Screen-Printed Carbon Electrode Modified with Carbon Nanotubes and Copper Film as a Simple Tool for Determination of Trace Concentrations of Lead Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. ASV Procedure of Lead Determination
2.4. Preparation of Certified Reference Materials and Real Samples
3. Results and Discussion
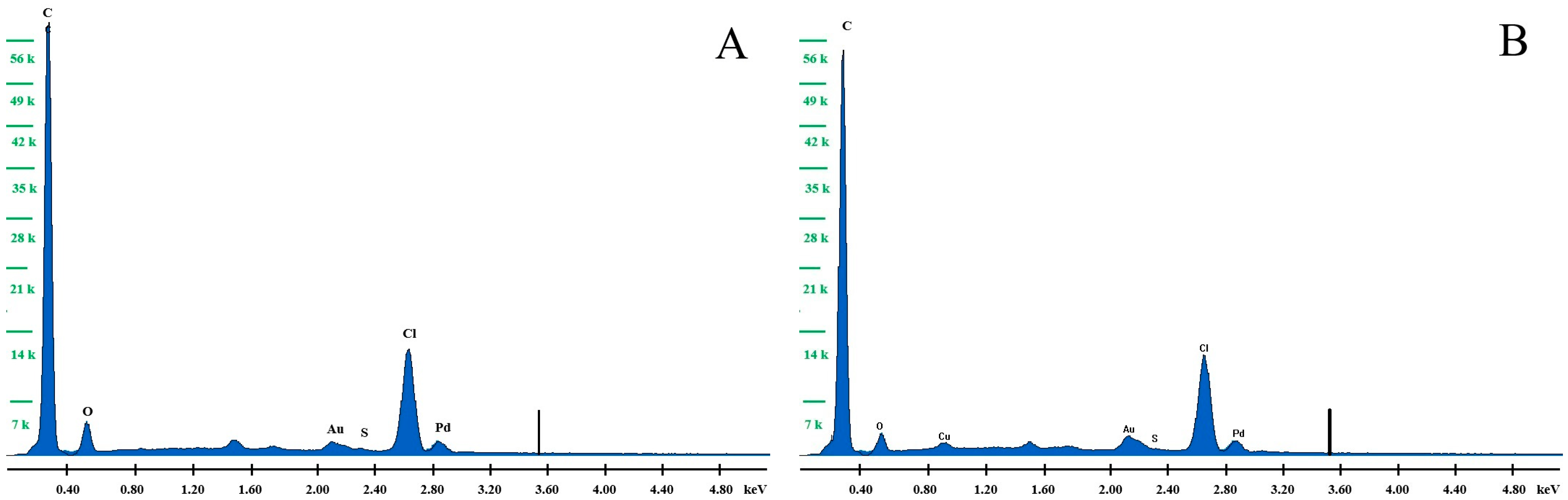
3.1. Morphology and Composition of the Electrode Material
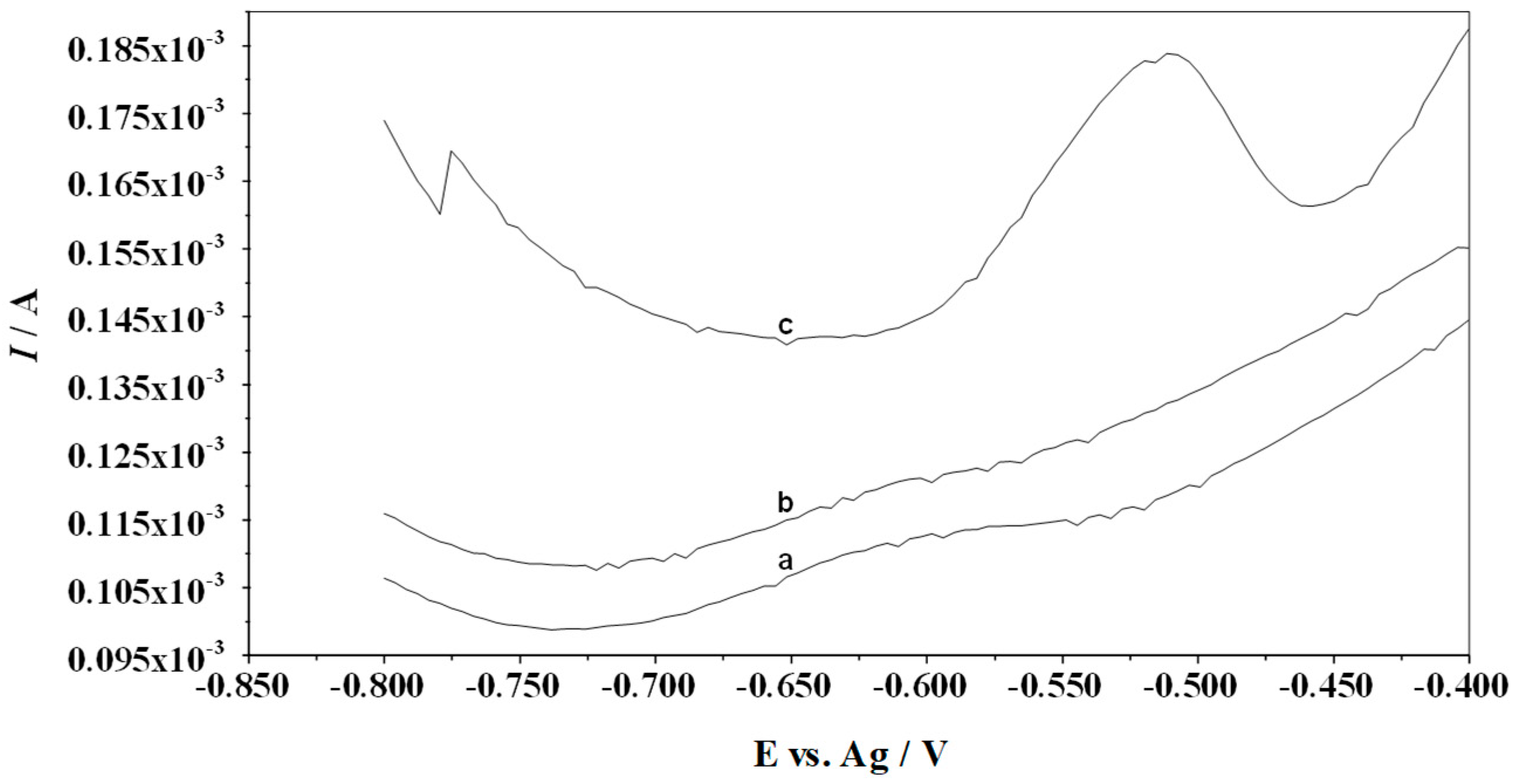
3.2. Influence of Supporting Electrolyte Concentration
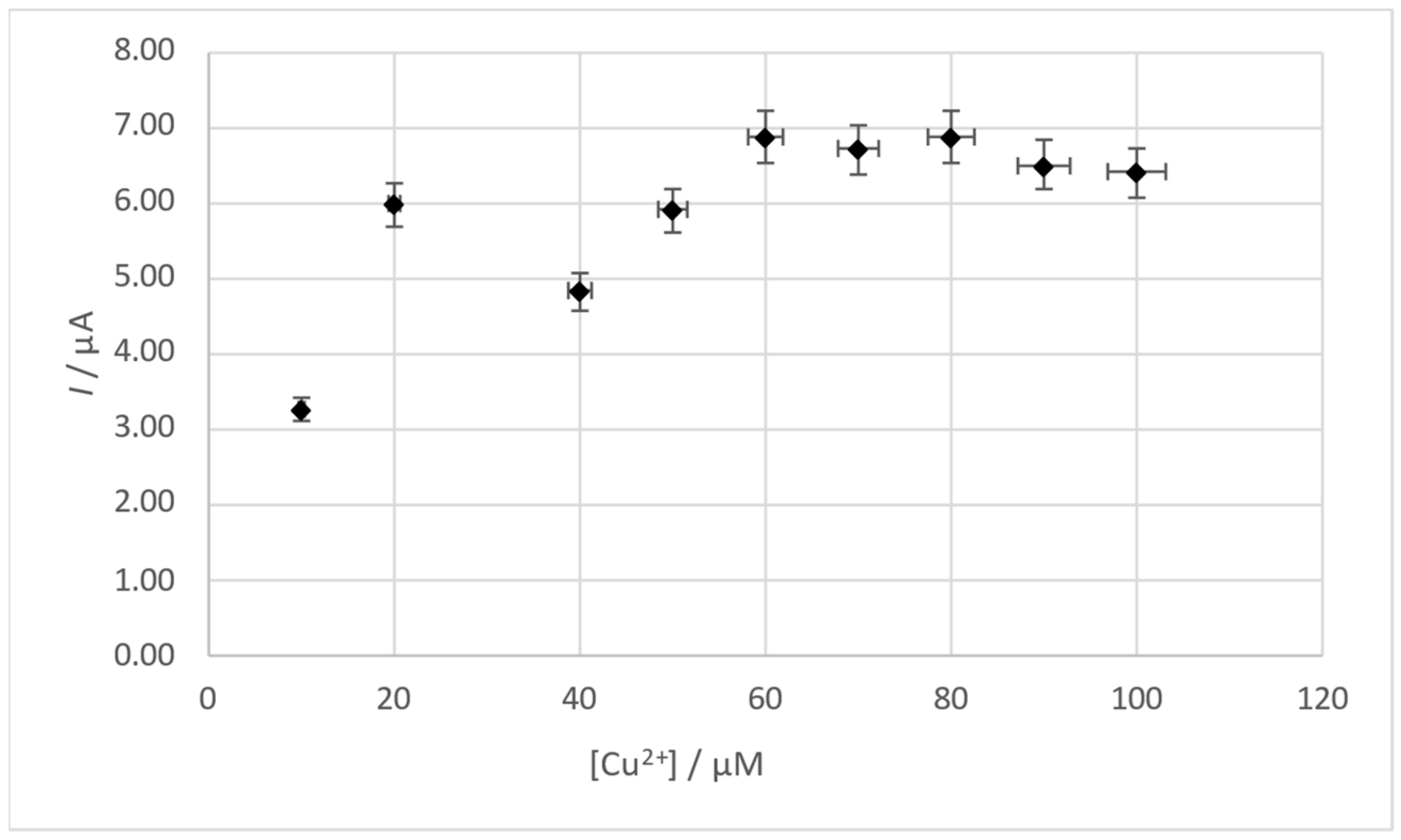
3.3. Influence of Cu(II) Concentration
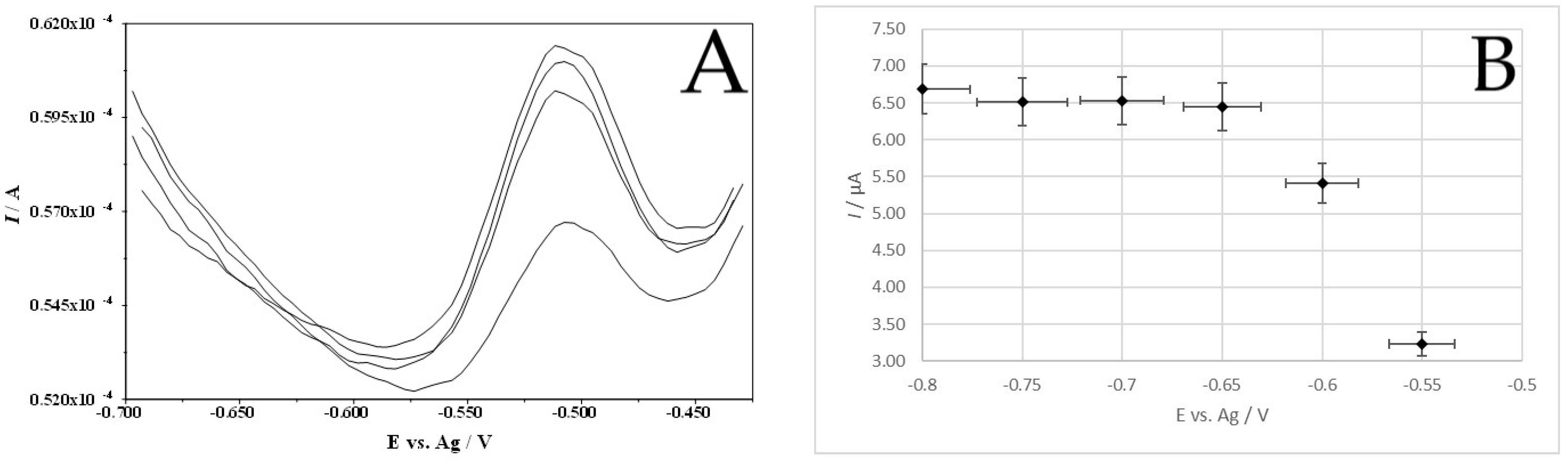
3.4. Conditions of Accumulation Potential and Time
3.5. Optimization of DPASV Parameters
3.6. Analytical Characteristics
3.7. Interferences
3.8. Analytical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pratiwi, N.H.; Azis, M.Y.; Setyorini, D.A.; Rahayu, R.S. Anodic Stripping Voltammetry for Simultaneous Determination of Lead and Cadmium using Bismuth-based Electrodes. Anal. Bioanal. Electrochem. 2022, 14, 331–347. [Google Scholar]
- Locsin, J.A.; Hood, K.M.; Doré, E.; Trueman, B.F.; Gagnon, G.A. Colloidal lead in drinking water: Formation, occurrence, and characterization. Crit. Rev. Environ. Sci. Technol. 2023, 53, 110–136. [Google Scholar] [CrossRef]
- Henn, A.S.; Frohlich, A.C.; Pedrotti, M.F.; Cuaduro, V.H.; Oliveira, M.L.S.; Flores, E.N.d.M.; Bizzi, C.A. Microvave-Assisted Solid Sampling Analysis Coupled to Flame Furnace Atomic Absorption Spectrometry for Cd and Pb Determination in Food-Contact Polymers. Sustainability 2022, 14, 291. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601. [Google Scholar] [CrossRef] [PubMed]
- Umaz, A.; Aydin, F.; Tokul Olmez, O.; Firat, M.; Ozturk, M.; Ertas, A.; Aydin, I. Determination and comparison of metal contents in simulated body fluid medium conditions of the plant species by flame atomic absorption spectrometry (FAAS). J. Res. Pharm. 2022, 26, 1281–1294. [Google Scholar] [CrossRef]
- Karasiński, J.; Bulska, E.; Halicz, L.; Tupys, A.; Wagner, B. Precise determination of lead isotope ratios by MC-ICP-MS without matrix separation exemplified by unique samples of diverse origin and history. J. Anal. At. Spectrom. 2023, 38, 2468–2476. [Google Scholar] [CrossRef]
- Jakkielska, D.; Dasteridis, I.; Kubicki, M.; Frankowski, M.; Zioła-Frankowska, A. Determination of Metal Content by Inductively Coupled Plasma-Mass Spectrometry in Polish Red and White Wine Samples in Relation to Their Type, Origin, Grape Variety and Health Risk Assessment. Foods 2023, 12, 3205. [Google Scholar] [CrossRef]
- Antunović, V.; Tripković, T.; Tomašević, B.; Baošić, R.; Jelić, D.; Lolić, A. Voltammetric Determination of Lead and Copper in Wine by Modified Glassy Carbon Electrode. Anal. Sci. 2021, 37, 353–358. [Google Scholar] [CrossRef]
- Zamarchi, F.; Silva, T.R.; Winiarski, J.P.; Santana, E.R.; Vieira, I.C. Polyethylenimine-Based Electrochemical Sensor for the Determination of Caffeic Acid in Aromatic Herbs. Chemosensors 2022, 10, 357. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef]
- Wan, X.; Du, H.; Tuo, D.; Qi, X.; Wang, T.; Wu, J.; Li, G. UiO-66/Carboxylated Multiwalled Carbon Nanotube Composites for Highly Efficient and Stable Voltammetric Sensors for Gatifloxacin. ACS Appl. Nano Mater. 2023, 6, 19403–19413. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Zhang, G.; Wang, S.; Li, K.; Wu, J.; Wan, X.; Liu, Y.; Li, Q. Low-cost voltammetric sensors for robust determination of toxic Cd(II) and Pb(II) in environment and food based on shuttle-like α-Fe2O3 nanoparticles decorated β-Bi2O3 microspheres. Microchem. J. 2022, 179, 107515. [Google Scholar] [CrossRef]
- Pedrosa Lisboa, T.; Vinicius de Faria, L.; Costa Matos, M.A.; Camargo Matos, R.; Arromba de Sousa, R. Simultaneous determination of cadmium, lead, and copper in the constituent parts of the illegal cigarettes by Square Wave Anodic Stripping Voltammetry. Microchem. J. 2019, 150, 104183. [Google Scholar] [CrossRef]
- Oularbi, L.; Turmine, M.; El Rhazi, M. Preparation of novel nanocomposite consisting of bismuth particles, polypyrrole and multi-walled carbon nanotubes for simultaneous voltammetric determination of cadmium(II) and lead(II). Synth. Met. 2019, 253, 1–8. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K. Metal film electrodes prepared with a reversibly deposited mediator in voltammetric analysis of metal ions. Curr. Opin. Electrochem. 2019, 17, 128–133. [Google Scholar] [CrossRef]
- Wasąg, J.; Grabarczyk, M. Application of adsorptive stripping voltammetry based on PbFE for the determination of trace aluminum released into the environment during the corrosion process. Talanta 2021, 233, 122565. [Google Scholar] [CrossRef]
- Nigović, B.; Hocevar, S.B. Antimony film electrode for direct cathodic measurement of sulfasalazine. Electrochim. Acta 2011, 58, 523–527. [Google Scholar] [CrossRef]
- Jovanovski, V.; Hrastnik, N.I.; Hočevar, S.B. Copper film electrode for anodic stripping voltammetric determination of trace mercury and lead. Electrochem. Commun. 2015, 57, 1–4. [Google Scholar] [CrossRef]
- Wasąg, J.; Grabarczyk, M. Copper Film Modified Glassy Carbon Electrode and Copper Film with Carbon Nanotubes Modified Screen-Printed Electrode for the Cd(II) Determination. Materials 2021, 14, 5148. [Google Scholar] [CrossRef]
- Hrastnik, N.I.; Jovanovski, V.; Hočevar, S.B. In-situ prepared copper film electrode for adsorptive stripping voltammetric detection of trace Ni(II). Sens. Actuators B Chem. 2019, 307, 127637. [Google Scholar] [CrossRef]
- Döll, E.G.; Santana, E.R.; Winiarski, J.P.; Baumgarten, L.G.; Vieira, I.C. Green Synthesis of Gold Nanoparticles Using Peach Extract Incorporated in Graphene for the Electrochemical Determination of Antioxidant Butylated Hydroxyanisole in Food Matrices. Biosensors 2023, 13, 1037. [Google Scholar] [CrossRef]
- GadelHak, Y.; Hafez, S.H.M.; Mohamed, H.F.M.; Abdel-Hady, E.E.; Mahmoud, R. Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: A review. Microchem. J. 2023, 193, 109043. [Google Scholar] [CrossRef]
- Buffa, A.; Erel, Y.; Mandler, D. Carbon Nanotube Based Flow-Through Electrochemical Cell for Electroanalysis. Anal. Chem. 2016, 88, 11007–11015. [Google Scholar] [CrossRef]
- Zhang, C.; Lai, Z.; Liu, X.; Ye, M.; Zhang, L.; Zhang, L.; Chen, X. Voltammetric Determination of Pb2+ in Water using Mn-doped MoS2/MWCNTs/Nafion Electrode Coupled with an Electrochemical Flow Analysis Device. Electroanalysis 2022, 34, 1638–1643. [Google Scholar] [CrossRef]
- Lai, Z.; Lin, F.; Huang, L.; Wang, Y.; Chen, X. Automated Determination of Cd2+ and Pb2+ in Natural Waters with Sequential Injection Analysis Device Using Differential Pulse Anodic Stripping Voltammetry. J. Anal. Test. 2021, 5, 60–68. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wlazłowska, E.; Wawruch, A. Adsorptive Stripping Voltammetry with Nanomaterials-based Electrode as an Advanced Technique in the Environmental Analysis of Trace Concentrations of Tin. ChemPhysChem 2023, 25, e202300633. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Wardak, C.; Wawruch, A. Investigation and elimination of surfactant-induced interferences in anodic stripping voltammetry for the determination of trace amounts of cadmium. Physicochem. Probl. Miner. Process. 2023, 59, 170717. [Google Scholar] [CrossRef]
- Kelíšková, P.; Matvieiev, O.; Janíková, L.; Šelešovská, R. Recent advances in the use of screen-printed electrodes in drug analysis: A review. Curr. Opin. Electrochem. 2023, 42, 101408. [Google Scholar] [CrossRef]
- Liang, G.; He, Z.; Zhen, J.; Tian, H.; Ai, L.; Pan, L.; Gong, W. Development of the screen-printed electrodes: A mini review on the application for pesticide detection. Environ. Technol. Innov. 2022, 28, 102922. [Google Scholar] [CrossRef]
- Available online: https://www.dropsens.com/en/screen_printed_electrodes_pag.html (accessed on 4 January 2024).
- Okiei, W.; Ogunlesi, M.; Adio-Adepoju, A.; Oluboyo, M. Determination of Copper and Lead in Water Samples from Zamfara State, Nigeria by Linear Sweep Anodic Stripping Voltammetry. Int. J. Electrochem. Sci. 2016, 11, 8280–8294. [Google Scholar] [CrossRef]
- Arantes, T.M.; Sardinha, A.; Baldan, M.R.; Cristovan, F.H.; Ferreira, N.G. Lead detection using micro/nanocrystalline boron-doped diamond by square-wave anodic stripping voltammetry. Talanta 2014, 128, 132–140. [Google Scholar] [CrossRef]
- Albalawi, I.; Hogan, A.; Alatawi, H.; Moore, E. A sensitive electrochemical analysis for cadmium and lead based on Nafion-Bismuth film in a water sample. Sens. Bio-Sens. Res. 2021, 34, 100454. [Google Scholar] [CrossRef]
- Honeychurch, K. Trace Voltammetric Determination of Lead at a Recycled Battery Carbon Rod Electrode. Sensors 2019, 19, 770. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Pan, D.; Li, Y.; Wang, J.; Wang, C. Stripping Voltammetric Determination of Lead in Coastal Waters with a Functional Micro-Needle Electrode. Front. Mar. Sci. 2020, 7, 196. [Google Scholar] [CrossRef]
- Cerovac, S.; Guzsvány, V.; Kónya, Z.; Ashrafi, A.M.; Švancara, I.; Rončević, S.; Kukovecz, Á.; Dalmacija, B.; Vytřas, K. Trace level voltammetric determination of lead and cadmium in sediment pore water by a bismuth-oxychloride particle-multiwalled carbon nanotube composite modified glassy carbon electrode. Talanta 2015, 134, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Valera, D.; Sanchez, M.; Dominguez, J.R.; Alvarado, J.; Espinoza-Montero, P.J.; Carrera, P.; Bonilla, P.; Manciati, C.; Gonzalez, G.; Fernandez, L. Electrochemical determination of lead in human blood serum and urine by anodic stripping voltammetry using glassy carbon electrodes covered with Ag–Hg and Ag–Bi bimetallic nanoparticles. Anal. Methods 2018, 10, 4114. [Google Scholar] [CrossRef]
- da Conceição, E.; Buffon, E.; Ramos Stradiotto, N. Lead signal enhancement in anodic stripping voltammetry using graphene oxide and pectin as electrode modifying agents for biofuel analysis. Fuel 2022, 325, 124906. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Huang, B.; Jia, N.; Tan, L.; Yao, S. Simultaneous determination of Cd(II) and Pb(II) using square wave anodic stripping voltammetry at a gold nanoparticle-graphene-cysteine composite modified bismuth film electrode. Electrochim. Acta 2014, 115, 471–477. [Google Scholar] [CrossRef]





| Electrode | Linear Range [nM] | LOD [nM] | Influence of the Tested Organic Matrix | Real Sample | References |
|---|---|---|---|---|---|
| GCE | - | - | - | water | [31] |
| BDND | - | - | - | - | [32] |
| Bi/NA/SPGE | 96.62–1449.28 | 14.49 | - | water | [33] |
| CREs | 13.53–531.40 | 13.53 | - | tap water | [34] |
| 3DrGO/F-BiNSs/ANE | 40.00–600.00 | 12.50 | - | coastal water | [35] |
| Nafion/MnCo2O4/GCE | 48.31–3864.73 | 8.07 | - | wine | [8] |
| HgF/GCE | 48.31–222.22 | 4.30 | - | tobacco | [13] |
| BiOCl/MWCNT/GCE | 24.15–241.55 | 2.75 | - | pore water | [36] |
| AgHgNpNf/GCE | 48.31–579.71 | 1.16 | - | human blood | [37] |
| Bi-PPy/MWCNT/CPE | 0.53–724.64 | 0.48 | - | tap water | [14] |
| GO-PEC/GCE | 1.00–1000.00 | 0.40 | - | biodiesel | [38] |
| Bi/Au-Gr-Cys/GCE | 2.42–193.24 | 0.24 | - | water | [39] |
| Cu/MWCNTs/SPCE | 0.50–500.00 | 0.12 | Triton X-100, SDS, CTAB, HA, FA, NOM | water | [this work] |
| Element | Percentage of Individual Elements for the Electrode before and after Modification with a Copper Film | |||
|---|---|---|---|---|
| Electrode before Modification | Electrode after Modification | |||
| Weight [%] | Atomic [%] | Weight [%] | Atomic [%] | |
| C | 85.18 | 90.91 | 87.20 | 93.14 |
| O | 8.47 | 6.79 | 5.21 | 4.18 |
| S | 0.15 | 0.06 | 0.15 | 0.06 |
| Cl | 6.19 | 2.27 | 6.97 | 2.52 |
| Cu | - | - | 0.47 | 0.09 |
| Certified Reference Material | Pb(II) Content [µg/L] | Pb(II) Found [µg/L] | Recovery [%] | RSD (n = 3) [%] | t-Test |
|---|---|---|---|---|---|
| SPS-WW1 | 100.0 ± 0.5 | 90.24 | 90.15 | 4.5 | 0.77 |
| 93.51 | 93.42 | 4.2 | 0.05 | ||
| 97.37 | 97.27 | 3.5 | 1.05 | ||
| TM-25.5 | 27.0 ± 2.4 | 24.38 | 92.36 | 3.0 | 1.21 |
| 26.65 | 100.95 | 5.3 | 0.93 | ||
| 25.00 | 94.68 | 4.3 | 0.31 |
| Samples | Pb(II) Added [nmol/L] | Pb(II) Found [nmol/L] | Recovery [%] | RSD (n = 3) [%] |
|---|---|---|---|---|
| Bystrzyca River | 10 | 9.13 | 91.25 | 4.9 |
| 9.20 | 92.04 | 5.1 | ||
| 8.78 | 87.79 | 4.1 | ||
| Lake Piaseczno | 9.48 | 94.79 | 3.3 | |
| 9.34 | 93.44 | 4.6 | ||
| 9.14 | 91.36 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabarczyk, M.; Wawruch, A. Screen-Printed Carbon Electrode Modified with Carbon Nanotubes and Copper Film as a Simple Tool for Determination of Trace Concentrations of Lead Ions. Membranes 2024, 14, 53. https://doi.org/10.3390/membranes14020053
Grabarczyk M, Wawruch A. Screen-Printed Carbon Electrode Modified with Carbon Nanotubes and Copper Film as a Simple Tool for Determination of Trace Concentrations of Lead Ions. Membranes. 2024; 14(2):53. https://doi.org/10.3390/membranes14020053
Chicago/Turabian StyleGrabarczyk, Malgorzata, and Agnieszka Wawruch. 2024. "Screen-Printed Carbon Electrode Modified with Carbon Nanotubes and Copper Film as a Simple Tool for Determination of Trace Concentrations of Lead Ions" Membranes 14, no. 2: 53. https://doi.org/10.3390/membranes14020053
APA StyleGrabarczyk, M., & Wawruch, A. (2024). Screen-Printed Carbon Electrode Modified with Carbon Nanotubes and Copper Film as a Simple Tool for Determination of Trace Concentrations of Lead Ions. Membranes, 14(2), 53. https://doi.org/10.3390/membranes14020053






