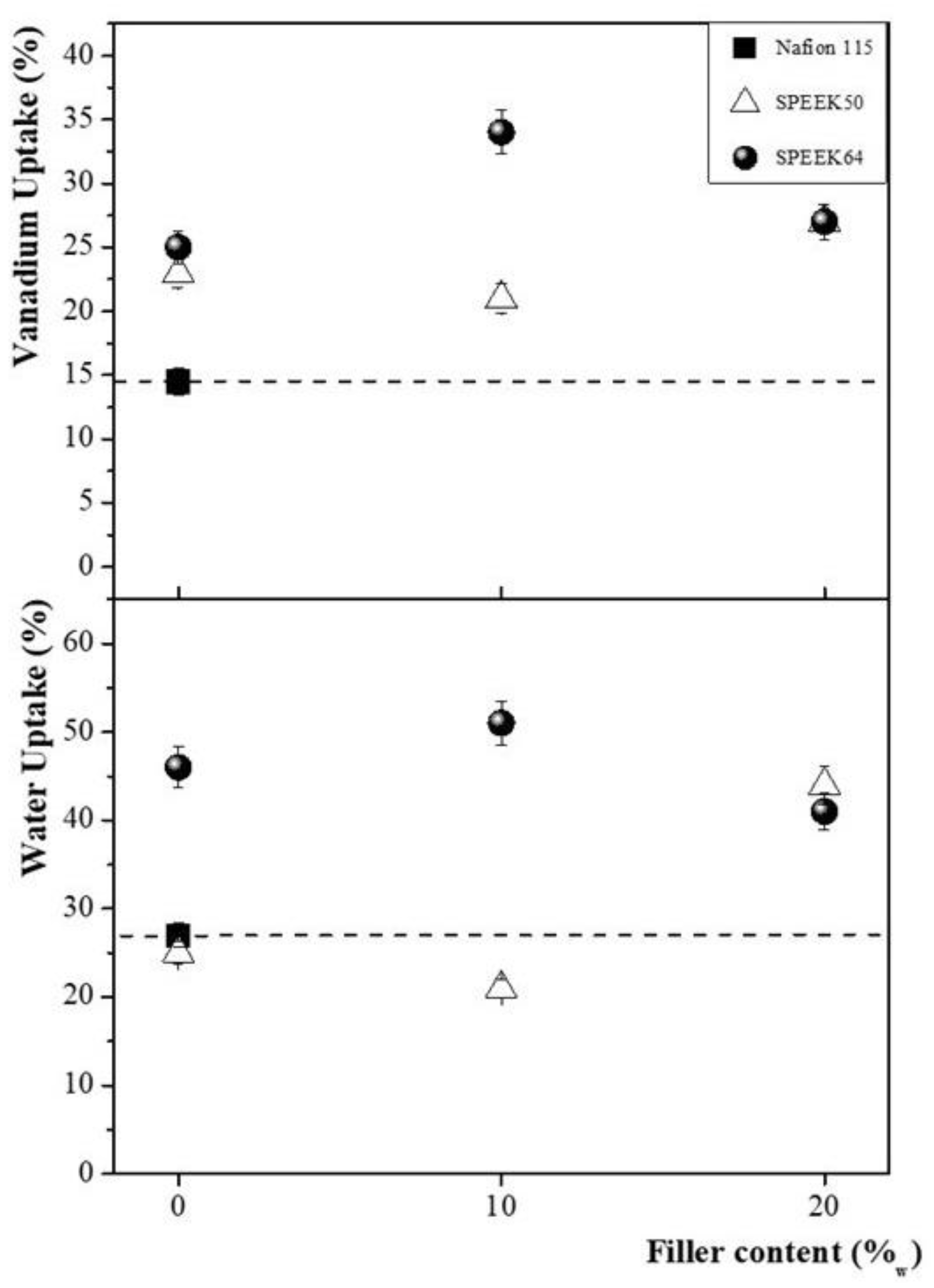
3.1. Membranes Characterization
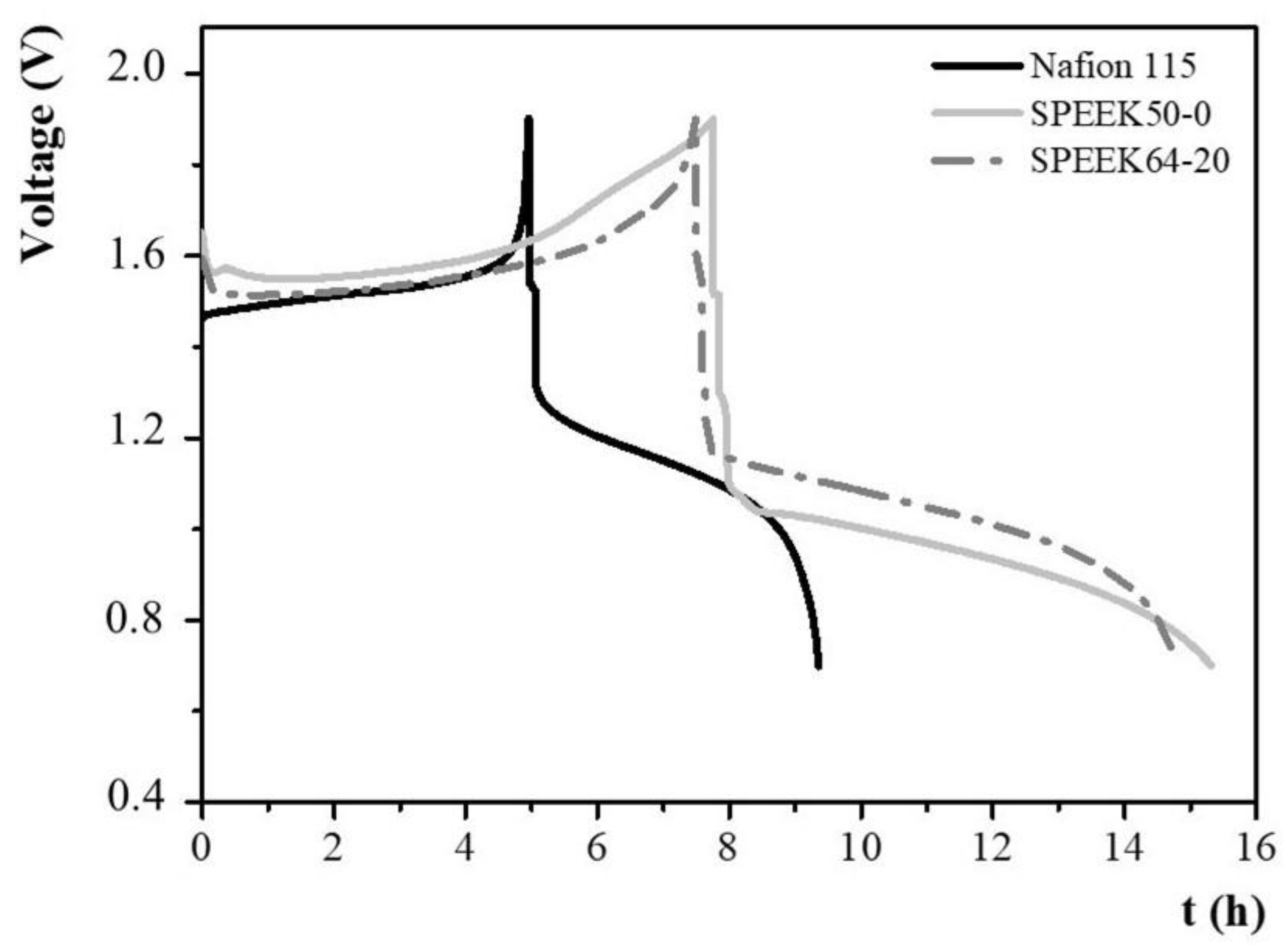
3.2. Single-Cell VRFB Tests
3.3. Optimization of Single-Cell VRFB
3.4. Five-Cell VRFB Stack System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabi, A.G.; Onumaegbu, C.; Wilberforce, T.; Ramadan, M.; Abdelkareem, M.A.; Al–Alami, A.H. Critical Review of Energy Storage Systems. Energy 2021, 214, 118987. [Google Scholar] [CrossRef]
- Zhang, Z.; Ding, T.; Zhou, Q.; Sun, Y.; Qu, M.; Zeng, Z.; Ju, Y.; Li, L.; Wang, K.; Chi, F. A Review of Technologies and Applications on Versatile Energy Storage Systems. Renew. Sustain. Energy Rev. 2021, 148, 111263. [Google Scholar] [CrossRef]
- Nadeem, F.; Hussain, S.M.S.; Tiwari, P.K.; Goswami, A.K.; Ustun, T.S. Comparative Review of Energy Storage Systems, Their Roles, and Impacts on Future Power Systems. IEEE Access 2019, 7, 4555–4585. [Google Scholar] [CrossRef]
- Jiang, H.R.; Sun, J.; Wei, L.; Wu, M.C.; Shyy, W.; Zhao, T.S. A High Power Density and Long Cycle Life Vanadium Redox Flow Battery. Energy Storage Mater. 2020, 24, 529–540. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium Redox Flow Batteries: A Review Oriented to Fluid-Dynamic Optimization. Energies 2021, 14, 176. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Redox Flow Batteries for Energy Storage: Their Promise, Achievements and Challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Huang, Z.; Mu, A.; Wu, L.; Yang, B.; Qian, Y.; Wang, J. Comprehensive Analysis of Critical Issues in All-Vanadium Redox Flow Battery. ACS Sustain. Chem. Eng. 2022, 10, 7786–7810. [Google Scholar] [CrossRef]
- Ye, J.; Yuan, D.; Ding, M.; Long, Y.; Long, T.; Sun, L.; Jia, C. A Cost-Effective Nafion/Lignin Composite Membrane with Low Vanadium Ion Permeation for High Performance Vanadium Redox Flow Battery. J. Power Sources 2021, 482, 229023. [Google Scholar] [CrossRef]
- Dassisti, M.; Cozzolino, G.; Chimienti, M.; Rizzuti, A.; Mastrorilli, P.; L’Abbate, P. Sustainability of Vanadium Redox-Flow Batteries: Benchmarking Electrolyte Synthesis Procedures. Int. J. Hydrogen Energy 2016, 41, 16477–16488. [Google Scholar] [CrossRef]
- Puleston, T.; Clemente, A.; Costa-Castelló, R.; Serra, M. Modelling and Estimation of Vanadium Redox Flow Batteries: A Review. Batteries 2022, 8, 121. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Crawford, A.J.; Thomsen, E.C.; Shamim, N.; Li, G.; Huang, Q.; Reed, D.M. An Overview of the Design and Optimized Operation of Vanadium Redox Flow Batteries for Durations in the Range of 4–24 Hours. Batteries 2023, 9, 221. [Google Scholar] [CrossRef]
- Maleki, M.; El-Nagar, G.A.; Bernsmeier, D.; Schneider, J.; Roth, C. Fabrication of an Efficient Vanadium Redox Flow Battery Electrode Using a Free-Standing Carbon-Loaded Electrospun Nanofibrous Composite. Sci. Rep. 2020, 10, 11153. [Google Scholar] [CrossRef]
- Zhang, L.; Yue, J.; Deng, Q.; Ling, W.; Zhou, C.-J.; Zeng, X.-X.; Zhou, C.; Wu, X.-W.; Wu, Y. Preparation of a Porous Graphite Felt Electrode for Advance Vanadium Redox Flow Batteries. RSC Adv. 2020, 10, 13374–13378. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Eifert, L.; Zeis, R. Characterization of Carbon Felt Electrodes for Vanadium Redox Flow Batteries—A Pore Network Modeling Approach. J. Energy Storage 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Chen, S.; Sun, C.; Zhang, H.; Yu, H.; Wang, W. Electrochemical Deposition of Bismuth on Graphite Felt Electrodes: Influence on Negative Half-Cell Reactions in Vanadium Redox Flow Batteries. Appl. Sci. 2024, 14, 3316. [Google Scholar] [CrossRef]
- Düerkop, D.; Widdecke, H.; Schilde, C.; Kunz, U.; Schmiemann, A. Polymer Membranes for All-Vanadium Redox Flow Batteries: A Review. Membranes 2021, 11, 214. [Google Scholar] [CrossRef]
- Cunha, Á.; Martins, J.; Rodrigues, N.; Brito, F.P. Vanadium Redox Flow Batteries: A Technology Review. Int. J. Energy Res. 2015, 39, 889–918. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An Efficient Barrier toward Vanadium Crossover in Redox Flow Batteries: The Bilayer [Nafion/(WO3)x] Hybrid Inorganic-Organic Membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Kear, G.; Shah, A.A.; Walsh, F.C. Development of the All-Vanadium Redox Flow Battery for Energy Storage: A Review of Technological, Financial and Policy Aspects. Int. J. Energy Res. 2012, 36, 1105–1120. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.; Yang, J.H.; Kim, H.-T. A Review of Vanadium Electrolytes for Vanadium Redox Flow Batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Feng, J.-K. Research Progress in Preparation of Electrolyte for All-Vanadium Redox Flow Battery. J. Ind. Eng. Chem. 2023, 118, 33–43. [Google Scholar] [CrossRef]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium Redox Flow Batteries: A Comprehensive Review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Jirabovornwisut, T.; Arpornwichanop, A. A Review on the Electrolyte Imbalance in Vanadium Redox Flow Batteries. Int. J. Hydrogen Energy 2019, 44, 24485–24509. [Google Scholar] [CrossRef]
- Ding, M.; Liu, T.; Zhang, Y.; Liu, H.; Pan, D.; Chen, L. Physicochemical and Electrochemical Characterization of Vanadium Electrolyte Prepared with Different Grades of V2O5 Raw Materials. Energies 2021, 14, 5958. [Google Scholar] [CrossRef]
- Choi, H.; Mandal, D.; Kim, H. Synthesis of a Low-Cost V3.5+ Electrolyte for Vanadium Redox Flow Batteries through the Catalytic Reduction of V2O5. ACS Sustain. Chem. Eng. 2022, 10, 17143–17150. [Google Scholar] [CrossRef]
- Yu, L.; Xi, J. Durable and Efficient PTFE Sandwiched SPEEK Membrane for Vanadium Flow Batteries. ACS Appl. Mater. Interfaces 2016, 8, 23425–23430. [Google Scholar] [CrossRef]
- Ding, M.; Ling, X.; Yuan, D.; Cheng, Y.; Wu, C.; Chao, Z.-S.; Sun, L.; Yan, C.; Jia, C. SPEEK Membrane of Ultrahigh Stability Enhanced by Functionalized Carbon Nanotubes for Vanadium Redox Flow Battery. Front. Chem. 2018, 6, 286. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; He, Z.; Das, R.; Dong, B.; Xie, X.; Guo, Z. An Overview of Amphoteric Ion Exchange Membranes for Vanadium Redox Flow Batteries. J. Mater. Sci. Technol. 2021, 69, 212–227. [Google Scholar] [CrossRef]
- Sharma, J.; Kulshrestha, V. Advancements in Polyelectrolyte Membrane Designs for Vanadium Redox Flow Battery (VRFB). Results Chem. 2023, 5, 100892. [Google Scholar] [CrossRef]
- Zhao, N.; Platt, A.; Riley, H.; Qiao, R.; Neagu, R.; Shi, Z. Strategy towards High Ion Selectivity Membranes for All-Vanadium Redox Flow Batteries. J. Energy Storage 2023, 72, 108321. [Google Scholar] [CrossRef]
- Thiam, B.G.; Vaudreuil, S. Review—Recent Membranes for Vanadium Redox Flow Batteries. J. Electrochem. Soc. 2021, 168, 070553. [Google Scholar] [CrossRef]
- Shi, Y.; Eze, C.; Xiong, B.; He, W.; Zhang, H.; Lim, T.M.; Ukil, A.; Zhao, J. Recent Development of Membrane for Vanadium Redox Flow Battery Applications: A Review. Appl. Energy 2019, 238, 202–224. [Google Scholar] [CrossRef]
- Wang, T.; Moon, S.J.; Hwang, D.-S.; Park, H.; Lee, J.; Kim, S.; Lee, Y.M.; Kim, S. Selective Ion Transport for a Vanadium Redox Flow Battery (VRFB) in Nano-Crack Regulated Proton Exchange Membranes. J. Membr. Sci. 2019, 583, 16–22. [Google Scholar] [CrossRef]
- Qian, P.; Zhou, W.; Zhang, Y.; Chao, D.; Song, M. Review and Perspectives of Sulfonated Poly(Ether Ether Ketone) Proton Exchange Membrane for Vanadium Flow Batteries. Energy Fuels 2023, 37, 17681–17707. [Google Scholar] [CrossRef]
- Prifti, H.; Parasuraman, A.; Winardi, S.; Lim, T.M.; Skyllas-Kazacos, M. Membranes for Redox Flow Battery Applications. Membranes 2012, 2, 275–306. [Google Scholar] [CrossRef]
- Minke, C.; Turek, T. Economics of Vanadium Redox Flow Battery Membranes. J. Power Sources 2015, 286, 247–257. [Google Scholar] [CrossRef]
- Winardi, S.; Raghu, S.C.; Oo, M.O.; Yan, Q.; Wai, N.; Lim, T.M.; Skyllas-Kazacos, M. Sulfonated Poly (Ether Ether Ketone)-Based Proton Exchange Membranes for Vanadium Redox Battery Applications. J. Membr. Sci. 2014, 450, 313–322. [Google Scholar] [CrossRef]
- Bai, E.; Zhu, H.; Sun, C.; Liu, G.; Xie, X.; Xu, C.; Wu, S. A Comparative Study of Nafion 212 and Sulfonated Poly(Ether Ether Ketone) Membranes with Different Degrees of Sulfonation on the Performance of Iron-Chromium Redox Flow Battery. Membranes 2023, 13, 820. [Google Scholar] [CrossRef]
- Carbone, A.; Castriciano, M.A.; Monsù Scolaro, L.; Gatto, I. Novel Polymeric Composite TPPS/s-PEEK Membranes for Low Relative Humidity PEFC. Polymers 2020, 12, 1431. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Liu, B.; Wang, H.; Shi, H. Sulfonated Polysulfone Proton Exchange Membrane Influenced by a Varied Sulfonation Degree for Vanadium Redox Flow Battery. J. Membr. Sci. 2019, 584, 173–180. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, Y.; Wang, H.; Ge, J.; Shi, H. Sulfonated Poly(Ether Ether Ketone) Hybrid Membranes with Amphoteric Graphene Oxide Nanosheets as Interfacial Reinforcement for Vanadium Redox Flow Battery. Energy Fuels 2020, 34, 2452–2461. [Google Scholar] [CrossRef]
- Ye, J.; Wu, C.; Qin, W.; Zhong, F.; Ding, M. Advanced Sulfonated Poly(Ether Ether Ketone)/Graphene-Oxide/Titanium Dioxide Nanoparticle Composited Membrane with Superior Cyclability for Vanadium Redox Flow Battery. J. Nanosci. Nanotechnol. 2020, 20, 4714–4721. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.; Oh, K.; Shanmugam, S. A Sulfonated Poly(Arylene Ether Ketone)/Polyoxometalate–Graphene Oxide Composite: A Highly Ion Selective Membrane for All Vanadium Redox Flow Batteries. Chem. Commun. 2017, 53, 917–920. [Google Scholar] [CrossRef]
- Lou, X.; Ye, J.; Xia, L.; Chang, S.; Zhao, X.; Wu, C.; Ding, M. Highly Efficient and Low Cost SPEEK/TiO2 Nanocomposite Membrane for Vanadium Redox Flow Battery. J. Nanosci. Nanotechnol. 2019, 19, 2247–2252. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid Inorganic-Organic Proton-Conducting Membranes Based on SPEEK Doped with WO3 Nanoparticles for Application in Vanadium Redox Flow Batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Palanisamy, G.; Oh, T.H. TiO2 Containing Hybrid Composite Polymer Membranes for Vanadium Redox Flow Batteries. Polymers 2022, 14, 1617. [Google Scholar] [CrossRef]
- Carbone, A.; Gatto, I.; Ohira, A.; Wu, L.; Passalacqua, E. Influence of Post-Casting Treatments on Sulphonated Polyetheretherketone Composite Membranes. J. Power Sources 2010, 195, 6037–6042. [Google Scholar] [CrossRef]
- Lee, D.; Yu, D.M.; Yoon, S.J.; Kim, S.; So, S.; Hong, Y.T. Aminopropyl Functionalized Silica Nanoparticle Dispersed Nafion Composite Membranes for Vanadium Redox Flow Batteries. Membr. J. 2020, 30, 307–318. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Saccà, A.; Gatto, I.; Passalacqua, E. Composite S-PEEK Membranes for Medium Temperature Polymer Electrolyte Fuel Cells. J. Power Sources 2008, 178, 661–666. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Portale, G.; Longo, A.; D’Ilario, L.; Passalacqua, E. Sulphonated Poly(Ether Ether Ketone) Membranes for Fuel Cell Application: Thermal and Structural Characterisation. J. Power Sources 2006, 163, 18–26. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Pedicini, R.; Gatto, I.; Passalacqua, E. Composite sPEEK Membranes for Vanadium Redox Batteries Application. Procedia Eng. 2012, 44, 1041–1043. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Pedicini, R.; Portale, G.; D’Ilario, L.; Longo, A.; Martorana, A.; Passalacqua, E. Structural and Electrochemical Investigation on Re-Cast Nafion Membranes for Polymer Electrolyte Fuel Cells (PEFCs) Application. J. Membr. Sci. 2006, 278, 105–113. [Google Scholar] [CrossRef]
- Semiz, L.; Demirci Sankir, N.; Sankir, M. Influence of the Basic Membrane Properties of the Disulfonated Poly(Arylene Ether Sulfone) Copolymer Membranes on the Vanadium Redox Flow Battery Performance. J. Membr. Sci. 2014, 468, 209–215. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Khastgir, D. Hybrid Composite Membranes of Chitosan/Sulfonated Polyaniline/Silica as Polymer Electrolyte Membrane for Fuel Cells. Carbohydr. Polym. 2018, 179, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, N.A.; Echeverri-Cuartas, C.E.; López, B.L. Composite Membranes Based on Functionalized Mesostructured Cellular Foam Particles and Sulfonated Poly(Ether Ether Sulfone) with Potential Application in Fuel Cells. Membranes 2022, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Ding, Y.; Liu, B.; Han, X.; Song, Y. Novel Sulfonated Poly (Ether Ether Keton)/Polyetherimide Acid-Base Blend Membranes for Vanadium Redox Flow Battery Applications. Electrochim. Acta 2014, 130, 90–96. [Google Scholar] [CrossRef]
- Shukla, G.; Shahi, V.K. Amine Functionalized Graphene Oxide Containing C16 Chain Grafted with Poly(Ether Sulfone) by DABCO Coupling: Anion Exchange Membrane for Vanadium Redox Flow Battery. J. Membr. Sci. 2019, 575, 109–117. [Google Scholar] [CrossRef]
- Chen, D.; Hickner, M.A. V5+ Degradation of Sulfonated Radel Membranes for Vanadium Redox Flow Batteries. Phys. Chem. Chem. Phys. 2013, 15, 11299–11305. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Schwenzer, B.; Kim, S.; Yang, Z.; Thevuthasan, S.; Liu, J.; Graff, G.L.; Hu, J. Investigation of Local Environments in Nafion–SiO2 Composite Membranes Used in Vanadium Redox Flow Batteries. Solid State Nucl. Magn. Reson. 2012, 42, 71–80. [Google Scholar] [CrossRef]



| Membrane | (cm2·s−1) | (106 S·s·cm−3) | σ (S·cm−1) | σafterVuptake (S·cm−1) | σloss (%) |
|---|---|---|---|---|---|
| Nafion 115 | 1.52 10−8 | 5.9 | 9.00·10−2 | 5.52·10−2 | 38 |
| SPEEK50-0 | 3.23 10−10 | 49.2 | 1.59·10−2 | 1.39·10−2 | 13 |
| SPEEK50-10 | 3.55 10−10 | 17.2 | 1.59·10−2 | 6.12·10−3 | 62 |
| SPEEK50-20 | 1.22 10−9 | 15.9 | 2.60·10−2 | 1.94·10−2 | 25 |
| SPEEK64-0 | 8.60 10−10 | 25.3 | 2.18·10−2 | 9.25·10−3 | 58 |
| SPEEK64-10 | 2.60 10−9 | 18.8 | 4.90·10−2 | 3.46·10−2 | 29 |
| SPEEK64-20 | 9.09 10−10 | 27.5 | 2.00·10−2 | 1.60·10−2 | 20 |
| Membrane | Cycle Time (h) | Mean Charge Voltage (V) | Mean Discharge Voltage (V) | Coulombic Efficiency (%) |
|---|---|---|---|---|
| Nafion 115 | 9.35 | 1.53 | 1.12 | 85.5 |
| SPEEK50-0 | 15.32 | 1.64 | 0.93 | 96.1 |
| SPEEK64-20 | 14.79 | 1.58 | 1.02 | 97.1 |
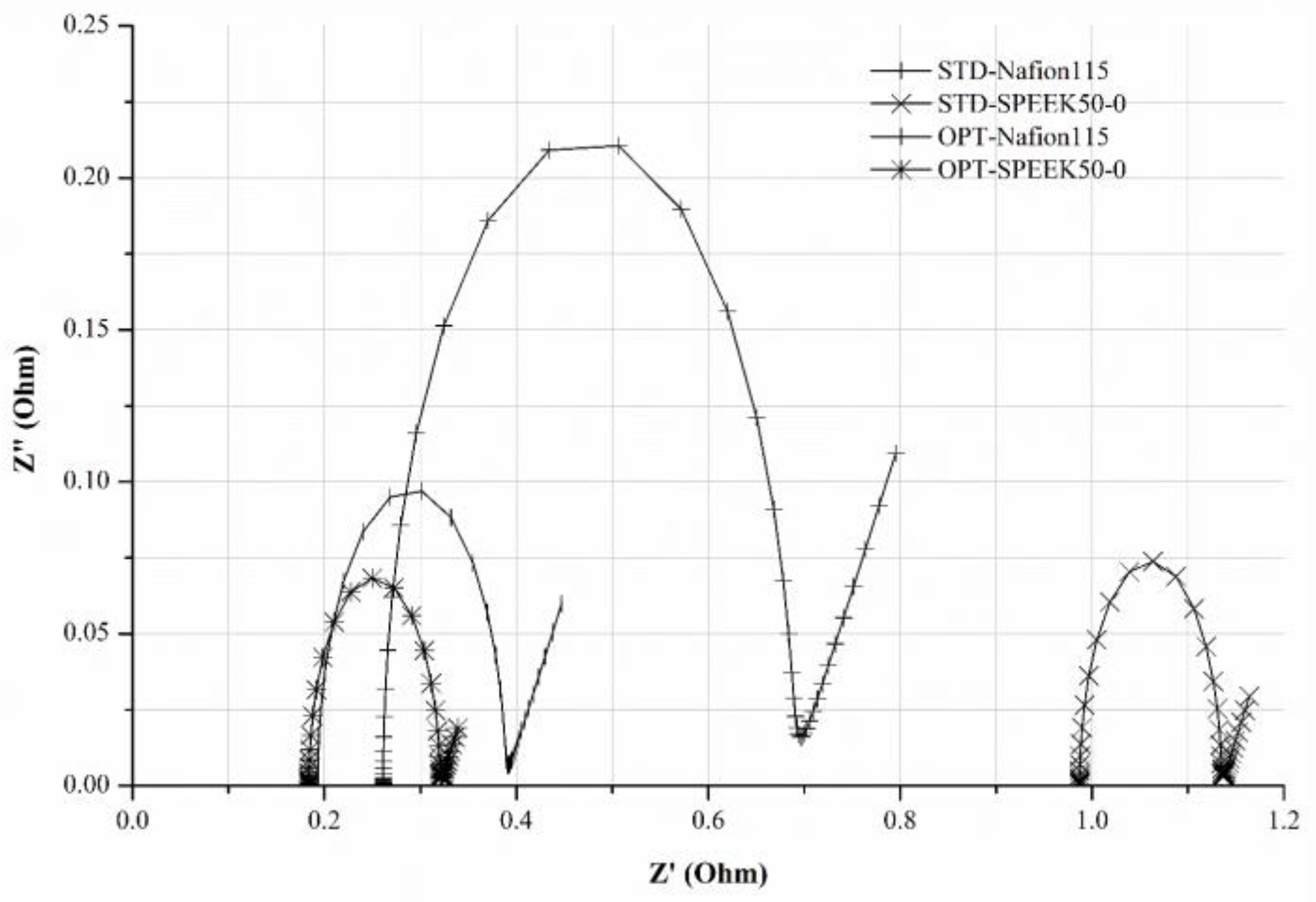
| Configuration | Rs (mΩ) | Rct (mΩ) | CPE (mF) | W (S s0.5) | Rtot (mΩ) |
|---|---|---|---|---|---|
| STD-Nafion115 | 261 | 425 | 8.5 | 36.5 | 686 |
| STD-SPEEK50-0 | 987 | 147 | 7.0 | 136 | 1134 |
| OPT-Nafion115 | 193 | 195 | 9.8 | 66.7 | 388 |
| OPT-SPEEK50-0 | 184 | 136 | 11.5 | 210 | 320 |
| Operating Parameter | Details |
|---|---|
| Electrolyte temperature | 25 ± 2 °C |
| Optimized composition of the electrolyte | [V3+] = 1.25 M, [VO2+] = 1.25 M [SO42−] = 2.50 M [Cl−] = 6.00 M |
| Anode face velocity | 1.5 cm·s−1 |
| Cathode face velocity | 1.5 cm·s−1 |
| Charge method | Constant current |
| Charge current density (cycle 1) | 20 mA·cm−2 |
| Charge current density (cycle > 1) | 20–60 mA·cm−2 |
| Top-of-charge cut-off voltage (per cell) | 1.7–2.0 V |
| Discharge method | Constant current |
| Discharge current density (cycle 1) | 20–100 mA·cm−2 |
| Discharge current density (cycle > 1) | 20–100 mA·cm−2 |
| Bottom-of-discharge cut-off voltage (per cell) | 0.7 V |
| Carbon felt | GFD SGL 4.6 |
| Compression rate | 0.2 |
| Electrode area | 459 cm2 (17 cm × 27 cm) |
| Bipolar Plate | PPG86 SGL |
| Membrane | Optimized SPEEK |
| Cycle | Current Density (mA/cm2) | Current (A) | Electrolyte Volume (L) | Discharge Capacity (Ah/L) | Coulombic Efficiency (%) | Mean Charge Voltage (V) | Mean Discharge Voltage (V) |
|---|---|---|---|---|---|---|---|
| 1 * | 20 | 9.2 | 3 | - | - | 8.7 | 3.9 |
| 2 | 20 | 9.2 | 3 | 6.7 | 94.5 | 8.6 | 3.9 |
| 3 | 50 | 23.0 | 3 | 7.1 | 87.1 | 8.8 | 3.8 |
| 4 | 50 | 23.0 | 5 | 6.2 | 87.3 | 8.8 | 3.8 |
| 5 | 80 | 36.7 | 5 | 5.5 | 79.5 | 8.9 | 3.6 |
| 6 | 100 | 44.3 | 5 | 4.9 | 74.0 | 9.6 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzuti, A.; Dilonardo, E.; Cozzolino, G.; Matera, F.; Carbone, A.; Musio, B.; Mastrorilli, P. Optimized Sulfonated Poly(Ether Ether Ketone) Membranes for In-House Produced Small-Sized Vanadium Redox Flow Battery Set-Up. Membranes 2024, 14, 176. https://doi.org/10.3390/membranes14080176
Rizzuti A, Dilonardo E, Cozzolino G, Matera F, Carbone A, Musio B, Mastrorilli P. Optimized Sulfonated Poly(Ether Ether Ketone) Membranes for In-House Produced Small-Sized Vanadium Redox Flow Battery Set-Up. Membranes. 2024; 14(8):176. https://doi.org/10.3390/membranes14080176
Chicago/Turabian StyleRizzuti, Antonino, Elena Dilonardo, Gennaro Cozzolino, Fabio Matera, Alessandra Carbone, Biagia Musio, and Piero Mastrorilli. 2024. "Optimized Sulfonated Poly(Ether Ether Ketone) Membranes for In-House Produced Small-Sized Vanadium Redox Flow Battery Set-Up" Membranes 14, no. 8: 176. https://doi.org/10.3390/membranes14080176
APA StyleRizzuti, A., Dilonardo, E., Cozzolino, G., Matera, F., Carbone, A., Musio, B., & Mastrorilli, P. (2024). Optimized Sulfonated Poly(Ether Ether Ketone) Membranes for In-House Produced Small-Sized Vanadium Redox Flow Battery Set-Up. Membranes, 14(8), 176. https://doi.org/10.3390/membranes14080176











