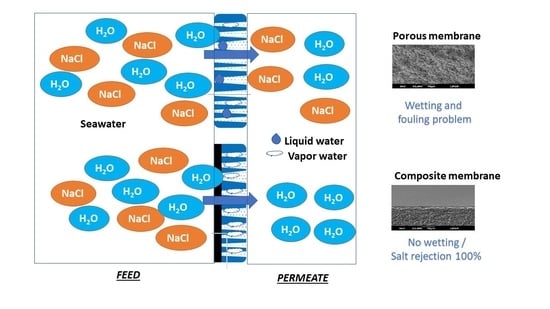
Design and Preparation a New Composite Hydrophilic/Hydrophobic Membrane for Desalination by Pervaporation
Abstract
1. Introduction
| MD | PV | |
|---|---|---|
| Membrane type | Porous and hydrophobic | Dense or molecular sieving hydrophilic or hydrophobic |
| Membrane role | Support medium for the vapor liquid interface Do not contribute to separation | Dense layer contributes to separation by interaction with water molecules:
|
| Mechanism | Knudsen diffusion Poiseuille flow (viscous flow), molecular diffusion | Solution-diffusion, Size exclusion Charge exclusion |
| Main Configurations | Direct contact MD, vacuum MD, sweeping gas MD, air gap MD | Vacuum PV, sweeping gas PV, air gap PV, direct contact PV (thermo-pervaporation) |
| Membrane material | PP, PVDF, PTFE | PVA, CTA |
| Applications | Concentration of juice, desalination, crystallization | Dehydration, recovery of organics, desalination |
| Challenges | Membrane fouling, membrane wetting and scaling, stability of permeation flux | Relatively lower permeation flux Membrane fouling and scaling |
| References | [26,27,28] | [10,21,29] |
2. Material and Methods
2.1. Chemicals
2.2. Membrane Preparation
2.3. Characterization Techniques
2.4. Permeation Experiment
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Fourier Transform InfraRed Spectroscopy (FTIR)
3.1.2. Surface Characterizations
3.1.3. Membrane Morphology
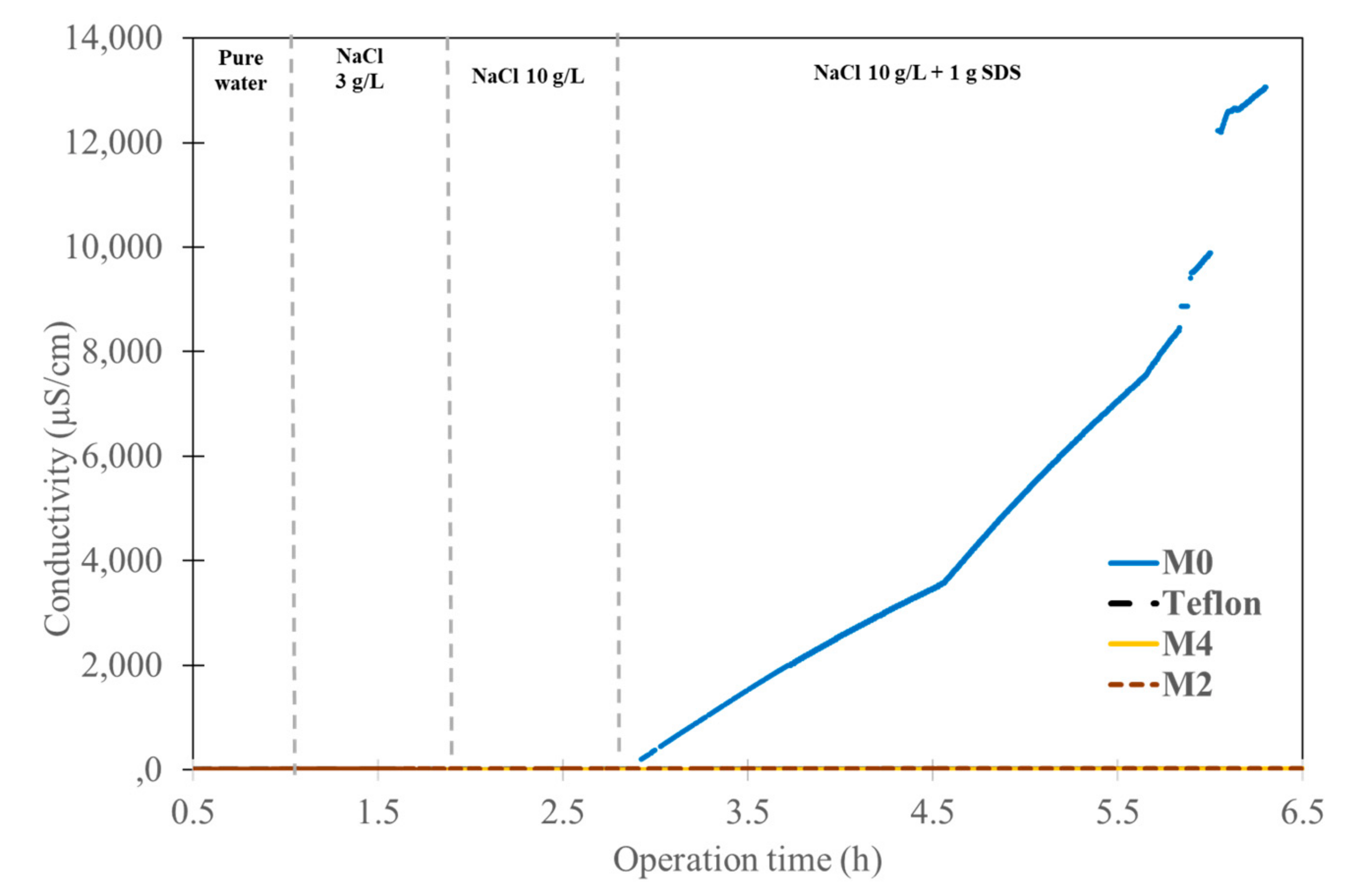
3.2. Membrane Performance with Synthetic Solution
3.3. Thickness Effect on Membrane Performance
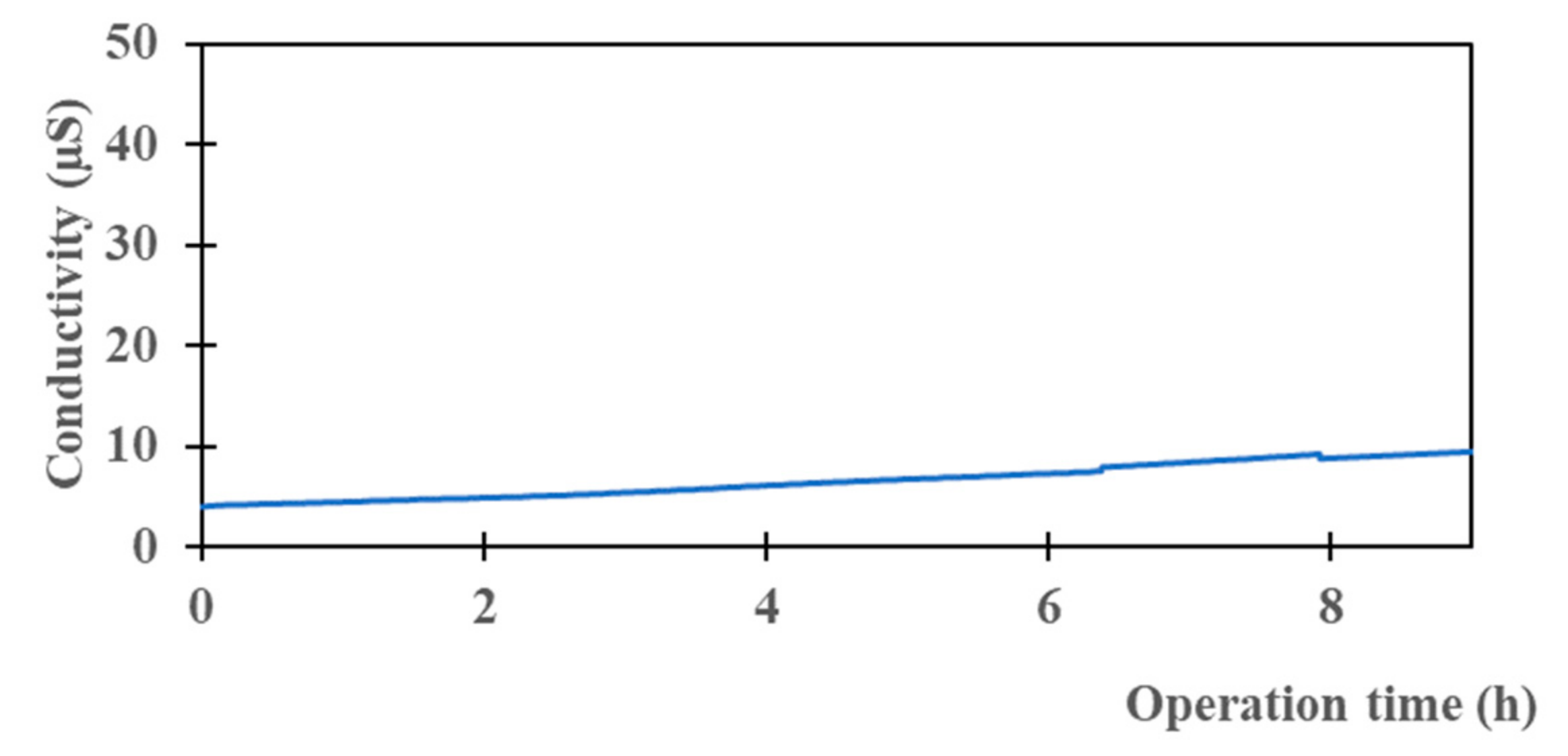
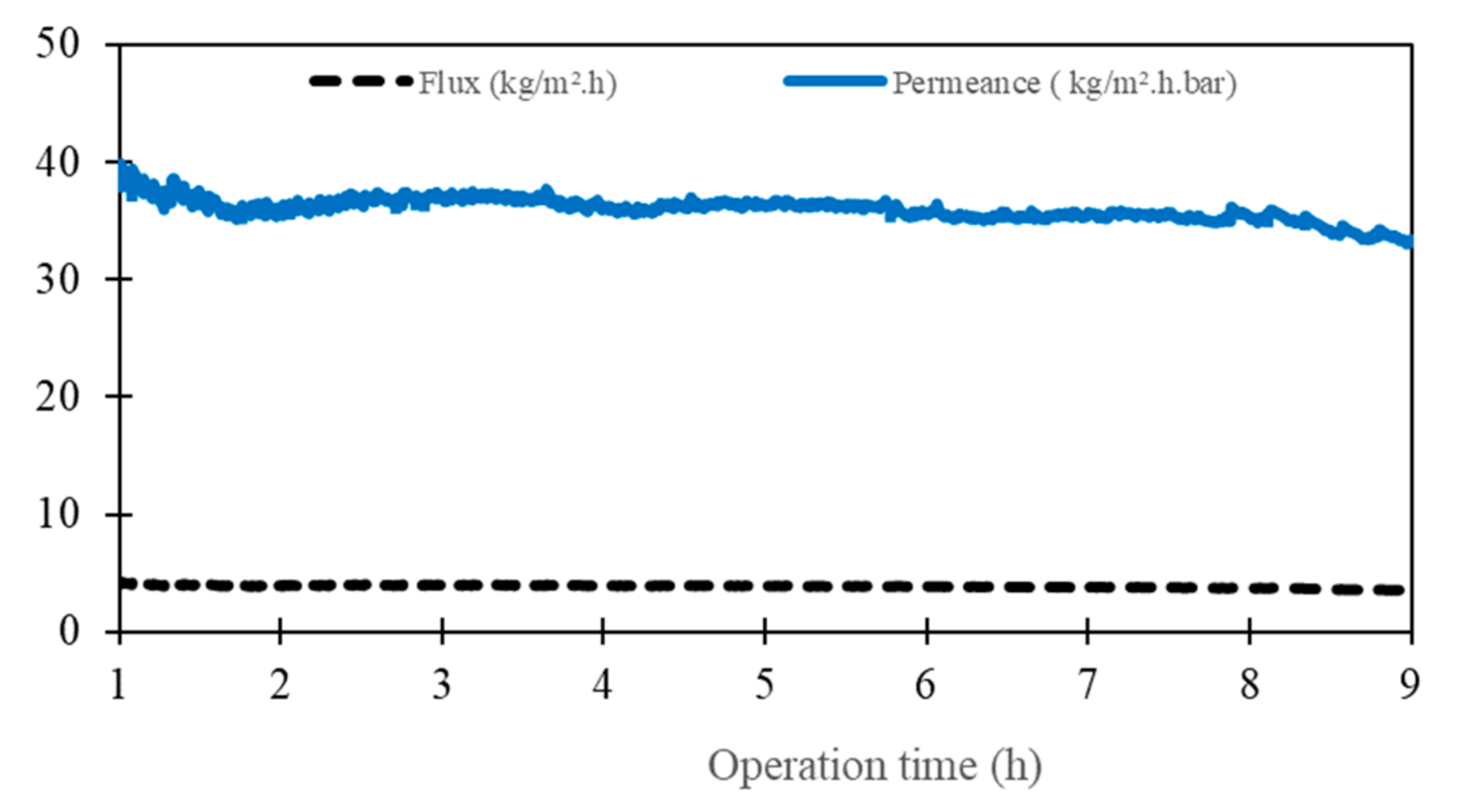
3.4. Raw Seawater Desalination
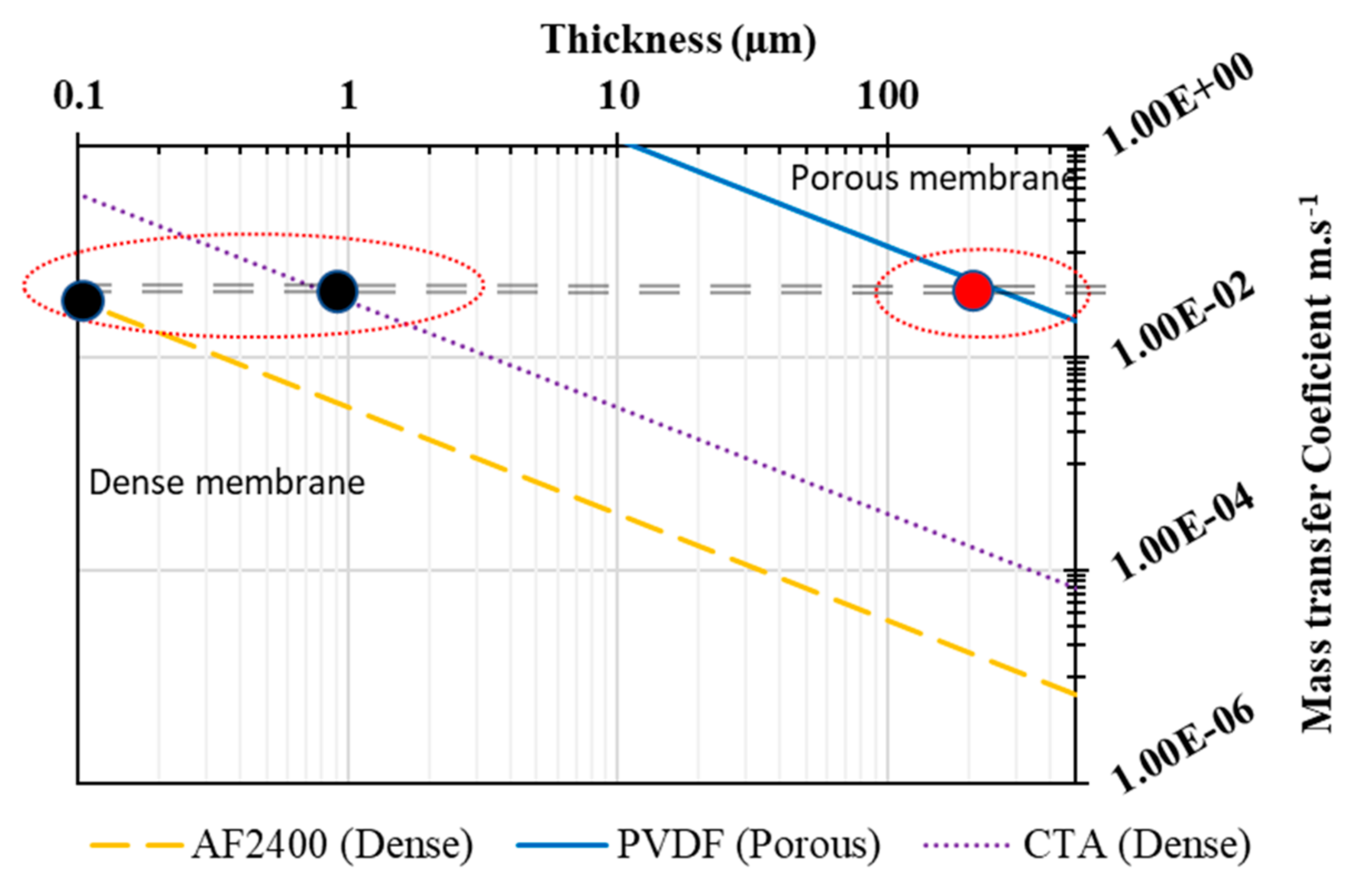
3.5. Theoretical Prediction and Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Bremere, I.; Kennedy, M.; Stikker, A.; Schippers, J. How water scarcity will effect the growth in the desalination market in the coming 25 years. Desalination 2001, 138, 7–15. [Google Scholar] [CrossRef]
- Feria-Díaz, J.; Maria Cristina, L.-M.; Sandoval-Herazo, L.; Correa Mahecha, F.; Rodriguez Miranda, J. Commercial Thermal Technologies for Desalination of Water from Renewable Energies: A State of the Art Review. Processes 2021, 9, 262. [Google Scholar] [CrossRef]
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.-M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2018, 657, 1343–1356. [Google Scholar] [CrossRef]
- Imbrogno, J.; Keating, J.J.; Kilduff, J.; Belfort, G. Critical aspects of RO desalination: A combination strategy. Desalination 2017, 401, 68–87. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Global Challenges in Energy and Water Supply: The Promise of Engineered Osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef]
- Eljaddi, T.; Mendez, D.L.M.; Favre, E.; Roizard, D. Development of new pervaporation composite membranes for desalination: Theoretical and experimental investigations. Desalination 2021, 507, 115006. [Google Scholar] [CrossRef]
- Elsaid, K.; Sayed, E.T.; Abdelkareem, M.A.; Baroutaji, A.; Olabi, A. Environmental impact of desalination processes: Mitigation and control strategies. Sci. Total Environ. 2020, 740, 140125. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wu, L.; Wei, G. Fabrication, Properties, Performances, and Separation Application of Polymeric Pervaporation Membranes: A Review. Polymers 2020, 12, 1466. [Google Scholar] [CrossRef]
- Golubev, G.; Eremeev, I.; Makaev, S.; Shalygin, M.; Vasilevsky, V.; He, T.; Drioli, E.; Volkov, A. Thin-film distillation coupled with membrane condenser for brine solutions concentration. Desalination 2021, 503, 114956. [Google Scholar] [CrossRef]
- Bindels, M.; Carvalho, J.; Gonzalez, C.B.; Brand, N.; Nelemans, B. Techno-economic assessment of seawater reverse osmosis (SWRO) brine treatment with air gap membrane distillation (AGMD). Desalination 2020, 489, 114532. [Google Scholar] [CrossRef]
- Kaminski, W.; Marszalek, J.; Tomczak, E. Water desalination by pervaporation–Comparison of energy consumption. Desalination 2018, 433, 89–93. [Google Scholar] [CrossRef]
- Franken, A.; Nolten, J.; Mulder, M.; Bargeman, D.; Smolders, C. Wetting criteria for the applicability of membrane distillation. J. Membr. Sci. 1987, 33, 315–328. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M. Membrane Distillation (MD). In Progress in Filtration and Separation; Academic Press: Cambridge, MA, USA, 2015; pp. 61–99. [Google Scholar] [CrossRef]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, Scaling, and Fouling in Membrane Distillation: State-of-the-Art Insights on Fundamental Mechanisms and Mitigation Strategies. ACS ES&T Eng. 2020, 1, 117–140. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 24. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, F. Potential of Pervaporation and Vapor Separation with Water Selective Membranes for an Optimized Production of Biofuels—A Review. Catalysts 2017, 7, 187. [Google Scholar] [CrossRef]
- Xie, Z.; Li, N.; Wang, Q.; Bolto, B. Desalination by pervaporation. Emerg. Technol. Sustain. Desalin. Handb. 2018, 6, 205–226. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Bolto, B.; Hoang, M.; Xie, Z. Desalination by pervaporation: A review. Desalination 2016, 387, 46–60. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Breakthroughs on tailoring pervaporation membranes for water desalination: A review. Water Res. 2020, 187, 116428. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Zhang, R.; Cao, B.; Li, P. Fabrication of pervaporation desalination membranes with excellent chemical resistance for chemical washing. J. Membr. Sci. 2020, 611, 118367. [Google Scholar] [CrossRef]
- Guan, K.; Liu, G.; Matsuyama, H.; Jin, W. Graphene-based membranes for pervaporation processes. Chin. J. Chem. Eng. 2020, 28, 1755–1766. [Google Scholar] [CrossRef]
- Yang, G.; Xie, Z.; Cran, M.; Wu, C.; Gray, S. Dimensional Nanofillers in Mixed Matrix Membranes for Pervaporation Separations: A Review. Membranes 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard, V.J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.-H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A review of membrane wettability for the treatment of saline water deploying membrane distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Nagy, E. Pervaporation. In Basic Equations Mass Transport through a Membrane Layer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 429–445. [Google Scholar] [CrossRef]
- You, M.; Yin, J.; Sun, R.; Cao, X.; Meng, J. Water/salt transport properties of organic/inorganic hybrid films based on cellulose triacetate. J. Membr. Sci. 2018, 563, 571–583. [Google Scholar] [CrossRef]
- Nakao, T.; Miura, Y.; Furuichi, K.; Yasukawa, M. Cellulose Triacetate (CTA) Hollow-Fiber (HF) Membranes for Sustainable Seawater Desalination: A Review. Membranes 2021, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Arnot, T.C.; Mattia, D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011, 370, 1–22. [Google Scholar] [CrossRef]
- Nguyen, T.P.N.; Jun, B.-M.; Kwon, Y.-N. The chlorination mechanism of integrally asymmetric cellulose triacetate (CTA)-based and thin film composite polyamide-based forward osmosis membrane. J. Membr. Sci. 2017, 523, 111–121. [Google Scholar] [CrossRef]
- Eljaddi, T.; Mendez, D.L.M.; Favre, E.; Roizard, D. Development of new pervaporation composite membranes for desalination: Membrane characterizations and experimental permeation data. Data Brief 2021, 35, 106943. [Google Scholar] [CrossRef]
- Azmi, R.; Goh, P.; Ismail, A.; Lau, W.; Ng, B.; Othman, N.; Noor, A.; Yusoff, M. Deacidification of crude palm oil using PVA-crosslinked PVDF membrane. J. Food Eng. 2015, 166, 165–173. [Google Scholar] [CrossRef]
- He, F.; Luo, B.; Yuan, S.; Liang, B.; Choong, C.; Pehkonen, S.O. PVDF film tethered with RGD-click-poly(glycidyl methacrylate) brushes by combination of direct surface-initiated ATRP and click chemistry for improved cytocompatibility. RSC Adv. 2013, 4, 105–117. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Bey, S.; Clarizia, G.; Mansouri, L.; Benamor, M. Gas permeation behavior of CTA polymer inclusion membrane (PIM) containing an acidic carrier for metal recovery (DEHPA). Sep. Purif. Technol. 2011, 80, 38–44. [Google Scholar] [CrossRef]
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative analysis of cellulose acetate with a high degree of substitution by FTIR and its application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Khongnakorn, W.; Bootluck, W.; Youravong, W. Surface Modification of CTA-FO Membrane by CO2 Plasma Treatment. J. Teknol. 2014, 70, 71–75. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Alizadeh, A.; Daraei, P.; Zinatizadeh, A.A.; Rahimpour, F. Separation of nitrophenols using cellulose acetate nanofiltration membrane: Influence of surfactant additives. Sep. Purif. Technol. 2012, 85, 147–156. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, M.; Li, X.; Wang, Y.; An, A.K.; Fang, J.; He, T. Anti-wetting behavior of negatively charged superhydrophobic PVDF membranes in direct contact membrane distillation of emulsified wastewaters. J. Membr. Sci. 2017, 535, 230–238. [Google Scholar] [CrossRef]
- Rezaei, M.; Samhaber, W. Wetting behaviour of superhydrophobic membranes coated with nanoparticles in membrane distillation. Chem. Eng. Trans. 2016, 47, 373–378. [Google Scholar] [CrossRef]
- Chen, G.Q.; Kanehashi, S.; Doherty, C.M.; Hill, A.J.; Kentish, S.E. Water vapor permeation through cellulose acetate membranes and its impact upon membrane separation performance for natural gas purification. J. Membr. Sci. 2015, 487, 249–255. [Google Scholar] [CrossRef]
- Mendez, D.L.M.; Castel, C.; Lemaitre, C.; Favre, E. Membrane distillation (MD) processes for water desalination applications. Can dense selfstanding membranes compete with microporous hydrophobic materials? Chem. Eng. Sci. 2018, 188, 84–96. [Google Scholar] [CrossRef]
- Liang, B.; Li, Q.; Cao, B.; Li, P. Water permeance, permeability and desalination properties of the sulfonic acid functionalized composite pervaporation membranes. Desalination 2018, 433, 132–140. [Google Scholar] [CrossRef]
- Makhloufi, C.; Lasseuguette, E.; Remigy, J.C.; Belaissaoui, B.; Roizard, D.; Favre, E. Ammonia based CO2 capture process using hollow fiber membrane contactors. J. Membr. Sci. 2014, 455, 236–246. [Google Scholar] [CrossRef]
- Ali, Z.; Wang, Y.; Ogieglo, W.; Pacheco, F.; Vovusha, H.; Han, Y.; Pinnau, I. Gas separation and water desalination performance of defect-free interfacially polymerized para-linked polyamide thin-film composite membranes. J. Membr. Sci. 2020, 618, 118572. [Google Scholar] [CrossRef]
- Huth, E.; Muthu, S.; Ruff, L.; Brant, J.A. Feasibility assessment of pervaporation for desalinating high-salinity brines. J. Water Reuse Desalination 2014, 4, 109–124. [Google Scholar] [CrossRef]
- Naim, M.; Elewa, M.; El-Shafei, A.; Moneer, A. Desalination of simulated seawater by purge-air pervaporation using an innovative fabricated membrane. Water Sci. Technol. 2015, 72, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, S.G.; Rajai, B.H.; Singh, P.S. Preparation of ultra-thin poly(vinyl alcohol) membranes supported on polysulfone hollow fiber and their application for production of pure water from seawater. Desalination 2015, 367, 272–284. [Google Scholar] [CrossRef]
- Zwijnenberg, H.J.; Koops, G.; Wessling, M. Solar driven membrane pervaporation for desalination processes. J. Membr. Sci. 2005, 250, 235–246. [Google Scholar] [CrossRef]


| Membrane | Contact Angle ° | Deviation | Image |
|---|---|---|---|
| M0 | 126 | 2.16 |  |
| M2 | 57 | 3.28 |  |
| Membrane | Dense Layer Thickness (µm ± 0.5 µm) | Cross-Section | Top Surface | Bottom Surface |
|---|---|---|---|---|
| M0 | without coating |  |  |  |
| M1 | 1.4 |  |  |  |
| M2 | 3.8 |  |  |  |
| M3 | 4.1 |  |  |  |
| M4 | 6.8 |  |  |  |
| Membrane | Dense Layer Thickness (µm) | Permeance (kg.m−2.h−1.bar−1) |
|---|---|---|
| M1 | 1.4 | 115 |
| M2 | 3.8 | 46 |
| M3 | 4.1 | 43 |
| M4 | 6.8 | 29 |
| Feed | Permeate | Retention % | |
|---|---|---|---|
| Salinity g/L | 38.3 | 0.3 | 99.2 |
| Conductivity (mS/cm) | 56.3 | 0.009 | 99.9 |
| TDS (g/L) | 40.8 | 0.005 | 99.9 |
| Support | Prediction for Composite Membranes b | ||
|---|---|---|---|
| PVDF (122 µm) a | CTA (0.1 µm) b | Teflon (0.1 µm) b | |
| Mass transfer coefficient (m/s) | 1.21 × 10−1 | 6.1 × 10−2 | 2.7 × 10−2 |
| Water permeance (kg·m−2·h−1·bar−1) | 323.47 | 163 | 72 |
| Membrane | NaCl (ppm) | Feed Temperature (°C) | Thickness (µm) | Flux kg/(m2·h) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|
| CTA | 100,000 | 50 | 10 | 2.3 | 99 | [48] |
| Polyester | 35,000 | 70 | 20–25 | 5.97–3.45 | 99.7 | [49] |
| PVA/MA | 30,000 | 70 | 0.1 | 7.4 | 99.9 | [50] |
| Polyether amide | 35,000 | 46–82 | 40 | 0.2 | 99.99 | [51] |
| S-PVA/PAN | 35,000 | 70 | 0.8 | 46.3 | 99.5 | [45] |
| CTA/PVDF | Seawater 37,300 | 50 | 4 | 4 | 99.5 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eljaddi, T.; Favre, E.; Roizard, D. Design and Preparation a New Composite Hydrophilic/Hydrophobic Membrane for Desalination by Pervaporation. Membranes 2023, 13, 599. https://doi.org/10.3390/membranes13060599
Eljaddi T, Favre E, Roizard D. Design and Preparation a New Composite Hydrophilic/Hydrophobic Membrane for Desalination by Pervaporation. Membranes. 2023; 13(6):599. https://doi.org/10.3390/membranes13060599
Chicago/Turabian StyleEljaddi, Tarik, Eric Favre, and Denis Roizard. 2023. "Design and Preparation a New Composite Hydrophilic/Hydrophobic Membrane for Desalination by Pervaporation" Membranes 13, no. 6: 599. https://doi.org/10.3390/membranes13060599
APA StyleEljaddi, T., Favre, E., & Roizard, D. (2023). Design and Preparation a New Composite Hydrophilic/Hydrophobic Membrane for Desalination by Pervaporation. Membranes, 13(6), 599. https://doi.org/10.3390/membranes13060599