Lyotropic Liquid Crystal (LLC)-Templated Nanofiltration Membranes by Precisely Administering LLC/Substrate Interfacial Structure
Abstract
1. Introduction
2. Fabrication of LLC Active Layer with Suitable Structure Retention
2.1. LLC Precursor on Porous Substrates
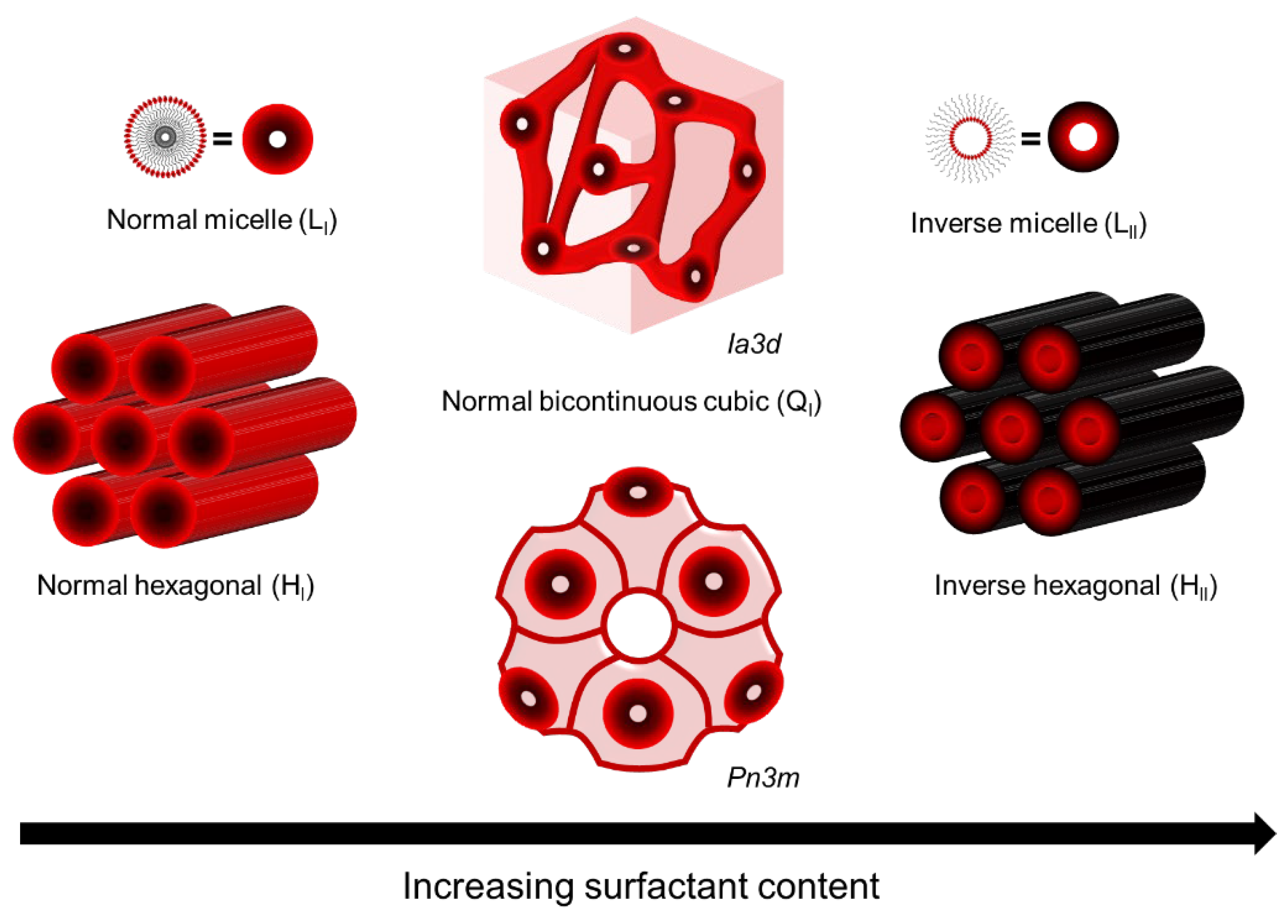
2.1.1. LLC Phases
2.1.2. LLC Phase Behavior
2.1.3. Polar Solvent
2.2. Ultrathin LLC Film Formation on Porous Substrates
2.2.1. LLC Templating
- Establishing strong enough thermodynamic interactions between the surfactant template and polymer;
- Increasing the system viscosity and chain entanglement by forming covalent and limiting species diffusion.
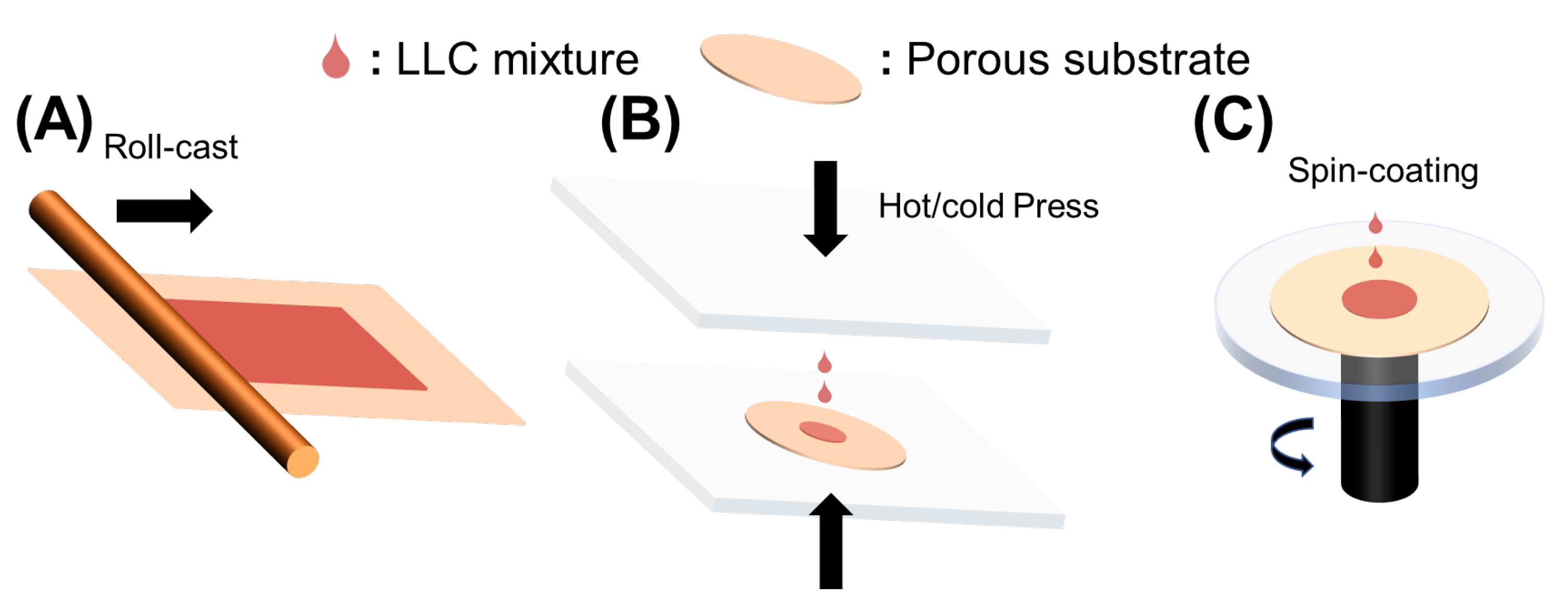
2.2.2. Fabrication Process
2.3. Performance of TFC Membranes with LLC as the Active Layer
3. Effect of Substrate on TFC’s Structures and Performance
3.1. Substrate’s Effect on Traditional TFC’s Structures and Performances
3.1.1. Pore Structures
3.1.2. Hydrophilicity
3.1.3. Roughness
3.2. Substrate’s Effect on LLC-Based Active Layer
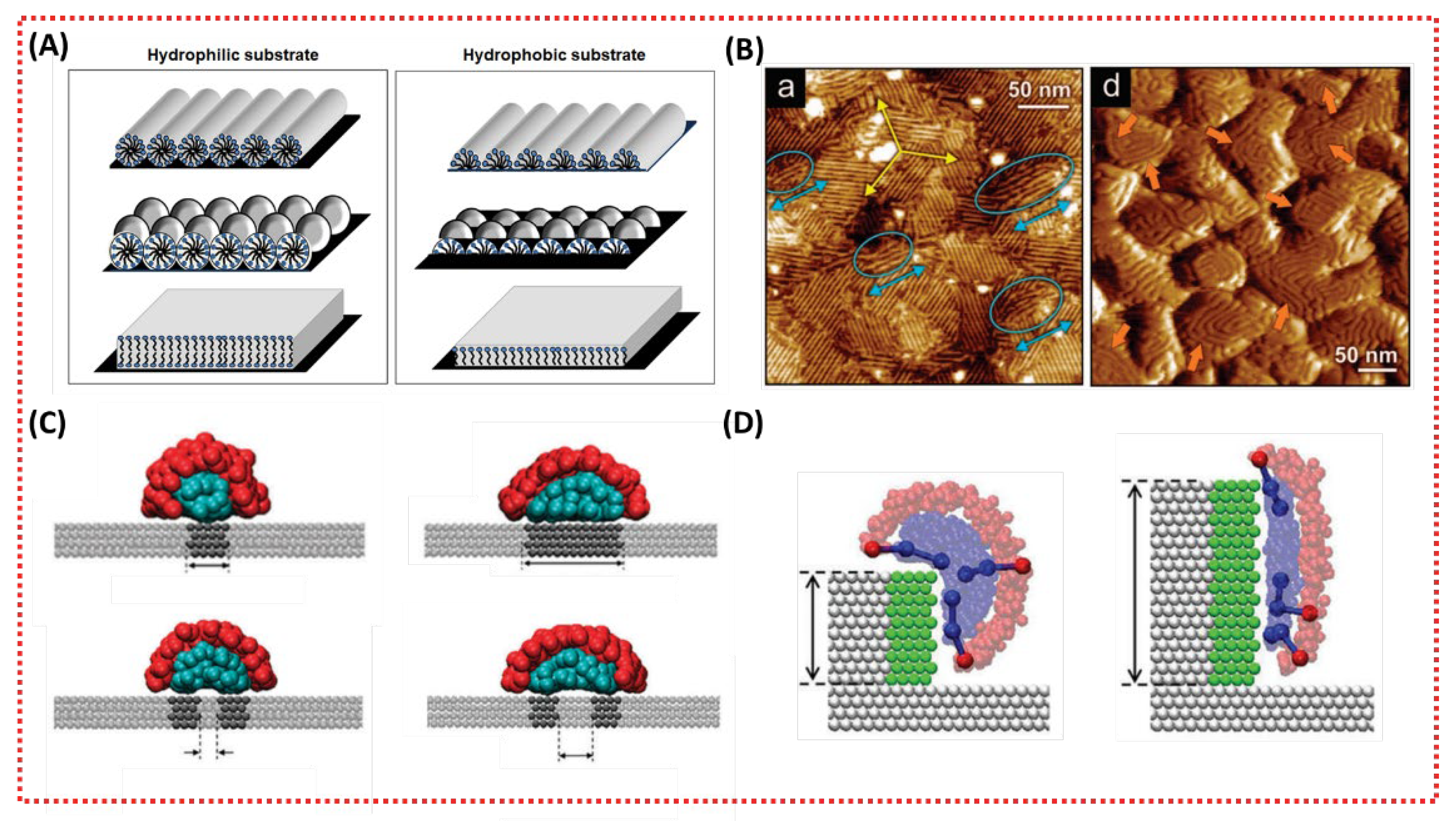
3.2.1. Effects of Substrate Hydrophilicity on the Structure of LLC Mesophases
3.2.2. Effects of Substrate Heterogeneity on the Structure of LLC Mesophases
4. Substrate Surface Modification
4.1. Ideal Substrate Surface Structure and Properties
4.2. Surface Modification
- Surface deposition;
- Surface grafting.
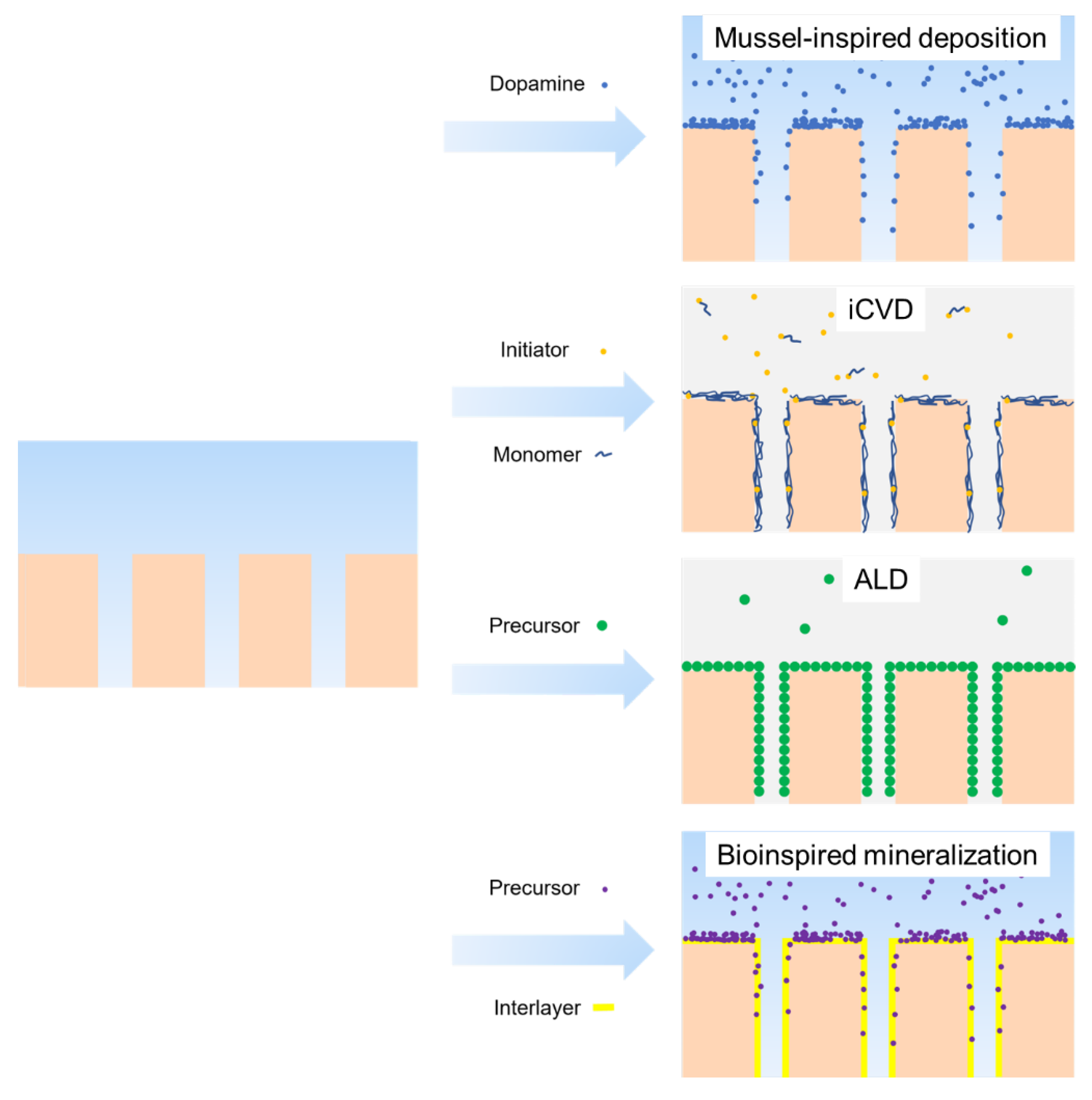
4.2.1. Surface Deposition
- The coating layer thickness can be easily adjusted by varying the deposition time and solvent concentration, which prevents pore size blocking, especially for UF [129];
- Further functionalization can be achieved since the deposition layer introduces amino and hydroxyl groups onto the membrane surface [130].
4.2.2. Surface Grafting
4.3. Interlayer
4.3.1. Polymeric Interlayer
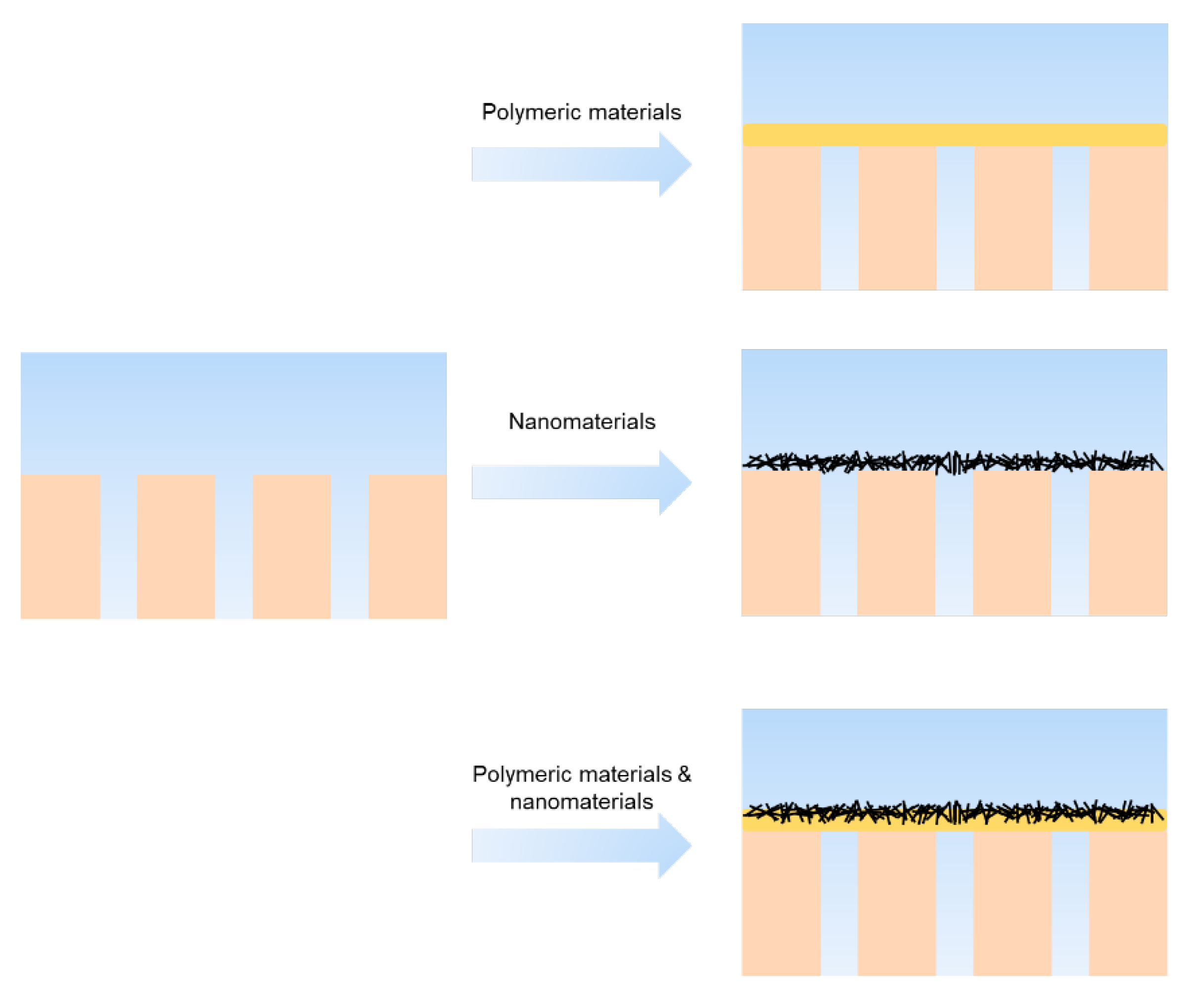
4.3.2. Nanomaterial as Interlayer
4.3.3. The Effect of Interlayer on the Interface of Interlayer/Substrate and Interlayer/Active Layer
- I.
- Reducing the surface pore size and roughness and increasing surface hydrophilicity can improve the dispersion of the pre-polymerization solvent, resulting in an active layer with minimal defects.
- II.
- Enhancing surface wettability and altering surface charge can control the diffusion of monomers and adjust the thickness and crosslinking degree of the active layer [31].
- III.
- Introducing an interlayer can improve the confinement effect for interfacial degassed nanobubbles, increasing the surface roughness of the active layer, and enhancing permeability.
- IV.
- Functional groups on the interlayer can participate in the formation reaction of the active layer, and thereby improving membrane rejection.
- V.
- Overall permeability can be improved by shortening the water path in the less-permeable active layer and increasing the water path in a more permeable interlayer [30].
4.3.4. Machine Learning for Interlayer Material Choose and Prediction of Modified Surface Performance
- Data preparation: Gathering a dataset that includes information about various interlayer materials, their properties, and the corresponding surface modifications process and substrate surface properties. These data should cover a diverse range of materials and surface characteristics.
- Data preprocessing and feature engineering: Cleaning and preprocessing the collected data. This involves handling missing values, normalizing or scaling the data, and encoding categorical variables if necessary, then extracting relevant features from the dataset that can effectively capture the characteristics of interlayer materials and their impact on surface properties.
- Model training: Selecting an appropriate machine learning algorithm, such as regression and classification, depending on the specific prediction task. Splitting the dataset into training and testing sets and training the model using the training data.
- Model evaluation: Evaluating the trained model’s performance using the testing dataset. Using appropriate evaluation metrics.
- Predictions and model optimization: Once the model is trained and evaluated, utilizing it to make predictions on new, unseen data. Inputting the relevant features of an interlayer material and modification process and the model will provide predictions for substrate surface properties. Then, fine-tuning and optimizing the machine learning model to enhance its predictive accuracy. This may involve a hyperparameter tuning model.
5. Characterization of LLC Phase/Substrate Interface
5.1. X-ray and Neutron Techniques for the Characterization of the Structures of LLC Films
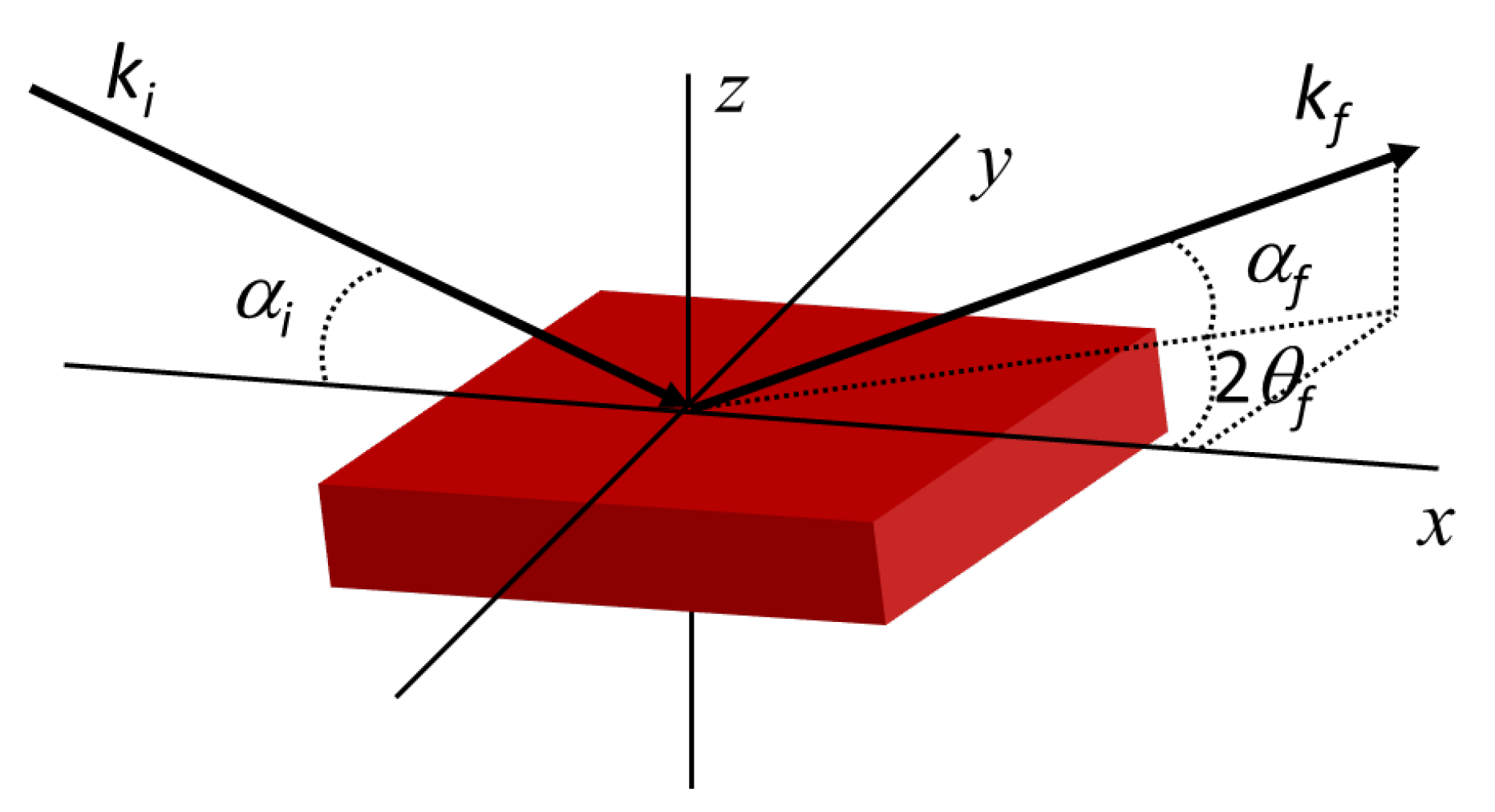
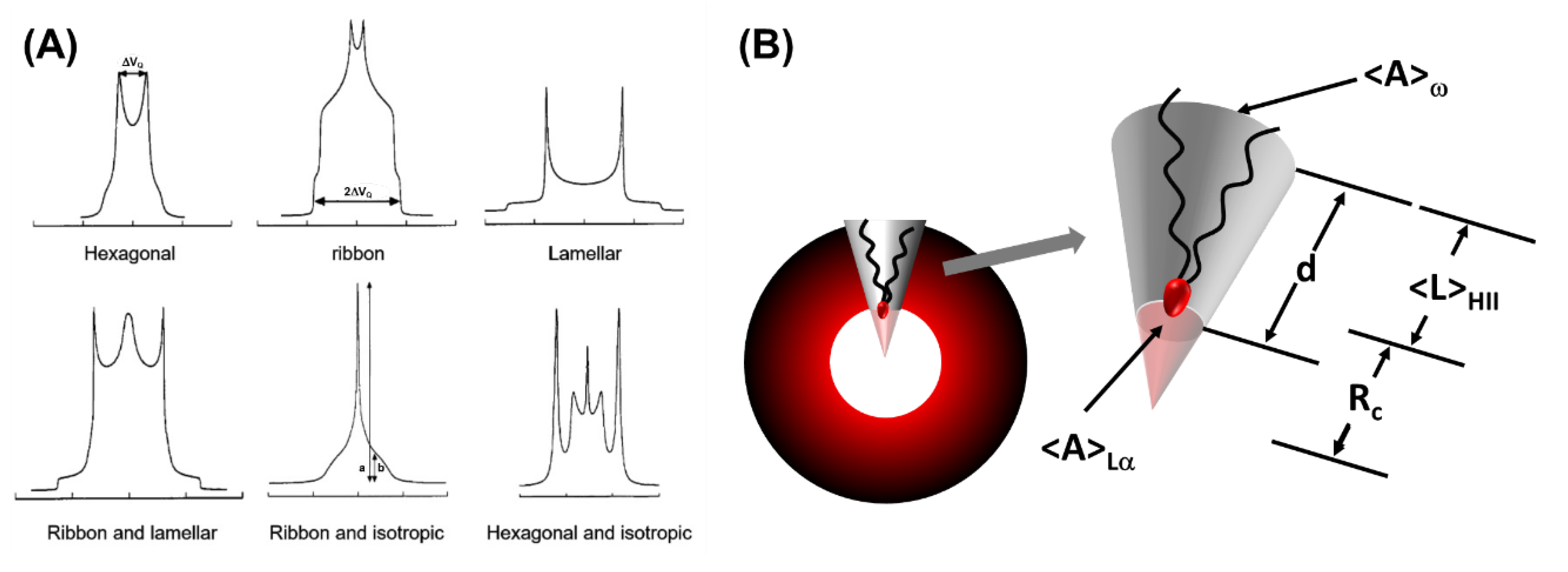
5.1.1. Small-Angle Scattering Technique
5.1.2. Small-Angle X-ray Scattering
5.1.3. Small-Angle Neutron Scattering
- Neutron is neutrally charged and non-destructive to costly samples, and they can deeply penetrate into the atom to interact directly with nuclei;
- The scattering power of neutrons is not related to the number of atoms, making lighter elements such as hydrogen more distinctive;
- Sample contrast can be altered to suit specific needs by partial deuteration.
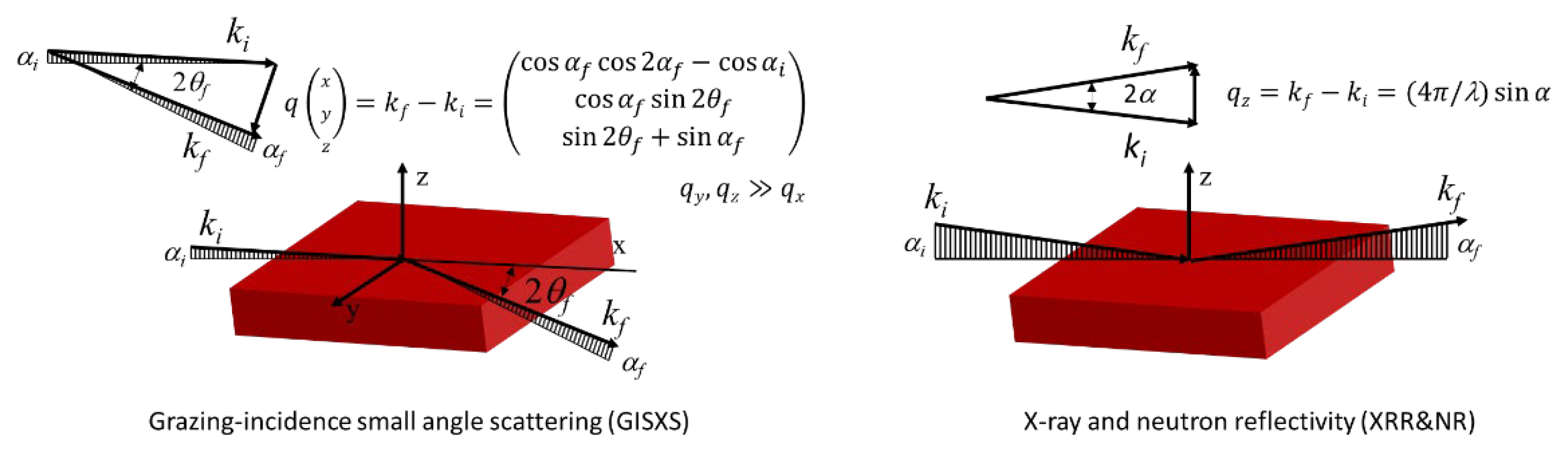
5.1.4. Grazing Incidence Small-Angle Scattering (GISAS)
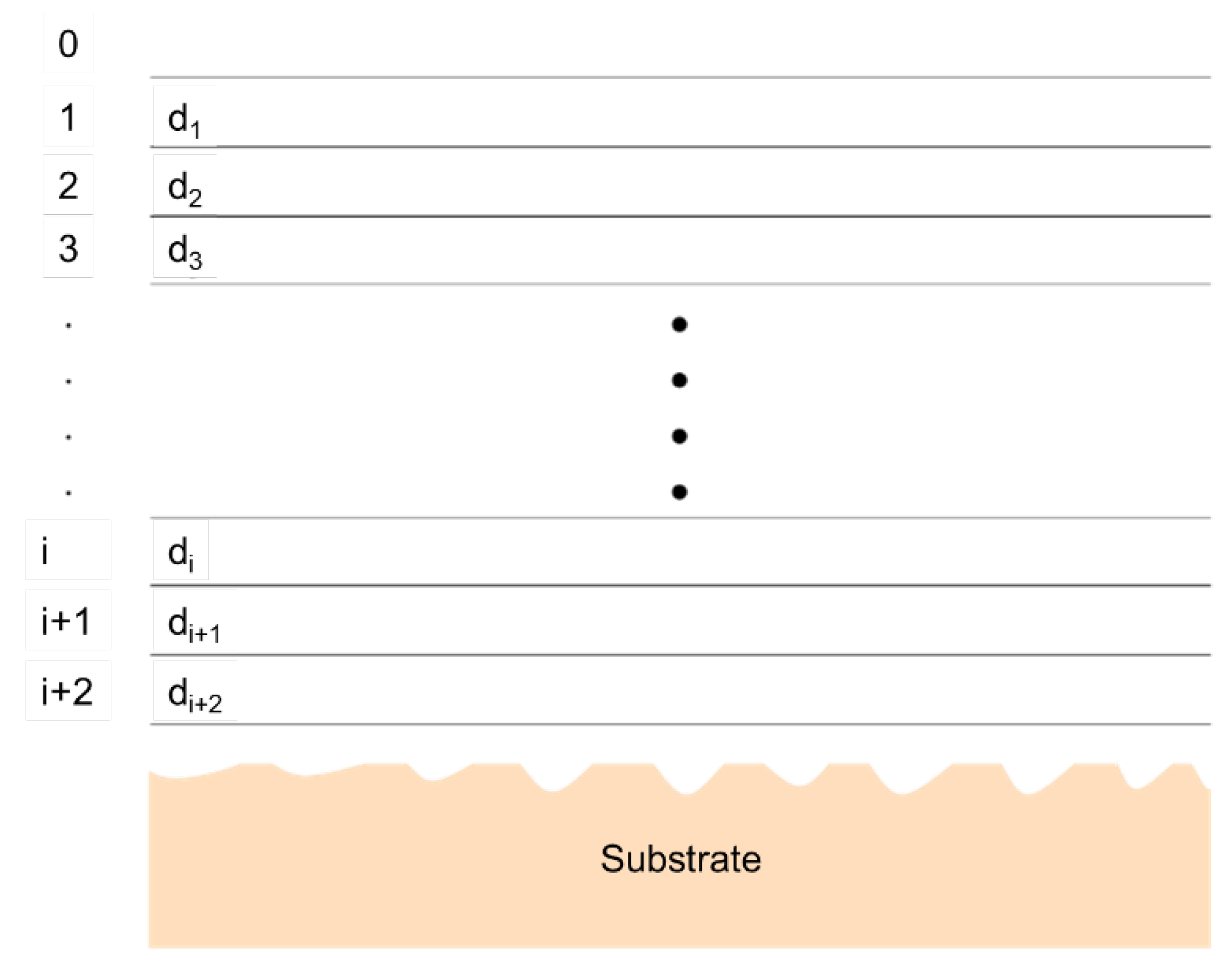
5.1.5. X-ray and Neutron Reflectivity
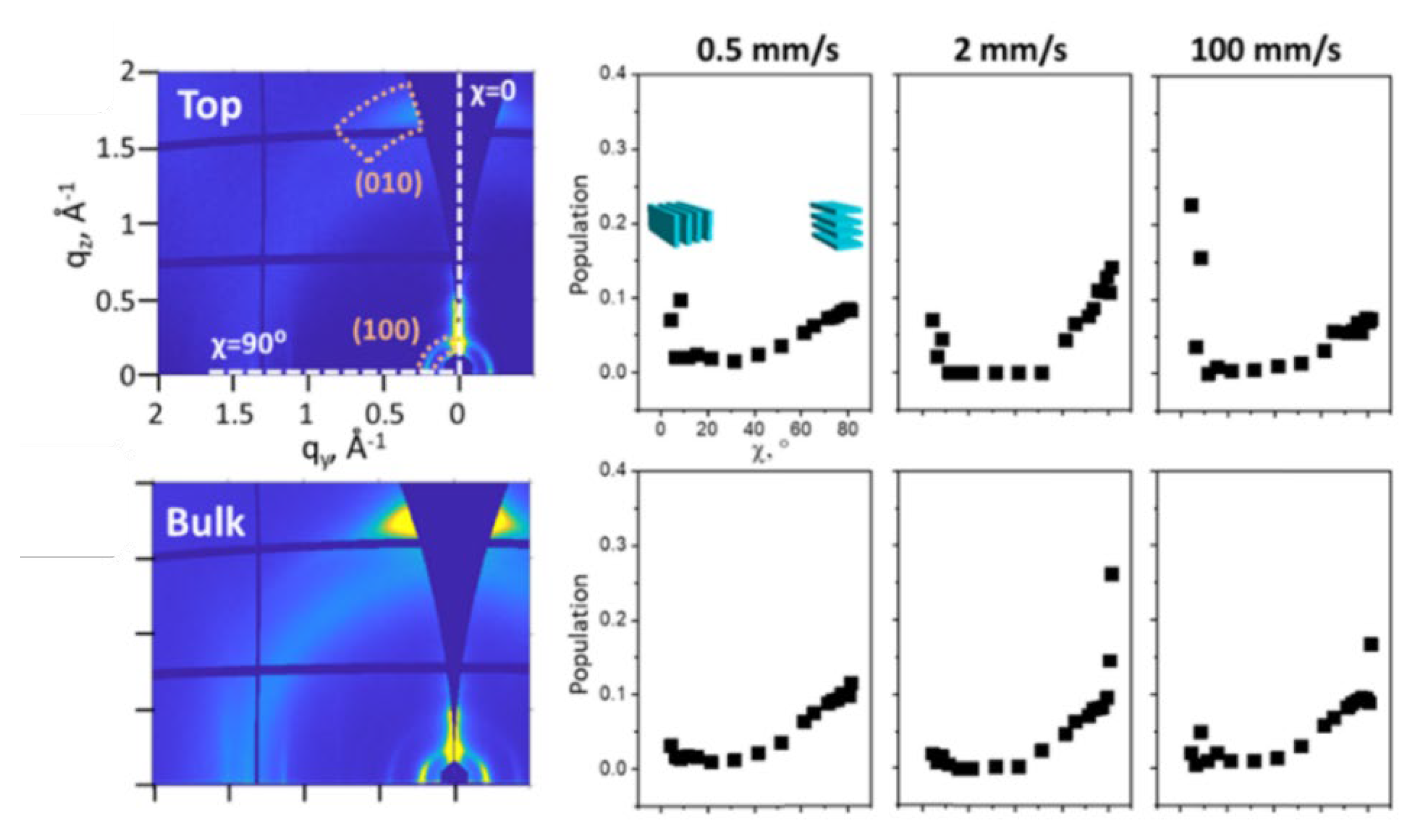
5.1.6. SAS Application in LLC Template Film
5.2. Deuterium NMR Spectroscopy
6. Conclusions and Future Perspectives
- Substrate’s effects on LLC phase structures: Although many studies have reported the potential effects of solid substrates on the types and shapes of surfactant mesophases, there has not been a comprehensive analysis of the LLC mesophase structures before and after polymerization at the LLC/substrate interface using SAXS and SANS techniques. To construct GI-mode measurements, ultrathin and uniform LLC membranes must be formed on substrates with varying surface hydrophilicity and roughness, and suitable conditions must be established (e.g., choosing a suitable beam incidence angle to detect the interfacial structures). To conduct neutron measurements, appropriate deuterated ingredients must be introduced to increase the system contrast;
- Structure retention of LLC parental structure on various substrates (substrate’s effect on polymerization): The retention of LLC parental structure is crucial to avoid phase separation, especially for the transcriptive templating system, and can be achieved by strong interaction between the surfactant template and polymers, as well as timely increase system viscosity and entanglement of polymer chains. In an individual system, the former factor is not easily affected by small changes in interfacial curvature induced by substrates, but changes can occur in the latter factor, which is determined by the polymerization rate. An increase in polymerization rate leads to an increase in the system’s viscosity and chains’ entanglement, which can counterbalance phase separation caused by the increase in free energy. However, the polymerization kinetics are determined by the segregation and diffusion behavior of monomers and initiators during polymerization. The curvature variation induced by substrates changes the LLC order, and for hydrophilic monomers, the polymerization rate increases with an increase in LLC order, while the rate decreases for hydrophobic monomers. In addition, the efficiency of a hydrophilic initiator decreases with an increase in LLC order, whereas it is the opposite for a hydrophobic initiator. Therefore, the substrate is indicated to influence the structure retention of LLC parental structure during polymerization, and a systematic study of this will undoubtedly help to fabricate the high-fidelity LLC-based TFC membranes;
- Substrate’s effect on reorientation: The substrate’s effect on the reorientation process of LLC cannot be ignored. Achieving less tortuosity in inner porous structures and high permeance of HLLC template membranes is possible through reorientation. However, the HLLC phase typically needs to be heated to a liquid-like isotropic phase during the reorientation process under a magnetic field and electric field, which causes the infusion of the LLC in the substrate. The surface properties of the substrate can interfere with the formation of the HLLC phase during this process. Therefore, designing the surface properties of the substrate suitably and studying the phase formation process in situ during the reorientation is essential for exploring LLC active layers with pores perpendicular to the surface;
- Substrate’s effect on mechanical properties: The mechanical properties of the LLC template material are significantly associated with the defects and durability of the membrane, which are determined by the width of the continuous phase and the crosslink density of the system. Curvature variation can lead to a wider continuous phase and decrease the crosslink density. Additionally, the substrate’s effect becomes more significant when the active layer’s thickness decreases. Therefore, to fabricate TFC with an ultrathin and flawless LLC active layer, it is essential to address the issue of maintaining or improving the robustness of the LLC template material.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A Review on Reverse Osmosis and Nanofiltration Membranes for Water Purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Garvey, C.J.; Zhao, H.; Huang, K.; Kong, L. Toward the fabrication of advanced nanofiltration membranes by controlling morphologies and mesochannel orientations of hexagonal lyotropic liquid crystals. Membranes 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Voutchkov, N. Energy use for membrane seawater desalination—Current status and trends. Desalination 2018, 431, 2–14. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication—A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Gin, D.L.; Bara, J.E.; Noble, R.D.; Elliott, B.J. Polymerized lyotropic liquid crystal assemblies for membrane applications. Macromol. Rapid Commun. 2008, 29, 367–389. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin film composite membrane for oily waste water treatment: Recent advances and challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Karki, S.; Ingole, P.G. Development of polymer-based new high performance thin-film nanocomposite nanofiltration membranes by vapor phase interfacial polymerization for the removal of heavy metal ions. Chem. Eng. J. 2022, 446, 137303. [Google Scholar] [CrossRef]
- Hatakeyama, E.S.; Gabriel, C.J.; Wiesenauer, B.R.; Lohr, J.L.; Zhou, M.; Noble, R.D.; Gin, D.L. Water filtration performance of a lyotropic liquid crystal polymer membrane with uniform, sub-1-nm pores. J. Membr. Sci. 2011, 366, 62–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, R.; Gabinet, U.R.; Poling-Skutvik, R.; Kim, N.K.; Lee, C.; Imran, O.Q.; Feng, X.; Osuji, C.O. Rapid Fabrication by Lyotropic Self-Assembly of Thin Nanofiltration Membranes with Uniform 1 Nanometer Pores. ACS Nano 2021, 15, 8192–8203. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, Y.; Liu, C.; Lee, K.-R.; Hung, W.-S.; Wang, Z.; Li, Y.; Elimelech, M.; Jin, J.; Lin, S. Polyamide nanofiltration membrane with highly uniform sub-nanometre pores for sub-1 Å precision separation. Nat. Commun. 2020, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Imran, Q.; Zhang, Y.; Sixdenier, L.; Lu, X.; Kaufman, G.; Gabinet, U.; Kawabata, K.; Elimelech, M.; Osuji, C.O. Precise nanofiltration in a fouling-resistant self-assembled membrane with water-continuous transport pathways. Sci. Adv. 2019, 5, eaav9308. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Kawabata, K.; Kaufman, G.; Elimelech, M.; Osuji, C.O. Highly selective vertically aligned nanopores in sustainably derived polymer membranes by molecular templating. ACS Nano 2017, 11, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tousley, M.E.; Cowan, M.G.; Wiesenauer, B.R.; Osuji, C.O. Scalable Fabrication of Polymer Membranes with Vertically Aligned 1 nm Pores by Magnetic Field Directed Self-Assembly. ACS Nano 2014, 8, 11977–11986. [Google Scholar] [CrossRef]
- Marets, N.; Kuo, D.; Torrey, J.R.; Sakamoto, T.; Kato, T. Highly Efficient Virus Rejection with Self-Organized Membranes Based on a Crosslinked Bicontinuous Cubic Liquid Crystal. Adv. Healthc. Mater. 2017, 6, 1700252. [Google Scholar] [CrossRef]
- Zhou, M.; Nemade, P.R.; Lu, X.; Zeng, X.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 2007, 129, 9574–9575. [Google Scholar] [CrossRef]
- Saadat, Y.; Imran, O.Q.; Osuji, C.O.; Foudazi, R. Lyotropic liquid crystals as templates for advanced materials. J. Mater. Chem. A 2021, 9, 21607–21658. [Google Scholar] [CrossRef]
- Striolo, A.; Grady, B.P. Surfactant assemblies on selected nanostructured surfaces: Evidence, driving forces, and applications. Langmuir 2017, 33, 8099–8113. [Google Scholar] [CrossRef]
- Peng, L.E.; Yang, Z.; Long, L.; Zhou, S.; Guo, H.; Tang, C.Y. A critical review on porous substrates of TFC polyamide membranes: Mechanisms, membrane performances, and future perspectives. J. Membr. Sci. 2022, 641, 119871. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub–10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Fang, W.; Wang, R.; Krantz, W.B. Effects of the support on the characteristics and permselectivity of thin film composite membranes. J. Membr. Sci. 2019, 580, 12–23. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hoek, E.M. Impacts of support membrane structure and chemistry on polyamide–polysulfone interfacial composite membranes. J. Membr. Sci. 2009, 336, 140–148. [Google Scholar] [CrossRef]
- Peng, L.E.; Yao, Z.; Yang, Z.; Guo, H.; Tang, C.Y. Dissecting the role of substrate on the morphology and separation properties of thin film composite polyamide membranes: Seeing is believing. Environ. Sci. Technol. 2020, 54, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gan, B.; Yang, Z.; Tang, C.Y.; Gao, C. Confined nanobubbles shape the surface roughness structures of thin film composite polyamide desalination membranes. J. Membr. Sci. 2019, 582, 342–349. [Google Scholar] [CrossRef]
- Park, S.-J.; Choi, W.; Nam, S.-E.; Hong, S.; Lee, J.S.; Lee, J.-H. Fabrication of polyamide thin film composite reverse osmosis membranes via support-free interfacial polymerization. J. Membr. Sci. 2017, 526, 52–59. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Guo, H.; Peng, L.E.; Ma, X.-H.; Song, X.-X.; Wang, Z.; Tang, C.Y. Mechanistic insights into the role of polydopamine interlayer toward improved separation performance of polyamide nanofiltration membranes. Environ. Sci. Technol. 2020, 54, 11611–11621. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, S.; Shi, W.; Zhu, J.; Van der Bruggen, B. Support membrane pore blockage (SMPB): An important phenomenon during the fabrication of thin film composite membrane via interfacial polymerization. Sep. Purif. Technol. 2019, 215, 670–680. [Google Scholar] [CrossRef]
- Dai, R.; Li, J.; Wang, Z. Constructing interlayer to tailor structure and performance of thin-film composite polyamide membranes: A review. Adv. Colloid Interface Sci. 2020, 282, 102204. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Xue, Y.-R.; Yang, H.-C.; Wu, J.; Xu, Z.-K. Surface and Interface Engineering of Polymer Membranes: Where We Are and Where to Go. Macromolecules 2022, 55, 3363–3383. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.-F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.-D.; Tang, C.Y. A critical review on thin-film nanocomposite membranes with interlayered structure: Mechanisms, recent developments, and environmental applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Yang, H.-C.; Du, Y.; Xu, Z.-K. Polyphenol coating as an interlayer for thin-film composite membranes with enhanced nanofiltration performance. ACS Appl. Mater. Interfaces 2016, 8, 32512–32519. [Google Scholar] [CrossRef] [PubMed]
- Müller-Buschbaum, P. GISAXS and GISANS as metrology technique for understanding the 3D morphology of block copolymer thin films. Eur. Polym. J. 2016, 81, 470–493. [Google Scholar] [CrossRef]
- Schindler, M.; Moulin, J.-F.; Müller-Buschbaum, P. Adhesive–adherent interfaces probed with grazing-incidence small-angle neutron scattering. J. Appl. Crystallogr. 2015, 48, 1047–1054. [Google Scholar] [CrossRef]
- Zhou, M.; Kidd, T.J.; Noble, R.D.; Gin, D.L. Supported lyotropic liquid-crystal polymer membranes: Promising materials for molecular-size-selective aqueous nanofiltration. Adv. Mater. 2005, 17, 1850–1853. [Google Scholar] [CrossRef]
- Hatakeyama, E.S.; Wiesenauer, B.R.; Gabriel, C.J.; Noble, R.D.; Gin, D.L. Nanoporous, bicontinuous cubic lyotropic liquid crystal networks via polymerizable gemini ammonium surfactants. Chem. Mater. 2010, 22, 4525–4527. [Google Scholar] [CrossRef]
- Firouzi, A.; Atef, F.; Oertli, A.; Stucky, G.; Chmelka, B. Alkaline lyotropic silicate—Surfactant liquid crystals. J. Am. Chem. Soc. 1997, 119, 3596–3610. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Bolisetty, S.; Mezzenga, R. Macroscopic Alignment of Lyotropic Liquid Crystals Using Magnetic Nanoparticles. Adv. Mater. 2011, 23, 3932–3937. [Google Scholar] [CrossRef]
- Vallooran, J.J.; Negrini, R.; Mezzenga, R. Controlling anisotropic drug diffusion in lipid-Fe3O4 nanoparticle hybrid mesophases by magnetic alignment. Langmuir 2013, 29, 999–1004. [Google Scholar] [CrossRef]
- Shen, C.; Matsubara, M.; Yabushita, M.; Maki, S.; Muramatsu, A.; Kanie, K. Magnetic field induced uniaxial alignment of the lyotropic liquid-crystalline PMMA-grafted Fe3O4 nanoplates with controllable interparticle interaction. Nanoscale Adv. 2020, 2, 814–822. [Google Scholar] [CrossRef]
- Ku, A.Y.; Saville, D.A.; Aksay, I.A. Electric-field-induced orientation of surfactant-templated nanoscopic silica. Langmuir 2007, 23, 8156–8162. [Google Scholar] [CrossRef]
- Trau, M.; Yao, N.; Kim, E.; Xia, Y.; Whitesides, G.; Aksay, I.A. Microscopic patterning of orientated mesoscopic silica through guided growth. Nature 1997, 390, 674–676. [Google Scholar] [CrossRef]
- Paineau, E.; Krapf, M.-E.M.; Amara, M.-S.; Matskova, N.V.; Dozov, I.; Rouzière, S.; Thill, A.; Launois, P.; Davidson, P. A liquid-crystalline hexagonal columnar phase in highly-dilute suspensions of imogolite nanotubes. Nat. Commun. 2016, 7, 10271. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Lu, X.; Zhang, Q.; Wu, J.; Wang, Y.; Bian, F.; Lu, Q.; Fei, Z.; Dyson, P.J. A facile approach for controlling the orientation of one-dimensional mesochannels in mesoporous titania films. J. Am. Chem. Soc. 2012, 134, 20238–20241. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Lu, X.; Lu, Q. A facile method to prepare macroscopically oriented mesostructured silica film: Controlling the orientation of mesochannels in multilayer films by air flow. J. Am. Chem. Soc. 2008, 130, 14356–14357. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, H.W.; Okubo, T.; Van Egmond, J.W.; Tsapatsis, M. Preparation of supported mesoporous silica layers in a continuous flow cell. Chem. Mater. 1997, 9, 1505–1507. [Google Scholar] [CrossRef]
- Hillhouse, H.W.; Van Egmond, J.W.; Tsapatsis, M. Highly oriented mesostructured thin films: Shear-induced deposition of optically anisotropic coatings of tungsten oxide/surfactant composites. Langmuir 1999, 15, 4544–4550. [Google Scholar] [CrossRef]
- Feng, X.; Nejati, S.; Cowan, M.G.; Tousley, M.E.; Wiesenauer, B.R.; Noble, R.D.; Elimelech, M.; Gin, D.L.; Osuji, C.O. Thin polymer films with continuous vertically aligned 1 nm pores fabricated by soft confinement. ACS Nano 2016, 10, 150–158. [Google Scholar] [CrossRef]
- Gruner, S.M. Stability of lyotropic phases with curved interfaces. J. Phys. Chem. 1989, 93, 7562–7570. [Google Scholar] [CrossRef]
- Kirk, G.L.; Gruner, S.M.; Stein, D. A thermodynamic model of the lamellar to inverse hexagonal phase transition of lipid membrane-water systems. Biochemistry 1984, 23, 1093–1102. [Google Scholar] [CrossRef]
- Mezzenga, R.; Seddon, J.M.; Drummond, C.J.; Boyd, B.J.; Schröder-Turk, G.E.; Sagalowicz, L. Nature-Inspired design and application of lipidic lyotropic liquid crystals. Adv. Mater. 2019, 31, 1900818. [Google Scholar] [CrossRef]
- Yaghmur, A.; Kriechbaum, M.; Amenitsch, H.; Steinhart, M.; Laggner, P.; Rappolt, M. Effects of Pressure and Temperature on the Self-Assembled Fully Hydrated Nanostructures of Monoolein−Oil Systems. Langmuir 2010, 26, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- De Campo, L.; Yaghmur, A.; Sagalowicz, L.; Leser, M.E.; Watzke, H.; Glatter, O. Reversible phase transitions in emulsified nanostructured lipid systems. Langmuir 2004, 20, 5254–5261. [Google Scholar] [CrossRef] [PubMed]
- Templer, R.; Seddon, J.; Warrender, N. Inverse bicontinuous cubic phases in fatty acid/phosphatidylcholine mixtures: The effects of pressure and lipid composition. Phys. Chem. Chem. Phys. 1999, 1, 887–893. [Google Scholar]
- Winter, R. Effects of hydrostatic pressure on lipid and surfactant phases. Curr. Opin. Colloid Interface Sci. 2001, 6, 303–312. [Google Scholar] [CrossRef]
- Okamoto, Y.; Masum, S.M.; Miyazawa, H.; Yamazaki, M. Low-pH-induced transformation of bilayer membrane into bicontinuous cubic phase in dioleoylphosphatidylserine/monoolein membranes. Langmuir 2008, 24, 3400–3406. [Google Scholar] [CrossRef] [PubMed]
- Awad, T.S.; Okamoto, Y.; Masum, S.M.; Yamazaki, M. Formation of cubic phases from large unilamellar vesicles of dioleoylphosphatidylglycerol/monoolein membranes induced by low concentrations of Ca2+. Langmuir 2005, 21, 11556–11561. [Google Scholar] [CrossRef]
- Hartnett, T.E.; Ladewig, K.; O’Connor, A.J.; Hartley, P.G.; McLean, K.M. Size and phase control of cubic lyotropic liquid crystal nanoparticles. J. Phys. Chem. B 2014, 118, 7430–7439. [Google Scholar] [CrossRef]
- Yaghmur, A.; De Campo, L.; Salentinig, S.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Oil-loaded monolinolein-based particles with confined inverse discontinuous cubic structure (Fd 3 m). Langmuir 2006, 22, 517–521. [Google Scholar] [CrossRef]
- Fong, W.-K.; Negrini, R.; Vallooran, J.J.; Mezzenga, R.; Boyd, B.J. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J. Colloid Interface Sci. 2016, 484, 320–339. [Google Scholar] [CrossRef]
- Iskandar, W.F.N.W.; Salim, M.; Hashim, R.; Zahid, N.I. Stability of cubic phase and curvature tuning in the lyotropic system of branched chain galactose-based glycolipid by amphiphilic additives. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126697. [Google Scholar] [CrossRef]
- Negrini, R.; Mezzenga, R. Diffusion, molecular separation, and drug delivery from lipid mesophases with tunable water channels. Langmuir 2012, 28, 16455–16462. [Google Scholar] [CrossRef] [PubMed]
- Auvray, X.; Perche, T.; Petipas, C.; Anthore, R.; Marti, M.; Rico, I.; Lattes, A. Influence of solvent-headgroup interactions on the formation of lyotropic liquid crystal phases of surfactants in water and nonaqueous protic and aprotic solvents. Langmuir 1992, 8, 2671–2679. [Google Scholar] [CrossRef]
- Carter, B.M.; Wiesenauer, B.R.; Hatakeyama, E.S.; Barton, J.L.; Noble, R.D.; Gin, D.L. Glycerol-based bicontinuous cubic lyotropic liquid crystal monomer system for the fabrication of thin-film membranes with uniform nanopores. Chem. Mater. 2012, 24, 4005–4007. [Google Scholar] [CrossRef]
- Greaves, T.L.; Weerawardena, A.; Fong, C.; Drummond, C.J. Many protic ionic liquids mediate hydrocarbon-solvent interactions and promote amphiphile self-assembly. Langmuir 2007, 23, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Worthington, K.S.; Baguenard, C.; Forney, B.S.; Guymon, C.A. Photopolymerization kinetics in and of self-assembling lyotropic liquid crystal templates. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 471–489. [Google Scholar] [CrossRef]
- Forney, B.S.; Baguenard, C.; Guymon, C.A. Effects of controlling polymer nanostructure using photopolymerization within lyotropic liquid crystalline templates. Chem. Mater. 2013, 25, 2950–2960. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Hill, A.J.; Cong, W.; She, F.H.; Gao, W.; Hoang, M.; Kong, L.X. Effects of a volatile solvent with low surface tension combining with the silica network reinforcement on retention of LLC structure in polymer matrix. Polym. Bull. 2018, 75, 581–595. [Google Scholar] [CrossRef]
- Qavi, S.; Lindsay, A.P.; Firestone, M.A.; Foudazi, R. Ultrafiltration membranes from polymerization of self-assembled Pluronic block copolymer mesophases. J. Membr. Sci. 2019, 580, 125–133. [Google Scholar] [CrossRef]
- Henmi, M.; Nakatsuji, K.; Ichikawa, T.; Tomioka, H.; Sakamoto, T.; Yoshio, M.; Kato, T. Self-organized liquid-crystalline nanostructured membranes for water treatment: Selective permeation of ions. Adv. Mater. 2012, 24, 2238–2241. [Google Scholar] [CrossRef]
- Vendamme, R.; Onoue, S.-Y.; Nakao, A.; Kunitake, T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat. Mater. 2006, 5, 494–501. [Google Scholar] [CrossRef]
- Hall, D.B.; Underhill, P.; Torkelson, J.M. Spin coating of thin and ultrathin polymer films. Polym. Eng. Sci. 1998, 38, 2039–2045. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, D.; Dong, R.; Feng, X.; Osuji, C.O. Tunable organic solvent nanofiltration in self-assembled membranes at the sub–1 nm scale. Sci. Adv. 2022, 8, eabm5899. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.-H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Engineering interface between bioenergy recovery and biogas desulfurization: Sustainability interplays of biochar application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Carter, B.M.; Wiesenauer, B.R.; Noble, R.D.; Gin, D.L. Thin-film composite bicontinuous cubic lyotropic liquid crystal polymer membranes: Effects of anion-exchange on water filtration performance. J. Membr. Sci. 2014, 455, 143–151. [Google Scholar] [CrossRef]
- Dischinger, S.M.; Rosenblum, J.; Noble, R.D.; Gin, D.L.; Linden, K.G. Application of a lyotropic liquid crystal nanofiltration membrane for hydraulic fracturing flowback water: Selectivity and implications for treatment. J. Membr. Sci. 2017, 543, 319–327. [Google Scholar] [CrossRef]
- Dischinger, S.M.; Rosenblum, J.; Noble, R.D.; Gin, D.L. Evaluation of a nanoporous lyotropic liquid crystal polymer membrane for the treatment of hydraulic fracturing produced water via cross-flow filtration. J. Membr. Sci. 2019, 592, 117313. [Google Scholar] [CrossRef]
- Thomas, M.; Corry, B. A computational assessment of the permeability and salt rejection of carbon nanotube membranes and their application to water desalination. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150020. [Google Scholar] [CrossRef]
- Tanganov, B. About sizes of the hydrated salt ions—The components of sea water. Eur. J. Nat. Hist. 2013, 1, 36–37. [Google Scholar]
- Kong, X.; Zhou, M.-Y.; Lin, C.-E.; Wang, J.; Zhao, B.; Wei, X.-Z.; Zhu, B.-K. Polyamide/PVC based composite hollow fiber nanofiltration membranes: Effect of substrate on properties and performance. J. Membr. Sci. 2016, 505, 231–240. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Kahrizi, M.; Gonzales, R.R.; Kong, L.; Matsuyama, H.; Lu, P.; Lin, J.; Zhao, S. Significant roles of substrate properties in forward osmosis membrane performance: A review. Desalination 2022, 528, 115615. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Wong, M.C.; Hoek, E.M. Transport through composite membrane, part 1: Is there an optimal support membrane? J. Membr. Sci. 2012, 415, 298–305. [Google Scholar] [CrossRef]
- Wijmans, J.; Hao, P. Influence of the porous support on diffusion in composite membranes. J. Membr. Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Dai, L.; Wang, H.; Li, S.; Zhang, S. Novel insights into the interplay between support and active layer in the thin film composite polyamide membranes. J. Membr. Sci. 2017, 537, 372–383. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.Y.; Helmer, B.; Chung, T.-S. Thin-film composite membranes and formation mechanism of thin-film layers on hydrophilic cellulose acetate propionate substrates for forward osmosis processes. Ind. Eng. Chem. Res. 2012, 51, 10039–10050. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Jung, S.G.; Kim, S.H. Structure-Motion-Performance Relationship of Flux-Enhanced Reverse Osmosis (RO) Membranes Composed of Aromatic Polyamide Thin Films. Environ. Sci. Technol. 2001, 35, 4334–4340. [Google Scholar] [CrossRef]
- Lin, L.; Lopez, R.; Ramon, G.Z.; Coronell, O. Investigating the void structure of the polyamide active layers of thin-film composite membranes. J. Membr. Sci. 2016, 497, 365–376. [Google Scholar] [CrossRef]
- Wong, M.C.; Lin, L.; Coronell, O.; Hoek, E.M.; Ramon, G.Z. Impact of liquid-filled voids within the active layer on transport through thin-film composite membranes. J. Membr. Sci. 2016, 500, 124–135. [Google Scholar] [CrossRef]
- Peng, L.E.; Jiang, Y.; Wen, L.; Guo, H.; Yang, Z.; Tang, C.Y. Does interfacial vaporization of organic solvent affect the structure and separation properties of polyamide RO membranes? J. Membr. Sci. 2021, 625, 119173. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Yao, Z.; Guo, H.; Xu, Z.; Tang, C.Y. Tuning roughness features of thin film composite polyamide membranes for simultaneously enhanced permeability, selectivity and anti-fouling performance. J. Colloid Interface Sci. 2019, 540, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Yao, Z.-K.; Yang, Z.; Guo, H.; Xu, Z.-L.; Tang, C.Y.; Elimelech, M. Nanofoaming of polyamide desalination membranes to tune permeability and selectivity. Environ. Sci. Technol. Lett. 2018, 5, 123–130. [Google Scholar] [CrossRef]
- Peng, L.E.; Yao, Z.; Liu, X.; Deng, B.; Guo, H.; Tang, C.Y. Tailoring polyamide rejection layer with aqueous carbonate chemistry for enhanced membrane separation: Mechanistic insights, chemistry-structure-property relationship, and environmental implications. Environ. Sci. Technol. 2019, 53, 9764–9770. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Karan, S.; Livingston, A.G. Water transport through ultrathin polyamide nanofilms used for reverse osmosis. Adv. Mater. 2018, 30, 1705973. [Google Scholar] [CrossRef]
- Singh, P.S.; Joshi, S.; Trivedi, J.; Devmurari, C.; Rao, A.P.; Ghosh, P. Probing the structural variations of thin film composite RO membranes obtained by coating polyamide over polysulfone membranes of different pore dimensions. J. Membr. Sci. 2006, 278, 19–25. [Google Scholar] [CrossRef]
- Ehsan Yakavalangi, M.; Rimaz, S.; Vatanpour, V. Effect of surface properties of polysulfone support on the performance of thin film composite polyamide reverse osmosis membranes. J. Appl. Polym. Sci. 2017, 134, 44444. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of support layer pore size on performance of thin film composite membranes for forward osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Choi, O.; Ingole, P.G.; Park, C.H. Precision-aiming tuning of membranes prepared by NIPS and its performance enhancement. J. Clean. Prod. 2022, 365, 132858. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Pellegrino, J.; Ding, Y. Fabrication and characterization of a surface-patterned thin film composite membrane. J. Membr. Sci. 2014, 452, 11–19. [Google Scholar] [CrossRef]
- Cui, H.-M.; Yan, X.; Chen, Y.; Xu, W.-Y.; Lang, W.-Z. Enhanced performance of forward osmosis membranes by incorporating PVDF substrates with hydrophilic nanofillers. Desalination Water Treat. 2019, 155, 1–14. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Lang, W.-Z.; Wang, Y. Improved performance of thin-film composite membrane with PVDF/PFSA substrate for forward osmosis process. J. Membr. Sci. 2017, 535, 188–199. [Google Scholar] [CrossRef]
- Lau, W.-J.; Lai, G.-S.; Li, J.; Gray, S.; Hu, Y.; Misdan, N.; Goh, P.-S.; Matsuura, T.; Azelee, I.W.; Ismail, A.F. Development of microporous substrates of polyamide thin film composite membranes for pressure-driven and osmotically-driven membrane processes: A review. J. Ind. Eng. Chem. 2019, 77, 25–59. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Ding, Y. Influence of substrate processing and interfacial polymerization conditions on the surface topography and permselective properties of surface-patterned thin-film composite membranes. J. Membr. Sci. 2016, 512, 50–60. [Google Scholar] [CrossRef]
- Shang, W.; Li, X.; Liu, W.; Yue, S.; Li, M.; von Eiff, D.; Sun, F.; An, A.K. Effective suppression of concentration polarization by nanofiltration membrane surface pattern manipulation: Numerical modeling based on LIF visualization. J. Membr. Sci. 2021, 622, 119021. [Google Scholar] [CrossRef]
- Hamon, J.; Tabor, R.F.; Striolo, A.; Grady, B.P. Directly probing surfactant adsorption on nanoscopic trenches and pillars. J. Colloid Interface Sci. 2020, 579, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Nagarajan, R. Modeling self-assembly of surfactants at solid–liquid interfaces. II. Hydrophilic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2000, 167, 21–30. [Google Scholar] [CrossRef]
- Johnson, R.A.; Nagarajan, R. Modeling self-assembly of surfactants at solid/liquid interfaces. I. Hydrophobic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2000, 167, 31–46. [Google Scholar] [CrossRef]
- Paria, S.; Khilar, K.C. A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv. Colloid Interface Sci. 2004, 110, 75–95. [Google Scholar] [CrossRef]
- Tiberg, F. Physical characterization of non-ionic surfactant layers adsorbed at hydrophilic and hydrophobic solid surfaces by time-resolved ellipsometry. J. Chem. Soc. Faraday Trans. 1996, 92, 531–538. [Google Scholar] [CrossRef]
- Tiberg, F.; Brinck, J.; Grant, L. Adsorption and surface-induced self-assembly of surfactants at the solid–aqueous interface. Curr. Opin. Colloid Interface Sci. 1999, 4, 411–419. [Google Scholar] [CrossRef]
- Grant, L.M.; Ducker, W.A. Effect of substrate hydrophobicity on surface—Aggregate geometry: Zwitterionic and nonionic surfactants. J. Phys. Chem. B 1997, 101, 5337–5345. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Shum, H.C.; Saville, D.A.; Aksay, I.A. Orientational order of molecular assemblies on rough surfaces. J. Phys. Chem. C 2008, 112, 14902–14906. [Google Scholar] [CrossRef]
- Suttipong, M.; Grady, B.P.; Striolo, A. Self-assembled surfactants on patterned surfaces: Confinement and cooperative effects on aggregate morphology. Phys. Chem. Chem. Phys. 2014, 16, 16388–16398. [Google Scholar] [CrossRef]
- Suttipong, M.; Grady, B.P.; Striolo, A. Surfactants adsorption on crossing stripes and steps. Soft Matter 2017, 13, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Striolo, A. Studying surfactants adsorption on heterogeneous substrates. Curr. Opin. Chem. Eng. 2019, 23, 115–122. [Google Scholar] [CrossRef]
- Klebes, J.; Finnigan, S.; Bray, D.J.; Anderson, R.L.; Swope, W.C.; Johnston, M.A.; Conchuir, B.O. The Role of Chemical Heterogeneity in Surfactant Adsorption at Solid–Liquid Interfaces. J. Chem. Theory Comput. 2020, 16, 7135–7147. [Google Scholar] [CrossRef]
- Tummala, N.R.; Grady, B.P.; Striolo, A. Lateral confinement effects on the structural properties of surfactant aggregates: SDS on graphene. Phys. Chem. Chem. Phys. 2010, 12, 13137–13143. [Google Scholar] [CrossRef]
- Gutig, C.; Grady, B.P.; Striolo, A. Experimental studies on the adsorption of two surfactants on solid–aqueous interfaces: Adsorption isotherms and kinetics. Langmuir 2008, 24, 4806–4816. [Google Scholar] [CrossRef]
- Shi, L.; Ghezzi, M.; Caminati, G.; Lo Nostro, P.; Grady, B.P.; Striolo, A. Adsorption isotherms of aqueous C12E6 and cetyltrimethylammonium bromide surfactants on solid surfaces in the presence of low molecular weight coadsorbents. Langmuir 2009, 25, 5536–5544. [Google Scholar] [CrossRef]
- Ji, C.; Zhai, Z.; Jiang, C.; Hu, P.; Zhao, S.; Xue, S.; Yang, Z.; He, T.; Niu, Q.J. Recent advances in high-performance TFC membranes: A review of the functional interlayers. Desalination 2021, 500, 114869. [Google Scholar] [CrossRef]
- Wu, M.-B.; Lv, Y.; Yang, H.-C.; Liu, L.-F.; Zhang, X.; Xu, Z.-K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, D.; Li, T.; Xiao, Y.; Shen, L.; Li, R.; Jiao, Y.; Lin, H. Manipulating the mussel-inspired co-deposition of tannic acid and amine for fabrication of nanofiltration membranes with an enhanced separation performance. J. Colloid Interface Sci. 2020, 565, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhang, Y.; Yang, H.; Fan, G.; Ding, A.; Liang, H.; Li, G.; Ren, N.; Van der Bruggen, B. Mussel-inspired polydopamine modification of polymeric membranes for the application of water and wastewater treatment: A review. Chem. Eng. Res. Des. 2020, 157, 195–214. [Google Scholar] [CrossRef]
- Tyszler, D.; Zytner, R.G.; Batsch, A.; Brügger, A.; Geissler, S.; Zhou, H.; Klee, D.; Melin, T. Reduced fouling tendencies of ultrafiltration membranes in wastewater treatment by plasma modification. Desalination 2006, 189, 119–129. [Google Scholar] [CrossRef]
- Ulbricht, M.; Belfort, G. Surface modification of ultrafiltration membranes by low temperature plasma II. Graft polymerization onto polyacrylonitrile and polysulfone. J. Membr. Sci. 1996, 111, 193–215. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface characteristics of a self-polymerized dopamine coating deposited on hydrophobic polymer films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Freeman, B.D. A bioinspired fouling-resistant surface modification for water purification membranes. J. Membr. Sci. 2012, 413–414, 82–90. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Nanofiltration membranes via co-deposition of polydopamine/polyethylenimine followed by cross-linking. J. Membr. Sci. 2015, 476, 50–58. [Google Scholar] [CrossRef]
- Yang, H.-C.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Wan, L.-S.; Xu, Z.-K. Mussel-inspired modification of a polymer membrane for ultra-high water permeability and oil-in-water emulsion separation. J. Mater. Chem. A 2014, 2, 10225–10230. [Google Scholar] [CrossRef]
- Yang, X.; Du, Y.; Zhang, X.; He, A.; Xu, Z.-K. Nanofiltration membrane with a mussel-inspired interlayer for improved permeation performance. Langmuir 2017, 33, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ou, Y.; Lei, W.X.; Wan, L.S.; Ji, J.; Xu, Z.K. CuSO4/H2O2-induced rapid deposition of polydopamine coatings with high uniformity and enhanced stability. Angew. Chem. Int. Ed. 2016, 55, 3054–3057. [Google Scholar] [CrossRef]
- Hong, S.H.; Hong, S.; Ryou, M.H.; Choi, J.W.; Kang, S.M.; Lee, H. Sprayable ultrafast polydopamine surface modifications. Adv. Mater. Interfaces 2016, 3, 1500857. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Oh, I.K.; Lee, H. Microwave-accelerated rapid, chemical oxidant-free, material-independent surface chemistry of poly (dopamine). Small 2017, 13, 1600443. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef]
- Yu, S.J.; Pak, K.; Kwak, M.J.; Joo, M.; Kim, B.J.; Oh, M.S.; Baek, J.; Park, H.; Choi, G.; Kim, D.H. Initiated chemical vapor deposition: A versatile tool for various device applications. Adv. Eng. Mater. 2018, 20, 1700622. [Google Scholar] [CrossRef]
- Tenhaeff, W.E.; Gleason, K.K. Initiated and oxidative chemical vapor deposition of polymeric thin films: iCVD and oCVD. Adv. Funct. Mater. 2008, 18, 979–992. [Google Scholar] [CrossRef]
- You, J.B.; Yoo, Y.; Oh, M.S.; Im, S.G. Simple and reliable method to incorporate the Janus property onto arbitrary porous substrates. ACS Appl. Mater. Interfaces 2014, 6, 4005–4010. [Google Scholar] [CrossRef]
- Yang, H.-C.; Waldman, R.Z.; Chen, Z.; Darling, S.B. Atomic layer deposition for membrane interface engineering. Nanoscale 2018, 10, 20505–20513. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, J.; Dai, J.; Yang, Y.; Chen, X.; Wang, Y. Hydrophilization of porous polypropylene membranes by atomic layer deposition of TiO2 for simultaneously improved permeability and selectivity. J. Membr. Sci. 2013, 448, 215–222. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Kong, L.; Zhang, J.; Zuo, W. Static adsorption of protein-polysaccharide hybrids on hydrophilic modified membranes based on atomic layer deposition: Anti-fouling performance and mechanism insight. J. Membr. Sci. 2018, 548, 470–480. [Google Scholar] [CrossRef]
- Chen, P.-C.; Wan, L.-S.; Xu, Z.-K. Bio-inspired CaCO3 coating for superhydrophilic hybrid membranes with high water permeability. J. Mater. Chem. 2012, 22, 22727–22733. [Google Scholar] [CrossRef]
- Yang, H.-C.; Pi, J.-K.; Liao, K.-J.; Huang, H.; Wu, Q.-Y.; Huang, X.-J.; Xu, Z.-K. Silica-decorated polypropylene microfiltration membranes with a mussel-inspired intermediate layer for oil-in-water emulsion separation. ACS Appl. Mater. Interfaces 2014, 6, 12566–12572. [Google Scholar] [CrossRef]
- Pi, J.-K.; Yang, H.-C.; Wan, L.-S.; Wu, J.; Xu, Z.-K. Polypropylene microfiltration membranes modified with TiO2 nanoparticles for surface wettability and antifouling property. J. Membr. Sci. 2016, 500, 8–15. [Google Scholar] [CrossRef]
- Yang, H.-C.; Chen, Y.-F.; Ye, C.; Jin, Y.-N.; Li, H.; Xu, Z.-K. Polymer membrane with a mineral coating for enhanced curling resistance and surface wettability. Chem. Commun. 2015, 51, 12779–12782. [Google Scholar] [CrossRef]
- Mortazavi, M.; Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]
- Steen, M.L.; Jordan, A.C.; Fisher, E.R. Hydrophilic modification of polymeric membranes by low temperature H2O plasma treatment. J. Membr. Sci. 2002, 204, 341–357. [Google Scholar] [CrossRef]
- Steen, M.L.; Butoi, C.I.; Fisher, E.R. Identification of Gas-Phase Reactive Species and Chemical Mechanisms Occurring at Plasma−Polymer Surface Interfaces. Langmuir 2001, 17, 8156–8166. [Google Scholar] [CrossRef]
- Steen, M.L.; Hymas, L.; Havey, E.D.; Capps, N.E.; Castner, D.G.; Fisher, E.R. Low temperature plasma treatment of asymmetric polysulfone membranes for permanent hydrophilic surface modification. J. Membr. Sci. 2001, 188, 97–114. [Google Scholar] [CrossRef]
- Chang, Y.; Ko, C.-Y.; Shih, Y.-J.; Quémener, D.; Deratani, A.; Wei, T.-C.; Wang, D.-M.; Lai, J.-Y. Surface grafting control of PEGylated poly (vinylidene fluoride) antifouling membrane via surface-initiated radical graft copolymerization. J. Membr. Sci. 2009, 345, 160–169. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Photografted thin polymer hydrogel layers on PES ultrafiltration membranes: Characterization, stability, and influence on separation performance. Langmuir 2007, 23, 7818–7830. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, H.; Crivello, J.V.; Belfort, G. Development of a novel photochemical technique for modifying poly (arylsulfone) ultrafiltration membranes. J. Membr. Sci. 1995, 105, 237–247. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, M.; Oechel, A.; Lehmann, C.; Tomaschewski, G.; Hicke, H.G. Gas-phase photoinduced graft polymerization of acrylic acid onto polyacrylonitrile ultrafiltration membranes. J. Appl. Polym. Sci. 1995, 55, 1707–1723. [Google Scholar] [CrossRef]
- Kim, M.; Saito, K. Radiation-induced graft polymerization and sulfonation of glycidyl methacrylate on to porous hollow-fiber membranes with different pore sizes. Radiat. Phys. Chem. 2000, 57, 167–172. [Google Scholar] [CrossRef]
- Shim, J.K.; Na, H.S.; Lee, Y.M.; Huh, H.; Nho, Y.C. Surface modification of polypropylene membranes by γ-ray induced graft copolymerization and their solute permeation characteristics. J. Membr. Sci. 2001, 190, 215–226. [Google Scholar] [CrossRef]
- Nasef, M.M.; Hegazy, E.-S.A. Preparation and applications of ion exchange membranes by radiation-induced graft copolymerization of polar monomers onto non-polar films. Prog. Polym. Sci. 2004, 29, 499–561. [Google Scholar] [CrossRef]
- Khabibullin, A.; Mastan, E.; Matyjaszewski, K.; Zhu, S. Surface-initiated atom transfer radical polymerization. In Controlled Radical Polymerization at and from Solid Surfaces; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–76. [Google Scholar]
- Mori, H.; Seng, D.C.; Zhang, M.; Müller, A.H. Hybrid nanoparticles with hyperbranched polymer shells via self-condensing atom transfer radical polymerization from silica surfaces. Langmuir 2002, 18, 3682–3693. [Google Scholar] [CrossRef]
- Ye, P.; Dong, H.; Zhong, M.; Matyjaszewski, K. Synthesis of binary polymer brushes via two-step reverse atom transfer radical polymerization. Macromolecules 2011, 44, 2253–2260. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Gong, X.; Zhang, Y.; Xu, M. Modulating pore size and surface properties of cellulose microporous membrane via thio-ene chemistry. Desalination 2013, 328, 58–66. [Google Scholar] [CrossRef]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Binder, W.H.; Sachsenhofer, R. ‘Click’chemistry in polymer and materials science. Macromol. Rapid Commun. 2007, 28, 15–54. [Google Scholar] [CrossRef]
- Fujimoto, K.; Takebayashi, Y.; Inoue, H.; Ikada, Y. Ozone-induced graft polymerization onto polymer surface. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 1035–1043. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, J.-H.; Choo, K.-H.; Lee, Y.-S.; Lee, C.-H. Hydrophilic modification of polypropylene microfiltration membranes by ozone-induced graft polymerization. J. Membr. Sci. 2000, 169, 269–276. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Gong, Y.; Wang, Z.; Fang, W.; Jin, J. Ultrathin polyamide nanofiltration membrane fabricated on brush-painted single-walled carbon nanotube network support for ion sieving. ACS Nano 2019, 13, 5278–5290. [Google Scholar] [CrossRef]
- Zhai, Z.; Jiang, C.; Zhao, N.; Dong, W.; Lan, H.; Wang, M.; Niu, Q.J. Fabrication of advanced nanofiltration membranes with nanostrand hybrid morphology mediated by ultrafast Noria–polyethyleneimine codeposition. J. Mater. Chem. A 2018, 6, 21207–21215. [Google Scholar] [CrossRef]
- Han, G.; Zhang, S.; Li, X.; Widjojo, N.; Chung, T.-S. Thin film composite forward osmosis membranes based on polydopamine modified polysulfone substrates with enhancements in both water flux and salt rejection. Chem. Eng. Sci. 2012, 80, 219–231. [Google Scholar] [CrossRef]
- Li, Y.; Su, Y.; Li, J.; Zhao, X.; Zhang, R.; Fan, X.; Zhu, J.; Ma, Y.; Liu, Y.; Jiang, Z. Preparation of thin film composite nanofiltration membrane with improved structural stability through the mediation of polydopamine. J. Membr. Sci. 2015, 476, 10–19. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Uliana, A.; Hou, J.; Li, J.; Li, X.; Tian, M.; Chen, Y.; Volodin, A.; Van der Bruggen, B. High-flux thin film composite membranes for nanofiltration mediated by a rapid co-deposition of polydopamine/piperazine. J. Membr. Sci. 2018, 554, 97–108. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, X.; Luo, X.; Liu, Y.; Xu, D.; Tang, X.; Gan, Z.; Yang, L.; Li, G.; Liang, H. Ultrathin thin-film composite polyamide membranes constructed on hydrophilic poly (vinyl alcohol) decorated support toward enhanced nanofiltration performance. Environ. Sci. Technol. 2020, 54, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Yang, Z.; Gan, Z.; Cheng, X.; Tang, X.; Luo, X.; Xu, D.; Li, G.; Liang, H. Toward tailoring nanofiltration performance of thin-film composite membranes: Novel insights into the role of poly (vinyl alcohol) coating positions. J. Membr. Sci. 2020, 614, 118526. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.-W.; Guo, H.; Yao, Z.; Ma, X.-H.; Song, X.; Feng, S.-P.; Tang, C.Y. Tannic acid/Fe3+ nanoscaffold for interfacial polymerization: Toward enhanced nanofiltration performance. Environ. Sci. Technol. 2018, 52, 9341–9349. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Yang, J.; Zhu, C.-Y.; Zhang, L.; Xu, Z.-K. Nanofiltration membranes with hydrophobic microfiltration substrates for robust structure stability and high water permeation flux. J. Membr. Sci. 2020, 593, 117444. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Wang, R.; Xiao, A.; Wang, Y. Ultra-permeable polyamide membranes harvested by covalent organic framework nanofiber scaffolds: A two-in-one strategy. Chem. Sci. 2019, 10, 9077–9083. [Google Scholar] [CrossRef]
- Gong, G.; Wang, P.; Zhou, Z.; Hu, Y. New insights into the role of an interlayer for the fabrication of highly selective and permeable thin-film composite nanofiltration membrane. ACS Appl. Mater. Interfaces 2019, 11, 7349–7356. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Boo, C.; Liu, Z.; Li, J.; Deng, L.; An, X. High-performance thin-film composite membrane with an ultrathin spray-coated carbon nanotube interlayer. Environ. Sci. Technol. Lett. 2018, 5, 243–248. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Gao, S.; Zhang, F.; Zhang, W.; Liu, Z.; Jin, J. Single-Walled Carbon Nanotube Film Supported Nanofiltration Membrane with a Nearly 10 nm Thick Polyamide Selective Layer for High-Flux and High-Rejection Desalination. Small 2016, 12, 5034–5041. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, H.-C.; Wu, M.-B.; Zhang, X.; Xu, Z.-K. Nanofiltration membranes with cellulose nanocrystals as an interlayer for unprecedented performance. J. Mater. Chem. A 2017, 5, 16289–16295. [Google Scholar] [CrossRef]
- Cheng, C.; Li, P.; Shen, K.; Zhang, T.; Cao, X.; Wang, B.; Wang, X.; Hsiao, B.S. Integrated polyamide thin-film nanofibrous composite membrane regulated by functionalized interlayer for efficient water/isopropanol separation. J. Membr. Sci. 2018, 553, 70–81. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, M.; Wu, H.; Liu, Y.; You, X.; Zhang, R.; Su, Y.; Yang, H.; Shen, J.; Jiang, Z. Covalent organic framework-modulated interfacial polymerization for ultrathin desalination membranes. J. Mater. Chem. A 2019, 7, 25641–25649. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2Tx mxene interlayered forward osmosis membranes for enhanced water purification and organic solvent recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Navarro, M.; Benito, J.; Paseta, L.; Gascon, I.; Coronas, J.; Tellez, C. Thin-film nanocomposite membrane with the minimum amount of MOF by the langmuir–schaefer technique for nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 1278–1287. [Google Scholar] [CrossRef]
- Wang, L.; Fang, M.; Liu, J.; He, J.; Li, J.; Lei, J. Layer-by-layer fabrication of high-performance polyamide/ZIF-8 nanocomposite membrane for nanofiltration applications. ACS Appl. Mater. Interfaces 2015, 7, 24082–24093. [Google Scholar] [CrossRef]
- Tsui, T.-H.; van Loosdrecht, M.C.; Dai, Y.; Tong, Y.W. Machine learning and circular bioeconomy: Building new resource efficiency from diverse waste streams. Bioresour. Technol. 2022, 369, 128445. [Google Scholar] [CrossRef]
- Tyler, A.I.; Law, R.V.; Seddon, J.M. X-ray diffraction of lipid model membranes. In Methods in Membrane Lipids; Springer: Berlin/Heidelberg, Germany, 2015; pp. 199–225. [Google Scholar]
- Wang, G. Toward an Advanced Monolithic Precursor Templeted from Hexagonal Lyotropic Liquid Crystals. Ph.D. Thesis, Deakin University, Geelong, Australia, 2017. [Google Scholar]
- Conn, C.E.; de Campo, L.; Whitten, A.E.; Garvey, C.J.; Krause-Heuer, A.M.; Van’t Hag, L. Membrane protein structures in lipid bilayers; small-angle neutron scattering with contrast-matched bicontinuous cubic phases. Front. Chem. 2021, 8, 619470. [Google Scholar] [CrossRef]
- Van’t Hag, L.; De Campo, L.; Tran, N.; Sokolova, A.; Trenker, R.; Call, M.E.; Call, M.J.; Garvey, C.J.; Leung, A.E.; Darwish, T.A. Protein-eye view of the in meso crystallization mechanism. Langmuir 2019, 35, 8344–8356. [Google Scholar] [CrossRef]
- Van’t Hag, L.; de Campo, L.; Garvey, C.J.; Feast, G.C.; Leung, A.E.; Yepuri, N.R.; Knott, R.; Greaves, T.L.; Tran, N.; Gras, S.L. Using SANS with contrast-matched lipid bicontinuous cubic phases to determine the location of encapsulated peptides, proteins, and other biomolecules. J. Phys. Chem. Lett. 2016, 7, 2862–2866. [Google Scholar] [CrossRef]
- Hamilton, W.A. Conformation, directed self-assembly and engineered modification: Some recent near surface structure determinations by grazing incidence small angle X-ray and neutron scattering. Curr. Opin. Colloid Interface Sci. 2005, 9, 390–395. [Google Scholar] [CrossRef]
- Lee, B.; Park, I.; Yoon, J.; Park, S.; Kim, J.; Kim, K.-W.; Chang, T.; Ree, M. Structural analysis of block copolymer thin films with grazing incidence small-angle X-ray scattering. Macromolecules 2005, 38, 4311–4323. [Google Scholar] [CrossRef]
- Müller-Buschbaum, P.; Cubitt, R.; Petry, W. Nanostructured diblock copolymer films: A grazing incidence small-angle neutron scattering study. Langmuir 2003, 19, 7778–7782. [Google Scholar] [CrossRef]
- Salditt, T.; Metzger, T.; Peisl, J.; Reinker, B.; Moske, M.; Samwer, K. Determination of the height-height correlation function of rough surfaces from diffuse X-ray scattering. EPL Europhys. Lett. 1995, 32, 331. [Google Scholar] [CrossRef]
- Russell, T. X-ray and neutron reflectivity for the investigation of polymers. Mater. Sci. Rep. 1990, 5, 171–271. [Google Scholar] [CrossRef]
- Singh, M.A.; Groves, M.N. Depth profiling of polymer films with grazing-incidence small-angle X-ray scattering. Acta Crystallogr. Sect. A Found. Crystallogr. 2009, 65, 190–201. [Google Scholar] [CrossRef]
- Qu, G.; Park, K.S.; Kafle, P.; Zhang, F.; Kwok, J.J.; Patel, B.B.; Smilgies, D.-M.; Thomsen, L.; McNeill, C.R.; Diao, Y. Lyotropic Liquid Crystalline Mesophase Governs Interfacial Molecular Orientation of Conjugated Polymer Thin Films. Chem. Mater. 2020, 32, 6043–6054. [Google Scholar] [CrossRef]
- Kjaer, K.; Als-Neilsen, J.; Heln, C.; Tippmann-Krayer, P.; Möhwald, H. An x-ray scattering study of lipid monolayers at the air-water interface and on solid supports. Thin Solid Film. 1988, 159, 17–28. [Google Scholar] [CrossRef]
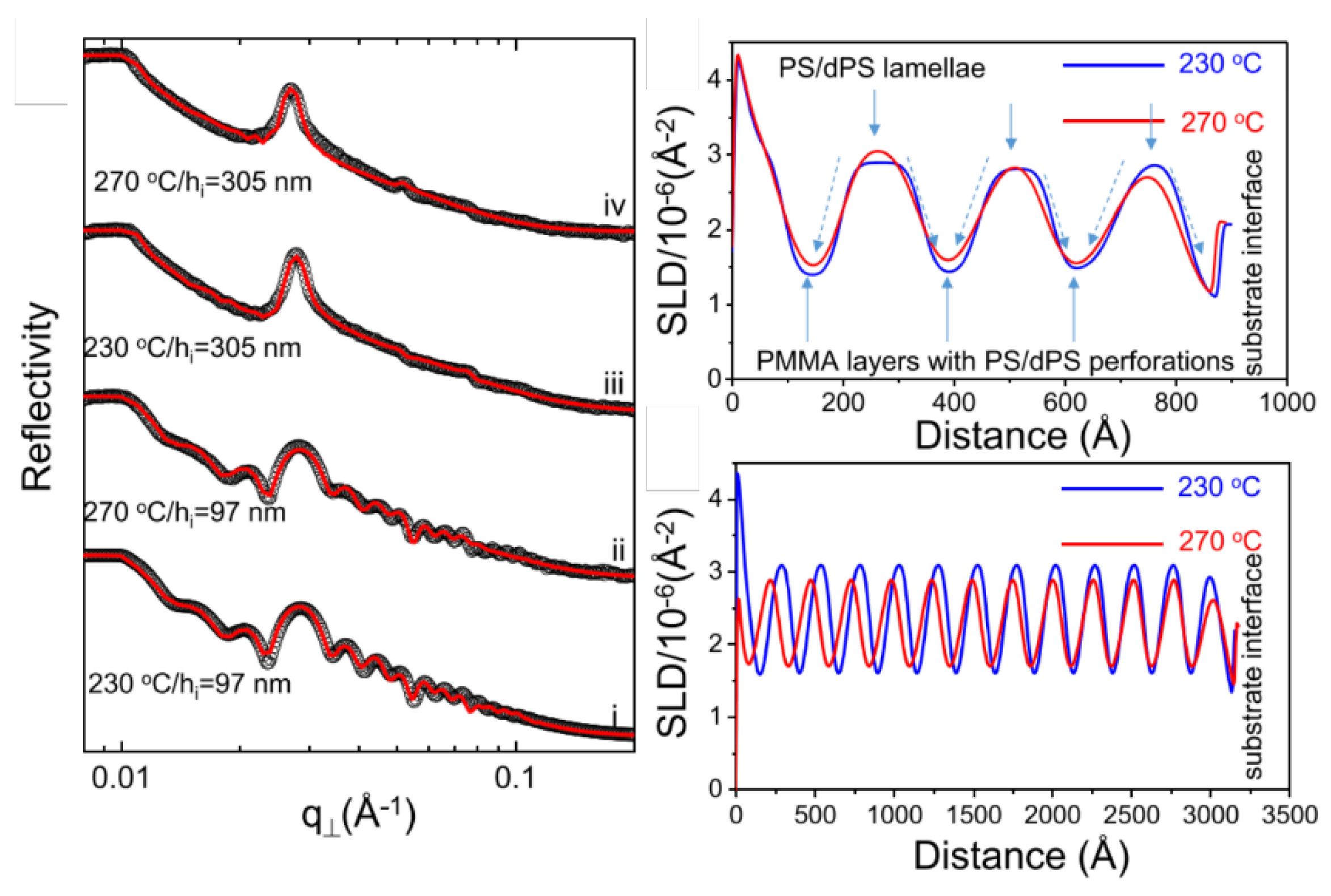
- Hong, J.-W.; Jian, Y.-Q.; Liao, Y.-P.; Hung, H.-H.; Huang, T.-Y.; Nelson, A.; Tsao, I.-Y.; Wu, C.-M.; Sun, Y.-S. Distributions of Deuterated Polystyrene Chains in Perforated Layers of Blend Films of a Symmetric Polystyrene-block-poly (methyl methacrylate). Langmuir 2021, 37, 13046–13058. [Google Scholar] [CrossRef]
- Tousley, M.E.; Feng, X.; Elimelech, M.; Osuji, C.O. Aligned nanostructured polymers by magnetic-field-directed self-assembly of a polymerizable lyotropic mesophase. ACS Appl. Mater. Interfaces 2014, 6, 19710–19717. [Google Scholar] [CrossRef]
- Wang, G.; Garvey, C.J.; Zhang, J.; O’Dell, L.A.; Krause-Heuer, A.M.; Forsyth, M.; Darwish, T.A.; Miloš, S.; Kong, L. Evolution of structural dimensions in mesoporous template precursor from hexagonal lyotropic liquid crystals. J. Phys. Condens. Matter 2019, 32, 75101. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.C.; Kilpatrick, P.K. Using deuterium NMR lineshapes to analyze lyotropic liquid crystalline phase transitions. Langmuir 1992, 8, 1679–1687. [Google Scholar] [CrossRef]
- Thurmond, R.L.; Lindblom, G.; Brown, M.F. Curvature, order, and dynamics of lipid hexagonal phases studied by deuterium NMR spectroscopy. Biochemistry 1993, 32, 5394–5410. [Google Scholar] [CrossRef] [PubMed]
- Juramy, M.; Chèvre, R.; Cerreia Vioglio, P.; Ziarelli, F.; Besson, E.; Gastaldi, S.; Viel, S.; Thureau, P.; Harris, K.D.; Mollica, G. Monitoring crystallization processes in confined porous materials by dynamic nuclear polarization solid-state nuclear magnetic resonance. J. Am. Chem. Soc. 2021, 143, 6095–6103. [Google Scholar] [CrossRef]




| Ref. | LLC Phase | Porous Substrate | Fabrication Process | Solvent | Reactive Temp (°C) | Pore Size in Diameter (nm) | Thickness | Pure Water Flux | Rejection (%) |
|---|---|---|---|---|---|---|---|---|---|
| [34] | HII | PSf MF | Roll-casting | H2O | RT | 1.2 | 0.6 μm | 0.053 Lm−2 h−1 bar−1 μm | Na-TSO (60) Na-NpSO (73) Na-AnSO (89) Na-PySO (94) PEG600 (25.7) PEG5000 (96.1) PEG20000 (99.6) |
| [16] | QI | PE MF | Hot-pressing | H2O | 65 | 0.75 | 40 μm | 0.086 Lm−2 h−1 bar−1 μm | Ethidium Red (99.9) PEG-600 (99.9) Sucrose (99.9) Glucose (96) Glycerol (53) EG (38) NaCl (95) MgCl2 (99.3) CaCl2 (99.3) |
| [35] | QI | PE MF | Hot-pressing | H2O | 60 | 0.86 | 40 μm | 0.054 Lm−2 h−1 bar−1 μm | NaCl (94) KCl (92) MgCl2 (95) CaCl2 (96.9) Sucrose (97.9) Glucose (95) Glycerol (45) Ethylene glycol (38) |
| [9] | QI | PE MF | Hot-pressing | H2O | 65 | 0.75 | 40 μm | Using Ref. [16]’s LLC membrane. Water filtration performances in between that of commercial RO membranes and NF membranes | |
| [63] | QI | PES UF | Rod-coating | Glycerol | 70 | 0.96 | 3 μm | 0.054 Lm−2 h−1 bar−1 μm | Sucrose (97) Glucose (87) Glycerol (45) EG (24) NaCl (98) MgCl2(99) |
| [74] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. Anion exchange in the pores can adjust the flux with little change in rejection performance | |
| [75] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. TFC QI possesses a similar performance as commercial RO and NF membranes in treating hydraulic fracturing flowback water. Controllable DOC recovery can be adjusted by pH | |
| [76] | QI | PES UF | Rod-coating | Glycerol | 70 | ≈1 | 3 μm | Using Ref. [63]’s LLC membrane. The 66 h cross-flow filtration of hydraulic fracturing produced water was conducted. Better performance than NF90 in portion of organic compounds, water flux, and fouling resistance | |
| [68] | HII Lα | PE MF (recovered) | Hot-pressing | H2O | RT (UV-curing) + 70 (thermal-curing) | 4 nm (HII) 3 nm (Lα) | 10 μm | These LLC membranes possess better permeability and antifouling performances than commercially UF membranes. BSA rejection higher than 95% | |
| [12] | HI | PAN UF | Pressing | H2O | RT | 1–2 nm | 3–30 μm | 10 Lm−2 h−1 bar−1 μm | Methylene blue (90) Crystal violet (90) Alcian blue (90) Charged solutes (~350 Da) Neutral solutes (~4 kDa) |
| [10] | HI | PAN UF and PVDF UF | Spin-coating | Glycerol | RT | ~1 nm | ~200 nm | 2 Lm−2 h−1 bar−1 μm | PEG600(>94) Methyl orange (91) Methylene blue (95) CaCl2 and MgCl2 (>80) LiCl, NaCl, and KCl (>40) |
| [72] | HI | PVDF UF | Spin-coating | Glycerol | RT | 0.6–1.5 nm | 170–200 nm | 10–30 Lm−2 h−1 bar in water 2–8 Lm−2 h−1 bar−1 μm in methanol | 4 HI membranes possess various performances. Only list the maximum performances here. PEG 600 (100%) Acid Fuchsin 585 Da (> 95%) Methyl Orange 327 Da (100%) |
| Physical Techniques | ||
|---|---|---|
| Techniques | Principle | Features |
| Plasma | Interactions between ionized gas with polymer surface atoms, inducing the homolytic bond cleavage, and creating free radicals. | |
| UV light | Activating the membrane surface and inducing radical formation by UV irradiation, forming hydrophilic groups with oxygen. |
|
| High-energy irradiation | Activating the membrane surface and inducing radical formation by X-ray, γ-ray and electron source [157,158]. |
|
| Chemical techniques | ||
| Natively initiating | Grafting small molecules with the left free amine and carboxylic acid groups on polyamide membrane. |
|
| Surface-initiated atom transfer radical polymerization (SI-ATRP) | Initiator moieties covalently attaches the substrate surface and reacts with the dormant species and induces the ATRP of monomers [160] (grafting from). |
|
| Click chemistry | Facilitating the alkyne-azide, thiol-vinyl addition, thiol-yne, etc., reaction to effectively functionalize the membrane surface (grafting to). | |
| Ozone oxidation | Ozone induces the peroxide formation on polymer surface, whose decomposition brings radicals to the surface. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Zhang, L.; de Campo, L.; O’Dell, L.A.; Wang, D.; Wang, G.; Kong, L. Lyotropic Liquid Crystal (LLC)-Templated Nanofiltration Membranes by Precisely Administering LLC/Substrate Interfacial Structure. Membranes 2023, 13, 549. https://doi.org/10.3390/membranes13060549
Gu S, Zhang L, de Campo L, O’Dell LA, Wang D, Wang G, Kong L. Lyotropic Liquid Crystal (LLC)-Templated Nanofiltration Membranes by Precisely Administering LLC/Substrate Interfacial Structure. Membranes. 2023; 13(6):549. https://doi.org/10.3390/membranes13060549
Chicago/Turabian StyleGu, Senlin, Liangliang Zhang, Liliana de Campo, Luke A. O’Dell, Dong Wang, Guang Wang, and Lingxue Kong. 2023. "Lyotropic Liquid Crystal (LLC)-Templated Nanofiltration Membranes by Precisely Administering LLC/Substrate Interfacial Structure" Membranes 13, no. 6: 549. https://doi.org/10.3390/membranes13060549
APA StyleGu, S., Zhang, L., de Campo, L., O’Dell, L. A., Wang, D., Wang, G., & Kong, L. (2023). Lyotropic Liquid Crystal (LLC)-Templated Nanofiltration Membranes by Precisely Administering LLC/Substrate Interfacial Structure. Membranes, 13(6), 549. https://doi.org/10.3390/membranes13060549







