Resourceful Treatment of Battery Recycling Wastewater Containing H2SO4 and NiSO4 by Diffusion Dialysis and Electrodialysis
Abstract
1. Introduction
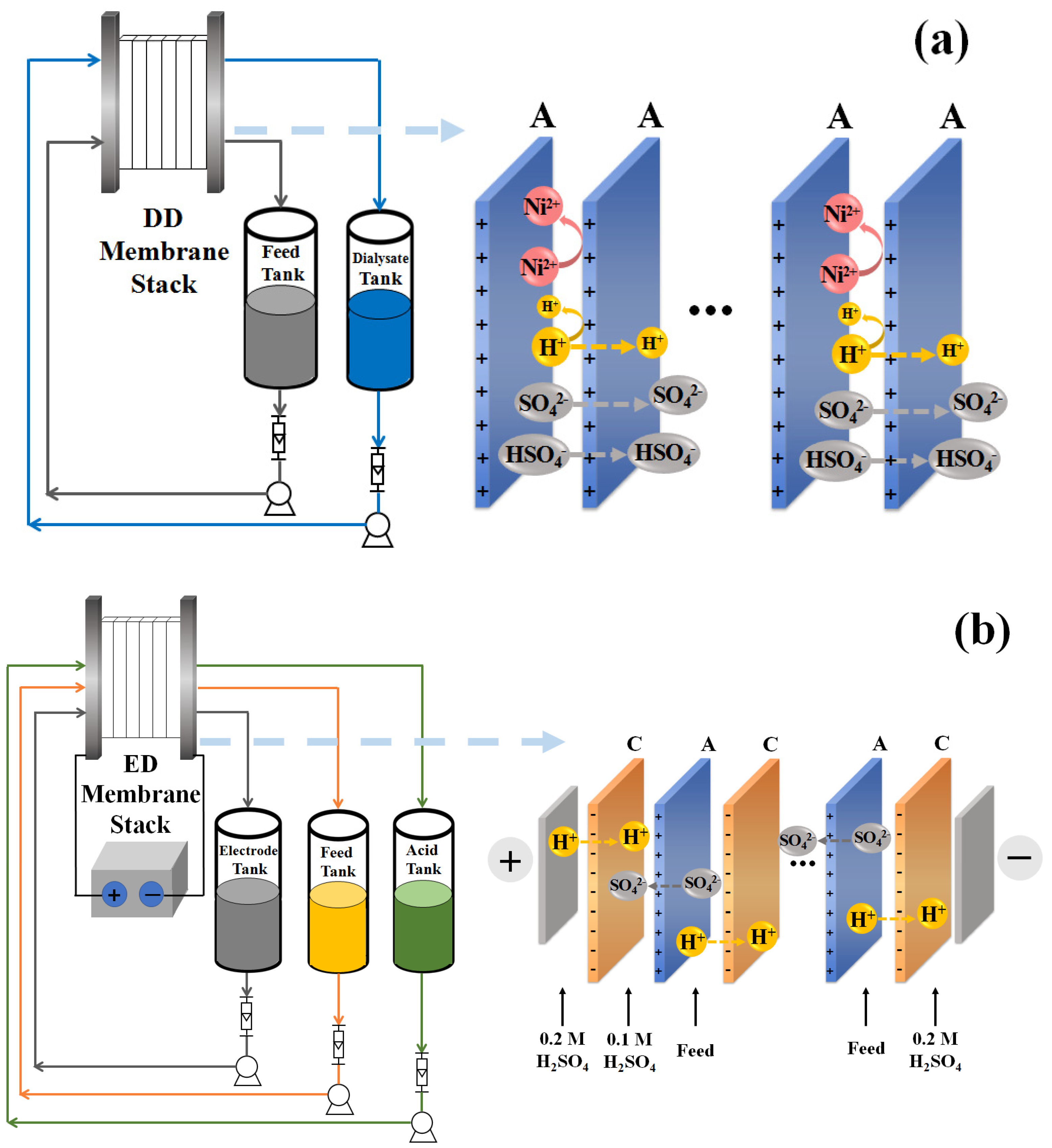
2. Materials and Methods
2.1. Materials
2.2. Analysis
3. Results
3.1. Diffusion Dialysis Process
3.1.1. Effect of Flow Rate
3.1.2. Effect of the Flow Rate Ratio
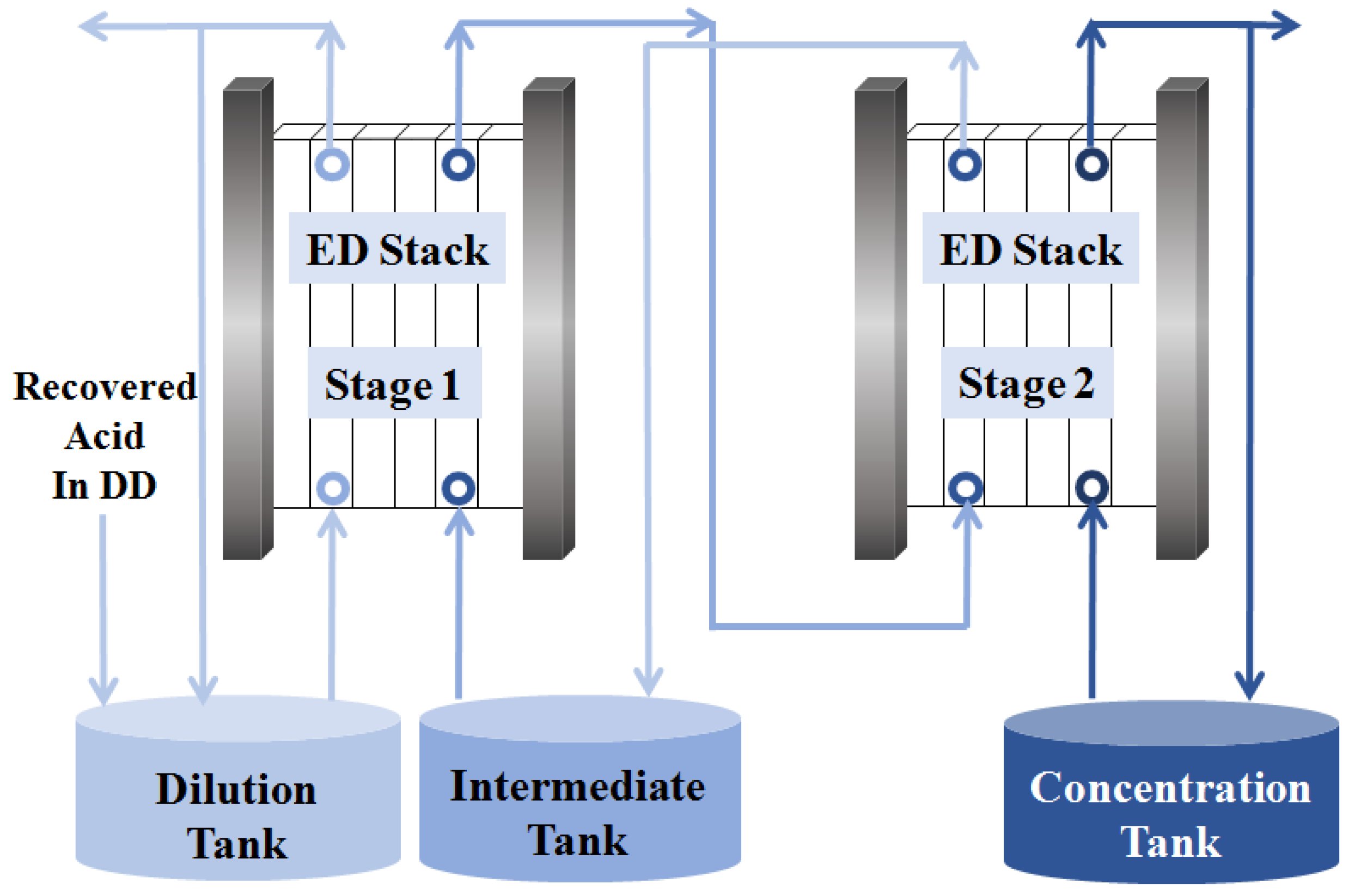
3.2. Electrodialysis Process
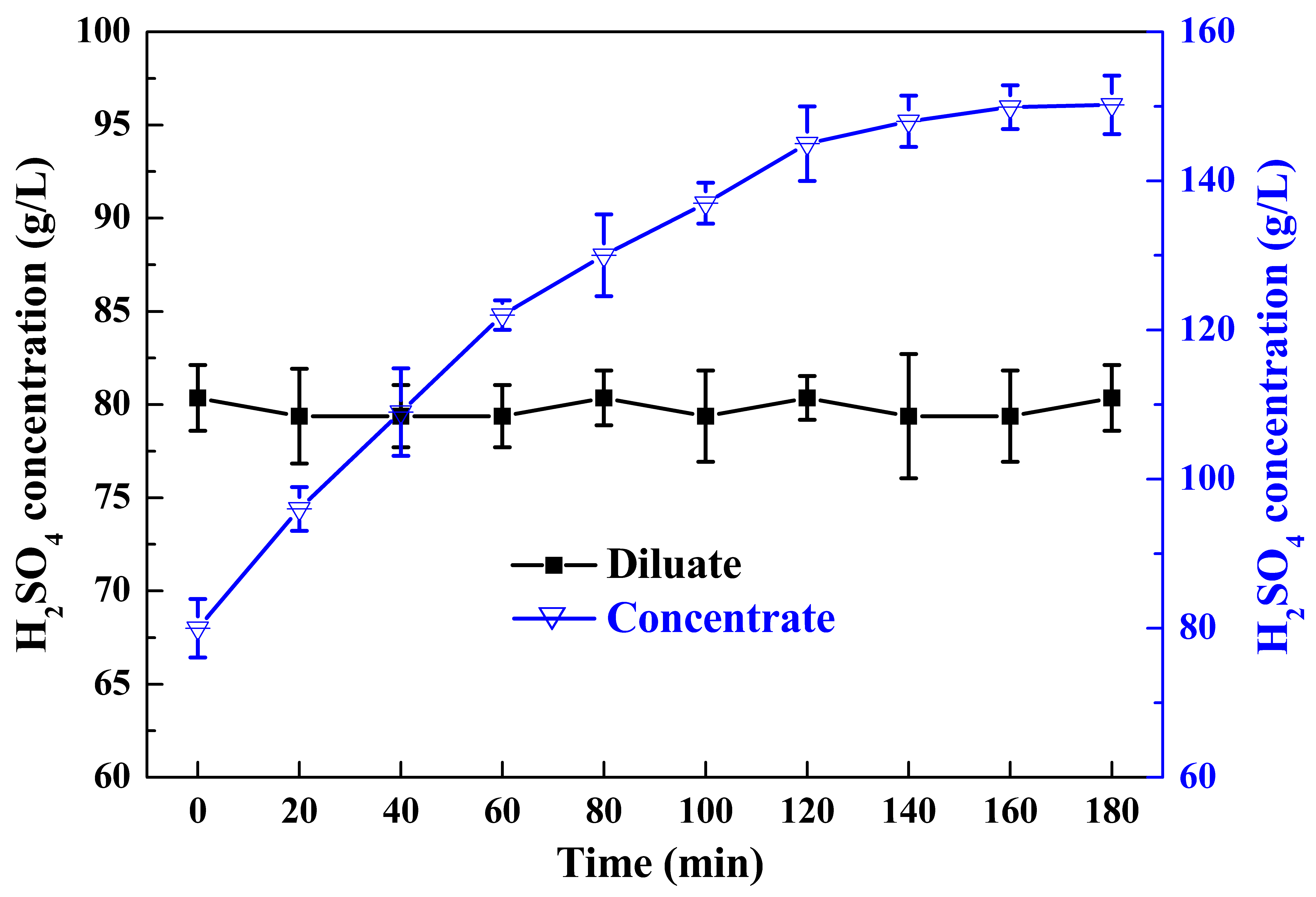
3.2.1. The First Stage of ED
3.2.2. The Second Stage of ED
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zheng, X.H.; Zhu, Z.W.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.B.; Sun, Z. A mini-review on metal recycling from spent lithium ion batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.X.; Li, M.; Chen, R.J.; Wu, F.; Amine, K.; Lu, J. The recycling of spent lithium-ion batteries: A review of current processes and technologies. Electrochem. Energ. Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J. A review of lithium supply and demand and a preliminary investigation of a room temperature method to recycle lithium ion batteries to recover lithium and other materials. Procedia CIRP 2014, 15, 289–293. [Google Scholar] [CrossRef]
- Lv, W.G.; Wang, Z.H.; Cao, H.B.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. Acs Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Chan, K.H.; Anawati, J.; Malik, M.; Azimi, G. Closed-Loop recycling of lithium, cobalt, nickel, and manganese from waste lithium-ion batteries of electric vehicles. ACS Sustain. Chem. Eng. 2021, 9, 4398–4410. [Google Scholar] [CrossRef]
- Chen, W.S.; Ho, H. Recovery of valuable metals from lithium-ion batteries NMC cathode waste materials by hydrometallurgical methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef]
- Li, S.; Dai, M.; Wu, Y.N.; Fu, H.; Hou, X.T.; Peng, C.S.; Luo, H.H. Resource utilization of electroplating wastewater: Obstacles and solutions. Environ. Sci. Water Res. Technol. 2022, 8, 484–509. [Google Scholar] [CrossRef]
- Agrawal, A.; Sahu, K.K. An overview of the recovery of acid from spent acidic solutions from steel and electroplating industries. J. Hazard. Mater. 2009, 171, 61–75. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Sanaeepur, H.; Amooghin, A.E.; Shirazi, M. Recovery of precious metals from industrial wastewater towards resource recovery and environmental sustainability: A critical review. Desalination 2022, 527, 115510. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Proc. Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Kesieme, U.; Chrysanthou, A.; Catulli, M.; Cheng, C.Y. A review of acid recovery from acidic mining waste solutions using solvent extraction. J. Chem. Technol. Biotechnol. 2018, 93, 5728. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.; Gontijo, D.; Amaral, M. One-step recycling of mineral acid from concentrated gold mining wastewater by high-temperature liquid–liquid extraction. Sep. Purif. Technol. 2022, 286, 120447. [Google Scholar] [CrossRef]
- Wei, Q.F.; Ren, X.L.; Guo, J.J.; Chen, Y.X. Recovery and separation of sulfuric acid and iron from dilute acidic sulfate effluent and waste sulfuric acid by solvent extraction and stripping. J. Hazard. Mater. 2016, 304, 1–9. [Google Scholar]
- Oden, M.K.; Sari-Erkan, H. Treatment of metal plating wastewater using iron electrode by electrocoagulation process: Optimization and process performance. Process Saf. Environ. 2018, 119, 207–217. [Google Scholar] [CrossRef]
- Nenov, V.; Dimitrova, N.; Dobrevsky, I. Recovery of sulphuric acid from waste aqueous solutions containing arsenic by ion exchange. Hydrometallurgy 1997, 44, 43–52. [Google Scholar] [CrossRef]
- López, J.; Gibert, O.; Cortina, J.L. Integration of membrane technologies to enhance the sustainability in the treatment of metal-containing acidic liquid wastes. An overview. Sep. Purif. Technol. 2021, 265, 118485. [Google Scholar] [CrossRef]
- Lopez, J.; Gibert, O.; Cortina, J.L. Evaluation of an extreme acid-resistant sulphonamide based nanofiltration membrane for the valorisation of copper acidic effluents. Chem. Eng. J. 2020, 405, 127015. [Google Scholar] [CrossRef]
- Culcasi, A.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Micale, G. Design of a novel membrane-integrated waste acid recovery process from pickling solution. J. Clean. Prod. 2019, 236, 117623. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, J.P. Recovery of sulfuric acid from a stone coal acid leaching solution by diffusion dialysis. Hydrometallurgy 2017, 173, 9–14. [Google Scholar] [CrossRef]
- Ruiz-Aguirre, A.; Lopez, J.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Cortina, J.L.; Micale, G. Diffusion dialysis for the treatment of H2SO4-CuSO4 solutions from electroplating plants: Ions membrane transport characterization and modelling. Sep. Purif. Technol. 2021, 266, 118215. [Google Scholar] [CrossRef]
- Strathmann, H. Ion Exchange Membrane Separation Processes. In Membrane Science and Technology Series; Elsevier B.V.: Amsterdam, The Netherlands, 2004; Volume 9, pp. 205–212. [Google Scholar]
- Xu, T.W. Ion exchange membranes: State of their development and perspective. J. Memb. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Chaimongkalayon, N.; Thanasupsin, S.P. Acidic recovery from wastewater of automotive battery plant using membrane technology. Appl. Environ. Res. 2016, 38, 33–41. [Google Scholar] [CrossRef]
- Juve, J.; Christensen, F.; Wang, Y.; Wei, Z.S. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Mohammadi, R.; Tang, W.; Sillanpaa, M. A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis. Desalination 2021, 498, 114626. [Google Scholar] [CrossRef]
- Yuzer, B.; Aydin, M.I.; Yildiz, H.; Hasancebi, B.; Selcuk, H.; Kadmi, Y. Optimal performance of electrodialysis process for the recovery of acid wastes in wastewater: Practicing circular economy in aluminum finishing industry. Chem. Eng. J. 2022, 434, 134755. [Google Scholar] [CrossRef]
- Chen, Q.B.; Zhang, G.; Liu, R.P.; Ji, Q.H.; Liu, H.J. Integration of concentration and electro-driven membrane system for effective water-saved acid recycling performance. Resour. Conserv. Recycl. 2023, 191, 106885. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.M.; Huang, J.; Zhu, X.B.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar]
- Gineste, J.L.; Pourcelly, G.; Lorrain, Y.; Persin, F.; Gavach, C. Analysis of factors limiting the use of bipolar membranes: A simplified model to determine trends. J. Memb. Sci. 1996, 112, 199–208. [Google Scholar] [CrossRef]
- Wang, K.; Xing, W.H.; Zhong, Z.X.; Fan, Y.Q. Study on adsorption phenomenon of diffusion dialysis for acid recovery. Sep. Purif. Technol. 2013, 110, 144–149. [Google Scholar] [CrossRef]
- Lopez, J.; Oliveira, R.R.; Reig, M.; Vecino, X.; Gibert, O.; Juan, A.; Cortina, J.L. Acid recovery from copper metallurgical process streams polluted with arsenic by diffusion dialysis. J. Environ. Chem. Eng. 2021, 9, 104692. [Google Scholar] [CrossRef]
- Xu, T.W.; Yang, W.H. Industrial recovery of mixed acid (HF + HNO3) from the titanium spent leaching solutions by diffusion dialysis with a new series of anion exchange membranes. J. Memb. Sci. 2003, 220, 89–95. [Google Scholar] [CrossRef]
- Lan, S.J.; Wen, X.M.; Zhu, Z.H.; Shao, F.; Zhu, C.L. Recycling of spent nitric acid solution from electrodialysis by diffusion dialysis. Desalination 2011, 278, 227–230. [Google Scholar] [CrossRef]
- Xuan, T.L. Concentration polarization and conductance of cation exchange membranes in sulfuric acid and alkaline sulfate media. J. Memb. Sci. 2012, 397, 66–79. [Google Scholar]
- Cerva, M.; Gurreri, L.; Tedesco, M.; Cipollina, A.; Ciofalo, M.; Tamburini, A.; Micale, G. Determination of limiting current density and current efficiency in electrodialysis units. Desalination 2018, 445, 138–148. [Google Scholar] [CrossRef]
- Yan, H.Y.; Wang, Y.M.; Wu, L.; Shehazd, M.; Jiang, C.X.; Fu, R.Q.; Liu, Z.M.; Xu, T.W. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Memb. Sci. 2019, 570, 245–257. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Lu, W.H.; Ren, H.Y.; Cong, W. Recovery of glutamic acid from isoelectric supernatant using electrodialysis. Sep. Purif. Technol. 2007, 55, 274–280. [Google Scholar] [CrossRef]
- Loza, S.; Loza, N.; Korzhov, A.; Romanyuk, N.; Kovalchuk, N.; Melnikov, S. Hybrid membrane technology for acid recovery from wastewater in coated steel wire production: A pilot scale study. Membranes 2022, 12, 1196. [Google Scholar] [CrossRef]




| Component | Concentration (g/L) |
|---|---|
| Ni | 46.85 |
| Fe | 1.74 |
| H2SO4 | 57.33 |
| H3PO4 | 1.45 |
| Membrane | Thickness (μm) | Area Resistance * (Ω·cm2) | Burst Strength (MPa) | pH | Tolerable Temperature (°C) | Used in |
|---|---|---|---|---|---|---|
| HWTT® DD-6 | 100 | 1.0 | ≥0.25 | 0–14 | 10–40 | DD |
| HWTT® A2N | 100 | 3.0 | ≥0.25 | 0–10 | 10–40 | ED-1 |
| LANCYTOM® CT-4 | 100 | 3.6 | ≥0.50 | 0–14 | 25–40 | ED-1/2 |
| LANCYTOM® ATD | 160 | 4.3 | ≥0.60 | 0–4 | 25–40 | ED-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhu, H.; Wu, Y.; Li, S.; Zhang, G.; Miao, Z. Resourceful Treatment of Battery Recycling Wastewater Containing H2SO4 and NiSO4 by Diffusion Dialysis and Electrodialysis. Membranes 2023, 13, 570. https://doi.org/10.3390/membranes13060570
Wu S, Zhu H, Wu Y, Li S, Zhang G, Miao Z. Resourceful Treatment of Battery Recycling Wastewater Containing H2SO4 and NiSO4 by Diffusion Dialysis and Electrodialysis. Membranes. 2023; 13(6):570. https://doi.org/10.3390/membranes13060570
Chicago/Turabian StyleWu, Sifan, Haitao Zhu, Yaqin Wu, Shuna Li, Gaoqi Zhang, and Zhiwei Miao. 2023. "Resourceful Treatment of Battery Recycling Wastewater Containing H2SO4 and NiSO4 by Diffusion Dialysis and Electrodialysis" Membranes 13, no. 6: 570. https://doi.org/10.3390/membranes13060570
APA StyleWu, S., Zhu, H., Wu, Y., Li, S., Zhang, G., & Miao, Z. (2023). Resourceful Treatment of Battery Recycling Wastewater Containing H2SO4 and NiSO4 by Diffusion Dialysis and Electrodialysis. Membranes, 13(6), 570. https://doi.org/10.3390/membranes13060570







