Theoretical Analysis of the Influence of Spacers on Salt Ion Transport in Electromembrane Systems Considering the Main Coupled Effects
Abstract
1. Introduction
1.1. Impact of the Dissociation/Recombination Reaction of Water Molecules on the Transport of Salt Ions
1.2. Effects of Electroconvection on Salt Ion Transport
1.3. Effects of Spacers on Salt Ion Transport
2. Materials and Methods
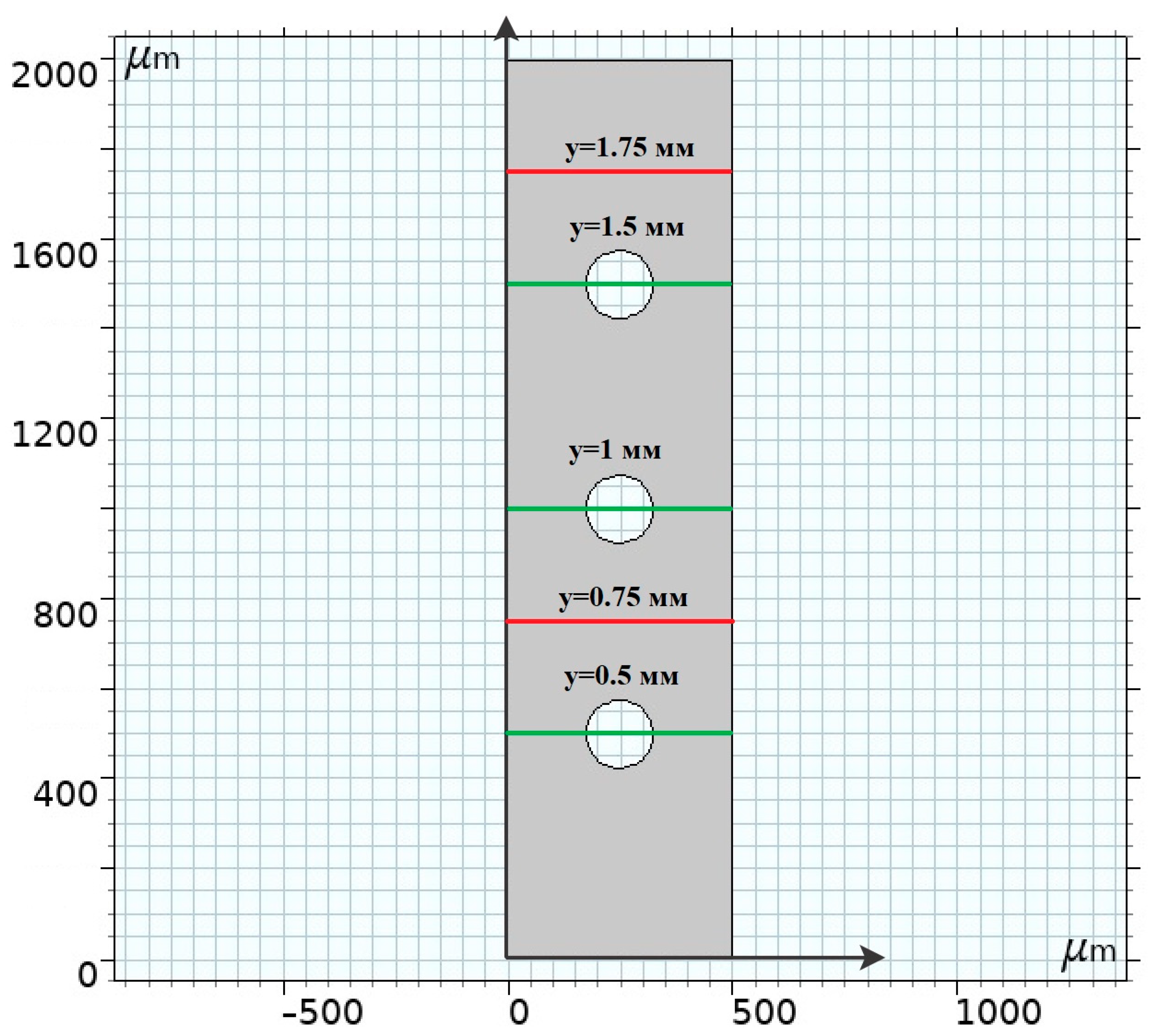
2.1. Geometry
2.2. Equations
2.3. Boundary and Initial Conditions
2.4. Numerical Solution Algorithm
3. Results
Formula for Calculating the CV Characteristics
4. Fundamental Regularities of Transfer
4.1. Methodology of Theoretical Study
- Spacers are present and the dissociation/recombination reaction of water molecules is considered;
- Spacers are absent and the dissociation/recombination reaction of water molecules is considered;
- Spacers are present and the reaction of dissociation/recombination of water molecules is not considered;
- Spacers are absent and the reaction of dissociation/recombination of water molecules is not considered.
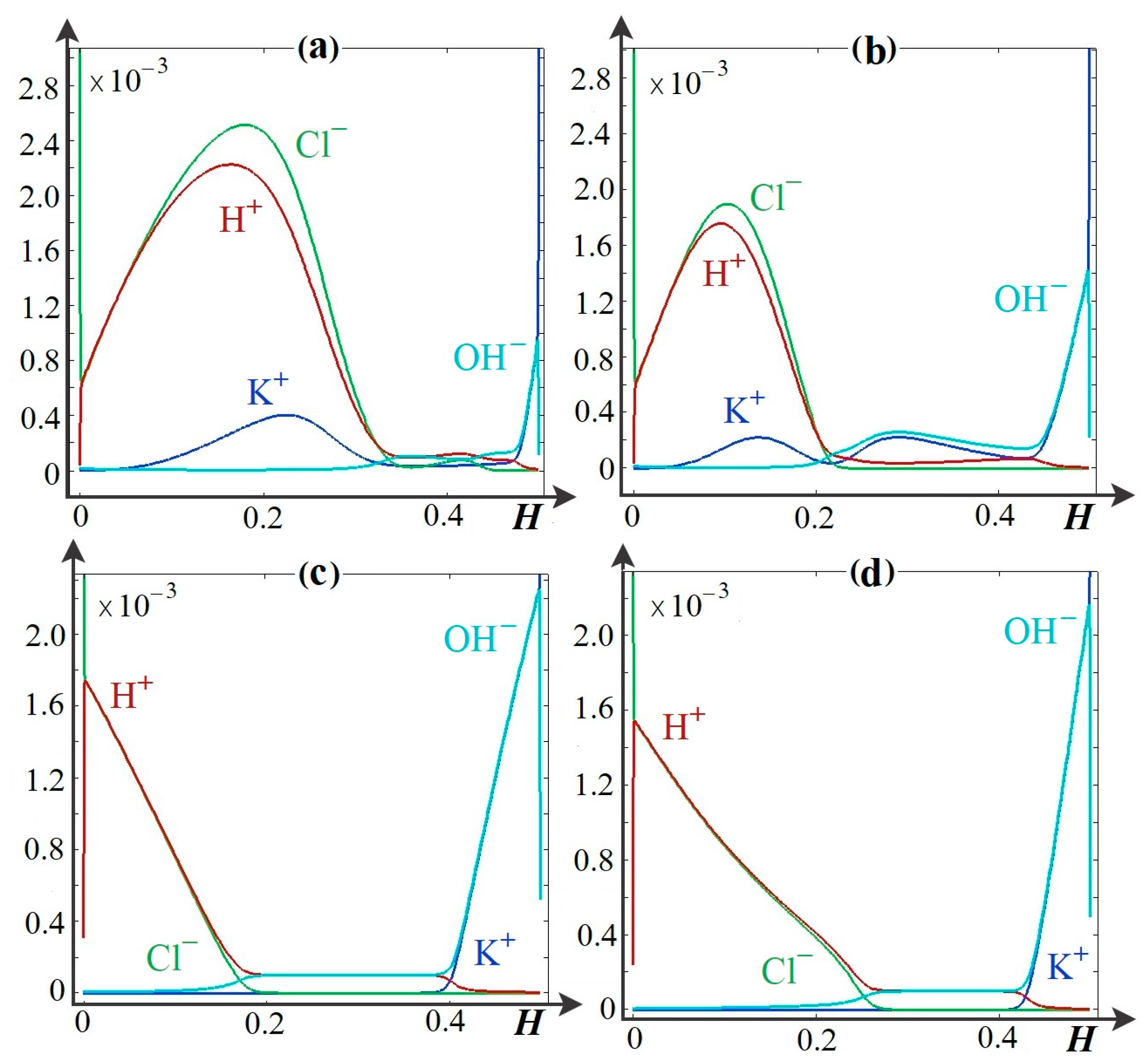
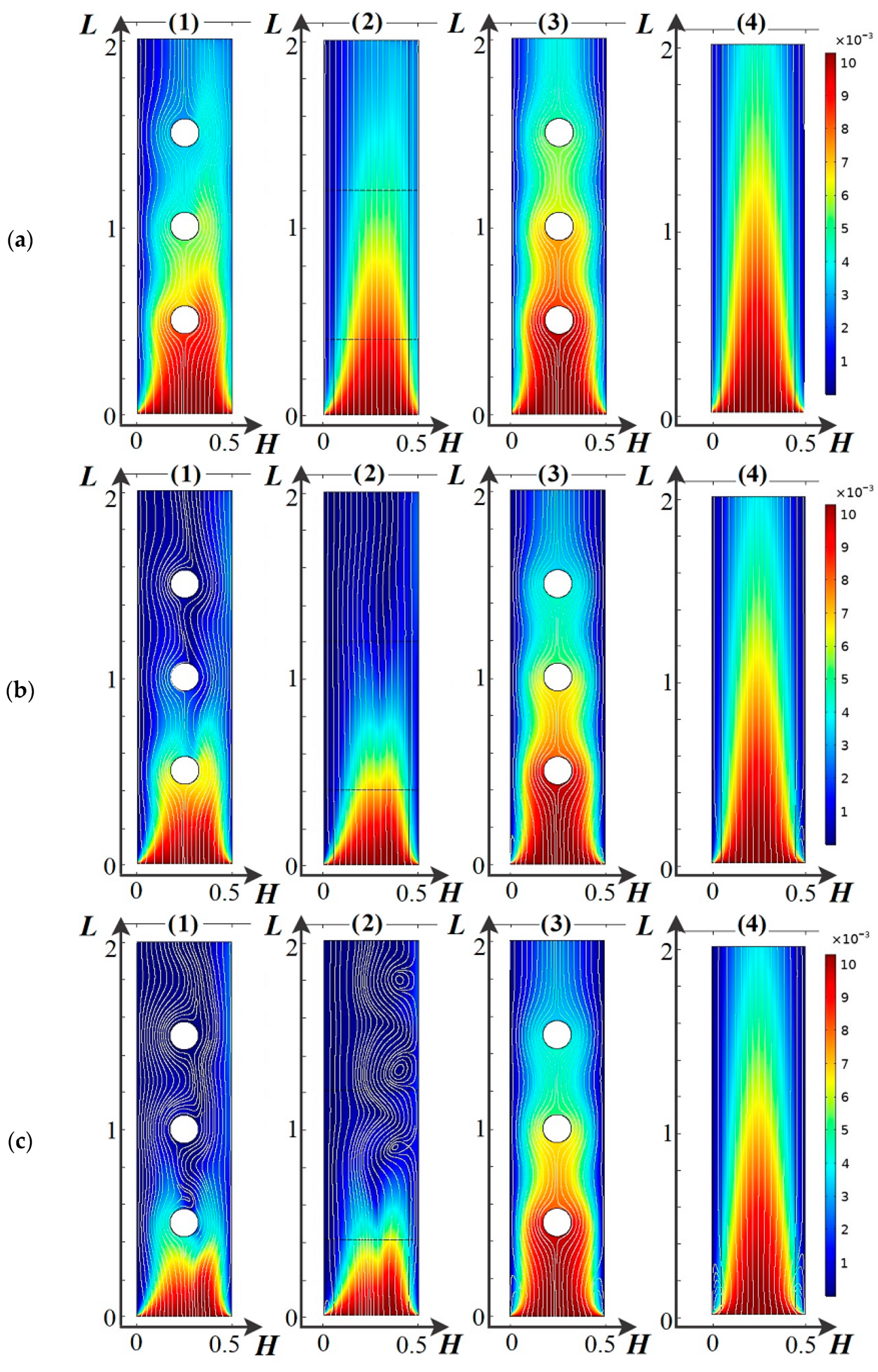
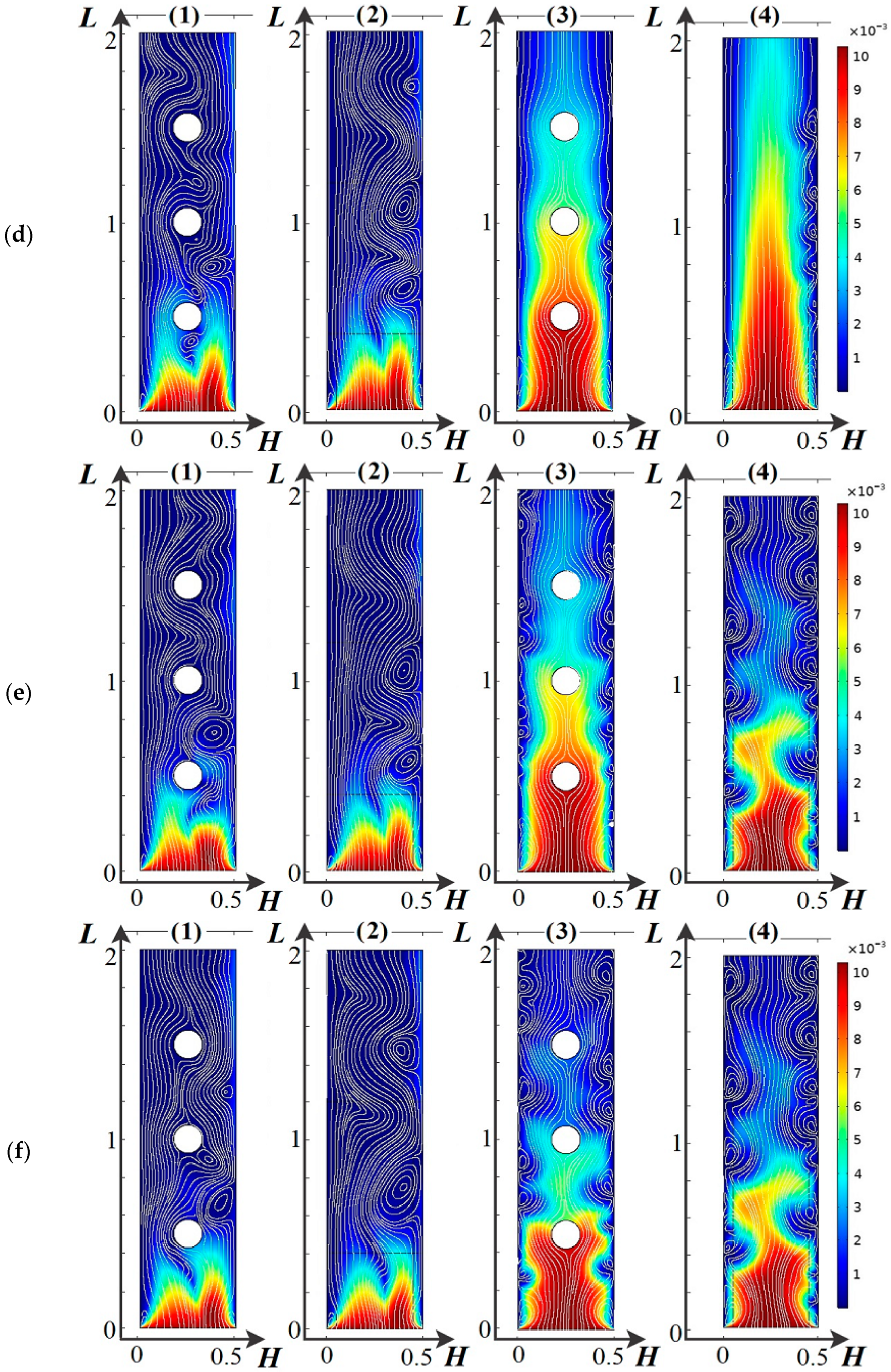
4.2. Basic Regularities of Spatial Charge Variation
4.3. Main Regularities of the Influence of the Dissociation/Recombination Reaction of Water Molecules on Salt Ion Transport
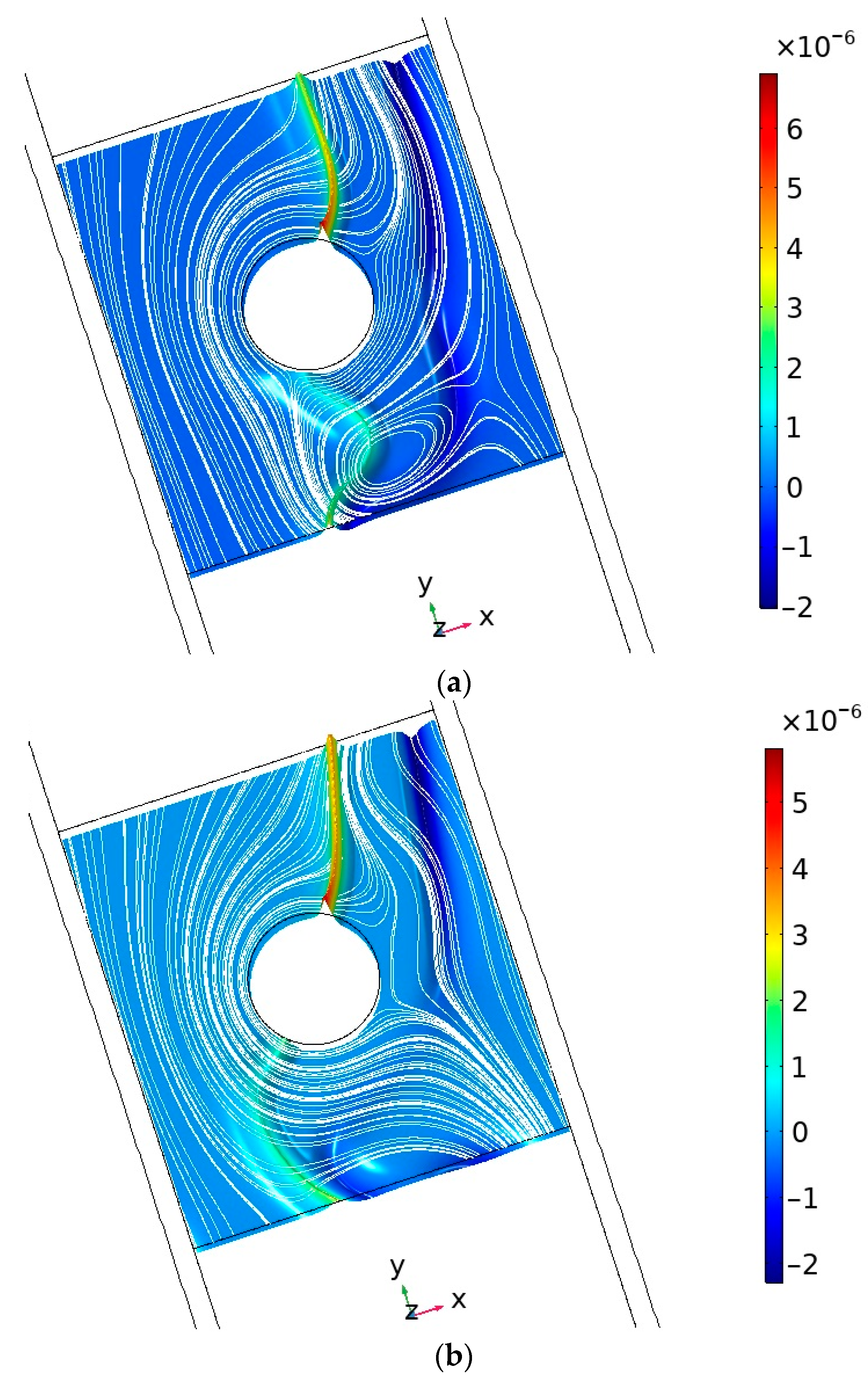
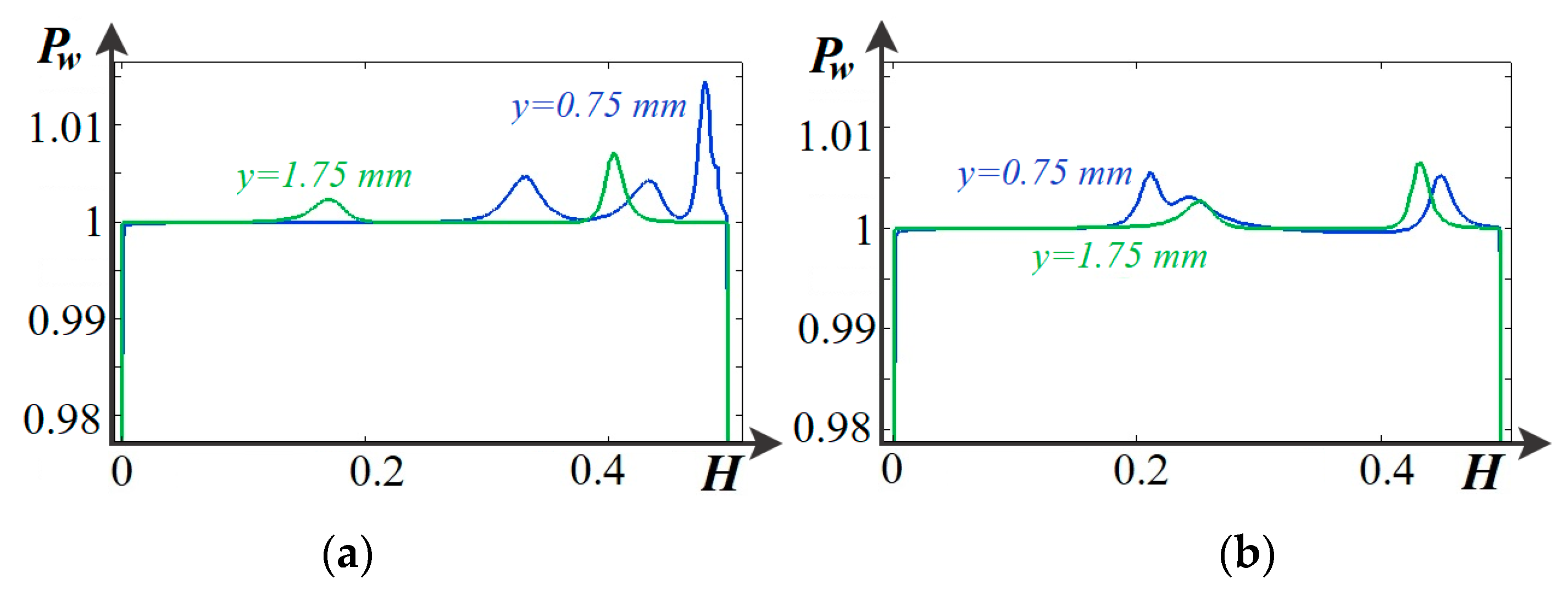
4.4. Basic Regularities of Occurrence and Development of Electroconvection
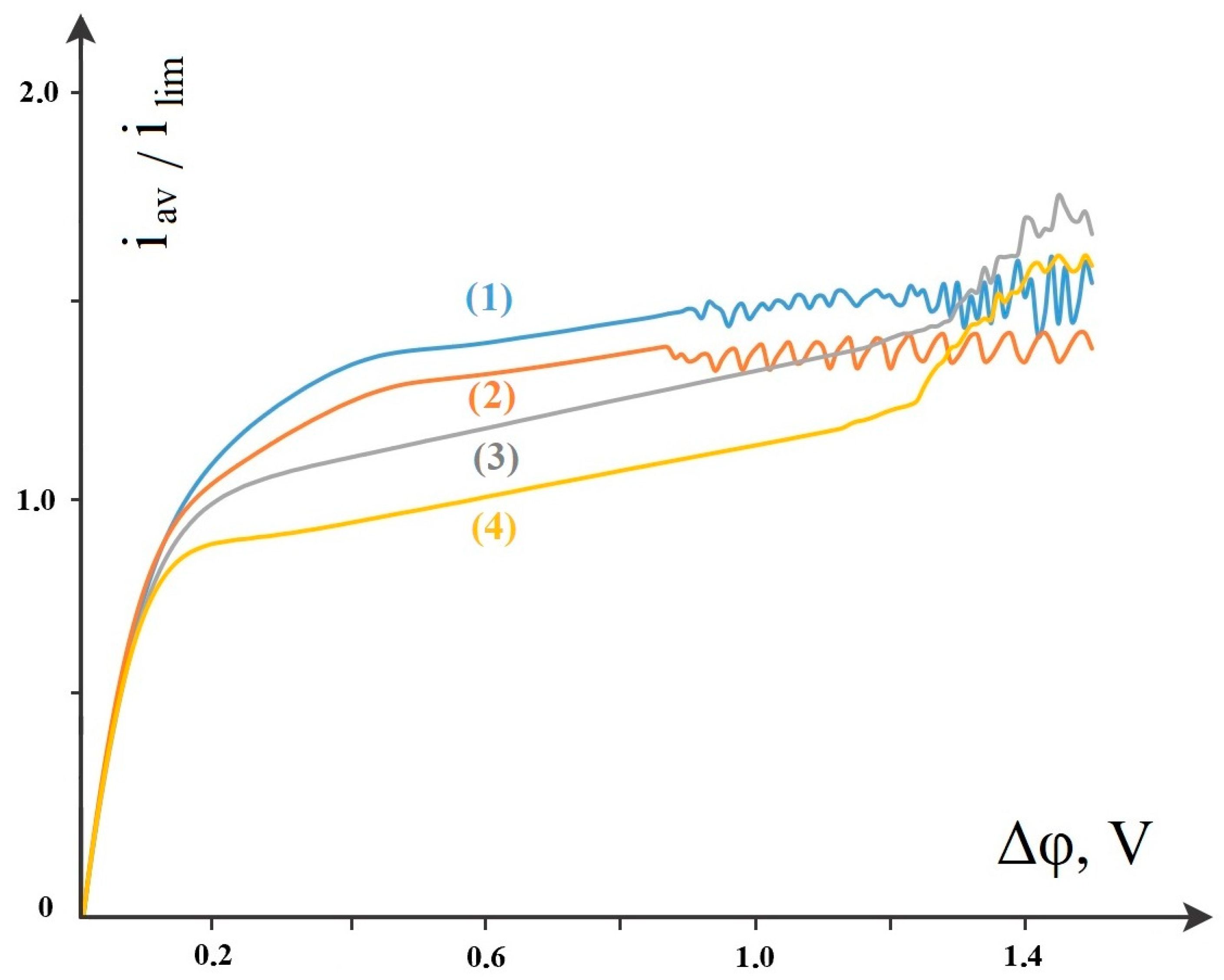
4.5. Main Patterns of Change in the Current–Voltage Characteristic
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hoekstra, A.Y.; Wiedmann, T.O. Humanity’s unsustainable environmental footprint. Science 2014, 344, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Heynen, M.; Pellicciotti, F. Freshwater Resources: Past, Present, Future. Int. Encycl. Geogr. 2016, 3, 1–12. [Google Scholar]
- Dinar, A.; Tieu, A.; Huynh, H. Water scarcity impacts on global food production. Glob. Food Secur. 2019, 23, 212–226. [Google Scholar] [CrossRef]
- UNESCO. UN-Water: United Nations World Water Development Report 2020: Water and Climate Change; UNESCO: Paris, France, 2020. [Google Scholar]
- Mekonnen, M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2016, 2, e1500323. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Djehdian, L.A.; Chini, C.M.; Marston, L.; Konar, M.; Stillwell, A.S. Exposure of urban food-energy-water (FEW) systems to water scarcity. Sustain. Cities Soc. 2019, 50, 101621. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Paranychianakis, N.V.; Angelakis, A.N. Water Supply and Water Scarcity. Water 2020, 12, 2347. [Google Scholar] [CrossRef]
- Doornbusch, G.; van der Wal, M.; Tedesco, M.; Post, J.; Nijmeijer, K.; Borneman, Z. Multistage electrodialysis for desalination of natural seawater. Desalination 2021, 505, 114973. [Google Scholar] [CrossRef]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of High Salinity Brine by Multi-Stage Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef]
- Joonhyeon, K.; Sangha, K.; Rhokyun, K. Controlling ion transport with pattern structures on ion exchange membranes in electrodialysis. Desalination 2021, 499, 114801. [Google Scholar]
- Kovalenko, A.; Evdochenko, E.; Stockmeier, F.; Köller, N.; Uzdenova, A.; Urtenov, M. Influence of spacers on mass transport in electromembrane desalination systems. J. Phys. Conf. Ser. 2021, 2131, 022011. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Uzdenova, A.M.; Ovsyannikova, A.V.; Urtenov, M.H.; Bostanov, R.A. Mathematical modeling of the effect of spacers on mass transfer in electromembrane systems. J. Samara State Tech. Univ. Ser. Phys. Math. Sci. 2022, 26, 520–543. [Google Scholar] [CrossRef]
- Kovalenko, A.V.; Wessling, M.; Nikonenko, V.V.; Mareev, S.A.; Moroz, I.A.; Evdochenko, E.; Urtenov, M.K. Space-Charge breakdown phenomenon and spatio-temporal ion concentration and fluid flow patterns in overlimiting current electrodialysis. J. Membr. Sci. 2021, 636, 119583. [Google Scholar] [CrossRef]
- Kovalenko, A.; Chubyr, N.; Uzdenova, A.; Urtenov, M. Theoretical Investigation of the Phenomenon of Space Charge Breakdown in Electromembrane Systems. Membranes 2022, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Varentsov, V.K.; Pevnitskaya, M.V. Transfer of ions through ion-exchange membranes during electrodialysis. Izv. SO AS USSR. Ser. Chem. Sci. 1973, 4, 134–138. [Google Scholar]
- Kononov, Y.A.; Vrevskiy, B.M. The role of water dissociation products in the transfer of electric current through ionic membranes. Zhurn. Priklykl. Chem. 1971, 44, 929–932. [Google Scholar]
- Sokirko, A.V.; Harkatz, Y.I. To the theory of the migration current exaltation effect with consideration of water dissociation. Electrochemistry 1988, 24, 1657–1663. [Google Scholar]
- Kharkats, Y.I. About the mechanism of the forbidden currents occurrence on the ion-exchange membrane/electrolyte boundary. Electrochemistry 1985, 21, 974–977. [Google Scholar]
- Kovalenko, A.V.; Nikonenko, V.V.; Chubyr, N.O.; Urtenov, M.K. Mathematical modeling of electrodialysis of a dilute solution with accounting for water dissociation-recombination reactions. Desalination 2023, 550, 116398. [Google Scholar] [CrossRef]
- Rubinstein, I.; Maletzki, F. Electroconvection at an electrically inhomogeneous permselective membrane surface. J. Chem. Soc. Faraday Trans. 1991, 87, 2079. [Google Scholar] [CrossRef]
- Belova, E.I.; Lopatkova, G.Y.; Pismenskaya, N.D.; Nikonenko, V.V.; Larchet, C.; Pourcelly, G. The effect of anion-exchange membrane surface properties on mechanisms of overlimiting mass transfer. J. Phys Chem B 2006, 110, 13458–13469. [Google Scholar] [CrossRef] [PubMed]
- Sharafan, M.V.; Zabolotskii, V.I.; Bugakov, V.V. Electric mass transport through homogeneous and surface-modified heterogeneous ion-exchange membranes at a rotating membrane disk. Rus. J. Electrochem. 2009, 45, 1162. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pismenskaya, N.; Han, J.; Bazinet, L. How physico-chemical and surface properties of cation-exchange membrane affect membrane scaling and electroconvective vortices: Influence on performance of electrodialysis with pulsed electric field. Desalination 2016, 393, 102–114. [Google Scholar] [CrossRef]
- Bazant, L.N.; Mikhaylin, S.; Bazinet, L. Voltage spike and electroconvective vortices generation during electrodialysis under pulsed electric field: Impact on demineralization process efficiency and energy consumption. Innov. Food Sci. Emerg. Technol. 2019, 52, 221–231. [Google Scholar]
- Bazant, M.Z.; Kilic, M.S.; Storey, B.D.; Ajdari, A. Towards an understanding of induced-charge electrokinetics at large applied voltages in concentrated solutions. Adv. Colloid Interface Sci. 2009, 152, 48–88. [Google Scholar] [CrossRef]
- Olesen, L.H.; Bruus, H.; Ajdari, A. Ac electrokinetic micropumps: The effect of geometrical confinement, Faradaic current injection, and nonlinear surface capacitance. Phys. Rev. E 2006, 73, 056313. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Stevens, A.L.; Han, J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 2005, 77, 4293. [Google Scholar] [CrossRef]
- Mani, A.; Bazant, M.Z. Deionization shocks in microstructures. Phys. Rev. E 2011, 84, 061504. [Google Scholar] [CrossRef]
- Yaroshchuk, A. Over-limiting currents and deionization shocks in current-induced polarization: Local equilibrium analysis. Adv. Colloid Interface Sci. 2012, 183–184, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wessling, M.; de Jong, J.; Lammertink, R.G.H. Membranes and microfluidics: A review. Lab Chip. 2006, 6, 1125. [Google Scholar]
- Slouka, Z.; Senapati, S.; Chang, H.C. Microfluidic Systems with Ion-Selective Membranes. Annu. Rev. Anal. Chem. 2014, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef]
- Zaltzman, B.; Rubinstein, I. Electro-osmotic slip and electroconvective instability. J. Fluid Mech. 2007, 579, 173. [Google Scholar] [CrossRef]
- Abu-Rjal, R.; Prigozhin, L.; Rubinstein, I.; Zaltzman, B. Equilibrium electro-convective instability in concentration polarization: The effect of non-equal ionic diffusivities and longitudinal flow. Russ. J. Electrochem. 2017, 53, 903–918. [Google Scholar] [CrossRef]
- Demekhin, E.A.; Shelistov, V.S.; Polyanskikh, S.V. Linear and nonlinear evolution and diffusion layer selection in electrokinetic instability. Phys. Rev. E. 2011, 84, 1722036318. [Google Scholar] [CrossRef]
- Demekhin, E.A.; Ganchenko, G.S.; Kalaydin, E.N. Transition to electrokinetic instability near imperfect charge-selective membranes. Phys. Fluids 2018, 30, 082006. [Google Scholar] [CrossRef]
- Kwak, R.; Pham, V.S.; Lim, K.M.; Han, J. Shear ow of an electrically charged uid by ion concentration polarization: Scaling laws for electroconvective vortices. Phys. Rev. Lett. 2013, 110, 114501. [Google Scholar] [CrossRef]
- Pham, S.V.; Kwon, H.; Kim, B.; White, J.K.; Lim, G.; Han, J. Helical vortex formation in three-dimensional electrochemical systems with ionselective Membranes. Phys. Review. E 2016, 93, 033114. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.; Wessling, M.; Mani, A. On the Dynamical Regimes of Pattern-Accelerated Electroconvection. Sci. Rep. 2016, 6, 22505. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Pérez, L.; Martí-Calatayud, M.C.; Montañés, M.T.; Pérez-Herranz, V. Interplay between Forced Convection and Electroconvection during the Overlimiting Ion Transport through Anion-Exchange Membranes: A Fourier Transform Analysis of Membrane Voltage Drops. Membranes 2023, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, A.V.; Yzdenova, A.M.; Sukhinov, A.I.; Chubyr, N.O.; Urtenov, M.K. Simulation of galvanic dynamic mode in membrane hydrocleaning systems taking into account space charge. AIP Conf. Proc. 2019, 2188, 050021. [Google Scholar] [CrossRef]
- Uzdenova, A.M.; Kovalenko, A.V.; Urtenov, M.K.; Nikonenko, V.V. Theoretical Analysis of the Effect of Ion Concentration in Solution Bulk and at Membrane Surface on the Mass Transfer at Overlimiting Currents. Russ. J. Electrochem. 2017, 53, 1254–1265. [Google Scholar] [CrossRef]
- Urtenov, M.K.; Kovalenko, A.V.; Sukhinov, A.I.; Chubyr, N.O.; Gudza, V.A. Model and numerical experiment for calculating the theoretical current-voltage characteristic in electro-membrane systems. IOP Conf. Ser. Mater. Sci. Eng. 2019, 680, 012030. [Google Scholar] [CrossRef]
- Winograd, Y.; Solan, A.; Toren, M. Mass transfer in narrow channels in the presence of turbulence promoters. Desalination 1973, 13, 171–186. [Google Scholar] [CrossRef]
- Kim, Y.; Walker, W.S.; Lawler, D.F. Electrodialysis with spacers: Effects of variation and correlation of boundary layer thickness. Desalination 2011, 274, 54–63. [Google Scholar] [CrossRef]
- La Cerva, M.L.; Di Liberto, M.; Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G.; Ciofalo, M. Coupling CFD with a one-dimensional model to predict the performance of reverse electrodialysis stacks. J. Memb. Sci. 2017, 541, 595–610. [Google Scholar] [CrossRef]
- Kim, B.; Choi, S.; Kwak, R.; Han, J. Energy efficiency enhancement of electromembrane desalination systems by local ow redistribution optimized for the asymmetry of cation/anion diffusivity. J. Membr. Sci. 2017, 524, 280–287. [Google Scholar] [CrossRef]
- Akberova, E.; Vasil’eva, V.I. Effect of the resin content in cation-exchange membranes on development of electroconvection. Electrochem. Commun. 2020, 111, 106659. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. How the fine structure of the electric double layer and the flow affect morphological instability in electrodeposition. Phys. Rev. Fluids 2023, 8, 093701. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. The effects of reaction kinetics upon the instabilities in cathodic electrodeposition. Curr. Opin. Colloid Interface Sci. 2022, 60, 101591. [Google Scholar] [CrossRef]
- Guan, Y.; Riley, J.; Novosselov, I. Three-dimensional electroconvective vortices in cross flow. Phys. Rev. E. 2020, 101, 033103. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalenko, A.; Urtenov, M.; Chekanov, V.; Kandaurova, N. Theoretical Analysis of the Influence of Spacers on Salt Ion Transport in Electromembrane Systems Considering the Main Coupled Effects. Membranes 2024, 14, 20. https://doi.org/10.3390/membranes14010020
Kovalenko A, Urtenov M, Chekanov V, Kandaurova N. Theoretical Analysis of the Influence of Spacers on Salt Ion Transport in Electromembrane Systems Considering the Main Coupled Effects. Membranes. 2024; 14(1):20. https://doi.org/10.3390/membranes14010020
Chicago/Turabian StyleKovalenko, Anna, Makhamet Urtenov, Vladimir Chekanov, and Natalya Kandaurova. 2024. "Theoretical Analysis of the Influence of Spacers on Salt Ion Transport in Electromembrane Systems Considering the Main Coupled Effects" Membranes 14, no. 1: 20. https://doi.org/10.3390/membranes14010020
APA StyleKovalenko, A., Urtenov, M., Chekanov, V., & Kandaurova, N. (2024). Theoretical Analysis of the Influence of Spacers on Salt Ion Transport in Electromembrane Systems Considering the Main Coupled Effects. Membranes, 14(1), 20. https://doi.org/10.3390/membranes14010020







