Polymeric Based Hydrogel Membranes for Biomedical Applications
Abstract
1. Introduction
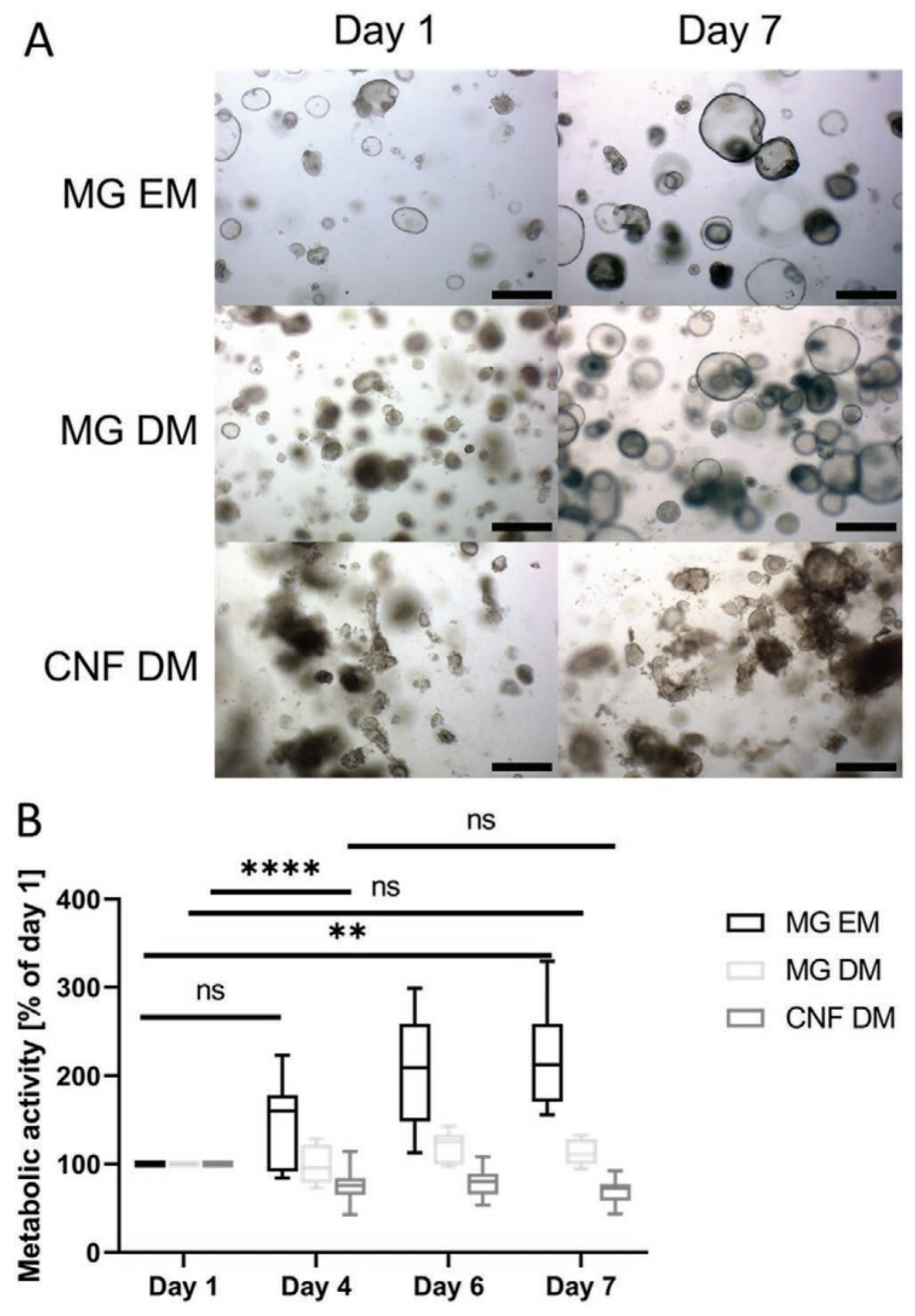
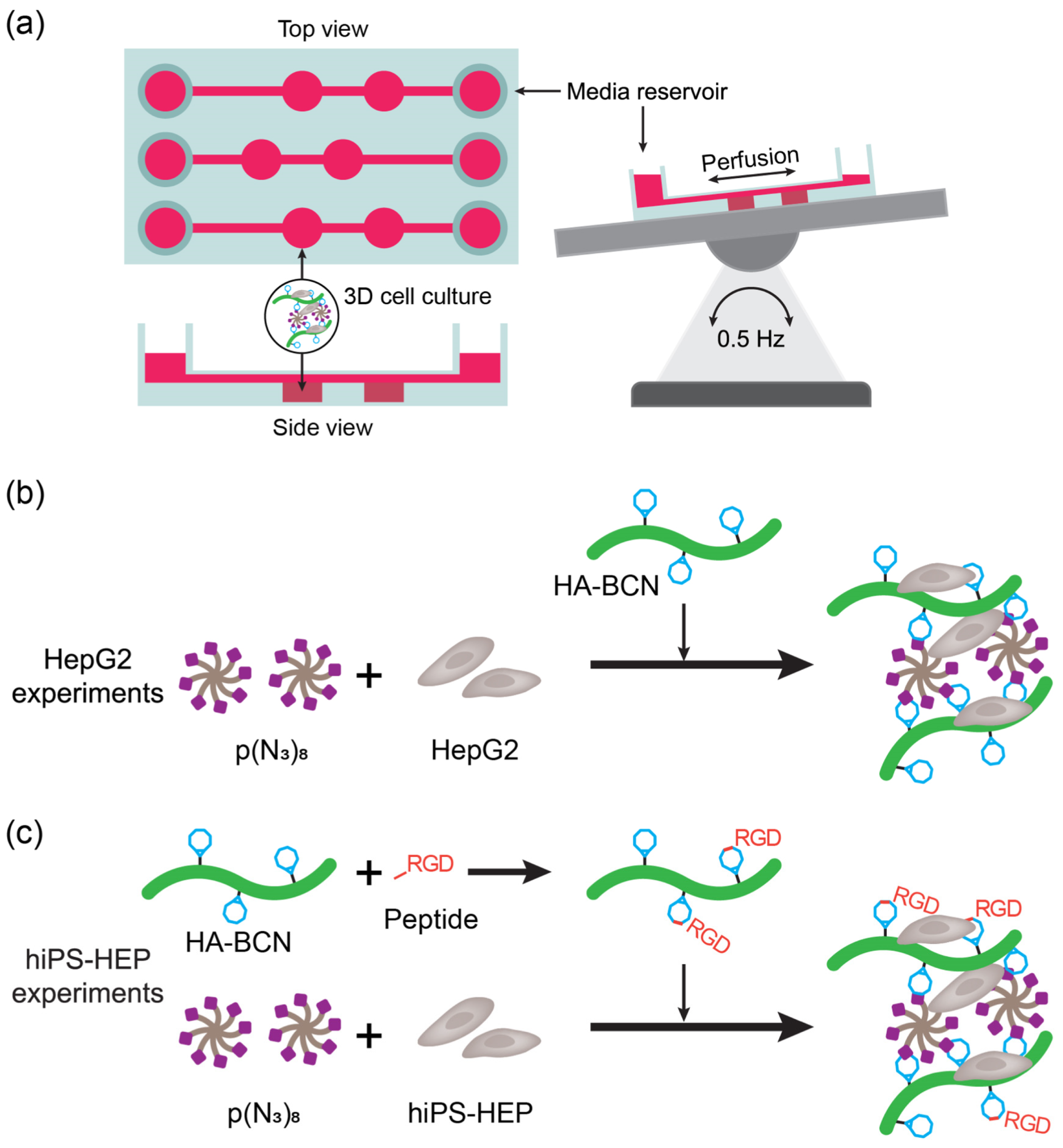
2. Liver
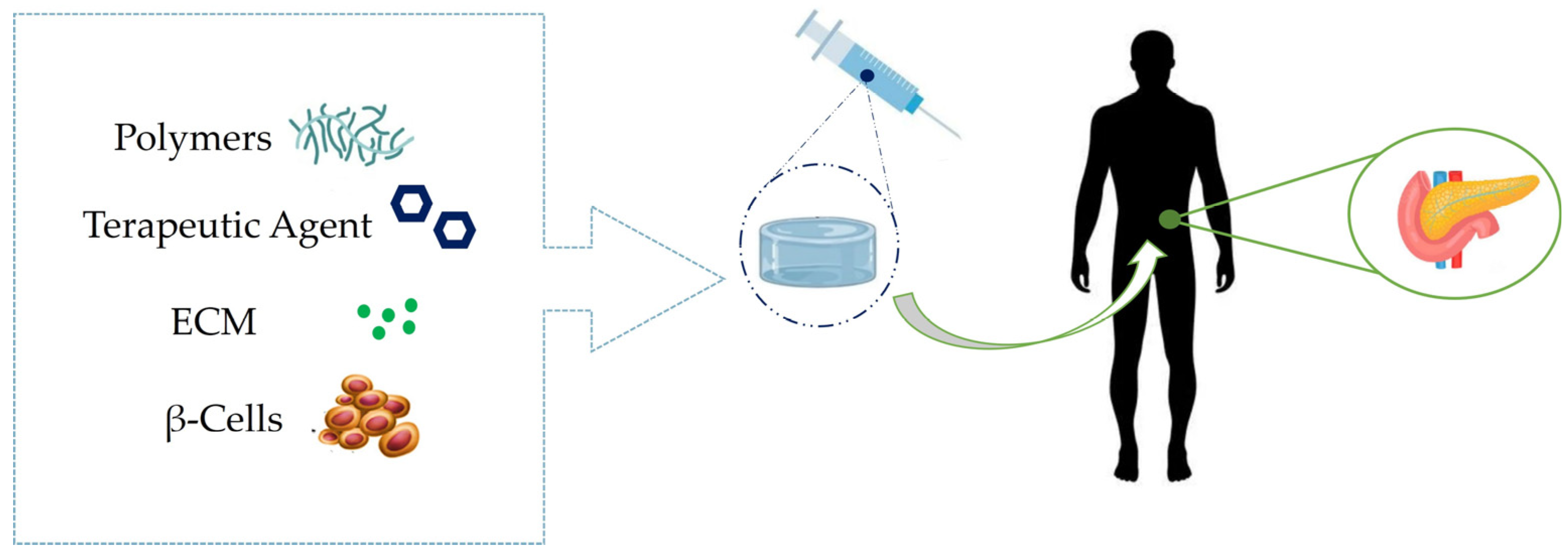
3. Pancreas Regeneration
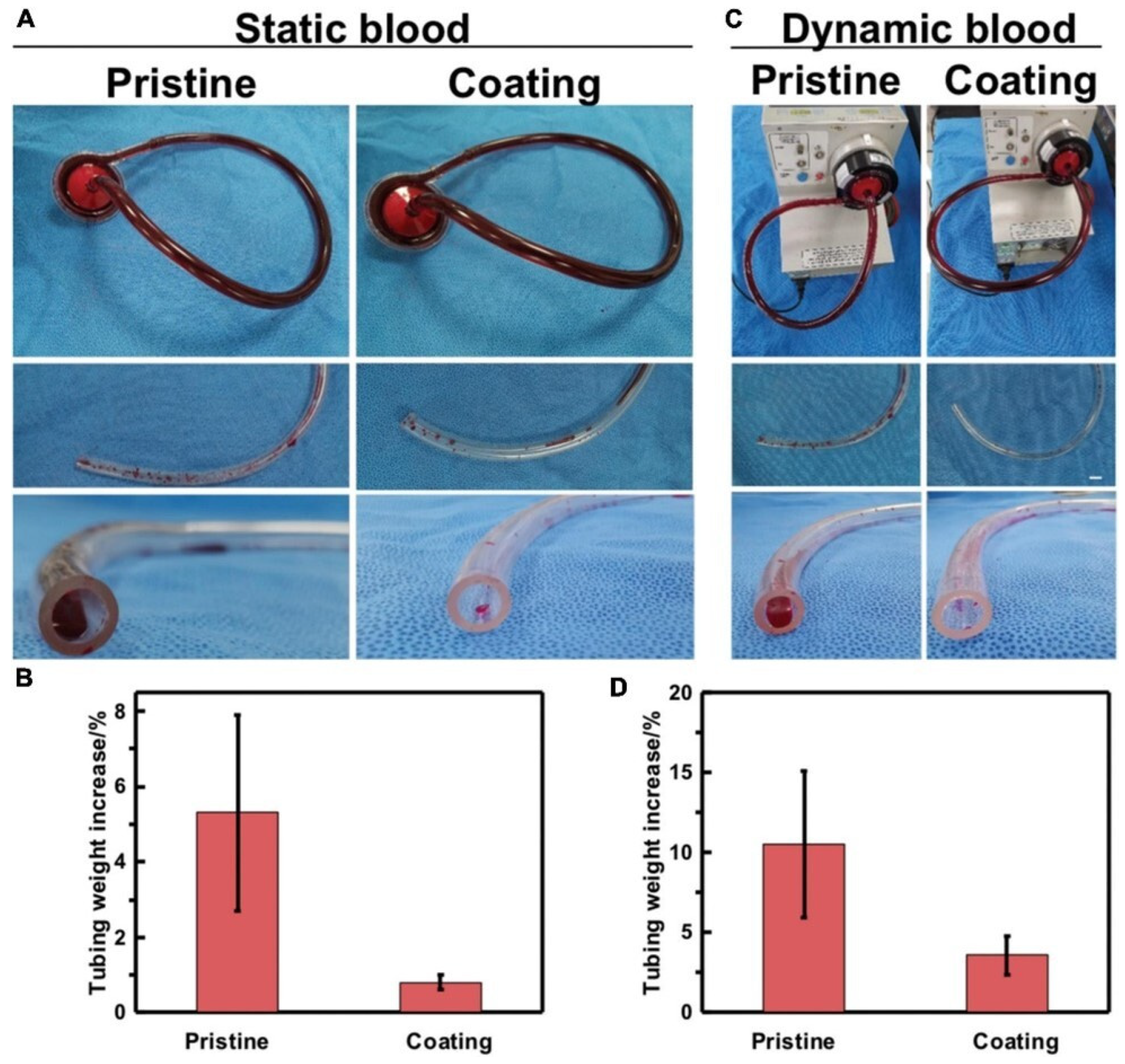
4. Artificial Oxygenators
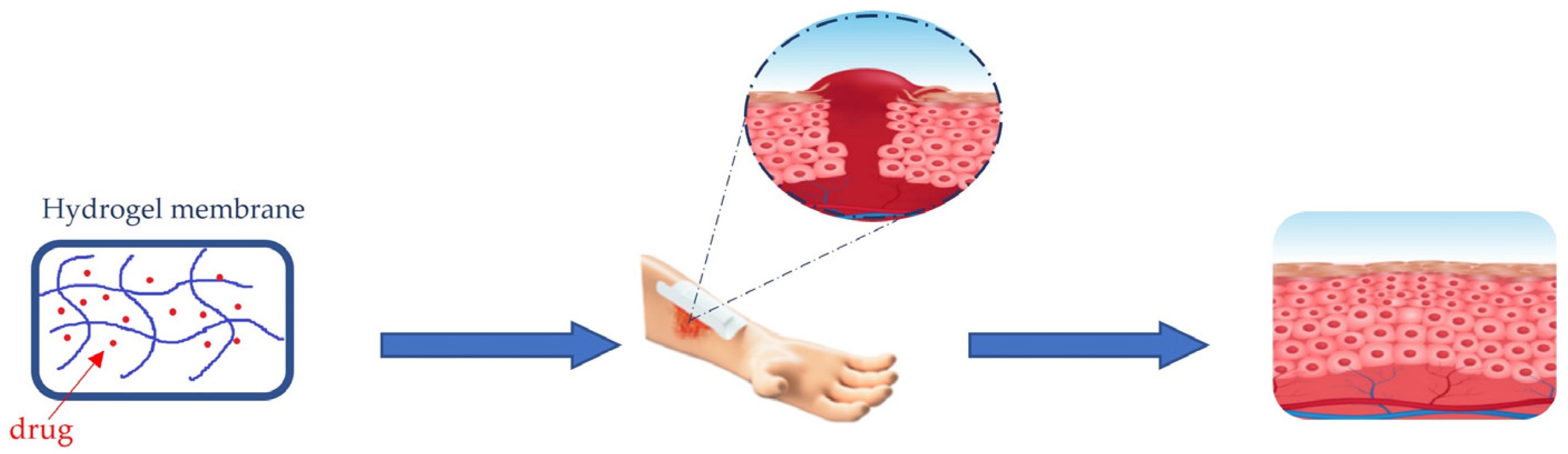
5. Wound Healing
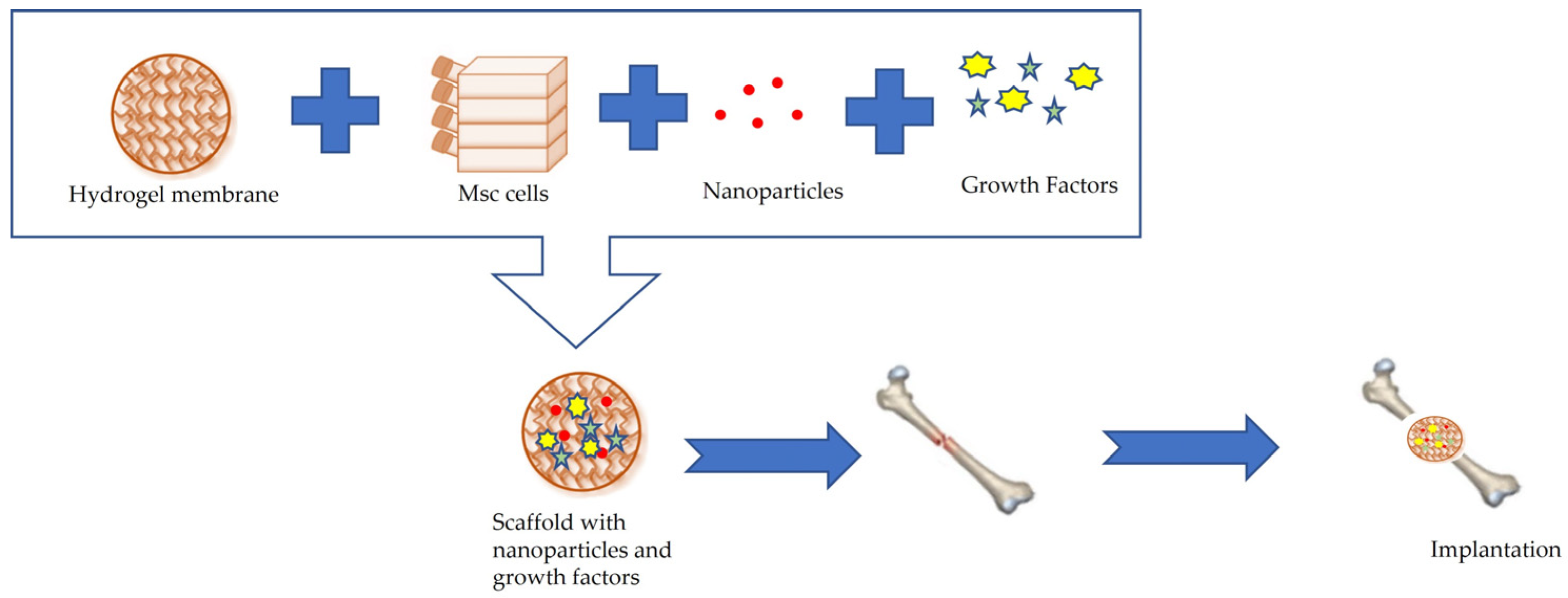
6. Bone Tissue Engineering
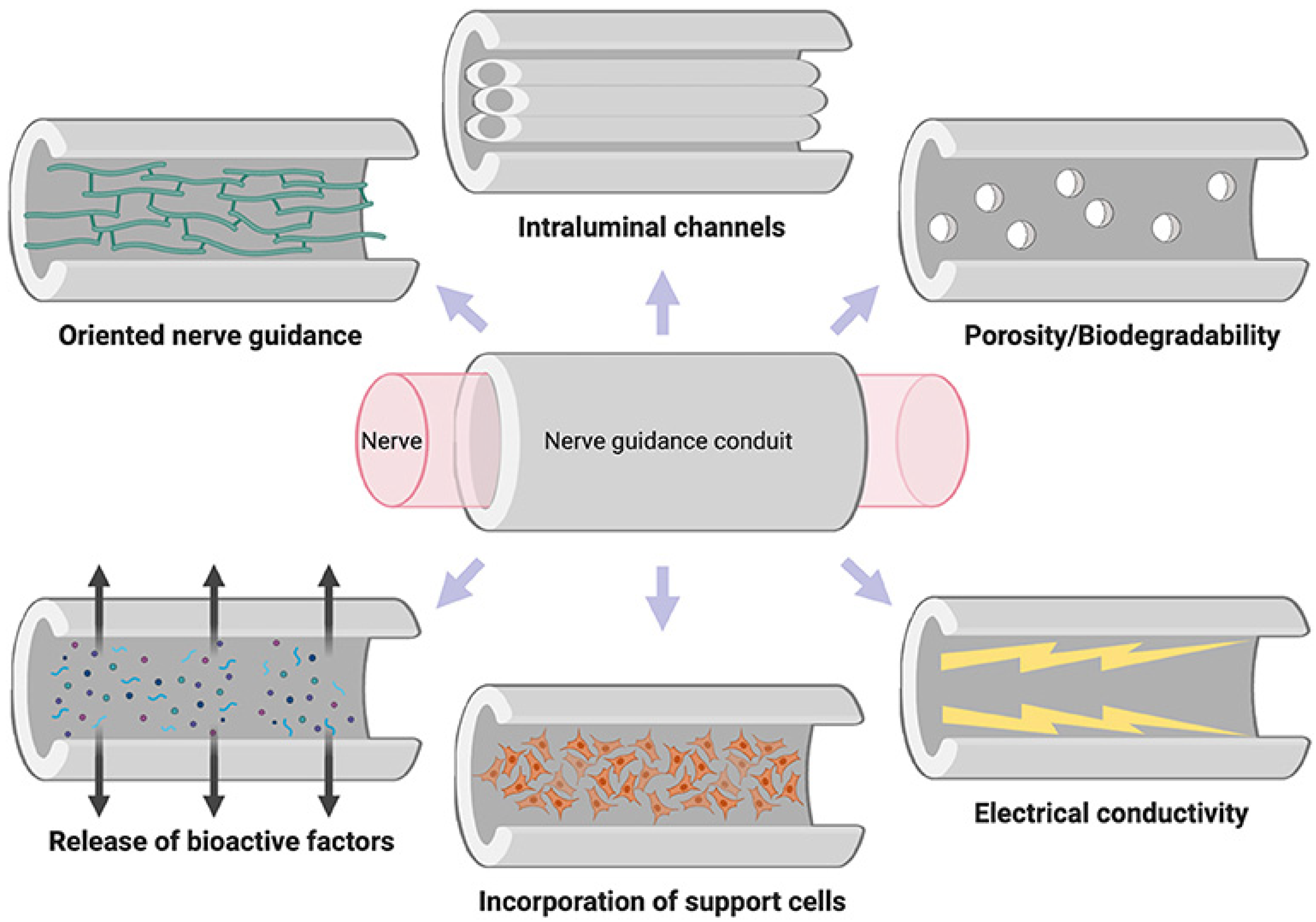
7. Neural Tissue Engineering
8. Drug Delivery
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Siddique, T.; Dutta, N.K.; Choudhury, N.R. Mixed-Matrix Membrane Fabrication for Water Treatment. Membranes 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Toniato, T.V.; Stocco, T.D.; Martins, D.D.S.; Santanna, L.B.; Tim, C.R.; Marciano, F.R.; Silva-Filho, E.C.; Campana-Filho, S.P.; Lobo, A. Hybrid chitosan/amniotic membrane-based hydrogels for articular cartilage tissue engineering application. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 961–970. [Google Scholar] [CrossRef]
- Arefi-Oskoui, S.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpour, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochemistry 2019, 58, 104633. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2018, 76, 4879–4901. [Google Scholar] [CrossRef]
- Mbituyimana, B.; Mao, L.; Hu, S.; Ullah, M.W.; Chen, K.; Fu, L.; Zhao, W.; Shi, Z.; Yang, G. Bacterial cellulose/glycolic acid/glycerol composite membrane as a system to deliver glycolic acid for anti-aging treatment. J. Bioresour. Bioprod. 2021, 6, 129–141. [Google Scholar] [CrossRef]
- Wsoo, M.A.; Razak, S.I.A.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef]
- Taemeh, M.A.; Shiravandi, A.; Korayem, M.A.; Daemi, H. Fabrication challenges and trends in biomedical applications of alginate electrospun nanofibers. Carbohydr. Polym. 2020, 228, 115419. [Google Scholar] [CrossRef]
- Donato, L. A Spasso con le Membrane; CNR Edizionii: Roma, Italy, 2021; pp. 21–22. [Google Scholar]
- Woźniak-Budych, M.J. Polymeric membranes for biomedical applications. Phys. Sci. Rev. 2021, 15, 619. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Bioinspired by cell membranes: Functional polymeric materials for biomedical applications. Mater. Chem. Front. 2020, 4, 750–774. [Google Scholar] [CrossRef]
- Wu, J.; Qu, Y.; Yu, Q.; Chen, H. Gold nanoparticle layer: A versatile nanostructured platform for biomedical applications. Mater. Chem. Front. 2018, 2, 2175–2190. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Zhang, X.; Deng, F.; Zhou, C.; Hui, J.; Liu, W.; Wei, Y. Interaction of tannic acid with carbon nanotubes: Enhancement of dispersibility and biocompatibility. Toxicol. Res. 2015, 4, 160–168. [Google Scholar] [CrossRef]
- Kiew, S.F.; Kiew, L.V.; Lee, H.B.; Imae, T.; Chung, L.Y. Assessing biocompatibility of graphene oxide-based nanocarriers: A review. J. Control. Release 2016, 226, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Parodi, A.; Molinaro, R.; Sushnitha, M.; Evangelopoulos, M.; Martinez, J.O.; Arrighetti, N.; Corbo, C.; Tasciotti, E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 2017, 147, 155–168. [Google Scholar] [CrossRef]
- Kim, Y.; Chortos, A.; Xu, W.; Liu, Y.; Oh, J.Y.; Son, D.; Kang, J.; Foudeh, A.M.; Zhu, C.; Lee, Y.; et al. A bioinspired flexible organic artificial afferent nerve. Science 2018, 360, 998–1003. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, L.; Jiang, L. Bioinspired smart asymmetric nanochannel membranes. Chem. Soc. Rev. 2018, 47, 322–356. [Google Scholar] [CrossRef]
- Clegg, J.R.; Wagner, A.M.; Shin, S.R.; Hassan, S.; Khademhosseini, A.; Peppas, N.A. Modular fabrication of intelligent material-tissue interfaces for bioinspired and biomimetic devices. Prog. Mater. Sci. 2019, 106, 100589. [Google Scholar] [CrossRef]
- Palivan, C.G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377–411. [Google Scholar] [CrossRef] [PubMed]
- Garni, M.; Thamboo, S.; Schoenenberger, C.-A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Huskens, J.; Pang, W.; Duan, X. Hypersonic poration of supported lipid bilayers. Mater. Chem. Front. 2019, 3, 782–790. [Google Scholar] [CrossRef]
- Biondi, M.; Ungaro, F.; Quaglia, F.; Netti, P.A. Controlled drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. 2020, 114, 111023. [Google Scholar] [CrossRef]
- Yang, Q.; Adrus, N.; Tomicki, F.; Ulbricht, M. Composites of functional polymeric hydrogels and porous membranes. J. Mater. Chem. 2011, 21, 2783–2811. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Khakpour, S.; Ahmed, H.M.M.; De Bartolo, L. Membrane-Based Bioartificial Liver Devices. In Biomedical Membranes and (Bio)Artificial Organs; World Scientific Series in Membrane Science and Technology: Biological and Biomimetic Applications, Energy and the Environment; World Scientific: Singapore, 2017; Volume 2, pp. 149–178. [Google Scholar]
- Zhang, K.; Zhang, L.; Liu, W.; Ma, X.; Cen, J.; Sun, Z.; Wang, C.; Feng, S.; Zhang, Z.; Yue, L.; et al. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell 2018, 23, 806–819.e4. [Google Scholar] [CrossRef]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e19. [Google Scholar] [CrossRef]
- Bedian, L.; Villalba-Rodríguez, A.M.; Hernández-Vargas, G.; Parra-Saldivar, R.; Iqbal, H.M.N. Bio-based materials with novel characteristics for tissue engineering applications—A review. Int. J. Biol. Macromol. 2017, 98, 837–846. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in Reproductive Tissue Engineering: A Review on Its Application as a Biomaterial for Fertility Preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A. A review on gradient hydrogel/fiber scaffolds for osteochondral regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1974–e1990. [Google Scholar] [CrossRef]
- Gradinaru, V.; Treweek, J.; Overton, K.; Deisseroth, K. Hydrogel-Tissue Chemistry: Principles and Applications. Annu. Rev. Biophys. 2018, 47, 355–376. [Google Scholar] [CrossRef]
- Ye, S.; Boeter, J.W.B.; Penning, L.C.; Spee, B.; Schneeberger, K. Hydrogels for Liver Tissue Engineering. Bioengineering 2019, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Heydari, Z.; Najimi, M.; Mirzaei, H.; Shpichka, A.; Ruoss, M.; Farzaneh, Z.; Montazeri, L.; Piryaei, A.; Timashev, P.; Gramignoli, R.; et al. Tissue Engineering in Liver Regenerative Medicine: Insights into Novel Translational Technologies. Cells 2020, 9, 304. [Google Scholar] [CrossRef]
- Krüger, M.; Oosterhoff, L.A.; van Wolferen, M.E.; Schiele, S.A.; Walther, A.; Geijsen, N.; De Laporte, L.; van der Laan, L.J.W.; Kock, L.M.; Spee, B. Cellulose Nanofibril Hydrogel Promotes Hepatic Differentiation of Human Liver Organoids. Adv. Healthc. Mater. 2020, 9, 1901658. [Google Scholar] [CrossRef]
- McCrary, M.W.; Bousalis, D.; Mobini, S.; Song, Y.H.; Schmidt, C.E. Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues. Acta Biomater. 2020, 111, 1–19. [Google Scholar] [CrossRef]
- Lu, S.; Cuzzucoli, F.; Jiang, J.; Liang, L.-G.; Wang, Y.; Kong, M.; Zhao, X.; Cui, W.; Li, J.; Wang, S. Development of a biomimetic liver tumor-on-a-chip model based on decellularized liver matrix for toxicity testing. Lab Chip 2018, 18, 3379–3392. [Google Scholar] [CrossRef]
- Damania, A.; Kumar, A.; Teotia, A.K.; Kimura, H.; Kamihira, M.; Ijima, H.; Sarin, S.K.; Kumar, A. Decellularized Liver Matrix-Modified Cryogel Scaffolds as Potential Hepatocyte Carriers in Bioartificial Liver Support Systems and Implantable Liver Constructs. ACS Appl. Mater. Interfaces 2017, 10, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Lotfi, H.; Salehi, R.; Mehdipour, A.; Zarghami, N.; Akbarzadeh, A.; Alizadeh, E. Hepatic cell-sheet fabrication of differentiated mesenchymal stem cells using decellularized extracellular matrix and thermoresponsive polymer. Biomed. Pharmacother. 2021, 134, 111096. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.H.; Park, K.-M.; Yu, L.; Kwak, H.-H.; Woo, H.-M. Decellularized hepatic extracellular matrix hydrogel attenuates hepatic stellate cell activation and liver fibrosis. Mater. Sci. Eng. 2020, 116, 111160. [Google Scholar] [CrossRef]
- Christoffersson, J.; Aronsson, C.; Jury, M.; Selegård, R.; Aili, D.; Mandenius, C.-F. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device. Biofabrication 2018, 11, 015013. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef]
- Christoffersson, J.; van Noort, D.; Mandenius, C.-F. Developing organ-on-a-chip concepts using bio-mechatronic design methodology. Biofabrication 2017, 9, 025023. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Mi, F.-L.; Lin, P.-Y.; Miao, Y.-B.; Huang, T.; Chen, K.-H.; Chen, C.-T.; Chang, Y.; Sung, H.-W. Strategies for improving diabetic therapy via alternative administration routes that involve stimuli-responsive insulin-delivering systems. Adv. Drug Deliv. Rev. 2019, 139, 71–82. [Google Scholar] [CrossRef]
- Dinnyes, A.; Schnur, A.; Muenthaisong, S.; Bartenstein, P.; Burcez, C.; Burton, N.; Cyran, C.; Gianello, P.; Kemter, E.; Nemeth, G.; et al. Integration of nano- and biotechnology for beta-cell and islet transplantation in type-1 diabetes treatment. Cell Prolif. 2020, 53, e12785. [Google Scholar] [CrossRef]
- Hwang, P.T.J.; Shah, D.K.; Garcia, J.A.; Bae, C.Y.; Lim, D.-J.; Huiszoon, R.C.; Alexander, G.C.; Jun, H.-W. Progress and challenges of the bioartificial pancreas. Nano Converg. 2016, 3, 28. [Google Scholar] [CrossRef]
- Ghasemi, A.; Akbari, E.; Imani, R. An Overview of Engineered Hydrogel-Based Biomaterials for Improved β-Cell Survival and Insulin Secretion. Front. Bioeng. Biotechnol. 2021, 9, 662084. [Google Scholar] [CrossRef]
- Haque, M.R.; Lee, D.Y.; Ahn, C.-H.; Jeong, J.-H.; Byun, Y. Local Co-Delivery of Pancreatic Islets and Liposomal Clodronate Using Injectable Hydrogel to Prevent Acute Immune Reactions in a Type 1 Diabetes. Pharm. Res. 2014, 31, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Orive, G.; Hernández, R.M.; Saenz del Burgo, L.; Pedraz, J. Review of Advanced Hydrogel-Based Cell Encapsulation Systems for Insulin Delivery in Type 1 Diabetes Mellitus. Pharmaceutics 2019, 11, 597. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Cheam, N.M.J.; Irvine, S.A.; Tan, N.S.; Venkatraman, S.; Tay, C.Y. Interpenetrating Network of Alginate–Human Adipose Extracellular Matrix Hydrogel for Islet Cells Encapsulation. Macromol. Rapid Commun. 2020, 41, 2000275. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Pei, Q.; Pu, C.; Chen, Y.; He, S.; Wang, B. Multifunctional Islet Transplantation Hydrogel Encapsulating A20 High-Expressing Islets. Drug Des. Dev. Ther. 2020, 14, 4021–4027. [Google Scholar] [CrossRef]
- Knobeloch, T.; Abadi, S.E.M.; Bruns, J.; Petrova Zustiak, S.; Kwon, G. Injectable polyethylene glycol hydrogel for islet encapsulation: An in vitro and in vivo characterization. Biomed. Phys. Eng. Express 2017, 3, 035022. [Google Scholar] [CrossRef]
- Abada, E.N.; Feinberg, B.J.; Roy, S. Evaluation of silicon membranes for extracorporeal membrane oxygenation (ECMO). Biomed. Microdevices 2018, 20, 86. [Google Scholar] [CrossRef]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- He, T.; He, J.; Wang, Z.; Cui, Z. Modification strategies to improve the membrane hemocompatibility in extracorporeal membrane oxygenator (ECMO). Adv. Compos. Hybrid Mater. 2021, 4, 847–864. [Google Scholar] [CrossRef]
- He, T.; Yu, S.; He, J.; Chen, D.; Li, J.; Hu, H.; Zhong, X.; Wang, Y.; Wang, Z.; Cui, Z. Membranes for extracorporeal membrane oxygenator (ECMO): History, preparation, modification and mass transfer. Chin. J. Chem. Eng. 2022, 49, 46–75. [Google Scholar] [CrossRef]
- Schmidt, M.; Tachon, G.; Devilliers, C.; Muller, G.; Hekimian, G.; Bréchot, N.; Merceron, S.; Luyt, C.E.; Trouillet, J.-L.; Chastre, J.; et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med. 2013, 39, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Schlanstein, P.C.; Limper, A.; Hesselmann, F.; Schmitz-Rode, T.; Steinseifer, U.; Arens, J. Experimental method to determine anisotropic permeability of hollow fiber membrane bundles. J. Membr. Sci. 2018, 546, 70–81. [Google Scholar] [CrossRef]
- Valencia, E.; Nasr, V.G. Updates in Pediatric Extracorporeal Membrane Oxygenation. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Lavery, K.S.; Rhodes, C.; Mcgraw, A.; Eppihimer, M.J. Anti-thrombotic technologies for medical devices. Adv. Drug Deliv. Rev. 2017, 112, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mi, M.Y.; Matthay, M.A.; Morris, A.H. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 379, 884–887. [Google Scholar] [CrossRef]
- Winnersbach, P.; Hosseinnejad, A.; Breuer, T.; Fechter, T.; Jakob, F.; Schwaneberg, U.; Rossaint, R.; Bleilevens, C.; Singh, S. Endogenous Nitric Oxide-Releasing Microgel Coating Prevents Clot Formation on Oxygenator Fibers Exposed to In Vitro Blood Flow. Membranes 2022, 12, 73. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2020, 8, nwaa254. [Google Scholar] [CrossRef]
- Naito, N.; Ukita, R.; Wilbs, J.; Wu, K.; Lin, X.; Carleton, N.M.; Roberts, K.; Jiang, S.; Heinis, C.; Cook, K.E. Combination of polycarboxybetaine coating and factor XII inhibitor reduces clot formation while preserving normal tissue coagulation during extracorporeal life support. Biomaterials 2021, 272, 120778. [Google Scholar] [CrossRef]
- Wang, W.; Lu, Y.; Zhu, H.; Cao, Z. Superdurable Coating Fabricated from a Double-Sided Tape with Long Term “Zero” Bacterial Adhesion. Adv. Mater. 2017, 29, 1606506. [Google Scholar] [CrossRef]
- Yao, X.; Liu, J.; Yang, C.; Yang, X.; Wei, J.; Xia, Y.; Gong, X.; Suo, Z. Hydrogel Paint. Adv. Mater. 2019, 31, 1903062. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, H.; Wu, X.; Yan, Z.; Tang, C.; Qin, Z.; Yao, M.; Che, P.; Yao, F.; Li, J. In Situ Clickable Purely Zwitterionic Hydrogel for Peritoneal Adhesion Prevention. Chem. Mater. 2020, 32, 6347–6357. [Google Scholar] [CrossRef]
- Xie, X.; Doloff, J.C.; Yesilyurt, V.; Sadraei, A.; McGarrigle, J.J.; Omami, M.; Veiseh, O.; Farah, S.; Isa, D.; Ghani, S.; et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat. Biomed. Eng. 2018, 2, 894–906. [Google Scholar] [CrossRef]
- Yao, M.; Wei, Z.; Li, J.; Guo, Z.; Yan, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Yao, F.; et al. Microgel reinforced zwitterionic hydrogel coating for blood-contacting biomedical devices. Nat. Commun. 2022, 13, 5339. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, H.; Liu, Y.; Tang, Q.; Wu, P.; Lin, T.; Li, T.; Sun, D. Sodium alginate-hydrogel coatings on extracorporeal membrane oxygenation for anticoagulation. Front. Cardiovasc. Med. 2022, 9, 966649. [Google Scholar] [CrossRef]
- Hyono, A.; Yonezawa, T.; Kawai, K.; Abe, S.; Fujihara, M.; Azuma, H.; Wakamoto, S. SEM observation of the live morphology of human red blood cells under high vacuum conditions using a novel RTIL. Surf. Interface Anal. 2014, 46, 425–428. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug Delivery Systems in Regenerative Medicine: An Updated Review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R. Hydrogels for Hydrophobic Drug Delivery. Classification, Synthesis and Applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In vitro and in vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.S.; Evans, G.R.D. Advances in Wound Healing: A Review of Current Wound Healing Products. Plast. Surg. Int. 2012, 2012, 190436. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Batool, S.; Hussain, Z.; Niazi, M.B.K.; Liaqat, U.; Afzal, M. Biogenic synthesis of silver nanoparticles and evaluation of physical and antimicrobial properties of Ag/PVA/starch nanocomposites hydrogel membranes for wound dressing application. J. Drug Deliv. Sci. Technol. 2019, 52, 403–414. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Truong-Dinh Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef]
- Price, R.D.; Myers, S.; Leigh, I.M.; Navsaria, H.A. The Role of Hyaluronic Acid in Wound Healing. Am. J. Clin. Dermatol. 2005, 6, 393–402. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Khan, S.; Minhas, M.U.; de Matas, M.; Sikstone, V.; Hussain, Z.; Abbasi, M.; Kousar, M. Biopolymer-based biomaterials for accelerated diabetic wound healing: A critical review. Int. J. Biol. Macromol. 2019, 139, 975–993. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers, Composites Renewable Resources; Gandini, A., Belgacem, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Chapter 5; pp. 517–542. [Google Scholar]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-functional hydrogel membranes loaded with chitosan nanoparticles for accelerated wound healing. Int. J. Biol. Macromol. 2021, 170, 207–221. [Google Scholar] [CrossRef]
- Singh, R.S.; Saini, G.K.; Kennedy, J.F. Pullulan: Microbial sources, production and applications. Carbohydr. Polym. 2008, 73, 515–531. [Google Scholar] [CrossRef]
- Tamer, T.M.; Hassan, M.A.; Omer, A.M.; Valachová, K.; Eldin, M.S.M.; Collins, M.N.; Šoltés, L. Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr. Polym. 2017, 169, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.S.; Iyappan, K.; Gayathri, V.S.; William, S.; Suguna, L. Influence of pullulan hydrogel on sutureless wound healing in rats. Wound Med. 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Pawar, V.; Dhanka, M.; Srivastava, R. Cefuroxime conjugated chitosan hydrogel for treatment of wound infections. Colloids Surf. B 2019, 173, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Paul, M.; Fraser, A.; Sarid, N.; Leibovici, L. Efficacy and safety of cefepime: A systematic review and meta-analysis. Lancet Infect. Dis. 2007, 7, 338–348. [Google Scholar] [CrossRef]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef]
- Mabrouk, M.; Rajendran, R.; Soliman, I.E.; Ashour, M.M.; Beherei, H.H.; Tohamy, K.M.; Thomas, S.; Kalarikkal, N.; Arthanareeswaran, G.; Das, D.B. Nanoparticle- and Nanoporous-Membrane-Mediated Delivery of Therapeutics. Pharmaceutics 2019, 11, 294. [Google Scholar] [CrossRef]
- Liu, F.; Wang, M.; Wang, X.; Wang, P.; Shen, W.; Ding, S.; Wang, Y. Fabrication and application of nanoporous polymer ion-track membranes. Nanotechnology 2018, 30, 052001. [Google Scholar] [CrossRef]
- Baptista, D.; Teixeira, L.M.; Birgani, Z.T.; van Riet, S.; Pasman, T.; Poot, A.; Stamatialis, D.; Rottier, R.J.; Hiemstra, P.S.; Habibović, P.; et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials 2021, 266, 120436. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- AI-Aql, Z.S.; Alagl, A.S.; Graves, D.T.; Gerstenfeld, L.C.; Einhorn, T.A. Molecular Mechanisms Controlling Bone Formation during Fracture Healing and Distraction Osteogenesis. J. Dent. Res. 2008, 87, 107–118. [Google Scholar] [CrossRef]
- Drago, L.; Clerici, P.; Morelli, I.; Ashok, J.; Benzakour, T.; Bozhkova, S.; Alizadeh, C.; Del Sel, H.; Sharma, H.K.; Peel, T.; et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) Procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and Other Implant-Related Infections. J. Clin. Med. 2019, 8, 933. [Google Scholar] [CrossRef] [PubMed]
- George, D.A.; Drago, L.; Scarponi, S.; Gallazzi, E.; Haddad, F.S.; Romano, C.L. Predicting lower limb periprosthetic joint infections: A review of risk factors and their classification. World J. Orthop. 2017, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Wang, X.; Li, J.; Deng, C.; Liu, Y.; Yao, Q.; Wang, L.; Chang, J.; Wu, C. 3D printing of Mo-containing scaffolds with activated anabolic responses and bi-lineage bioactivities. Theranostics 2018, 8, 4372–4392. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the Repair of Articular Cartilage Defects. Tissue Eng. 2011, 17, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, P.; Chen, Y.; Li, M.; Chen, C.; Lu, H. 3D-Printed Extracellular Matrix/Polyethylene Glycol Diacrylate Hydrogel Incorporating the Anti-inflammatory Phytomolecule Honokiol for Regeneration of Osteochondral Defects. Am. J. Sport. Med. 2020, 48, 2808–2818. [Google Scholar] [CrossRef]
- Yuan, H.; Zheng, X.; Liu, W.; Zhang, H.; Shao, J.; Yao, J.; Mao, C.; Hui, J.; Fan, D. A novel bovine serum albumin and sodium alginate hydrogel scaffold doped with hydroxyapatite nanowires for cartilage defects repair. Colloids Surf. B 2020, 192, 111041. [Google Scholar] [CrossRef]
- Madhusudanan, P.; Raju, G.; Shankarappa, S. Hydrogel systems and their role in neural tissue engineering. J. R. Soc. Interface 2020, 17, 20190505. [Google Scholar] [CrossRef]
- Silver, D.J.; Silver, J. Contributions of chondroitin sulfate proteoglycans to neurodevelopment, injury, and cancer. Curr. Opin. Neurobiol. 2014, 27, 171–178. [Google Scholar] [CrossRef]
- Buchli, A.D.; Schwab, M.E. Inhibition of Nogo: A key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann. Med. 2005, 37, 556–567. [Google Scholar] [CrossRef] [PubMed]
- McKerracher, L.; Rosen, K.M. MAG, myelin and overcoming growth inhibition in the CNS. Front. Mol. Neurosci. 2015, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Madhusudanan, P.; Reade, S.; Shankarappa, S.A. Neuroglia as targets for drug delivery systems: A review. Nanomedicine 2017, 13, 667–679. [Google Scholar] [CrossRef]
- Franze, K.; Gerdelmann, J.; Weick, M.; Betz, T.; Pawlizak, S.; Lakadamyali, M.; Bayer, J.; Rillich, K.; Gögler, M.; Lu, Y.-B.; et al. Neurite Branch Retraction Is Caused by a Threshold-Dependent Mechanical Impact. Biophys. J. 2009, 97, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Georges, P.C.; Miller, W.J.; Meaney, D.F.; Sawyer, E.S.; Janmey, P.A. Matrices with Compliance Comparable to that of Brain Tissue Select Neuronal over Glial Growth in Mixed Cortical Cultures. Biophys. J. 2006, 90, 3012–3018. [Google Scholar] [CrossRef]
- Goganau, I.; Sandner, B.; Weidner, N.; Fouad, K.; Blesch, A. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp. Neurol. 2018, 300, 247–258. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Boukhaddaoui, H.; Campo, B.; Al-Jumaily, M.; Mayeux, V.; Greuet, D.; Valmier, J.; Scamps, F. Axotomy-Induced Expression of Calcium-Activated Chloride Current in Subpopulations of Mouse Dorsal Root Ganglion Neurons. J. Neurophysiol. 2003, 90, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Wei, C.; Chow, J.K.; Nguy, L.; Nguyen, H.K.; Schmidt, C.E. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J. Neural Eng. 2013, 10, 046011. [Google Scholar] [CrossRef]
- Lee, J.M.; Moon, J.Y.; Kim, T.H.; Lee, S.W.; Ahrberg, C.D.; Chung, B.G. Conductive hydrogel/nanowire micropattern-based sensor for neural stem cell differentiation. Sens. Actuators B 2018, 258, 1042–1050. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, S.; Lei, T.; Kim, Y.; Niu, S.; Wang, H.; Wang, X.; Foudeh, A.M.; Tok, J.B.H.; et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019, 3, 58–68. [Google Scholar] [CrossRef]
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for Neural Tissue Engineering. Front. Nanotechnol. 2021, 3, 643507. [Google Scholar] [CrossRef]
- Zou, L.-B.; Gong, J.-Y.; Ju, X.-J.; Liu, Z.; Wang, W.; Xie, R.; Chu, L.-Y. Smart membranes for biomedical applications. Chin. J. Chem. Eng. 2022, 49, 34–45. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Pan, H.; Min, L.; Zheng, H.; Zhu, H.; Liu, G.; Yang, W.; Chen, X.; Hou, X. Bioinspired liquid gating membrane-based catheter with anticoagulation and positionally drug release properties. Sci. Adv. 2020, 6, eabb4700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ju, X.-J.; Wang, W.; Xie, R.; Jiang, L.; Chen, Q.; Zhang, Y.-Q.; Wu, J.-F.; Chu, L.-Y. Stimuli-Responsive Capsule Membranes for Controlled Release in Pharmaceutical Applications. Curr. Pharm. Des. 2017, 23, 295–301. [Google Scholar] [CrossRef]
- Chu, L.; Xie, R.; Ju, X. Stimuli-responsive Membranes: Smart Tools for Controllable Mass-transfer and Separation Processes. Chin. J. Chem. Eng. 2011, 19, 891–903. [Google Scholar] [CrossRef]
- Wise, D.L. Handbook of Pharmaceutical Controlled Release Technology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Misra, A.; Jogani, V.; Jinturkar, K.; Vyas, T. Recent Patents Review on Intranasal Administration for CNS Drug Delivery. Recent PAt. Drug Deliv. Formul. 2008, 2, 25–40. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Xie, R.; Ju, X.-J.; Chu, L.-Y. Stimuli-responsive smart gating membranes. Chem. Soc. Rev. 2016, 45, 460–475. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Zhang, P.; Qiu, L.-D.; Chen, T.; Wang, W.; Chu, L.-Y. Controllable microfluidic fabrication of microstructured functional materials. Biomicrofluidics 2020, 14, 061501. [Google Scholar] [CrossRef]
- Zhang, M.-J.; Wang, W.; Xie, R.; Ju, X.-J.; Liu, L.; Gu, Y.-Y.; Chu, L.-Y. Microfluidic fabrication of monodisperse microcapsules for glucose-response at physiological temperature. Soft Matter 2013, 9, 4150. [Google Scholar] [CrossRef]
- Wang, Y.; Boero, G.; Zhang, X.; Brugger, J. Thermal and pH Sensitive Composite Membrane for On-Demand Drug Delivery by Applying an Alternating Magnetic Field. Adv. Mater. Interfaces 2020, 7, 2000733. [Google Scholar] [CrossRef]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced anti-cancer activity by localized delivery of curcumin form PVA/CNCs hydrogel membranes: Preparation and in vitro bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Fahmy, A.; Taha, T.H.; El-Fakharany, E.M.; Makram, M.; Soliman, H.M.A.; Shehata, H. Thermo-and pH-sensitive hydrogel membranes composed of poly(N-isopropylacrylamide)-hyaluronan for biomedical applications: Influence of hyaluronan incorporation on the membrane properties. Int. J. Biol. Macromol. 2018, 106, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Nagarjuna, G.; Babu, P.; Maruthi, Y.; Parandhama, A.; Madhavi, C.; Subha, M.; Chowdojirao, K. Interpenetrating Polymer Network Hydrogel Membranes of Karayagum and Sodium Alginate for Control Release of Flutamide Drug. J. Appl. Pharm. Sci. 2016, 6, 011–019. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombino, S.; Sole, R.; Curcio, F.; Cassano, R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Membranes 2023, 13, 576. https://doi.org/10.3390/membranes13060576
Trombino S, Sole R, Curcio F, Cassano R. Polymeric Based Hydrogel Membranes for Biomedical Applications. Membranes. 2023; 13(6):576. https://doi.org/10.3390/membranes13060576
Chicago/Turabian StyleTrombino, Sonia, Roberta Sole, Federica Curcio, and Roberta Cassano. 2023. "Polymeric Based Hydrogel Membranes for Biomedical Applications" Membranes 13, no. 6: 576. https://doi.org/10.3390/membranes13060576
APA StyleTrombino, S., Sole, R., Curcio, F., & Cassano, R. (2023). Polymeric Based Hydrogel Membranes for Biomedical Applications. Membranes, 13(6), 576. https://doi.org/10.3390/membranes13060576









