Influence of Iron and Magnesium on Fouling Properties of Organic Matter Solution in Membrane Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Solution Preparation and Characterization
2.2. Filtration Tests
2.3. Fouling Mechanism Analysis
2.3.1. Estimation of Fouling Resistances
2.3.2. Fouling Layer Properties
2.3.3. SEM Imaging
3. Results and Discussion
3.1. Characterization of SROM Feed Solution with Different Fe2+ and Mg2+ Concentrations
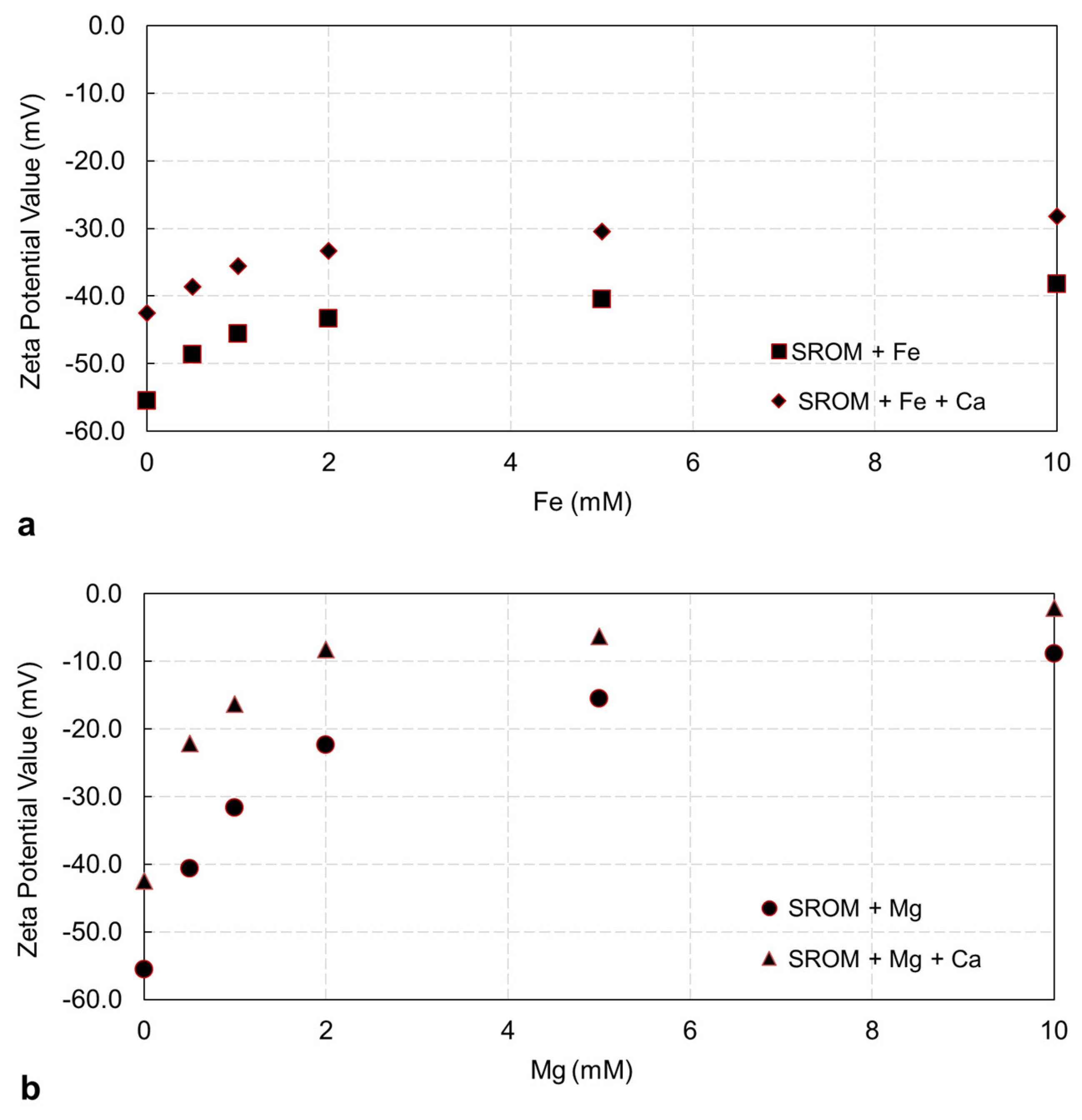
3.1.1. Zeta Potential of Feed Solutions
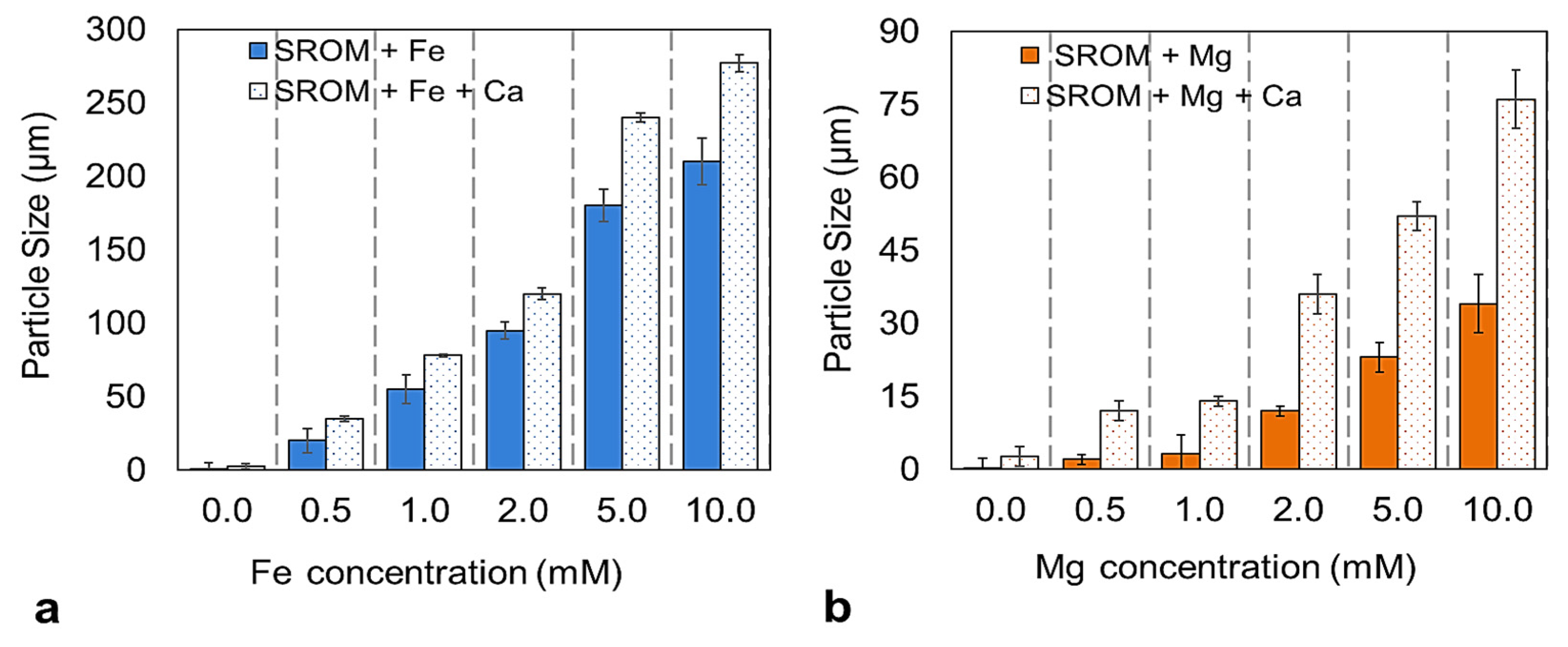
3.1.2. Particle Size Analysis of Feed Solutions
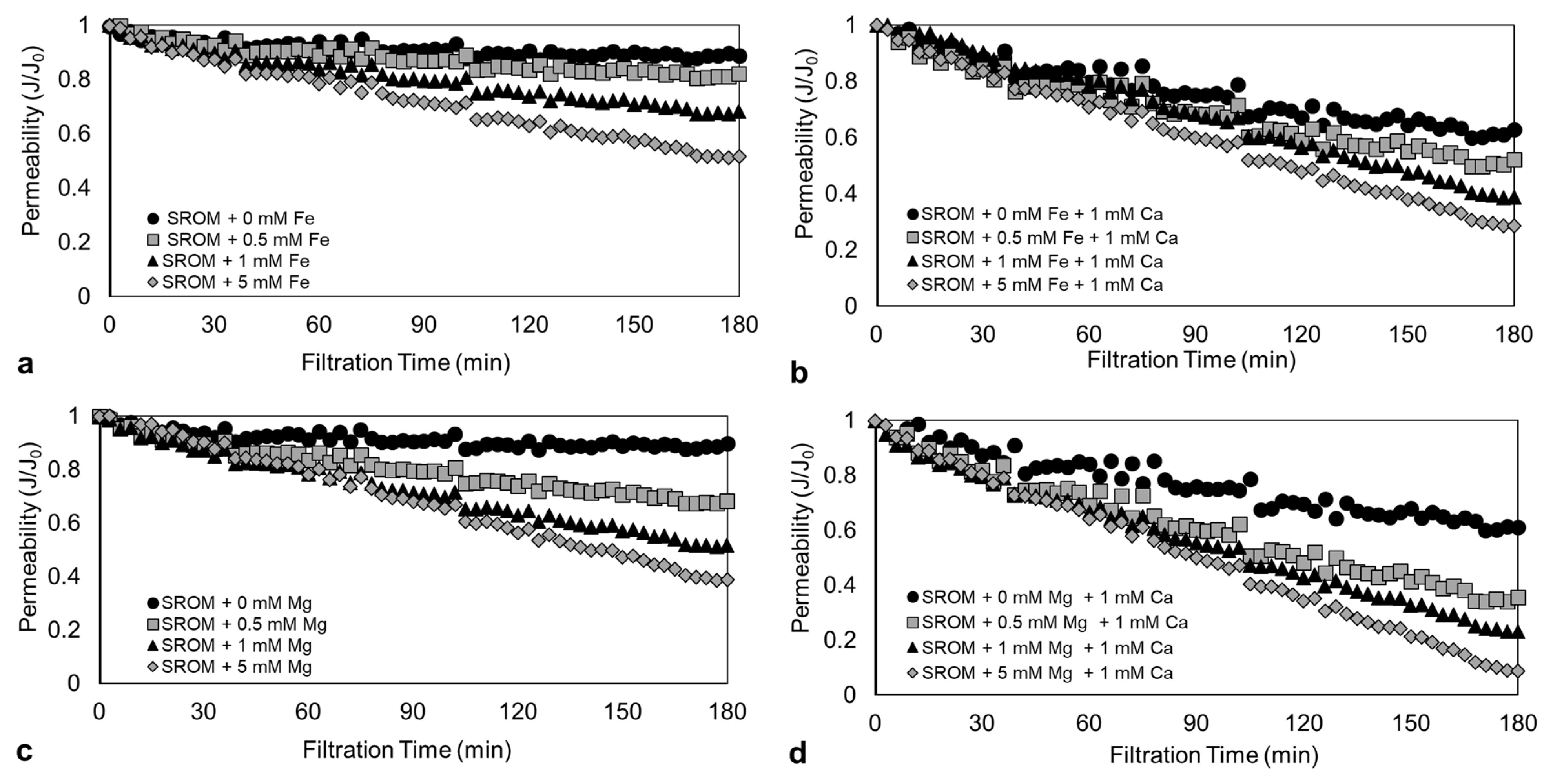
3.2. Effect of Fe2+ and Mg2+ Concentration on the Filtration Performance of SROM
3.3. Analysis of SROM Fouling with Different Fe2+ and Mg2+ Concentrations
3.3.1. Cake Layer and Pore Blocking Resistances
3.3.2. Fouling Layer Properties
3.3.3. SEM Analysis of Fouled Membranes
4. Conclusions
- Fe2+ and Mg2+ ions have a pronounced effect of the zeta potential of SROM, which resulted in less stable particles.
- Increasing the concentration of Fe2+ and Mg2+ from 0–5 mM promoted SROM fouling, and resulted in an increased flux decline of up to 33% and 58%, respectively.
- For SROM only, 75% of fouling was due to pore blocking, whereas the remaining 25% was due to cake filtration. The cake layer resistance became more dominant with the addition of Fe2+ and Mg2+, and was responsible for more than 60% of the fouling. Mg2+, however, caused higher internal pore blocking and facilitated the formation of a less permeable cake layer compared to Fe2+. This was evident in the analysis of the cake layer properties and the visualization of the fouling layer.
- In all cases, SROM fouling with Fe2+ and Mg2+ worsened in the presence of Ca2+ ions due to charge neutralization and aggregation of SROM; hence, it has a high fouling tendency.
- The results of the study indicate the importance of understanding the interaction between organic matter and Fe2+ and Mg2+, which would provide useful insights on their fouling mechanism and control. Future studies on the fouling behavior of other positively-charged ions and heavy metals from different water and wastewater sources in membrane processes are recommended.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Zularisam, A.W.; Ahmad, A.; Sakinah, M.; Ismail, A.F.; Matsuura, T. Role of natural organic matter (NOM), colloidal particles, and solution chemistry on ultrafiltration performance. Sep. Purif. Technol. 2011, 78, 189–200. [Google Scholar] [CrossRef]
- Akhondi, E.; Wicaksana, F.; Fane, A.G. Evaluation of fouling deposition, fouling reversibility and energy consumption of submerged hollow fiber membrane systems with periodic backwash. J. Membr. Sci. 2014, 452, 319–331. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, J.; Wang, Z.; Osterhus, S. Backpulsing technology applied in MF and UF processes for membrane fouling mitiga-tion: A review. J. Membr. Sci. 2019, 587, 117136. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Karabelas, A.; Mappas, V. Membrane Fouling Due to Protein—Polysaccharide Mixtures in Dead-End Ultra-filtration; the Effect of Permeation Flux on Fouling Resistance. Membranes 2019, 9, 21. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Organic fouling and chemical cleaning of nanofiltration membranes: Measurements and mechanisms. Environ. Sci. Technol. 2004, 38, 4683–4693. [Google Scholar] [CrossRef] [PubMed]
- Melián-Martel, N.; Alonso, J.J.S.; Ruiz-García, A. Combined silica and sodium alginate fouling of spiral-wound reverse osmosis membranes for seawater desalination. Desalination 2018, 439, 25–30. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; Elimelech, M. Complexation between dissolved silica and alginate molecules: Implications for reverse osmosis membrane fouling. J. Membr. Sci. 2020, 605, 118109. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, J.; Li, Y.; Chen, Q.; Xie, W.; Wang, J. Characterizing membrane fouling formation during ultrafiltration of high-salinity organic wastewater. Chemosphere 2022, 287, 132057. [Google Scholar] [CrossRef]
- Taheri, A.; Sim, L.; Haur, C.; Akhondi, E.; Fane, A. The fouling potential of colloidal silica and humic acid and their mixtures. J. Membr. Sci. 2013, 433, 112–120. [Google Scholar] [CrossRef]
- Philippe, A.; Schaumann, G. Interactions of dissolved organic matter with natural and engineered inorganic colloids: A review. Environ. Sci. Technol. 2014, 48, 8946–8962. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Soltani, A.; Zheng, X.; Ernst, M. Effect of inorganic colloidal water constituents on combined low-pressure mem-brane fouling with natural organic matter (NOM). J. Membr. Sci. 2016, 507, 154–164. [Google Scholar] [CrossRef]
- Li, Q.; Elimelech, M. Synergistic effects in combined fouling of a loose Nanofiltration membrane by colloidal materials and natural organic matter. J. Membr. Sci. 2006, 278, 72–82. [Google Scholar] [CrossRef]
- Springer, F.; Laborie, S.; Guigui, C. Removal of SiO2 nanoparticles from industry wastewaters and subsurface waters by ultra-filtration: Investigation of process efficiency, deposit properties and fouling mechanism. Sep. Purif. Technol. 2013, 108, 6–14. [Google Scholar] [CrossRef]
- Alresheedi, M. Understanding Protein and Polysaccharide Fouling with Silicon Dioxide and Aluminum Oxide in Low-Pressure Membranes. Membranes 2023, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tian, Y.; Li, Z.; Wang, H.; Chen, L. Microfiltration (MF) membrane fouling potential evaluation of protein with different ion strengths and divalent cations based on extended DLVO theory. Desalination 2013, 331, 62–68. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Ohmukai, Y.; Matsuyama, H.; Maruyama, T. Effect of metal ions on the protein fouling of hollow-fiber ultrafiltration membranes. Sep. Purif. Technol. 2013, 111, 137–144. [Google Scholar] [CrossRef]
- Mewes, A.; Langer, G.; de Nooijer, L.J.; Bijma, J.; Reichart, G.-J. Effect of different seawater Mg2+ concentrations on calcification in two benthic foraminifers. Mar. Micropaleontol. 2014, 113, 56–64. [Google Scholar] [CrossRef]
- Lin, M.; Hu, X.; Pan, D.; Han, H. Determination of iron in seawater: From the laboratory to in situ measurements. Talanta 2018, 188, 135–144. [Google Scholar] [CrossRef]
- Meng, S.; Wang, R.; Meng, X.; Wang, Y.; Fan, W.; Liang, D.; Zhang, M.; Liao, Y.; Tang, C. Reaction heterogeneity in the bridging effect of divalent cations on polysaccharide fouling. J. Membr. Sci. 2022, 641, 119933. [Google Scholar] [CrossRef]
- Sioutopoulos, D.; Yiantsios, S.; Karabelas, A. Relation between fouling characteristics of RO and UF membranes in experiments with colloidal organic and inorganic species. J. Membr. Sci. 2010, 350, 62–82. [Google Scholar] [CrossRef]
- Davidkova, D.; Graham, M.; Castrillón, S.; Semião, A. Influence of colloidal iron oxide and natural organic matter fouling on nanofiltration membrane performance: Role of feed composition and membrane properties. Environ. Sci. Water Res. Technol. 2023, 9, 2942–2953. [Google Scholar] [CrossRef]
- Xin, Y.; Bligh, M.; Kinsela, A.; Waite, D. Effect of iron on membrane fouling by alginate in the absence and presence of calcium. J. Membr. Sci. 2016, 497, 289–299. [Google Scholar] [CrossRef]
- Wang, L.-F.; He, D.-Q.; Chen, W.; Yu, H.-Q. Probing the roles of Ca2+ and Mg2+ in humic acids-induced ultrafiltration membrane fouling using an integrated approach. Water Res. 2015, 81, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liang, D.; Liu, X.; Fan, W.; Meng, S.; Caib, W. Effect of magnesium ion on polysaccharide fouling. Chem. Eng. J. 2020, 379, 122351. [Google Scholar] [CrossRef]
- Zou, H.; Huang, J.; Zhang, M.; Lin, H.; Teng, J.; Huang, Z. Mitigation of protein fouling by magnesium ions and the related mechanisms in ultrafiltration process. Chemosphere 2023, 310, 136817. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Hermia, J. Blocking Filtration. Application to Non-Newtonian Fluids. In Mathematical Models and Design Methods in Solid-Liquid Separation; Rushton, A., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 83–89. [Google Scholar]
- Chellam, S.; Wendong, X. Blocking laws analysis of dead-end constant flux microfiltration of compressible cakes. J. Colloid Interf. Sci. 2006, 301, 248–257. [Google Scholar] [CrossRef]
- Foley, G. A review of factors affecting filter cake properties in dead-end microfiltration of microbial suspensions. J. Membr. Sci. 2006, 274, 38–46. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Shen, L.; Liao, B.; Wu, X.; Li, R. Effect of calcium ions on fouling properties of alginate solution and its mechanisms. J. Membr. Sci. 2017, 525, 320–329. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, X.; Wang, B.; Song, L. Calcium ion on membrane fouling reduction and bio-flocculation promotion in mem-brane bioreactor at high salt shock. Bioresour. Technol. 2016, 200, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.; Nakhla, G. Impact of calcium on the membrane fouling in membrane bioreactors. J. Membr. Sci. 2008, 314, 134–142. [Google Scholar] [CrossRef]
- Mahlangu, T.; Thwala, J.; Mamba, B.; D’Haese, A.; Verliefde, A. Factors governing combined fouling by organic and colloidal foulants in cross-flow nanofiltration. J. Membr. Sci. 2015, 491, 53–62. [Google Scholar] [CrossRef]
- Alresheedi, M.; Basu, O. Investigation into the temperature effect on NOM fouling and cleaning in submerged polymeric membrane systems. Desalination Water Treat. 2019, 142, 104–113. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, C.; Hu, J.; Ji, B.; Shao, S. Presence of iron oxide particles can reduce the cake fouling in UF systems. Sep. Purif. Technol. 2024, 342, 127015. [Google Scholar] [CrossRef]
- Alresheedi, M.; Barbeau, B.; Basu, O. Comparisons of NOM fouling and cleaning of ceramic and polymeric membranes during water treatment. Sep. Purif. Technol. 2019, 209, 452–460. [Google Scholar] [CrossRef]
- Alresheedi, M.; Basu, O.; Barbeau, B. Chemical cleaning of ceramic ultrafiltration membranes–Ozone versus conventional cleaning chemicals. Chemosphere 2019, 226, 668–677. [Google Scholar] [CrossRef]
- Mo, H.; Tay, K.G.; Ng, H.Y. Fouling of reverse osmosis membrane by protein (BSA): Effects of pH, calcium, magnesium, ionic strength and temperature. J. Membr. Sci. 2008, 315, 28–35. [Google Scholar] [CrossRef]


| Foulant | Specific Cake Resistance αc (m/gC), (×103) | Cake Compressibility Index (n) |
|---|---|---|
| SROM + 0 mM Fe2+ | 1.56 | 0.28 |
| SROM + 0.5 mM Fe2+ | 2.22 | 0.41 |
| SROM + 1 mM Fe2+ | 4.14 | 0.55 |
| SROM + 5 mM Fe2+ | 5.36 | 0.68 |
| SROM + 0 mM Fe2+ + 1 mM Ca2+ | 3.52 | 0.44 |
| SROM + 0.5 mM Fe2+ + 1 mM Ca2+ | 6.54 | 0.63 |
| SROM + 1 mM Fe2+ + 1 mM Ca2+ | 8.21 | 0.76 |
| SROM + 5 mM Fe2+ + 1 mM Ca2+ | 9.53 | 0.88 |
| Foulant | Specific Cake Resistance αc (m/gC), (×103) | Cake Compressibility Index (n) |
|---|---|---|
| SROM + 0 mM Mg2+ | 1.56 | 0.28 |
| SROM + 0.5 mM Mg2+ | 3.22 | 0.46 |
| SROM + 1 mM Mg2+ | 5.44 | 0.65 |
| SROM + 5 mM Mg2+ | 6.89 | 0.72 |
| SROM + 0 mM Mg2++ 1 mM Ca2+ | 3.52 | 0.44 |
| SROM + 0.5 mM Mg2+ + 1 mM Ca2+ | 7.88 | 0.71 |
| SROM + 1 mM Mg2+ + 1 mM Ca2+ | 9.13 | 0.85 |
| SROM + 5 mM Mg2+ + 1 mM Ca2+ | 9.96 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alresheedi, M.T. Influence of Iron and Magnesium on Fouling Properties of Organic Matter Solution in Membrane Process. Membranes 2024, 14, 150. https://doi.org/10.3390/membranes14070150
Alresheedi MT. Influence of Iron and Magnesium on Fouling Properties of Organic Matter Solution in Membrane Process. Membranes. 2024; 14(7):150. https://doi.org/10.3390/membranes14070150
Chicago/Turabian StyleAlresheedi, Mohammad T. 2024. "Influence of Iron and Magnesium on Fouling Properties of Organic Matter Solution in Membrane Process" Membranes 14, no. 7: 150. https://doi.org/10.3390/membranes14070150
APA StyleAlresheedi, M. T. (2024). Influence of Iron and Magnesium on Fouling Properties of Organic Matter Solution in Membrane Process. Membranes, 14(7), 150. https://doi.org/10.3390/membranes14070150





