Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods
Abstract
:1. Introduction
2. Preparation of MOF/PVA Composite Film
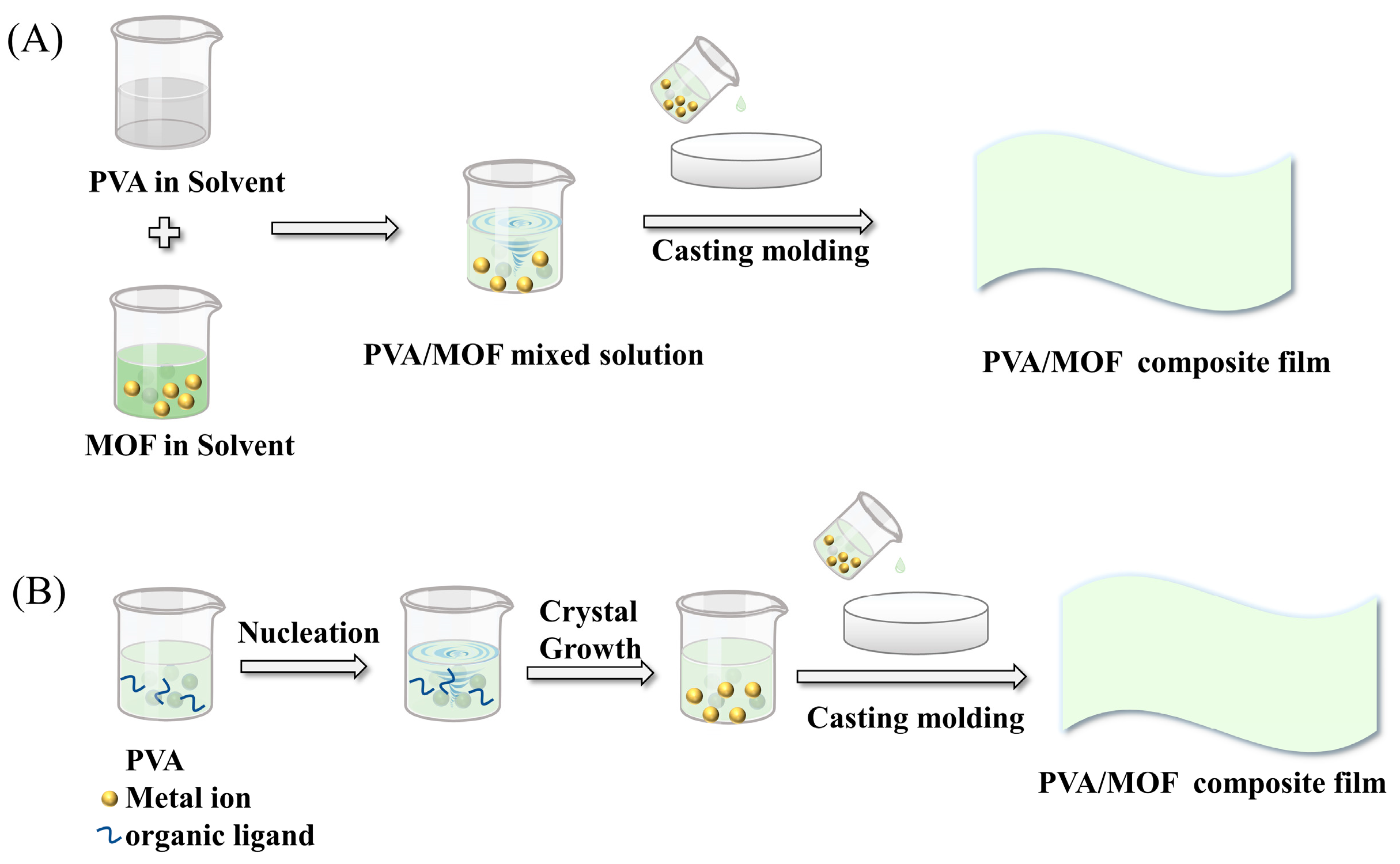
2.1. Solution Casting
2.1.1. Blending
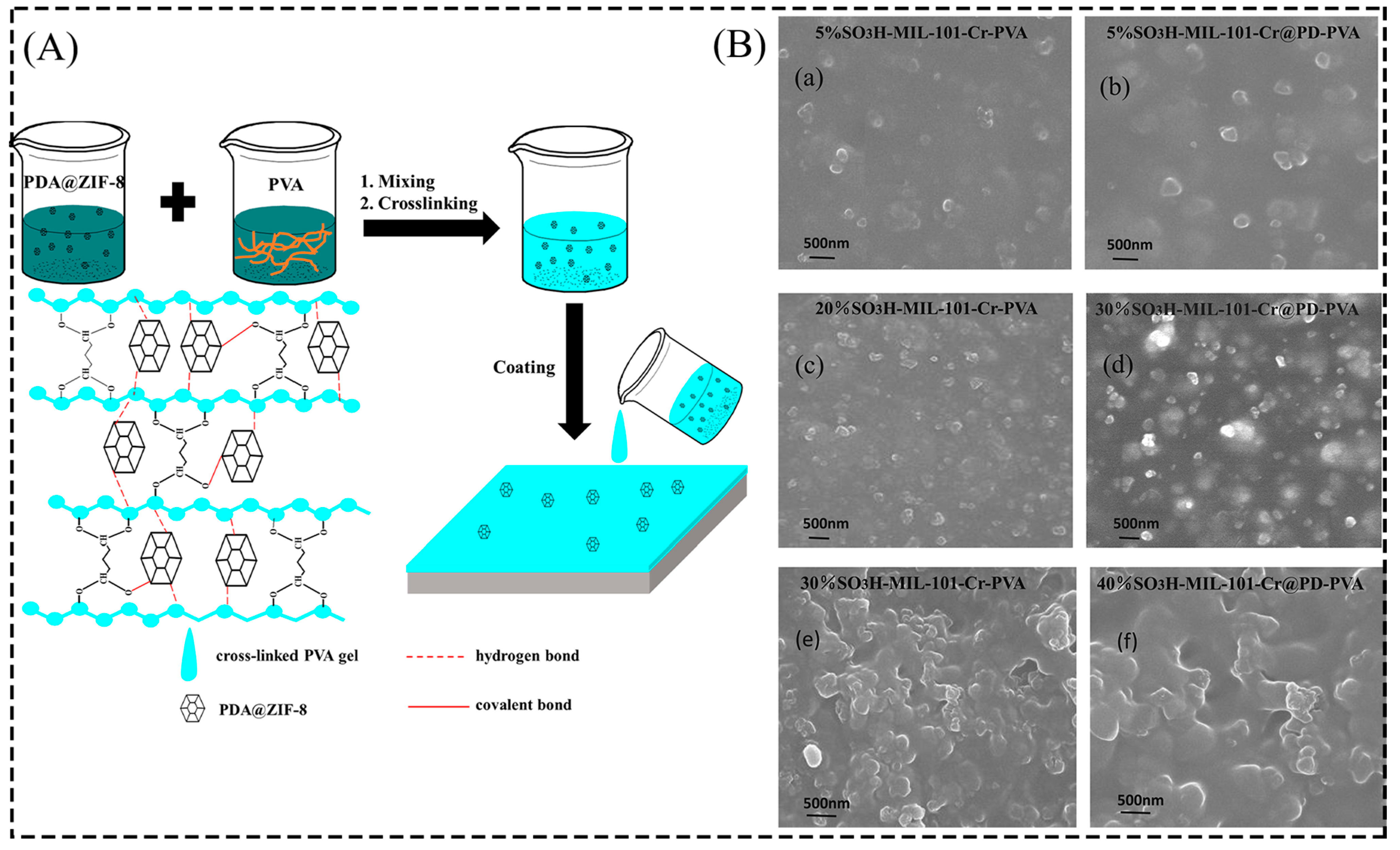
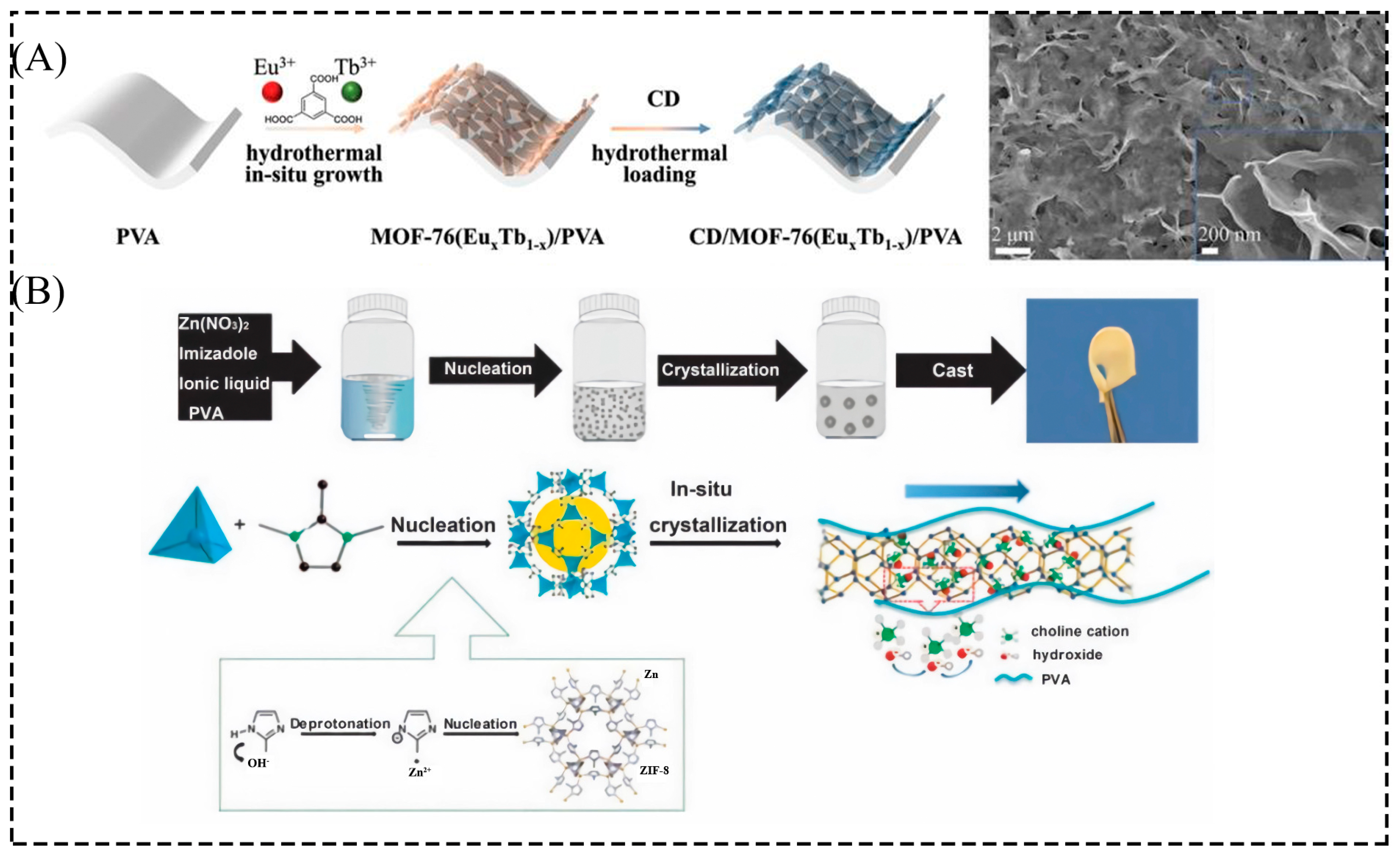
2.1.2. In Situ Growth
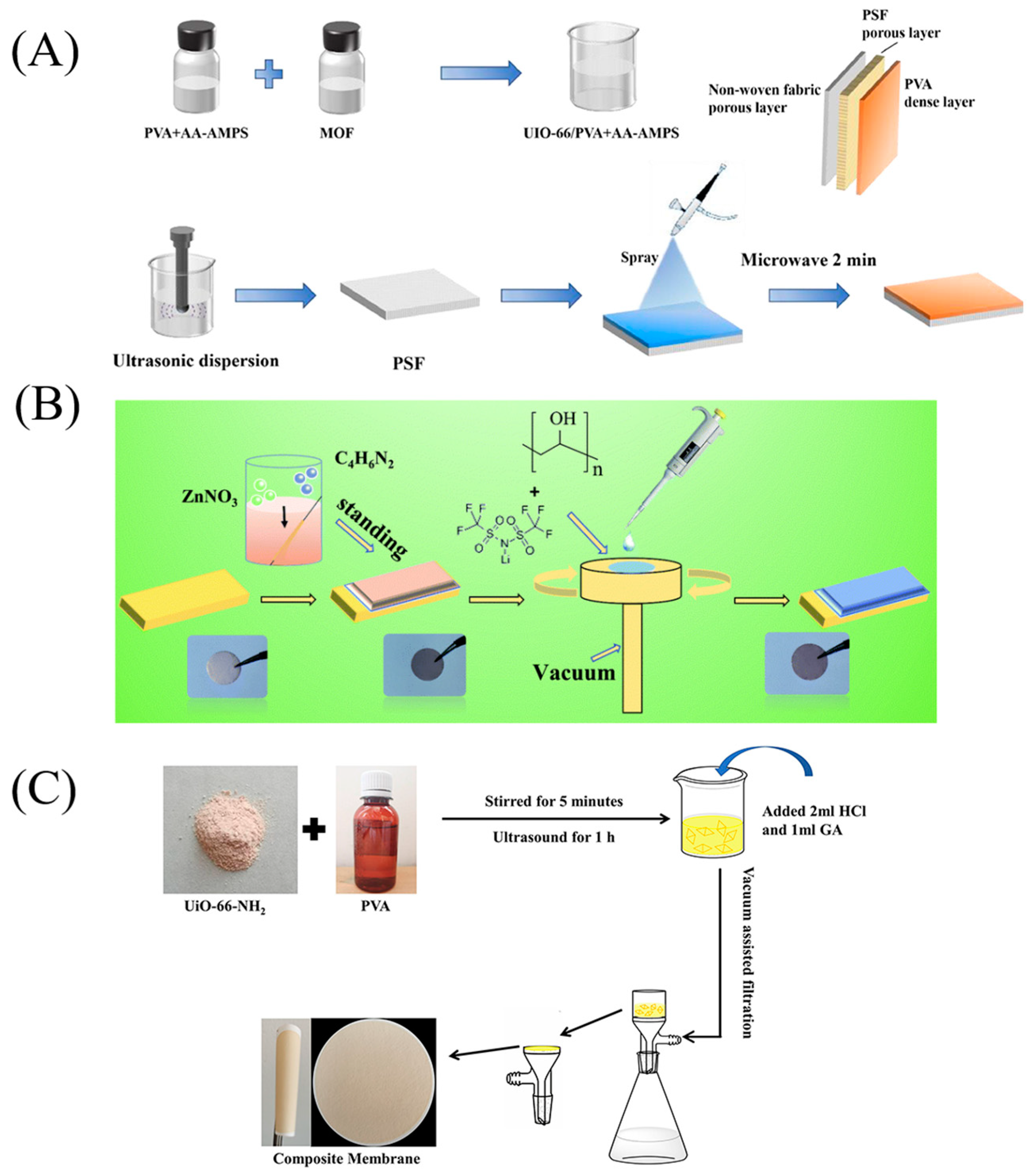
2.2. Electrostatic Spinning
2.2.1. Blending
2.2.2. Layer-by-Layer Assembly
2.3. Alternative Methods
3. Applications
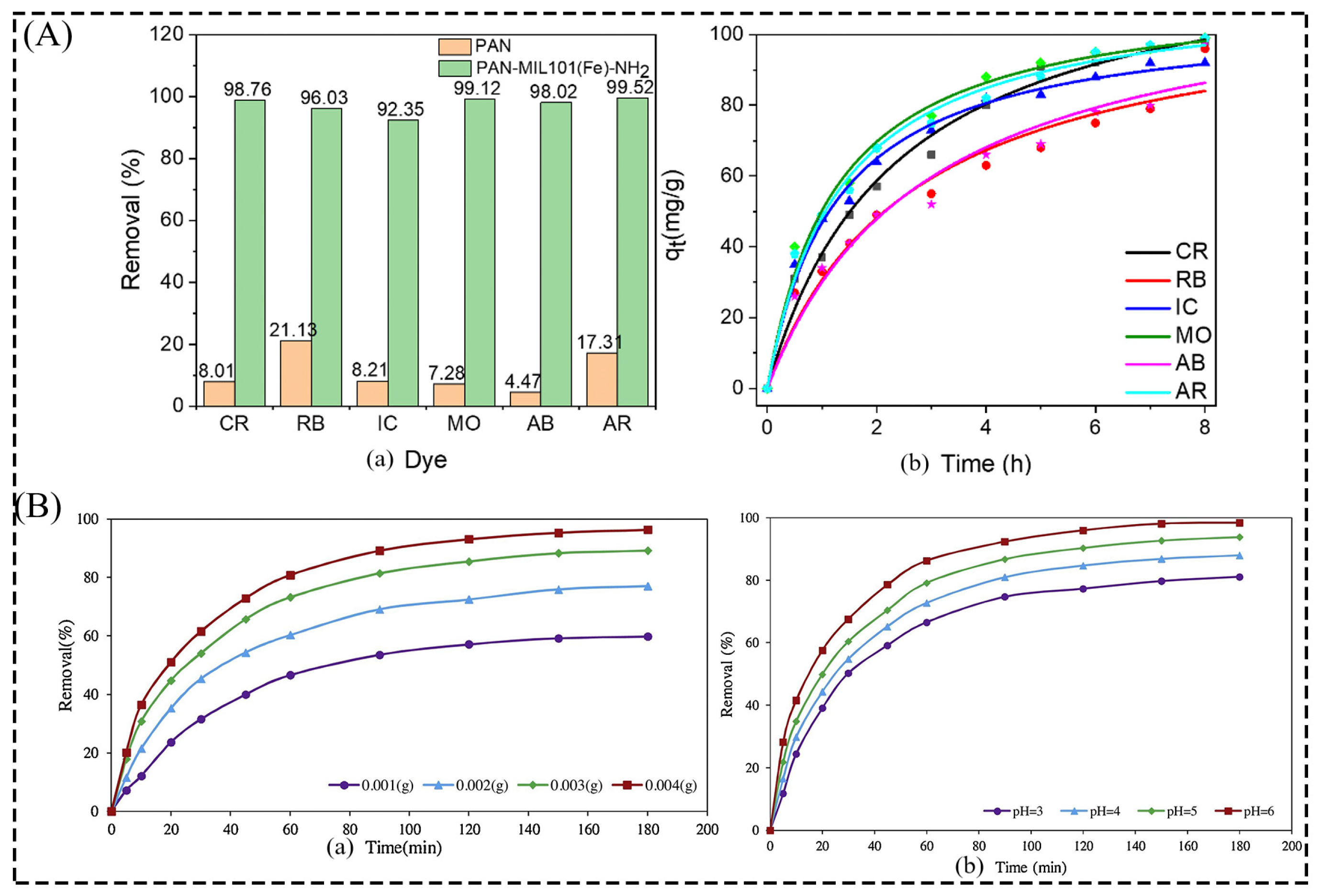
3.1. Water Treatment
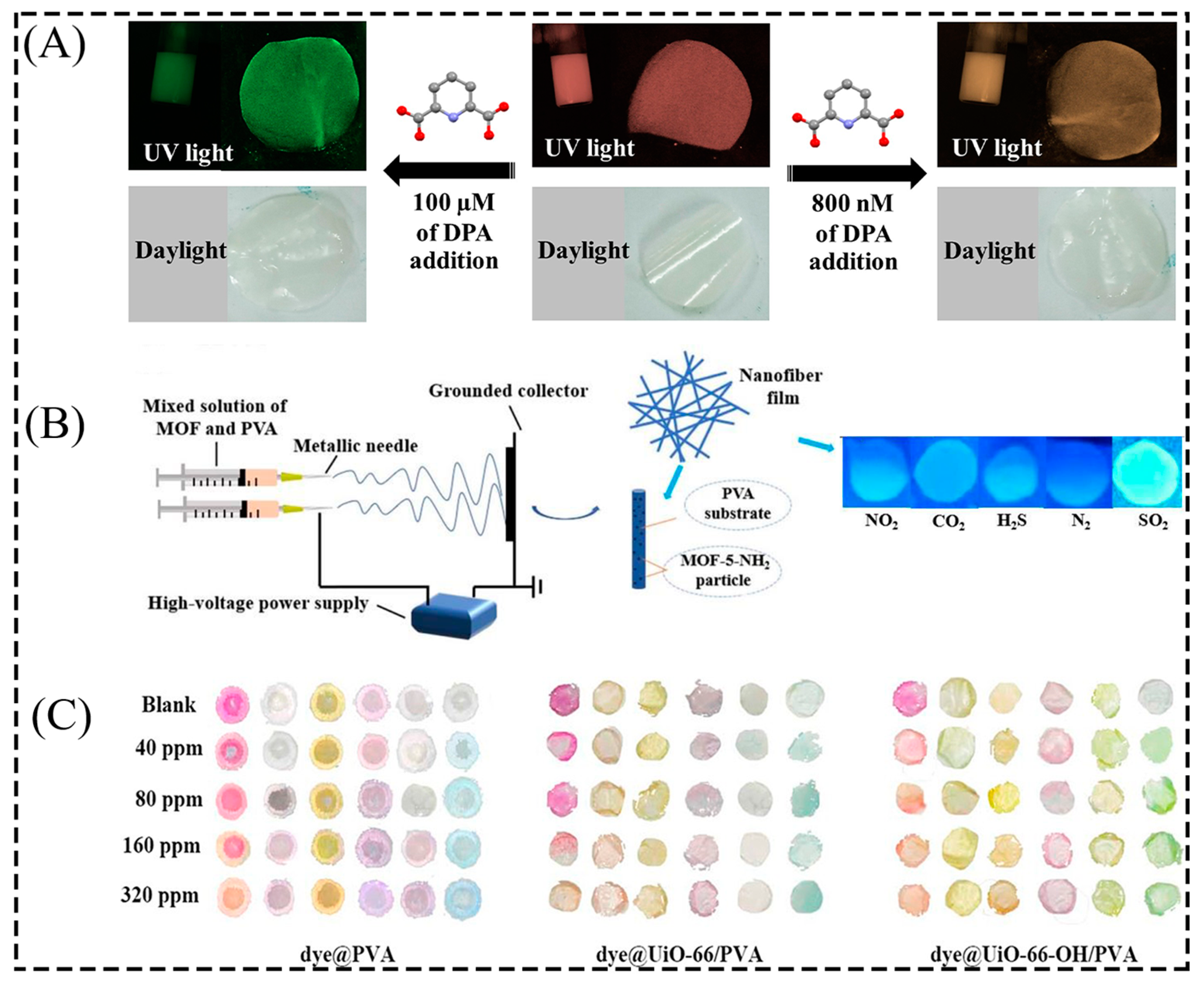
3.2. Sensing
3.3. Air Purification
3.4. Separation
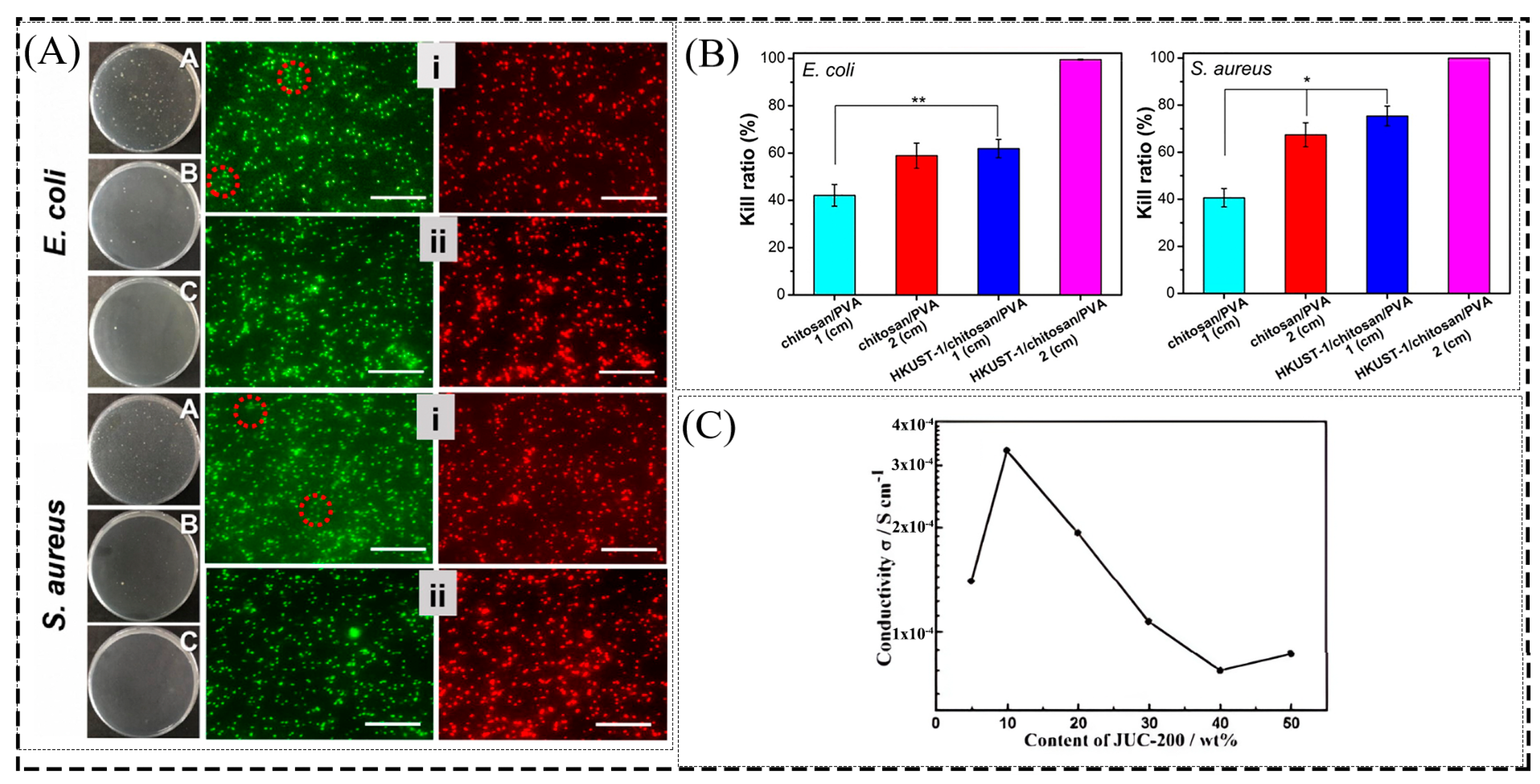
3.5. Antibacterial
3.6. Other Applications
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Yang, Z.; Wang, Z.; Liu, X.; Lu, Y.; Ji, L.; Wang, Z.L.; Cheng, J. Energy from greenhouse plastic films. Nano Energy 2021, 89, 106328. [Google Scholar] [CrossRef]
- Gündoğdu, R.; Önder, D.; Gündoğdu, S.; Gwinnett, C. Plastics derived from disposable greenhouse plastic films and irrigation pipes in agricultural soils: A case study from Turkey. Environ. Sci. Pollut. Res. Int. 2022, 29, 87706–87716. [Google Scholar] [CrossRef] [PubMed]
- Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Bakar Siddique, M.A.; Harrison, S.M.; Brunton, N.P.; Carpes, S.T. Development of a biodegradable plastic film extruded with the addition of a Brazilian propolis by-product. LWT 2022, 157, 113124. [Google Scholar] [CrossRef]
- Andrade, J.; Gonzalez-Martinez, C.; Chiralt, A. Antimicrobial PLA-PVA multilayer films containing phenolic compounds. Food Chem. 2022, 375, 131861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiong, Y.-C.; Li, F.-M.; Wang, R.-Y.; Qiang, S.-C.; Yao, T.-F.; Mo, F. Plastic film mulch for half growing-season maximized WUE and yield of potato via moisture-temperature improvement in a semi-arid agroecosystem. Agric. Water Manag. 2012, 104, 68–78. [Google Scholar] [CrossRef]
- Jones, H.; Black, T.A.; Jassal, R.S.; Nesic, Z.; Johnson, M.S.; Smukler, S. Characterization of shortwave and longwave properties of several plastic film mulches and their impact on the surface energy balance and soil temperature. Sol. Energy 2021, 214, 457–470. [Google Scholar] [CrossRef]
- Noble, R.D. Perspectives on mixed matrix membranes. J. Membr. Sci. 2011, 378, 393–397. [Google Scholar] [CrossRef]
- Khan, A.L.; Cano-Odena, A.; Gutiérrez, B.; Minguillón, C.; Vankelecom, I.F.J. Hydrogen separation and purification using polysulfone acrylate–zeolite mixed matrix membranes. J. Membr. Sci. 2010, 350, 340–346. [Google Scholar] [CrossRef]
- Anson, M.; Marchese, J.; Garis, E.; Ochoa, N.; Pagliero, C. ABS copolymer-activated carbon mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2004, 243, 19–28. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: II. Modeling permeation behavior. J. Membr. Sci. 2003, 211, 335–348. [Google Scholar] [CrossRef]
- Sherugar, P.; Naik, N.S.; Padaki, M.; Nayak, V.; Gangadharan, A.; Nadig, A.R.; Déon, S. Fabrication of zinc doped aluminium oxide/polysulfone mixed matrix membranes for enhanced antifouling property and heavy metal removal. Chemosphere 2021, 275, 130024. [Google Scholar] [CrossRef] [PubMed]
- Kudo, Y.; Mikami, H.; Tanaka, M.; Isaji, T.; Odaka, K.; Yamato, M.; Kawakami, H. Mixed matrix membranes comprising a polymer of intrinsic microporosity loaded with surface-modified non-porous pearl-necklace nanoparticles. J. Membr. Sci. 2020, 597, 117627. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.D.; Chung, T.S.; Zhang, S.; Zhao, D. Advanced Porous Materials in Mixed Matrix Membranes. Adv. Mater. 2018, 30, 1802401. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ping, L.J.; Wu, J.; Li, Y.Y.; Zhou, X.P. Increasing the Stability of Metal-Organic Frameworks by Coating with Poly(tetrafluoroethylene). Inorg. Chem. 2022, 61, 5092–5098. [Google Scholar] [CrossRef]
- Liu, L.; Du, S.; Guo, X.; Xiao, Y.; Yin, Z.; Yang, N.; Bao, Y.; Zhu, X.; Jin, S.; Feng, Z.; et al. Water-Stable Nickel Metal-Organic Framework Nanobelts for Cocatalyst-Free Photocatalytic Water Splitting to Produce Hydrogen. J. Am. Chem. Soc. 2022, 144, 2747–2754. [Google Scholar] [CrossRef]
- Li, P.-X.; Yan, X.-Y.; Song, X.-M.; Li, J.-J.; Ren, B.-H.; Gao, S.-Y.; Cao, R. Zirconium-Based Metal–Organic Framework Particle Films for Visible-Light-Driven Efficient Photoreduction of CO2. ACS Sustain. Chem. Eng. 2021, 9, 2319–2325. [Google Scholar] [CrossRef]
- Lohe, M.R.; Rose, M.; Kaskel, S. Metal-organic framework (MOF) aerogels with high micro- and macroporosity. Chem. Commun. 2009, 40, 6056–6058. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, C.; Kaneti, Y.V.; Eguchi, M.; Lin, J.; Yamauchi, Y.; Na, J. Practical MOF Nanoarchitectonics: New Strategies for Enhancing the Processability of MOFs for Practical Applications. Langmuir 2020, 36, 4231–4249. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Fang, M.; Wang, T.; Yu, L.; Li, J. ZIF-7/PDMS mixed matrix membranes for pervaporation recovery of butanol from aqueous solution. Sep. Purif. Technol. 2016, 163, 39–47. [Google Scholar] [CrossRef]
- Denny, M.S., Jr.; Cohen, S.M. In Situ Modification of Metal-Organic Frameworks in Mixed-Matrix Membranes. Angew. Chem. Int. Ed. Engl. 2015, 54, 9029–9032. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Chen, X.; Tang, K.; Meng, Q.; Shen, C.; Zhang, G. Zeolite Imidazolate Framework Membranes on Polymeric Substrates Modified with Poly(vinyl alcohol) and Alginate Composite Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 12605–12612. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, Y.; Wei, X.Y.; Zheng, L.W.; Li, Z.Q.; Zhang, K.G.; Yuan, C.G. Electrospun metal-organic frameworks hybrid nanofiber membrane for efficient removal of As(III) and As(V) from water. Ecotoxicol. Environ. Saf. 2021, 228, 112990. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, J.; Rauf, A.; Zhang, S.; Wang, G.; Shi, S.; Ning, G. A flexible fibrous membrane based on copper(ii) metal-organic framework/poly(lactic acid) composites with superior antibacterial performance. Biomater. Sci. 2021, 9, 3851–3859. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Y.; Li, J.; Yue, Z.; Zhou, J.; Wang, X. Degradable, antibacterial and ultrathin filtrating electrospinning membranes of Ag-MOFs/poly(l-lactide) for air pollution control and medical protection. Int. J. Biol. Macromol. 2022, 212, 182–192. [Google Scholar] [CrossRef]
- Zheng, Q.; Xiong, L.; Yu, L.; Wu, D.; Yang, C.; Xiao, Y. An enzyme-free fluorescent sensing platform for the detection of uric acid in human urine. J. Lumin. 2021, 236, 118076. [Google Scholar] [CrossRef]
- Wen, Y.; Dai, R.; Li, X.; Zhang, X.; Cao, X.; Wu, Z.; Lin, S.; Tang, C.Y.; Wang, Z. Metal-organic framework enables ultraselective polyamide membrane for desalination and water reuse. Sci. Adv. 2022, 8, eabm4149. [Google Scholar] [CrossRef]
- Sorribas, S.; Gorgojo, P.; Tellez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal-organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, X.; Yu, H.; Lu, X.; Liu, J.; Zhang, L.; Wang, G.; Ye, J.; Ning, G. Silver(I) metal-organic framework-embedded polylactic acid electrospun fibrous membranes for efficient inhibition of bacteria. Dalton Trans. 2022, 51, 6673–6681. [Google Scholar] [CrossRef]
- Premkumar, P.S. Preparation and electrical studies on pure and oxygen plasma treated polyvinyl alcohol films. J. Mater. Res. Technol. 2019, 8, 2232–2237. [Google Scholar] [CrossRef]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of ultrasonication duration of polyvinyl alcohol (PVA) gel on characterizations of PVA film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, D.; Pochat-Bohatier, C.; Gassara, S.; Cambedouzou, J.; Bechelany, M.; Miele, P. Development of novel h-BNNS/PVA porous membranes via Pickering emulsion templating. Green. Chem. 2018, 20, 4319–4329. [Google Scholar] [CrossRef]
- Ceia, T.F.; Silva, A.G.; Ribeiro, C.S.; Pinto, J.V.; Casimiro, M.H.; Ramos, A.M.; Vital, J. PVA composite catalytic membranes for hyacinth flavour synthesis in a pervaporation membrane reactor. Catal. Today 2014, 236, 98–107. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Shen, J.; Lee, H.P.; Yan, X. Sound absorption performance and mechanism of flexible PVA microperforated membrane. Appl. Acoust. 2022, 185, 108420. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, B. Structure and Sound Absorption Properties of Spiral Vane Electrospun PVA/PEO Nanofiber Membranes. Appl. Sci. 2018, 8, 296. [Google Scholar] [CrossRef]
- Xie, M.; Wang, J.; Zhao, H. A PVA film for detecting lipid oxidation intended for food application. Sens. Actuators B Chem. 2018, 273, 260–263. [Google Scholar] [CrossRef]
- Cao, S.-g.; Liu, Z.-f.; Hu, B.-h.; Liu, H.-q. Stabilization of electrospun poly(vinyl alcohol) nanofibrous mats in aqueous solutions. Chin. J. Polym. Sci. 2010, 28, 781–788. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Zhou, L.; Du, H.; Zhu, Z.; Wen, Y. Electrospun pullulan/PVA nanofibers integrated with thymol-loaded porphyrin metal-organic framework for antibacterial food packaging. Carbohydr. Polym. 2021, 270, 118391. [Google Scholar] [CrossRef]
- Patil, A.S.; Waghmare, R.D.; Pawar, S.P.; Salunkhe, S.T.; Kolekar, G.B.; Sohn, D.; Gore, A.H. Photophysical insights of highly transparent, flexible and re-emissive PVA @ WTR-CDs composite thin films: A next generation food packaging material for UV blocking applications. J. Photochem. Photobiol. A Chem. 2020, 400, 112647. [Google Scholar] [CrossRef]
- Kassenova, N.; Kalybekkyzy, S.; Kahraman, M.V.; Mentbayeva, A.; Bakenov, Z. Photo and thermal crosslinked poly(vinyl alcohol)-based nanofiber membrane for flexible gel polymer electrolyte. J. Power Sources 2022, 520, 230896. [Google Scholar] [CrossRef]
- Holloway, J.L.; Lowman, A.M.; VanLandingham, M.R.; Palmese, G.R. Chemical grafting for improved interfacial shear strength in UHMWPE/PVA-hydrogel fiber-based composites used as soft fibrous tissue replacements. Compos. Sci. Technol. 2013, 85, 118–125. [Google Scholar] [CrossRef]
- Jia, S.; Ji, D.; Wang, L.; Qin, X.; Ramakrishna, S. Metal–Organic Framework Membranes: Advances, Fabrication, and Applications. Small Struct. 2022, 3, 2100222. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Regmi, C.; Friess, K. Interfacial Design of Mixed Matrix Membranes via Grafting PVA on UiO-66-NH2 to Enhance the Gas Separation Performance. Membranes 2021, 11, 419. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Tang, Q.; Zhang, Z.; Yu, C.; Li, H.; Xu, L.; Zhang, S.; Zou, Z. Enhanced mechanical, thermal, and UV-shielding properties of poly(vinyl alcohol)/metal-organic framework nanocomposites. RSC Adv. 2018, 8, 38681–38688. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, W.; Liu, Q.; Wang, H.; Li, J.; Wu, C.; Li, Y.; Wei, Z. Intensification of water/ethanol separation by PVA hybrid membrane with different functional ligand UiO-66-X nanochannels in pervaporation process. Sep. Purif. Technol. 2021, 256, 117802. [Google Scholar] [CrossRef]
- Lu, C.; Xiao, H.; Chen, X. MOFs/PVA hybrid membranes with enhanced mechanical and ion-conductive properties. e-Polymers 2021, 21, 160–165. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sudarshan, K.; Pujari, P.K. Unraveling the sub-nanoscopic structure at interphase in a poly(vinyl alcohol)-MOF nanocomposite, and its role on thermo-mechanical properties. Phys. Chem. Chem. Phys. 2016, 18, 25434–25442. [Google Scholar] [CrossRef]
- Zhu, T.; Zhao, X.; Yi, M.; Xu, S.; Wang, Y. Ternary cross-linked PVA-APTES-ZIF-90 membrane for enhanced ethanol dehydration performance. Adv. Compos. Hybrid. Mater. 2021, 5, 91–103. [Google Scholar] [CrossRef]
- Fu, W.; Chen, J.; Li, C.; Jiang, L.; Qiu, M.; Li, X.; Wang, Y.; Cui, L. Enhanced flux and fouling resistance forward osmosis membrane based on a hydrogel/MOF hybrid selective layer. J. Colloid. Interface Sci. 2021, 585, 158–166. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, Y.; Ma, J.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Mixed matrix membranes incorporated with polydopamine-coated metal-organic framework for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2017, 527, 8–17. [Google Scholar] [CrossRef]
- Neufeld, M.J.; Lutzke, A.; Jones, W.M.; Reynolds, M.M. Nitric Oxide Generation from Endogenous Substrates Using Metal-Organic Frameworks: Inclusion within Poly(vinyl alcohol) Membranes To Investigate Reactivity and Therapeutic Potential. ACS Appl. Mater. Interfaces 2017, 9, 35628–35641. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, M.; Yang, Y.; Meng, H.; Wang, Q.; Li, C.; Li, G. Luminescence-colour-changing sensing toward neurological drug carbamazepine in water and biofluids based on white light-emitting CD/Ln-MOF/PVA test papers. J. Mater. Chem. C 2021, 9, 8683–8693. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, Q.; Wu, C.; Wang, H.; Wang, H. Viscosity-driven in situ self-assembly strategy to fabricate cross-linked ZIF-90/PVA hybrid membranes for ethanol dehydration via pervaporation. Sep. Purif. Technol. 2018, 201, 256–267. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, G.; Zhao, C.; Li, X.; Li, M.; Na, H. MOFs synthesized by the ionothermal method addressing the leaching problem of IL-polymer composite membranes. Chem Commun 2014, 50, 14121–14124. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.-W.; Zhang, X.; Wang, Y.; Zhang, X.; Shiu, B.-C.; Li, T.-T.; Lin, J.-H. Study on the preparation and performance of flexible sulfur dioxide gas sensors based on metal-organic framework. J. Polym. Res. 2022, 29, 114. [Google Scholar] [CrossRef]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef]
- Zhu, J.; Qiu, W.; Yao, C.; Wang, C.; Wu, D.; Pradeep, S.; Yu, J.; Dai, Z. Water-stable zirconium-based metal-organic frameworks armed polyvinyl alcohol nanofibrous membrane with enhanced antibacterial therapy for wound healing. J. Colloid. Interface Sci. 2021, 603, 243–251. [Google Scholar] [CrossRef]
- Truong, Y.B.; Choi, J.; Mardel, J.; Gao, Y.; Maisch, S.; Musameh, M.; Kyratzis, I.L. Functional Cross-Linked Electrospun Polyvinyl Alcohol Membranes and Their Potential Applications. Macromol. Mater. Eng. 2017, 302, 1700024. [Google Scholar] [CrossRef]
- Xing, Y.; Xue, Y.; Qin, D.; Zhao, P.; Li, P. Microwave-induced ultrafast crosslinking of Poly (vinyl alcohol) blended with nanoparticles as wave absorber for pervaporation desalination. J. Membr. Sci. Lett. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Fan, L.; Guo, Z.; Zhang, Y.; Wu, X.; Zhao, C.; Sun, X.; Yang, G.; Feng, Y.; Zhang, N. Stable artificial solid electrolyte interphase films for lithium metal anode via metal–organic frameworks cemented by polyvinyl alcohol. J. Mater. Chem. A 2020, 8, 251–258. [Google Scholar] [CrossRef]
- Chen, L.; Li, F.; Jiang, L.; He, F.; Wei, Y. UiO-66-NH2/PVA composite Janus membrane with a dense hydrophilic surface layer for strong resistance to fouling and wettability in membrane distillation. J. Water Process Eng. 2022, 48, 102887. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of pearl necklace-like ZIF-8@chitosan/PVA nanofiber with synergistic effect for recycling aqueous dye removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef] [PubMed]
- Othong, J.; Boonmak, J.; Kielar, F.; Hadsadee, S.; Jungsuttiwong, S.; Youngme, S. Self-calibrating sensor with logic gate operation for anthrax biomarker based on nanoscaled bimetallic lanthanoid MOF. Sens. Actuators B Chem. 2020, 316, 128156. [Google Scholar] [CrossRef]
- Ma, P.; Xu, W.; Teng, Z.; Luo, Y.; Gong, C.; Wang, Q. An Integrated Food Freshness Sensor Array System Augmented by a Metal-Organic Framework Mixed-Matrix Membrane and Deep Learning. ACS Sens. 2022, 7, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Fan, Y.; Cen, X.; Wang, Y.; Shiu, B.C.; Ren, H.T.; Peng, H.K.; Jiang, Q.; Lou, C.W.; Lin, J.H. Polypropylene/Polyvinyl Alcohol/Metal-Organic Framework-Based Melt-Blown Electrospun Composite Membranes for Highly Efficient Filtration of PM2.5. Nanomaterials 2020, 10, 2025. [Google Scholar] [CrossRef]
- Wu, G.; Jiang, M.; Zhang, T.; Jia, Z. Tunable Pervaporation Performance of Modified MIL-53(Al)-NH2/Poly(vinyl Alcohol) Mixed Matrix Membranes. J. Membr. Sci. 2016, 507, 72–80. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sadatnia, B. Poly(vinyl alcohol)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2014, 469, 1–10. [Google Scholar] [CrossRef]
- Singbumrung, K.; Motina, K.; Pisitsak, P.; Chitichotpanya, P.; Wongkasemjit, S.; Inprasit, T. Preparation of Cu-BTC/PVA Fibers with Antibacterial Applications. Fibers Polym. 2018, 19, 1373–1378. [Google Scholar] [CrossRef]
- Lin, X.; Li, N.; Xiao, Q.; Guo, Y.; Wei, J.; Jiao, T.; Chen, Q.; Chen, Q.; Chen, X. Polyvinyl alcohol/starch-based film incorporated with grape skin anthocyanins and metal-organic framework crystals for colorimetric monitoring of pork freshness. Food Chem. 2022, 395, 133613. [Google Scholar] [CrossRef]
- Cai, K.; Sun, F.; Liang, X.; Liu, C.; Zhao, N.; Zou, X.; Zhu, G. An acid-stable hexaphosphate ester based metal–organic framework and its polymer composite as proton exchange membrane. J. Mater. Chem. A 2017, 5, 12943–12950. [Google Scholar] [CrossRef]
- Qi, Z.; Zhang, S.; Liu, B.; Li, M.; Mei, D. Performance Research of PVA (Polyvinyl alcohol) Based on HKUST-1 as Additive. Chem. Lett. 2022, 51, 658–661. [Google Scholar] [CrossRef]
- Anjum, M.W.; Vermoortele, F.; Khan, A.L.; Bueken, B.; De Vos, D.E.; Vankelecom, I.F. Modulated UiO–66 based Mixed Matrix Membranes for CO2 Separation. ACS Appl. Mater. Interfaces 2015, 7, 25193–25201. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Ouyang, J.; Liu, T.; Li, Z.; Li, Z.; Liu, Y.; Wang, S.; Wu, H.; Jiang, Z.; Cao, X. Enhanced interfacial interaction and CO2 separation performance of mixed matrix membrane by incorporating polyethylenimine-decorated metal-organic frameworks. ACS Appl. Mater. Interfaces 2015, 7, 1065–1077. [Google Scholar] [CrossRef]
- Sabetghadam, A.; Seoane, B.; Keskin, D.; Duim, N.; Rodenas, T.; Shahid, S.; Sorribas, S.; Le Guillouzer, C.; Clet, G.; Tellez, C.; et al. Metal Organic Framework Crystals in Mixed-Matrix Membranes: Impact of the Filler Morphology on the Gas Separation Performance. Adv. Funct. Mater. 2016, 26, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Laínez, J.; Zornoza, B.; Mayoral, Á.; Berenguer-Murcia, Á.; Cazorla-Amorós, D.; Téllez, C.; Coronas, J. Beyond the H2/CO2 upper bound: One-step crystallization and separation of nano-sized ZIF-11 by centrifugation and its application in mixed matrix membranes. J. Mater. Chem. A 2015, 3, 6549–6556. [Google Scholar] [CrossRef]
- Lu, G.; Li, S.; Guo, Z.; Farha, O.K.; Hauser, B.G.; Qi, X.; Wang, Y.; Wang, X.; Han, S.; Liu, X.; et al. Imparting functionality to a metal-organic framework material by controlled nanoparticle encapsulation. Nat. Chem. 2012, 4, 310–316. [Google Scholar] [CrossRef]
- Hunley, M.T.; Long, T.E. Electrospinning functional nanoscale fibers: A perspective for the future. Polym. Int. 2008, 57, 385–389. [Google Scholar] [CrossRef]
- Lannutti, J.; Reneker, D.; Ma, T.; Tomasko, D.; Farson, D. Electrospinning for tissue engineering scaffolds. Mater. Sci. Eng. C 2007, 27, 504–509. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, W.; Kaiser, A. Electrospinning of Metal-Organic Frameworks for Energy and Environmental Applications. Adv. Sci. 2020, 7, 1902590. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal–organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.; Kang, X.; Zhang, L.; Wu, C.; Zhang, J.; Han, B. In situ synthesis of sub-nanometer metal particles on hierarchically porous metal-organic frameworks via interfacial control for highly efficient catalysis. Chem. Sci. 2018, 9, 1339–1343. [Google Scholar] [CrossRef]
- Farha, O.K.; Yazaydin, A.O.; Eryazici, I.; Malliakas, C.D.; Hauser, B.G.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Li, B.; Wang, H.; Hu, T.; Bao, Z.; Chen, B. Control of interpenetration in a microporous metal-organic framework for significantly enhanced C2H2/CO2 separation at room temperature. Chem. Commun. 2016, 52, 3494–3496. [Google Scholar] [CrossRef]
- Ma, D.; Li, Z.; Zhu, J.; Zhou, Y.; Chen, L.; Mai, X.; Liufu, M.; Wu, Y.; Li, Y. Inverse and highly selective separation of CO2/C2H2 on a thulium–organic framework. J. Mater. Chem. A 2020, 8, 11933–11937. [Google Scholar] [CrossRef]
- Yu, Z.; Anstine, D.M.; Boulfelfel, S.E.; Gu, C.; Colina, C.M.; Sholl, D.S. Incorporating Flexibility Effects into Metal-Organic Framework Adsorption Simulations Using Different Models. ACS Appl. Mater. Interfaces 2021, 13, 61305–61315. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, L.; Liu, X.; Tan, G.; Yuan, F.; Nezamzadeh-Ejhieh, A.; Qi, N.; Liu, J.; Peng, Y. Fluorescence detection platform of metal-organic frameworks for biomarkers. Colloids Surf. B: Biointerfaces 2023, 229, 113455. [Google Scholar] [CrossRef]
- Luo, D.; Huang, J.; Jian, Y.; Singh, A.; Kumar, A.; Liu, J.; Pan, Y.; Ouyang, Q. Metal–organic frameworks (MOFs) as apt luminescent probes for the detection of biochemical analytes. J. Mater. Chem. B 2023, 11, 6802–6822. [Google Scholar] [CrossRef]
- Dong, J.; Yu, Y.; Pei, Y.; Pei, Z. pH-responsive aminotriazole doped metal organic frameworks nanoplatform enables self-boosting reactive oxygen species generation through regulating the activity of catalase for targeted chemo/chemodynamic combination therapy. J. Colloid. Interface Sci. 2022, 607, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Peng, Z.; Peng, Y.; Li, B.; Pan, Y.; Ouyang, Q.; Sakiyama, H.; Muddassir, M.; Liu, J. Construction of Fe-doped ZIF-8/DOX nanocomposites for ferroptosis strategy in the treatment of breast cancer. J. Mater. Chem. B 2023, 11, 6335–6345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-M.; Xie, L.-H.; Wu, Y. Recent advances in the shaping of metal–organic frameworks. Inorg. Chem. Front. 2020, 7, 2840–2866. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 separation. Sep. Purif. Technol. 2019, 224, 85–94. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, Y.; Yu, K.; Wang, S.; Zhao, D.; Chen, B. MOF-Nanocomposite Mixed-Matrix Membrane for Dual-Luminescence Ratiometric Temperature Sensing. Adv. Opt. Mater. 2021, 9, 2100945. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, Z.; Zhao, Y.; Qiao, L.; Zhang, H.; Li, X. Advanced porous PBI membranes with tunable performance induced by the polymer-solvent interaction for flow battery application. Energy Storage Mater. 2018, 10, 40–47. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Q.; Jiang, J. A molecular simulation protocol for swelling and organic solvent nanofiltration of polymer membranes. J. Membr. Sci. 2019, 573, 639–646. [Google Scholar] [CrossRef]
- Farrokhi, A.; Jafarpour, M.; Alipour, M. Solar-driven advanced oxidation process catalyzed by metal–organic frameworks for water depollution. Polyhedron 2019, 170, 325–333. [Google Scholar] [CrossRef]
- Song, K.; Qian, X.; Zhu, X.; Li, X.; Hong, X. Fabrication of mechanical robust keratin film by mesoscopic molecular network reconstruction and its performance for dye removal. J. Colloid. Interface Sci. 2020, 579, 28–36. [Google Scholar] [CrossRef]
- Alqadami, A.A.; Khan, M.A.; Siddiqui, M.R.; Alothman, Z.A. Development of citric anhydride anchored mesoporous MOF through post synthesis modification to sequester potentially toxic lead (II) from water. Microporous Mesoporous Mater. 2018, 261, 198–206. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Shariati, J. Synthesis of a nanostructured pillar MOF with high adsorption capacity towards antibiotics pollutants from aqueous solution. J. Hazard. Mater. 2019, 366, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, J.; Cai, J.; Song, R.; Cui, Y.; Yang, Y.; Chen, B.; Qian, G. A porous metal–organic framework with –COOH groups for highly efficient pollutant removal. Chem. Commun. 2014, 50, 14455–14458. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Xu, Y.; Lv, J.; Song, Y.; Zhou, X.; Tian, C.; Hong, X.; Cai, Y.; Zhao, C.; Lin, Z. Efficient removal of low-concentration organoarsenic by Zr-based metal–organic frameworks: Cooperation of defects and hydrogen bonds. Environ. Sci. Nano 2019, 6, 3590–3600. [Google Scholar] [CrossRef]
- Saiz, P.G.; Iglesias, N.; Gonzalez Navarrete, B.; Rosales, M.; Quintero, Y.M.; Reizabal, A.; Orive, J.; Fidalgo Marijuan, A.; Larrea, E.S.; Lopes, A.C.; et al. Chromium Speciation in Zirconium-Based Metal-Organic Frameworks for Environmental Remediation. Chemistry 2020, 26, 13861–13872. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Jia, J.; Wu, H.; Xu, L.; Dong, F.; Jia, Y.; Liu, X. Removal of Acidic Organic Ionic Dyes from Water by Electrospinning a Polyacrylonitrile Composite MIL101(Fe)-NH2 Nanofiber Membrane. Molecules 2022, 27, 2035. [Google Scholar] [CrossRef]
- Cao, X.T.; Vo, T.K.; An, T.N.M.; Nguyen, T.D.; Kabtamu, D.M.; Kumar, S. Enhanced Dye Adsorption of Mixed-Matrix Membrane by Covalent Incorporation of Metal-Organic Framework with Poly(styrene-alt-maleic anhydride). ChemistrySelect 2021, 6, 4689–4697. [Google Scholar] [CrossRef]
- Basu, S.; Balakrishnan, M. Polyamide thin film composite membranes containing ZIF-8 for the separation of pharmaceutical compounds from aqueous streams. Sep. Purif. Technol. 2017, 179, 118–125. [Google Scholar] [CrossRef]
- Huang, J.; Huang, D.; Zeng, F.; Ma, L.; Wang, Z. Photocatalytic MOF fibrous membranes for cyclic adsorption and degradation of dyes. J. Mater. Sci. 2020, 56, 3127–3139. [Google Scholar] [CrossRef]
- Karimi, A.; Vatanpour, V.; Khataee, A.; Safarpour, M. Contra-diffusion synthesis of ZIF-8 layer on polyvinylidene fluoride ultrafiltration membranes for improved water purification. J. Ind. Eng. Chem. 2019, 73, 95–105. [Google Scholar] [CrossRef]
- Low, Z.-X.; Razmjou, A.; Wang, K.; Gray, S.; Duke, M.; Wang, H. Effect of addition of two-dimensional ZIF-L nanoflakes on the properties of polyethersulfone ultrafiltration membrane. J. Membr. Sci. 2014, 460, 9–17. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, F.; Kang, D.J.; Pang, H. A Review of Metal-Organic Framework-based Compounds for Environmental Applications. Energy Environ. Mater. 2022, 6, e12414. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of metal organic framework in wastewater treatment. Green. Energy Environ. 2022, 8, 698–721. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Sheikhshoaie, I.; Nugraha, A.S.; Jang, H.W.; Yamauchi, Y.; Shokouhimehr, M. Performance of metal–organic frameworks in the electrochemical sensing of environmental pollutants. J. Mater. Chem. A 2021, 9, 8195–8220. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Gong, Y.N.; Huang, Y.L.; Jiang, L.; Lu, T.B. A luminescent microporous metal-organic framework with highly selective CO2 adsorption and sensing of nitro explosives. Inorg. Chem. 2014, 53, 9457–9459. [Google Scholar] [CrossRef]
- Butler, C.; Goetz, S.; Fitchett, C.M.; Kruger, P.E.; Gunnlaugsson, T. Spontaneous formation of novel luminescent dinuclear lanthanide complexes that emit in the visible and near-IR regions. Inorg. Chem. 2011, 50, 2723–2725. [Google Scholar] [CrossRef] [PubMed]
- Sousaraei, A.; Queiros, C.; Moscoso, F.G.; Lopes-Costa, T.; Pedrosa, J.M.; Silva, A.M.G.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Sub-ppm Amine Detection via Absorption and Luminescence Turn-On Caused by Ligand Exchange in Metal Organic Frameworks. Anal. Chem. 2019, 91, 15853–15859. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, X.-Y.; Cheng, C.; Shao, Z.-S.; Wang, H.-S. Strategies to fabricate metal–organic framework (MOF)-based luminescent sensing platforms. J. Mater. Chem. C 2019, 7, 10743–10763. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H.; Wei, X.; Lin, Z.; Guo, L.; Qiu, B.; Chen, G. Metal-organic framework (MOF): A novel sensing platform for biomolecules. Chem. Commun. 2013, 49, 1276–1278. [Google Scholar] [CrossRef]
- Lin, X.-M.; Niu, J.-L.; Wen, P.-X.; Pang, Y.; Hu, L.; Cai, Y.-P. A Polyhedral Metal–Organic Framework Based on Supramolecular Building Blocks: Catalysis and Luminescent Sensing of Solvent Molecules. Cryst. Growth Des. 2016, 16, 4705–4710. [Google Scholar] [CrossRef]
- Wang, S.; Sun, B.; Su, Z.; Hong, G.; Li, X.; Liu, Y.; Pan, Q.; Sun, J. Lanthanide-MOFs as multifunctional luminescent sensors. Inorg. Chem. Front. 2022, 9, 3259–3266. [Google Scholar] [CrossRef]
- Ali, A.; AlTakroori, H.H.D.; Greish, Y.E.; Alzamly, A.; Siddig, L.A.; Qamhieh, N.; Mahmoud, S.T. Flexible Cu3(HHTP)2 MOF Membranes for Gas Sensing Application at Room Temperature. Nanomaterials 2022, 12, 913. [Google Scholar] [CrossRef]
- GBD Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Oliveri Conti, G.; Zanobetti, A.; Baccarelli, A.; Fiore, M.; Ferrante, M. Effect of particulate matter-bound metals exposure on prothrombotic biomarkers: A systematic review. Environ. Res. 2019, 177, 108573. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A.R. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Aguado, S. Removal of pollutants from indoor air using zeolite membranes. J. Membr. Sci. 2004, 240, 159–166. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic gas removal--metal-organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Wang, R.; Wang, S.; Yao, C.; Ren, W.; Chen, C.; Zhang, L. Metal–organic framework-based nanofiber filters for effective indoor air quality control. J. Mater. Chem. A 2018, 6, 15807–15814. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Guo, Y.; Ma, X.; Li, Z.; Ying, W.; Peng, X. Porous cellulose nanofiber stringed HKUST-1 polyhedron membrane for air purification. Appl. Mater. Today 2019, 14, 96–101. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. 2004, 116, 6456–6461. [Google Scholar] [CrossRef]
- Hupp, J.T.; Poeppelmeier, K.R. Chemistry. Better living through nanopore chemistry. Science 2005, 309, 2008–2009. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553–8557. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal-organic frameworks for air purification of toxic chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef]
- Huang, C.Y.; Song, M.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Probing the adsorption characteristic of metal-organic framework MIL-101 for volatile organic compounds by quartz crystal microbalance. Environ. Sci. Technol. 2011, 45, 4490–4496. [Google Scholar] [CrossRef]
- Yang, K.; Sun, Q.; Xue, F.; Lin, D. Adsorption of volatile organic compounds by metal–organic frameworks MIL-101: Influence of molecular size and shape. J. Hazard. Mater. 2011, 195, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Huang, S.; Xia, Q.; Li, Z. Adsorption and Diffusion of Benzene on Chromium-Based Metal Organic Framework MIL-101 Synthesized by Microwave Irradiation. Ind. Eng. Chem. Res. 2011, 50, 2254–2261. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Feng, X.; Li, H.; Zhou, J.; Wang, B. Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control. J. Am. Chem. Soc. 2016, 138, 5785–5788. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, D.; Geng, Q.; Yang, X.; Wu, H.; Xie, Y.; Wang, L.; Ning, X.; Ming, J. MOF-Embedded Bifunctional Composite Nanofiber Membranes with a Tunable Hierarchical Structure for High-Efficiency PM0.3 Purification and Oil/Water Separation. ACS Appl. Mater. Interfaces 2021, 13, 39831–39843. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chai, Y.; Li, P.; Wang, B. Metal-Organic Framework Films and Their Potential Applications in Environmental Pollution Control. Acc. Chem. Res. 2019, 52, 1461–1470. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, X.; Feng, S.; Zhong, Z.; Yao, J.; Yao, Z. ZIF-8@SiO2 composite nanofiber membrane with bioinspired spider web-like structure for efficient air pollution control. J. Membr. Sci. 2019, 581, 252–261. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, D.; Son, D.; Doh, S.J.; Kim, M.; Lee, H.; Yoon, K.R. Rational Process Design for Facile Fabrication of Dual Functional Hybrid Membrane of MOF and Electrospun Nanofiber towards High Removal Efficiency of PM2.5 and Toxic Gases. Macromol. Rapid Commun. 2022, 43, 2100648. [Google Scholar] [CrossRef]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Zereshki, S.; Figoli, A.; Madaeni, S.S.; Simone, S.; Esmailinezhad, M.; Drioli, E. Effect of polymer composition in PEEKWC/PVP blends on pervaporation separation of ethanol/cyclohexane mixture. Sep. Purif. Technol. 2010, 75, 257–265. [Google Scholar] [CrossRef]
- Wee, S.-L.; Tye, C.-T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Xu, Y.M.; Chung, T.-S. High-performance UiO-66/polyimide mixed matrix membranes for ethanol, isopropanol and n-butanol dehydration via pervaporation. J. Membr. Sci. 2017, 531, 16–26. [Google Scholar] [CrossRef]
- Chen, S.-H.; Liou, R.-M.; Lai, C.-L.; Hung, M.-Y.; Tsai, M.-H.; Huang, S.-L. Embedded nano-iron polysulfone membrane for dehydration of the ethanol/water mixtures by pervaporation. Desalination 2008, 234, 221–231. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Membr. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- An, Q.-F.; Ang, M.B.M.Y.; Huang, Y.-H.; Huang, S.-H.; Chiao, Y.-H.; Lai, C.-L.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Wu, Y.-P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, Z.; Zhang, C.; Yuan, S.; Zhu, Y.; Wang, J.; Wang, S. PVAm-PIP/PS Composite Membrane with High Performance for CO2/N2 Separation. AIChE J. 2013, 59, 215–228. [Google Scholar] [CrossRef]
- Wang, S.-N.; Huang, Z.; Wang, J.-T.; Ru, X.-F.; Teng, L.-J. PVA/UiO-66 mixed matrix membranes for n-butanol dehydration via pervaporation and effect of ethanol. Sep. Purif. Technol. 2023, 313, 123487. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; Iglesia, Ó.d.l.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Membr. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal-Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Xu, X.; Nikolaeva, D.; Hartanto, Y.; Luis, P. MOF-based membranes for pervaporation. Sep. Purif. Technol. 2021, 278, 119233. [Google Scholar] [CrossRef]
- Khan, A.; Ali, M.; Ilyas, A.; Naik, P.; Vankelecom, I.F.J.; Gilani, M.A.; Bilad, M.R.; Sajjad, Z.; Khan, A.L. ZIF-67 filled PDMS mixed matrix membranes for recovery of ethanol via pervaporation. Sep. Purif. Technol. 2018, 206, 50–58. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Geng, Y.; Lu, X.; Jia, Z. Adjustable pervaporation performance of Zr-MOF/poly(vinyl alcohol) mixed matrix membranes. J. Chem. Technol. Biotechnol. 2019, 94, 973–981. [Google Scholar] [CrossRef]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Nwagwu, C.; Anyim, O.; Ekweremadu, C.; Kim, S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int. Immunopharmacol. 2021, 90, 107247. [Google Scholar] [CrossRef]
- Chai, Z.; Tian, Q.; Ye, J.; Zhang, S.; Wang, G.; Qi, Y.; Che, Y.; Ning, G. Hierarchical magnesium oxide microspheres for removal of heavy ions from water and efficient bacterial inactivation. J. Mater. Sci. 2020, 55, 4408–4419. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, S.; Liu, Y.; Yin, W.; Gu, Z.; Zhao, Y. An overview of the use of nanozymes in antibacterial applications. Chem. Eng. J. 2021, 418, 129431. [Google Scholar] [CrossRef]
- Sun, Y.; Qin, H.; Yan, Z.; Zhao, C.; Ren, J.; Qu, X. Combating Biofilm Associated Infection In Vivo: Integration of Quorum Sensing Inhibition and Photodynamic Treatment based on Multidrug Delivered Hollow Carbon Nitride Sphere. Adv. Funct. Mater. 2019, 29, 1808222. [Google Scholar] [CrossRef]
- Li, R.; Chen, T.; Pan, X. Metal-Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Diendorf, J.; Köller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Yao, S.; Chi, J.; Wang, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Zn-MOF Encapsulated Antibacterial and Degradable Microneedles Array for Promoting Wound Healing. Adv. Heal. Healthc. Mater. 2021, 10, e2100056. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Shakya, S.; Mittal, P.; Ren, X.; Guo, T.; Bello, M.G.; Wu, L.; Li, H.; Zhu, W.; Regmi, B.; et al. Co-delivery of superfine nano-silver and solubilized sulfadiazine for enhanced antibacterial functions. Int. J. Pharm. 2020, 584, 119407. [Google Scholar] [CrossRef] [PubMed]
- Seyedpour, S.F.; Rahimpour, A.; Najafpour, G. Facile in-situ assembly of silver-based MOFs to surface functionalization of TFC membrane: A novel approach toward long-lasting biofouling mitigation. J. Membr. Sci. 2019, 573, 257–269. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zheng, X.; Xie, Z. Zirconium-Based Nanoscale Metal-Organic Framework/Poly(epsilon-caprolactone) Mixed-Matrix Membranes as Effective Antimicrobials. ACS Appl. Mater. Interfaces 2017, 9, 41512–41520. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Spry, D.B.; Fayer, M.D. Water dynamics and proton transfer in nafion fuel cell membranes. Langmuir 2008, 24, 3690–3698. [Google Scholar] [CrossRef]
- Yang, S.-L.; Sun, P.-P.; Yuan, Y.-Y.; Zhang, C.-X.; Wang, Q.-L. High proton conduction behavior in 12-connected 3D porous lanthanide–organic frameworks and their polymer composites. CrystEngComm 2018, 20, 3066–3073. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, P.; Lv, P.; Lemstra, P.J.; Cai, X.; Yang, W.; Dong, W.; Chen, M.; Liu, T.; Du, M.; et al. Excellent UV Resistance of Polylactide by Interfacial Stereocomplexation with Double-Shell-Structured TiO2 Nanohybrids. ACS Appl. Mater. Interfaces 2020, 12, 49090–49100. [Google Scholar] [CrossRef]




| MOF | Preparation Methods | Applications | Ref. |
|---|---|---|---|
| UiO-66-NH2 | Solution casting | Gas separation | [44] |
| HKUST-1 | Solution casting | UV blocking | [45] |
| UiO-66-X | Solution casting | Separation | [46] |
| ZIF-8 | Solution casting | Ionic dynamics | [47] |
| ZIF-8 | Solution casting | --- | [48] |
| ZIF-90 | Solution casting | Separation | [49] |
| ZIF-8 | Solution casting | Water treatment | [50] |
| SO3H-MIL-101-Cr | Solution casting | Separation | [51] |
| Cu-BTTri | Solution casting | Healthcare | [52] |
| MOF-76(EuxTb1−x) | Solution casting | Sensing | [53] |
| ZIF-90 | Solution casting | Separation | [54] |
| ZIF-8 | Solution casting | Electrochemistry | [55] |
| MOF-5-NH2 | Electrospinning | Sensing | [56] |
| HKUST-1 | Electrospinning | Antibacterial | [57] |
| UiO-66-NH2 | Electrospinning | Antibacterial | [58] |
| HKUST-1 | Electrospinning | Air purification | [59] |
| UiO-66 | Spraying method | Water treatment | [60] |
| Zn-MOF | Spin coating | Electrochemistry | [61] |
| UiO-66-NH2 | Vacuum filtration | Water treatment | [62] |
| ZIF-8 | Electrospinning | Water treatment | [63] |
| Ln-MOF | Solution casting | Sensing | [64] |
| UiO-66-X | Solution casting | Sensing | [65] |
| ZIF-8 | Electrospinning | Air purification | [66] |
| MIL-53(Al)-NH2 | Solution casting | Separation | [67] |
| ZIF-8 | Solution casting | Separation | [68] |
| Cu-BTC | Electrospinning | Antibacterial | [69] |
| MIL-101 | Solution casting | Antibacterial | [70] |
| JUC-200 | Solution casting | Electrochemistry | [71] |
| HKUST-1 | Solution casting | UV blocking | [72] |
| Synthesis Method | Advantages | Disadvantages | |
|---|---|---|---|
| Solution Casting | Blending | Convenient and fast | Agglomerates easily |
| Low operation cost | |||
| In situ growth | Even distribution Good interface compatibility | High MOF loads are difficult to achieve | |
| Electrospinning | Blending | High specific surface area, high flux, high porosity | Low efficiency in mass production |
| Layer-by-layer assembly | High specific surface area and high active site Multi-layer assembly, better performance | The interface between layers may be defective Time consuming | |
| Spraying | Blending | Even distribution Convenient and fast Large-scale production | High dispersion requirements for suspension Uniformity and thickness are difficult to control |
| Spin coating | In situ growth | The airflow during the rotation is conducive to uniform drying | Low concentration solution is difficult to coat Not suitable for mass production |
| Vacuum filtration | Blending | Convenient operation Easy to control film thickness | Not suitable for mass production The film is easily damaged during the separation process |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, S.; Li, M.; Wang, Y.; Mei, D. Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods. Membranes 2023, 13, 755. https://doi.org/10.3390/membranes13090755
Liu B, Zhang S, Li M, Wang Y, Mei D. Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods. Membranes. 2023; 13(9):755. https://doi.org/10.3390/membranes13090755
Chicago/Turabian StyleLiu, Binyan, Shuhua Zhang, Ming Li, Yu Wang, and Dajiang Mei. 2023. "Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods" Membranes 13, no. 9: 755. https://doi.org/10.3390/membranes13090755
APA StyleLiu, B., Zhang, S., Li, M., Wang, Y., & Mei, D. (2023). Metal–Organic Framework/Polyvinyl Alcohol Composite Films for Multiple Applications Prepared by Different Methods. Membranes, 13(9), 755. https://doi.org/10.3390/membranes13090755





