2.1. Simulation Approach
Successful approaches for simulating the properties of extended photonic crystals involve solving the light transport as a plane wave in each spatial direction and then combining the probabilities for each spatial direction to obtain the overall superposition [
6]. However, this concept is only applicable when the object is much larger than the wavelength and exhibits strong, large-scale regularity, which is not the case for the lens mitochondrion.
Another computationally expensive approach is to solve the light transport for a polarized collimated source attached to a unit cell of interest and calculate the transport across an assembly of unit cells by integrating over their surfaces rather than their volume. While this approach is suitable for finite-sized unit cell assemblies, it cannot be directly applied to the spherical boundary of the mitochondrion due to the large fraction of partial unit cells. For instance, if we consider a diameter of 5 unit cells (2 µm over a 400 nm lattice), we expect approximately
unit cells within the confined volume and
at the surface (the actual number of fractional unit cells according to the simulator is 52). Consequently, solving the unit cell becomes of limited use, and efficient computational approaches designed for three-dimensional photonic crystals [
7] are not readily applicable in this scenario.
Structures with a complexity similar in magnitude to the wavelength are typically solved accurately and reliably using FDTD solvers. Representing the membrane as an FDTD structure is straightforward: the volume of the cubic membrane is converted into the solver. This conversion allows the entire spectral response to be proved with a single broadband pulse, eliminating the need for simulating the transmission for each monochromatic wavelength and subsequently combining the results.
However, the multilayer membrane consists of structures on the scale of one nanometer, resulting in enormous meshes. Even with a grid resolution of 2 nm, considerable deviations from the rigorous solution are observed. To assess the limitations and accuracy of the approach, we rigorously solved the reflection/transmission curve for a flattened, extended membrane of the original thickness [
8] and compared it to the results obtained using FDTD. While the errors for a 1 nm mesh are acceptable, the stability of the method is not satisfactory.
Meshing a 2 µm mitochondrion results in an overwhelming number of approximately 2 billion mesh entries, pushing the limits of our computational capabilities. Nevertheless, we conducted simulations for the 2-micron case using both ray tracing and finite difference time domain (FDTD) methods. The discrepancies observed in the results highlight the limitations of both approaches, but they also qualitatively support our initial hypothesis. Thus, in this study, we focus on the ray tracing approach since it enables proper modeling of the enclosed mitochondrion without disregarding its boundary.
Considering the 12-layer membrane and the homogeneous interstitial compartments as fundamental building blocks, we proposed a straightforward solution by replacing the membrane structure with an equivalent homogeneous material. This substitution conveniently scales the cubic membrane of the mitochondrion to a size comparable to well-studied photonic crystals. However, rotating the membrane stack at various aspect angles reveals the non-existence of such a material. Similarly, replacing the membrane with the average refractive index yields no significant photonic properties. Thus, we can confidently assert that the cubic membrane present in the lens mitochondrion cannot be considered a proper photonic crystal. Instead, a reasonably accurate approximation can be achieved by replacing the membrane with a thin sandwich material characterized by steeply changing refractive indices (RIs). This adjustment brings the model closer to the scenario elegantly solved by Momeni et al. [
7]. Moreover, it simplifies the mesh volume significantly and reduces its size.
Overall, these challenges and limitations arise when utilizing various simulation approaches to investigate the unique characteristics of the lens mitochondrion, including its confined size and complex structure. The computational constraints associated with meshing a 2 µm mitochondrion emphasize the need for careful consideration. However, by employing ray tracing, we were able to effectively model the enclosed mitochondrion while accounting for its boundary. To address these challenges, we explored alternative approaches and introduced a thin sandwich material with steeply changing refractive indices (RIs) as a promising approximation. This adjustment simplifies the mesh volume and demonstrates qualitative agreement with our initial hypotheses. We emphasize the importance of further refinement and exploration to achieve more accurate results in the study of lens mitochondria.
2.2. Closed Bilayer Approximation
For a well-defined multi-layer system with known materials and geometry, the reflectance dependents on the wavelength and angle () of incidence.
To approximate the multi-layer, we minimized the reflectance difference between it and the closed bilayer. This involves optimizing the four parameters of the bilayer, denoted as:
, which represent the outer reflective index, the core reflective index, and their respective thicknesses.
where
, and
are defined by the reflection coefficients, depending on the direction of polarization.
The wavelength λ is sampled within visible light from 400 to 800 nm in 5 nm steps. The incident angle is sampled from 0 to 90 degree stepped in single-degree steps. The optimization algorithm “interior-point” was used to establish the parameters of the closest bilayer in Matlab.
The obtained results presented in the
Table 1 demonstrate a satisfactory level of accuracy while also revealing some unexpected findings. Firstly, it can be observed that the average RI and overall thickness of the structure are maintained closely, indicating a successful approximation. Furthermore, the introduced sandwich structure exhibits improved performance in handling total internal reflection compared to the homogeneous material approach (
Table 1).
Remarkably, it was discovered that the core material within the sandwich structure is identical to the interstitial material, effectively resulting in two photonic structures nested within each other. Although some maximum errors are experienced at high angles, the deviations remain within the low percentage range. The presence of thick sandwich layers allows for a coarser meshing approach compared to the original structure, offering computational advantages. Additionally, since the mitochondrion contains fewer than five repeats of the unit cell, errors at high angles do not accumulate superlinearly. To balance between accurately modeling the rigorous solution presented in
Table 1 and to ensure computational feasibility within the memory constraints of our largest compute node, we employed a compromise meshing strategy. The curvature of the membrane necessitated smaller mesh cells of approximately 5 nm between material transitions, while no such limitation exists within homogeneous materials. As a result, the mesh size was reduced by about 2.5 orders of magnitude, and the entire simulation could be completed within a week of compute time for all wavelengths.
2.3. Alternative Approach
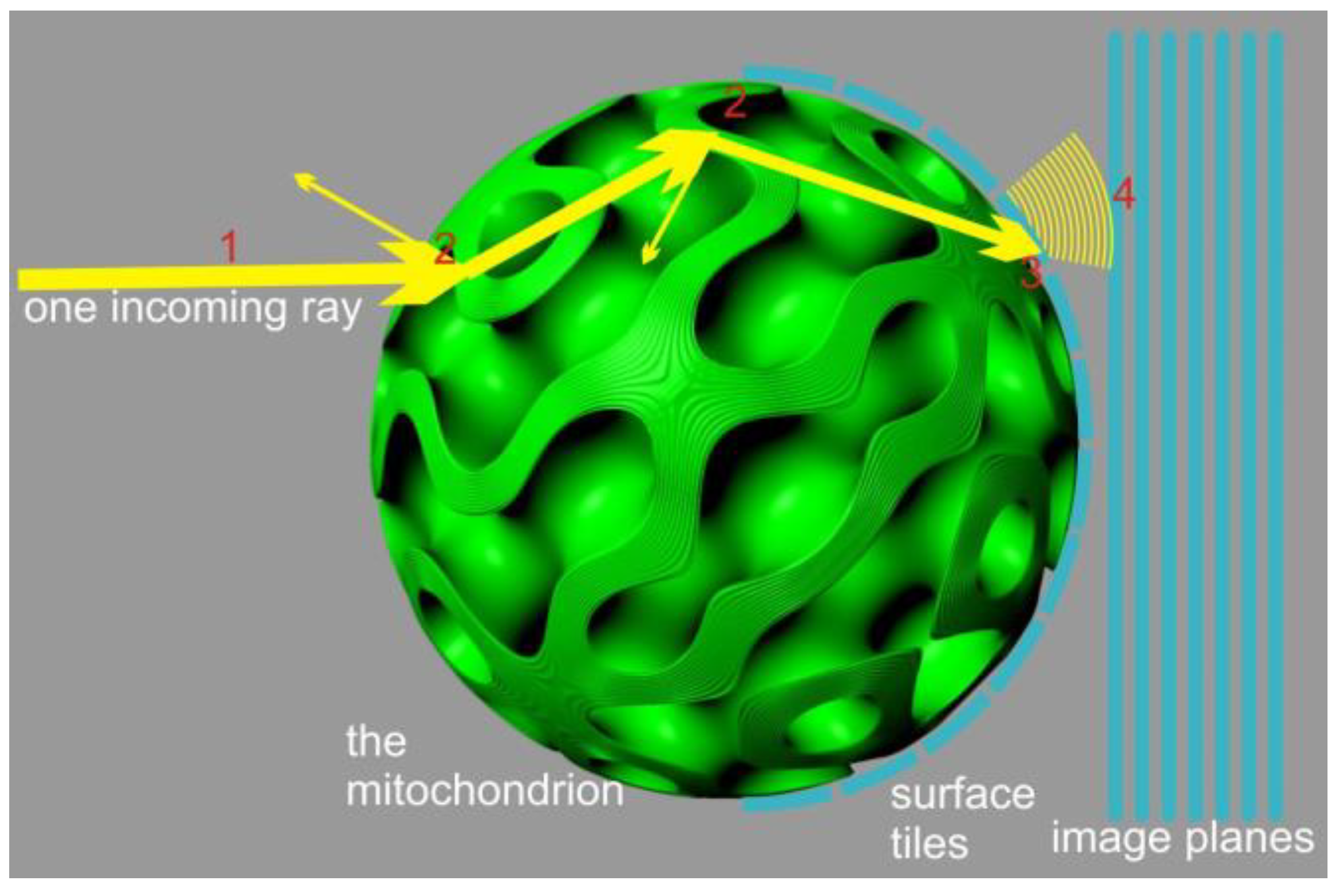
An alternative approach can be devised by neglecting diffraction effects and focusing solely on energy transport and polarization. This approach reduces the problem to a local ray-tracing method which sacrifices the accuracy of light distribution geometry but ensures localized energy preservation and rigorous treatment of interference contributions, limited by the numerical accuracy of sampling and integration (
Figure 2). To implement this method, we employed a Monte Carlo process in which a finite number of rays, sharing the same frequency, polarization, phase, and direction (parallel to the
x-axis), were emitted from a 2D grid in the y-z plane. The grid was arranged with offsets to cover a circular region centered around the y-z origin, matching the radius (R) of the mitochondrion. This arrangement guaranteed that all fired rays would intersect with the virtual mitochondrion.
The splitting condition for the Monte Carlo process was defined as the transition between different membrane compartments or the entry/exit points of the mitochondrion. The compartments were separated by a sandwich structure composed of lipid bilayers, with cytosolic layers of comparable thickness (each layer approximately 5–8 nm wide). In the tree shrew’s mitochondrion, there are 12 lipid bilayers separating the compartments. Although the sandwich wall between the compartments is not thin compared to the compartment volume (approximately 120+ nm versus a lattice of 400–500 nm), we opted to model the membrane transition “sandwich” as a locally flat stratified medium with 25 layers, including the contacting compartments. This simplification was possible due to the significantly smaller size of the individual layers compared to the wavelength.
This simplification greatly reduces the complexity of the problem by leveraging well-established investigations on transitions through stratified media [
9]. We adopted Hecht’s simplification [
8] to solve the transition at any angle, extending it to cover arbitrary polarization cases.
To achieve this, we divided our complex light packet into two components: a forward component with polarization in the plane of incidence onto the membrane transition and a lateral component with polarization orthogonal to that plane. Each fraction was treated independently, and their results were merged after the scattering process.
The Hecht solution considers each stratified layer as an element that alters the phase, direction of propagation, and fractions of propagated and reflected components. It describes this transition using a 2 × 2 matrix,
where index I marks the fields entering medium 1 from medium 0 and index II the fields entering medium 2 from medium 1. The direction of propagation of light enters the medium
a (with thickness
and a refractive index of
) at an angle
and hence has the apparent thickness
in the direction of propagation.
Here, if the electrical field is parallel to the plane of incidence (polarization s state). When the electrical field is perpendicular to the plane of incidence (polarization p state), .
Hecht’s solution demands the continuity of the tangential fields across an interface and strictly linear dielectric properties .
For a polarized electrical field in
s state, the following equations will hold as a boundary condition,
While in the
p state,
with
representing the amplitudes incoming from the medium 0 to medium 1 and reflected at the medium 1, respectively,
representing the transmission into the next medium 2,
representing the angles of incidence into medium 1 and within medium 1 into the next medium, respectively. Finally, the refractive indexes of the medium before entering medium 1 are
and the subsequent one,
.
The propagation through many layers is again given by the product of all matrices, and the transmission and reflection coefficients are defined by:
For a polarization
s state, introducing the auxiliary ratios
and
, the coefficients will be:
and
While in the
p state, setting
and
, the coefficients will become:
and
This addition accounts for the parallel polarized case, which is the only extension we made over Hecht’s approach. The general polarization is split into these two components at each scatter process, and after reflection and transmission, both components are merged again. Therefore, the scatter process incorporates the possibility of a polarization change.
The ratio of transmitted and reflected power is deterministic and depends on factors such as the wavelength, incident angle, polarization, and refractive index change. However, the diffractive process is simulated using a stochastic component that accounts for the variability in the propagation direction. Instead of directly simulating the propagating wave, this approach involves substituting it with one or several rays. These rays’ directions and relative powers are modeled based on the probability of propagation. This simulation technique resembles how incident light decomposes from a continuous wave into individual photon interactions.
Thus, in the stochastic process, the transition is assumed to occur precisely between the two compartments and is considered infinitely thin. However, diffraction, polarization change, reflection, and phase alteration are still calculated based on the original structure’s width. Thus, the transition acts as a black box that transmits, bends, reflects light, and modifies its polarization depending on the wavelength, polarization, and angle of incidence. However, the stochastic splitting is computed only once per transition.
We used the same physics as described in [
6], but we no longer relied on the simplifications concerning the
z-axis and polarization. Additionally, at this stage, we did not need to consider shape inaccuracies or tolerances. Therefore, our pure interference model was limited to a single transition, and we employed scattering between transitions to transport the electric field from one event to the next. Total internal reflection can occur at either the interface of the first layer or at the exit from the last layer, and we verified its occurrence. However, we simplified all cases by using the center approximation, as depicted in
Figure 3. When total internal reflection occurs, no interference transition is calculated, and it is always reduced to a perfect reflection.
We explored several mechanisms for implementing the scattering process, referred to as “photon splitting”:
(i) Only the transmitted or reflected portion survives, with a probability based on the relative energy of the two fractions. This ensures algorithm termination and requires a large number of initial rays for numerical accuracy.
(ii) Both portions are pursued, starting with the weaker portion. If a portion’s energy falls below a fraction ϵ (chosen as one billionth of the initial energy), it is discarded. This approach uses minimal memory as the weaker portion loses energy exponentially, and it provides stable results with a fraction of the original rays. We found convergence with 4000 or more original rays, and the fraction of lost “photons” can be controlled by adjusting ϵ to keep the total lost energy below 1%.
(iii) Splitting the scattered portion into multiple packets to account for diffraction. However, this approach did not yield smoother outcomes or require fewer rays compared to case (ii) in the small set of transitions tested. Consequently, we abandoned this approach in favor of faster computation.