Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatment of MWCNTs
2.2. Characterization
2.3. Supercapacitors
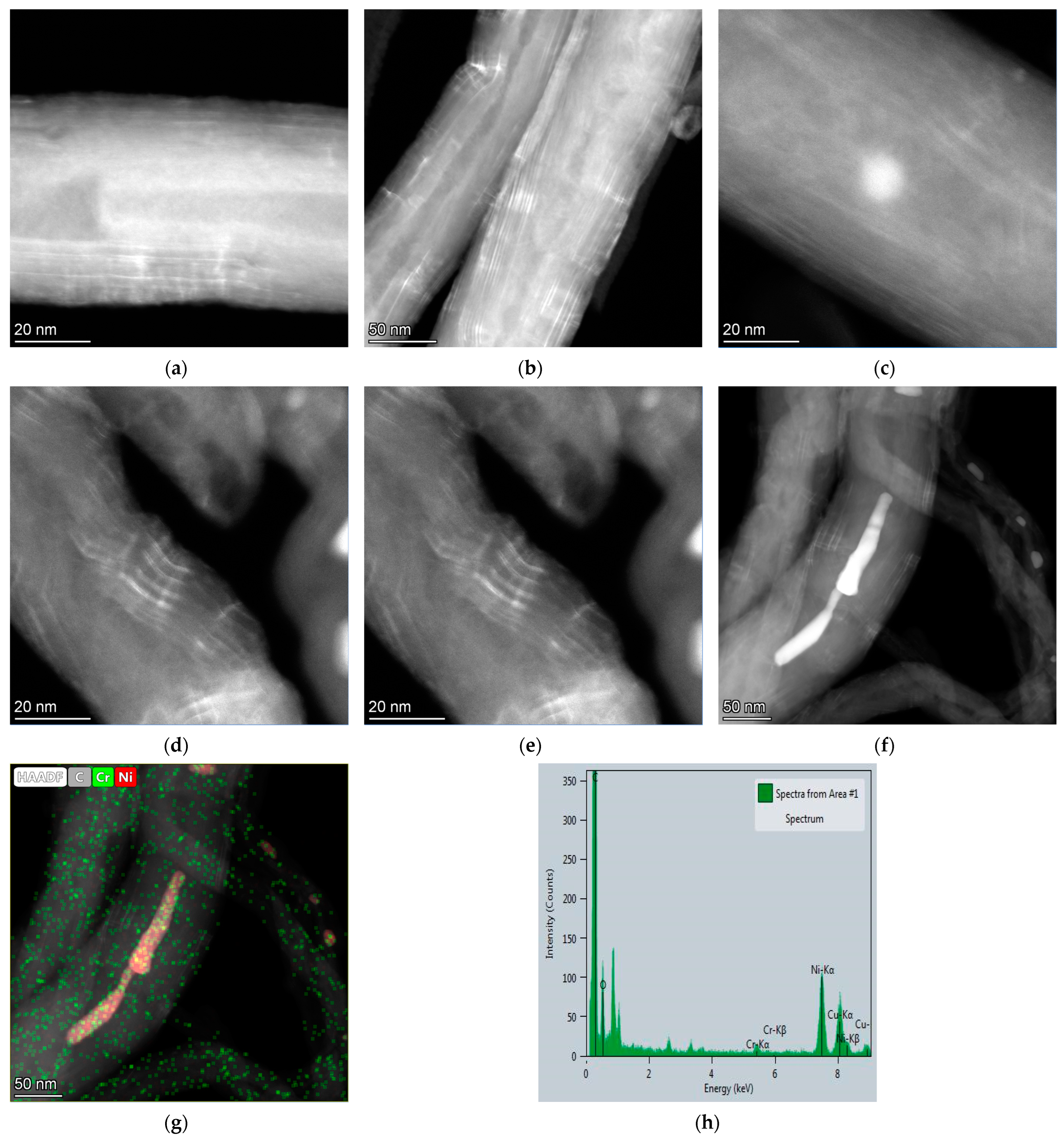
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shchegolkov, A.V.; Burakova, E.A.; Dyachkova, T.P.; Orlova, N.V.; Komarov, F.F.; Lipkin, M.S. Synthesis and functionalization of carbon nanotubes for supercapacitor electrodes. ChemChemTech 2020, 63, 74–81. [Google Scholar] [CrossRef]
- Golovakhin, V.V.; Kim, E.Y.; Novgorodtseva, O.N.; Bannov, A.G. Effect of chemical treatment of multi-walled carbon nanotubes on the specific capacitance of supercapacitors. Chim. Techno Acta 2022, 9, 1–5. [Google Scholar] [CrossRef]
- Mofokeng, T.P.; Tetana, Z.N.; Ozoemena, K.I. Defective 3D nitrogen-doped carbon nanotube-carbon fibre networks for high-performance supercapacitor: Transformative role of nitrogen-doping from surface-confined to diffusive kinetics. Carbon 2020, 169, 312–326. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Z.; Zheng, S.; Mi, B. Polyamide-crosslinked graphene oxide membrane for forward osmosis. J. Memb. Sci. 2018, 545, 11–18. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2021, 19, 1457–1475. [Google Scholar] [CrossRef]
- Omoriyekomwan, J.E.; Tahmasebi, A.; Dou, J.; Wang, R.; Yu, J. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass. Fuel Process. Technol. 2021, 214, 106686. [Google Scholar] [CrossRef]
- Xiong, Z.Y.; Zhang, B.Y.; Wang, L.; Yu, J.; Guo, Z.X. Modeling the electrical percolation of mixed carbon fillers in polymer blends. Carbon 2014, 70, 233–240. [Google Scholar] [CrossRef]
- Dong, Y.; Ni, Q.Q.; Li, L.; Fu, Y. Novel vapor-grown carbon nanofiber/epoxy shape memory nanocomposites prepared via latex technology. Mater. Lett. 2014, 132, 206–209. [Google Scholar] [CrossRef]
- Su, D.S.; Centi, G. A perspective on carbon materials for future energy application. J. Energy Chem. 2013, 22, 151–173. [Google Scholar] [CrossRef]
- Saad, R.; Gamal, A.; Zayed, M.; Ahmed, A.M.; Shaban, M.; Binsabt, M.; Rabia, M.; Hamdy, H. Fabrication of ZnO/CNTs for application in CO2 sensor at room temperature. Nanomaterials 2021, 11, 3087. [Google Scholar] [CrossRef] [PubMed]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef]
- Sharma, S.; Sengupta, K.; Islam, S.S. Deposition of pristine and functionalized MWCNTs in alumina matrix by sol-gel technique and investigation of their ammonia sensing properties. Nanomater. Nanotechnol. 2012, 2, 4. [Google Scholar] [CrossRef]
- Santidrián, A.; Sanahuja, O.; Villacampa, B.; Diez, J.L.; Benito, A.M.; Maser, W.K.; Muñoz, E.; Ansón-Casaos, A. Chemical Postdeposition Treatments to Improve the Adhesion of Carbon Nanotube Films on Plastic Substrates. ACS Omega 2019, 4, 2804–2811. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized carbon nanotubes: Synthesis, properties and applications in water purification, drug delivery, and material and biomedical sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Saito, T.; Matsushige, K.; Tanaka, K. Chemical treatment and modification of multi-walled carbon nanotubes. Phys. B Condens. Matter 2002, 323, 280–283. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of multiwalled carbon nanotubes by nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, J.; Cui, S. Sulfonitric treatment of Multiwalled carbon nanotubes and their dispersibility in water. Materials 2018, 11, 2442. [Google Scholar] [CrossRef]
- Zeng, L.; Alemany, L.B.; Edwards, C.L.; Barron, A.R. Demonstration of covalent sidewall functionalization of single wall carbon nanotubes by NMR spectroscopy: Side chain length dependence on the observation of the sidewall sp3 carbons. Nano Res. 2008, 1, 72–88. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Gul, A.; Khaligh, N.G.; Julkapli, N.M. Surface modification of Carbon-Based Nanoadsorbents for the Advanced Wastewater Treatment. J. Mol. Struct. 2021, 1235, 130148. [Google Scholar] [CrossRef]
- Han, M.; Kim, J.K.; Kang, S.W.; Jung, D. Post-treatment effects on the gas sensing performance of carbon nanotube sheets. Appl. Surf. Sci. 2019, 481, 597–603. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, M.; Leu, F.C.; Hsu, S.Y.; Wang, S.C.; Shi, S.C.; Chen, C.F. Purification of multi-walled carbon nanotubes by microwave digestion method. Diam. Relat. Mater. 2004, 13, 1182–1186. [Google Scholar] [CrossRef]
- Uvarov, N.F.; Mateyshina, Y.G.; Ulihin, A.S.; Yusin, S.I.; Varentsova, V.I.; Varentsov, V.K. Surface Electrochemical Treatment of Carbon Materials for Supercapacitors. ECS Trans. 2010, 25, 11. [Google Scholar] [CrossRef]
- Bannov, A.G.; Varentsov, V.K.; Chukanov, I.S.; Gorodilova, E.V.; Kuvshinov, G.G. Comparative analysis of methods of oxidative modification of carbon nanofibers. Prot. Met. Phys. Chem. Surf. 2012, 48, 199–206. [Google Scholar] [CrossRef]
- Kónya, Z.; Zhu, J.; Niesz, K.; Mehn, D.; Kiricsi, I. End morphology of ball milled carbon nanotubes. Carbon 2004, 42, 2001–2008. [Google Scholar] [CrossRef]
- Bannov, A.G.; Uvarov, N.F.; Ukhina, A.V.; Chukanov, I.S.; Dyukova, K.D.D.; Kuvshinov, G.G. Structural changes in carbon nanofibers induced by ball milling. Carbon 2012, 50, 1090–1098. [Google Scholar] [CrossRef]
- Pierard, N.; Fonseca, A.; Colomer, J.F.; Bossuot, C.; Benoit, J.M.; Van Tendeloo, G.; Pirard, J.P.; Nagy, J.B. Ball milling effect on the structure of single-wall carbon nanotubes. Carbon 2004, 42, 1691–1697. [Google Scholar] [CrossRef]
- Kuvshinov, G.G.; Chukanov, I.S.; Krutsky, Y.L.; Ochkov, V.V.; Zaikovskii, V.I.; Kuvshinov, D.G. Changes in the properties of fibrous nanocarbons during high temperature heat treatment. Carbon 2009, 47, 215–225. [Google Scholar] [CrossRef]
- Lv, X.; Yang, S.; Jin, J.; Zhang, L.; Li, G.; Jiang, J. Preparation and electromagnetic properties of carbon nanofiber/epoxy composites. J. Macromol. Sci. Part B Phys. 2010, 49, 355–365. [Google Scholar] [CrossRef]
- Costa, R.S.; Soares, O.S.G.P.; Vilarinho, R.; Moreira, J.A.; Pereira, M.F.R.; Pereira, A.; Pereira, C. Unveiling the role of oxidative treatments on the electrochemical performance of carbon nanotube-based cotton textile supercapacitors. Carbon Trends 2021, 5, 100137. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, E. Functionalized and tip-open carbon nanotubes for high-performance symmetric supercapacitors. Dalt. Trans. 2021, 50, 12982–12989. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Bae, J.; Yoon, S.-H. A study on the effect of surface treatment of carbon nanotubes for liquid crystalline epoxide–carbon nanotube composites. J. Mater. Chem. 2003, 13, 676–681. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. 2000, 61, 95–107. [Google Scholar] [CrossRef]
- Barnakov, C.N.; Khokhlova, G.P.; Popova, A.N.; Sozinov, S.A.; Ismagilov, Z.R. XRD characterization of the structure of graphites and carbon materials obtained by the low-temperature graphitization of coal tar pitch. Eurasian Chem. J. 2015, 17, 87–93. [Google Scholar] [CrossRef]
- Brester, A.E.; Golovakhin, V.V.; Novgorodtseva, O.N.; Lapekin, N.I.; Shestakov, A.A.; Ukhina, A.V.; Prosanov, I.Y.; Maksimovskii, E.A.; Popov, M.V.; Bannov, A.G. Chemically Treated Carbon Nanofiber Materials for Supercapacitors. Dokl. Chem. 2021, 501, 264–269. [Google Scholar] [CrossRef]
- Yusin, S.I.; Bannov, A.G. Synthesis of composite electrodes for supercapacitors based on carbon materials and the metal oxide/metal hydroxide system. Prot. Met. Phys. Chem. Surf. 2017, 53, 475–482. [Google Scholar] [CrossRef]
- Bannov, A.G.; Yusin, S.I.; Timofeeva, A.A.; Dyukova, K.D.; Ukhina, A.V.; Maksimovskii, E.A.; Popov, M.V. Synthesis of exfoliated graphite and its use as an electrode in supercapacitors. Prot. Met. Phys. Chem. Surf. 2016, 52, 645–652. [Google Scholar] [CrossRef]
- Zhao, H.; Chang, Y.; Liu, C. Electrochemical behavior of electrodes modified with metalloporphyrin and multiwalled carbon nanotubes for the reduction of oxygen, proton and carbon dioxide. J. Porphyr. Phthalocyanines 2013, 17, 259–263. [Google Scholar] [CrossRef]
- Shukla, A.K.; Banerjee, A.; Ravikumar, M.K.; Jalajakshi, A. Electrochemical capacitors: Technical challenges and prognosis for future markets. Electrochim. Acta 2012, 84, 165–173. [Google Scholar] [CrossRef]
- Suhasini. Effect of deposition method and the surfactant on high capacitance of electrochemically deposited MnO2 on stainless steel substrate. J. Electroanal. Chem. 2013, 690, 13–18. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Bannov, A.G.; Popov, M.V.; Yun, J.-W.; Nguyen, A.D.; Kim, Y.S. High-temperature-treated multiwall carbon nanotubes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2018, 43, 6526–6531. [Google Scholar] [CrossRef]
- Lapekin, N.I.; Golovakhin, V.V.; Kim, E.Y.; Bannov, A.G. NO2 Sensing Behavior of Compacted Chemically Treated Multi-Walled Carbon Nanotubes. Micromachines 2022, 13, 1495. [Google Scholar] [CrossRef]
- Mortazavi, S.Z.; Novinrooz, A.J.; Reyhani, A.; Mirershadi, S. Effects of acid treatment duration and sulfuric acid molarity on purification of multi-walled carbon nanotubes. Cent. Eur. J. Phys. 2010, 8, 940–946. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Combined role of water and surface chemistry in reactive adsorption of ammonia on graphite oxides. Langmuir 2010, 26, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liao, J.L.; Wang, S.Y.; Chiang, W.H. Intercalation-assisted longitudinal unzipping of carbon nanotubes for green and scalable synthesis of graphene nanoribbons. Sci. Rep. 2016, 6, 22755. [Google Scholar] [CrossRef] [PubMed]
- Mordkovich, V.Z.; Baxendale, M.; Chang, R.P.H.; Yoshimura, S. Intercalation into carbon nanotubes without breaking the tubular structure. Synth. Met. 1997, 86, 2049–2050. [Google Scholar] [CrossRef]
- Madrona, C.; Vila, M.; Oropeza, F.E.; de la Peña O’Shea, V.A.; Vilatela, J.J. Macroscopic yarns of FeCl3-intercalated collapsed carbon nanotubes with high doping and stability. Carbon 2021, 173, 311–321. [Google Scholar] [CrossRef]
- Zhou, O.; Gao, B.; Bower, C.; Fleming, L.; Shimoda, H. Structure and Electrochemical Properties of Carbon Nanotube Intercalation Compounds. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2000, 340, 541–546. [Google Scholar] [CrossRef]
- Shiozawa, H.; Pichler, T.; Pfeiffer, R.; Kuzmany, H. Single-Walled Carbon Nanotubes: Functionalization by Intercalation. Encycl. Inorg. Bioinorg. Chem. 2015, 1–24. [Google Scholar] [CrossRef]
- Pullini, D.; Siong, V.; Tamvakos, D.; Lobato Ortega, B.; Sgroi, M.F.; Veca, A.; Glanz, C.; Kolaric, I.; Pruna, A. Enhancing the capacitance and active surface utilization of supercapacitor electrode by graphene nanoplatelets. Compos. Sci. Technol. 2015, 112, 16–21. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Rahmam, S.; Mohamed, N.M.; Sufian, S. Effect of acid treatment on the multiwalled carbon nanotubes. Mater. Res. Innov. 2014, 18, S6–S196. [Google Scholar] [CrossRef]
- Pumera, M.; Šmíd, B.; Veltruská, K. Influence of nitric acid treatment of carbon nanotubes on their physico-chemical properties. J. Nanosci. Nanotechnol. 2009, 9, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Torres, J.C.; Wirtz, L.; Pichler, T. Raman spectroscopy of graphite intercalation compounds: Charge transfer, strain, and electron–phonon coupling in graphene layers. Phys. Status Solidi 2014, 251, 2337–2355. [Google Scholar] [CrossRef]
- Yousefi, M.; Arami, S.M.; Takallo, H.; Hosseini, M.; Radfard, M.; Soleimani, H.; Mohammadi, A.A. Modification of pumice with HCl and NaOH enhancing its fluoride adsorption capacity: Kinetic and isotherm studies. Hum. Ecol. Risk Assess. 2019, 25, 1508–1520. [Google Scholar] [CrossRef]
- Ionescu, R.; Espinosa, E.H.; Sotter, E.; Llobet, E.; Vilanova, X.; Correig, X.; Felten, A.; Bittencourt, C.; Van Lier, G.; Charlier, J.-C.C.; et al. Oxygen functionalisation of MWNT and their use as gas sensitive thick-film layers. Sens. Actuators B Chem. 2006, 113, 36–46. [Google Scholar] [CrossRef]
- Manakhov, A.; Michlíček, M.; Felten, A.; Pireaux, J.-J.; Nečas, D.; Zajíčková, L. XPS depth profiling of derivatized amine and anhydride plasma polymers: Evidence of limitations of the derivatization approach. Appl. Surf. Sci. 2017, 394, 578–585. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Kovtun, A.; Jones, D.; Dell’Elce, S.; Treossi, E.; Liscio, A.; Palermo, V. Accurate chemical analysis of oxygenated graphene-based materials using X-ray photoelectron spectroscopy. Carbon 2019, 143, 268–275. [Google Scholar] [CrossRef]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Salvi, A.M.; Castle, J.E.; Watts, J.F.; Desimoni, E. Peak fitting of the chromium 2p XPS spectrum. Appl. Surf. Sci. 1995, 90, 333–341. [Google Scholar] [CrossRef]
- Rahman, A.; Mohamed, M.H.; Ahmed, M.; Aitani, A.M. Characterization of chromia/alumina catalysts by X-ray photoelectron spectroscopy, proton induced X-ray emission and thermogravimetric analysis. Appl. Catal. A Gen. 1995, 121, 203–216. [Google Scholar] [CrossRef]
- Mittal, J.; Konno, H.; Inagaki, M. Synthesis of graphite intercalation compounds with CrVI compounds using CrO3 and HCl at room temperature. Synth. Met. 1998, 96, 103–108. [Google Scholar] [CrossRef]
- Kobets, A.A.; Iurchenkova, A.A.; Asanov, I.P.; Okotrub, A.V.; Fedorovskaya, E.O. Redox Processes in Reduced Graphite Oxide Decorated by Carboxyl Functional Groups. Phys. Status Solidi Basic Res. 2019, 256, 1800700. [Google Scholar] [CrossRef]
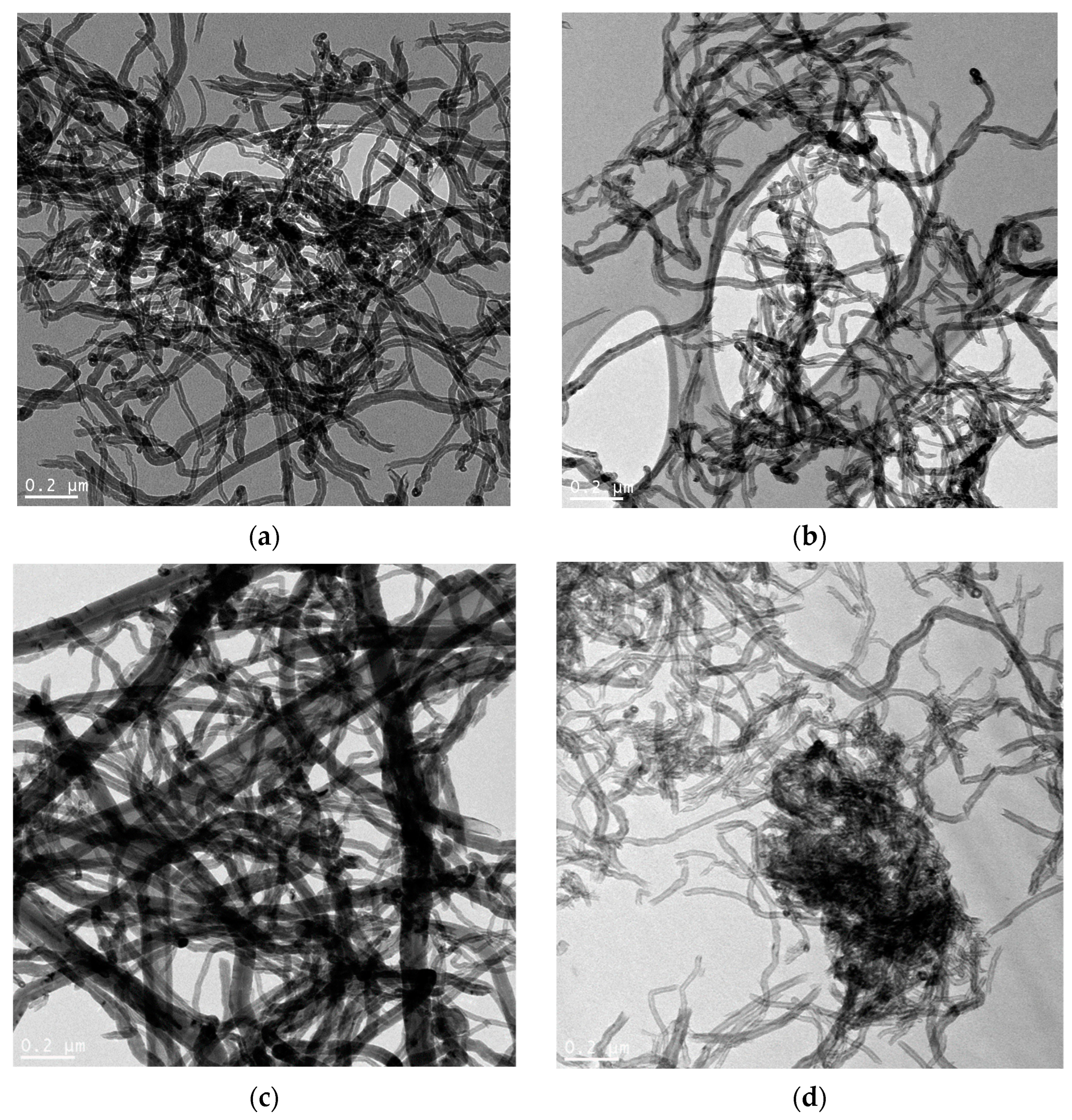
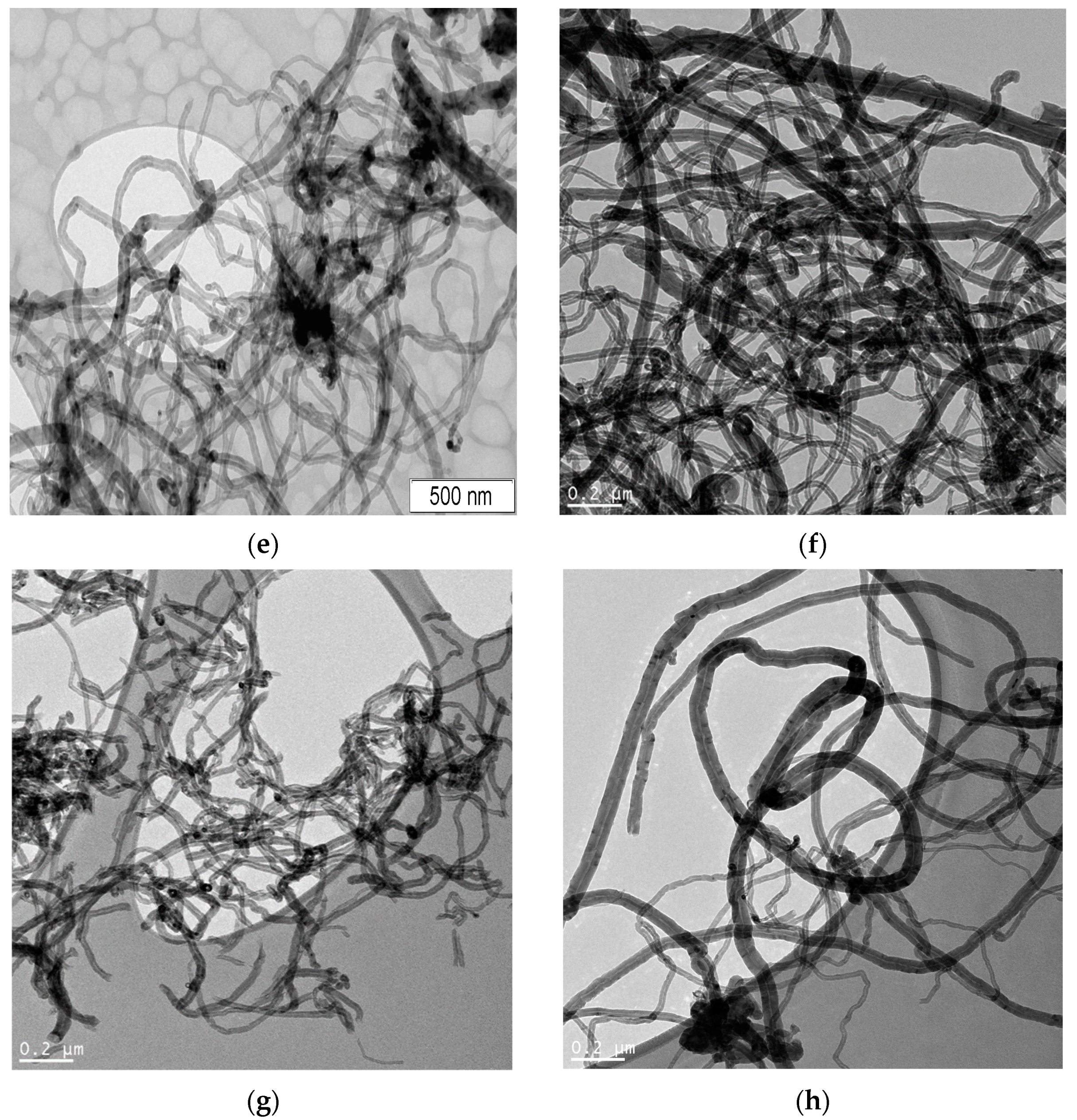
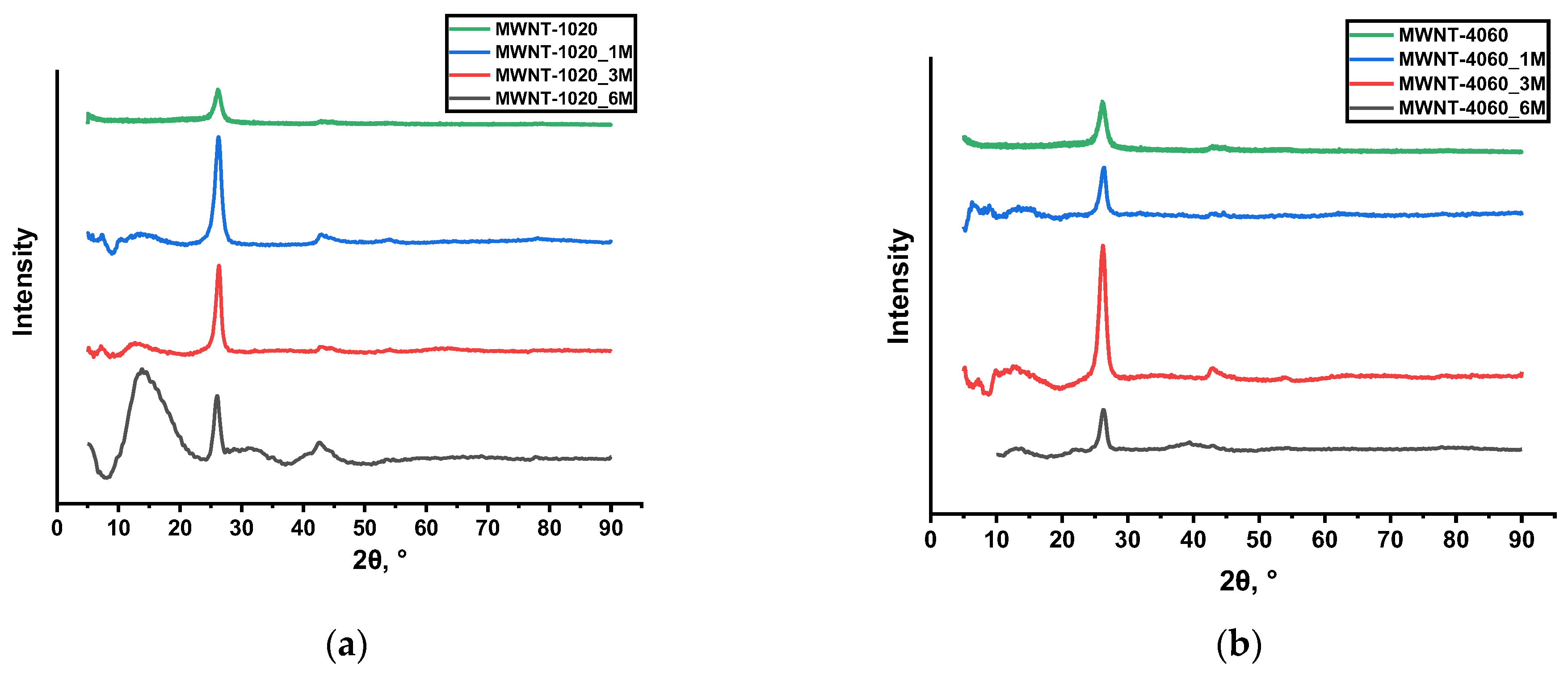
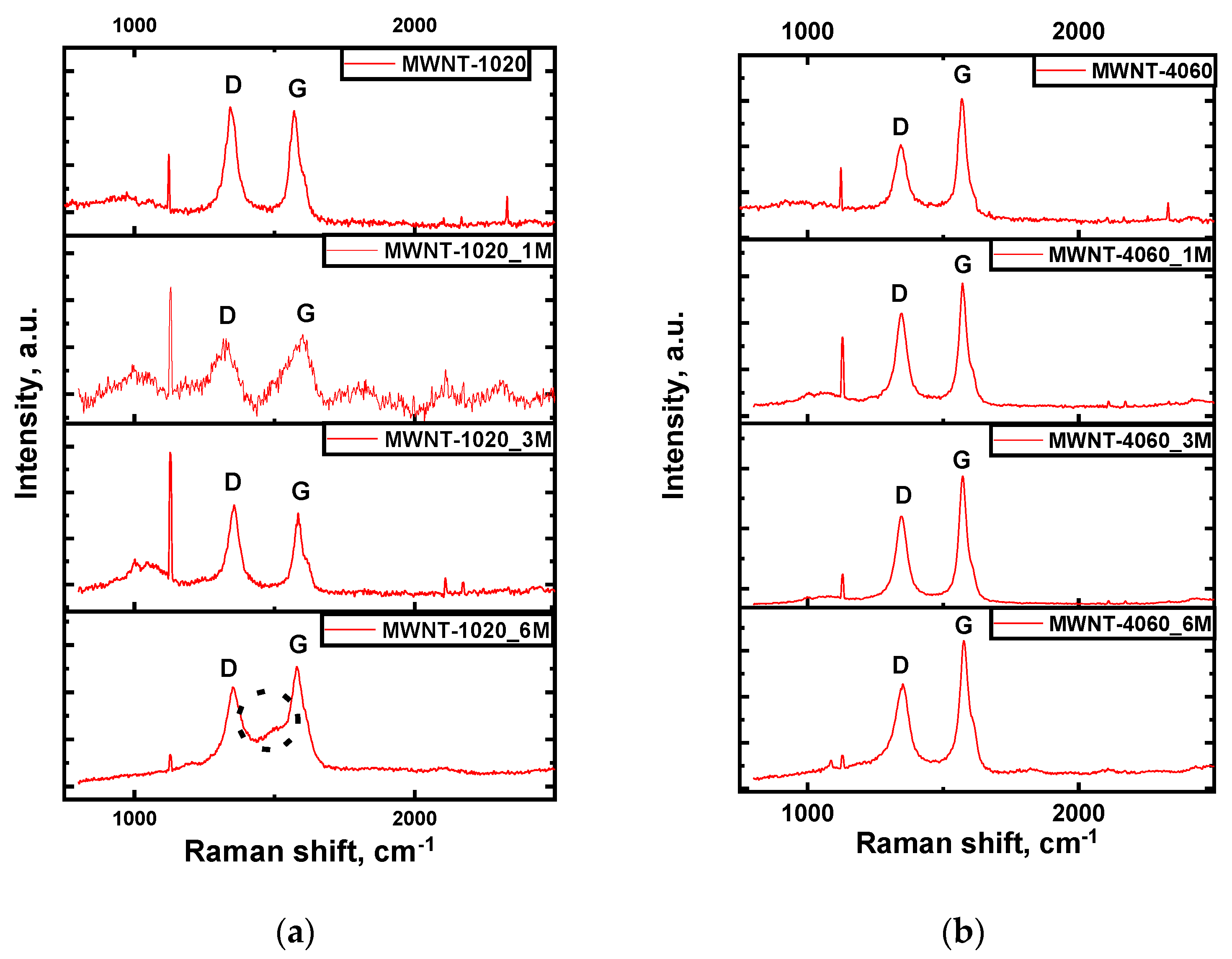
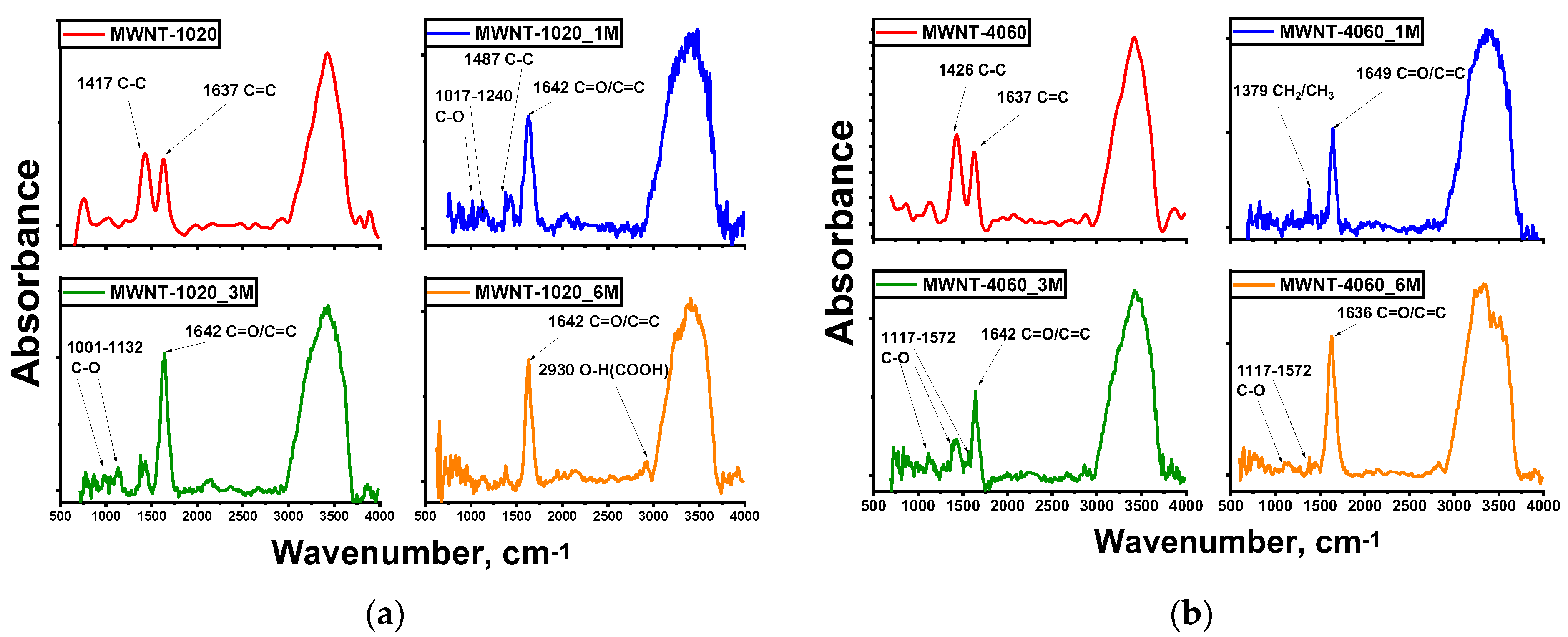
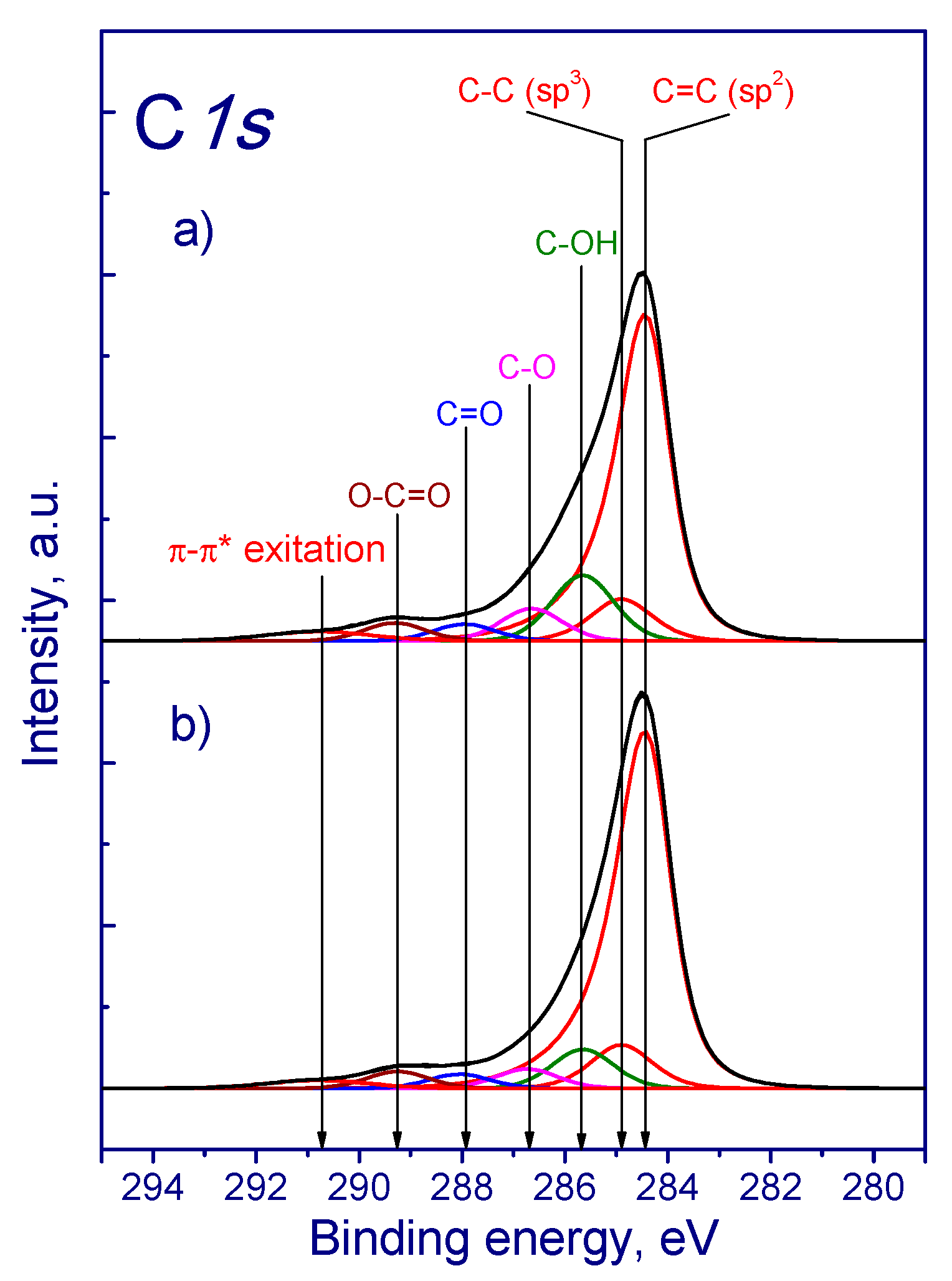
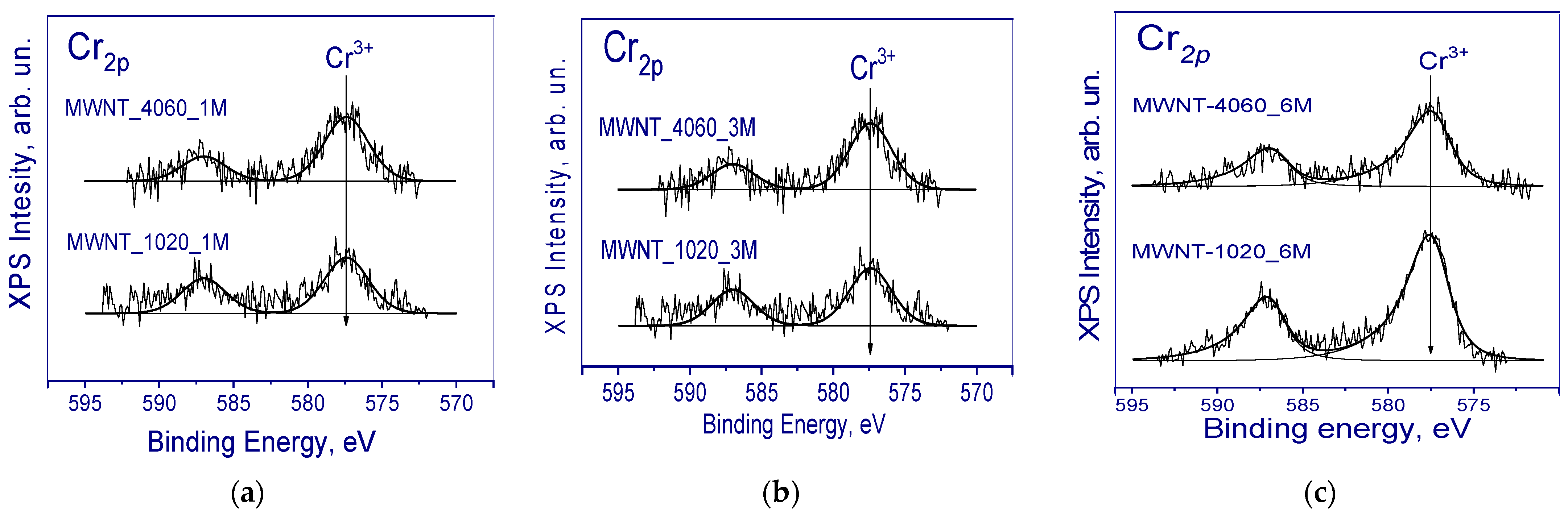
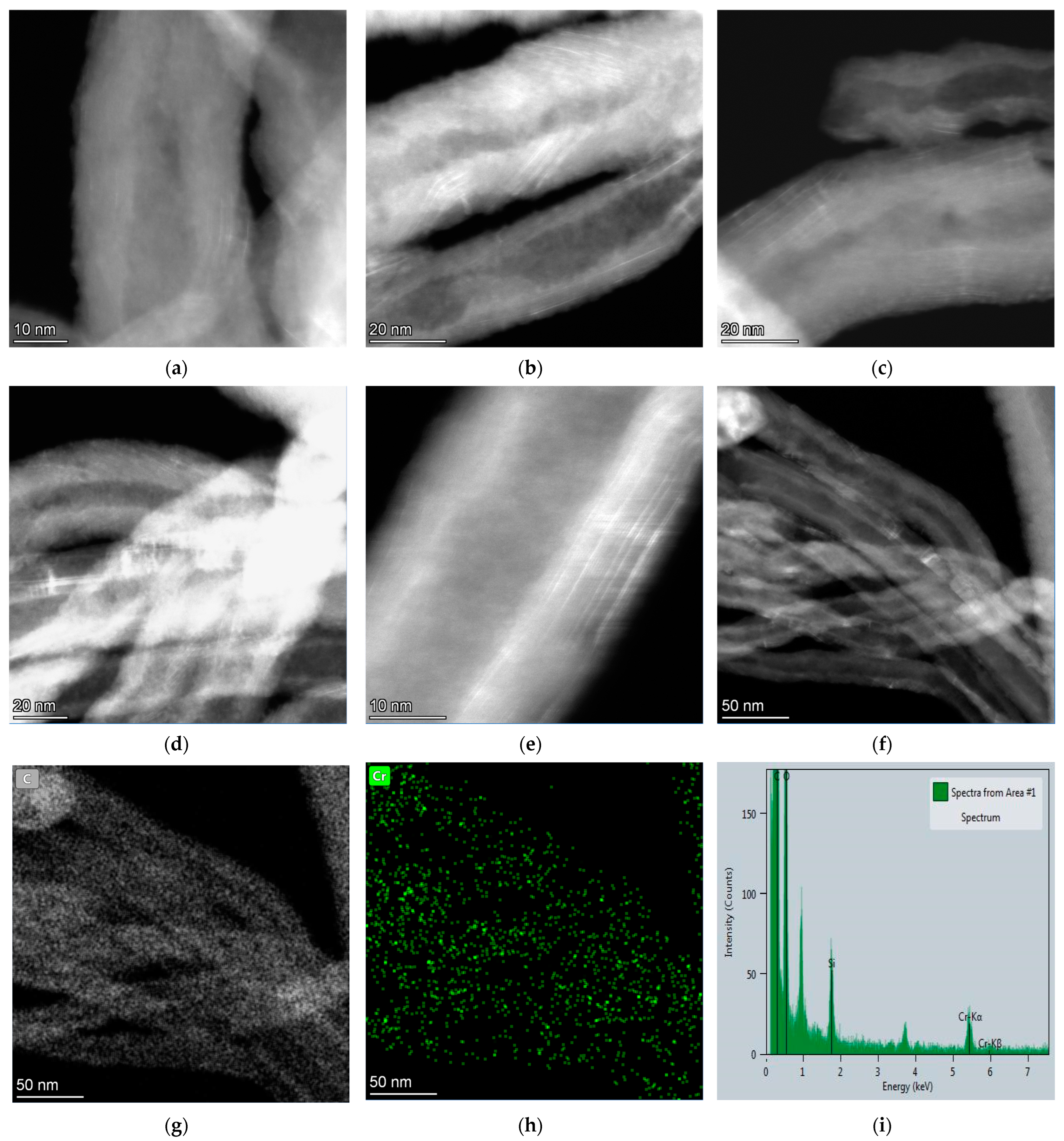

| Sample | Concentration of CrO3, mol‧L−1 |
|---|---|
| MWNT-1020 | - |
| MWNT-1020_1M | 1 |
| MWNT-1020_3M | 3 |
| MWNT-1020_6M | 6 |
| MWNT-4060 | - |
| MWNT-4060_1M | 1 |
| MWNT-4060_3M | 3 |
| MWNT-4060_6M | 6 |
| Sample | Average Diameter of CNTs, nm |
|---|---|
| MWNT-1020 | 26 ± 0.82 |
| MWNT-1020_1M | 25.6 ± 0.75 |
| MWNT-1020_3M | 23.9 ± 1.42 |
| MWNT-1020_6M | 21.4 ± 0.55 |
| MWNT-4060 | 36 ± 1.51 |
| MWNT-4060_1M | 27.1 ± 1.27 |
| MWNT-4060_3M | 23.2 ± 1.82 |
| MWNT-4060_6M | 22.6 ± 1.20 |
| Sample | Raman Spectroscopy | XRD | Surface Area (BET), m2/g | |||
|---|---|---|---|---|---|---|
| FWHM, cm−1 (D) | FWHM, cm−1 (G) | I(D)/I(G) | d002, nm | Y, % | ||
| MWNT-1020 | 52.4 | 50.6 | 1.03 | 0.3400 | 46.5 | 128 |
| MWNT-1020_1M | 89 | 78 | 1.28 | 0.3380 | 43.5 | 123 |
| MWNT-1020_3M | 53.6 | 53.9 | 1.16 | 0.3382 | 67 | 119 |
| MWNT-1020_6M | 61.8 | 73 | 0.78 | 0.3414 | 30 | 111 |
| MWNT-4060 | 50.7 | 42.5 | 0.54 | 0.3387 | 61.6 | 68 |
| MWNT-4060_1M | 52 | 44.4 | 0.70 | 0.3385 | 62.3 | 65 |
| MWNT-4060_3M | 54.6 | 44.4 | 0.78 | 0.3367 | 84 | 63 |
| MWNT-4060_6M | 59 | 51.7 | 0.65 | 0.3388 | 61 | 61 |
| Sample | Concentration of Components of C1s Photoelectron Peak (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| C=C (sp2) 284.5 eV | C-C (sp3) 284.9 eV | C-OH 285.7 eV | C-O 286.7 eV | C=O 288.0 eV | O-C=O 289.3 eV | π-π* 290.8 eV | |
| MWNT-1020 | 65.4 | 8.0 | 13.2 | 6.0 | 2.2 | 2.8 | 2.4 |
| MWNT-1020_1M | 62.1 | 8.1 | 17.4 | 4.4 | 2.5 | 4.2 | 1.4 |
| MWNT-1020_3M | 60.5 | 16.0 | 9.0 | 4.7 | 3.1 | 3.3 | 3.5 |
| MWNT-1020_6M | 72.4 | 8.3 | 7.3 | 3.6 | 2.6 | 3.2 | 2.6 |
| MWNT-4060 | 57.0 | 4.2 | 23.0 | 7.9 | 2.8 | 3.9 | 1.2 |
| MWNT-4060_1M | 59.9 | 11.8 | 14.8 | 5.2 | 2.5 | 3.7 | 2.1 |
| MWNT-4060_3M | 63.7 | 6.1 | 17.1 | 4.4 | 2.8 | 3.8 | 2.0 |
| MWNT-4060_6M | 63.4 | 9.9 | 12.3 | 5.0 | 3.0 | 3.4 | 3.1 |
| Sample | EDX | XPS | ||
|---|---|---|---|---|
| C:O | Impurities, at.% | C:O | Impurities, at.% | |
| MWNT-1020 | - | 0 | 13.1 | 0 |
| MWNT-1020_1M | 22 | Cr (0.1) | 7.3 | Cr (0.16) |
| MWNT-1020_3M | 14 | Cr (0.31) | 7.7 | Cr (0.29) |
| MWNT-1020_6M | 10 | Cr (1.68) | 8.54 | Cr (0.19) |
| MWNT-4060 | - | 0 | 9.4 | 0 |
| MWNT-4060_1M | 73 | Ni (0.13) Cr (0.12) | 8.5 | Cr (0.16) |
| MWNT-4060_3M | 33 | Ni (0.11) Cr (0.15) | 8.53 | Cr (0.35) |
| MWNT-4060_6M | 18 | Cr (0.48) | 6.4 | Cr (0.11) |
| Sample | Specific Capacitance Csp, F g−1 | ||
|---|---|---|---|
| 10 mV s−1 | 5 mV s−1 | 2 mV s−1 | |
| MWNT-1020 | 0.3 | 0.5 | 0.7 |
| MWNT-1020_1M | 38 | 42 | 60 |
| MWNT-1020_3M | 35 | 43 | 58 |
| MWNT-1020_6M | 76 | 89 | 111 |
| MWNT-4060 | 0.16 | 0.19 | 0.3 |
| MWNT-4060_1M | 66 | 86 | 114 |
| MWNT-4060_3M | 71 | 97 | 141 |
| MWNT-4060_6M | 28 | 33 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golovakhin, V.; Kim, E.Y.; Novgorodtseva, O.N.; Maksimovskiy, E.A.; Ukhina, A.V.; Ishchenko, A.V.; Bannov, A.G. Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation. Membranes 2023, 13, 729. https://doi.org/10.3390/membranes13080729
Golovakhin V, Kim EY, Novgorodtseva ON, Maksimovskiy EA, Ukhina AV, Ishchenko AV, Bannov AG. Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation. Membranes. 2023; 13(8):729. https://doi.org/10.3390/membranes13080729
Chicago/Turabian StyleGolovakhin, Valeriy, Ekaterina Yu. Kim, Oksana N. Novgorodtseva, Evgene A. Maksimovskiy, Arina V. Ukhina, Arcady V. Ishchenko, and Alexander G. Bannov. 2023. "Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation" Membranes 13, no. 8: 729. https://doi.org/10.3390/membranes13080729
APA StyleGolovakhin, V., Kim, E. Y., Novgorodtseva, O. N., Maksimovskiy, E. A., Ukhina, A. V., Ishchenko, A. V., & Bannov, A. G. (2023). Treatment of Multi-Walled Carbon Nanotubes with Dichromic Acid: Oxidation and Appearance of Intercalation. Membranes, 13(8), 729. https://doi.org/10.3390/membranes13080729










