Abstract
Synthesis and study of materials based on bismuth cerates and titanates were carried out. Complex oxides Bi1.6Y0.4Ti2O7 were synthesized by the citrate route; Bi2Ce2O7 and Bi1.6Y0.4Ce2O7—by the Pechini method. The structural characteristics of materials after conventional sintering at 500–1300 °C were studied. It is demonstrated that the formation of a pure pyrochlore phase, Bi1.6Y0.4Ti2O7, occurs after high-temperature calcination. Complex oxides Bi2Ce2O7 and Bi1.6Y0.4Ce2O7 have a pyrochlore structure formed at low temperatures. Yttrium doping of bismuth cerate lowers the formation temperature of the pyrochlore phase. As a result of calcination at high temperatures, the pyrochlore phase transforms into the CeO2-like fluorite phase enriched by bismuth oxide. The influence of radiation-thermal sintering (RTS) conditions using e-beams was studied as well. In this case, dense ceramics are formed even at sufficiently low temperatures and short processing times. The transport characteristics of the obtained materials were studied. It has been shown that bismuth cerates have high oxygen conductivity. Conclusions are drawn about the oxygen diffusion mechanism for these systems. The materials studied are promising for use as oxygen-conducting layers in composite membranes.
1. Introduction
Oxides and solid solutions with the pyrochlore structure A2B2O7 (or A2B2O6O’, where A and B are rare earth or transition elements) attracted a lot of attention as materials for many applications such as oxygen [1,2,3,4] and hydrogen [5,6] separation membranes, solid oxide fuel cell/electrolyzer electrolytes [1,7,8,9] and electrodes [8,10,11], catalysts for various transformation processes [12,13], pigments [14,15], etc. [16,17,18]. The prospects of using pyrochlores in various electrochemical devices are provided by their high ionic or mixed ionic-electronic conductivity, depending on their composition and synthesis conditions [1,8,9,10,11,19,20].
The transport characteristics of pyrochlores are affected by their structural and defect features. In the real pyrochlore structure, various defects are present, including antistructural cation disordering:
Frenkel anion disordering:
partial ordering of the structure via association of defects, etc. [21,22,23,24,25]. In the A2B2O6O’ structure, O (48f Wyckoff positions) and O’ (8a Wyckoff positions) are non-equivalent and belong to two different sublattices (B2O6 and A2O’, respectively); however, while studying oxide ionic transport, these forms are sometimes undistinguishable, thus suggesting some kind of cooperative migration involving both O and O’ anions [20,26,27]. For some pyrochlores, the oxygen forms differing by their mobility are distinguishable; however, their fractions’ ratio differs from 6:1, hence probably supporting the abovementioned assumption or making evidence of other effects on the oxygen mobility such as different bonding energies of oxide anions in A–O–A, A–O–B and B–O–B chains [27,28]. The features of grain boundaries enhancing [28,29] or blocking [30] oxygen transport are reported as well.
As is known, the thermal instability of Bi2Ti2O7 at temperatures above 612 °C [31] is due to an unfavorable size factor (the ratio of bismuth and titanium cations), thus limiting the possibility of obtaining it in the form of dense ceramics for practical use. The stability of bismuth titanate pyrochlore can be achieved by replacing part of the bismuth atoms with atoms of other elements with a smaller ionic radius [32,33]. Doped bismuth titanates were previously studied and showed good transport properties with doping both A and B sites with various cations such as Co, Zn, etc., enhancing both ionic and total conductivity, which was probably associated with disordering of dopant cation distribution between A- and B-sublattices (Equation (1)) [20,26]. Hence, doped bismuth titanates can be assumed to be promising in electrochemical applications, such as components of permselective layers of oxygen separation membranes.
Bismuth cerates were previously studied as photocatalysts [13] and inorganic pigments [14,15]. Unfortunately, there is a lack of information on their transport properties; however, they probably possess good transport characteristics, especially oxygen mobility, due to the redox activity of Ce4+/Ce3+ cations and a high oxygen vacancy content [34,35,36]. Hence, they are of potential interest in electrochemical applications as well.
Obtaining functional ceramics (for solid oxide fuel cells and permselective membranes) with the required morphological and transport properties is a separate problem in materials science, where sintering is the most important procedure. With traditional thermal sintering in a furnace, long-term processing, and high temperatures are required to obtain ceramics with desired properties, such as gas tightness, homogeneous phase composition, etc. To solve this problem, radiation-thermal sintering (e-beam processing) is proposed [37,38,39,40,41]. Using the main advantages of radiation-thermal reactions—lowering the treatment temperature and a high reaction rate—will reduce the processing time while also significantly reducing internal thermal stresses. This technique was successfully applied for sintering functional layers of solid oxide fuel cells (thin layers of electrolytes such as Y or Sc + Ce -doped zirconia, Gd-doped ceria, etc. on anode substrates, nanocomposite cathode layers such as LSM–ScCeSZ, PrNiCoO-GDC, etc.) and asymmetrically supported oxygen separation membranes (thin and dense permselective layers of mixed ionic-electronic conductors comprised of complex oxides with perovskite, fluorite, spinel, etc. structures or their nanocomposites, such as LFN-GDC, LFC-GDC, La0.3Bi0.7MnOx–Bi1.5Y0.3Sm0.2O3, etc.) using unique equipment of the Budker Institute of Nuclear Physics [37,38,39,40,41]. Disordering of nanodomains by electron beams leads to their easy sintering at moderate temperatures without increasing their sizes. This results in a developed network of domain boundaries, which, for nanocomposites [37,38,39,40,41] or even some complex oxides (such as molybdates of lanthanoids [42], etc.), provide fast oxygen diffusion channels described by the so-called 2D model of oxygen diffusion. For solid oxide fuel cells and asymmetric oxygen separation membranes on metallic substrates, due to the decreased temperature and duration of sintering as compared with conventional sintering methods, such negative phenomena as a variation of functional layer phase composition, cracking, and damage to metallic substrates were prevented. This also allowed for maximum power densities of thin-film solid oxide fuel cells with nanocomposite perovskite-fluorite cathode layers operating on wet H2 as fuel and air as oxidant up to 500 mV/cm2 at 700 °C, which is promising for practical application. For an asymmetric membrane comprised of LaBiMnO-YSmBiO layers supported on a binary Ni-Al foam substrate, the oxygen flux under the air/He gradient was up to 5 mL O2 at 950 °C, which is really high [41]. However, at such high temperatures, Bi can be evaporated from these oxides; hence, to deal with this problem, doped Bi cerates and titanates known to be more chemically stable were studied in this work.
This work aimed at studying the structural and oxide ionic transport properties of bismuth titanates and cerates, including Y-doped ones, and the effects of such processing as radiation-thermal sintering for pyrochlores. Its realization will provide the basis for manufacturing materials for solid oxide fuel cells or oxygen-conducting membranes. The effect of doping with Y on the structural stability and phase composition of these materials was studied. The sinterability of samples was investigated in order to check the adaptability of these materials to obtain gas-tight ceramics for electrochemical applications, including radiation-thermal sintering by e-beams. The oxygen transport properties were studied by the temperature-programmed isotope exchange of oxygen with C18O2 in the flow reactor to acquire data on the oxygen diffusivity required for these applications.
2. Materials and Methods
Bi2Ce2O7 and Bi1.6Y0.4Ce2O7 were synthesized via the modified Pechini technique, as described elsewhere [43]. For the Bi1.6Y0.4Ti2O7 sample synthesized by the citrate method, corresponding salts in the required ratios were added to a solution of citric acid in water (1;5 mole ratio), while the Me:citric acid ratio was equal to 1:2. Bi(NO3)3·5H2O (>99%), Ce(NO3)3·6H2O (>99%), Ti (OCH(CH3)2)4 (>99%), and Y(NO3)3·6H2O (>99%) were used as initial reagents. The xerogels obtained were dried in a drying oven at 110 °C for 12 h, then calcined at 500 °C for 3 h. As-prepared powders were pressed into pellets and sintered at 700 °C for 3 h, at 900 °C for 3 h, at 1100 °C for 10 h, and at 1300 °C for 8 h using conventional sintering.
Radiation-thermal sintering was carried out using an accelerator, ILU-6 (BINP SB RAS, Russia). Electron pulses with 2.4 MeV energy, 328 mA pulse beam current, a pulse duration ~600 s, a narrow scan, and up to 25 Hz pulse frequency were used. The temperature of the samples was controlled using a Pt-Pt-Rh thermocouple and FildPoint (National Instruments, USA) controlling module. Power adjustment was carried out by changing the frequency of pulses. The heating rate was 50 C min−1, and after achieving 1100 °C, samples were sintered for 30 min.
X-ray diffraction (XRD) studies were performed using a Bruker D8 Advance diffractometer with the Lynx-Eye detector using CuKα radiation. XRD patterns were recorded in the 2θ range of 15–95° with a step of 0.05°. Rietveld refinement for quantitative analysis and calculation of lattice parameters was carried out using the software package Topas V.4.2.
Infrared (IR) spectra (4000–250 cm−1, 32 scans, resolution 4 cm−1) were acquired using a Cary 660 (Agilent Technologies, Santa Clara, CA, USA) spectrometer with the GladiATR PIKE Technologies console.
Scanning electron microscopy studies were carried out using a dual-beam scanning electron microscope, the Tescan Solaris FE/SEM (Tescan, Brno, Czech Republic). The experiments were performed in the secondary electron mode at an accelerating voltage of 20 kV.
Oxygen mobility and surface reactivity were studied by the temperature-programmed isotope exchange (TPIE) of oxygen with C18O2 in the flow reactor. Samples (0.25–0.5 mm fraction) were loaded into quartz tube reactors (with an inner diameter of 3 mm). Pretreatment was carried out in He + 1% O2 flow (25 mL min−1) at 700 °C for 30 min. The isotope exchange was carried out in He + 1% C18O2 + 1% Ar flow (25 mL min−1) while heating from 50 to 800 °C with a constant ramp of 5 °C min−1. 18O atomic fraction (α) and C16O18O atomic fraction (f16–18) responses were analyzed in order to estimate isotope exchange kinetic parameters.
3. Results and Discussion
3.1. Structural Features
Compositions and lattice parameters of all prepared samples are presented in Table 1.

Table 1.
Structural properties of prepared pyrochlores.
According to the XRD data, for all cerate samples after calcination at 500–700 °C, there is an admixture of bismuth oxide (Figure 1). For bismuth cerates’ samples, the cubic fluorite phase forms after sintering at low temperatures. Metastable bismuth cerate is proposed to form the solid solution in the ceria-yttria complex oxide (Figure 1b). Similar behavior was observed for the undoped Bi cerate: metastable Bi cerate forms after synthesis, and at further sintering, it decomposes into the oxide mixture, followed by forming the solid solution (Figure 1a). The introduction of Y3+ into Bi2Ce2O7 decreases the lattice constant from 5.413 Å to 5.407 Å, thus evidencing substitution of ions in the lattice with contraction as expected, since the ionic radius of Y3+ (r = 1.01 Å, CN = 8) is smaller than that of Bi3+ (r = 1.18 Å, CN = 8) (Table 1). With increasing the temperature of sintering, there is a decrease in the lattice constants for the yttrium-doped bismuth cerates, suggesting a higher disordering of their structure. After sintering at 1300 °C, the XRD pattern for Bi1.6Y0.4Ce2O7 contains peaks attributed to the disordered CeO2 fluorite phase, which is visible according to the peaks’ shift.
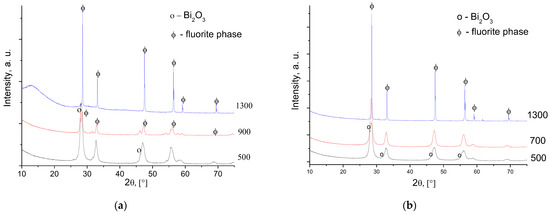
Figure 1.
XRD patterns of Bi2Ce2O7 (a) and Bi1.6Y0.4Ce2O7 (b) obtained by conventional sintering at various temperatures.
According to IR spectroscopy data for both Bi cerate samples, the bands at 1631 cm−1 and 3401 cm−1 observed in IR spectra correspond to –OH symmetric vibrations appearing due to water adsorption (Figure 2). The increasing temperature of sintering leads to the removal of chemisorbed water from the surface. The most intense band at 1383 cm−1 corresponds to Bi–O bond vibrations [12,44,45]. The peak corresponding to the C–O functional group at 2367 cm−1 is shown in Figure 2 [33]. The absorption band that appears at wavelengths below 500 cm−1 presents the stretching vibration in the structure of the cerium oxide (Ce-O-Bi) mode [46].
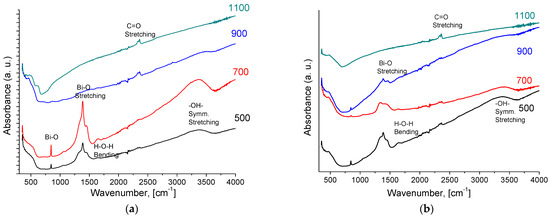
Figure 2.
IR spectra of Bi2Ce2O7 (a) and Bi1.6Y0.4Ce2O7 (b) obtained by conventional sintering at various temperatures.
The Bi1.6Y0.4Ti2O7 samples after sintering at 500 and 900 °C are not single-phased (Figure 3a). According to the XRD data, for titanate sample as in the case of cerates after calcination at 500–700 °C, there is an admixture of bismuth oxide (Figure 3a). Bismuth titanates, along with the pyrochlore phase corresponding to the cubic Bi1.74Ti2O6.624 [PDF 089-4732] phase, contain admixtures of the tetragonal Bi4Ti3O12 [047-0398] and tetragonal Bi2O3 [PDF 071-0465] phases.
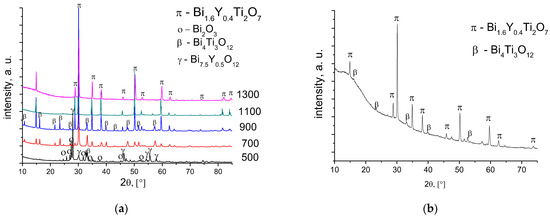
Figure 3.
XRD patterns of Bi1.6Y0.4Ti2O7 sintered at various temperatures using conventional sintering (a) and radiation-thermal sintering at 1100 °C (b).
The Y-doped Bi titanate sintered at 1100–1300 °C contains no admixtures. From the point of view of the phase composition, the RTS conditions used in this work did not allow us to obtain a single-phase sample (Figure 3b). The content of the impurity phase, Bi4Ti3O12, was 8%.
As in the case of bismuth cerates’ samples, the bands observed at 1631 cm−1 and 3401 cm−1 are explained by –OH symmetric vibrations appearing due to water adsorption (Figure 4a). The characteristic feature of the IR spectra of pyrochlores is the presence of an intense band in the range of 400–600 cm−1 [12].
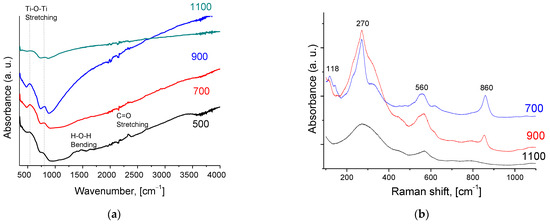
Figure 4.
IR (a) and RAMAN (b) spectra of Bi1.6Y0.4Ti2O7 samples sintered at various temperatures using conventional sintering.
RAMAN spectra for Bi1.6Y0.4Ti2O7 samples are given in Figure 4b. Typical vibration modes present in the spectra demonstrate the formation of the pyrochlore phase only after high-temperature calcination, in good agreement with the XRD data [12,47]. According to the XRD data, the Bi4Ti3O12 phase is present for doped bismuth titanate after calcination at 700–900 °C. In the low-frequency region of the spectrum, the lines at 50–150 cm−1 correspond to Bi oscillations relative to oxygen octahedra. Modes in the frequency range of 200–400 cm−1 correspond to deformation vibrations of O–Ti–O bonds, and high-frequency bands in the range of 500–850 cm−1 correspond to valence vibrations. There is also a band corresponding to the full-symmetric valence oscillation of O–Ti-O bonds of octahedra with a frequency of 843 cm−1.
Figure 5 demonstrates SEM micrographs of Bi2Ce2O7 and Bi1.6Y0.4Ce2O7 after conventional sintering at 1100 °C. For both samples, pores have an irregular shape, with their size varying from a micrometer to a few micrometers. Conventionally sintered bismuth titanates’ samples have larger particles compared to the samples after RTS at 1100 °C (Figure 6). A similar tendency was demonstrated in [37] for lanthanide tungstates and molybdates. Using the traditional sintering of bismuth titanate at 1100 °C, the average grain size ranges from 1 to 10 microns. The different morphology of the particles visible here is due to the admixture of the Bi4Ti3O12 phase. Sintering at this temperature for 10 h was not sufficient since the presence of pores is visible (Figure 6c,d). The porosity and average pore size values are given in Table 2. Figure 6e,f, showing SEM images of RTS Bi1.6Y0.4Ti2O7, do not contain microcracks and pores, which demonstrates the optimal sintering conditions. The difference in particle size is apparently caused by the effects of the sintering technique (conventional or radiation-thermal), sintering temperature, and duration, which affect the particles’ aggregation and growth. Hence, it was demonstrated that RTS allows for carrying out sintering processes for shorter times and at lower temperatures compared to those for conventional sintering. Such a difference in sintering temperature and duration required to obtain the desired gas-tightness for radiation–thermal sintering and conventional sintering is apparently related to the dissipation of radiation energy in heterogeneous structures, thermal-diffusional stimulation of mass transfer, and other features of the RTS process [37].

Figure 5.
SEM micrographs of Bi2Ce2O7 (a) and Bi1.6Y0.4Ce2O7 (b) obtained by conventional sintering at 1100 °C.
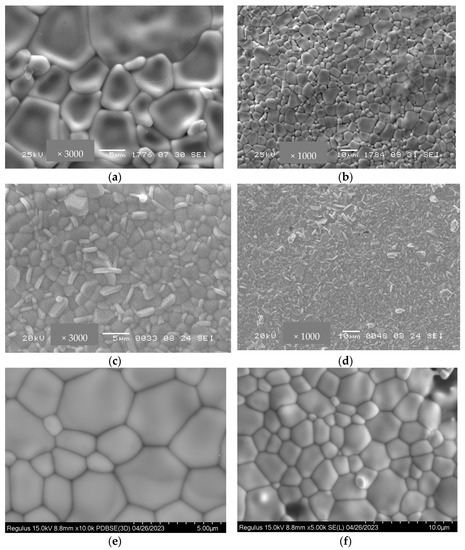
Figure 6.
SEM micrographs of Bi1.6Y0.4Ti2O7 obtained by conventional sintering at 1300 °C for 10 h (a,b), at 1100 °C for 10 h (c,d), and radiation-thermal sintering at 1100 °C for 30 min (e,f).

Table 2.
The pore parameters for Bi2−xYxM2O7 (M = Ce, Ti) samples.
3.2. Oxygen Transport Features
According to the TPIE with C18O2 data for the bismuth cerate sample, a few peaks in the TPIE curve are visible, providing evidence of strong nonuniformity of oxygen mobility (Figure 7). According to the numeric analysis, the first peak (70 °C) is related to the substitution of the surface oxygen and is characterized by a surface exchange effective activation energy of 150 kJ mol−1. The most clearly expressed peak (150 °C) is determined by fast oxygen diffusion, which involves about 1/3 of the total oxygen content. This is probably oxygen-bound cerium. The rate of substitution of the rest of the oxygen is characterized by less-expressed peaks. For the description, the model including a single diffusion channel across the most mobile oxygen of Ce–O–Ce chains with subsequent exchange with neighboring more strongly bound oxide anions in the lattice was used [48]. The mean integral exchange coefficient (β) is 0.012 min−1 for bismuth cerate. The calculated parameters of the isotope exchange are given in Table 3. The Arrhenius plots of the oxygen tracer diffusion coefficient are given in Figure 8.
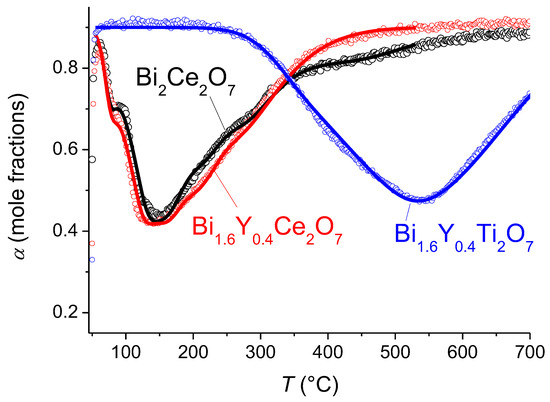
Figure 7.
Temperature-programmed isotope exchange of oxygen with C18O2 in a flow reactor for bismuth cerate and titanate samples sintered at 700 °C. Points—experiment, lines—modeling.

Table 3.
The values of surface heteroexchange rate (R), tracer diffusion coefficient normalized by mean diffusion pathway (D*/L2), bulk oxygen exchange coefficient (β) at 120 °C, and their effective activation energies (ER, ED, Eβ, respectively) calculated according to TPIE data modeling.
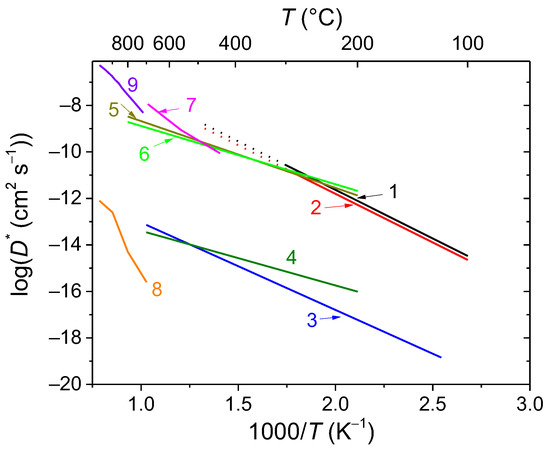
Figure 8.
Arrhenius plots for oxygen tracer diffusion coefficients acquired by TPIE data modeling for Bi2Ce2O7 (1), Bi1.6Y0.4Ce2O7 (2), and Bi1.6Y0.4Ti2O7 (3) samples sintered at 700 °C compared to other oxide materials: Bi1.6Sc0.2Ti2O7−δ (4) [26], Bi1.6Mg0.2Ti2O7−δ (5) [26], Bi1.6Zn0.2Ti2O7−δ (6) [20], Zr0.84Y0.16O1.92 (7) [49], La0.8Sr0.2MnO3−δ (8) [50], La0.5Sr0.5Fe0.7Co0.3O3−δ (9) [51].
For the Y-doped Bi cerate sample, a similar behavior of isotope substitution dynamics is observed. The same model as that for the undoped sample [48] was used. The difference in the diffusion rate via the Ce–O–Ce channel is insignificant; however, the exchange with other forms of oxygen is significantly faster. The mean integral exchange coefficient (β) is 0.017 min−1 for Y-doped bismuth cerate (Table 3).
For Y-doped Bi titanate, the oxygen substitution rate is significantly lower than that for Bi cerates. The isotope propagation rate is described by the uniform diffusion model with an identical oxygen tracer diffusion coefficient within the entire volume.
Such a difference in the oxygen mobility of doped Bi titanate and Bi cerates is probably related to the content of oxygen vacancies participating in oxide ions’ migration via M–O–M channels. The redox activity of Ce4+/Ce3+ cations [34,35,36] is probably responsible for the higher oxygen vacancies’ content for Bi cerates compared to that for Bi titanates. The possible evidence of this is the frequency and intensity of bands corresponding to the H–O–H bending in IR spectra for Bi cerates and titanates (Figure 2 and Figure 4), since these bands appear due to water adsorption with the participation of oxygen vacancies [33]:
While compared with other Bi titanate-based pyrochlores, the oxygen tracer diffusion coefficient values of Y-doped Bi titanate are slightly lower than those for Sc-doped Bi titanate and significantly lower than those for Mg- and Zn-doped Bi titanates (Figure 8, curves 4–6) previously studied by authors [18,24]. This is probably due to the difference in cation size and charge as well as A-site stoichiometry (the samples from works [20,26] are A-site deficient) and, hence, oxygen vacancy content and space in the lattice for oxygen migration.
It is difficult to compare the Bi cerate-based pyrochlores studied in this work with similar materials since there is a lack of information on the oxygen mobility of Bi cerates in the literature. However, it is comparable to or higher than that for Mg- and Zn-doped Bi titanates (Figure 8, curves 4–6) [20,26], exceeding that for commonly used ionic-conducting and MIEC materials for oxygen separation membrane components such as YSZ, LSM, and LSFC (Figure 8, curves 7–9) [49,50,51].
As mentioned in the Introduction, pyrochlore-like oxide materials can be utilized in catalytic reactors based on oxygen [1,2] and hydrogen [5,6] separation membranes for hydrogen and syngas production via fuel transformation reactions. For such reactors based on oxygen separation membranes, a high oxygen mobility (along with a high mixed ionic-electronic conductivity) allows for high oxygen permeation fluxes across the membrane from the air side onto the fuel side, thereby providing efficient performance in fuel transformation reactions [1,2,52,53,54]. For catalytic membrane reactors based on hydrogen separation membranes, high oxygen mobility is also desirable for the membrane materials. This is due to some proton transport mechanisms being mediated by oxygen transport, such as the vehicle mechanism [52,55,56], as well as the vehicular transport of structurally bound water proposed for some oxides [57,58]. Moreover, the presence of oxide-ionic conductivity in the hydrogen separation membrane allows for additional hydrogen yield due to the water splitting reaction while humidifying the purge side feed [59,60]. Finally, the application of triple (protonic—oxide-ionic—electronic) conducting materials in membrane reactors allows for enhance the reactor performance in various catalytic reactions and to improve gas separation characteristics due to coupled transport of electrons/holes, oxide anions/vacancies, and protons, forcing any of these species to be transported due to their chemical potential gradient [61,62,63].
Hence, undoped and Y-doped Bi cerates studied in the current work possessing a high oxygen mobility (Table 3, Figure 8) meet the criteria for use in oxygen separation membrane-based reactors for hydrogen and syngas production [1,2,52,53,54]. The Y-doped Bi titanate involved in this work possesses moderate oxygen mobility (Table 2, Figure 8) and may be used in oxygen separation membranes as well; however, in order to achieve a high oxygen permeation flux, additional modification and/or use as a component of composite membranes can be recommended. It is to be noted that the materials studied can probably be used in hydrogen separation membrane-based reactors as well due to their oxygen transport properties, which enable them to predict good proton transport properties [5,47,56]. However, since these materials are initially intended for potential application in oxygen separation membranes, investigating the proton transport properties of these Bi cerates and titanates is outside the scope of this work and requires a separate study.
4. Conclusions
Complex oxides Bi1.6Ti0.4Ce2O7, Bi2Ce2O7, and Bi1.6Y0.4Ce2O7 were synthesized using Pechini and citrate methods, and the structural characteristics after calcination using conventional and e-beam sintering were studied. It was shown that the formation of the pure pyrochlore phase Bi1.6Y0.4Ti2O7 occurs after high-temperature calcination at 1100 °C. In addition, the complex oxides Bi2Ce2O7 and Bi1.6Y0.4Ce2O7 have a fluorite structure with negligible amounts of Bi2O3 formed at low temperatures. As a result of calcination at high temperatures, the pyrochlore phase turns into a fluorite CeO2 phase enriched with bismuth oxide. RTS of bismuth titanate made it possible to obtain fine-grained ceramics in a minimum processing time and at a much lower temperature. The transport characteristics of pyrochlore samples were studied. It has been shown that bismuth cerates have high oxygen conductivity. Conclusions are drawn about the mechanism of oxygen diffusion in these systems. The obtained materials can potentially be used as oxygen-conducting layers of catalytic membranes.
Author Contributions
Conceptualization, Y.B. and V.S.; methodology, Y.B., E.S. (Ekaterina Sadovskaya), M.M. and M.K.; formal analysis, E.S. (Ekaterina Sadovskaya) and T.K.; investigation, T.K., O.B. and E.S. (Evgenii Suprun); data curation, E.S. (Ekaterina Sadovskaya), Y.B. and T.K.; writing—original draft preparation, N.E. and Y.B.; writing—review and editing, V.S.; visualization, E.S. (Evgenii Suprun) and T.K.; supervision, V.S.; project administration, Y.B. and V.S.; funding acquisition, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-73-00045.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shlyakhtina, A.V.; Shcherbakova, L.G. New solid electrolytes of the pyrochlore family. Russ. J. Electrochem. 2012, 48, 1–25. [Google Scholar] [CrossRef]
- Julbe, A.; Farrusseng, D.; Guizard, C. Limitations and potentials of oxygen transport dense and porous ceramic membranes for oxidation reactions. Catal. Today 2005, 104, 102–113. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Zeng, L.; He, Y.; Yu, P.; Luo, H. Effects of Bi Substitution on the cobalt-free 60wt.%Ce0.9Pr0.1O2−δ-40wt.%Pr0.6Sr0.4Fe1−xBixO3−δ oxygen transport membranes. Processes 2021, 9, 1767. [Google Scholar] [CrossRef]
- Sunarso, J.; Motuzas, J.; Liuc, S.; Diniz da Costa, J.C. Bi-doping effects on the structure and oxygen permeation properties of BaSc0.1Co0.9O3−δ perovskite membranes. J. Membr. Sci. 2010, 361, 120–125. [Google Scholar] [CrossRef]
- Phair, J.W.; Badwal, S.P.S. Materials for separation membranes in hydrogen and oxygen production and future power generation. Sci. Tech. Adv. Mater. 2006, 7, 792–805. [Google Scholar] [CrossRef]
- Phair, J.W.; Badwal, S.P.S. Review of proton conductors for hydrogen separation. Ionics 2006, 12, 103–115. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide electrolysis cells: A review. Int. J. Hydrog. Energy 2020, 45, 24203–24218. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Abrantes, J.C.C.; Gomes, E.; Lyskov, N.V.; Konysheva, E.Y.; Chernyak, S.A.; Kharitonova, E.P.; Karyagina, O.K.; Kolbanev, I.V.; Shcherbakova, L.G. Evolution of oxygen–ion and proton conductivity in Ca-doped Ln2Zr2O7 (Ln = Sm, Gd), located near pyrochlore–fluorite phase boundary. Materials 2019, 12, 2452. [Google Scholar] [CrossRef]
- Anantharaman, A.P.; Hari, P.D. Potential of pyrochlore structure materials in solid oxide fuel cell applications. Ceram. Int. 2021, 47, 4367–4388. [Google Scholar] [CrossRef]
- Zhong, F.; Yang, S.; Chen, C.; Fang, H.; Chen, K.; Zhou, C.; Lin, L.; Luo, Y.; Au, C.; Jiang, L. Defect-induced pyrochlore Pr2Zr2O7 cathode rich in oxygen vacancies for direct ammonia solid oxide fuel cells. J. Power Sources 2022, 520, 230847. [Google Scholar] [CrossRef]
- Esposito, V.; Luong, B.H.; Di Bartolomeo, E.; Wachsmann, E.D.; Traversa, E. Bi2Ru2O7 Pyrochlore electrodes for Bi2O3 based electrolyte for IT-SOFC applications. ECS Trans. 2006, 1, 263–277. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, R.; Kumar, S.; Kaur, M.; Sharma, J.D. Enhancement in the photocatalytic activity of Bi2Ti2O7 nanopowders synthesised via Pechini vs co-precipitation method. Ceram. Int. 2019, 45, 20386–20395. [Google Scholar] [CrossRef]
- Saha, D.; Madras, G.; Guru Row, T.N. Synthesis and structure of Bi2Ce2O7: A new compound exhibiting high solar photocatalytic activity. Dalton Trans. 2012, 41, 9598–9600. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sreena, T.S.; Aju Thara, T.R. Pigmentary colors from yellow to red in Bi2Ce2O7 by rare earth ion substitutions as possible high NIR reflecting pigments. Dyes Pigm. 2019, 160, 177–187. [Google Scholar] [CrossRef]
- Těšitelová, K.; Šulcová, P. Synthesis and study of Bi2Ce2O7 as inorganic pigment. J. Therm. Anal. Calorim. 2012, 125, 1047–1052. [Google Scholar] [CrossRef]
- Hardy, A.; Van Elshocht, S.; Hadermann, J.; Pourtois, G.; De Gendt, S.; Afanas’ev, V.V.; Van Bael, M.K. Properties and thermal stability of solution processed ultrathin, high-k bismuth titanate (Bi2Ti2O7) films. Mater. Res. Bull. 2012, 47, 511–517. [Google Scholar] [CrossRef]
- Cho, K.H.; Kang, M.G.; Jang, H.W.; Shin, H.Y.; Kang, C.Y.; Yoon, S.J. Significantly reduced leakage currents in organic thin film transistors with Mn-doped Bi2Ti2O7 high-k gate dielectrics. Phys. Status Solidi-Rapid Res. Lett. 2012, 6, 208–210. [Google Scholar] [CrossRef]
- Chezhina, N.V.; Piir, I.V.; Krasnov, A.G.; Koroleva, M.S.; Kellerman, D.G.; Semenov, V.G.; Shalaeva, E.V.; Leonidov, I.O.; Shein, I.R. Structure and magnetic properties of a nanosized iron-doped bismuth titanate pyrochlore. Inorg. Chem. 2022, 61, 13369–13378. [Google Scholar] [CrossRef]
- Díaz-guillén, J.A.; Díaz-guillén, M.R.; Padmasree, K.P.; Fuentes, A.F.; Santamaría, J.; León, C. High ionic conductivity in the pyrochlore-type Gd2−yLayZr2O7 solid solution (0 ≤ y ≤ 1). Solid State Ion. 2008, 179, 2160–2164. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Koroleva, M.S.; Piir, I.V.; Chezhina, N.V.; Korolev, D.A.; Skriabin, P.I.; Krasnov, A.V.; Sadovskaya, E.M.; Eremeev, N.F.; Nekipelov, S.V.; et al. Structural and transport properties of doped bismuth titanates and niobates. Solid State Ion. 2018, 315, 33–39. [Google Scholar] [CrossRef]
- Talanov, M.V.; Talanov, V.M. Structural diversity of ordered pyrochlores. Chem. Mater. 2021, 33, 2706–2725. [Google Scholar] [CrossRef]
- Kocevski, V.; Pilania, G.; Uberuaga, B.P. Modeling disorder in pyrochlores and other anion-deficient fluorite structural derivative oxides. Front. Chem. 2021, 9, 712543. [Google Scholar] [CrossRef] [PubMed]
- Talanov, M.V.; Talanov, V.M. Formation of breathing pyrochlore lattices: Structural, thermodynamic and crystal chemical aspects. CrystEngComm. 2020, 2, 1176–1187. [Google Scholar] [CrossRef]
- Lang, M.; Zhang, F.; Zhang, J.; Wang, J.; Lian, J.; Weber, W.J.; Schuster, B.; Trautmann, C.; Neumann, R.; Ewing, R.C. Review of A2B2O7 pyrochlore response to irradiation and pressure. Nucl. Instrum. Methods Phys. Res. B Nucl. Instrum. Meth. B 2010, 268, 2951–2959. [Google Scholar] [CrossRef]
- Cai, L.; Arias, A.L.; Nino, J.C. The tolerance factors of the pyrochlore crystal structure. J. Mater. Chem. 2011, 2, 3611–3618. [Google Scholar] [CrossRef]
- Krasnov, A.G.; Piir, I.V.; Koroleva, M.S.; Sekushin, N.A.; Ryabkov, Y.I.; Piskaykina, M.M.; Sadykov, V.A.; Sadovskaya, E.M.; Pelipenko, V.V.; Eremeev, N.F. The conductivity and ionic transport of doped bismuth titanate pyrochlore Bi1.6MxTi2O7−δ (M—Mg, Sc, Cu). Solid State Ion. 2017, 302, 118–125. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Pigalskiy, K.S.; Belov, D.A.; Lyskov, N.V.; Kharitonova, E.P.; Kolbanev, I.V.; Borunova, A.B.; Karyagina, O.K.; Sadovskaya, E.M.; Sadykov, V.A.; et al. Proton and oxygen ion conductivity in the pyrochlore/fluorite family of Ln2−xCaxScMO7−δ (Ln = La, Sm, Ho, Yb; M = Nb, Ta; x = 0, 0.05, 0.1) niobates and tantalates. Dalton Trans. 2018, 47, 2376–2392. [Google Scholar] [CrossRef]
- Sadykov, V.; Shlyakhtina, A.; Lyskov, N.; Sadovskaya, E.; Cherepanova, S.; Eremeev, N.; Skazka, V.; Goncharov, V.; Kharitonova, E. Oxygen diffusion in Mg-doped Sm and Gd zirconates with pyrochlore structure. Ionics 2020, 26, 4621–4633. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V. Morphotropy, isomorphism, and polymorphism of Ln2M2O7-based (Ln = La-Lu, Y, Sc; M = Ti, Zr, Hf, Sn) oxides. Cryst. Rep. 2013, 58, 548–562. [Google Scholar] [CrossRef]
- Huo, D.; Baldinozzi, G.; Siméone, D.; Khodja, H.; Surblé, S. Grain size—Dependent electrical properties of La1.95Sr0.05Zr2O7−δ as potential proton ceramic fuel cell electrolyte. Solid State Ion. 2016, 298, 35–43. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, J.R.; Hinojosa, B.B.; Nino, J.C.; Esquivel-Elizondo, J.R. Bi2Ti2O7: It is not what you have read. Chem. Mater. 2011, 23, 4965–4974. [Google Scholar] [CrossRef]
- Krasnov, A.V.; Shein, I.R.; Piir, I.V.; Ryabkov, Y.I. Bismuth titanate pyrochlores doped by alkaline earth elements: First-principles calculations and experimental study. Solid State Ion. 2018, 317, 183–189. [Google Scholar] [CrossRef]
- Anu; Yadav, K.; Gaur, A.; Haldar, K.K. Effect of oxygen vacancies, lattice distortions and secondary phase on the structural, optical, dielectric and ferroelectric properties in Cd-doped Bi2Ti2O7 nanoparticles. Mater. Res. Bull. 2021, 141, 111373. [Google Scholar] [CrossRef]
- Uniyal, S.; Atri, S.; Uma, S.; Nagarajan, R. Microstructural changes caused by Ba and Pr doping in nanosized Bi2Ce2O7 leading to interesting optical, magnetic, and catalytic property. CrystEngComm. 2021, 33, 986–999. [Google Scholar] [CrossRef]
- Li, G.; Mao, Y.; Li, L.; Feng, S.; Wang, M.; Yao, X. Solid solubility and transport properties of nanocrystalline (CeO2)1−x(BiO1.5)x by hydrothermal conditions. Chem. Mater. 1999, 11, 1259–1266. [Google Scholar] [CrossRef]
- Li, Z.C.; Zhang, H.; Bergman, B. Synthesis and characterization of nanostructured Bi2O3-doped cerium oxides fabricated by PVA polymerization process. Ceram. Int. 2008, 34, 1949–1953. [Google Scholar] [CrossRef]
- Sadykov, V.; Bespalko, Y.; Sadovskaya, E.; Krieger, T.; Belyaev, V.; Eremeev, N.; Mikhailenko, M.; Bryazgin, A.; Korobeynikov, M.; Ulihin, A.; et al. Structural and transport properties of e-beam sintered lanthanide tungstates and tungstates-molybdates. Nanomaterials 2022, 12, 3282. [Google Scholar] [CrossRef]
- Lysenko, E.; Vlasov, V.; Nikolaev, E.; Surzhikov, A.; Ghyngazov, S. Technological Aspects of Lithium-Titanium Ferrite Synthesis by Electron-Beam Heating. Materials 2023, 16, 604. [Google Scholar] [CrossRef]
- Ghyngazov, S. Zirconia ceramics processing by intense electron and ion beams. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 435, 190–193. [Google Scholar] [CrossRef]
- Sadykov, V.; Usoltsev, V.; Fedorova, Y.; Mezentseva, N.; Krieger, T.; Eremeev, N.; Arapova, M.; Ishchenko, A.; Salanov, A.; Pelipenko, V.; et al. Advanced Sintering Techniques in Design of Planar IT SOFC and Supported Oxygen Separation Membranes. In Sintering of Ceramics-New Emerging Technologies, 1st ed.; Lakshmanan, A., Ed.; InTech-Open: Rijeka, Croatia, 2012; pp. 121–140. [Google Scholar]
- Sadykov, V.; Usoltsev, V.; Yeremeev, N.; Mezentseva, N.; Pelipenko, V.; Krieger, T.; Belyaev, V.; Sadovskaya, E.; Muzykantov, V.; Fedorova, Y.; et al. Functional Nanoceramics for Intermediate Temperature Solid Oxide Fuel Cells and Oxygen Separation Membranes. J. Europ. Ceram. Soc. 2013, 33, 2241–2250. [Google Scholar] [CrossRef]
- Sadykov, V.; Sadovskaya, E.; Eremeev, N.; Skazka, V.; Goncharov, V. 2D diffusion of oxygen in Ln10Mo2O21 (Ln = Nd, Ho) oxides. Solid State Ion. 2020, 346, 115229. [Google Scholar] [CrossRef]
- Stoyanovskii, V.O.; Vedyagin, A.A.; Volodin, A.M.; Bespalko, Y.N. Effect of carbon shell on stabilization of single-phase lanthanum and praseodymium hexaaluminates prepared by a modified Pechini method. Ceram. Int. 2020, 46, 29150–29159. [Google Scholar] [CrossRef]
- Henderson, S.J.; Shebanova, O.; Hector, A.L.; McMillan, P.F.; Weller, M.T. Structural Variations in pyrochlore-structured Bi2Hf2O7, Bi2Ti2O7 and Bi2Hf2−xTixO7 solid solutions as a function of composition and temperature by neutron and X-ray diffraction and Raman spectroscopy. Chem. Mater. 2007, 19, 1712–1722. [Google Scholar] [CrossRef]
- Fang, W.; Zhou, L.; Shen, B.; Zhou, Y.; Yi, Q.; Xing, M.; Zhang, J. Advanced Bi2O2.7/Bi2Ti2O7 composite film with enhanced visible-light-driven activity for the degradation of organic dyes. Res. Chem. Intermed. 2018, 44, 4609–4618. [Google Scholar] [CrossRef]
- Prekajski, M.; Fruth, V.; Andronescu, C.; Trandafilović, L.; Pantić, J.; Kremenović, A.; Matović, B. Thermal stability of Ce1−xBixO2−δ (x = 0.1 – 0.5) solid solution. J. Alloys Comp. 2013, 578, 26–31. [Google Scholar] [CrossRef]
- Leite, E.R.; Nobre, M.A.L.; Cerqueira, M.; Longo, E.; Valera, J.A. Particle growth during calcination of polycation oxides synthesized by the polymeric precursors method. J. Am. Ceram. Soc. 1997, 80, 2649–2657. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Filonova, E.A.; Eremeev, N.F.; Belyaev, V.D.; Tsvinkinberg, V.A.; Pikalova, E.Y. Oxide ionic transport features in Gd-doped La nickelates. Solid State Ion. 2020, 357, 115462. [Google Scholar] [CrossRef]
- Kilner, J.A. Fast oxygen transport in acceptor doped oxides. Solid State Ion. 2000, 129, 13–23. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A.; Walker, J.F. A SIMS study of oxygen tracer diffusion and surface exchange in La0.8Sr0.2MnO3+δ. Mater. Lett. 2000, 43, 43–52. [Google Scholar] [CrossRef]
- Geffroy, P.-M.; Blond, E.; Richet, N.; Chartier, T. Understanding and identifying the oxygen transport mechanisms through a mixed-conductor membrane. Chem. Eng. Sci. 2017, 162, 245–261. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Dewangan, N.; Li, Z.; Das, S.; Pati, S.; Li, Z.; Lin, J.Y.S.; Kawi, S. Catalytic mixed conducting ceramic membrane reactors for methane conversion. React. Chem. Eng. 2020, 5, 1868–1891. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Gao, B. Ceramic membranes originated from cost-effective and abundant natural minerals and industrial wastes for broad applications—A review. Desalin. Water Treat. 2020, 201, 121–138. [Google Scholar] [CrossRef]
- Han, N.; Shen, Z.; Zhao, X.; Chen, R.; Thakur, V.K. Perovskite oxides for oxygen transport: Chemistry and material horizons. Sci. Total Environ. 2022, 806, 151213. [Google Scholar] [CrossRef] [PubMed]
- Poetzsch, D.; Merkle, R.; Maier, J. Proton conductivity in mixed-conducting BSFZ perovskite from thermogravimetric relaxation. Phys. Chem. Chem. Phys. 2014, 16, 16446–16453. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. Dual-phase mixed protonic-electronic conducting hydrogen separation membranes: A review. Membranes 2022, 12, 647. [Google Scholar] [CrossRef] [PubMed]
- Animitsa, I.; Neiman, A.; Sharafutdinov, A.; Nochrin, S. Strontium tantalates with perovskite-related structure. Solid State Ion. 2000, 136–137, 265–271. [Google Scholar] [CrossRef]
- Stub, S.Ø.; Vøllestad, E.; Norby, T. Mechanisms of protonic surface transport in porous oxides: Example of YSZ. J. Phys. Chem. C 2017, 121, 12817–12825. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Haugsrud, R. On the ionic character of H2 separation through mixed conducting Nd5.5W0.5Mo0.5O11.25−δ membrane. Int. J. Hydrog. Energy 2017, 42, 11392–11399. [Google Scholar] [CrossRef]
- Papac, M.; Stevanović, V.; Zakutayev, A.; O’Hayre, R. Triple ionic–electronic conducting oxides for next-generation electrochemical devices. Nat. Mater. 2020, 20, 301–313. [Google Scholar] [CrossRef]
- Virkar, A.V. Transport of H2, O2 and H2O through single-phase, two-phase and multi-phase mixed proton, oxygen ion, and electron hole conductors. Solid State Ion. 2001, 140, 275–283. [Google Scholar] [CrossRef]
- Sanders, M.D.; O’Hayre, R.P. Coupled transport and uphill permeation of steam and oxygen in a dense ceramic membrane. J. Membr. Sci. 2011, 376, 96–101. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).