1. Introduction
Several thousand species of stinging ants are known, being insects belonging to the family Formicidae and the order Hymenoptera [
1]. They are equipped with a venom that contains a true cocktail of bioactive substances [
2]. Much like other venomous animals, it is generally assumed that toxins in ant venom are employed for different purposes, such as predation and defense, being designed against bigger animals as well as much smaller microbial pathogens [
3]. The observed biological activities associated with ant toxins are impressively diverse and include paralytic, cytolytic, hemolytic, allergenic, pro-inflammatory, insecticidal, antimicrobial, and pain-producing pharmacologic activities [
4]. Non-toxic functions have also been reported and include a role in chemical communication involving sex pheromones and deterrents. Antimicrobial peptides (AMPs) are very well known, such as bicarinalin from the ant
Tetramorium bicarinatum [
5] and pilosulin from the ant
Myrmecia pilosula [
6]. They are often characterized by a broad-spectrum activity against Gram-positive and Gram-negative bacteria and against fungal pathogens. Ponericins from the neo-tropical ant
Neoponera commutata are another example, which are active as anthelmintic agents [
6]. Ant venoms are particular and distinct from other animal venoms since they are not only rich in linear and disulfide-rich peptides but also in volatile and non-volatile small molecules such as alkaloids and hydrocarbons. This is highlighted by stingless ants from the subfamily Formicinae, which spray their venoms through a special opening called the acidopore, with the well-known formic acid molecule as a key example, and its name is derived from this (sub)family [for a review, see [
4]].
Ant venom peptides have previously been classified based on their structure into three main groups: linear, dimeric or inhibitor cystine knot (ICK)-like peptides [for a review, see [
2]]. An alternative approach for classification follows their biological activity, with two main characteristics: cytolytic or neurotoxic. The wide prevalence of small linear peptides devoid of disulfide bonds, smaller than 35 residues, and with cytolytic activity has clearly been demonstrated in ant venoms. They often act as amphipathic, helical structures forming pores through biological membranes, disturbing the cellular integrity and facilitating the passage of other disulfide-rich neurotoxins to their molecular targets [
7]. In this manner, cytolytic peptides act synergistically with neurotoxins, a phenomenon also observed in spiders. As compared to other venomous animals, studies investigating neurotoxic ant venom peptides are relatively scarce. The best-known examples are (
i) poneratoxin, a small 25-residue peptide derived from the bullet ant
Paraponera clavate; it has been shown to modulate voltage-gated sodium (Nav) channels of both vertebrates and invertebrates, blocking synaptic transmission in the insect CNS [
8,
9], and (
ii) ectatomin Et-1, from the ant
Ectatomma tuberculatum [
10], which is a voltage-gated calcium (Cav) channel blocker and also a pore-forming peptide cytotoxic to vertebrate and invertebrate cells [
11,
12]. This dual pharmacological feature is an interesting observation and will be discussed further, given its relevance for this work. Different from pilosulins and ectatomins, myrmexins have been described as heterodimeric peptides isolated from the venom of
Pseudomyrmex triplarinus with an anti-inflammatory function [
13]. In a transcriptome analysis of the venom glands of the giant ant
Dinoponera quadriceps, another structural class of ant venom peptides was discovered: ICK-like peptides [
14]. They contain three disulfide bonds forming a pseudo knot and are very stable. They are also often present in the venoms of cone snails and spiders and typically have neurotoxic properties. They have proven to be great leads in drug discovery [
15]. Unfortunately, in the case of several disulfide-rich ant venom peptides, their role and biological target remain unknown at present. Apart from the presence of peptides in ant venom, accumulating data become available indicating the presence and importance of a number of proteins that, among other effects, cause neurotoxicity [for a review, see [
4]]. For example, in the venom of the fire ant
Solenopsis invicta, a protein homologous to U5-ctenitoxin-Pk1a-like protein, found in the venom of the spider
Phoneutria nigriventer, has been reported [
16] and is involved in causing spastic paralysis in mice [
17]. Furthermore, phospholipases have been described as important larger proteins present in several hymenopteran venoms and are considered potent neurotoxic, cytotoxic and allergenic proteins.
In more recent studies published since 2020, Touchard et al., using an integrated transcriptomic and proteomic approach, characterized the venom peptidome of the European red ant,
Manica rubida. They identified 13 myrmicitoxins that share sequence similarities with previously identified ant venom peptides, one of them being identified as an EGF-like toxin. Insecticidal assays of reversed-phase HPLC venom fractions on the blowfly
Lucilia caesar enabled the authors to identify six myrmicitoxins with insecticidal activity. It was concluded that
M. rubida employs a paralytic venom rich in linear insecticidal peptides, which likely act by disrupting cell membranes [
18]. In 2021, Robinson et al. characterized an O-linked glycopeptide, Mg7a, as a major component of the venom of the ant
Myrmecia gulosa. The authors showed that Mg7a is paralytic and lethal to insects and triggers pain behavior and inflammation in mammals, which it achieves through a membrane-targeting mode of action. Interestingly, the deglycosylation of Mg7a renders it insoluble in aqueous solution, suggesting a key solubilizing role of the O-glycans [
19]. Last year, Barassé et al. studied peptide U
11 from
Tetramorium bicarinatum, a 34-amino-acid peptide, with in-depth insecticidal, structural and pharmacological experiments [
20]. They concluded that U
11 is one of the most paralytic peptides ever reported from ant venoms against blowflies and is also capable of paralyzing honeybees. An NMR spectroscopy of U
11 uncovered a unique scaffold featuring a compact triangular ring helix structure stabilized by a single disulfide bond. Pharmacological assays using
Drosophila S2 cells demonstrated that U
11 is not cytotoxic but suggest that it may modulate potassium conductance, which structural data seem to corroborate [
20].
3. Results
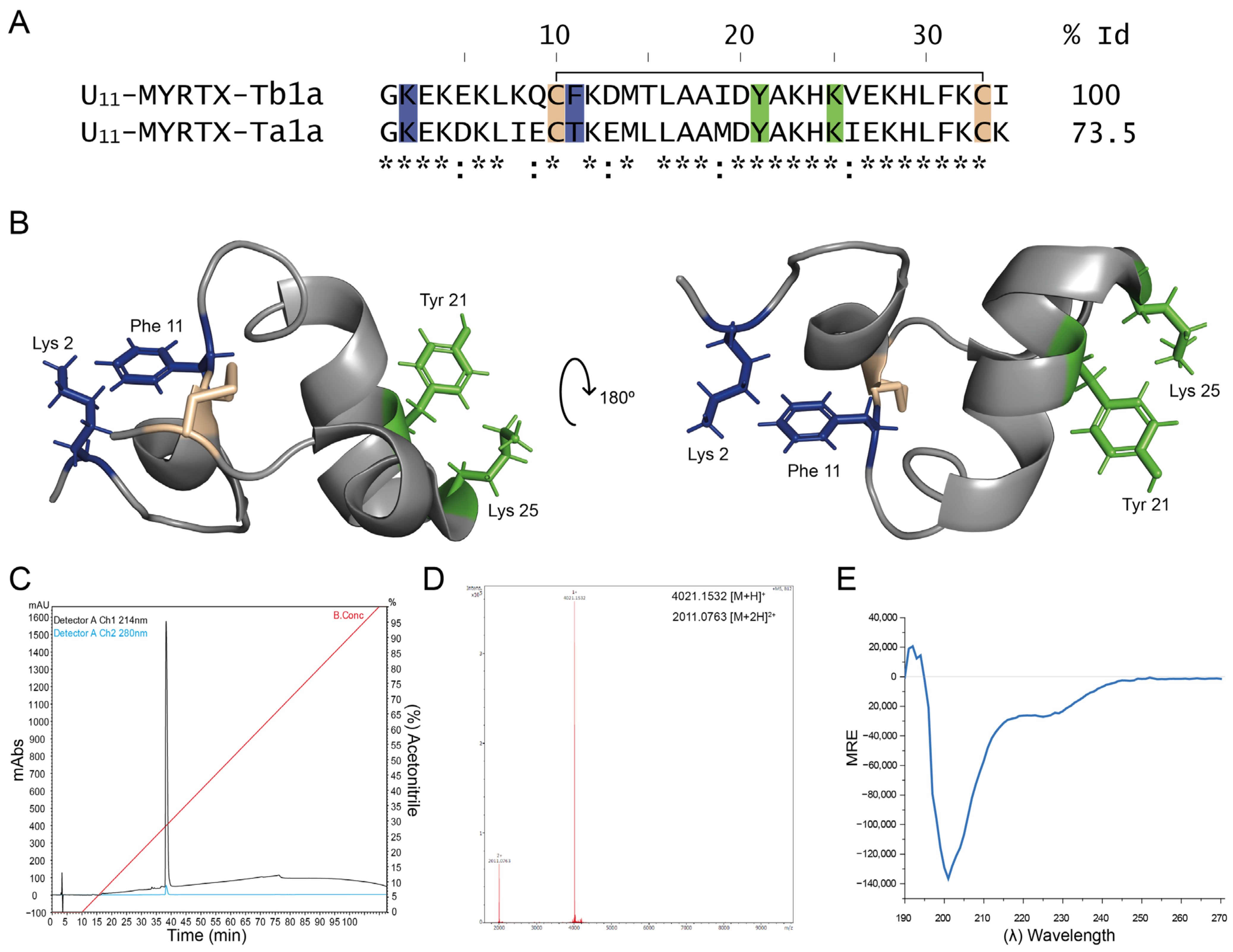
Peptide U
11-MYRTX-Tb1a, abbreviated as U
11 with ‘U’ referring to ‘unknown function’, from the venom of the ant
Tetramorium bicarinatum, is a 34-amino-acid peptide containing a single disulfide bond. A closely related peptide from the ant
Tetramorium africanum was also reported [
23], named peptide U
11-MYRTX-Ta1a, with 73.5% identity to U
11 and investigated by us here (
Figure 1A). NMR spectroscopy carried out by Barassé et al. (2023) revealed a unique scaffold for U
11 with a compact triangular ring helix structure stabilized by this disulfide bridge (
Figure 1B). Interestingly, several pore-blocking peptides of voltage-gated K channels have been described from different venomous animals, where two crucial residues, commonly known as ‘functional dyad’, take part in physically occluding the pore of these channels [
24]; this has also been shown by our group—see [
25]. The dyad is composed of a basic amino acid, lysine, protruding into the selectivity filter of the K channel and stabilized by a hydrophobic aromatic amino acid, usually a tyrosine or phenylalanine residue. Barassé et al. (2023) [
20] have noticed in their work the presence of two potential dyads in U
11: Lys2-Phe11 and Lys25-Tyr21. Residues of these functional dyads are illustrated in a ribbon presentation in blue and green (
Figure 1B). Since residue Phe11 is not conserved between the two
Tetramorium species, Barassé et al. (2023) [
20] suggested that Lys25-Tyr21 appears more likely as the putative pharmacophore. This hypothesis was further strengthened by the observation that it superimposes the dyad in the sea anemone peptide BgK (PDB: 1BGK), with a similar distance of approximately 7.2 Å between the two residues. BgK is a peptide with the ShKT fold that is widely distributed in nature. Many of these peptides block voltage-gated K channels with interesting selectivity and/or impressive affinity [
26]. The obvious presence of such putative pharmacophore, as hypothesized by Barassé et al. (2023) [
20], was the rationale for us to check whether U
11 is indeed able to block voltage-gated K channels, and if so, which one(s) and how potently? In the next section, we have therefore undertaken an extensive screening of many types of voltage-gated K channels.
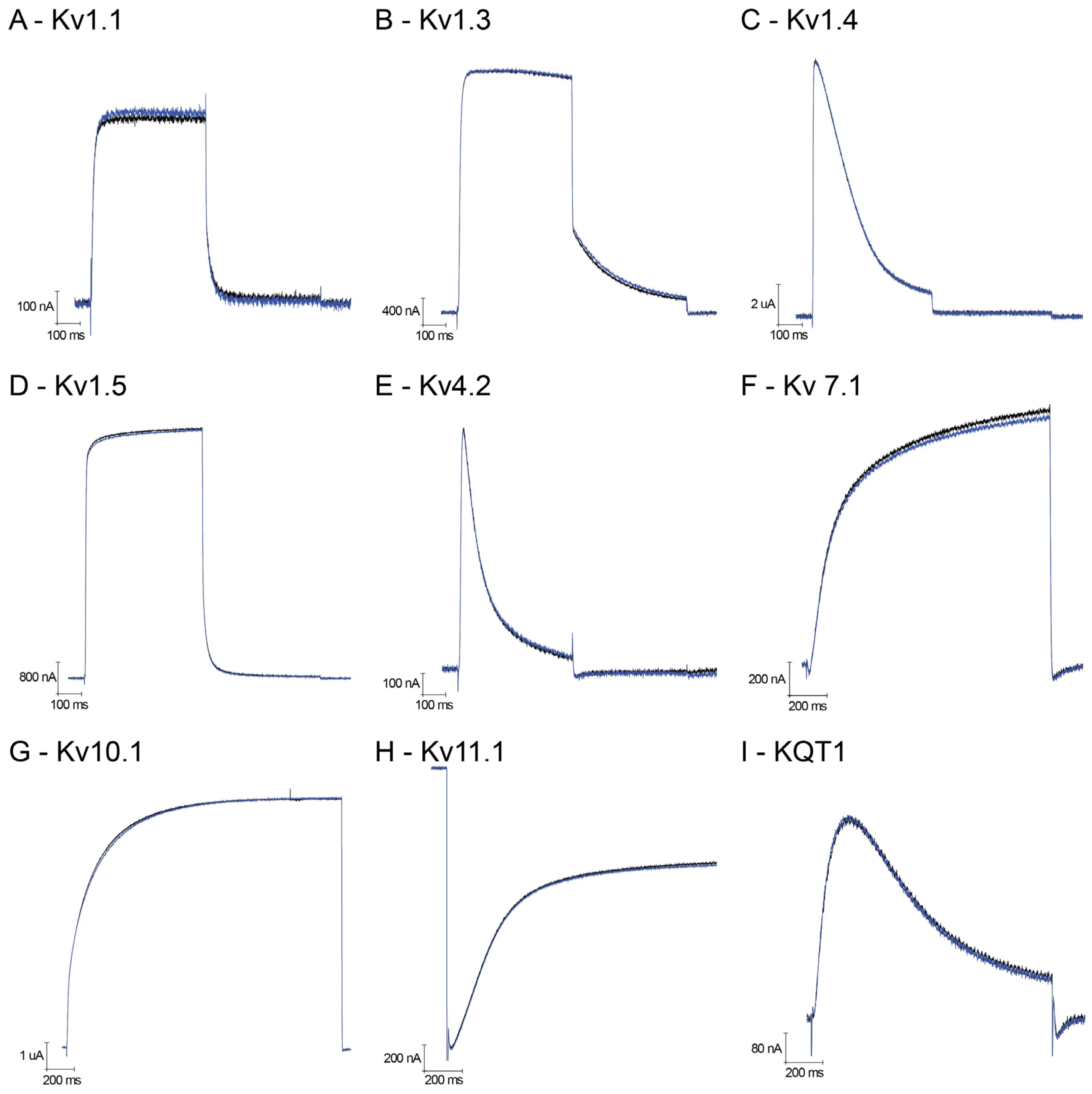
Using the heterologous
Xenopus laevis expression system, we have been able to functionally study voltage-gated potassium channels belonging to different families: from the Shaker family, Kv1.1, Kv1.3, Kv1.4, Kv1.5 and
Shaker IR (‘Inactivation Removed’); from the
Shal family, Kv4.2; and together with Kv10.1 (known as
EAG), Kv11.1 (known as
ERG) and KQT-1.
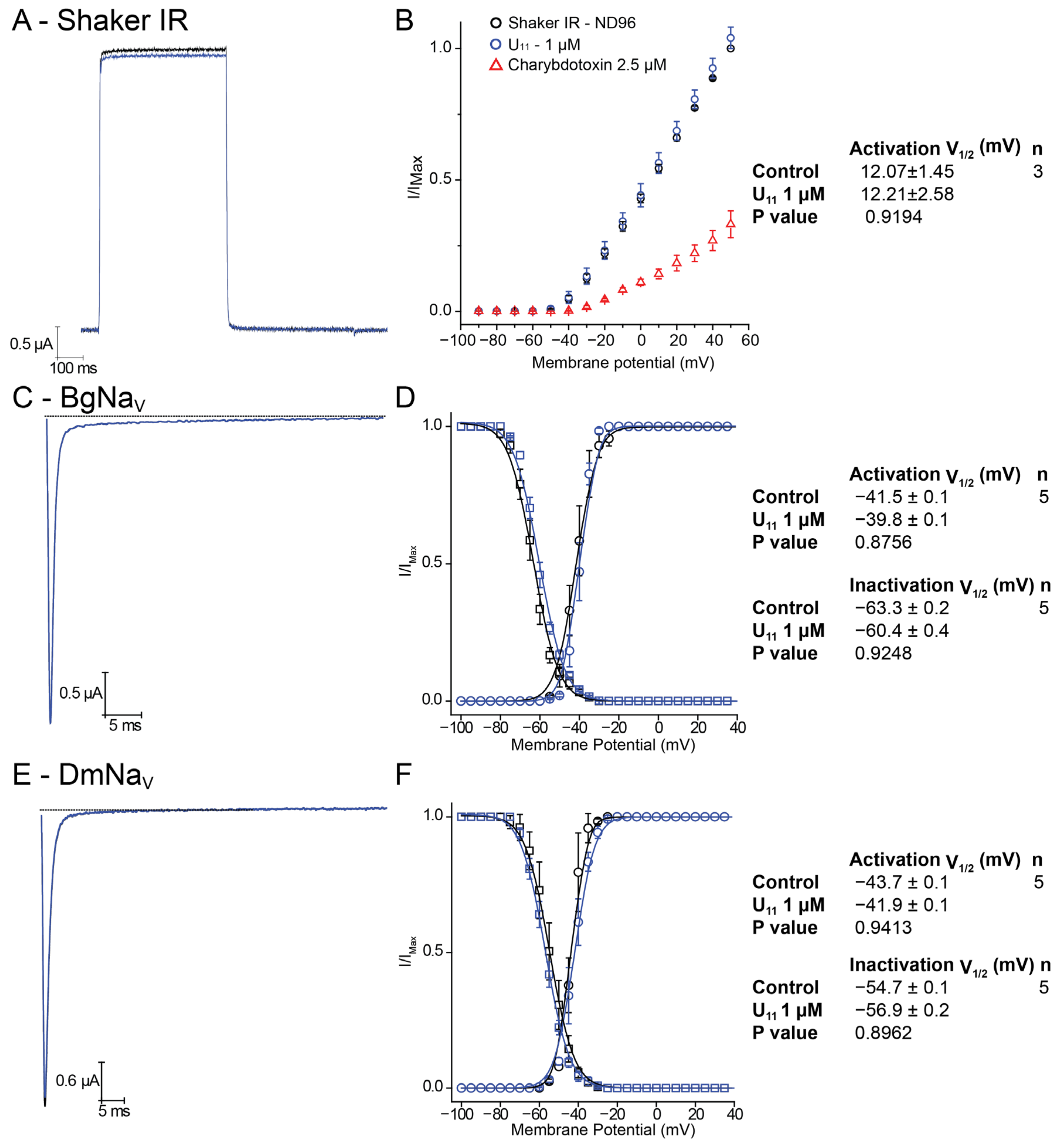
Figure 2A–I show representative traces of outward potassium currents evoked during step depolarizations, where some of these channels inactivate during the test pulse; for Kv11.1, inward tail potassium currents are shown, representing recovery from inactivation typically seen with this channel. In all cases, the presence of 1 μM U
11 did not induce any effect (neither a block nor an increase in the K current). As a control, we have compared the lack of effect of U
11, again at 1 μM on the
Shaker IR channel with the well-known charybdotoxin at 2.5 μM: as expected, a clear block of
Shaker IR currents is seen in the presence of charybdotoxin in contrast to U
11 (
Figure 3A).
Since Barassé et al. (2023) stated that U
11, in their hands, is the most paralytic peptide ever reported from ant venoms against blowflies, is also capable of paralyzing honeybees and it has received little attention yet holds great promise for the discovery of novel insecticidal molecules, we have also checked whether the observed paralysis seen in blowflies and honeybees could be explained via modulation of voltage-gated sodium channels. Historically, it is well known that voltage-gated sodium channels are a key target for several classes of insecticides [for a review, see [
27]].
Figure 3 shows representative inward sodium currents evoked by a step depolarization of the insect cockroach sodium channel BgNav1 (panel A) and the fruit fly DmNav1 (panel C). Application of 1 μM U
11 again did not show any effect. A careful analysis of the steady-state Boltzmann activation and inactivation curves also did not reveal any shift or change (panels B and D).
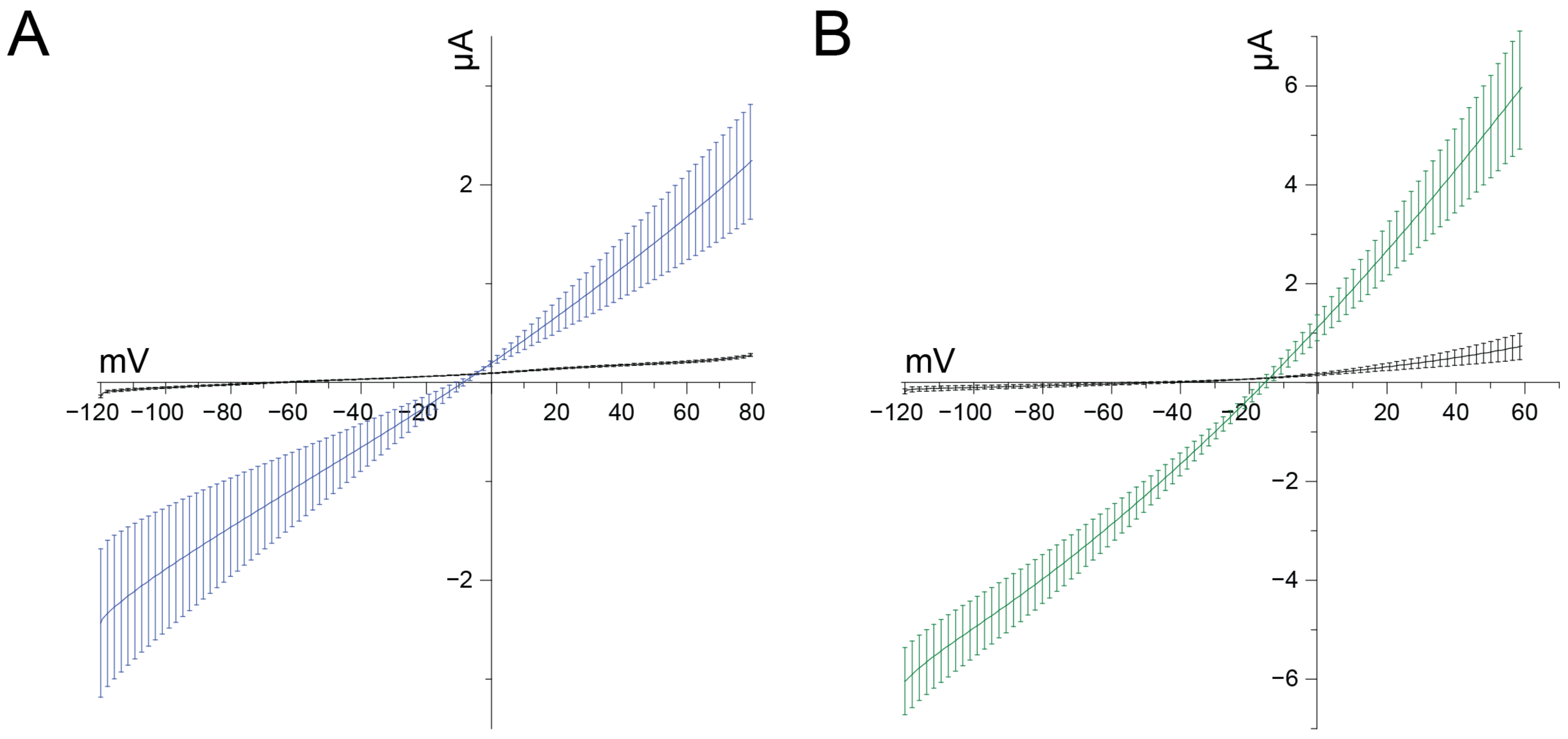
In the course of our screenings, we noticed that upon application of concentrations of U
11 higher than 1 μM, the oocytes quickly became leaky and died soon after. Such a phenomenon is indicative of cytolysis or cytotoxicity and may be explained by pore formation. To investigate this in detail, we compared the effect of 10 μM U
11 with crude venom of the honey bee
Apis mellifera since this bee venom is well known for bioactive substances causing cytolysis via a process of pore formation, among which is the peptide melittin [for a review, see [
28]]. In non-injected control oocytes, the current evoked by a voltage ramp is minimal in amplitude, and the slope is minimal (small chord conductance) (
Figure 4). A reversal potential of −67.5 mV can be noticed, indicating a healthy condition of the oocyte. The application of 10 μM U
11 quickly and irreversibly caused two clear effects: (1) a shift of the reversal potential in the depolarizing direction to −12.63 mV, and (2) a significant increase in slope (increased chord conductance). Interestingly, a similar effect was obtained with crude venom from
Apis mellifera, with a shift of the reversal potential to −14.96 mV and a dramatic increase in slope. These observations can be explained by a putative pore-forming mechanism caused by U
11 and bee venom, and its relevance in the context of U
11 and its insecticidal action will be discussed in detail below.
4. Discussion
Stinging ants of different subfamilies, such as Myrmicinae, possess complex venom mixtures rich in hyaluronidase, phospholipases, peptidyl toxins, histamine and further low-molecular-mass compounds [for a review, see [
7]]. All these molecules interact together, resulting in extremely rapid immobilization and/or killing of prey or aggressor. Most proteomic studies on ant venoms have confirmed the prevalence of small linear peptides (devoid of disulfide bonds) with fewer than 35 residues [for a review, see [
4]]. These small peptides are often cytolytic with insecticidal, hemolytic and/or antimicrobial properties. Examples include ponericins from the ant
Neoponera goeldii that exhibit hemolytic activity and antibacterial activity against both Gram-positive and Gram-negative bacteria, as well as insecticidal activity. Additional homologous toxins include the bicarinalins from
Tetramorium bicarinatum (Myrmicinae), the species of our study. Another group of ant venom peptides are pilosulins from
Myrmecia pilosula (Myrmeciinae). For instance, pilosulin 1 is a 57-amino-acid-long linear peptide with hemolytic and cytolytic activities. Although several of these peptides are presented as allergens, their exact biological function and mechanism of action often remain unknown. In spider and scorpion venoms, cytolytic peptides are believed to act as membrane-disrupting agents, commonly known as pore-forming peptides, facilitating the passage of other bioactive molecules through cellular barriers. Currently, several mechanisms promoting pore formation have been proposed in the literature, with the most well-documented being the toroidal model, the barrel-stave model, the carpet model and the detergent model [
29,
30]. Pore formation leads to the quick breakdown of the transmembrane potential by the loss of ion gradients, resulting in cellular death, on the basis of which these peptides are called cytolytic or cytotoxic. The type of pore formed and its dimensions are relevant in this respect. Different strategies exist that can be used to estimate pore dimensions formed by the peptide [
29], such as liposome leakage experiments, advanced microscopy, neutron or X-ray scattering and electrophysiological techniques, with the last strategy being used by us and explained further.
Since our results obtained with U
11 point in the direction of putative pore formation, let us now elaborate first on this aspect below. Parker and Feil (2005) state that pore-forming toxins (PFTs) are one of nature’s most potent biological weapons [
31]. A unique feature is the remarkable property that PFTs can exist either in a stable water-soluble state (without much toxicity involved) or as an integral membrane pore (with clear toxicity). In order to convert from the water-soluble to the membrane state, the toxin undergoes a large conformational change driven by the proximity and composition of a barrier, such as a phospholipid cell membrane. Notwithstanding the fact that PFTs differ markedly in their primary, secondary and tertiary structures, they can be classified into one of two families based on the types of pores they form: alpha-PFTs or beta-PFTs. Two points of view need to be discussed: qualitative and quantitative.
From a qualitative point of view, the observation that U
11 can form putative pores is novel, given its 3-dimensional structure. We have no evidence whether or not U
11 needs to polymerize in order to form a pore, but the HPLC profile does not indicate the presence of multimers in the presence of the solutions used in the chromatography (
Figure 1C). Evidently, on the one hand, this does not exclude possible multimerization in case U
11 is exposed to a phospholipid membrane, but on the other hand, it seems unlikely that a significant conformational change would take place in the case of U
11 due to its rigid and cysteine-stabilized scaffold, in contrast to what is observed with cysteine-free linear cytolytic peptides. Interestingly, the presence in U
11 of two 3
10-helical segments (Leu7 to Gln9 and Glu27 to Leu30) located at either end of the alpha-helical stretch (Ala17 to His24) mimics a conformational preference also found in pore-forming molecules such as alamethicin [
32]. The preference consists of the short bits of a 3
10-helix tightening up the ends of the alpha-helix by moving the related peptide groups nearer the axis. Barassé et al. (2023) indicated in their work that residues Cys10 to Cys33 delineate an almost planar triangular monocycle closed by the disulfide bridge [
20]. Future studies are needed to investigate whether this structural feature is possibly involved in a phenomenon of U
11’s multimerization, leading to pore formation once a critical concentration is reached. Based on the HPLC-based purity check we carried out of our synthetic U
11, we have no indication that possible degradation products would be responsible for the observed pore formation.
From a quantitative point of view, we have found an interesting correlation between the reported neurotoxicity by Barassé et al. (2023) on the insect species and cells they have used and the putative pore formation we observe: Barassé et al. report that 60% of blowflies were affected 24 h post ingestion with a dose of 8 mg/mL (i.e., 350 nmol·g−1). Taking into account the molecular mass of U11 (4018.1), this is 2 μM. Interestingly, we have used in our oocyte assay either 1 μM or 10 μM: with 1 μM, no effects were observed on the ion channels tested, and with 10 μM, a clear cytolytic effect was present. In other words, the 2 μM reported by Barassé et al. falls in between and confirms that we have used the same range of concentrations. Moreover, it is striking that the percentage paralysis observed by Barassé et al. in both L. caesar and A. mellifera insects, expressed as 50% paralyzing dose (PD50), matches exactly with the concentration range at which U11 seems to be able to form pores. The weak effect seen with U11 in the experiments by Barassé et al. in the aphid A. pisum is difficult to compare, in our view, with the effect seen in the other two insects, since the injection route was different: intrathoracic injection vs. intra-abdominal injection. It is not illogical that the route of administration crucially determines the final effect. In this way, we acknowledge that it is difficult to compare the doses/concentrations/duration of U11 used in Barassé’s insect experiments and those used here on Xenopus oocytes. The smaller effect on relative fluorescence induced by (a high concentration of) 10 μM U11 using Drosophila S2 cells, as seen by Barassé et al., could be explained by the fact that U11 already provokes a membrane depolarization due to pore formation prior to the application of 50 mM KCl, resulting in a lower relative fluorescence signal.
At this stage, we cannot exclude the possibility that the membrane composition of oocytes of
Xenopus laevis is so much different from the ones in insects, and as such, that tissue specificity plays a role here as an explanation of why we see a pore-forming effect at 10 μM U
11 and higher, in contrast to Barassé et al. (2023), who attribute neurotoxic features to the pharmacology of U
11 despite the absence of any demonstrated molecular target, such as voltage-gated potassium channels. It is precisely because Barassé et al. have hypothesized the presence of a functional dyad in U
11, putatively responsible for blocking a potassium conductance and, as such, explaining a neurotoxic mechanism of action for the observed paralysis, that we have thoroughly checked this possibility by testing a wide array of voltage-gated potassium channels. As illustrated in
Figure 2, we could not find any neurotoxic effect caused by potassium channel blockade, nor did we find any effect on voltage-gated sodium channels. Since these two types of voltage-gated ion channels are crucially involved in muscle activity, we doubt whether U
11 exerts its neurotoxic effect via modulation of these channels. Instead, our results favor interpreting the observed paralysis due to pore formation. To substantiate this further, we have compared the well-known pore-forming capacity of bee venom (
Apis mellifera) in our
Xenopus laevis bioassay. The effect evoked by the bee venom is very similar, if not identical, to the effect caused by U
11. This result makes the composition of the membrane of the oocyte implausible as an explanation for the difference in interpretation of U
11’s effect between Barassé et al. (2023) (i.e., U
11 being neurotoxic) and our work presented here (i.e., U
11 as a pore-forming peptide). An additional advantage of our electrophysiological comparison of U
11 with
Apis mellifera venom is the quantitative analysis of the shift in reversal potential made possible: as illustrated in
Figure 4, the depolarizing shift of the reversal potential induced by U
11 is not significantly different from the one induced by
Apis mellifera. Based on the amphipathic property of melittin, assumed to be the most important pore-forming peptide in
Apis mellifera venom, and its easy insertion into membranes by disrupting both natural and synthetic phospholipid bilayers yielding non-selective pores, it can be surmised that U
11 acts in a similar manner with pores that are also non-selective (or very slightly selective) for the flux of ions they allow. It should be emphasized that the measurement of a reversal potential using a voltage clamp is an accurate and reliable methodology to assess the ion selectivity of an ion channel or pore. Furthermore, the observation that the chord conductance in the presence of both bee venom and U
11 has increased dramatically indicates that the pore-forming molecules, in both cases, have generated a kind of ‘big gate’ rather than a ‘small door’. This helps to explain the fast kinetics of cytolysis observed.
As several ant venoms have paralytic effects on arthropods, it is generally believed that they also contain neurotoxins inducing paralysis. The two best-characterized neurotoxins from ants are (
i) poneratoxin, a 25-amino-acid linear peptide from
Paraponera clavata (Paraponerinae), which is a modulator of voltage-gated sodium channels [
4]; and (
ii) ectatomin Et-1 from the ant
Ectatomma tuberculatum, a voltage-gated calcium channel blocker but also a pore-forming peptide [
11]. In the case of
Tetramorium bicarinatum, this most likely is also the case, based on the number of venom peptides found by Barassé et al. (2023) (U
2–U
15) with hitherto unknown function(s) but with clear paralysis-inducing effects when screened in a blowfly (
L. caesar) paralytic assay. With this in mind, we do not claim that venom from the
Tetramorium bicarinatum does not indeed contain one or more neuroactive peptides. Undoubtedly, future experiments will unravel the pharmacological effects contained in ant venom peptides, such as U
2–U
15 found in
Tetramorium bicarinatum [
33].