Composite Anion Exchange Membranes Based on Quaternary Ammonium-Functionalized Polystyrene and Cerium(IV) Phosphate with Improved Monovalent-Ion Selectivity and Antifouling Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials and Equipment
2.2. Procedures for the Synthesis and Study of Materials
2.2.1. Synthesis of Hybrid Membranes
2.2.2. Synthesis of Dopants and Hybrid Ion Exchangers
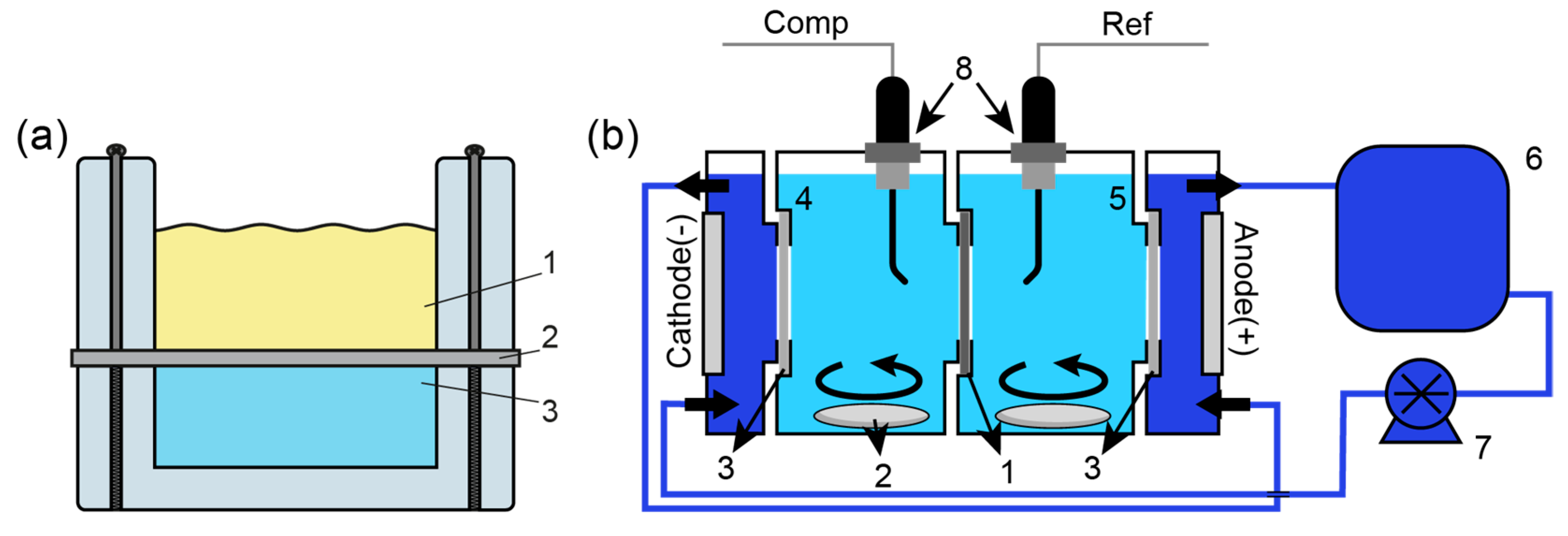
2.2.3. Instrumental Investigation Methods
2.2.4. Characterization of Dopants, Hybrid Ion Exchangers and Membranes
3. Results and Discussion
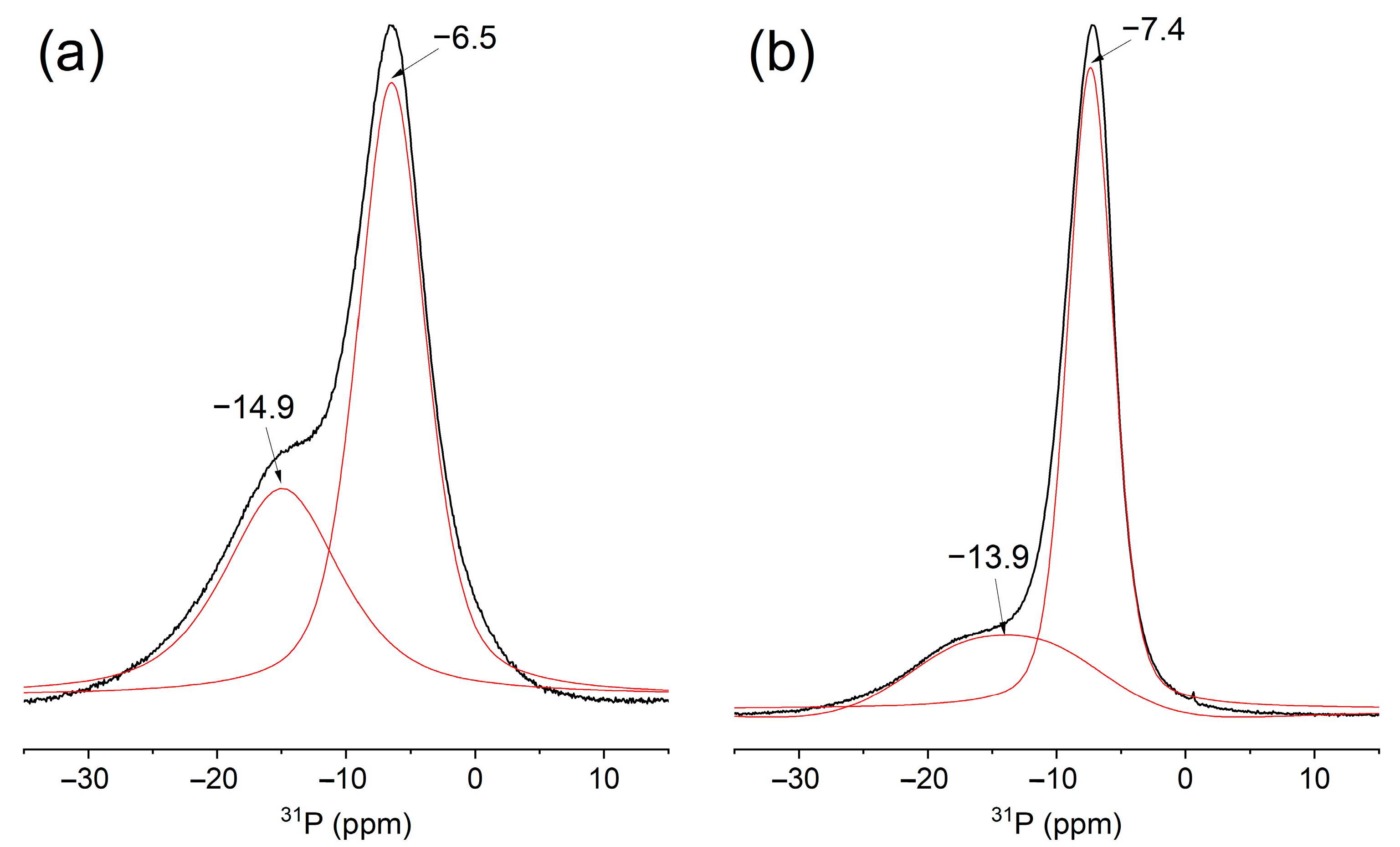
3.1. Structure and Properties of Cerium Phosphates
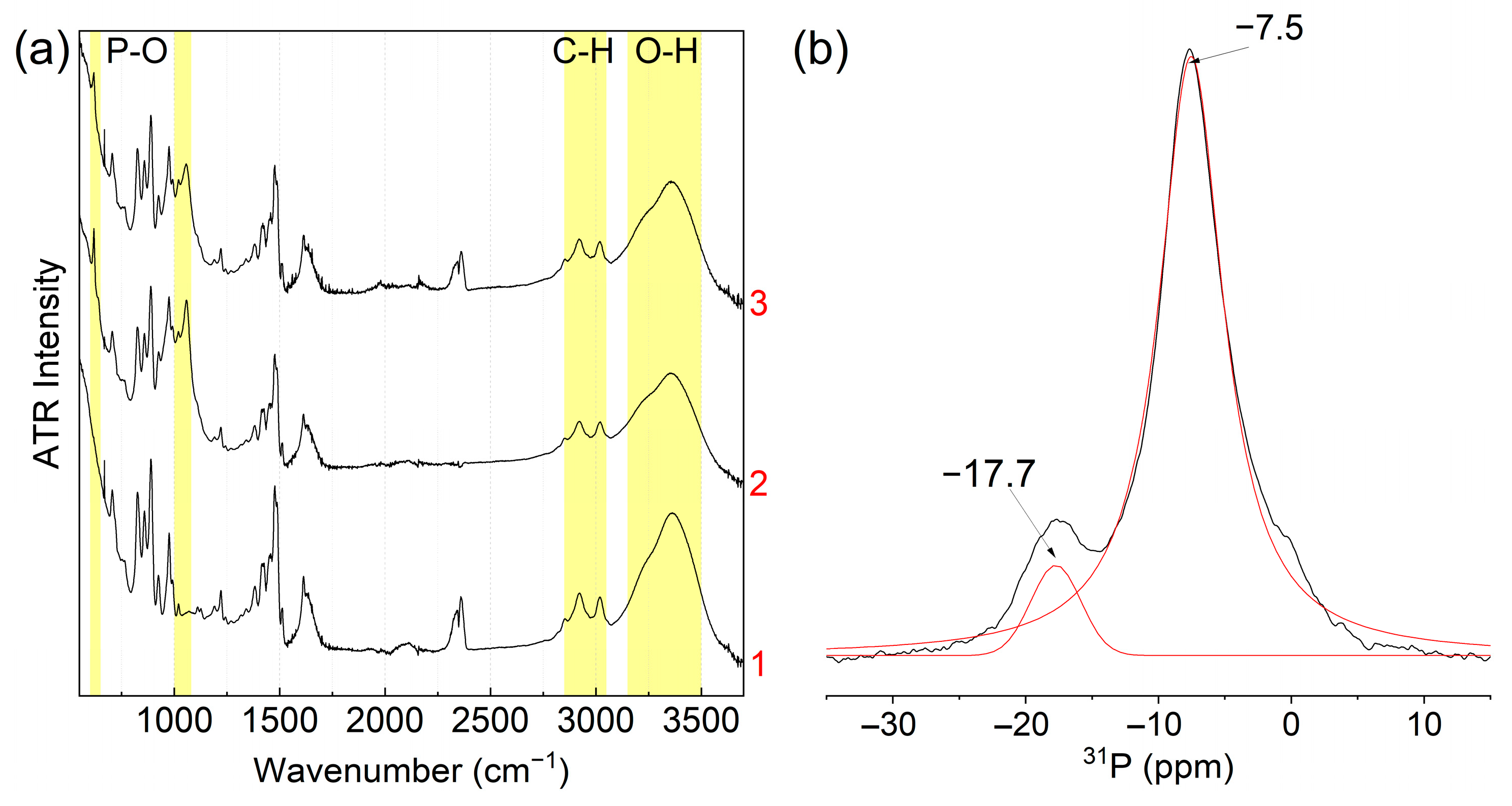
3.2. Structure and Properties of Ion Exchange Resins
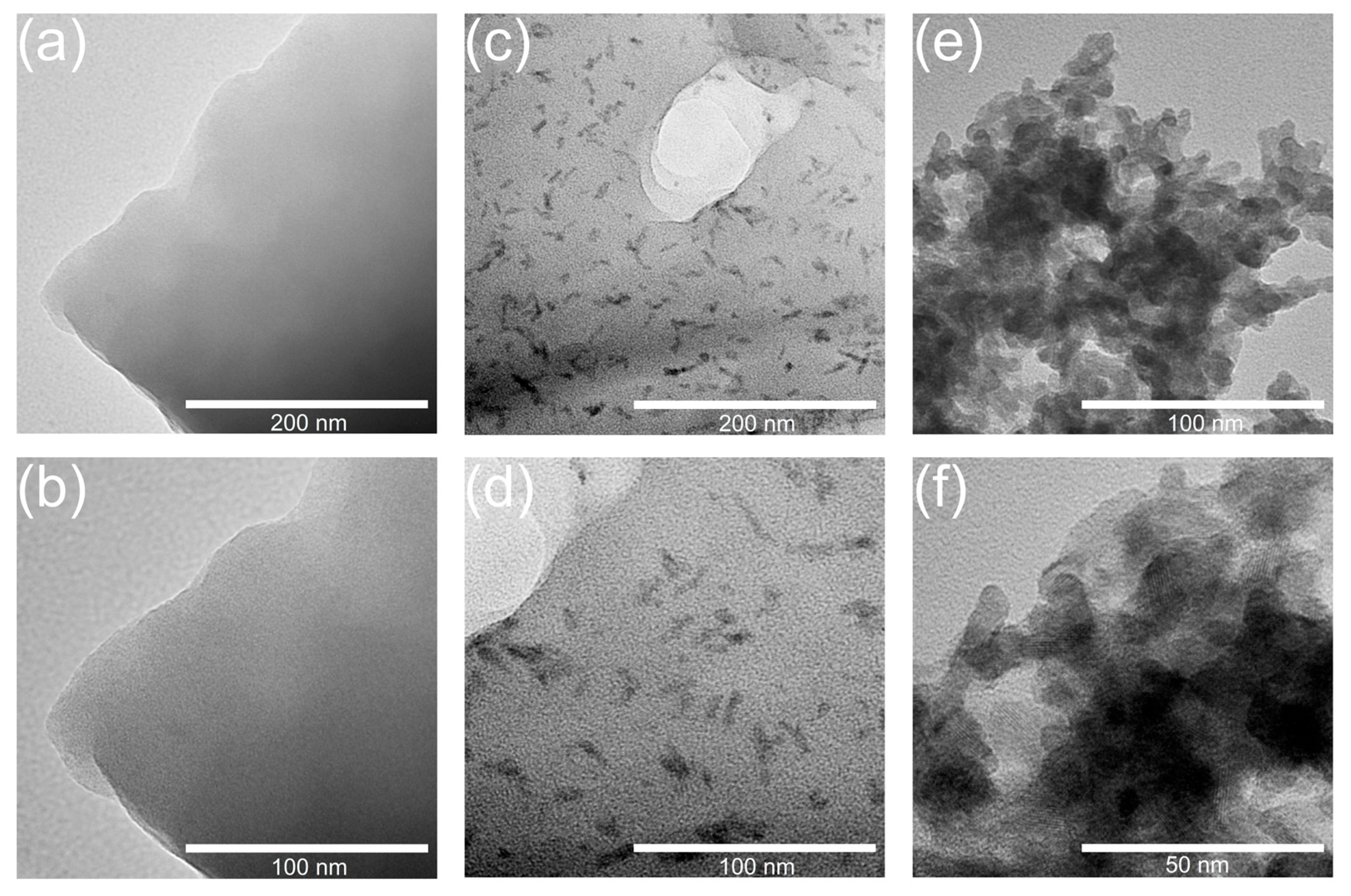
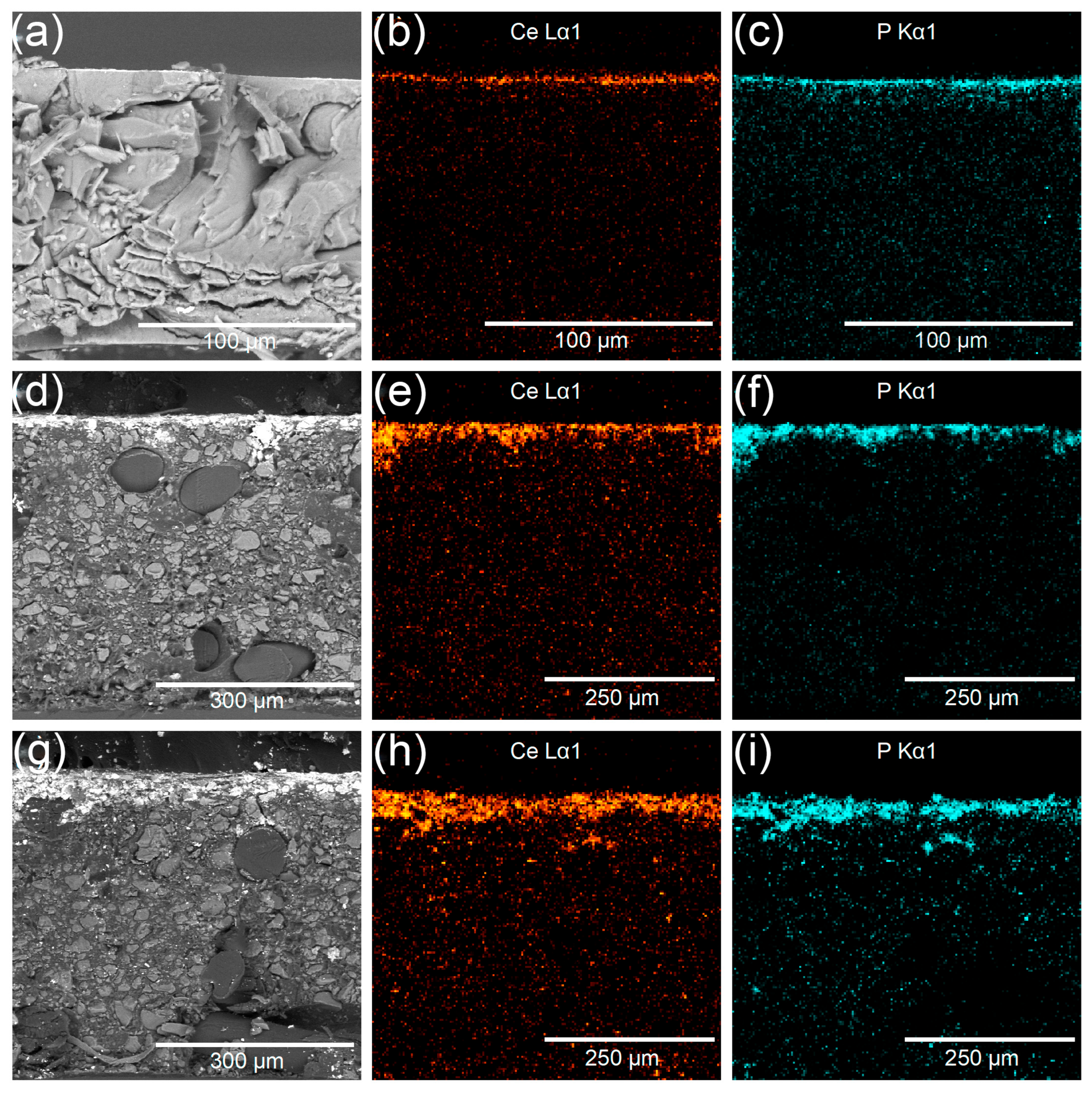
3.3. Structure of Hybrid Membranes
3.4. Membrane Properties
3.4.1. Membrane Physicochemical Properties
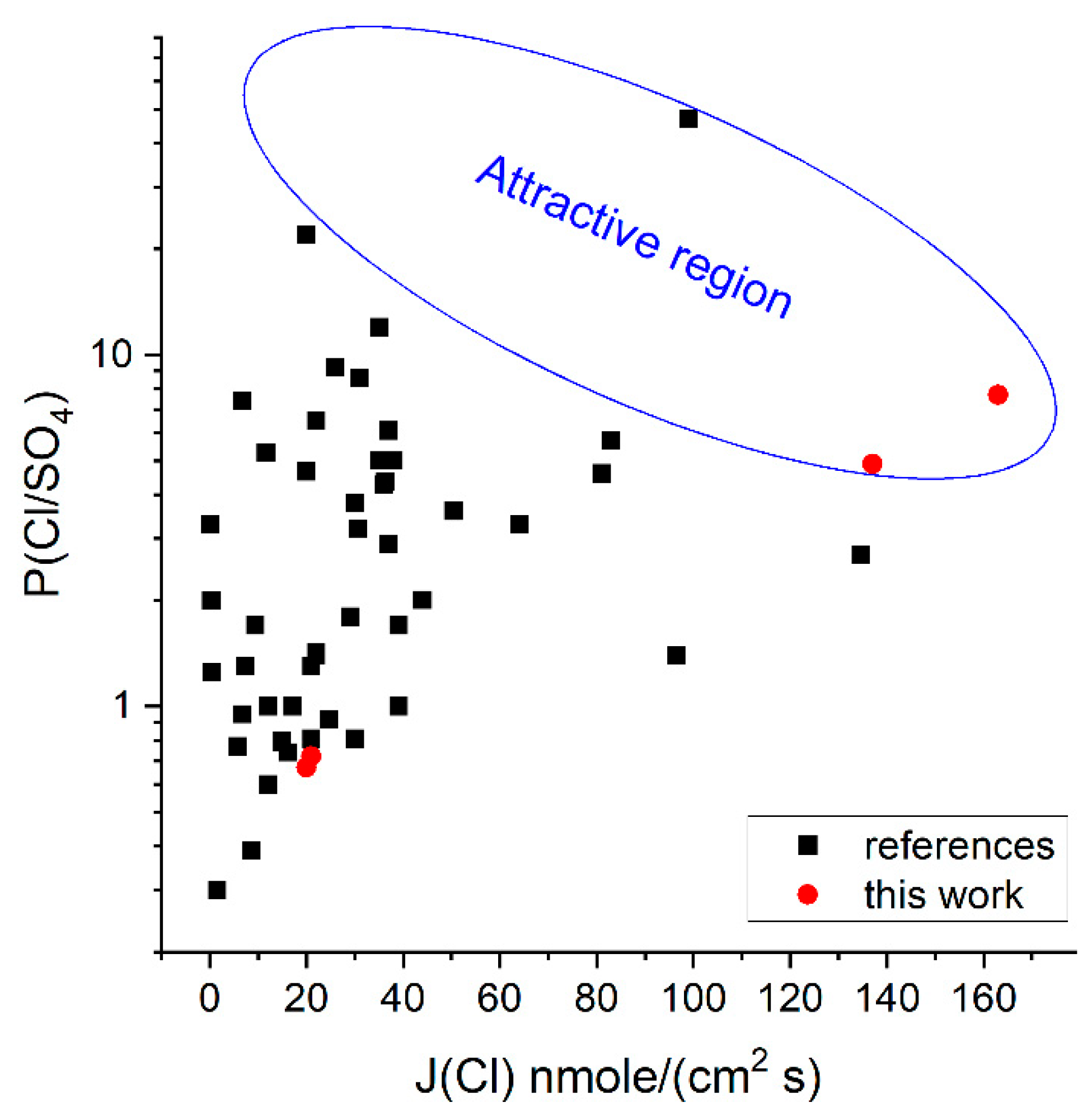
3.4.2. Selectivity during ED Desalination
3.4.3. Antifouling Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arana Juve, J.M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for Metal Removal and Recovery: A Review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Román-Hidalgo, C.; Martín-Valero, M.J.; López-Pérez, G.; Villar-Navarro, M. Green Method for the Selective Electromembrane Extraction of Parabens and Fluoroquinolones in the Presence of NSAIDs by Using Biopolymeric Chitosan Films. Membranes 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Kowalik-Klimczak, A.; Gajewska-Midziałek, A.; Buczko, Z.; Łożyńska, M.; Życki, M.; Barszcz, W.; Ciciszwili, T.; Dąbrowski, A.; Kasierot, S.; Charasińska, J.; et al. Circular Economy Approach in Treatment of Galvanic Wastewater Employing Membrane Processes. Membranes 2023, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. The Application of Polyethersulfone Ultrafiltration Membranes for Separation of Car Wash Wastewaters: Experiments and Modelling. Membranes 2023, 13, 321. [Google Scholar] [CrossRef]
- Fan, H.; Yip, N.Y. Donnan Dialysis Desalination with a Thermally Recoverable Solute. ACS EST Eng. 2022, 2, 2076–2085. [Google Scholar] [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Karagunduz, A. Recent Developments of Electromembrane Desalination Processes. Environ. Technol. Rev. 2018, 7, 199–219. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-Exchange Membranes in the Chemical Process Industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing Miscellaneous Heavy Metals by All-in-One Ion Exchange-Nanofiltration Membrane. Water Res. 2022, 222, 118888. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.; Jiao, L.; Waheed, M.; Sun, Z.; Zhang, L. Separation Mechanism, Selectivity Enhancement Strategies and Advanced Materials for Mono-/Multivalent Ion-Selective Nanofiltration Membrane. Adv. Membr. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Frioui, S.; Oumeddour, R.; Lacour, S. Highly Selective Extraction of Metal Ions from Dilute Solutions by Hybrid Electrodialysis Technology. Sep. Purif. Technol. 2017, 174, 264–274. [Google Scholar] [CrossRef]
- Drioli, E.; Cassano, A. Membranes and Integrated Membrane Operations as Clean Technologies in the Leather Industry. Clean Technol. 2023, 5, 274–296. [Google Scholar] [CrossRef]
- Kumar, A.; Naidu, G.; Fukuda, H.; Du, F.; Vigneswaran, S.; Drioli, E.; Lienhard, J.H. Metals Recovery from Seawater Desalination Brines: Technologies, Opportunities, and Challenges. ACS Sustain. Chem. Eng. 2021, 9, 7704–7712. [Google Scholar] [CrossRef]
- Havelka, J.; Fárová, H.; Jiríček, T.; Kotala, T.; Kroupa, J. Electrodialysis-Based Zero Liquid Discharge in Industrial Wastewater Treatment. Water Sci. Technol. 2019, 79, 1580–1586. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent Cation Perm-Selective Membranes (MCPMs): New Developments and Perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Tang, C.; Bruening, M.L. Ion Separations with Membranes. J. Polym. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent Advances on Separator Membranes for Lithium-Ion Battery Applications: From Porous Membranes to Solid Electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Sprague, L.A.; Hirsch, R.M.; Aulenbach, B.T. Nitrate in the Mississippi River and Its Tributaries, 1980 to 2008: Are We Making Progress? Environ. Sci. Technol. 2011, 45, 7209–7216. [Google Scholar] [CrossRef]
- Castaldo, G.; Visser, A.; Fogg, G.E.; Harter, T. Effect of Groundwater Age and Recharge Source on Nitrate Concentrations in Domestic Wells in the San Joaquin Valley. Environ. Sci. Technol. 2021, 55, 2265–2275. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Nichka, V.S.; Geoffroy, T.R.; Nikonenko, V.; Bazinet, L. Impacts of Flow Rate and Pulsed Electric Field Current Mode on Protein Fouling Formation during Bipolar Membrane Electroacidification of Skim Milk. Membranes 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, S.; Kakihana, Y.; Abo, T.; Yuan, Q.; Higa, M. Power Generation Performance of a Pilot-Scale Reverse Electrodialysis Using Monovalent Selective Ion-Exchange Membranes. Membranes 2021, 11, 27. [Google Scholar] [CrossRef]
- Güler, E.; Van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-Ion-Selective Membranes for Reverse Electrodialysis. J. Membr. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Tsygurina, K.; Sarapulova, V.; Kirichenko, E.; Kirichenko, K. Monovalent Selective Membrane for Electrodialysis Desalination of Water for Agriculture. IOP Conf. Ser. Earth Environ. Sci. 2022, 996, 012019. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of Ion Exchange Membranes: A Review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of Transport Processes in Ion-Exchange Membranes: Relationship with the Structure and Methods for Its Improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef] [PubMed]
- DuChanois, R.M.; Porter, C.J.; Violet, C.; Verduzco, R.; Elimelech, M. Membrane Materials for Selective Ion Separations at the Water–Energy Nexus. Adv. Mater. 2021, 33, 2101312. [Google Scholar] [CrossRef]
- Gorobchenko, A.D.; Gil, V.V.; Nikonenko, V.V.; Sharafan, M.V. Mathematical Modeling of the Selective Transport of Singly Charged Ions Through Multilayer Composite Ion-Exchange Membrane during Electrodialysis. Membr. Membr. Technol. 2022, 4, 423–432. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical Modeling of the Effect of Pulsed Electric Field on the Specific Permselectivity of Ion-Exchange Membranes. Membranes 2021, 11, 115. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566. [Google Scholar] [CrossRef]
- Butylskii, D.; Dammak, L.; Larchet, C.; Pismenskaya, N.; Nikonenko, V. Selective Recovery and Re-Utilization of Lithium: Prospects for the Use of Membrane Methods. Russ. Chem. Rev. 2023, 92, 5074. [Google Scholar] [CrossRef]
- Ding, D.; Yaroshchuk, A.; Bruening, M.L. Electrodialysis through Nafion Membranes Coated with Polyelectrolyte Multilayers Yields 99% Pure Monovalent Ions at High Recoveries. J. Membr. Sci. 2022, 647, 120294. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. Effect of Current Density, Concentration of Ternary Electrolyte and Type of Cations on the Monovalent Ion Selectivity of Surface-Sulfonated Graft Anion-Exchange Membranes: Modelling and Experiment. J. Membr. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]
- Achoh, A.R.; Zabolotsky, V.I.; Lebedev, K.A.; Sharafan, M.V.; Yaroslavtsev, A.B. Electrochemical Properties and Selectivity of Bilayer Ion-Exchange Membranes in Ternary Solutions of Strong Electrolytes. Membr. Membr. Technol. 2021, 3, 52–71. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yuan, S.; Zhu, J.; Houtmeyers, S.; Li, J.; Dewil, R.; Gao, C.; Van der Bruggen, B. A Chemically Assembled Anion Exchange Membrane Surface for Monovalent Anion Selectivity and Fouling Reduction. J. Mater. Chem. A 2019, 7, 6348–6356. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Tang, K.; Jin, Y.; Pan, J.; Der Bruggen, B.V.; Shen, J.; Gao, C. Mimicking the Cell Membrane: Bio-Inspired Simultaneous Functions with Monovalent Anion Selectivity and Antifouling Properties of Anion Exchange Membrane. Sci. Rep. 2016, 6, 37285. [Google Scholar] [CrossRef] [PubMed]
- Tekinalp, Ö.; Zimmermann, P.; Burheim, O.S.; Deng, L. Designing Monovalent Selective Anion Exchange Membranes for the Simultaneous Separation of Chloride and Fluoride from Sulfate in an Equimolar Ternary Mixture. J. Membr. Sci. 2023, 666, 121148. [Google Scholar] [CrossRef]
- Ahmad, M.; Tang, C.; Yang, L.; Yaroshchuk, A.; Bruening, M.L.; States, U. Layer-by-Layer Modification of Aliphatic Polyamide Anion-Exchange Membranes to Increase Cl−/SO42− Selectivity. J. Membr. Sci. 2019, 578, 209–219. [Google Scholar] [CrossRef]
- Mondal, R.; Sarkar, S.; Patnaik, P.; Chatterjee, U. Preparation of a Monovalent-Selective Anion-Exchange Membrane: Effect of Alkyl Chain Length and Crystallinity. ACS Appl. Polym. Mater. 2023, 5, 2513–2524. [Google Scholar] [CrossRef]
- Sarkar, S.; Patnaik, P.; Mondal, R.; Chatterjee, U. Cross-Linked, Monovalent Selective Anion Exchange Membrane: Effect of Prealkylation and Co-Ions on Selectivity. ACS Appl. Polym. Mater. 2023, 5, 1977–1988. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Liao, J.; Li, J.; Xu, J.; Wang, T.; Tang, Y.; Xu, Y.; Ruan, H.; Shen, J. Subnanometer Ion Channel Anion Exchange Membranes Having a Rigid Benzimidazole Structure for Selective Anion Separation. ACS Nano 2022, 16, 4629–4641. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Xiao, P.; Ruan, H.; Liao, J.; Gao, C.; Van der Bruggen, B.; Shen, J. Mussel-Inspired Surface Functionalization of AEM for Simultaneously Improved Monovalent Anion Selectivity and Antibacterial Property. Membranes 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chen, Q.; Pan, N.; Yu, X.; Gao, X.; Shen, J.; Gao, C. Amphoteric Blend Ion-Exchange Membranes for Separating Monovalent and Bivalent Anions in Electrodialysis. Sep. Purif. Technol. 2020, 242, 116793. [Google Scholar] [CrossRef]
- Goel, P.; Bhuvanesh, E.; Mandal, P.; Shahi, V.K.; Bandyopadhyay, A.; Chattopadhyay, S. Di-Quaternized Graphene Oxide Based Multi-Cationic Cross-Linked Monovalent Selective Anion Exchange Membrane for Electrodialysis. Sep. Purif. Technol. 2021, 276, 119361. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Nemati, M.; Jeddi, F.; Salehi, E.; Khodabakhshi, A.R.; Madaeni, S.S. Fabrication of Mixed Matrix Heterogeneous Cation Exchange Membrane Modified by Titanium Dioxide Nanoparticles: Mono/Bivalent Ionic Transport Property in Desalination. Desalination 2015, 359, 167–175. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Gholami, A.; Koranian, P.; Nemati, M.; Madaeni, S.S.; Moghadassi, A.R. Electrochemical Characterization of Mixed Matrix Heterogeneous Cation Exchange Membrane Modified by Aluminum Oxide Nanoparticles: Mono/Bivalent Ionic Transportation. J. Taiwan Inst. Chem. Eng. 2014, 45, 1241–1248. [Google Scholar] [CrossRef]
- Khodabakhshi, A.R.; Madaeni, S.S.; Hosseini, S.M. Preparation and Characterization of Monovalent Ion-Selective Poly(Vinyl Chloride)-Blend-Poly(Styrene-Co-Butadiene) Heterogeneous Anion-Exchange Membranes. Polym. Int. 2011, 60, 466–474. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Madaeni, S.S.; Heidari, A.R.; Amirimehr, A. Preparation and Characterization of Ion-Selective Polyvinyl Chloride Based Heterogeneous Cation Exchange Membrane Modified by Magnetic Iron–Nickel Oxide Nanoparticles. Desalination 2012, 284, 191–199. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yurova, P.A.; Novak, L.; Achoh, A.R.; Zabolotsky, V.I.; Yaroslavtsev, A.B. Improvement of Ion Conductivity and Selectivity of Heterogeneous Membranes by Sulfated Zirconia Modification. Colloid Polym. Sci. 2021, 299, 719–728. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Manin, A.D.; Wang, Y.; Xu, T.; Yaroslavtsev, A.B. The Way to Increase the Monovalent Ion Selectivity of FujiFilm® Anion-Exchange Membranes by Cerium Phosphate Modification for Electrodialysis Desalination. Desalination 2022, 531, 115719. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Karavanova, Y.A.; Melnikov, S.S.; Achoh, A.R.; Pourcelly, G.; Yaroslavtsev, A.B. An Approach to Increase the Permselectivity and Mono-Valent Ion Selectivity of Cation-Exchange Membranes by Introduction of Amorphous Zirconium Phosphate Nanoparticles. J. Membr. Sci. 2018, 563, 777–784. [Google Scholar] [CrossRef]
- Kozlova, T.O.; Baranchikov, A.E.; Ivanov, V.K. Cerium(IV) Orthophosphates (Review). Russ. J. Inorg. Chem. 2021, 66, 1761–1778. [Google Scholar] [CrossRef]
- Ahmad, M.; Ahmed, M. Characterization and Applications of Ion-Exchange Membranes and Selective Ion Transport through Them: A Review. J. Appl. Electrochem. 2023. [Google Scholar] [CrossRef]
- Kusumoto, K.; Mizutani, Y. New Anion-Exchange Membrane Resistant to Organic Fouling. Desalination 1975, 17, 121–130. [Google Scholar] [CrossRef]
- Kusumoto, K.; Mizumoto, Y.; Mizutani, Y. Modification of Anion Exchange Membranes by Oxidation of Selected Chemical Sites for the Purpose of Preventing Fouling during Dialysis. Desalination 1975, 17, 303–311. [Google Scholar] [CrossRef]
- Yurova, P.A.; Tabachkova, N.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Properties of Ceria Nanoparticles with Surface Modified by Acidic Groups. J. Nanopart. Res. 2020, 22, 318. [Google Scholar] [CrossRef]
- Clavier, N.; Mesbah, A.; Szenknect, S.; Dacheux, N. Monazite, Rhabdophane, Xenotime & Churchite: Vibrational Spectroscopy of Gadolinium Phosphate Polymorphs. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2018, 205, 85–94. [Google Scholar] [CrossRef]
- Kozlova, T.O.; Vasilyeva, D.N.; Kozlov, D.A.; Teplonogova, M.A.; Baranchikov, A.E.; Simonenko, N.P.; Ivanov, V.K. Synthesis and Thermal Behavior of KCe2(PO4)3, a New Full-Member in the AIMIV 2 (PO4)3 Family. Nanosyst. Phys. Chem. Math. 2023, 14, 112–119. [Google Scholar] [CrossRef]
- Shekunova, T.O.; Baranchikov, A.E.; Ivanova, O.S.; Skogareva, L.S.; Simonenko, N.P.; Karavanova, Y.A.; Lebedev, V.A.; Borilo, L.P.; Ivanov, V.K. Cerous Phosphate Gels: Synthesis, Thermal Decomposition and Hydrothermal Crystallization Paths. J. Non-Cryst. Solids 2016, 447, 183–189. [Google Scholar] [CrossRef]
- Stenina, I.A.; Il’in, A.B.; Kirik, S.D.; Zhilyaeva, N.A.; Yurkov, G.Y.; Yaroslavtsev, A.B. Catalytic Properties of Composite Materials Based on Mesoporous Silica and Zirconium Hydrogen Phosphate. Inorg. Mater. 2014, 50, 586–591. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic Mobility in Ion-Exchange Membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Safronova, E.Y.; Lysova, A.A.; Novikova, S.A.; Stenina, I.A.; Volkov, V.I. Ion Conductivity of Hybrid Ion Exchange Membranes Incorporating Nanoparticles. Desalination Water Treat. 2011, 35, 202–208. [Google Scholar] [CrossRef]
- Mulyati, S.; Takagi, R.; Fujii, A.; Ohmukai, Y.; Matsuyama, H. Simultaneous Improvement of the Monovalent Anion Selectivity and Antifouling Properties of an Anion Exchange Membrane in an Electrodialysis Process, Using Polyelectrolyte Multilayer Deposition. J. Membr. Sci. 2013, 431, 113–120. [Google Scholar] [CrossRef]
- Gierke, T.D.; Munn, G.E.; Wilson, F.C. The Morphology in Nafion® Perfluorinated Membrane Products, as Determined by Wide- and Small-Angle x-Ray Studies. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1687–1704. [Google Scholar] [CrossRef]
- Kononenko, N.; Nikonenko, V.; Grande, D.; Larchet, C.; Dammak, L.; Fomenko, M.; Volfkovich, Y. Porous Structure of Ion Exchange Membranes Investigated by Various Techniques. Adv. Colloid Interface Sci. 2017, 246, 196–216. [Google Scholar] [CrossRef] [PubMed]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of Ion-Exchange Membrane Materials: Properties vs Structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Helfferich, F. Ion Exchange; McGraw-Hill Book Company: New York, NY, USA, 1962. [Google Scholar]
- Inanuddin, L.M.; Luqman, M. (Eds.) Ion Exchange Technology I—Theory and Materials; Springer: Dordrecht, The Netherlands, 2012; ISBN 978-94-007-1699-5. [Google Scholar]
- Epsztein, R.; Shaulsky, E.; Qin, M.; Elimelech, M. Activation Behavior for Ion Permeation in Ion-Exchange Membranes: Role of Ion Dehydration in Selective Transport. J. Membr. Sci. 2019, 580, 316–326. [Google Scholar] [CrossRef]
- Pan, J.; Ding, J.; Tan, R.; Chen, G.; Zhao, Y.; Gao, C.; Der Bruggen, B.V.; Shen, J. Preparation of a Monovalent Selective Anion Exchange Membrane through Constructing a Covalently Crosslinked Interface by Electro-Deposition of Polyethyleneimine. J. Membr. Sci. 2017, 539, 263–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Brugge, B.; Shen, J.; Gao, C. Electric-Pulse Layer-by-Layer Assembled of Anion Exchange Membrane with Enhanced Monovalent Selectivity. J. Membr. Sci. 2018, 548, 81–90. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; Van der Bruggen, B.; Shen, J. A Facile Avenue to Modify Polyelectrolyte Multilayers on Anion Exchange Membranes to Enhance Monovalent Selectivity and Durability Simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]




| Designation * | c(CAN), M | Precipitant |
|---|---|---|
| Membranes | ||
| N-0 (pristine) | - | - |
| N-15-NH | 0.15 | NH4H2PO4 |
| R-0 (pristine) | - | - |
| R-15-NH | 0.15 | NH4H2PO4 |
| R-15-HP | H3PO4 | |
| R-30-NH | 0.3 | NH4H2PO4 |
| R-60-NH | 0.6 | |
| Ion exchangers | ||
| I-0 (pristine) | - | - |
| I-15-NH | 0.15 | NH4H2PO4 |
| I-15-HP | H3PO4 | |
| I-60-NH | 0.6 | NH4H2PO4 |
| Dopants | ||
| CeP-NH | 0.15 | NH4H2PO4 |
| CeP-HP | H3PO4 | |
| Membrane | ωd, % | T ± 1, µm | ωw ± 1, wt. % | IEC ± 0.03, mg-eq./g | σ(NaCl), mS/cm | σ(Na2SO4), mS/cm | U(Cl−)/ U(SO42−) | |
|---|---|---|---|---|---|---|---|---|
| N-0 | - | 153 | 19 | 1.44 | 1.9 | 1.1 | 1.9 | 96.3 |
| N-15-NH | 0.5 | 148 | 20 | 1.44 | 1.6 | 1.0 | 1.4 | 96.4 |
| R-0 | - | 566 | 56 | 2.12 | 3.1 | 2.2 | 1.4 | 94.5 |
| R-15-NH | 0.6 | 567 | 48 | 1.87 | 2.3 | 1.8 | 1.2 | 94.5 |
| R-30-NH | 2.00 | 615 | 54 | 2.07 | 3.0 | 2.2 | 1.3 | 94.0 |
| R-60-NH | 2.00 | 612 | 52 | 2.12 | 3.2 | 2.3 | 1.3 | 93.3 |
| R-15-HP | 1.0 | 598 | 52 | 2.11 | 3.0 | 2.2 | 1.4 | 94.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manin, A.; Golubenko, D.; Novikova, S.; Yaroslavtsev, A. Composite Anion Exchange Membranes Based on Quaternary Ammonium-Functionalized Polystyrene and Cerium(IV) Phosphate with Improved Monovalent-Ion Selectivity and Antifouling Properties. Membranes 2023, 13, 624. https://doi.org/10.3390/membranes13070624
Manin A, Golubenko D, Novikova S, Yaroslavtsev A. Composite Anion Exchange Membranes Based on Quaternary Ammonium-Functionalized Polystyrene and Cerium(IV) Phosphate with Improved Monovalent-Ion Selectivity and Antifouling Properties. Membranes. 2023; 13(7):624. https://doi.org/10.3390/membranes13070624
Chicago/Turabian StyleManin, Andrey, Daniel Golubenko, Svetlana Novikova, and Andrey Yaroslavtsev. 2023. "Composite Anion Exchange Membranes Based on Quaternary Ammonium-Functionalized Polystyrene and Cerium(IV) Phosphate with Improved Monovalent-Ion Selectivity and Antifouling Properties" Membranes 13, no. 7: 624. https://doi.org/10.3390/membranes13070624
APA StyleManin, A., Golubenko, D., Novikova, S., & Yaroslavtsev, A. (2023). Composite Anion Exchange Membranes Based on Quaternary Ammonium-Functionalized Polystyrene and Cerium(IV) Phosphate with Improved Monovalent-Ion Selectivity and Antifouling Properties. Membranes, 13(7), 624. https://doi.org/10.3390/membranes13070624








