Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective
Abstract
1. Introduction
2. Organic Interlayers
2.1. Polyphenols
2.2. Ionic Polymers
2.3. Polymeric Organic Acid
2.4. Other Organic Interlayers
3. Nanomaterial Interlayer
3.1. Nanoparticles
3.1.1. Inorganic NPs
3.1.2. Organic NPs
3.1.3. Organic-Inorganic NPs
3.2. One-Dimensional Nanomaterials
3.2.1. Inorganic 1D Nanomaterials
3.2.2. Organic 1D Nanomaterials
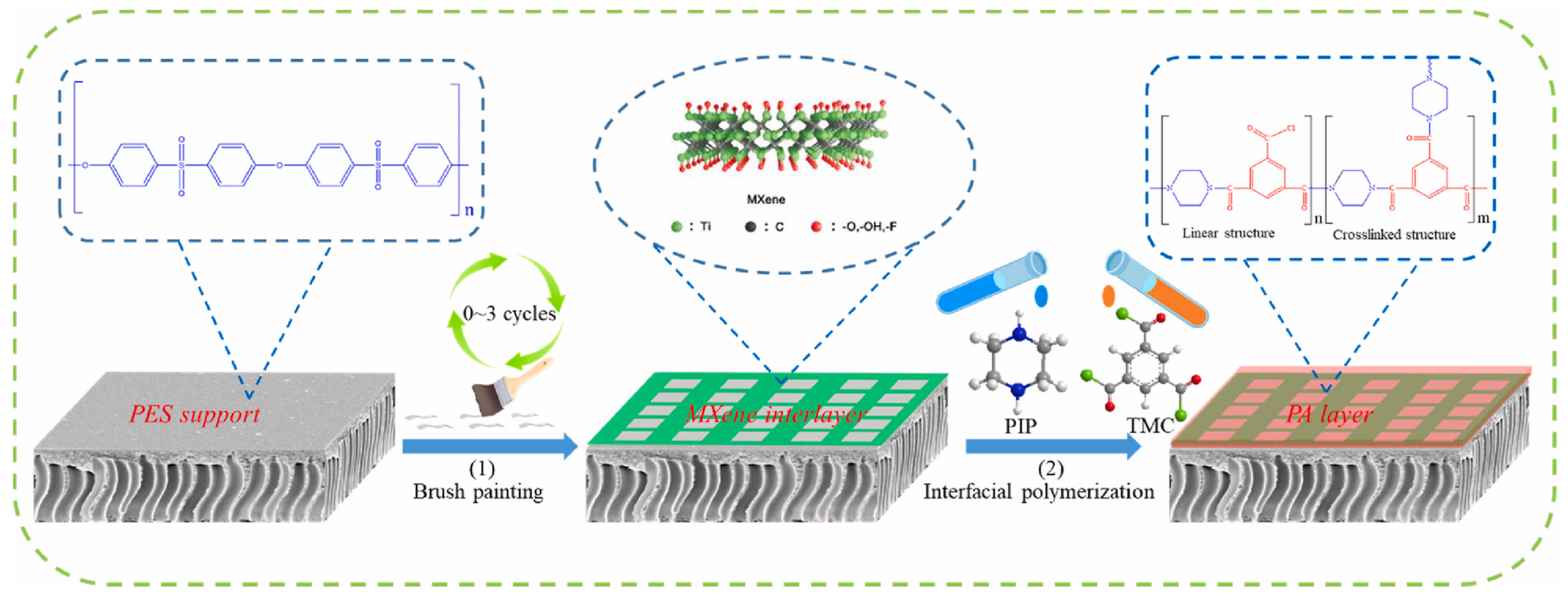
3.3. Two-Dimensional Nanomaterials
3.3.1. Inorganic 2D Nanomaterials
3.3.2. Organic 2D Nanomaterials
3.3.3. Inorganic-Organic 2D Nanomaterials
| Category | Nanomaterial Used | Porous Substrate | IP Condition (Optimum) | Polyamide Thickness (nm) | Water Flux (L m−2 h−1 bar−1) | Salt Rejection | Year [Ref] |
|---|---|---|---|---|---|---|---|
| NPs | ZIF-8-PSS | PES MF substrate (0.22 μm) | 2 w/v% PIP/water, 0.13 w/v% TMC/n-hexane 1 min reaction | 235 | 9.6 | 91% Na2SO4 | 2021 [49] |
| NPs | SiO2 | PSF UF substrate (Mw 30,000) | 0.2 w/v% PIP/water, 0.1 w/v% TMC/n-hexane 30 s reaction | 15 | 14.5 | 98.7% Na2SO4 | 2021 [46] |
| NPs | COF | PSF UF substrate | 0.1 wt% PIP/water, 0.1 wt% TMC/n-hexane 1 min reaction | 44 | 35.7 | 98.9% Na2SO4 | 2022 [48] |
| NPs | PDA@SiO2 | PMIA substrate | 1 wt% PIP/water, 0.1 w/v% TMC/n-hexane 30 s reaction | 20.9 | 31.37 | 97% Na2SO4 | 2022 [47] |
| 1D | PDA/SWCNT (5–30 μm × Φ < 2 nm) | PES MF substrate (0.4 μm) | 0.025 w/v% PIP/water, 0.02 w/v% TMC/n-hexane 30 s reaction | 12 | 32 | 95.9% Na2SO4 | 2016 [54] |
| 1D | MWCNTs (50 μm × Φ 8–15 nm) | PSF UF substrate (Mw 30,000) | 0.15 w/v% PIP/water, 0.02 w/v% TMC/n-hexane 30 s reaction | NA | 17.57 | 95% Na2SO4 | 2016 [16] |
| 1D | Cellulose nanocrystal (0.2–0.5 μm × Φ 20–50 nm) | PES MF substrate (0.22 μm) | 0.5 mg/mL PIP/water 0.5 mg/mL TMC/n hexane 2 min reaction | 45 | 34 | 97.7% Na2SO4 | 2017 [53] |
| 1D | PDA@ZIF-8/PDA@SWCNTs | PES MF substrate | 2.5 mg/mL PIP/water 2 mg/mL TMC/n-hexane 30 s reaction | NA | 53.5 | 95% Na2SO4 | 2018 [55] |
| 1D | SWCNT (5~30 μm × Φ 1–2 nm) | PES MF substrate (0.45 μm) | 0.125 w/v% PIP/water, 0.1 w/v% TMC/n-hexane 30 s reaction | 15 | 40 | 96.5% Na2SO4 | 2019 [51] |
| 1D | PDA/SWCNTs (5–30 μm × Φ < 2 nm) | PES MF substrate (0.22 μm) | 0.05 wt% PIP/water, 0.02 wt% TMC/n-hexane 30 s reaction | 29 | 21 | 98.5% Na2SO4 | 2019 [56] |
| 1D | SWCNT (1~3 μm × Φ 1–2 nm) | PES MF substrate (0.22 μm) | 0.15 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 42.6 | 18.24 | 97.88% Na2SO4 | 2021 [52] |
| 1D | Polyaniline nanofibers | PES MF substrate (0.45 μm) | 1 w/v% PIP/water, 0.15 w/v% TMC/n-hexane 30 s reaction | 11 | 49.7 | 98.7% Na2SO4 | 2022 [60] |
| 1D | sulfonated polyaniline nanofibers (0.58 μm × Φ 80 nm) | PES MF substrate (0.45 μm) | 1 w/v% PIP/water, 0.15 w/v% TMC/n-hexane 30 s reaction | 48 | 29.35 | 98.92% Na2SO4 | 2022 [61] |
| 1D | Cellulose nanofibers | PSF UF (Mw 50,000) | 0.3 w/v% PEI/water, 0.1 w/v% TMC/n-hexane 1min reaction | 105.4 | 13.3 | 96.8%MgCl2 | 2022 [59] |
| 1D | MWCNTs | PES UF substrate (Mw 20,000) | 0.2 wt% PIP/water, 0.1 wt% TMC/n-hexane 2 min reaction | NA | 13 | NA | 2022 [21] |
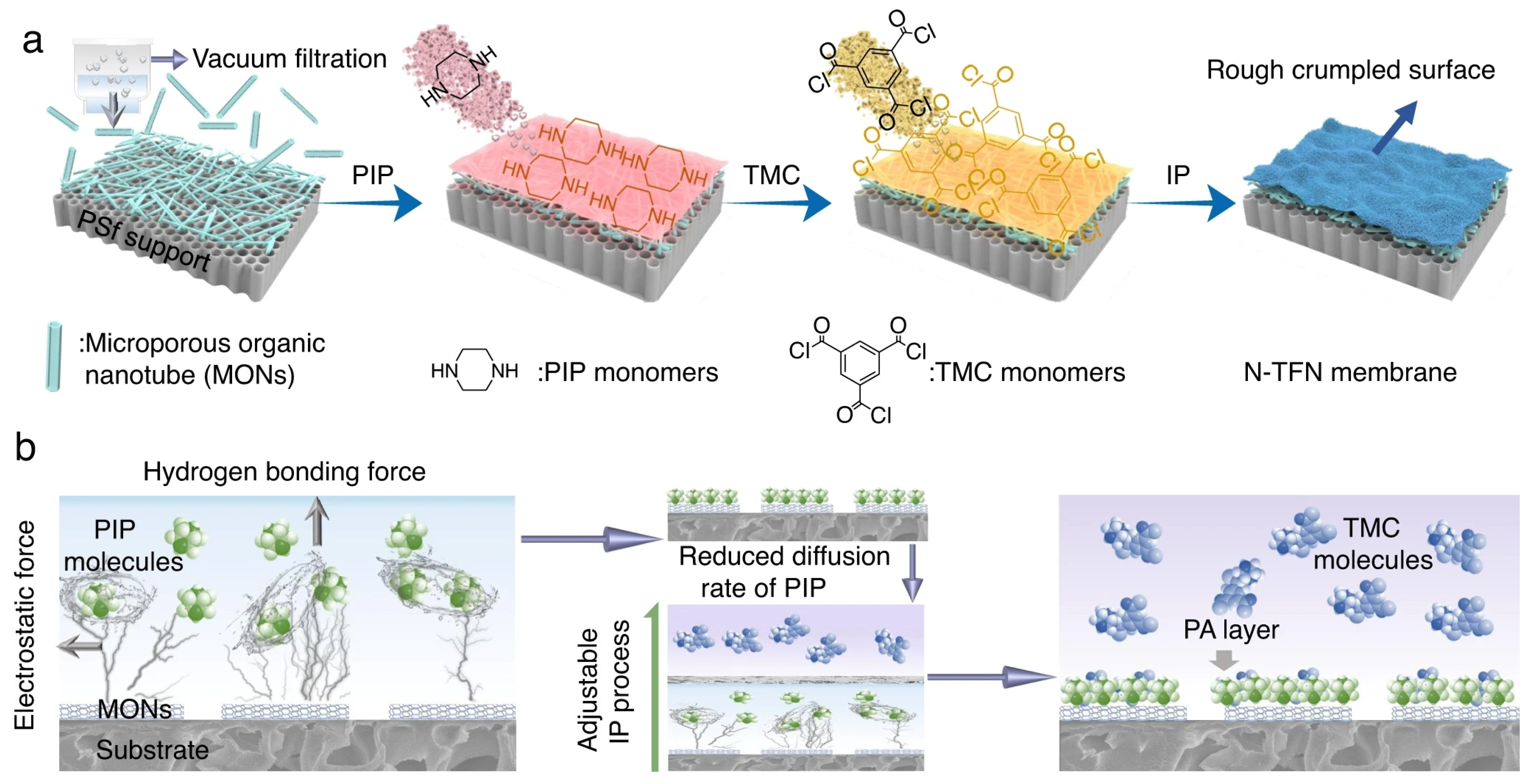
| 1D | Microporous organic nanotubes | PSF substrate | 0.1 wt% PIP/water, 0.1 wt% TMC/n-hexane 1 min reaction | 15 | 41.7 | 98.7% Na2SO4 | 2022 [63] |
| 1D | sulfonated polyaniline | PES MF substrate (0.45 μm) | 1 wt% PIP/water, 0.15 wt% TMC/n-hexane 30 s reaction | 43 | 35.35 | 98.95% Na2SO4 | 2023 [62] |
| 2D | PDA-COF nanosheets (lateral size 60–130 nm) | PAN UF substrate (Mw100,000) | 0.1 w/v% PIP/water, 0.1 w/v% TMC/n-hexane 2 min reaction | 11 | 20.71 | 93.4% Na2SO4 | 2019 [84] |
| 2D | COF nanosheets (lateral size 250 nm) | PES MF substrate (0.10 μm) | 0.15 wt% PIP/water, 0.15 wt% TMC/n-hexane 2 min reaction | 7 | 53.55 | 94.3% Na2SO4 | 2019 [85] |
| 2D | GO nanosheets | Nylon MF substrate (0.22 μm) | 0.5 w/v% PIP/water, 0.5 w/v% TMC/n-hexane 2 min reaction | NA | 30.3 | 93.56% Na2SO4 | 2020 [87] |
| 2D | MXene nanosheets | PES UF substrate | 1 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 15 | 27.8 | 99.9% Na2SO4 | 2021 [75] |
| 2D | MXene nanosheets | PES MF substrate (0.22 μm) | 0.2 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 20 | 45.7 | 96% Na2SO4 | 2021 [72] |
| 2D | GO@PVA/GA | PSF substrate | 0.3 wt% PIP/water, 0.06 wt% TMC/n-hexane 30 s reaction | 15 | 15.8 | 99.7% Na2SO4 | 2021 [69] |
| 2D | MOFs nanosheets | PES UF substrate (Mw 20,000) | 0.15 wt% PIP/water, 0.1 wt% TMC/n-hexane 1 min reaction | 23.5 | 32.7 | 99.7% Na2SO4 | 2022 [86] |
| 2D | MoS2 (average size of 2 μm) | PES UF substrate (Mw 150,000) | 1 wt% PIP/water, 0.15 wt% TMC/n-hexane 1 min reaction | 72.7 | 15.9 | 96.8% Na2SO4 | 2022 [76] |
| 2D | TiO2 nanosheets (lateral size 0.6–1.2 μm)/TiO2 NPs | PVDF MF substrate (0.45 μm) | 1 wt% PIP/water, 0.1 wt% TMC/n-hexane 15 s reaction | 30 | 36.3 | NA | 2023 [88] |
| 2D | MXene-TiO2 | PSF UF substrate (Mw 20,000) | 0.75 wt% PIP/water, 0.038 wt% TMC/n-hexane 2 min reaction | 30.25 | 11.10 | 98.29% MgSO4 | 2023 [79] |
| 2D | MXene Nanosheets/Fe3O4 NPs | PSF UF substrate (Mw 20,000) | 0.75 wt% PIP/water, 0.038 wt% TMC/n-hexane 2 min reaction | 22 | 9.48 | >97% MgSO4 | 2023 [78] |
4. Comparison of Performance of TFCi NF Membranes
4.1. Comparison of Performance of TFCi NF Membranes with TFC0 NF Membranes
4.2. Comparison of Performance of TFCi NF Membranes Prepared Using Different Interlayer Materials
5. Concluding Remarks and Future Perspectives
- Interlayer materials. Considering that 1D nanomaterials are most effective in improving membrane flux, and organic polymers and 2D nanomaterials are good at improving salt rejection ability, composite materials such as organic/1D nanomaterials and 2D/1D nanomaterials may be candidate materials for preparing high-performance NF membranes. Some researchers have introduced PDA/CNT as an interlayer for the preparation of NF membranes [54,55,56]. In addition to enhancing the selectivity of the NF membrane, the function of PDA can also serve as an adhesive to encapsulate CNTs, thereby improving the stability of the TFC NF membrane. Some other researchers have directly used organic 1D nanomaterials (such as Polyaniline nanofibers [60] and microporous organic nanotubes [63]) as interlayers to prepare NF membranes with not only extremely high flux (>40 L m2− h−1 bar−1) but also high salt rejection ability (rejection of Na2SO4 > 98%) (see Table 2). Organic 1D nanomaterials have more hydrophilic functional groups and better chemical stability than inorganic 1D nanomaterials (such as CNTs), combining the advantages of both inorganic 1D nanomaterials and organic polymers. Moreover, to date, there have been no reports on 2D/1D nanomaterials used as interlayer materials for preparing NF membranes, although 2D/1D nanomaterials (such as MXene/CNTs [89]) have been reported to be used for preparing high-performance 2D membranes. Given the effectiveness of these materials as interlayers, it is necessary to further explore the possibility of using other composite materials (such as organic polymer/1D nanomaterials, 2D/1D nanomaterials) and organic 1D nanomaterials as interlayers for preparing high-performance NF membranes. The selection of interlayer materials must also consider factors such as compatibility with the substrate, preparation difficulty, stability under operating conditions, and cost-effectiveness.
- Current studies in membrane technology have largely focused on enhancing permeability, but there is also a pressing need for membranes that exhibit high selectivity towards specific solutes. Interlayers have shown promise in addressing this need by allowing for the customization of membrane properties to selectively adsorb or repel certain solutes, thereby enhancing selectivity. Notably, Zhu et al. [38] introduced positively charged quaternized cross-linked microgels (PNI6) as an interlayer to improve Mg2+ removal, demonstrating the ability of interlayers to significantly impact the selectivity of PA membranes. An exciting avenue for future research is exploring how the unique chemical properties of interlayer materials can be harnessed to enhance selectivity towards specific pollutants [90,91,92].
- Another important aspect of developing interlayer-based TFC NF membranes is the need for scalable and cost-effective manufacturing methods. While many promising results have been reported in the literature, most of these methods are still confined to the laboratory scale and may not be suitable for large-scale production. Therefore, further research is necessary to develop scalable methods that can be seamlessly integrated into existing membrane manufacturing processes.
- Moreover, it is crucial to conduct comprehensive investigations into the long-term stability and durability of interlayer-based TFC NF membranes under diverse operating conditions. Although several studies have reported positive outcomes in terms of membrane performance, few have explored the impact of contamination, chemical degradation, and other factors on membrane performance over extended periods of use. Hence, more extensive research is necessary to evaluate the effects of these factors on membrane performance over time.
- Current studies on the preparation of nanofiltration membranes with an interlayer primarily focus on achieving a balance between permeability and selectivity, and there is limited research on the interlayer’s impact on anti-pollution properties. However, the impact of the interlayer on anti-fouling performance is essential in the development of nanofiltration membranes. Moving forward, we expect that further research in this area will lead to the development of novel interlayer materials and strategies that can improve both permeability/selectivity and pollution resistance simultaneously.
- During the preparation of TFC NF membranes, controlling the dispersion of nanomaterials is crucial to avoid aggregation and ensure optimal performance. Existing literature has used various methods such as surface modification (carboxylation treatment of MWCNTs [16]), dispersion in solutions such as PDA [54,56], polyvinyl alcohol sodium [69], dodecyl benzene sulfonate [51], sodium dodecylbenzene sulfonate [56], Tris-HCl buffer [47], and ultrasound treatment [21,47,51,52,53,54,56,69,72,75,87] to prevent the agglomeration of nanomaterials. While there are many methods available to avoid the agglomeration of nanomaterials, there is always room for improvement and the development of new methods. This is because the properties and behavior of nanomaterials can be complex and difficult to predict, and different applications may require different strategies for preventing agglomeration. Additionally, as new types of nanomaterials are developed and used in various applications, new methods for avoiding agglomeration may need to be developed as well. Therefore, continued research and development in this area is necessary to optimize the performance of nanomaterials in various applications.
- In conclusion, while there are significant challenges in the application of interlayer materials in the preparation of TFC PA NF membranes, this field holds enormous potential for enhancing membrane performance and extending its scope of applications. Through sustained research and development in this area, we may witness substantial progress in membrane technology in the forthcoming years.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, R.; Li, J.; Wang, Z. Constructing interlayer to tailor structure and performance of thin-film composite polyamide membranes: A review. Colloid Interface Sci. 2020, 282, 102204. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef]
- Lau, W.J.; Ismail, A.F.; Misdan, N.; Kassim, M.A. A recent progress in thin film composite membrane: A review. Desalination 2012, 287, 190–199. [Google Scholar] [CrossRef]
- Choi, W.; Gu, J.-E.; Park, S.-H.; Kim, S.; Bang, J.; Baek, K.-Y.; Park, B.; Lee, J.S.; Chan, E.P.; Lee, J.-H. Tailor-Made Polyamide Membranes for Water Desalination. ACS Nano 2015, 9, 345–355. [Google Scholar] [CrossRef]
- Freger, V. Nanoscale heterogeneity of polyamide membranes formed by interfacial polymerization. Langmuir 2003, 19, 4791–4797. [Google Scholar] [CrossRef]
- Freger, V.; Srebnik, S. Mathematical model of charge and density distributions in interfacial polymerization of thin films. J. Appl. Polym. Sci. 2003, 88, 1162–1169. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Nowbahar, A.; Mansard, V.; Mecca, J.M.; Paul, M.; Arrowood, T.; Squires, T.M. Measuring interfacial polymerization kinetics using microfluidic interferometry. J. Am. Chem. Soc. 2018, 140, 3173–3176. [Google Scholar] [CrossRef]
- Liang, Y.; Li, C.; Li, S.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Graphene quantum dots (GQDs)-polyethyleneimine as interlayer for the fabrication of high performance organic solvent nanofiltration (OSN) membranes. Chem. Eng. J. 2020, 380, 122462. [Google Scholar] [CrossRef]
- Geise, G.M.; Paul, D.R.; Freeman, B.D. Fundamental water and salt transport properties of polymeric materials. Prog. Polym. Sci. 2014, 39, 1–42. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–768. [Google Scholar] [CrossRef]
- Wu, M.-B.; Lv, Y.; Yang, H.-C.; Liu, L.-F.; Zhang, X.; Xu, Z.-K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, R.; Yuan, S.; Li, J.; Tian, M.; Wang, P.; Zhang, Y.; Volodin, A.; Van der Bruggen, B. Rapid water transport through controllable, ultrathin polyamide nanofilms for high-performance nanofiltration. J. Mater. Chem. A 2018, 6, 15701–15709. [Google Scholar] [CrossRef]
- Lau, W.-J.; Lai, G.-S.; Li, J.; Gray, S.; Hu, Y.; Misdan, N.; Goh, P.-S.; Matsuura, T.; Azelee, I.W.; Ismail, A.F. Development of microporous substrates of polyamide thin film composite membranes for pressure-driven and osmotically-driven membrane processes: A review. J. Ind. Eng. Chem. 2019, 77, 25–59. [Google Scholar] [CrossRef]
- Yang, X.; Du, Y.; Zhang, X.; He, A.; Xu, Z.K. Nanofiltration membrane with a mussel-inspired interlayer for improved permeation performance. Langmuir 2017, 33, 2318–2324. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Guo, H.; Peng, L.E.; Ma, X.H.; Song, X.X.; Wang, Z.; Tang, C.Y. Mechanistic insights into the role of polydopamine interlayer toward improved separation performance of polyamide nanofiltration membranes. Environ. Sci. Technol. 2020, 54, 11611–11621. [Google Scholar] [CrossRef]
- Long, L.; Wu, C.; Yang, Z.; Tang, C.Y. Carbon nanotube interlayer enhances water permeance and antifouling performance of nanofiltration membranes: Mechanisms and experimental evidence. Environ. Sci. Technol. 2022, 56, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, P.F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Polymer coating technology for high performance applications: Fundamentals and advances. J. Macromol. Sci. A 2018, 55, 440–448. [Google Scholar] [CrossRef]
- Ulbricht, M. Design and synthesis of organic polymers for molecular separation membranes. Curr. Opin. Chem. Eng. 2020, 28, 60–65. [Google Scholar] [CrossRef]
- Zhai, Z.; Jiang, C.; Zhao, N.; Dong, W.; Lan, H.; Wang, M.; Niu, Q.J. Fabrication of advanced nanofiltration membranes with nanostrand hybrid morphology mediated by ultrafast Noria–polyethyleneimine codeposition. J. Mater. Chem. A 2018, 6, 21207–21215. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of catechol/polyethyleneimine on porous membranes for efficient decolorization of dye water. J. Mater. Chem. A 2015, 3, 14438–14444. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, X.; Luo, X.; Liu, Y.; Xu, D.; Tang, X.; Gan, Z.; Yang, L.; Li, G.; Liang, H. Ultrathin thin-Film composite polyamide membranes constructed on hydrophilic poly(vinyl alcohol) decorated support toward enhanced nanofiltration performance. Environ. Sci. Technol. 2020, 54, 6365–6374. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Yang, H.C.; Waldman, R.Z.; Wu, M.B.; Hou, J.; Chen, L.; Darling, S.B.; Xu, Z.K. Dopamine: Just the Right Medicine for Membranes. Adv. Funct. Mater. 2018, 28, 1705327. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Yang, H.-C.; Du, Y.; Xu, Z.-K. Polyphenol coating as an interlayer for thin-film composite membranes with enhanced nanofiltration performance. ACS Appl. Mater Inter. 2016, 8, 32512–32519. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Wang, M.; Tao, L.; Hou, Y.; Niu, Q.J. Rapid in-situ covalent crosslinking to construct a novel azo-based interlayer for high-performance nanofiltration membrane. Sep. Purif. Technol. 2021, 258, 118029. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Z.-w.; Guo, H.; Yao, Z.; Ma, X.-h.; Song, X.; Feng, S.-P.; Tang, C.Y. Tannic acid/Fe3+ nanoscaffold for interfacial polymerization: Toward enhanced nanofiltration performance. Environ. Sci. Technol. 2018, 52, 9341–9349. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, S.; Lan, H.; Xie, T.; Wang, H.; Chen, Y.; Li, P.; Sun, H.; Niu, Q.J.; Yang, C. Dual-electric layer nanofiltration membranes based on polyphenol/PEI interlayer for highly efficient Mg2+/Li+ separation. J. Membr. Sci. 2022, 660, 120860. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Luo, X.; Chen, Y.; Jiang, C.; Zhai, Z.; Niu, Q.J. Fabrication of thin-film composite polyamide nanofiltration membrane based on polyphenol intermediate layer with enhanced desalination performance. Desalination 2020, 488, 114525. [Google Scholar] [CrossRef]
- Tian, B.; Hu, P.; Zhao, S.; Wang, M.; Hou, Y.; Niu, Q.J.; Li, P. Nanofiltration membrane combining environmental-friendly polycarboxylic interlayer prepared from catechol for enhanced desalination performance. Desalination 2021, 512, 115118. [Google Scholar] [CrossRef]
- Hu, P.; Tian, B.; Xu, Z.; Niu, Q.J. Fabrication of high performance nanofiltration membrane on a coordination-driven assembled interlayer for water purification. Sep. Purif. Technol. 2020, 235, 116192. [Google Scholar] [CrossRef]
- Deng, M.; Pei, T.; Ge, P.; Zhu, A.; Zhang, Q.; Liu, Q. Ultrathin sulfonated mesoporous interlayer facilitates to prepare highly-permeable polyamide nanofiltration membranes. J. Membr. Sci. 2022, 652, 120507. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, S.; Fan, W.; Nie, J.; Zhu, L.; Du, B. Preparation of high-performance nanofiltration membranes with quaternized cross-linked microgels as intermediate layer. Desalination 2023, 549, 116310. [Google Scholar] [CrossRef]
- Song, Q.; Lin, Y.; Zhou, S.; Istirokhatun, T.; Wang, Z.; Shen, Q.; Mai, Z.; Guan, K.; Matsuyama, H. Highly permeable nanofilms with asymmetric multilayered structure engineered via amine-decorated interlayered interfacial polymerization. J. Membr. Sci. 2023, 670, 121377. [Google Scholar] [CrossRef]
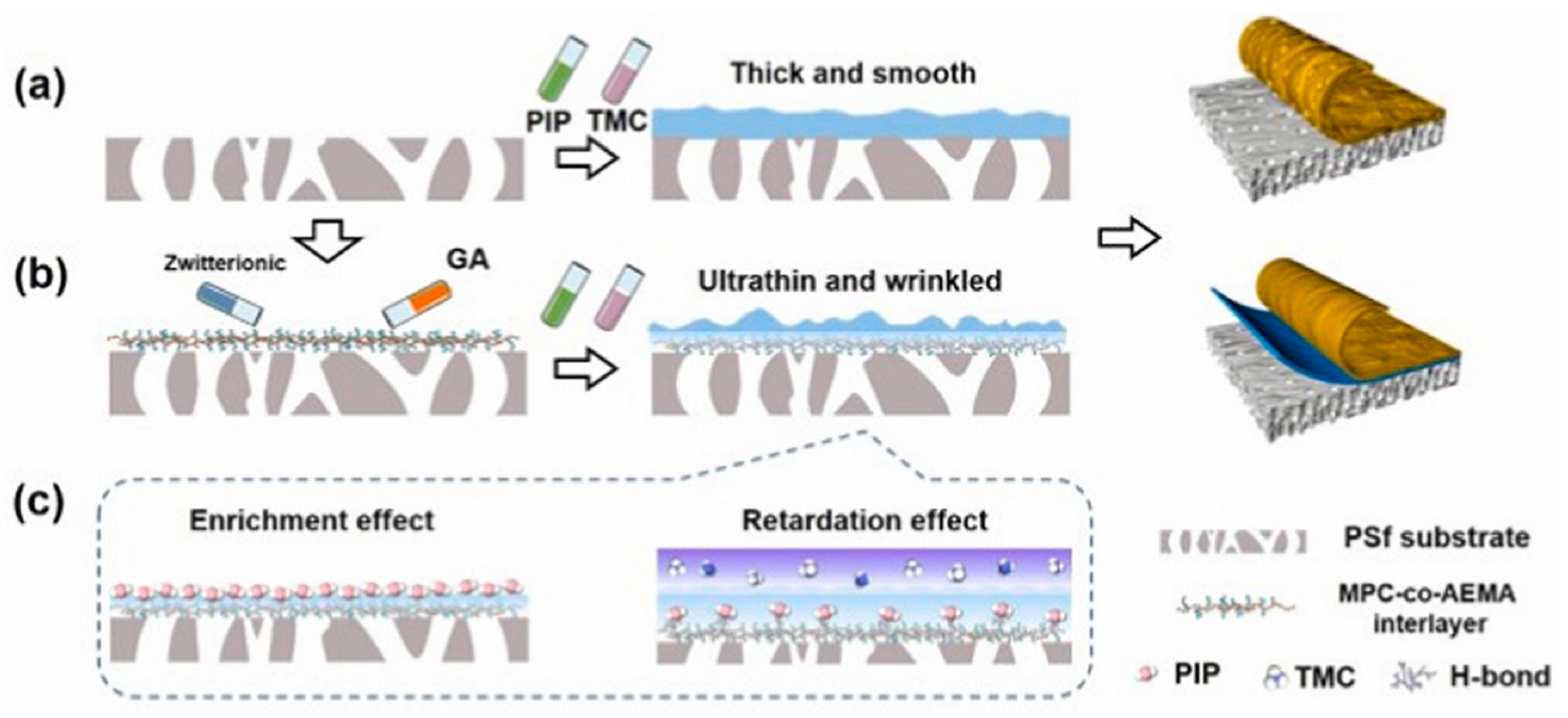
- Song, Q.; Lin, Y.; Ueda, T.; Shen, Q.; Lee, K.-R.; Yoshioka, T.; Matsuyama, H. A zwitterionic copolymer-interlayered ultrathin nanofilm with ridge-shaped structure for ultrapermeable nanofiltration. J. Membr. Sci. 2022, 657, 120679. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.; Fu, J.; Wang, A.; Ding, M.; Zhang, L.; Han, L.; Gao, L. Hyaluronic acid-modified nanofiltration membrane for ultrahigh water permeance and efficient rejection of PFASs. Process Saf. Environ. Prot. 2022, 166, 214–221. [Google Scholar] [CrossRef]
- Wang, X.-L.; Xue, Y.-X.; Dong, S.-Q.; Wang, Q.; Yu, J.-T.; Wang, H.-C.; Zhang, H.; Wang, W.; Wei, J.-F. Poly(caffeic acid) as interlayer to enhance nanofiltration performance of polyamide composite membrane. Desalination 2023, 545, 116168. [Google Scholar] [CrossRef]
- Lan, H.; Li, P.; Wang, H.; Wang, M.; Jiang, C.; Hou, Y.; Li, P.; Jason Niu, Q. Construction of a gelatin scaffold with water channels for preparing a high performance nanofiltration membrane. Sep. Purif. Technol. 2021, 264, 118391. [Google Scholar] [CrossRef]
- Liu, Z.; An, Z.; Mi, Z.; Wang, Z.; Zhu, Q.; Zhang, D.; Wang, J.; Liu, J.; Zhang, J. Thin-film composite nanofiltration membranes with poly (amidoxime) as organic interlayer for effective desalination. J. Environ. Chem. Eng. 2022, 10, 107015. [Google Scholar] [CrossRef]
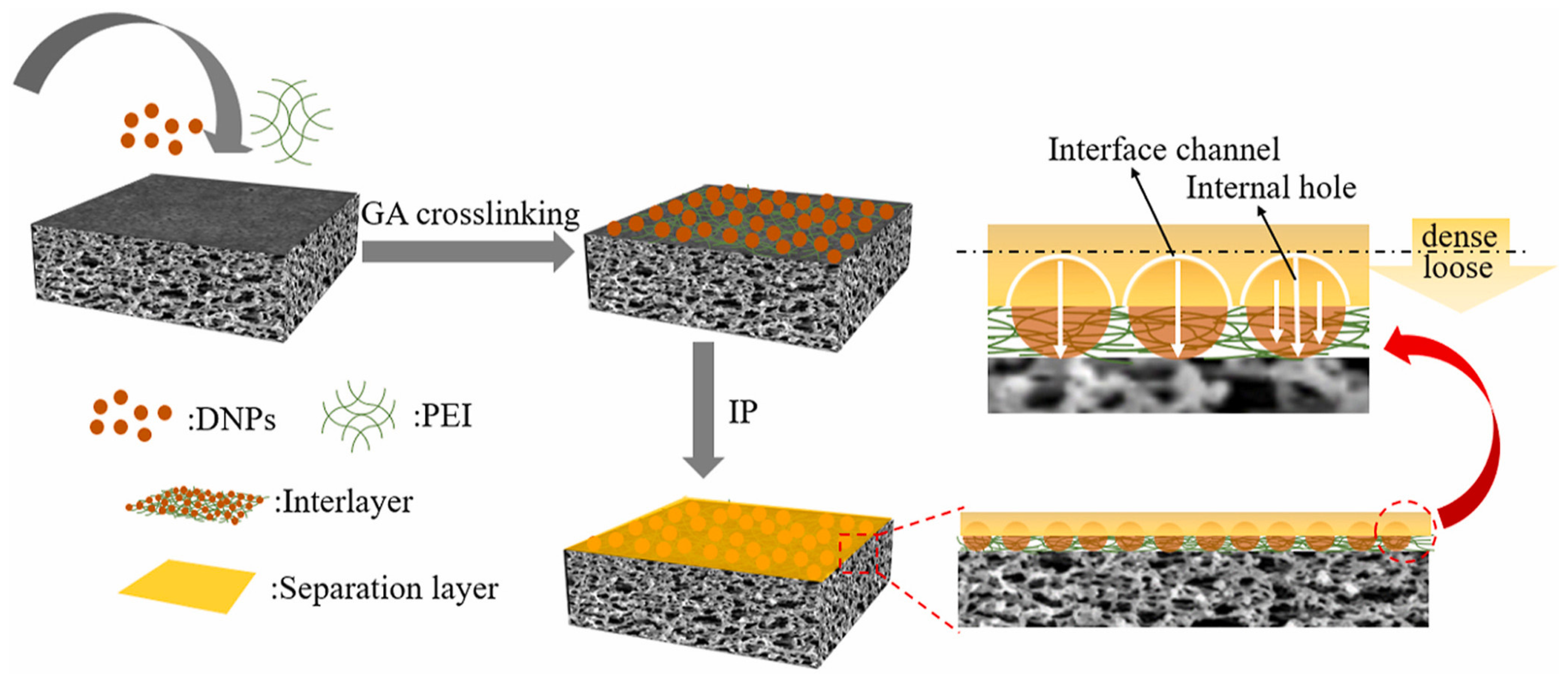
- Chen, Y.; Sun, H.; Tang, S.; Feng, H.; Zhang, H.; Chen, K.; Li, P.; Niu, Q.J. Nanofiltration membranes with enhanced performance by constructing an interlayer integrated with dextran nanoparticles and polyethyleneimine coating. J. Membr. Sci. 2022, 654, 120537. [Google Scholar] [CrossRef]
- Song, Q.; Lin, Y.; Ueda, T.; Istirokhatun, T.; Shen, Q.; Guan, K.; Yoshioka, T.; Matsuyama, H. Mechanism insights into the role of the support mineralization layer toward ultrathin polyamide nanofilms for ultrafast molecular separation. J. Mater. Chem. A 2021, 9, 26159–26171. [Google Scholar] [CrossRef]
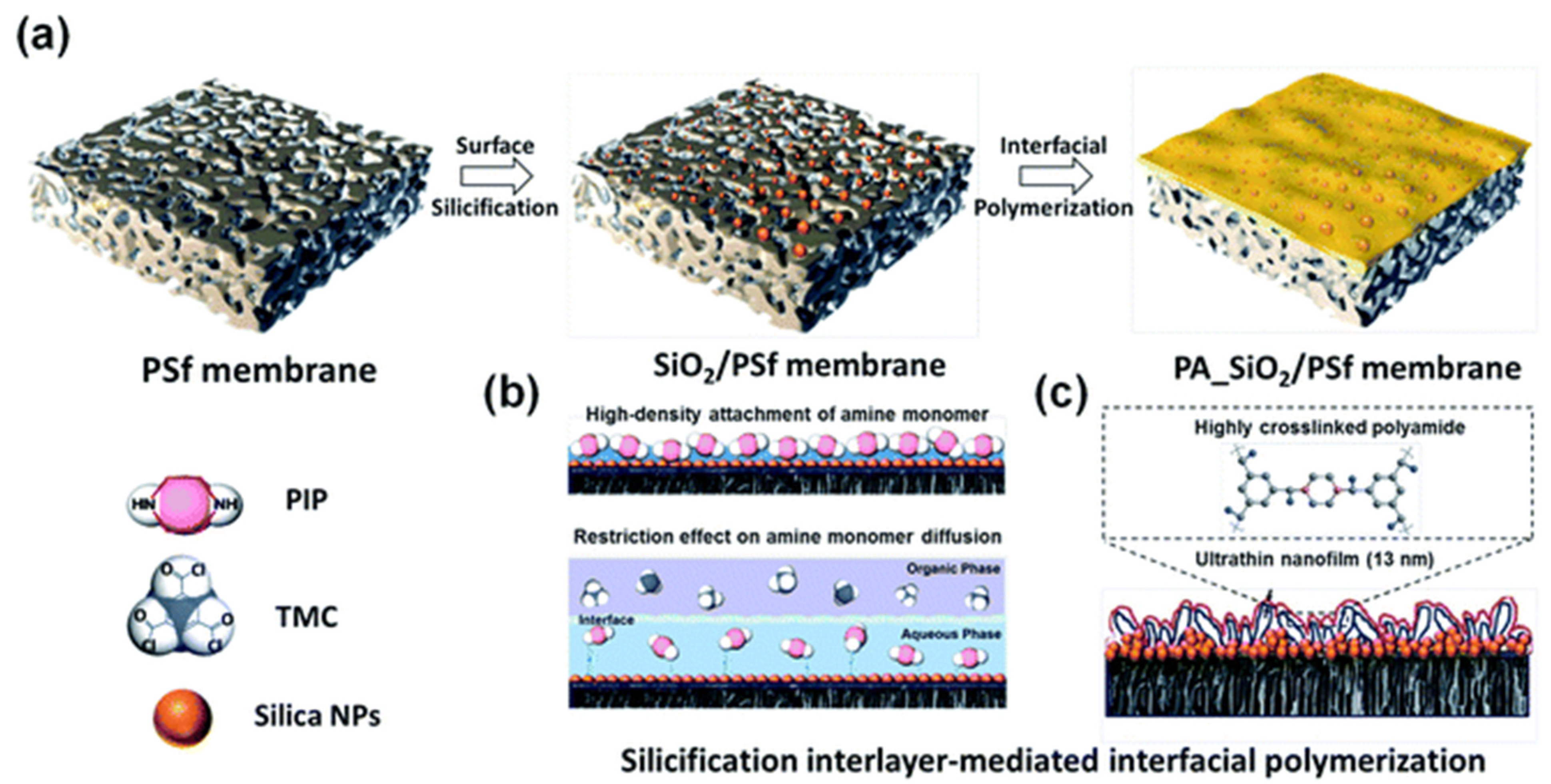
- Wang, Y.; Wang, T.; Li, S.; Zhao, Z.; Zheng, X.; Zhang, L.; Zhao, Z. Novel Poly(piperazinamide)/poly(m-phenylene isophthalamide) composite nanofiltration membrane with polydopamine coated silica as an interlayer for the splendid performance. Sep. Purif. Technol. 2022, 285, 120390. [Google Scholar] [CrossRef]
- Han, S.; Mai, Z.; Wang, Z.; Zhang, X.; Zhu, J.; Shen, J.; Wang, J.; Wang, Y.; Zhang, Y. Covalent organic framework-mediated thin-film composite polyamide membranes toward precise ion sieving. ACS Appl. Mater Inter. 2022, 14, 3427–3436. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, Z.; Wang, H.; Wang, L.; Qian, Y.; Long, X.; Ma, C.; Zhang, Z.; Li, J.; Zhang, H. Enhanced water permeance of a polyamide thin-film composite nanofiltration membrane with a metal-organic framework interlayer. J. Membr. Sci. 2021, 625, 119154. [Google Scholar] [CrossRef]
- Irigoyen, J.; Laakso, T.; Politakos, N.; Dahne, L.; Pihlajamaki, A.; Manttari, M.; Enrique Moya, S. Design and Performance Evaluation of Hybrid Nanofiltration Membranes Based on Multiwalled Carbon Nanotubes and Polyelectrolyte Multilayers for Larger Ion Rejection and Separation. Macromol. Chem. Phys. 2016, 217, 804–811. [Google Scholar] [CrossRef]
- Gao, S.; Zhu, Y.; Gong, Y.; Wang, Z.; Fang, W.; Jin, J. Ultrathin polyamide nanofiltration membrane fabricated on brush-painted single-walled carbon nanotube network support for ion sieving. ACS Nano 2019, 13, 5278–5290. [Google Scholar] [CrossRef]
- Park, M.J.; Wang, C.; Seo, D.H.; Gonzales, R.R.; Matsuyama, H.; Shon, H.K. Inkjet printed single walled carbon nanotube as an interlayer for high performance thin film composite nanofiltration membrane. J. Membr. Sci. 2021, 620, 118901. [Google Scholar] [CrossRef]
- Wang, J.-J.; Yang, H.-C.; Wu, M.-B.; Zhang, X.; Xu, Z.-K. Nanofiltration membranes with cellulose nanocrystals as an interlayer for unprecedented performance. J. Mater. Chem. A 2017, 5, 16289–16295. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Gao, S.; Zhang, F.; Zhang, W.; Liu, Z.; Jin, J. Single-walled carbon nanotube film supported nanofiltration membrane with a nearly 10 nm thick polyamide selective layer for high-flux and high-rejection desalination. Small 2016, 12, 5034–5041. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat.Commun. 2018, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Wang, P.; Zhou, Z.; Hu, Y. New insights into the role of an interlayer for the fabrication of highly selective and permeable thin-film composite nanofiltration membrane. ACS Appl. Mater. Inter. 2019, 11, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, R. Recent progress and perspectives on the toxicity of carbon nanotubes at organism, organ, cell, and biomacromolecule levels. Environ. Int. 2012, 40, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Xie, F.; Ding, H.-Z.; Huang, W.; Ma, X.-H.; Xu, Z.-L. Effects of locations of cellulose nanofibers in membrane on the performance of positively charged membranes. J. Membr. Sci. 2022, 652, 120464. [Google Scholar] [CrossRef]
- Ji, C.; Lin, C.-W.; Zhang, S.; Guo, Y.; Yang, Z.; Hu, W.; Xue, S.; Niu, Q.J.; Kaner, R.B. Ultrapermeable nanofiltration membranes with tunable selectivity fabricated with polyaniline nanofibers. J. Mater. Chem. A 2022, 10, 4392–4401. [Google Scholar] [CrossRef]
- Guo, Y.; Ji, C.; Ye, Y.; Chen, Y.; Yang, Z.; Xue, S.; Niu, Q.J. High performance nanofiltration membrane using self-doping sulfonated polyaniline. J. Membr. Sci. 2022, 652, 120441. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, S.; Chen, Y.; Ye, H.; Xue, S.; Niu, Q.J. Sulfonated polyaniline interlayer with controllable doping conditions for high-performance nanofiltration. J. Membr. Sci. 2023, 672, 121478. [Google Scholar] [CrossRef]
- Han, S.; Zhu, J.; Uliana, A.A.; Li, D.; Zhang, Y.; Zhang, L.; Wang, Y.; He, T.; Elimelech, M. Microporous organic nanotube assisted design of high performance nanofiltration membranes. Nat. Commun. 2022, 13, 7954. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.-M.; Ji, C.-H.; Xu, Z.-L.; Tang, Y.-J.; Li, R.-H. Chlorine resistant TFN nanofiltration membrane incorporated with octadecylamine-grafted GO and fluorine-containing monomer. J. Membr. Sci. 2018, 545, 185–195. [Google Scholar] [CrossRef]
- Medhekar, N.V.; Ramasubramaniam, A.; Ruoff, R.S.; Shenoy, V.B. Hydrogen Bond Networks in Graphene Oxide Composite Paper: Structure and Mechanical Properties. ACS Nano 2010, 4, 2300–2306. [Google Scholar] [CrossRef]
- Lim, M.-Y.; Choi, Y.-S.; Kim, J.; Kim, K.; Shin, H.; Kim, J.-J.; Shin, D.M.; Lee, J.-C. Cross-linked graphene oxide membrane having high ion selectivity and antibacterial activity prepared using tannic acid-functionalized graphene oxide and polyethyleneimine. J. Membr. Sci. 2017, 521, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Chen, J.; Yin, Y.; Wu, H. Structurally stable graphene oxide-based nanofiltration membranes with bioadhesive polydopamine coating. Appl. Surf. Sci. 2018, 427, 1092–1098. [Google Scholar] [CrossRef]
- Aburabie, J.; Peinemann, K.-V. Crosslinked poly(ether block amide) composite membranes for organic solvent nanofiltration applications. J. Membr. Sci. 2017, 523, 264–272. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Ren, D.; Li, H.; Lv, X.; Han, L.; Su, B. Fabrication of ultra-smooth thin-film composite nanofiltration membrane with enhanced selectivity and permeability on interlayer of hybrid polyvinyl alcohol and graphene oxide. Sep. Purif. Technol. 2021, 268, 118649. [Google Scholar] [CrossRef]
- Wang, S.; Mahalingam, D.; Sutisna, B.; Nunes, S.P. 2D-dual-spacing channel membranes for high performance organic solvent nanofiltration. J. Mater. Chem. A 2019, 7, 11673–11682. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; Liu, Y.; Wu, Y.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 2020, 3, 296–302. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, X.; Luo, X.; Guo, Y.; Liu, Y.; Yang, L.; Tang, X.; Li, G.; Liang, H. MXene Nanosheet templated nanofiltration membranes toward ultrahigh water transport. Environ. Sci. Technol. 2021, 55, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ling, R.; Chen, J.P.; Reinhard, M. Quantitative assessment of the iron-catalyzed degradation of a polyamide nanofiltration membrane by hydrogen peroxide. J. Membr. Sci. 2019, 588, 117154. [Google Scholar] [CrossRef]
- Ling, R.; Yu, L.; Thi Phuong Thuy, P.; Shao, J.; Chen, J.P.; Reinhard, M. Catalytic effect of iron on the tolerance of thin-film composite polyamide reverse osmosis membranes to hydrogen peroxide. J. Membr. Sci. 2018, 548, 91–98. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Li, J.; Luo, X.; Xu, D.; Wu, D.; Wang, W.; Cheng, X.; Li, G.; Liang, H. Crumple-textured polyamide membranes via MXene nanosheet-regulated interfacial polymerization for enhanced nanofiltration performance. J. Membr. Sci. 2021, 635, 119536. [Google Scholar] [CrossRef]
- Cao, S.; Deshmukh, A.; Wang, L.; Han, Q.; Shu, Y.; Ng, H.Y.; Wang, Z.; Lienhard, J.H. Enhancing the permselectivity of thin-film composite membranes interlayered with MoS2 nanosheets via precise thickness control. Environ. Sci. Technol. 2022, 56, 8807–8818. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, C. A model-based analysis of water transport dynamics and fouling behaviors of osmotic membrane. Chem. Eng. J. 2015, 266, 289–298. [Google Scholar] [CrossRef]
- Wang, A.; Xu, H.; Fu, J.; Lin, T.; Ma, J.; Ding, M.; Gao, L. Enhanced high-salinity brines treatment using polyamide nanofiltration membrane with tunable interlayered MXene channel. Sci. Total Environ. 2022, 856, 158434. [Google Scholar] [CrossRef]
- Fu, J.; Xu, H.; Lin, T.; Wang, A.; Wang, A.; Yao, C.; Chen, W.; Ding, M.; Geng, C.; Gao, L. Tailoring the crumpled structures of a polyamide membrane with a heterostructural MXene-TiO2 interlayer for high water permeability. Desalination 2023, 549, 116352. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, M.; Tang, Y.; Zhu, J. TiO2 nanoparticle modified organ-like Ti3C2 MXene nanocomposite encapsulating hemoglobin for a mediator-free biosensor with excellent performances. Biosens. Bioelectron. 2015, 74, 1022–1028. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Luo, S.; Luo, Z.; Zhang, X.; Huang, R.; Luo, M. In situ modification of cobalt on MXene/TiO2 as composite photocatalyst for efficient nitrogen fixation. J. Colloid Interf. Sci. 2021, 585, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hu, Y.; Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2017, 1, 0056. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chandra, S.; Kandambeth, S.; Lukose, B.; Heine, T.; Banerjeet, R. Mechanochemical Synthesis of Chemically Stable Isoreticular Covalent Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 5328–5331. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yuan, J.; Wu, H.; Su, Y.; Yang, H.; You, X.; Zhang, R.; He, X.; Khan, N.A.; Kasher, R.; et al. Ultrathin nanofiltration membrane with polydopamine-covalent organic framework interlayer for enhanced permeability and structural stability. J. Membr. Sci. 2019, 576, 131–141. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, M.; Wu, H.; Liu, Y.; You, X.; Zhang, R.; Su, Y.; Yang, H.; Shen, J.; Jiang, Z. Covalent organic framework-modulated interfacial polymerization for ultrathin desalination membranes. J. Mater. Chem. A 2019, 7, 25641–25649. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Y.; Wang, X.; Fan, K.; Li, P.; Xia, S. Regulating interfacial polymerization via constructed 2D metal-organic framework interlayers for fabricating nanofiltration membranes with enhanced performance. Desalination 2022, 544, 116134. [Google Scholar] [CrossRef]
- Kang, X.; Liu, X.; Liu, J.; Wen, Y.; Qi, J.; Li, X. Spin-assisted interfacial polymerization strategy for graphene oxide-polyamide composite nanofiltration membrane with high performance. Appl. Surf. Sci. 2020, 508, 145198. [Google Scholar] [CrossRef]
- Xue, S.; Lin, C.W.; Ji, C.; Guo, Y.; Liu, L.; Yang, Z.; Zhao, S.; Cai, X.; Niu, Q.J.; Kaner, R.B. Thin-film composite membranes with a hybrid dimensional titania interlayer for ultrapermeable nanofiltration. Nano Lett. 2022, 22, 1039–1046. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Chen, W.; Kong, Q.; Lin, T.; Tao, H.; Zhang, K.; Liu, Q.; Zhang, K.; Xie, Z. Construction of a hierarchical carbon nanotube/MXene membrane with distinct fusiform channels for efficient molecular separation. J. Mater. Chem. A 2020, 8, 22666–22673. [Google Scholar] [CrossRef]
- Guo, H.; Deng, Y.; Tao, Z.; Yao, Z.; Wang, J.; Lin, C.; Zhang, T.; Zhu, B.; Tang, C.Y. Does Hydrophilic Polydopamine Coating Enhance Membrane Rejection of Hydrophobic Endocrine-Disrupting Compounds? Environ. Sci. Technol. Lett. 2016, 3, 332–338. [Google Scholar] [CrossRef]
- Guo, H.; Yao, Z.; Yang, Z.; Ma, X.; Wang, J.; Tang, C.Y. A One-Step Rapid Assembly of Thin Film Coating Using Green Coordination Complexes for Enhanced Removal of Trace Organic Contaminants by Membranes. Environ. Sci. Technol. 2017, 51, 12638–12643. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Deng, Y.; Yao, Z.; Yang, Z.; Wang, J.; Lin, C.; Zhang, T.; Zhu, B.; Tang, C.Y. A highly selective surface coating for enhanced membrane rejection of endocrine disrupting compounds: Mechanistic insights and implications. Water Res. 2017, 121, 197–203. [Google Scholar] [CrossRef] [PubMed]

| Category | Organic Material | Porous Substrate | IP Condition (Optimum) | Polyamide Thickness (nm) | Water Flux (L m−2 h−1 bar−1) | Salt Rejection | Year [Ref] |
|---|---|---|---|---|---|---|---|
| Polyphenols | TA-Fe nano scaffold | PSF UF substrate (Mw 35,000) | 0.2 wt% PIP/water, 0.15 wt% TMC/n-hexane 1 min reaction | 54.9 | 19.6 | 20.6 ± 2.8 α (NaCl/MgSO4) | 2018 [32] |
| Noria–PEI solution | PSF UF substrate | 1 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 39.7 | 28 | >96% Na2SO4 | 2018 [25] | |
| TTSBI-PEI solution | PSF UF substrate(Mw 70,000) | 1 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 28.6 | 23.7 | 99.4% Na2SO4 | 2020 [34] | |
| PDA | PSF substrate (Mw 35,000) | 0.2 wt% PIP/water, 0.15 wt% TMC/n-hexane 1 min reaction | 116 | 14.8 | 34.4 ± 1.0 α (NaCl/MgSO4) | 2020 [20] | |
| TA-DDS | PSF UF substrate | 0.1 w/v% PIP/water, 0.1 wt% TMC/n-hexane 25 s reaction | 42 | 17.4 | >99% Na2SO4 | 2021 [31] | |
| Catechol-SA | PSF UF substrate | 1 mg/mL PIP/water 0.1 mg/mL TMC/n hexane 30 s reaction | 110 | 13.71 | 99.15% Na2SO4 | 2021 [35] | |
| Catechol-PEI | PSF UF substrate | 10 mg/mL PIP/water 1 mg/mL TMC/n hexane 30 s reaction | 35 | 18.4 | 50.7 α(Mg2+/Li+) | 2022 [33] | |
| Ionic polymers | PSS-Ca2+ | PSF UF substrate | 1 w/v% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 38.4 | 22.15 | 99.3% Na2SO4 | 2020 [36] |
| P[MPC-co-AEMA]-GA | PSF substrate | 0.2 w/v% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 12 | 20.4 | 96.8% Na2SO4 | 2022 [40] | |
| SPEK-C | PTFE MF substrate (0.22 μm) | 0.1 wt% PIP/water, 0.025 w/v% TMC/n-hexane 1 min reaction | 25 | 36.3 | 98.5% Na2SO4 | 2022 [37] | |
| Quaternized crosslinked microgels | PAN UF substrate (Mw 50,000) | 1.5 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 44 | 10.1 | 93.4% Na2SO4 | 2023 [38] | |
| PAH-GA | PSF UF substrate (Mw 600,000) | 0.1 wt% PIP/water, 0.05 wt% TMC/n-hexane30 s reaction | 8 | 12.5 | 98.9% Na2SO4 | 2023 [39] | |
| Polymeric organic acid | Hyaluronic acid | PES MF substrate (0.10 μm) | 0.5 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 41.5 | 29.53 | 94.9% Na2SO4 | 2022 [41] |
| Poly(caffeic acid) | PAN UF substrate (Mw 80,000) | NA | 284 | 17.7 | 98.5% Na2SO4 | 2023 [42] | |
| Other organic interlayers | PVA-GA | PES UF substrate(Mw 150,000) | 0.5 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 9.6 | 31.4 | 99.4% Na2SO4 | 2020 [27] |
| Gelatin-GA | PSF UF substrate | 1 w/v% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 45 | 16.95 | 99.3% Na2SO4 | 2021 [43] | |
| Poly (amidoxime) | PES UF substrate (Mw 58,000) | 0.2 wt% PIP/water, 0.1 wt% TMC/n-hexane 1 min reaction | 18.2 | 25.2 | 99.2% Na2SO4 | 2022 [44] | |
| PEI/DNPs-GA | PSF UF substrate | 1 wt% PIP/water, 0.1 wt% TMC/n-hexane 30 s reaction | 33.3 | 31.33 | 99.1% Na2SO4 | 2022 [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zhang, L.; Geng, N. Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective. Membranes 2023, 13, 497. https://doi.org/10.3390/membranes13050497
Liu M, Zhang L, Geng N. Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective. Membranes. 2023; 13(5):497. https://doi.org/10.3390/membranes13050497
Chicago/Turabian StyleLiu, Mingxiang, Lei Zhang, and Nannan Geng. 2023. "Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective" Membranes 13, no. 5: 497. https://doi.org/10.3390/membranes13050497
APA StyleLiu, M., Zhang, L., & Geng, N. (2023). Effect of Interlayer Construction on TFC Nanofiltration Membrane Performance: A Review from Materials Perspective. Membranes, 13(5), 497. https://doi.org/10.3390/membranes13050497





