Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance
Abstract
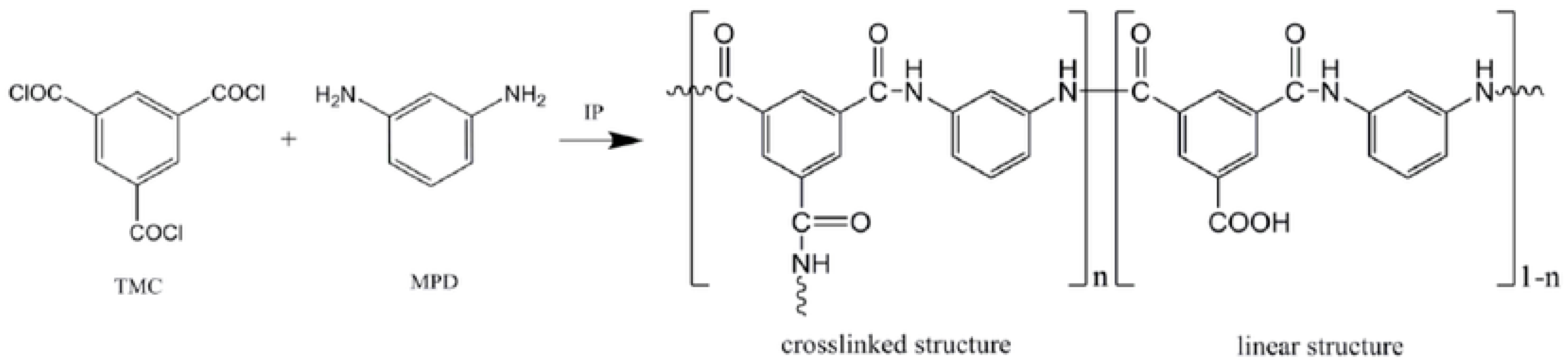
1. Introduction
2. Thin-Film Nanocomposite Membranes
2.1. Incorporation of Inorganic Nanofiller
| Nanofiller | Average Size (nm) | Loading at Best Performance | Feed Solution Conc. | Pressure (Bar) | Membrane Type | PWP LMH/Bar (From → to) Best Performance | % Salt Rejection (From → to) Best Performance | Reference | Notes |
|---|---|---|---|---|---|---|---|---|---|
| Inorganic Nanofillers | |||||||||
| TiO2 nanoparticles (NPs) | 10 | 0.0125 wt/v% (O) | 2000 ppm NaCl | 15.2 | RO | 1.41 → 1.60 | 97.9 → 97.7 | [43] | Thermal stability and anti-biofouling |
| Fluorinated SiO2 NPs | 150–200 | 0.12 wt/v% (O) | 2000 ppm NaCl | 15.5 | RO | 2.66 → 2.50 | 96.0 → 98.6 | [47] | Hydrophobic NPs to improve salt rejection |
| CeO2 NPs | 54 | 0.01 wt/v% 100 mg/L (A) | 2000 ppm NaCl | 16.0 | RO | 1.84 → 2.75 | 98.7 → 98.0 | [44] | Rougher surfaces and thinner PA layers—antifouling |
| CeO2 NPs | -- | 0.2 wt/v% (O) | 2000 ppm NaCl | 20.0 | NF | 1.66 → 2.06 | ~ 85.5 → 94.8 | [48] | Antifouling |
| FeO | 50 | 0.2 wt/v% (O) | 2000 ppm NaCl | 10.3 | NF | 1.7 → 2.3 | 66.2 → 92.1 | [49] | Antifouling |
| Ag NPs | -- | 0.2 wt/v% Preloaded on PSf | 2000 ppm NaCl | 20.0 | RO | 0.93 → 2.50 | 97.4 → 99.1 | [41] | Formation of nanochannels around the Ag NPs |
| Halloysite nanotubes (HNT)-COOH | 28 (inner diameter) | 0.05 wt/v% (O) | 3000 ppm NaCl | 20 | RO | 1.31 → 2.48 | 99.1 → 99.1 | [50] | Leaching test |
| Cu NPs nanovoids | 10–21 | Preloaded on PSf | 2000 ppm NaCl | 20 | RO | 0.76 → 1.26 | 96.7 → 95 | [51] | Removed by acid etching |
| MgFe2O4 NPs | 21 | 0.005 wt/v% (A) | 2000 ppm NaCl | 10 | NF | 3.71 → 5.05 | 51.0 → 69.1 | [52] | Antibacterial |
| Si NPs | 50 | 0.07 wt/v% (O) | 1000 ppm Na2SO4 | 6 | NF | 6.98 → 9.85 | 98.5 → 98.3 | [53] | Antifouling BSA |
| Si NPs | -- | 0.02% w/v (O) | 2000 ppm NaCl | 5–30 | RO | 4.80 → 8.20 | 98.0 → 98.0 | [54] | In-situ prep. |
| Aminophenyl-modified MSN (AMSN) | 40 | 0.025% w/v (A) | 32,000 ppm NaCl | 55.2 | RO | 0.83 → 1.00 | 99.3 → 99.0 | [55] | Lower flux than expected |
| Aminated TiO2 NPs | 123 | 0.3% w/v (A) | 1000 ppm Na2SO4, NaCl | 5 | NF | 3.90 → 10.40 | 32.1 → 18.6 (NaCl) 97.8 → 98.0 (Na2SO4) | [56] | Monovalent/Divalent salt separation |
| TiO2 NPs | <100 nm | 0.1% w/v (O) | 2000 ppm NaCl | 20 | RO | 1.93 → 3.14 | 97.2 → 97.0 | [57] | Antifouling (humic acid); antibacterial |
| ZnO NPs | 15–20 | 0.02% w/v (A) | 2000 ppm NaCl | 20 | RO | 0.72 → 1.19 | 99.2 → 97.0 | [58] | Antifouling (humic acid); antibacterial |
| Alkyl-silica NPs | 20 | 0.5 wt/v% (O) | 2000 ppm NaCl | 15.5 | RO | 3.4 → 3.57 | 99.5 → 99.6 | [46] | Boron removal |
| 32,000 ppm NaCl | 55.0 | 98.6 → 99.4 | |||||||
| Amine-rich synthetic talc (NHST) nanosheets | --- | 0.5 wt/v% (A) | 2000 ppm Na2SO4 | 5 | NF | 16.0 → 24.45 | 87.00 → 98.96 | [59] | Antifouling (BSA); antibacterial |
| TiO2 | 5–10 | 0.015 wt/v% (A) | 1000 ppm Na2SO4 | 5 | NF | 12.5 → 20.3 | 95.5 → 91.1 | [60] | Electrospray |
| Hierarchical nanosized zeolite | 200–800 | 0.005 wt/v% preloaded on PSf | 1000 ppm Na2SO4 | 4 | NF | 13.1 → 23.2 | 98.5 → 97.5 | [61] | * Vacuum filtration * Stability |
| Flower-like MnO2 NPs | 200–500 | 0.5 wt/v% (A) | 20 ppm Rhodamine B dye | 10 | NF | 7.72 → 11.88 Methanol permeability | 99.2 → 96.94 | [62] | OSN |
| Modified silica (m-silica) NPs | --- | 0.4 wt/v% (A) | 1000 ppm Na2SO4 | 10 | NF | 3.63 → 6.16 | 23.32 → 97.96 | [45] | Stability |
| Carbon-based Nanofillers | |||||||||
| N-GO quantum dots (QD) | 3–8 | 0.02 wt/v% (A) | 2000 ppm NaCl | 15.0 | RO | 0.62 → 1.66 | ~ 93.0 → 93.0 | [63] | Improved thermal stability |
| Na-C QDs | 2–6 | 1 wt/v% (A) | 2000 ppm NaCl | 15.0 | RO | 1.74 → 2.56 | 97.7 → 97.7 | [64] | Stable at 23 bar |
| Cellulose nanocrystals (CNCs) | 15 | 0.1 wt/v% (O) | 3000 ppm NaCl | 20 | RO | 1.50 → 3.15 | 98.5 → 97.8 | [65] | |
| Graphene oxide (GO) | -- | 0.00087 wt/v% Preloaded on PES | 1000 ppm Na2SO4 | 8.0 | NF | 3.11 → 4.04 | 94 → 95.8 | [66] | Vacuum filtration; antifouling test (dyes) |
| GO | -- | 0.0013 w/v % Preloaded on PES | 1000 ppm Na2SO4 | 8.0 | NF | 1.80 → 4.13 | 95.0 → 96.0 | [67] | Vacuum filtration; antifouling BSA, RB5 |
| Hollow porous carbon spheres (HPCSs) | 150 | 0.02 wt/v% (A) | 20 ppm Rhodamine B dye | 10 | NF | 8.09 → 11.51 Methanol permeability | 99.0 → 97.50 | [68] | OSN |
| Acrylic-acid-coated GO | --- | 0.0013 wt/v% Preloaded on PSf | 1000 ppm Na2SO4 | 8 | NF | 9.15 → 8.41 | 97.00 → 98.69 | [69] | * Prepared at 55 °C * Antifouling BSA * Textile saline |
| Liposomes | 130–160 | 0.02 wt/v% (A) | 1000 ppm MgCl2 | 2 | NF | 11.17 → 18.21 | 90.1 → 95.9 | [19] | Ion selectivity |
| Nanodiamond (ND) | 50 | 0.02 wt/v% (A) | 1420 ppm Na2SO4 (10 mM) | 6 | NF | 4.7 → 15.0 | 98.0 → 97.3 | [3] | |
| Carboxylated cellulose nanocrystals (C–CNCs) | 100–500 | 0.05 wt/v% (A) | 2000 ppm Na2SO4 | 6 | NF | 8.2 → 10.4 | 95.0 → 98.3 | [70] | |
| Carboxylated multiwalled carbon nanotubes (MWCNTs) | 50–200 | 0.01 wt/v% (A) | 1169 ppm NaCl (20 mM) | 2.5 | NF | [71] | FO | ||
| Hybrid Nanofillers | |||||||||
| Metal organic frameworks (MOFs)-UiO-66 | 145 | 0.005 wt/v% (O) | 1000 ppm NaCl | 2 | FO | 1.87 → 4.47 | 96.3 → 96.7 | ||
| MOF/UiO-66 | 50 | 0.05 wt/v% (O) | 2000 ppm NaCl | 15.5 | RO | 2.37 → 3.67 | 99.1 → 99.4 | [72] | Boron removal |
| 32,000 ppm NaCl | 55.0 | RO | 51.46 → 61.32 | 99.05 → 99.27 | Doping in hexane before TMC | ||||
| MOF/UiO-66-NH2 | 200 | 0.01 wt/v% (A) | 1500 ppm Na2SO4 | 4 | NF | 14.50 → 30.80 | 99.0 → 97.5 | [73] | Vacuum filtration |
| MOF/UiO-66-NH2 | 100 | 0.02 wt/v% (A) | 100-ppm malachite green dye | 10 | NF | 1.11 → 1.33 | 85.3 → 91.9 | [74] | Antifouling (humic acid); antibacterial |
| MOF/ZIF-8 | 150 | 0.2 wt/v% (A) | 2000 ppm NaCl | 15.5 | RO | 2.76 → 3.95 | 98.9 → 99.2 | [75] | Investigated dispersion of ZIF-8 in organic and aq. solvents |
| MOF/ZIF-8 | 60 | 0.05 wt/v% Preloaded on PSf | 2000 ppm NaCl | 15.5 | RO | 2.86 → 3.72 | 96.6 → 97.8 | [76] | Spray coating |
| MOF/ZIF-8 | 70 | Preloaded by dip coating | 20 ppm Sunset Yellow/Methanol | 20 | NF | 5.8 → 8.7 | 96.5 → 90.0 | [77] | |
| MOF/ZIF-67 | 240 | Preloaded by dip coating | 20 ppm Sunset Yellow/Methanol | 20 | NF | 5.8 → 4.8 | 96.5 → 79.3 | [77] | |
| MOF/ZIF-93 | 67 | 0.2% wt/v% (O) | 1 ppm Diclofenac | 20 | NF | 6.8 → 24.2 | 99.3 → 99 | [78] | Interfacial synthesis |
| MOF/HKUST | 800 | 0.2% wt/v% (O) | 1 ppm Diclofenac | 20 | NF | 6.8 → 33.1 | 99.3 → 99.5 | [78] | Interfacial synthesis |
| (Polydopamine) PD/MOF/ZIF-8 | 150 | 0.005 wt/v% Preloaded on PSf | 1000 ppm Na2SO4 | 4 | NF | 33.8 → 53.5 | 99.2 → 95.3 | [79] | Also used ZIF-67 and CaCO3 as sacrificial template |
| Covalent organic framework nanosheets (CONs) | 250 nm (lateral size) | 0.01 wt/v% Preloaded on PSf | 1000 ppm Na2SO4 | 2 | NF | 15.7 → 53.6 | 85 → 94.3 | [80] | Vacuum filtration; thinner skin layer to sub 10 nm |
| Camphor- Al2O3 NPs | 11–20 | 0.5 wt/v% (O) | 2000 ppm Na2SO4, NaCl | 10.0 | NF | 3.02 → 7.88 | 96.5%, 92.4% | [81] | Long term stability of membrane for 10.5 h |
| PMSA * -g-GO (Zwitterion-GO) | -- | 0.02 wt% (A) | 1000 ppm NaCl | 12 | RO | 0.85 → 1.47 | 93.8 → 94.8 | [82] | Antifouling |
| Ag/carbon nanotube (CNT)/PDA | -- | Preloaded on PES | 2000 ppm Na2SO4, | 10.0 | NF | 0.54 → 1.16 | 99.4 → 94.1 | [83] | Vacuum filtration |
| Al2O3/CNT/PDA | -- | Preloaded on PES | 2000 ppm Na2SO4, | 10.0 | NF | 0.54 → 1.17 | 99.4 → 95.0 | ||
| Fe2O3/CNT/PDA | -- | Preloaded on PES | 2000 ppm Na2SO4, | 10.0 | NF | 0.54 → 1.05 | 99.4 → 96.7 | ||
| TiO2/CNT/PDA | -- | Preloaded on PES | 2000 ppm Na2SO4, | 10.0 | NF | 0.54 → 0.96 | 99.4 → 96.9 | ||
| Single-wall carbon nanotube (SWCNT)/PDA | CNT diameter, <2 nm effective surface area: 12.56 cm2 | 0.01 wt% Preloaded on PES Vacuum filtration | 2000 ppm Na2SO4 100 ppm Methyl orange (MO) and other dyes | 5.0 | NF | 6.5 → 21 | RMO, 82 → 91.5 RNa2SO4, 98.5 | [84] | Selectivity of Cl−/SO42− is ~85.5 Selectivity of NaCl/MV is >123.5 |
| Ag-ZnO | 37 | 1.00 wt/v% (A) | 5.0 ppm 2,4-DCP | 11.0 | NF | 0.90 → 1.90 | 58.0 → 85.0 | [85] | Antifouling silver leaching studies |
| MOF/MIL-53 (Al) | 12 | 0.20 wt/v% (O) | 584.4 ppm NaCl | 5.0 | FO | 0.39 → 1.74 | 80.3 → 92.1 | [86] | Doxycycline removal |
| Cu-Al layered double hydroxide (LDH) nanofillers | -- | 0.10 wt/v% (O) | 1000 ppm Na2SO4 | 7.0 | NF | 3.18 → 7.01 | 98.0 → 98.0 | [87] | Antifouling CTAB |
| PDA-SiNPs | 60 | 0.07 wt/v% (O) | 1000 ppm Na2SO4 | 6 | NF | 6.98 → 20.00 | 95.0 → 94.5 | [88] | Antifouling BSA |
| Ni-MOFs | -- | 0.015% w/v (A) | 4000 ppm NaCl | 20 | RO | 1.03 → 2.52 | 99.3 → 99.2 | [89] | Antifouling (humic acid) |
| Ag-MOFs | 439 | 0.01% w/v (A) | 10 ppm Na2SO4 | 2 | NF | 9.2 → 14.3 | 54.0 → 84.1 | [90] | Antibacterial |
| 10 ppm RB dye | 9.2 → 14.3 | 92.8 → 99.3 | |||||||
| ZIF-8 | 100 | 0.05 wt/v% Preloaded on PSf | 2000 ppm NaCl | 15.5 | RO | 0.95 → 3.74 | 98.47 → 99.03 | [17] | |
| GO@CS | 200 | 0.01 wt/v% (A) | 2000 ppm NaCl | 20 | RO | 0.73 → 1.58 | 98.35 → 99.10 | [91] | Stability |
| Ag@ZnO-OAc | 8 | 0.3 wt/v% (O) | 1000 ppm Na2SO4 | 5 | NF | 7.2 → 5.5 | 94.5 → 98.8 | [92] | Antifouling BSA |
| Palygorskite/Ag clay nanotubes | 30–60 | 0.00075 wt/v% (A) | 2000 ppm NaCl | 16 | RO | 1.5 → 2.5 | 98.6 → 98.3 | [93] | Antibacterial * Stability |
2.2. Incorporation of Carbon-Based Nanofillers
2.3. Incorporation of Hybrid Nanofillers
3. Membrane Characterization
3.1. Structural and Elemental Analysis
3.1.1. X-ray Diffraction (XRD)
3.1.2. X-ray Photoelectron Spectrometer (XPS)
3.1.3. Energy-Dispersive X-ray Spectroscopy (EDX/EDS)
3.2. Surface and Morphology Analysis
3.2.1. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
3.2.2. Atomic-Force Microscopy (AFM)
3.2.3. Contact Angle Measurements
3.2.4. Zeta Potential Measurements
3.2.5. Nitrogen Gas Adsorption
3.3. Compositional Analysis
3.3.1. Fourier-Transform Infrared (FTIR)
3.3.2. Thermal Gravimetric Analysis (TGA)
3.3.3. Positron Annihilation Spectroscopy (PAS)
3.4. Mechanical Properties
4. Performance of TFN Membranes in Desalination and Water Treatment Applications
4.1. Water Flux
4.2. Salt Rejection
4.3. Antifouling
4.4. Chlorine Resistance
4.5. Antibacterial Activity
4.6. Thermal Stability
4.7. Dye Removal
5. Conclusions and Future Outlook
- Nanomaterials used: While a wide variety of nanomaterials have been employed, as reported in various studies, they represent a small portion of materials which could be used. As more nanomaterials are investigated and used, developing and establishing methodologies for the successful and reproducible incorporation of these different nanofillers within TFN membranes will represent a need of particular significance. More specifically, methodologies for the good dispersion, the controlled orientation, and the possibility of the precise positioning of nanofillers will be needed. Additionally, tailoring the surface properties of nanomaterials for better compatibility with membrane polymer matrices is an area of current research interest that will continue to expand. This will become more relevant with the need to establish an understanding of the role nanofiller interactions with membrane matrices play in membrane performance. Furthermore, enhancing the interactions between nanofillers and polymer matrices would increase the stability and lifespan of TFN membranes, potentially addressing concerns about their environmental impact.
- Membrane characterization: While membrane characterization is now more consistently conducted and reported on, a better comprehension of the relation between membrane structure and morphology on the one hand, and membrane performance on the other is very much needed. Furthermore, the impact of the nanofiller presence within membrane matrices on the membrane structure and morphology is still to be consistently investigated and understood. This will necessitate the complementary use of several techniques such as SEM, TEM, FTIR, porometer, and other gas adsorption techniques for pore structure determinations.
- Long-term performance and impact: The establishment of TFN membranes as viable alternatives to currently used membranes in desalination and water purification applications is so far limited by a number of factors which will need to be addressed. These include: (i) reproducibility, as producing TFN membranes with consistent performance is still a challenge, and there is still a lack of understanding of the factors that affect reproducibility; (ii) durability—while incorporating nanoparticles in TFN membranes provides increased durability and resistance to fouling, the long-term durability, and, thus, performance, of these membranes is still unknown and needs to be studied; (iii) environmental impact—the leaching of nanomaterials from TFN membranes is a concern for their potential adverse environmental and health implications, and quantifying the leaching rate, toxicity, and exposure risks is very much needed.
- Scaling-up production and cost: Scaling up the production of TFN membranes to meet the demands of large-scale water treatment applications would be needed. To this end, the economic viability of TFN membrane production needs to be evaluated for an acceptable cost–benefit balance, as, while TFN membranes are relatively inexpensive to produce compared to other advanced membrane technologies, they are still more expensive than traditional polymeric membranes, which can limit their widespread adoption.
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- You, H.; Zhang, X.; Zhu, D.; Yang, C.; Chammingkwan, P.; Taniike, T. Advantages of polydopamine coating in the design of ZIF-8-filled thin-film nanocomposite (TFN) membranes for desalination. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127492. [Google Scholar] [CrossRef]
- Qin, D.; Huang, G.; Terada, D.; Jiang, H.; Ito, M.M.; Gibbons, A.H.; Igarashi, R.; Yamaguchi, D.; Shirakawa, M.; Sivaniah, E.; et al. Nanodiamond mediated interfacial polymerization for high performance nanofiltration membrane. J. Membr. Sci. 2020, 603, 118003. [Google Scholar] [CrossRef]
- Lu, X.; Elimelech, M. Fabrication of desalination membranes by interfacial polymerization: History, current efforts, and future directions. Chem. Soc. Rev. 2021, 50, 6290–6307. [Google Scholar] [CrossRef]
- Gallardo, M.R.; Ang, M.B.M.Y.; Millare, J.C.; Huang, S.H.; Tsai, H.A.; Lee, K.R. Vacuum-Assisted Interfacial Polymerization Technique for Enhanced Pervaporation Separation Performance of Thin-Film Composite Membranes. Membranes 2022, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.X.; Liu, Q.; Shamsaei, E.; Zhang, X.; Wang, H. Preparation and Characterization of Thin-Film Composite Membrane with Nanowire-Modified Support for Forward Osmosis Process. Membranes 2015, 5, 136–149. [Google Scholar] [CrossRef]
- Gohil, J.M.; Ray, P. A review on semi-aromatic polyamide TFC membranes prepared by interfacial polymerization: Potential for water treatment and desalination. Sep. Purif. Technol. 2017, 181, 159–182. [Google Scholar] [CrossRef]
- Berezkin, A.V.; Kudryavtsev, Y.V. Effect of cross-linking on the structure and growth of polymer films prepared by interfacial polymerization. Langmuir 2015, 31, 12279–12290. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef]
- Wei, J.; Liu, X.; Qiu, C.; Wang, R.; Tang, C.Y. Influence of monomer concentrations on the performance of polyamide-based thin film composite forward osmosis membranes. J. Membr. Sci. 2011, 381, 110–117. [Google Scholar] [CrossRef]
- Zhan, Z.M.; Xu, Z.L.; Zhu, K.K.; Tang, Y.J. How to understand the effects of heat curing conditions on the morphology and performance of polypiperazine-amide NF membrane. J. Membr. Sci. 2020, 597, 117640. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef]
- Gu, J.E.; Lee, S.; Stafford, C.M.; Lee, J.S.; Choi, W.; Kim, B.Y.; Baek, K.-Y.; Chan, E.P.; Chung, J.Y.; Bang, J.; et al. Molecular Layer-by-Layer Assembled Thin-Film Composite Membranes for Water Desalination. Adv. Mater. 2013, 25, 4778–4782. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, M.; Zhong, Z.; Wang, Y. Atomic-layer-deposition-enabled thin-film composite membranes of polyimide supported on nanoporous anodized alumina. J. Membr. Sci. 2017, 535, 56–62. [Google Scholar] [CrossRef]
- Maruf, S.H.; Greenberg, A.R.; Ding, Y. Influence of substrate processing and interfacial polymerization conditions on the surface topography and permselective properties of surface-patterned thin-film composite membranes. J. Membr. Sci. 2016, 512, 50–60. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347–1351. [Google Scholar] [CrossRef]
- Hu, P.; Yuan, B.; Jason Niu, Q.; Wang, N.; Zhao, S.; Cui, J.; Jiang, J. In situ assembled zeolite imidazolate framework nanocrystals hybrid thin film nanocomposite membranes for brackish water desalination. Sep. Purif. Technol. 2022, 293, 121134. [Google Scholar] [CrossRef]
- Behdarvand, F.; Valamohammadi, E.; Tofighy, M.A.; Mohammadi, T. Polyvinyl alcohol/polyethersulfone thin-film nanocomposite membranes with carbon nanomaterials incorporated in substrate for water treatment. J. Environ. Chem. Eng. 2021, 9, 104650. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Goh, K.; Tan, C.H.; Wang, R. Liposomes-assisted fabrication of high performance thin film composite nanofiltration membrane. J. Membr. Sci. 2021, 620, 118833. [Google Scholar] [CrossRef]
- Jeong, B.H.; Hoek, E.M.V.; Yan, Y.; Subramani, A.; Huang, X.; Hurwitz, G.; Ghosh, A.K.; Jawor, A. Interfacial polymerization of thin film nanocomposites: A new concept for reverse osmosis membranes. J. Membr. Sci. 2007, 294, 1–7. [Google Scholar] [CrossRef]
- Bhaskar, V.V.; Kaleekkal, N.J. Next-generation thin-film composite nanofiltration membranes for water remediation: A review. Emergent Mater. 2021, 5, 1373–1390. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; Arafat, H.A. Recent Developments in the Rational Fabrication of Thin Film Nanocomposite Membranes for Water Purification and Desalination. ACS Omega 2020, 5, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Kadhom, M. A review on the polyamide thin film composite (TFC) membrane used for desalination: Improvement methods, current alternatives, and challenges. Chem. Eng. Res. Des. 2023, 191, 472–492. [Google Scholar] [CrossRef]
- Nambi Krishnan, J.; Venkatachalam, K.R.; Ghosh, O.; Jhaveri, K.; Palakodeti, A.; Nair, N. Review of Thin Film Nanocomposite Membranes and Their Applications in Desalination. Front. Chem. 2022, 10, 781372. [Google Scholar] [CrossRef]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Han, X.; Liu, Y.; Wang, C.; Yan, F.; Wang, J. Regulating the interfacial polymerization process toward high-performance polyamide thin-film composite reverse osmosis and nanofiltration membranes: A review. J. Membr. Sci. 2021, 640, 119765. [Google Scholar] [CrossRef]
- Kim, A.; Moon, S.J.; Kim, J.H.; Patel, R. Review on thin-film nanocomposite membranes with various quantum dots for water treatments. J. Ind. Eng. Chem. 2023, 118, 19–32. [Google Scholar] [CrossRef]
- Zhao, D.L.; Feng, F.; Shen, L.; Huang, Z.; Zhao, Q.; Lin, H.; Chung, T.S. Engineering metal–organic frameworks (MOFs) based thin-film nanocomposite (TFN) membranes for molecular separation. Chem. Eng. J. 2023, 454, 140447. [Google Scholar] [CrossRef]
- Zhang, N.; Song, X.; Jiang, H.; Tang, C.Y. Advanced thin-film nanocomposite membranes embedded with organic-based nanomaterials for water and organic solvent purification: A review. Sep. Purif. Technol. 2021, 269, 118719. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Zheng, J.; Wang, X.; Xia, S.; Van der Bruggen, B. A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms. J. Membr. Sci. 2022, 661, 120952. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, P.F.; Li, X.; Gan, B.; Wang, L.; Song, X.; Park, H.D.; Tang, C.Y. A Critical Review on Thin-Film Nanocomposite Membranes with Interlayered Structure: Mechanisms, Recent Developments, and Environmental Applications. Environ. Sci. Technol. 2020, 54, 15563–15583. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Li, F.; Chen, K.; Zhao, S.; Chen, Y.; Sun, H.; Li, P.; Niu, Q.J. Fabrication of novel thin-film nanocomposite polyamide membrane by the interlayer approach: A review. Desalination 2023, 554, 116509. [Google Scholar] [CrossRef]
- Ng, Z.C.; Lau, W.J.; Matsuura, T.; Ismail, A.F. Thin film nanocomposite RO membranes: Review on fabrication techniques and impacts of nanofiller characteristics on membrane properties. Chem. Eng. Res. Des. 2021, 165, 81–105. [Google Scholar] [CrossRef]
- Zhu, T.; Xia, Q.; Zuo, J.; Liu, S.; Yu, X.; Wang, Y. Recent advances of thin film composite membranes for pervaporation applications: A comprehensive review. Adv. Membr. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Feng, X.; Peng, D.; Zhu, J.; Wang, Y.; Zhang, Y. Recent advances of loose nanofiltration membranes for dye/salt separation. Sep. Purif. Technol. 2022, 285, 120228. [Google Scholar] [CrossRef]
- Karami, P.; Khorshidi, B.; McGregor, M.; Peichel, J.T.; Soares, J.B.P.; Sadrzadeh, M. Thermally stable thin film composite polymeric membranes for water treatment: A review. J. Clean. Prod. 2020, 250, 119447. [Google Scholar] [CrossRef]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Tehrani-Bagha, A.; Sadrzadeh, M. Surface characterization of thin-film composite membranes using contact angle technique: Review of quantification strategies and applications. Adv. Colloid Interface Sci. 2022, 299, 102524. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhao, Q.; Chung, T.S. Fabrication of defect-free thin-film nanocomposite (TFN) membranes for reverse osmosis desalination. Desalination 2021, 516, 115230. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhao, Q.; Lin, H.; Chen, S.B.; Chung, T.S. Pressure-assisted polydopamine modification of thin-film composite reverse osmosis membranes for enhanced desalination and antifouling performance. Desalination 2022, 530, 115671. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Yao, Z.K.; Mei, Y.; Tang, C.Y. Hydrophilic Silver Nanoparticles Induce Selective Nanochannels in Thin Film Nanocomposite Polyamide Membranes. Environ. Sci. Technol. 2019, 53, 5301–5308. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, A.M.A. Improvement of reverse osmosis performance of polyamide thin-film composite membranes using TiO2 nanoparticles. Desalination Water Treat. 2014, 55, 2939–2950. [Google Scholar]
- Khorshidi, B.; Biswas, I.; Ghosh, T.; Thundat, T.; Sadrzadeh, M. Robust fabrication of thin film polyamide-TiO2 nanocomposite membranes with enhanced thermal stability and anti-biofouling propensity. Sci. Rep. 2018, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, B.; Li, S.; Jin, B.; Yue, Q.; Wang, Z. Cerium oxide doped nanocomposite membranes for reverse osmosis desalination. Chemosphere 2019, 218, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Al-Gamal, A.Q.; Satria, M.; Alghunaimi, F.I.; Aljuryyed, N.W.; Saleh, T.A. Synthesis of thin-film nanocomposite membranes using functionalized silica nanoparticles for water desalination with drastically improved properties. React. Funct. Polym. 2022, 181, 105433. [Google Scholar] [CrossRef]
- Wu, B.; Wang, S.; Wang, J.; Song, X.; Zhou, Y.; Gao, C. Facile Fabrication of High-Performance Thin Film Nanocomposite Desalination Membranes Imbedded with Alkyl Group-Capped Silica Nanoparticles. Polymers 2020, 12, 1415. [Google Scholar] [CrossRef]
- Pang, R.; Zhang, K. Fabrication of hydrophobic fluorinated silica-polyamide thin film nanocomposite reverse osmosis membranes with dramatically improved salt rejection. J. Colloid Interface Sci. 2018, 510, 127–132. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Cerium oxide nanoparticles embedded thin-film nanocomposite nanofiltration membrane for water treatment. Sci. Rep. 2018, 8, 4976. [Google Scholar] [CrossRef]
- Lakhotia, S.R.; Mukhopadhyay, M.; Kumari, P. Iron oxide (FeO) nanoparticles embedded thin-film nanocomposite nanofiltration (NF) membrane for water treatment. Sep. Purif. Technol. 2019, 211, 98–107. [Google Scholar] [CrossRef]
- Asempour, F.; Akbari, S.; Bai, D.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Improvement of stability and performance of functionalized halloysite nano tubes-based thin film nanocomposite membranes. J. Membr. Sci. 2018, 563, 470–480. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Ma, X.; Zhou, Z.; Guo, H.; Yao, Z.; Feng, S.P.; Tang, C.Y. Fabrication of a novel and green thin-film composite membrane containing nanovoids for water purification. J. Membr. Sci. 2019, 570–571, 314–321. [Google Scholar] [CrossRef]
- Nambikkattu, J.; Kaleekkal, N.J.; Jacob, J.P. Metal ferrite incorporated polysulfone thin-film nanocomposite membranes for wastewater treatment. Environ. Sci. Pollut. Res. 2021, 28, 11915–11927. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.B.M.Y.; Pereira, J.M.; Trilles, C.A.; Aquino, R.R.; Huang, S.H.; Lee, K.R.; Lai, J.Y. Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres. Sep. Purif. Technol. 2019, 210, 521–529. [Google Scholar] [CrossRef]
- Shen, H.; Wang, S.; Xu, H.; Zhou, Y.; Gao, C. Preparation of polyamide thin film nanocomposite membranes containing silica nanoparticles via an in-situ polymerization of SiCl4 in organic solution. J. Membr. Sci. 2018, 565, 145–156. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Gao, X.; Tian, X.; Wei, Y.; Cao, Z.; Guo, C.; Zhang, H.; Ma, Z.; Zhang, Y. Surface modification of mesoporous silica nanoparticle with 4-triethoxysilylaniline to enhance seawater desalination properties of thin-film nanocomposite reverse osmosis membranes. Front. Environ. Sci. Eng. 2020, 14, 6. [Google Scholar] [CrossRef]
- Wei, S.; Chen, Y.; Hu, X.; Wang, C.; Huang, X.; Liu, D.; Zhang, Y. Monovalent/Divalent salts separation via thin film nanocomposite nanofiltration membrane containing aminated TiO2 nanoparticles. J. Taiwan Inst. Chem. Eng. 2020, 112, 169–179. [Google Scholar] [CrossRef]
- Al Mayyahi, A. TiO2 polyamide thin film nanocomposite reverses osmosis membrane for water desalination. Membranes 2018, 8, 66. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Kumar, M.; Chetty, R. Morphological effect of ZnO nanostructures on desalination performance and antibacterial activity of thin-film nanocomposite (TFN) membrane. Desalination 2020, 495, 114673. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Thakare, N.R.; Pawar, R.R.; Hazarika, S.; Ingole, P.G. Building rapid water transport channels within thin-film nanocomposite membranes based on 2D mesoporous nanosheets. Desalination 2023, 547, 116222. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.; Fu, X.; Lin, H.; Zhang, W.; Wang, X.; Liu, F. Electrosprayed thin film nanocomposite polyamide nanofiltration with homogeneous distribution of nanoparticles for enhanced separation performance. Desalination 2023, 546, 116206. [Google Scholar] [CrossRef]
- Wang, D.; Tian, M.; Han, S.; Ding, K.; Yin, L.; Zhu, J.; Zhang, Y.; Han, L. Enhanced performance of thin-film nanocomposite membranes achieved by hierarchical zeolites for nanofiltration. J. Membr. Sci. 2023, 671, 121405. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Xu, P.; Ma, S.; Peng, H.; Wang, D.; Zhou, H.; Chen, C. Flower-like MnO2 nanoparticles modified thin film nanocomposite membranes for efficient organic solvent nanofiltration. Compos. Commun. 2023, 38, 101515. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Tien, H.N.; Khivantsev, K.; Song, Z.; Zhou, F.; Yu, M. Polyamide/nitrogen-doped graphene oxide quantum dots (N-GOQD) thin film nanocomposite reverse osmosis membranes for high flux desalination. Desalination 2019, 451, 125–132. [Google Scholar] [CrossRef]
- Gai, W.; Zhao, D.L.; Chung, T.S. Thin film nanocomposite hollow fiber membranes comprising Na+-functionalized carbon quantum dots for brackish water desalination. Water Res. 2019, 154, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Asempour, F.; Emadzadeh, D.; Matsuura, T.; Kruczek, B. Synthesis and characterization of novel Cellulose Nanocrystals-based Thin Film Nanocomposite membranes for reverse osmosis applications. Desalination 2018, 439, 179–187. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Tan, Y.H.; Ng, B.C.; Ismail, A.F. A novel interfacial polymerization approach towards synthesis of graphene oxide-incorporated thin film nanocomposite membrane with improved surface properties. Arab. J. Chem. 2019, 12, 75–87. [Google Scholar] [CrossRef]
- He, H.; Xu, P.; Wang, X.; Ma, S.; Liu, Z.; Peng, H.; Wang, D.; Zhou, H.; Chen, C. Hollow porous carbon spheres (HPCSs) doped thin-film nanocomposite membrane for efficient organic solvent nanofiltration. J. Environ. Chem. Eng. 2023, 11, 109252. [Google Scholar] [CrossRef]
- Seah, M.Q.; Lau, W.J.; Goh, P.S.; Ooi, B.S.; Lai, G.S.; Ismail, A.F. Improving properties of thin film nanocomposite membrane via temperature-controlled interfacial polymerization for nanofiltration process. Desalination 2023, 545, 116091. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, L.; Zhu, X.; Xu, D.; Li, G.; Liang, H.; Wiesner, M.R. The role of carboxylated cellulose nanocrystals placement in the performance of thin-film composite (TFC) membrane. J. Membr. Sci. 2021, 617, 118581. [Google Scholar] [CrossRef]
- Rashed, A.O.; Esawi, A.M.K.; Ramadan, A.R. Novel Polysulfone/Carbon Nanotube-Polyamide Thin Film Nanocomposite Membranes with Improved Water Flux for Forward Osmosis Desalination. ACS Omega 2020, 5, 14427–14436. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, X.; Qi, S.; Li, R.; Zhang, X.; Song, X.; Gao, C. Thin film nanocomposite reverse osmosis membrane incorporated with UiO-66 nanoparticles for enhanced boron removal. J. Memb. Sci. 2019, 23–33, 101–109. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Yuan, S.; Zhao, Y.; Li, Y.; Zhang, R.; Tian, M.; Li, J.; Wang, J.; Van Der Bruggen, B. MOF-positioned polyamide membranes with a fishnet-like structure for elevated nanofiltration performance. J. Mater. Chem. A 2019, 7, 16313–16322. [Google Scholar] [CrossRef]
- Borpatra Gohain, M.; Karki, S.; Yadav, D.; Yadav, A.; Thakare, N.R.; Hazarika, S.; Lee, H.K.; Ingole, P.G. Development of Antifouling Thin-Film Composite/Nanocomposite Membranes for Removal of Phosphate and Malachite Green Dye. Membranes 2022, 12, 768. [Google Scholar] [CrossRef]
- Lee, T.H.; Oh, J.Y.; Hong, S.P.; Lee, J.M.; Roh, S.M.; Kim, S.H.; Park, H.B. ZIF-8 particle size effects on reverse osmosis performance of polyamide thin-film nanocomposite membranes: Importance of particle deposition. J. Memb. Sci. 2019, 570–571, 23–33. [Google Scholar] [CrossRef]
- Lee, T.H.; Park, I.; Oh, J.Y.; Jang, J.K.; Park, H.B. Facile Preparation of Polyamide Thin-Film Nanocomposite Membranes Using Spray-Assisted Nanofiller Predeposition. Ind. Eng. Chem. Res. 2019, 58, 4248–4256. [Google Scholar] [CrossRef]
- Sarango, L.; Paseta, L.; Navarro, M.; Zornoza, B.; Coronas, J. Controlled deposition of MOFs by dip-coating in thin film nanocomposite membranes for organic solvent nanofiltration. J. Ind. Eng. Chem. 2018, 59, 8–16. [Google Scholar] [CrossRef]
- 110th Anniversary-Polyamide Metal−Organic Framework Bilayered Thin Film Composite Membranes for the Removal of Pharmaceutical Compounds from Water.pdf. Available online: https://digital.csic.es/bitstream/10261/201903/1/110water.pdf (accessed on 1 April 2023).
- Wang, Z.; Wang, Z.; Lin, S.; Jin, H.; Gao, S.; Zhu, Y.; Jin, J. Nanoparticle-templated nanofiltration membranes for ultrahigh performance desalination. Nat. Commun. 2018, 9, 2004. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, M.; Wu, H.; Liu, Y.; You, X.; Zhang, R.; Su, Y.; Yang, H.; Shen, J.; Jiang, Z. Covalent organic framework-modulated interfacial polymerization for ultrathin desalination membranes. J. Mater. Chem. A 2019, 7, 25641–25649. [Google Scholar] [CrossRef]
- Kotp, Y.H. High-flux TFN nanofiltration membranes incorporated with Camphor-Al2O3 nanoparticles for brackish water desalination. Chemosphere 2021, 265, 128999. [Google Scholar] [CrossRef]
- Mahdavi, H.; Rahimi, A. Zwitterion functionalized graphene oxide/polyamide thin film nanocomposite membrane: Towards improved anti-fouling performance for reverse osmosis. Desalination 2018, 433, 94–107. [Google Scholar] [CrossRef]
- Al Aani, S.; Haroutounian, A.; Wright, C.J.; Hilal, N. Thin Film Nanocomposite (TFN) membranes modified with polydopamine coated metals/carbon-nanostructures for desalination applications. Desalination 2018, 427, 60–74. [Google Scholar] [CrossRef]
- Gong, G.; Wang, P.; Zhou, Z.; Hu, Y. New Insights into the Role of an Interlayer for the Fabrication of Highly Selective and Permeable Thin-Film Composite Nanofiltration Membrane. ACS Appl. Mater. Interfaces 2019, 11, 7349–7356. [Google Scholar] [CrossRef] [PubMed]
- Kotlhao, K.; Lawal, I.A.; Moutloali, R.M.; Klink, M.J. Antifouling properties of silver-zinc oxide polyamide thin film composite membrane and rejection of 2-chlorophenol and 2,4-dichlorophenol. Membranes 2019, 9, 96. [Google Scholar] [CrossRef]
- Samsami, S.; Sarrafzadeh, M.H.; Ahmadi, A. Surface modification of thin-film nanocomposite forward osmosis membrane with super-hydrophilic MIL-53 (Al) for doxycycline removal as an emerging contaminant and membrane antifouling property enhancement. Chem. Eng. J. 2022, 431, 133469. [Google Scholar] [CrossRef]
- Tajuddin, M.H.; Yusof, N.; Wan Azelee, I.; Wan Salleh, W.N.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Nagai, K.; Razali, N.F. Development of copper-aluminum layered double hydroxide in thin film nanocomposite nanofiltration membrane for water purification process. Front. Chem. 2019, 7, 3. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Trilles, C.A.; De Guzman, M.R.; Pereira, J.M.; Aquino, R.R.; Huang, S.H.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Improved performance of thin-film nanocomposite nanofiltration membranes as induced by embedded polydopamine-coated silica nanoparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Zong, Z.; Lin, R.; Zhang, X.; Chen, F.; Ding, W.; Zhang, L.; Meng, X.; Hou, J. Thin film nanocomposite membrane incorporated with 2D-MOF nanosheets for highly efficient reverse osmosis desalination. J. Membr. Sci. 2022, 653, 120520. [Google Scholar] [CrossRef]
- Tan, Z.K.; Gong, J.L.; Fang, S.Y.; Li, J.; Cao, W.C.; Chen, Z.P. Outstanding anti-bacterial thin-film composite membrane prepared by incorporating silver-based metal–organic framework (Ag-MOF) for water treatment. Appl. Surf. Sci. 2022, 590, 153059. [Google Scholar] [CrossRef]
- Qian, X.; Wang, X.; Gao, X.; Cao, W.; Gao, C. Effects of GO@CS core-shell nanomaterials loading positions on the properties of thin film nanocomposite membranes. J. Membr. Sci. 2021, 624, 119102. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Feng, X.; Hu, X.; Zhang, Y.; Liu, L. Incorporation of oleic acid-modified Ag@ZnO core-shell nanoparticles into thin film composite membranes for enhanced antifouling and antibacterial properties. J. Membr. Sci. 2020, 602, 117956. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, W.; Gao, B.; Wang, Z. Palygorskite/silver nanoparticles incorporated polyamide thin film nanocomposite membranes with enhanced water permeating, antifouling and antimicrobial performance. Chemosphere 2019, 236, 124396. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Safarpour, M.; Khataee, A.; Zarrabi, H.; Yekavalangi, M.E.; Kavian, M. A thin film nanocomposite reverse osmosis membrane containing amine-functionalized carbon nanotubes. Sep. Purif. Technol. 2017, 184, 135–143. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, S.; Wu, L.; Zhang, L.; Chen, H.; Gao, C. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Chew, Y.T.; Yong, W.F. Recent advances of thin film nanocomposite membranes: Effects of shape/structure of nanomaterials and interfacial polymerization methods. Chem. Eng. Res. Des. 2021, 172, 135–158. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Fry, B. Chapter 5: Characterization Techniques for Nanomaterials. In Nanotechnology-Enabled Sensors; Taylor & Francis Group: Abingdon, UK, 2008; pp. 211–281. [Google Scholar]
- Dai, R.; Zhang, X.; Liu, M.; Wu, Z.; Wang, Z. Porous metal organic framework CuBDC nanosheet incorporated thin-film nanocomposite membrane for high-performance forward osmosis. J. Membr. Sci. 2019, 573, 46–54. [Google Scholar] [CrossRef]
- Bagherzadeh, M.; Bayrami, A.; Shekari, Z.; Amini, M. High-performance thin-film nanocomposite (TFN) forward osmosis (FO) membranes incorporated with porous hydrophobic-core/hydrophilic-shell nanoparticles. Desalination 2021, 515, 115181. [Google Scholar] [CrossRef]
- Lee, H.S.; Im, S.J.; Kim, J.H.; Kim, H.J.; Kim, J.P.; Min, B.R. Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination 2008, 219, 48–56. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Kim, S.H.; Kim, S.S. Hybrid Organic/Inorganic Reverse Osmosis ( RO ) Membrane for Preparation and Characterization of TiO2 Nanoparticle Self-Assembled Aromatic Polyamide Membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Li, C.; Li, S.; Tian, L.; Zhang, J.; Su, B.; Hu, M.Z. Covalent organic frameworks (COFs)-incorporated thin film nanocomposite (TFN) membranes for high-flux organic solvent nanofiltration (OSN). J. Membr. Sci. 2019, 572, 520–531. [Google Scholar] [CrossRef]
- Liao, Z.; Fang, X.; Xie, J.; Li, Q.; Wang, D.; Sun, X.; Wang, L.; Li, J. Hydrophilic Hollow Nanocube-Functionalized Thin Film Nanocomposite Membrane with Enhanced Nanofiltration Performance. ACS Appl. Mater. Interfaces 2019, 11, 5344–5352. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kwak, S.Y.; Suzuki, T. Positron Annihilation Spectroscopic Evidence to Demonstrate the Flux-Enhancement Mechanism in Morphology-Controlled Thin-Film-Composite (TFC) Membrane. Environ. Sci. Technol. 2005, 39, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, Y.Y.; Kim, S.R.; Elimelech, M.; Hu, S.; Kim, J.H. Controlled TiO2 Growth on Reverse Osmosis and Nanofiltration Membranes by Atomic Layer Deposition: Mechanisms and Potential Applications. Environ. Sci. Technol. 2018, 52, 14311–14320. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cheng, C.; Zhang, T.; Wang, X. High performance polyamide composite nanofiltration membranes via reverse interfacial polymerization with the synergistic interaction of gelatin interlayer and trimesoyl chloride. J. Membr. Sci. 2019, 588, 117192. [Google Scholar] [CrossRef]
- Park, H.J.; Bhatti, U.H.; Nam, S.C.; Park, S.Y.; Lee, K.B.; Baek, I.H. Nafion/TiO2 nanoparticle decorated thin film composite hollow fiber membrane for efficient removal of SO2 gas. Sep. Purif. Technol. 2019, 211, 377–390. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing Morphology and Separation Performance of Polyamide 6,6 Membranes By Minimal Incorporation of Silver Decorated Graphene Oxide Nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef]
- Li, M.P.; Zhang, X.; Zhang, H.; Liu, W.L.; Huang, Z.H.; Xie, F.; Ma, X.H.; Xu, Z.L. Hydrophilic yolk-shell ZIF-8 modified polyamide thin-film nanocomposite membrane with improved permeability and selectivity. Sep. Purif. Technol. 2020, 247, 116990. [Google Scholar] [CrossRef]
- Akin, O.; Temelli, F. Probing the hydrophobicity of commercial reverse osmosis membranes produced by interfacial polymerization using contact angle, XPS, FTIR, FE-SEM and AFM. Desalination 2011, 278, 387–396. [Google Scholar] [CrossRef]
- Urper-Bayram, G.M.; Bossa, N.; Warsinger, D.M.; Koyuncu, I.; Wiesner, M. Comparative impact of SiO2 and TiO2 nanofillers on the performance of thin-film nanocomposite membranes. J. Appl. Polym. Sci. 2020, 137, 49382. [Google Scholar] [CrossRef]
- Karami, P.; Aktij, S.A.; Khorshidi, B.; Firouzjaei, M.D.; Asad, A.; Elliott, M.; Rahimpour, A.; Soares, J.B.P.; Sadrzadeh, M. Nanodiamond-decorated thin film composite membranes with antifouling and antibacterial properties. Desalination 2022, 522, 115436. [Google Scholar] [CrossRef]
- Tayel, A.; Ramadan, A.R.; El Seoud, O.A. Titanium dioxide/graphene and titanium dioxide/graphene oxide nanocomposites: Synthesis, characterization and photocatalytic applications for water decontamination. Catalysts 2018, 8, 491. [Google Scholar] [CrossRef]
- Liao, Z.; Fang, X.; Li, Q.; Xie, J.; Ni, L.; Wang, D.; Sun, X.; Wang, L.; Li, J. Resorcinol-formaldehyde nanobowls modified thin film nanocomposite membrane with enhanced nanofiltration performance. J. Membr. Sci. 2020, 594, 117468. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene Quantum Dots-Doped Thin Film Nanocomposite Polyimide Membranes with Enhanced Solvent Resistance for Solvent-Resistant Nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shah, A.A.; Nam, S.E.; Park, Y.I.; Park, H. Thin-film composite membranes comprising ultrathin hydrophilic polydopamine interlayer with graphene oxide for forward osmosis. Desalination 2019, 449, 41–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, H.; Guo, C.; Liao, J.; Shen, J.; Gao, C. Thin-film nanocomposite reverse osmosis membranes with enhanced antibacterial resistance by incorporating p-aminophenol-modified graphene oxide. Sep. Purif. Technol. 2020, 234, 116017. [Google Scholar] [CrossRef]
- Tian, L.; Jiang, Y.; Li, S.; Han, L.; Su, B. Graphene oxide interlayered thin-film nanocomposite hollow fiber nanofiltration membranes with enhanced aqueous electrolyte separation performance. Sep. Purif. Technol. 2020, 248, 117153. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.; Liu, Y.; Jian, M.; Gao, L.; Wang, H.; Zhang, X. Thin-Film Nanocomposite Forward-Osmosis Membranes on Hydrophilic Microfiltration Support with an Intermediate Layer of Graphene Oxide and Multiwall Carbon Nanotube. ACS Appl. Mater. Interfaces 2018, 10, 34464–34474. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, L.; Borjigin, B.; Liu, Q.; Zhao, C.; Hou, D. Fabrication of thin-film composite nanofiltration membranes with improved performance using β-cyclodextrin as monomer for efficient separation of dye/salt mixtures. Appl. Surf. Sci. 2021, 539, 148284. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Wang, Y.; Huang, M.; Gul, S.; Jiang, H. Nanocomposite membranes embedded with dopamine-melanin nanospheres for enhanced interfacial compatibility and nanofiltration performance. Sep. Purif. Technol. 2020, 242, 116816. [Google Scholar] [CrossRef]
- Abadikhah, H.; Naderi Kalali, E.; Khodi, S.; Xu, X.; Agathopoulos, S. Multifunctional Thin-Film Nanofiltration Membrane Incorporated with Reduced Graphene Oxide@TiO2@Ag Nanocomposites for High Desalination Performance, Dye Retention, and Antibacterial Properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545. [Google Scholar] [CrossRef]
- Tra, C.; Pihlajama, A.; Huisman, I.H.; Tra, G. Determining the zeta-potential of ceramic microfiltration membranes using the electroviscous effect. J. Membr. Sci. 1998, 147, 187–194. [Google Scholar]
- Tian, E.; Wang, X.; Wang, X.; Ren, Y.; Zhao, Y.; An, X. Preparation and Characterization of Thin-Film Nanocomposite Membrane with High Flux and Antibacterial Performance for Forward Osmosis. Ind. Eng. Chem. Res. 2019, 58, 897–907. [Google Scholar] [CrossRef]
- Sun, H.; Wu, P. Tuning the functional groups of carbon quantum dots in thin film nanocomposite membranes for nanofiltration. J. Membr. Sci. 2018, 564, 394–403. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Zhu, J.; Van der Bruggen, B. High-flux, antibacterial composite membranes via polydopamine-assisted PEI-TiO2/Ag modification for dye removal. Chem. Eng. J. 2019, 373, 275–284. [Google Scholar] [CrossRef]
- Saeedi-Jurkuyeh, A.; Jafari, A.J.; Kalantary, R.R.; Esrafili, A. A novel synthetic thin-film nanocomposite forward osmosis membrane modified by graphene oxide and polyethylene glycol for heavy metals removal from aqueous solutions. React. Funct. Polym. 2020, 146, 104397. [Google Scholar] [CrossRef]
- Lari, S.; Parsa, S.A.M.; Akbari, S.; Emadzadeh, D.; Lau, W.J. Fabrication and evaluation of nanofiltration membrane coated with amino-functionalized graphene oxide for highly efficient heavy metal removal. Int. J. Environ. Sci. Technol. 2022, 19, 4615–4626. [Google Scholar] [CrossRef]
- Gai, W.; Zhao, D.L.; Chung, T.S. Novel thin film composite hollow fiber membranes incorporated with carbon quantum dots for osmotic power generation. J. Membr. Sci. 2018, 551, 94–102. [Google Scholar] [CrossRef]
- Li, Y.; Bi, R.; Su, Y.; Li, Y.; Yang, C.; You, X.; Shen, J.; Yuan, J.; Zhang, R.; Jiang, Z. Tuning the pore size of graphene quantum dots composite nanofiltration membranes by P-aminobenzoic acid for enhanced dye/salt separation. Sep. Purif. Technol. 2021, 263, 118372. [Google Scholar] [CrossRef]
- Rajaeian, B.; Rahimpour, A.; Tade, M.O.; Liu, S. Fabrication and characterization of polyamide thin film nanocomposite (TFN) nanofiltration membrane impregnated with TiO2 nanoparticles. Desalination 2013, 313, 176–188. [Google Scholar] [CrossRef]
- Liu, S.; Low, Z.X.; Hegab, H.M.; Xie, Z.; Ou, R.; Yang, G.; Simon, G.P.; Zhang, X.; Zhang, L.; Wang, H. Enhancement of desalination performance of thin-film nanocomposite membrane by cellulose nanofibers. J. Membr. Sci. 2019, 592, 117363. [Google Scholar] [CrossRef]
- Gogoi, M.; Goswami, R.; Ingole, P.G.; Hazarika, S. Selective permeation of L-tyrosine through functionalized single-walled carbon nanotube thin film nanocomposite membrane. Sep. Purif. Technol. 2020, 233, 116061. [Google Scholar] [CrossRef]
- Ren, Y.; Zhu, J.; Cong, S.; Wang, J.; Van der Bruggen, B.; Liu, J.; Zhang, Y. High flux thin film nanocomposite membranes based on porous organic polymers for nanofiltration. J. Membr. Sci. 2019, 585, 19–28. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.S.; Ali, F.A.A.; Mishra, U.; Hamid, A.A. Thin-Film Nanocomposite Membrane Incorporated with Porous Zn-Based Metal-Organic Frameworks: Toward Enhancement of Desalination Performance and Chlorine Resistance. ACS Appl. Mater. Interfaces 2021, 13, 28818–28831. [Google Scholar] [CrossRef]
- Farhadi, R.; Aroon, M.A.; Ebrahimian Pirbazari, A.; Safarpour, M.; Matsuura, T.; Seirafi, P. Simultaneous separation and degradation of methylene blue by a thin film nanocomposite membrane containing TiO2/MWCNTs nanophotocatalyst. Environ. Technol. 2023, 44, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, C.; Verbeke, R.; Pfanmöller, M.; Koschine, T.; Dickmann, M.; Timpel-Lindner, T.; Egger, W.; Bals, S.; Vankelecom, I.F.J. The role of MOFs in Thin-Film Nanocomposite (TFN) membranes. J. Membr. Sci. 2018, 563, 938–948. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, C.; Wang, C.; Liu, H.; Huang, N. Preparation and characterization of a novel thermally stable thin film composite nanofiltration membrane with poly (m-phenyleneisophthalamide) (PMIA) substrate. J. Membr. Sci. 2018, 550, 36–44. [Google Scholar] [CrossRef]
- Huang, H.; Yu, J.; Guo, H.; Shen, Y.; Yang, F.; Wang, H.; Liu, R.; Liu, Y. Improved antifouling performance of ultrafiltration membrane via preparing novel zwitterionic polyimide. Appl. Surf. Sci. 2018, 427, 38–47. [Google Scholar] [CrossRef]
- Bi, R.; Zhang, Q.; Zhang, R.; Su, Y.; Jiang, Z. Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property. J. Membr. Sci. 2018, 553, 17–24. [Google Scholar] [CrossRef]
- Inurria, A.; Cay-Durgun, P.; Rice, D.; Zhang, H.; Seo, D.K.; Lind, M.L.; Perreault, F. Polyamide thin-film nanocomposite membranes with graphene oxide nanosheets: Balancing membrane performance and fouling propensity. Desalination 2019, 451, 139–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayel, A.; Abdelaal, A.B.; Esawi, A.M.K.; Ramadan, A.R. Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance. Membranes 2023, 13, 477. https://doi.org/10.3390/membranes13050477
Tayel A, Abdelaal AB, Esawi AMK, Ramadan AR. Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance. Membranes. 2023; 13(5):477. https://doi.org/10.3390/membranes13050477
Chicago/Turabian StyleTayel, Amr, Ahmed B. Abdelaal, Amal M. K. Esawi, and Adham R. Ramadan. 2023. "Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance" Membranes 13, no. 5: 477. https://doi.org/10.3390/membranes13050477
APA StyleTayel, A., Abdelaal, A. B., Esawi, A. M. K., & Ramadan, A. R. (2023). Thin-Film Nanocomposite (TFN) Membranes for Water Treatment Applications: Characterization and Performance. Membranes, 13(5), 477. https://doi.org/10.3390/membranes13050477







