Different Approaches for the Preparation of Composite Ionic Liquid-Based Membranes for Proton Exchange Membrane Fuel Cell Applications—Recent Advancements
Abstract
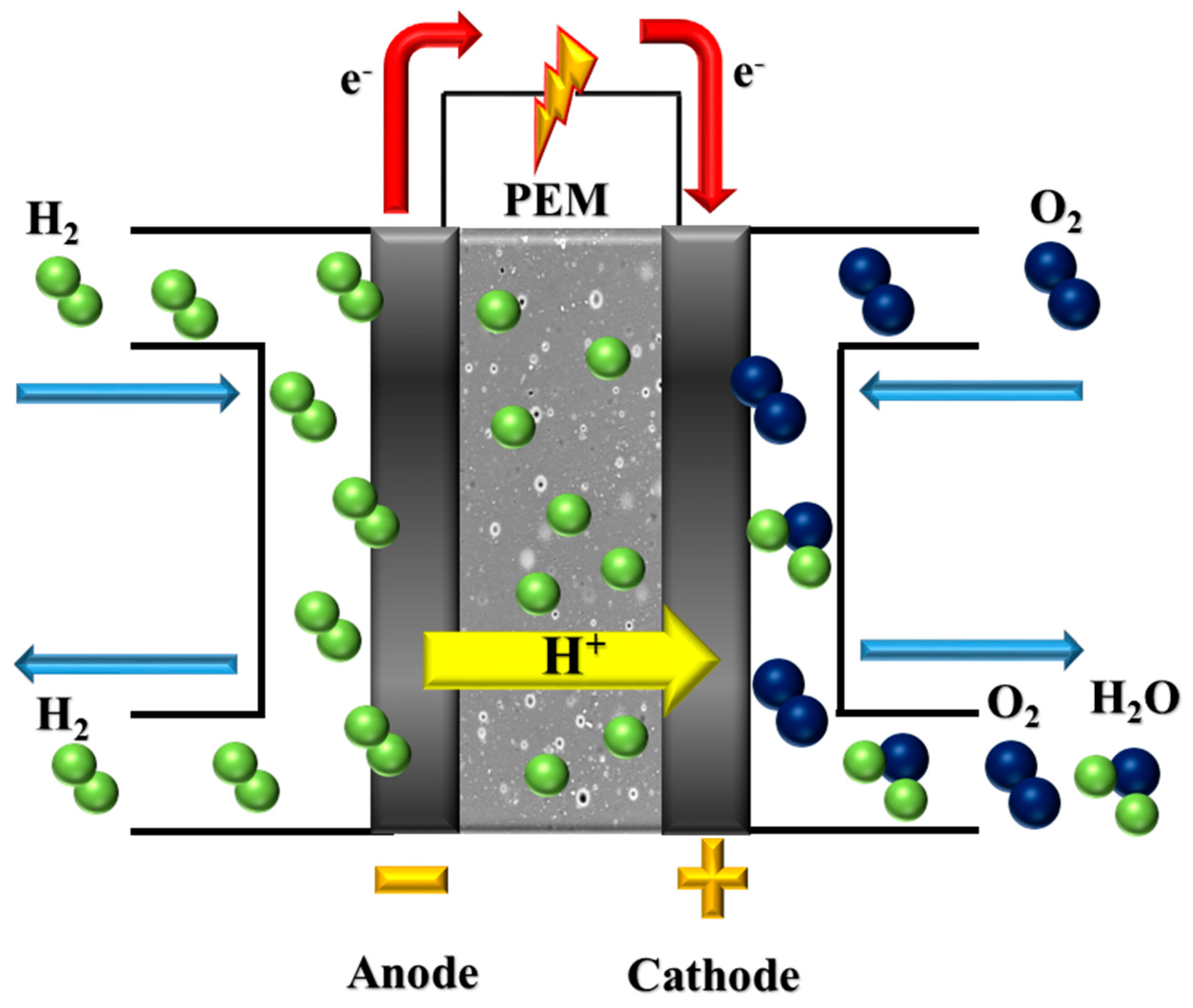
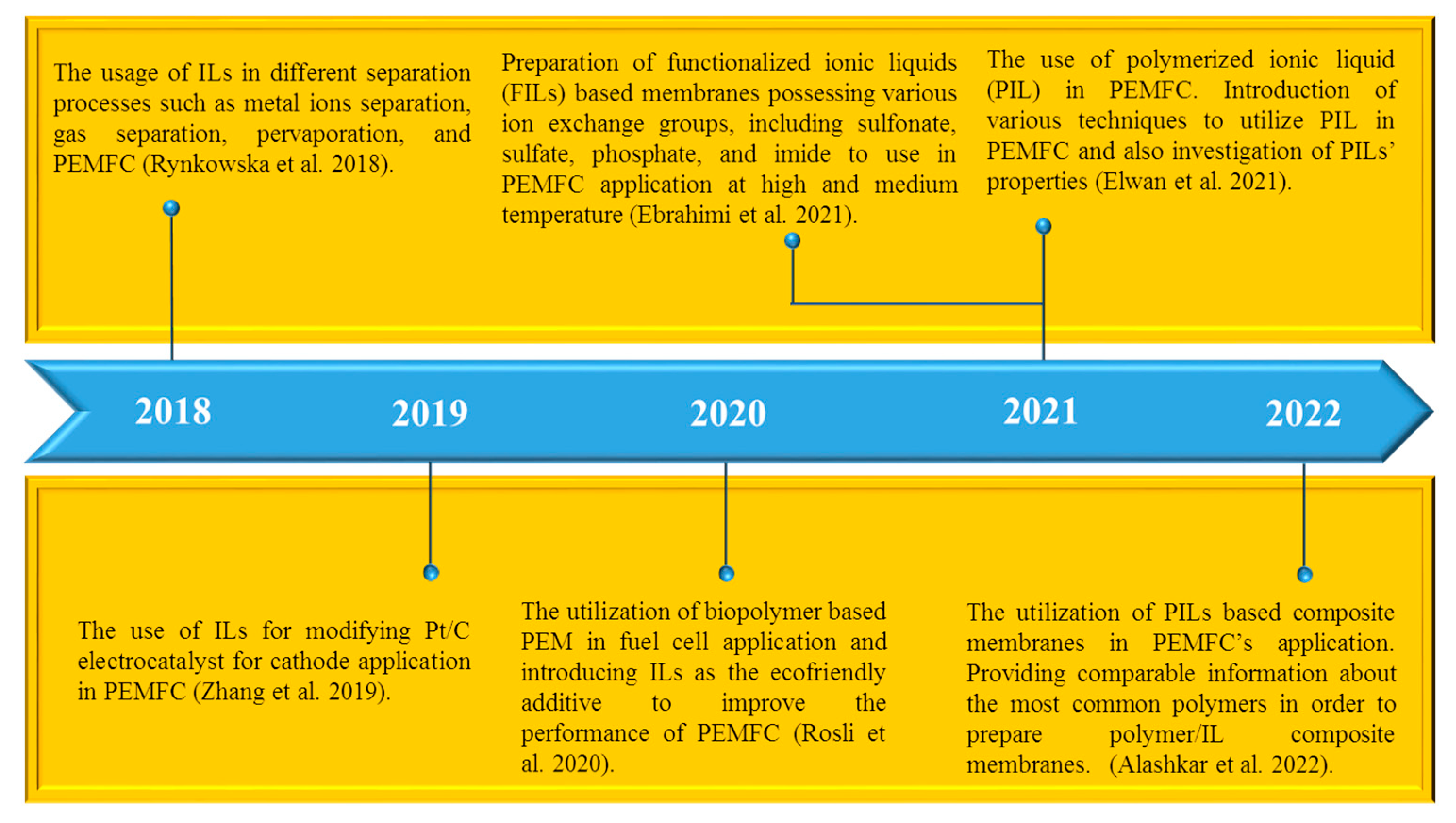
1. Introduction
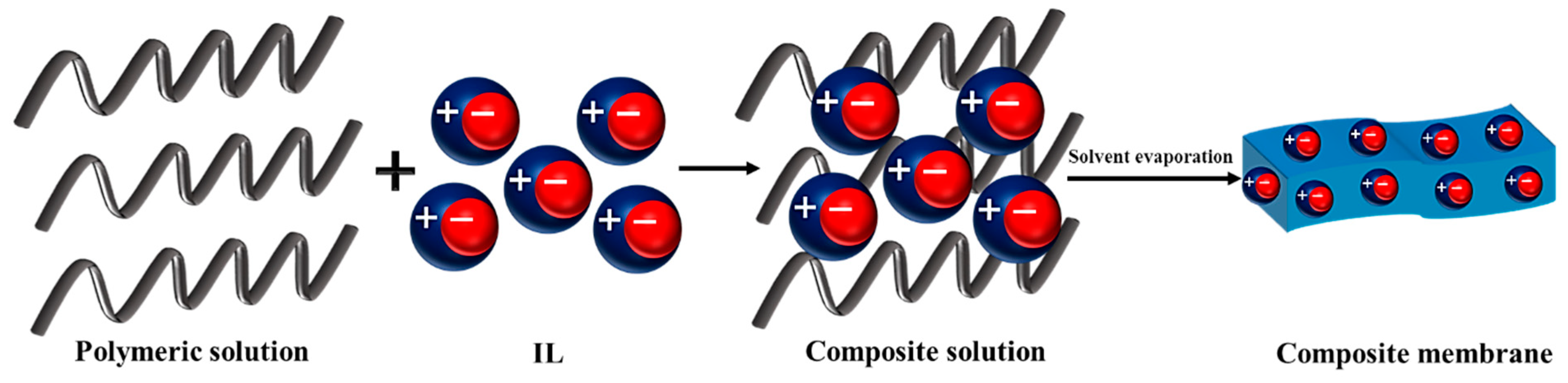
2. Incorporation of IL into a Polymer Solution
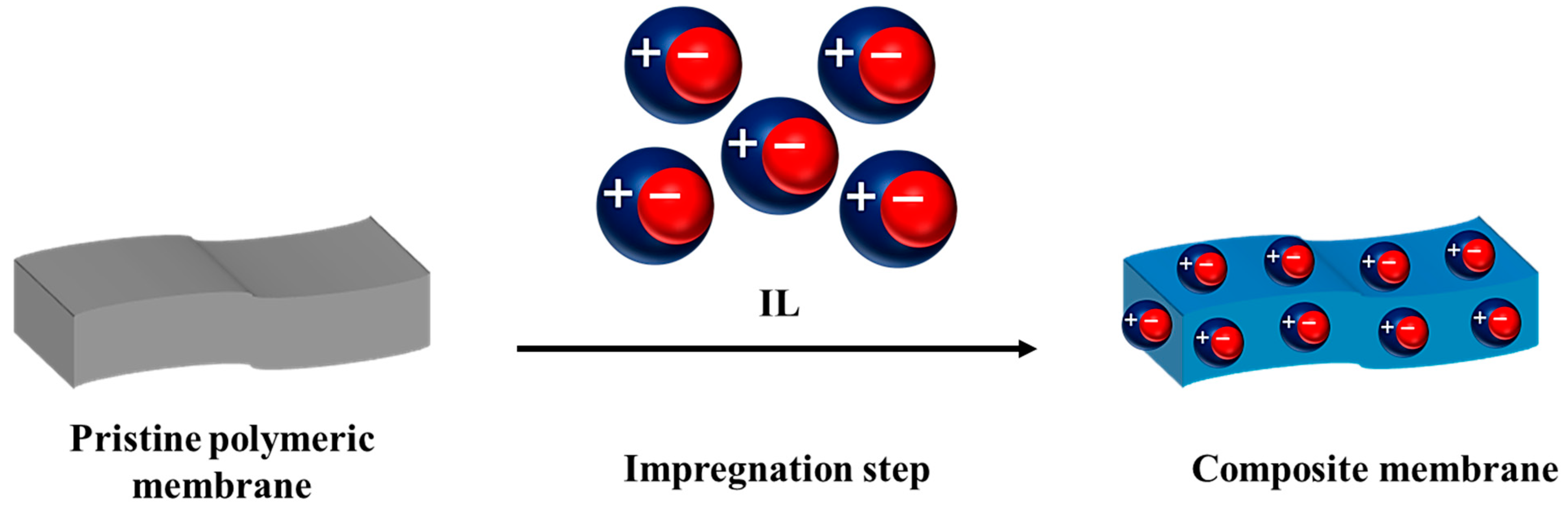
3. Impregnation of the Polymer with IL
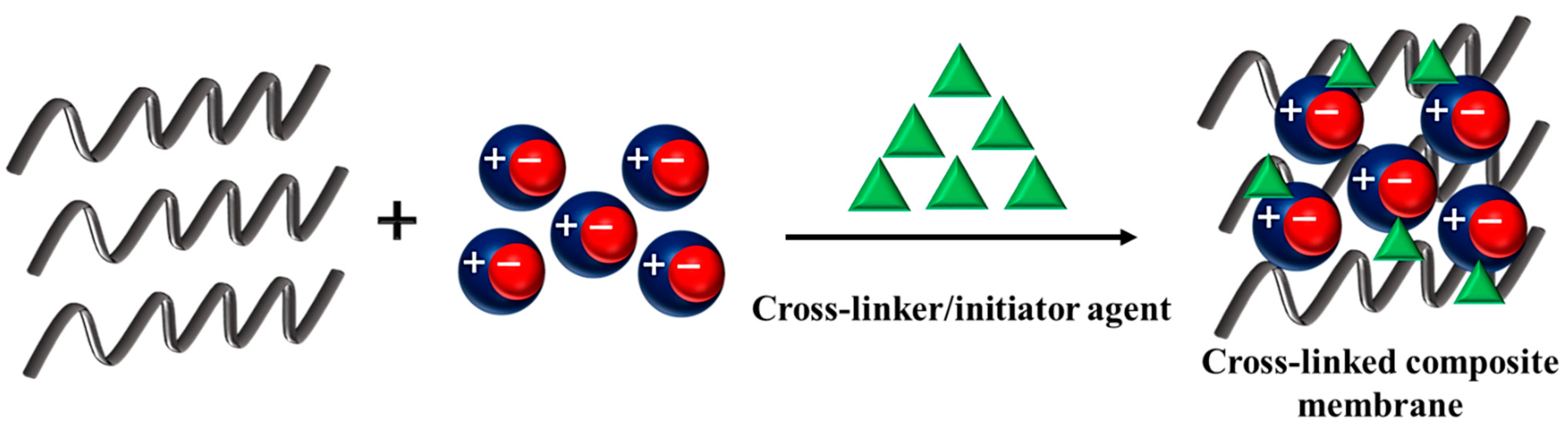
4. Cross-Linking
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFC | Alkaline fuel cell |
| AIBN | Azobis-(isobutyronitrile) |
| DMA | N,N-dimethylacetamide |
| DMAC | Dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMFC | Direct methanol fuel cell |
| DMSO | Dimethyl sulfoxide |
| ETFE | Ethylene-co-tetrafluoroethylene |
| fd | Freeze drying |
| FFC | Field flow channel |
| FIL | Functionalized ionic liquid |
| FTIR | Fourier-transform infrared spectroscopy |
| GDL | Gas diffusion layer |
| hPFSVE | Hydrolyzed perfluoro-3,6-dioxa-4-methyl-7-octene sulfonyl fluoride |
| IEC | Ion exchange capacity |
| IL | Ionic liquid |
| LbL | Layer by layer |
| MCFC | Molten carbonate fuel cell |
| Meq | Milliequivalent |
| MMA | Methyl methacrylate |
| MOF | Metal organic framework |
| Nb | Norbornene |
| NMP | N-methyl-2-pyrrolidinone |
| NMPC | N-methylene phosphonic chitosan |
| OCNT | Carbon nanotube oxide |
| PA | Phosphoric acid |
| PAFC | Phosphoric acid fuel cell |
| PAN | Propyl ammonium nitrate |
| PBI | Polybenzimidazole |
| PEM | Polymer electrolyte membrane |
| PEMFC | Proton exchange membrane fuel cell |
| PI | Polyimide |
| PIL | Polymerized ionic liquid |
| PrIL | Protic ionic liquid |
| PSAN | Poly(styrene/acrylonitrile) |
| PSP | Perfluoro-sulfonated polymers |
| PTFE | Polytetrafluoroethylene |
| PU | Polyurethane |
| PVA | Poly(vinyl alcohol) |
| PVP | Polyvinylpyrrolidone |
| RH | Relative humidity |
| SBA-15 | Santa Barbara Amorphous-15 |
| SBES | Polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene |
| SEM | Scanning electron microscope |
| SILM | Supported ionic liquid membrane |
| SiO2 | Silicon dioxide |
| SOFC | Solid oxide fuel cell |
| SPEEK | Sulfonated poly ether ether ketone |
| SPSU | Sulfonated polysulfone |
| TGA | Thermogravimetric analysis |
| TIL | Triazole-based IL |
| ZrP | Zirconium phosphate |
References
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Derwent, R. Global warming consequences of replacing natural gas with hydrogen in the domestic energy sectors of future low-carbon economies in the United Kingdom and the United States of America. Int. J. Hydrog. Energy 2021, 46, 30190–30203. [Google Scholar] [CrossRef]
- Asiedu, B.A.; Hassan, A.A.; Bein, M.A. Renewable energy, non-renewable energy, and economic growth: Evidence from 26 European countries. Environ. Sci. Pollut. Res. 2021, 28, 11119–11128. [Google Scholar] [CrossRef] [PubMed]
- Cevik, E.I.; Yıldırım, D.Ç.; Dibooglu, S. Renewable and non-renewable energy consumption and economic growth in the US: A Markov-Switching VAR analysis. Energy Environ. 2020, 32, 519–541. [Google Scholar] [CrossRef]
- Montoya, M.A.; Allegretti, G.; Sleimann Bertussi, L.A.; Talamini, E. Renewable and Non-renewable in the energy-emissions-climate nexus: Brazilian contributions to climate change via international trade. J. Clean. Prod. 2021, 312, 127700. [Google Scholar] [CrossRef]
- Sharma, P.; Jamwal, A.; Sharma, N.; Agrawal, R. Opportunities and Issues with Clean Renewable Energy Development in India: A Review. In Advances in Fluid and Thermal Engineering; Springer: Singapore, 2021; pp. 527–537. [Google Scholar]
- Tanaka, S.; Nagumo, K.; Yamamoto, M.; Chiba, H.; Yoshida, K.; Okano, R. Fuel cell system for Honda CLARITY fuel cell. ETransportation 2020, 3, 100046. [Google Scholar] [CrossRef]
- Karaaslan, A.; Çamkaya, S. The relationship between CO2 emissions, economic growth, health expenditure, and renewable and non-renewable energy consumption: Empirical evidence from Turkey. Renew. Energy 2022, 190, 457–466. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Sher, F.; Curnick, O.; Azizan, M.T. Sustainable conversion of renewable energy sources. Sustainability 2021, 13, 2940. [Google Scholar] [CrossRef]
- Bundschuh, J.; Kaczmarczyk, M.; Ghaffour, N.; Tomaszewska, B. State-of-the-art of renewable energy sources used in water desalination: Present and future prospects. Desalination 2021, 508, 115035. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Abdelkareem, M.A. Fuel cell application in the automotive industry and future perspective. Energy 2021, 214, 118955. [Google Scholar] [CrossRef]
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Du, Z.; Liu, C.; Zhai, J.; Guo, X.; Xiong, Y.; Su, W.; He, G. A Review of Hydrogen Purification Technologies for Fuel Cell Vehicles. Catalysts 2021, 11, 393. [Google Scholar] [CrossRef]
- Fan, L.; Tu, Z.; Chan, S.H. Recent development of hydrogen and fuel cell technologies: A review. Energy Rep. 2021, 7, 8421–8446. [Google Scholar] [CrossRef]
- Muthukumar, M.; Rengarajan, N.; Velliyangiri, B.; Omprakas, M.; Rohit, C.; Raja, U.K. The development of fuel cell electric vehicles—A review. Mater. Today Proc. 2021, 45, 1181–1187. [Google Scholar] [CrossRef]
- Sazali, N.; Wan Salleh, W.N.; Jamaludin, A.S.; Mhd Razali, M.N. New perspectives on fuel cell technology: A brief review. Membranes 2020, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Hemmat Esfe, M.; Afrand, M. A review on fuel cell types and the application of nanofluid in their cooling. J. Therm. Anal. Calorim. 2020, 140, 1633–1654. [Google Scholar] [CrossRef]
- Haider, R.; Wen, Y.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Selection of proton exchange membrane fuel cell for transportation. Int. J. Hydrog. Energy 2021, 46, 30625–30640. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Prasad, A.K.; Advani, S.G. High durability sulfonated poly (ether ether ketone)-ceria nanocomposite membranes for proton exchange membrane fuel cell applications. J. Membr. Sci. 2018, 556, 12–22. [Google Scholar] [CrossRef]
- Wang, X.X.; Swihart, M.T.; Wu, G. Achievements, challenges and perspectives on cathode catalysts in proton exchange membrane fuel cells for transportation. Nat. Catal. 2019, 2, 578–589. [Google Scholar] [CrossRef]
- Bhosale, A.C.; Ghosh, P.C.; Assaud, L. Preparation methods of membrane electrode assemblies for proton exchange membrane fuel cells and unitized regenerative fuel cells: A review. Renew. Sustain. Energy Rev. 2020, 133, 110286. [Google Scholar] [CrossRef]
- Liu, Q.; Lan, F.; Chen, J.; Zeng, C.; Wang, J. A review of proton exchange membrane fuel cell water management: Membrane electrode assembly. J. Power Sources 2022, 517, 230723. [Google Scholar] [CrossRef]
- Shangguan, Z.; Li, B.; Ming, P.; Zhang, C. Understanding the functions and modifications of interfaces in membrane electrode assemblies of proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 15111–15139. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K.; Kujawa, J. A review on ionic liquids-based membranes for middle and high temperature polymer electrolyte membrane fuel cells (PEM FCs). Int. J. Mol. Sci. 2021, 22, 5430. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, P.C.; Belgacem, I.B.; Emori, W.; Uzoma, P.C. Nafion degradation mechanisms in proton exchange membrane fuel cell (PEMFC) system: A review. Int. J. Hydrog. Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Oh, K.; Kwon, O.; Son, B.; Lee, D.H.; Shanmugam, S. Nafion-sulfonated silica composite membrane for proton exchange membrane fuel cells under operating low humidity condition. J. Membr. Sci. 2019, 583, 103–109. [Google Scholar] [CrossRef]
- Elumalai, V.; Ganesh, T.; Selvakumar, C.; Sangeetha, D. Phosphonate ionic liquid immobilised SBA-15/SPEEK composite membranes for high temperature proton exchange membrane fuel cells. Mater. Sci. Energy Technol. 2018, 1, 196–204. [Google Scholar] [CrossRef]
- Saniei, N.; Ghasemi, N.; Zinatizadeh, A.; Zinadini, S.; Ramezani, M.; Derakhshan, A. Electricity generation enhancement in microbial fuel cell via employing a new SPEEK based proton exchange membrane modified by goethite nanoparticles functionalized with tannic acid and sulfanilic acid. Environ. Technol. Innov. 2022, 25, 102168. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A critical review on the use of ionic liquids in proton exchange membrane fuel cells. Membranes 2022, 12, 178. [Google Scholar]
- Elwan, H.A.; Mamlouk, M.; Scott, K. A review of proton exchange membranes based on protic ionic liquid/polymer blends for polymer electrolyte membrane fuel cells. J. Power Sources 2021, 484, 229197. [Google Scholar]
- Elwan, H.A.; Thimmappa, R.; Mamlouk, M.; Scott, K. Applications of poly ionic liquids in proton exchange membrane fuel cells: A review. J. Power Sources 2021, 510, 230371. [Google Scholar]
- Niu, B.; Luo, S.; Lu, C.; Yi, W.; Liang, J.; Guo, S.; Wang, D.; Zeng, F.; Duan, S.; Liu, Y.; et al. Polybenzimidazole and ionic liquid composite membranes for high temperature polymer electrolyte fuel cells. Solid State Ion. 2021, 361, 115569. [Google Scholar]
- Kesava, M.; Dinakaran, K. SnO2 nanoparticles dispersed carboxylated Poly (arylene ether sulfones) nanocomposites for proton exchange membrane fuel cell (PEMFC) applications. Int. J. Hydrog. Energy 2021, 46, 1121–1132. [Google Scholar]
- Li, X.; Ma, H.; Wang, P.; Liu, Z.; Peng, J.; Hu, W.; Jiang, Z.; Liu, B. Construction of high-performance, high-temperature proton exchange membranes through incorporating SiO2 nanoparticles into novel cross-linked polybenzimidazole networks. ACS Appl. Mater. Interfaces 2019, 11, 30735–30746. [Google Scholar] [CrossRef]
- Esmaeili, N.; Gray, E.M.; Webb, C.J. Non-Fluorinated Polymer Composite Proton Exchange Membranes for Fuel Cell Applications—A Review. ChemPhysChem 2019, 20, 2016–2053. [Google Scholar] [PubMed]
- Li, G.; Kujawski, W.; Rynkowska, E. Advancements in proton exchange membranes for high-performance high-temperature proton exchange membrane fuel cells (HT-PEMFC). Rev. Chem. Eng. 2022, 38, 327–346. [Google Scholar]
- Goh, J.T.E.; Abdul Rahim, A.R.; Masdar, M.S.; Shyuan, L.K. Enhanced Performance of Polymer Electrolyte Membranes via Modification with Ionic Liquids for Fuel Cell Applications. Membranes 2021, 11, 395. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [PubMed]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar]
- Flieger, J.; Flieger, M. Ionic Liquids Toxicity—Benefits and Threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar]
- Nguyen, V.; Blum, L. Reversible fuel cells. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 115–145. [Google Scholar]
- Gabbasa, M.; Sopian, K.; Fudholi, A.; Asim, N. A review of unitized regenerative fuel cell stack: Material, design and research achievements. Int. J. Hydrog. Energy 2014, 39, 17765–17778. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar]
- Zhang, H.; Liang, J.; Xia, B.; Li, Y.; Du, S. Ionic liquid modified Pt/C electrocatalysts for cathode application in proton exchange membrane fuel cells. Front. Chem. Sci. Eng. 2019, 13, 695–701. [Google Scholar]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of chitosan-based polymers as proton exchange membranes and roles of chitosan-supported ionic liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef] [PubMed]
- da Trindade, L.G.; Zanchet, L.; Souza, J.C.; Leite, E.R.; Martini, E.M.A.; Pereira, E.C. Enhancement of sulfonated poly(ether ether ketone)-based proton exchange membranes doped with different ionic liquids cations. Ionics 2020, 26, 5661–5672. [Google Scholar] [CrossRef]
- da Trindade, L.G.; Borba, K.M.; Zanchet, L.; Lima, D.W.; Trench, A.B.; Rey, F.; Diaz, U.; Longo, E.; Bernardo-Gusmão, K.; Martini, E.M. SPEEK-based proton exchange membranes modified with MOF-encapsulated ionic liquid. Mater. Chem. Phys. 2019, 236, 121792. [Google Scholar]
- YILMAZOĞLU, M.; KORKMAZ, Ş. Development of 1, 2, 3-Triazole Based Ionic Liquid Doped Sulfonated Polysulfone (SPSU) Electrolytes for Anhydrous Proton Exchange Membrane Applications. El-Cezeri 2022, 9, 584–597. [Google Scholar]
- Rogalsky, S.; Bardeau, J.-F.; Makhno, S.; Tarasyuk, O.; Babkina, N.; Cherniavska, T.; Filonenko, M.; Fatyeyeva, K. New polymer electrolyte membrane for medium-temperature fuel cell applications based on cross-linked polyimide Matrimid and hydrophobic protic ionic liquid. Mater. Today Chem. 2021, 20, 100453. [Google Scholar] [CrossRef]
- da Trindade, L.G.; Zanchet, L.; Martins, P.C.; Borba, K.M.; Santos, R.D.; Paiva, R.d.S.; Vermeersch, L.A.; Ticianelli, E.A.; de Souza, M.O.; Martini, E.M. The influence of ionic liquids cation on the properties of sulfonated poly (ether ether ketone)/polybenzimidazole blends applied in PEMFC. Polymer 2019, 179, 121723. [Google Scholar] [CrossRef]
- Skorikova, G.; Rauber, D.; Aili, D.; Martin, S.; Li, Q.; Henkensmeier, D.; Hempelmann, R. Protic ionic liquids immobilized in phosphoric acid-doped polybenzimidazole matrix enable polymer electrolyte fuel cell operation at 200 °C. J. Membr. Sci. 2020, 608, 118188. [Google Scholar]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Lee, T.K.; Ahmad, A. Phosphorylated chitosan/poly (vinyl alcohol) based proton exchange membranes modified with propylammonium nitrate ionic liquid and silica filler for fuel cell applications. Int. J. Hydrog. Energy 2022, 47, 19217–19236. [Google Scholar] [CrossRef]
- Escorihuela, J.; García-Bernabé, A.; Montero, Á.; Sahuquillo, Ó.; Giménez, E.; Compañ, V. Ionic liquid composite polybenzimidazol membranes for high temperature PEMFC applications. Polymers 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fernández, I.; Raghibi, M.; Bouzina, A.; Timperman, L.; Bigarré, J.; Anouti, M. Protic ionic liquids/poly (vinylidene fluoride) composite membranes for fuel cell application. J. Energy Chem. 2021, 53, 197–207. [Google Scholar] [CrossRef]
- Nair, M.G.; Mohapatra, S.R.; Garda, M.-R.; Patanair, B.; Saiter-Fourcin, A.; Thomas, S. Role of protic ionic liquid concentration in proton conducting polymer electrolytes for improved electrical and thermal properties. Mater. Res. Express 2020, 7, 064005. [Google Scholar] [CrossRef]
- Zanchet, L.; da Trindade, L.G.; Bariviera, W.; Nobre Borba, K.M.; Santos, R.D.M.; Paganin, V.A.; de Oliveira, C.P.; Ticianelli, E.A.; Martini, E.M.A.; de Souza, M.O. 3-Triethylammonium propane sulfonate ionic liquids for Nafion-based composite membranes for PEM fuel cells. J. Mater. Sci. 2020, 55, 6928–6941. [Google Scholar] [CrossRef]
- Fatyeyeva, K.; Rogalsky, S.; Makhno, S.; Tarasyuk, O.; Soto Puente, J.A.; Marais, S. Polyimide/ionic liquid composite membranes for middle and high temperature fuel cell application: Water sorption behavior and proton conductivity. Membranes 2020, 10, 82. [Google Scholar]
- Dahi, A.; Fatyeyeva, K.; Langevin, D.; Chappey, C.; Rogalsky, S.P.; Tarasyuk, O.P.; Marais, S. Polyimide/ionic liquid composite membranes for fuel cells operating at high temperatures. Electrochim. Acta 2014, 130, 830–840. [Google Scholar]
- Kobzar, Y.; Fatyeyeva, K.; Lobko, Y.; Yakovlev, Y.; Hrbek, T.; Marais, S. New ionic liquid-based polyoxadiazole electrolytes for hydrogen middle- and high-temperature fuel cells. J. Membr. Sci. 2021, 640, 119774. [Google Scholar]
- Kobzar, Y.L.; Azzouz, G.; Albadri, H.; Levillain, J.; Dez, I.; Gaumont, A.-C.; Lecamp, L.; Chappey, C.; Marais, S.; Fatyeyeva, K. Novel Ionic Conducting Composite Membrane Based on Polymerizable Ionic Liquids. Polymers 2021, 13, 3704. [Google Scholar] [CrossRef]
- Al-Othman, A.; Nancarrow, P.; Tawalbeh, M.; Ka'ki, A.; El-Ahwal, K.; El Taher, B.; Alkasrawi, M. Novel composite membrane based on zirconium phosphate-ionic liquids for high temperature PEM fuel cells. Int. J. Hydrog. Energy 2021, 46, 6100–6109. [Google Scholar]
- Zakeri, M.; Abouzari-Lotf, E.; Nasef, M.M.; Ahmad, A.; Miyake, M.; Ting, T.M.; Sithambaranathan, P. Fabrication and characterization of supported dual acidic ionic liquids for polymer electrolyte membrane fuel cell applications. Arab. J. Chem. 2019, 12, 1011–1023. [Google Scholar] [CrossRef]
- Javed, R.M.N.; Al-Othman, A.; Nancarrow, P.; Tawalbeh, M. Zirconium silicate-ionic liquid membranes for high-temperature hydrogen PEM fuel cells. Int. J. Hydrog. Energy 2022, in press. [Google Scholar]
- Tawalbeh, M.; Al-Othman, A.; Ka'ki, A.; Farooq, A.; Alkasrawi, M. Lignin/zirconium phosphate/ionic liquids-based proton conducting membranes for high-temperature PEM fuel cells applications. Energy 2022, 260, 125237. [Google Scholar] [CrossRef]
- Hunger, K.; Schmeling, N.; Jeazet, H.B.T.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation. Membranes 2012, 2, 727–763. [Google Scholar] [PubMed]
- Liu, F.; Wang, S.; Wang, D.; Liu, G.; Cui, Y.; Liang, D.; Wang, X.; Yong, Z.; Wang, Z. Multifunctional poly (ionic liquid) s cross-linked polybenzimidazole membrane with excellent long-term stability for high temperature-proton exchange membranes fuel cells. J. Power Sources 2021, 494, 229732. [Google Scholar]
- Liu, F.; Wang, S.; Li, J.; Wang, X.; Yong, Z.; Cui, Y.; Liang, D.; Wang, Z. Novel double cross-linked membrane based on poly (ionic liquid) and polybenzimidazole for high-temperature proton exchange membrane fuel cells. J. Power Sources 2021, 515, 230637. [Google Scholar] [CrossRef]
- Lin, B.; Yuan, W.; Xu, F.; Chen, Q.; Zhu, H.; Li, X.; Yuan, N.; Chu, F.; Ding, J. Protic ionic liquid/functionalized graphene oxide hybrid membranes for high temperature proton exchange membrane fuel cell applications. Appl. Surf. Sci. 2018, 455, 295–301. [Google Scholar]
- Chen, H.; Wang, S.; Li, J.; Liu, F.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Novel cross-linked membranes based on polybenzimidazole and polymeric ionic liquid with improved proton conductivity for HT-PEMFC applications. J. Taiwan Inst. Chem. Eng. 2019, 95, 185–194. [Google Scholar]
- Ortiz-Martínez, V.M.; Ortiz, A.; Fernández-Stefanuto, V.; Tojo, E.; Colpaert, M.; Ameduri, B.; Ortiz, I. Fuel cell electrolyte membranes based on copolymers of protic ionic liquid [HSO3-BVIm][TfO] with MMA and hPFSVE. Polymer 2019, 179, 121583. [Google Scholar]
- Liu, F.; Ma, S.; Wang, S.; Li, J.; Wang, X.; Yong, Z.; Cui, Y.; Liang, D.; Wang, Z. Diazoniabicyclo-type poly (ionic liquid) cross-linked polybenzimidazole membrane with improved phosphoric acid retention for HT-PEMFCs. Int. J. Hydrog. Energy 2022, 47, 22522–22531. [Google Scholar]
- Duan, X.; Jia, J.; Wang, N.; Song, D.; Liu, K.; Feng, Y.; Che, Q. Enhancing proton conductivity of phosphoric acid-doped Kevlar nanofibers membranes by incorporating polyacrylamide and 1-butyl-3-methylimidazolium chloride. Int. J. Energy Res. 2020, 44, 11772–11782. [Google Scholar]
- Che, Q.; Fan, H.; Duan, X.; Feng, F.; Mao, W.; Han, X. Layer by layer self-assembly fabrication of high temperature proton exchange membrane based on ionic liquids and polymers. J. Mol. Liq. 2018, 269, 666–674. [Google Scholar]
- Zhao, J.; Song, D.; Jia, J.; Wang, N.; Liu, K.; Zuo, T.; Che, Q. Constructing proton exchange membranes with high and stable proton conductivity at subzero temperature through vacuum assisted flocculation technique. Appl. Surf. Sci. 2022, 585, 152579. [Google Scholar]
- Song, D.; Liu, K.; Zuo, T.; Wei, X.; Hu, S.; Che, Q. Immobilizing imidazolium ionic liquid in flexible proton exchange membranes to modify microstructure fracture. Int. J. Hydrog. Energy 2023, 48, 3065–3077. [Google Scholar]
| Membrane Composition | Solvent | Operating Temperature (°C) | Highest Proton Conductivity (S·cm−1) | Observations | Ref. |
|---|---|---|---|---|---|
| [BMI][HSO4]/SPEEK [Im][HSO4]/SPEEK [MI][HSO4]/SPEEK | NMP | 25 and 80 | 150·10−3 | It was found that by adding ILs, the surface roughness was reduced. The [MI][HSO4]/SPEEK composite membrane with 5 wt.% [MI][HSO4] showed the best proton conductivity. | [50] |
| Phosphonated IL-SBA-15/SPEEK | NMP | 60 to 140 | 10.2·10−3 | The composite membrane containing 6 wt.% of IL-SBA exhibited the highest tensile strength of 23 MPa. The maximum proton conductivity value was observed for the composite membrane with 6 wt.% of IL-SBA. | [30] |
| [TEA-PS][HSO4]/MOF/SPEEK [BImH][HSO4]/MOF/SPEEK [BMI][HSO4]/MOF/SPEEK | DMA | 25 and 80 | 140·10−3 | The [TEA-PS][HSO4]/MOF/SPEEK composite membrane with 2.5 wt.% of IL showed the highest conductivity at both operating temperatures (25 and 80 °C). | [51] |
| TIL1/SPSU TIL2/SPSU TIL3/SPSU | NMP | 105 to 175 | 5.81·10−2 | The membranes showed acceptable thermal stability. The highest conductivity value was measured for TIL3/SPSU with 1 mole ratio of TIL3 at 175 °C. | [52] |
| [BAIM][TFSI]/PI | Methylene chloride | 25 to 160 | 1.0·10−2 | The resultant membranes were thermally stable up to 350 °C. [BAIM][TFSI] demonstrated excellent conductivity of 5.6·10−2 S·cm-1 at 140 °C. It was found that the composite membrane containing 30 wt.% of IL showed the greatest tensile strength of 72.5 MPa. | [53] |
| [TEA-PS][HSO4]/PBI/SPEEK [BImH][HSO4]/PBI/SPEEK | DMA | 25 and 80 | 101·10−3 | The composite membrane containing 5 wt.% of [TEA-PS][HSO4] presented the best conductivity at 80 °C and 60% RH. The [TEA-PS][HSO4]/PBI/SPEEK composite membrane with 5 wt.% of IL exhibited the highest OCP and current density (0.97 V and 1.83 A·cm−2, respectively). | [54] |
| [dema][NTf2]/PBI [HHTMG][NTf2]/PBI [dema][NTf2]/PA/PBI [HHTMG][NTf2]/PA/PBI | DMAC | 80 to 180 | 60·10−3 | [HHTMG][NTf2]/PA/PBI presented the highest conductivity at 180 °C. The resultant composite membrane demonstrated great thermal stability up to 250 °C. However, the pristine PBI membrane showed better mechanical properties as compared to composite membranes. | [55] |
| NMPC/PVA PAN IL/NMPC/PVA SiO2/NMPC/PVA PAN IL/SiO2/NMPC/PVA | Water | 25 to 100 | 1.54·10−3 | The PAN IL/NMPC/PVA composite membrane with 20 wt.% PAN IL revealed the highest conductivity at 100 °C. The membranes were thermally stable up to 100 °C. The NMPC/PVA/SiO2 membrane showed better mechanical features as compared to PAN IL/NMPC/PVA membranes. | [56] |
| [C1Im][NTf2]/PBI [dema][TfO]/PBI [emim][TfO]/PBI [HOemim][NTf2]/PBI | DMF | 100 to 250 | 108.9·10−3 | The resultant IL/PBI composite membranes presented great thermal stability (between 310 and 383 °C). The maximum conductivity was observed for the [dema][TfO]/PBI composite membrane at 250 °C. The [dema][TfO]/PBI composite membrane demonstrated the best tensile strength of 7.8 MPa. | [35] |
| [BMIM][NCS]/PBI [BMIM][Cl]/PBI [BMIM][NTf2]/PBI [BMIM][I]/PBI [BMIM][PF6]/PBI | DMAC | 0 to 200 | 9.4·10−2 | The composite membranes showed good thermal stability at 200 °C. The [BMIM][BF4]/PBI composite membrane showed the highest proton conductivity at 200 °C. The [BMIM][Cl]/PBI membrane demonstrated the highest Young’s modulus (3.7 GPa) and tensile stress (141 MPa). | [57] |
| [EHNH2][H2PO4]/PVDF [Im][Hex]/PVDF | DMSO, DMC | 20 and 60 | 0.15 | The [EHNH2][H2PO4]/PVDF composite membrane showed the highest proton conductivity at 20 °C. By increasing the temperature, the proton conductivity of the imidazolium-based IL increased, whereas the proton conductivity of phosphated-based membranes decreased. | [58] |
| [dema][TfO]/PA/PVDF-HFP | Acetone | RT | 6.3·10−4 | The [dema][TfO]/PA/PVDF-HFP composite membrane with 40 wt.% of Il showed the maximum value for proton conductivity. Leakage of IL at high concentrations resulted in proton conductivity reduction. | [59] |
| [TEA-PS][HSO4]/Nafion® [TEA-PS][BF4]/Nafion® [TEA-PS][CF3SO3]/Nafion® | DMF | 25 and 80 | 1.59 | [TEA-PS][HSO4]/Nafion® containing 5 wt.% of IL demonstrated the highest proton conductivity at 25 and 80 °C. The rise in operating temperature from 25 to 80 °C led to an increase in the IL leaching for all composite membranes. | [60] |
| Membrane Composition | Operating Temperature (°C) | Highest Proton Conductivity (S·cm−1) | Observations | Ref. |
|---|---|---|---|---|
| [MIM][TFSI]/Matrimid® [EIM][TFSI]/Matrimid® [PIM][TFSI]/Matrimid® [BIM][TFSI]/Matrimid® | 25 to 150 | 1·10−3 | The composite films containing [MIM][TFSI] showed the maximum proton conductivity value at 150 °C. The composite films exhibited the greatest thermal stability in the temperature range from 260 to 290 °C. The mechanical features of the composite membranes were better than those of pure Matrimid®. | [61] |
| [C4im][BEHP]/PI [C4im][DBP]/PI [C1im][DBP]/PI | 25 to 115 | 2.0·10−2 | The resultant composite membranes demonstrated great retention ability against IL leaching. It was found that [C4im][DBP])/PI composite film presented the highest proton conductivity at 115 °C. | [62] |
| [MeIm][Tf]/Polyoxadiazole/PVP | 40 to 120 | 1.3·10−3 | The [MeIm][Tf]/polyoxadiazole/PVP composite membranes showed a good degree of IL impregnation of 297%. The membrane samples demonstrated great thermal stability up to 350 °C. The composite films showed acceptable mechanical features. | [63] |
| [Vim][Tf]/PI [AIm][Tf]/PI [MIm][Tf]/PI [PVim][Tf]/PI [Vim][Tf]/[PVim][Tf]/PI | 30 to 150 | 1.0·10−4 | The [Vim][Tf]/[PVim][Tf]/PI composite membrane showed the highest Young’s modulus and elongation at break of 1371 MPa and 271%, respectively. The highest impregnation content was observed for the [Vim][Tf]/[PVim][Tf]/PI membrane (276 ± 16 wt.%). The composite films exhibited tunable thermal stability up to 300 °C. | [64] |
| [EMIM][ESO4]/ZrP/PTFE | 200 | 0.061 | The composite membrane showed great proton conductivity at 200 °C and non-humid conditions. The composite membrane was thermally stable as only 20% of weight loss was observed at 500 °C. | [65] |
| Dual acidic IL/ETFE | 30 to 95 | 259·10−3 | The membrane sample with higher IEC demonstrated better proton conductivity at the same operating condition. The composite membrane with the IEC of 3.4 meq·g−1 showed the highest conductivity at 95 °C. The resultant membranes were thermally stable up to 280 °C. | [66] |
| [HMIM][TCM]/ZrSi/GLY/PTFE [BMIM][SCN]/ZrSi/GLY/PTFE | 25 and 200 | 0.196 | The composite membrane showed higher proton conductivity than that of Nafion® at 25 °C. It was found that the membrane proton conductivity increased by water uptake increasing. The composite membranes exhibited great thermal stability up to 200 °C. | [67] |
| ZrP/PTFE Lignin/ZrP/PTFE [HMIM][C4N3]/Lignin/ZrP/PTFE [DMEA][OMS]/Lignin/ZrP/PTFE [EMIM][CH3O3S]/Lignin/ZrP/PTFE | 25 to 150 | 1.0·10−1 | The hexyl-based, IL-based membrane showed the highest proton conductivity at 25 °C. The resultant composite membranes showed a good potential to be used in low-temperature PEMFC. | [68] |
| Membrane Composition | Chemical Agents | Operating Temperature (°C) | Highest Proton Conductivity (S·cm−1) | Observations | Ref. |
|---|---|---|---|---|---|
| P[VBIm][Cl]/NbPBI P[MPIm][Br]/NbPBI P[TPAm][Br]/NbPBI | Solvent and initiator: DMAC, AIBN | 110 to 170 | 0.074 | FTIR analysis confirmed that PILs are chemically cross-linked to polymer. The NbPBI/P[MPIm]Br composite film demonstrated the highest proton conductivity at 170 °C. The composite film showed good thermal stability (∼220 °C). | [70] |
| P[TSPDO][BrCl]/NbPBI | Solvent and initiator: DMSO, AIBN | 110 to 170 | 0.061 | Modified membranes demonstrated higher thermal stability (∼300 °C) as compared to pure membranes (∼250 °C). The composite polymer film with 30 wt.% of P[TSPDO][BrCl] showed the greatest conductivity at 170 °C. The cross-linked composite membranes exhibited good mechanical properties. | [71] |
| [APMIm][Br]-GO/[MIm][TfO]/PSAN | Solvent and photo-initiator: [MIm][TfO], benzoin isobutyl ether | 100 to 160 | 1.48·10−2 | The membrane containing 1 wt.% of [APMIm][Br]-GO demonstrated the best proton conductivity at 160 °C. It was found that the addition of [APMIm][Br]-GO caused a decrease in the membrane mechanical properties. The leaching test showed that by increasing the concentration of [APMIm][Br]-GO, leaching of PrIL was reduced. | [72] |
| PIL(PBI-BF4)/PBI PIL(PBI-BF4)/PA/PBI | Solvent and cross-linker: DMAC, γ-(2, 3-epoxypropoxy) propyltrimethoxysilane | 110 to 170 | 0.117 | To increase the conductivity of membranes, some samples were immersed in a PA solution. The membrane containing 40 wt.% of PIL exhibited the maximum value of proton conductivity at 170 °C. An increase in concentration of PIL caused a decrease in the mechanical features of composite films. | [73] |
| [HSO3-BVIm][TfO]/MMA [HSO3-BVIm][TfO]/hPFSVE | Cross-linker and photo-initiator: glycerol dimethacrylate, 2-hydroxy-2-methyl propiophenone | 25 to 90 | 1.0·10−2 | FTIR confirmed the photochemical copolymerization reaction between IL and both hPFSVE and MMA. Membranes revealed acceptable proton conductivity in both dry and wet states. The membranes represented acceptable thermal stability (≥200 °C). | [74] |
| [CPDOc]Br2/PBI [CPDOc]Br2/PA/PBI | Solvent and initiator: DMSO, AIBN | 110 to 170 | 0.121 | The [CPDOc]Br2/PA/PBI composite membrane with 30 wt.% of IL showed the best conductivity at 170 °C. The resultant films were thermally stable up to 200–250 °C. | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, M.; Fatyeyeva, K.; Kujawski, W. Different Approaches for the Preparation of Composite Ionic Liquid-Based Membranes for Proton Exchange Membrane Fuel Cell Applications—Recent Advancements. Membranes 2023, 13, 593. https://doi.org/10.3390/membranes13060593
Ebrahimi M, Fatyeyeva K, Kujawski W. Different Approaches for the Preparation of Composite Ionic Liquid-Based Membranes for Proton Exchange Membrane Fuel Cell Applications—Recent Advancements. Membranes. 2023; 13(6):593. https://doi.org/10.3390/membranes13060593
Chicago/Turabian StyleEbrahimi, Mohammad, Kateryna Fatyeyeva, and Wojciech Kujawski. 2023. "Different Approaches for the Preparation of Composite Ionic Liquid-Based Membranes for Proton Exchange Membrane Fuel Cell Applications—Recent Advancements" Membranes 13, no. 6: 593. https://doi.org/10.3390/membranes13060593
APA StyleEbrahimi, M., Fatyeyeva, K., & Kujawski, W. (2023). Different Approaches for the Preparation of Composite Ionic Liquid-Based Membranes for Proton Exchange Membrane Fuel Cell Applications—Recent Advancements. Membranes, 13(6), 593. https://doi.org/10.3390/membranes13060593








