Anti-Viral Surfaces in the Fight against the Spread of Coronaviruses
Abstract
1. Background
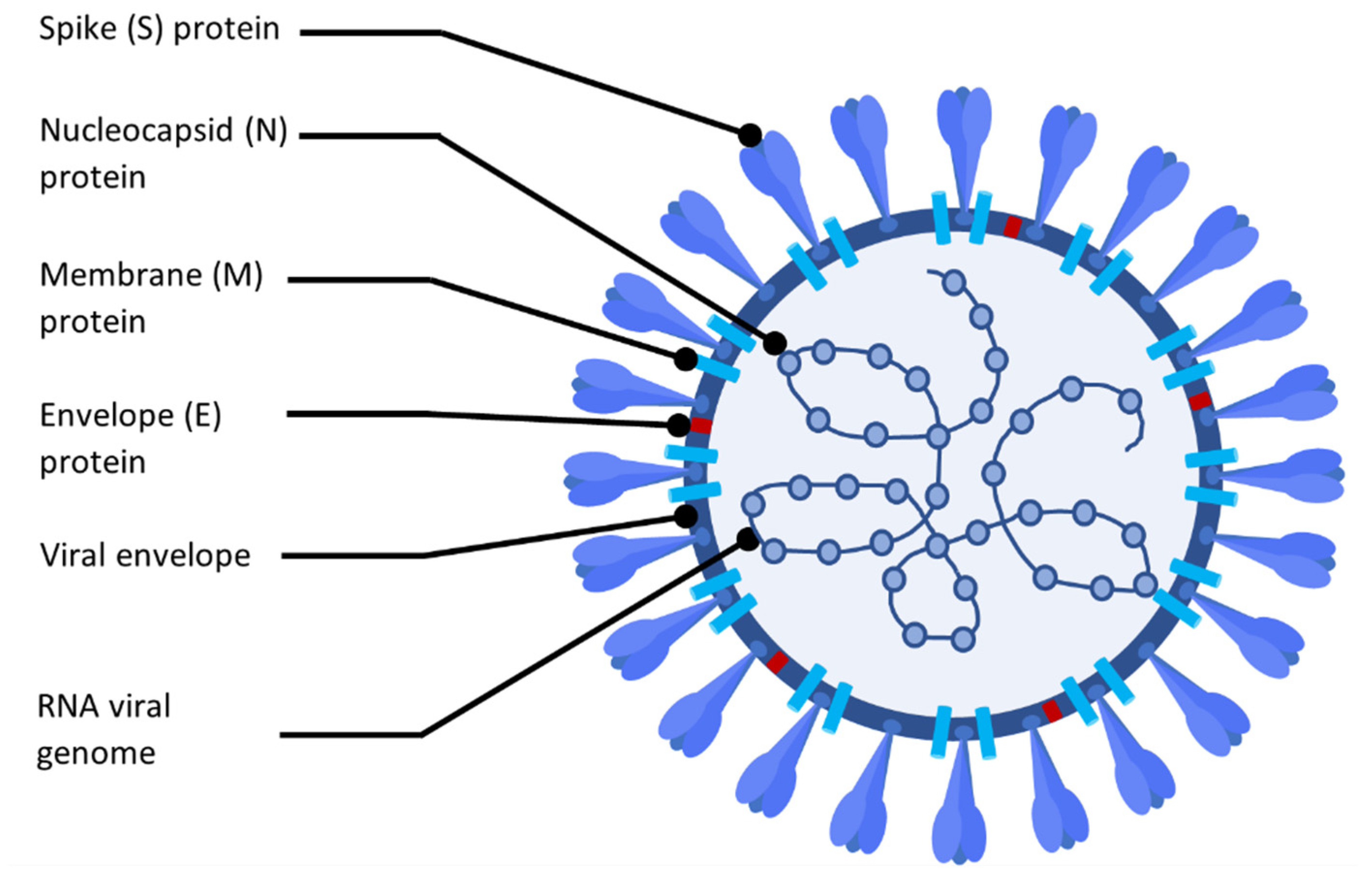
2. Coronavirus Disease Characteristics
3. Detection of Predictor Parameters for Prognosis of Patients with COVID-19
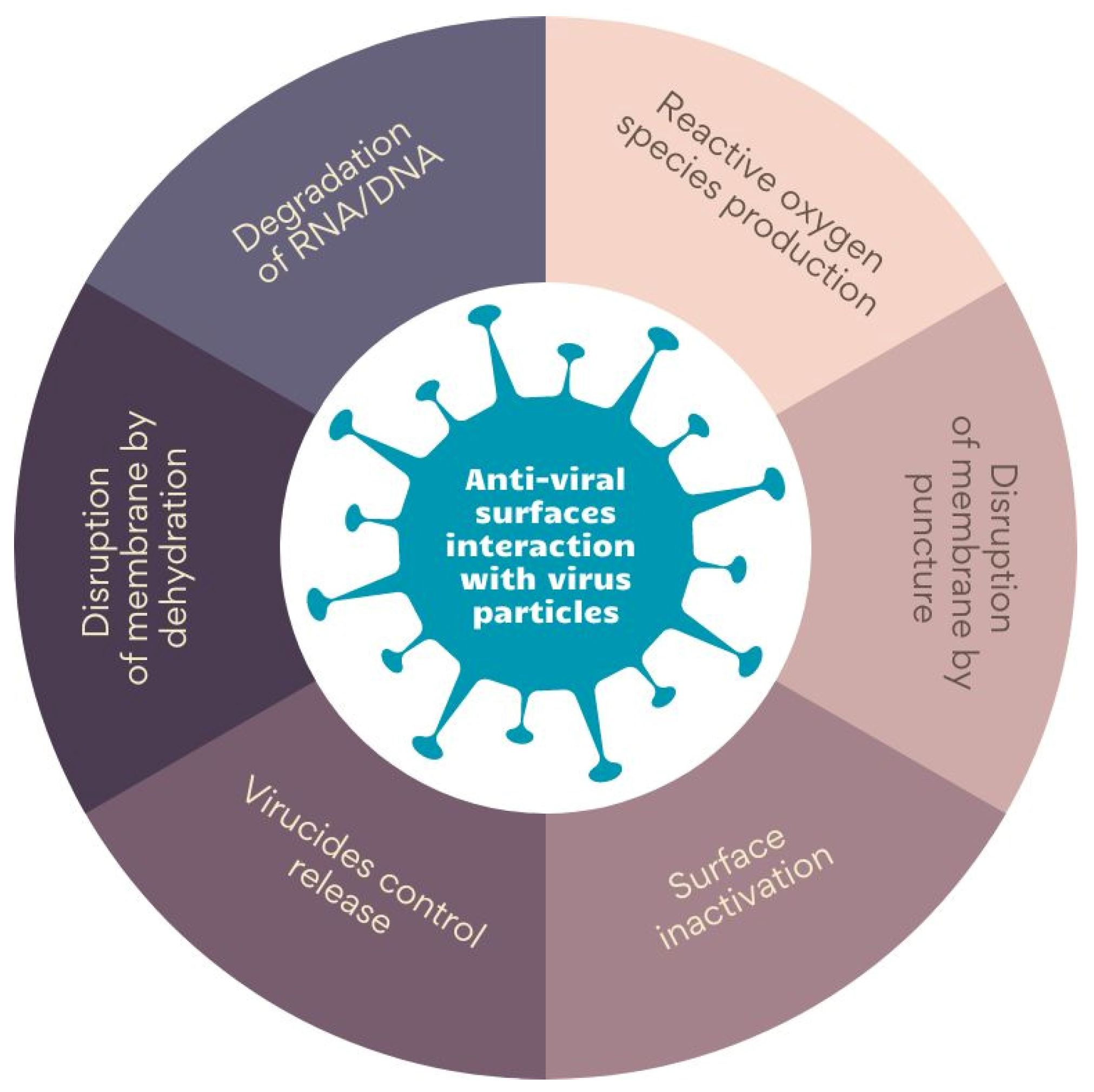
4. Anti-Viral Surfaces and Coatings and Their Mechanisms of Action
4.1. Detection of Viruses on the Surfaces
4.2. Physico-Chemical Surface Characteristics and Anti-Viral Properties
4.3. Anti-Viral Materials
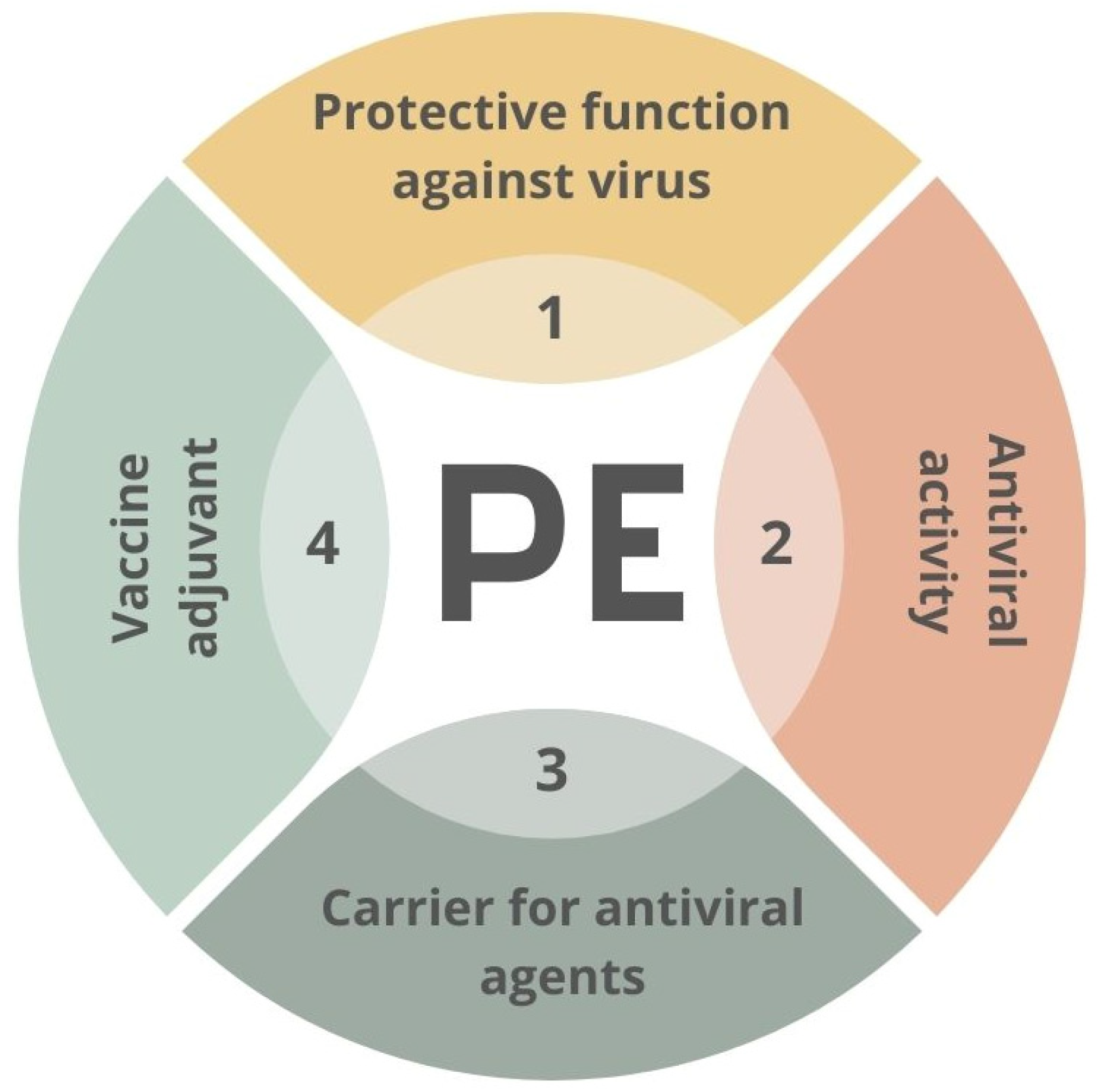
4.4. Other Applications of PE for Viral/Bacterial Binding and Potential Usage
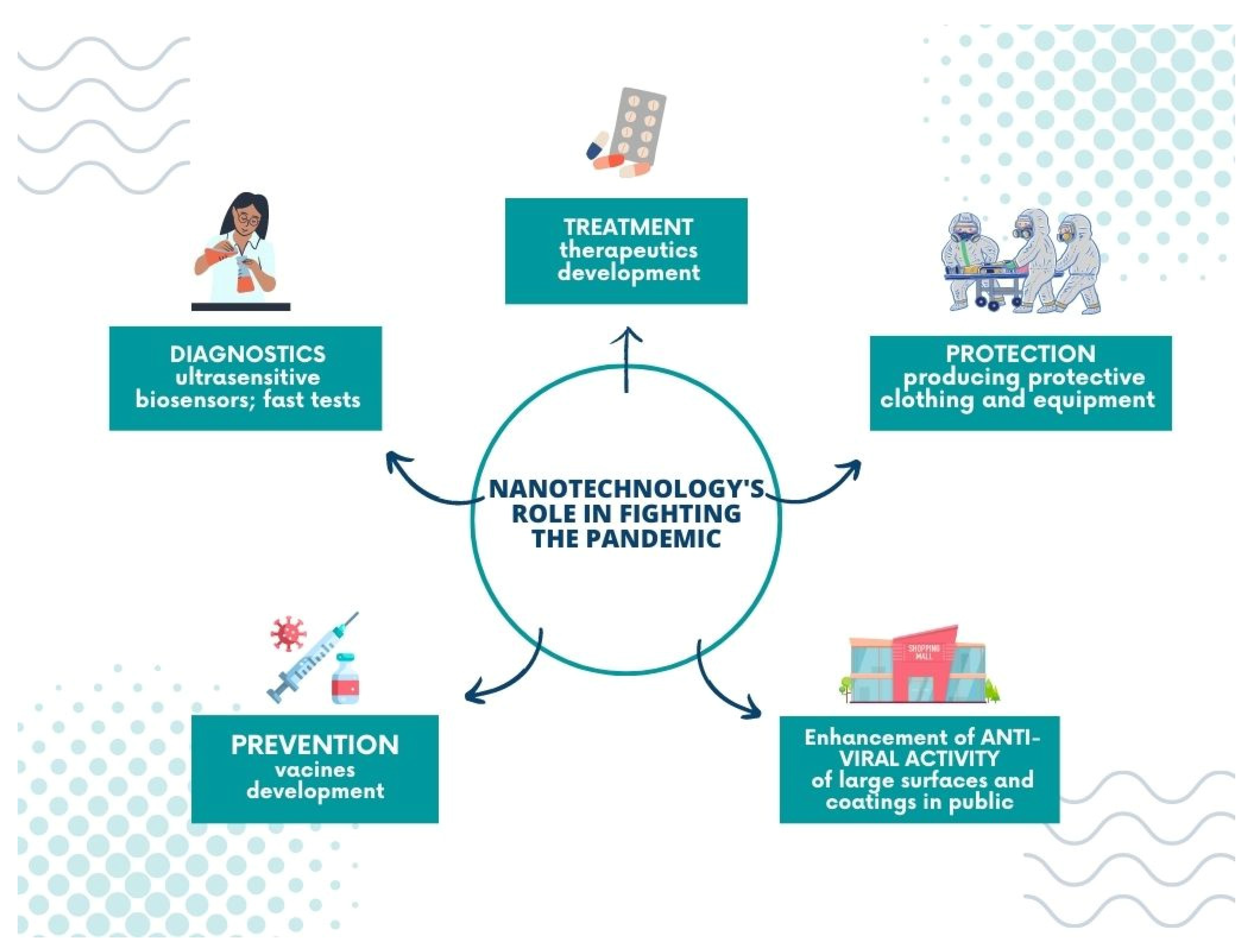
5. Nanotechnology’s Role in Fighting the Pandemic
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wagner, C.S.; Cai, X.; Zhang, Y.; Fry, C.V. One-year in: COVID-19 research at the international level in CORD-19 data. PLoS ONE 2022, 17, e0261624. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Bonde, S.; Yadav, A.; Bhowmik, A.; Rathod, S.; Ingle, P.; Gade, A. Nanotechnology as a Shield against COVID-19: Current Advancement and Limitations. Viruses 2021, 13, 1224. [Google Scholar] [CrossRef]
- Biswas, M.; Rahaman, S.; Biswas, T.K.; Haque, Z.; Ibrahim, B. Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology 2021, 64, 36–47. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Ahmad Malik, J.; Ahmed, S.; Shinde, M.; Almermesh, M.H.S.; Alghamdi, S.; Hussain, A.; Anwar, S. The Impact of COVID-19 On Comorbidities: A Review of Recent Updates For Combating It. Saudi J. Biol. Sci. 2022, 29, 3586–3599. [Google Scholar] [CrossRef]
- Ogungbe, O.; Kumbe, B.; Fadodun, O.A.; Latha, T.; Meyer, D.; Asala, A.F.; Davidson, P.M.; Dennison Himmelfarb, C.R.; Post, W.S.; Commodore-Mensah, Y. Subclinical myocardial injury, coagulopathy, and inflammation in COVID-19: A meta-analysis of 41,013 hospitalized patients. IJC Heart Vasc. 2022, 40, 100950. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, A.; Conway, F.; Della Rocca, D.G.; Biondi-Zoccai, G.; De Felice, F.; Musto, C.; Picichè, M.; Martuscelli, E.; Natale, A.; Versaci, F. COVID-19, Acute Myocardial Injury, and Infarction. Card. Electrophysiol. Clin. 2022, 14, 29–39. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Reynbakh, O.; Braunstein, E.D.; Hsu, M.; Ellis, J.; Crosson, L.; Lenane, J.; Krumerman, A.; Di Biase, L.; Ferrick, K.J. Arrhythmia patterns during and after hospitalization for COVID-19 infection detected via patch-based mobile cardiac telemetry. Am. Heart J. Plus: Cardiol. Res. Pract. 2022, 13, 100084. [Google Scholar] [CrossRef]
- Berg, D.D.; Alviar, C.L.; Bhatt, A.S.; Baird-Zars, V.M.; Barnett, C.F.; Daniels, L.B.; Defilippis, A.P.; Fagundes, A.; Katrapati, P.; Kenigsberg, B.B.; et al. Epidemiology of Acute Heart Failure in Critically Ill Patients With COVID-19: An Analysis from the Critical Care Cardiology Trials Network. J. Card. Fail. 2022, 28, 675–681. [Google Scholar] [CrossRef]
- Zuin, M.; Engelen, M.M.; Barco, S.; Spyropoulos, A.C.; Vanassche, T.; Hunt, B.J.; Vandenbriele, C.; Verhamme, P.; Kucher, N.; Rashidi, F.; et al. Incidence of venous thromboembolic events in COVID-19 patients after hospital discharge: A systematic review and meta-analysis. Thromb. Res. 2022, 209, 94–98. [Google Scholar] [CrossRef]
- Zuin, M.; Cervellati, C.; Rigatelli, G.; Zuliani, G.; Roncon, L. Reduction of venous thromboembolic events in COVID-19 patients: Which role for IL-6 antagonists? Thromb. Res. 2021, 208, 170–172. [Google Scholar] [CrossRef]
- Mandal, P.; Shah, S.; Chamlagain, R.; Rawal, L.; Gami, R.; Kartikey, A.; Singh, A.K.; Sah, S.K.; Joshi, A.; Acharya, S. Late onset ST-elevation myocardial infarction (STEMI) in patient with COVID-19: A case report from Nepal. Ann. Med. Surg. 2022, 78, 103764. [Google Scholar] [CrossRef]
- Sung, J.G.; Sobieszczyk, P.S.; Bhatt, D.L. Acute Myocardial Infarction Within 24 Hours After COVID-19 Vaccination. Am. J. Cardiol. 2021, 156, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, F.; Prasad, K.; Kumar, V. COVID-19 associated nervous system manifestations. Sleep Med. 2022, 91, 231–236. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef]
- Babazadeh, A.; Barary, M.; Ebrahimpour, S.; Sio, T.T.; Mohseni Afshar, Z. Non-arteritic anterior ischemic optic neuropathy as an atypical feature of COVID-19: A case report. J. Fr. Ophtalmol. 2022, 45, e171–e173. [Google Scholar] [CrossRef]
- Sohal, S.; Mansur, M. COVID-19 Presenting with Seizures. IDCases 2020, 20, e00782. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Hazzaa, N.M. Neurological complications associated with coronavirus disease-2019 (COVID-19): MRI features. Heliyon 2021, 7, e07879. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported Olfactory and Taste Disorders in Patients with Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef]
- Roy, D.; Ghosh, R.; Dubey, S.; Dubey, M.J.; Benito-Leon, J.; Kanti Ray, B. Neurological and Neuropsychiatric Impacts of COVID-19 Pandemic. Can. J. Neurol. Sci. 2021, 48, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Qusti, S.; Alshammari, E.M.; Gyebi, G.A.; Batiha, G.E.S. COVID-19-Induced Dysautonomia: A Menace of Sympathetic Storm. ASN Neuro 2021, 13, 17590914211057635. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.; Kelly, D.; Wood, G.K.; McCausland, B.M.; Ellul, M.A.; Varatharaj, A.; Galea, I.; Thomas, R.H.; Michael, B.D.; Gallen, B. COVID-19 Encephalitis with SARS-CoV-2 Detected in Cerebrospinal Fluid Presenting as a Stroke Mimic. J. Stroke Cerebrovasc. Dis. 2021, 30, 105915. [Google Scholar] [CrossRef] [PubMed]
- Toljan, K. Letter to the Editor Regarding the Viewpoint “Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanism. ” ACS Chem. Neurosci. 2020, 11, 1192–1194. [Google Scholar] [CrossRef]
- Krisanova, N.; Pozdnyakova, N.; Pastukhov, A.; Dudarenko, M.; Shatursky, O.; Gnatyuk, O.; Afonina, U.; Pyrshev, K.; Dovbeshko, G.; Yesylevskyy, S.; et al. Amphiphilic anti-SARS-CoV-2 drug remdesivir incorporates into the lipid bilayer and nerve terminal membranes influencing excitatory and inhibitory neurotransmission. Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183945. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, R.; Mo, Y.; Zhang, J. The blood-brain barrier in health, neurological diseases, and COVID-19. Fundam. Res. 2022, 2, 817–826. [Google Scholar] [CrossRef]
- Thakkar, K.; Goldberg, A.; Gomez, J.; Suboc, T.M.; de Lavallaz, J.D.F.; Bouroukas, A.; Volgman, A.S.; Williams, K.A.; Aggarwal, N.T. Clinical characteristics of neurological disease and COVID-19. J. Am. Coll. Cardiol. 2022, 79, 2122. [Google Scholar] [CrossRef]
- Romagnolo, A.; Imbalzano, G.; Artusi, C.A.; Balestrino, R.; Ledda, C.; De Rosa, F.G.; Riccardini, F.; Montanaro, E.; Bozzali, M.; Rizzone, M.G.; et al. Neurological comorbidities and COVID-19-related case fatality: A cohort study. J. Neurol. Sci. 2021, 428, 117610. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Taghizadeh, F.; Mojtahedi, H.; Zargar Balaye Jame, S.; Markazi Moghaddam, N. The effects of Alzheimer’s and Parkinson’s disease on 28-day mortality of COVID-19. Rev. Neurol. 2022, 178, 129–136. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Davis, P.B.; Gurney, M.E.; Xu, R. COVID-19 and dementia: Analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers. Dement. 2021, 17, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Khaki, A.R.; Wise-Draper, T.M.; French, B.; Hennessy, C.; Hsu, C.Y.; Shyr, Y.; Li, X.; Choueiri, T.K.; Painter, C.A.; et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: A report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021, 32, 787. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Borsingh Wann, S.; Kalita, J.; Manna, P. Is diabetes mellitus a wrongdoer to COVID-19 severity? Diabetes Res. Clin. Pract. 2021, 178, 108936. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Daoud, A.; Nso, N.; Medina, L.; Ghernautan, V.; Bhangoo, H.; Nyein, A.; Mohamed, M.; Alqassieh, A.; Soliman, K.; et al. Diabetes Mellitus and COVID-19: Review Article. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102268. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhadada, S.K. COVID-19 and diabetes mellitus: An unholy interaction of two pandemics. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; McKiernan, F. Iatrogenic Cushing syndrome and adrenal insufficiency during concomitant therapy with ritonavir and fluticasone. Springerplus 2015, 4, 455. [Google Scholar] [CrossRef]
- Sharma, P.; Behl, T.; Sharma, N.; Singh, S.; Grewal, A.S.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S. COVID-19 and diabetes: Association intensify risk factors for morbidity and mortality. Biomed. Pharmacother. 2022, 151, 113089. [Google Scholar] [CrossRef]
- Basin, S.; Valentin, S.; Maurac, A.; Poussel, M.; Pequignot, B.; Brindel, A.; Poupet, G.; Robert, C.; Baumann, C.; Luc, A.; et al. Progression to a severe form of COVID-19 among patients with chronic respiratory diseases. Respir. Med. Res. 2022, 81, 100880. [Google Scholar] [CrossRef]
- Bhinder, O.S.; Swarnim, S.; Mantan, M.; Dabas, A.; Ahlawat, R.S. Chronic Kidney Disease and COVID-19: Outcomes of hospitalised adults from a tertiary care centre in North India. Med. J. Armed Forces India 2022, in press. [CrossRef] [PubMed]
- Nakamura, T.; Isoda, N.; Sakoda, Y.; Harashima, H. Strategies for fighting pandemic virus infections: Integration of virology and drug delivery. J. Control. Release 2022, 343, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Coronavirus Structure, Vaccine and Therapy Development. Available online: https://www.biophysics.org/blog/coronavirus-structure-vaccine-and-therapy-development (accessed on 27 March 2022).
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 2020, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Tortorici, M.A.; Veesler, D. Structural insights into coronavirus entry. Adv. Virus Res. 2019, 105, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Qu, J.-M.; Cao, B.; Chen, R.-C. Clinical features of COVID-19. COVID-19 2021, 3, 13–39. [Google Scholar] [CrossRef]
- Smail, S.W.; Babaei, E.; Amin, K. Hematological, Inflammatory, Coagulation, and Oxidative/Antioxidant Biomarkers as Predictors for Severity and Mortality in COVID-19: A Prospective Cohort-Study. Int. J. Gen. Med. 2023, 16, 565–580. [Google Scholar] [CrossRef]
- Thungthienthong, M.; Vattanavanit, V. Platelet-to-White Blood Cell Ratio as a Predictor of Mortality in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Infect. Drug Resist. 2023, 16, 445–455. [Google Scholar] [CrossRef]
- Routes of Transmission. Available online: https://www.aaha.org/aaha-guidelines/infection-control-configuration/routes-of-transmission/ (accessed on 3 July 2022).
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of Superoxide Dismutase Activity in Tumor Tissue. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 457–464. [Google Scholar] [CrossRef]
- Eslamijouybari, M.; Heydari, K.; Maleki, I.; Moosazadeh, M.; Hedayatizadeh-Omran, A.; Vahedi, L.; Ghasemian, R.; Sharifpour, A.; Alizadeh-Navaei, R. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in COVID-19 patients and control group and relationship with disease prognosis. Casp. J. Intern. Med. 2020, 11, 531. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, C.; Ni, W.; Shen, H.; Qiu, M.; Zhao, Y. Peripheral blood inflammatory markers in predicting prognosis in patients with COVID-19. Some differences with influenza A. J. Clin. Lab. Anal. 2021, 35, e23657. [Google Scholar] [CrossRef]
- Ertekin, B.; Yortanlı, M.; Özelbaykal, O.; Doğru, A.; Girişgin, A.S.; Acar, T. The Relationship between Routine Blood Parameters and the Prognosis of COVID-19 Patients in the Emergency Department. Emerg. Med. Int. 2021, 2021, 7489675. [Google Scholar] [CrossRef]
- Erdogan, A.; Can, F.E.; Gönüllü, H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J. Med. Virol. 2021, 93, 5555–5559. [Google Scholar] [CrossRef]
- Seyit, M.; Avci, E.; Nar, R.; Senol, H.; Yilmaz, A.; Ozen, M.; Oskay, A.; Aybek, H. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID-19. Am. J. Emerg. Med. 2021, 40, 110–114. [Google Scholar] [CrossRef]
- Bilge, M.; Akilli, I.K.; Karaayvaz, E.B.; Yesilova, A.; Kart Yasar, K. Comparison of systemic immune-inflammation index (SII), early warning score (ANDC) and prognostic nutritional index (PNI) in hospitalized patients with malignancy, and their influence on mortality from COVID-19. Infect. Agent. Cancer 2021, 16, 60. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Louten, J. Virus Transmission and Epidemiology. Essent. Hum. Virol. 2016, 5, 71–92. [Google Scholar] [CrossRef]
- Li, Y. Basic routes of transmission of respiratory pathogens—A new proposal for transmission categorization based on respiratory spray, inhalation, and touch. Indoor Air 2021, 31, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.S.; Liu, L.; Zhang, Z. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 3 July 2022).
- ISO 21702:2019; Measurement of Antiviral Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/71365.html (accessed on 3 July 2022).
- ASTM E1482-04; Standard Test Method for Neutralization of Virucidal Agents in Virucidal Efficacy Evaluations. ASTM: New York, NY, USA, 2012. Available online: https://webstore.ansi.org/Standards/ASTM/astme148204 (accessed on 3 July 2022).
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Owen, L.; Shivkumar, M.; Cross, R.B.M.; Laird, K. Porous surfaces: Stability and recovery of coronaviruses. Interface Focus 2021, 12, 20210039. [Google Scholar] [CrossRef]
- Edwards, T.; Kay, G.A.; Aljayyoussi, G.; Owen, S.I.; Harland, A.R.; Pierce, N.S.; Calder, J.D.F.; Fletcher, T.E.; Adams, E.R. SARS-CoV-2 viability on sports equipment is limited, and dependent on material composition. Sci. Rep. 2022, 12, 1416. [Google Scholar] [CrossRef]
- Chatterjee, S.; Murallidharan, J.S.; Agrawal, A.; Bhardwaj, R. Why coronavirus survives longer on impermeable than porous surfaces. Phys. Fluids 2021, 33, 021701. [Google Scholar] [CrossRef]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: Role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005, 43, 105–114. [Google Scholar] [CrossRef]
- Fukuda, M.; Islam, M.S.; Shimizu, R.; Nassar, H.; Rabin, N.N.; Takahashi, Y.; Sekine, Y.; Lindoy, L.F.; Fukuda, T.; Ikeda, T.; et al. Lethal Interactions of SARS-CoV-2 with Graphene Oxide: Implications for COVID-19 Treatment. ACS Appl. Nano Mater. 2021, 4, 11881–11887. [Google Scholar] [CrossRef]
- Ziganshyna, S.; Szczepankiewicz, G.; Kuehnert, M.; Schulze, A.; Liebert, U.G.; Pietsch, C.; Eulenburg, V.; Werdehausen, R. Photodynamic Inactivation of SARS-CoV-2 Infectivity and Antiviral Treatment Effects In Vitro. Viruses 2022, 14, 1301. [Google Scholar] [CrossRef]
- Conrado, P.C.V.; Sakita, K.M.; Arita, G.S.; Galinari, C.B.; Gonçalves, R.S.; Lopes, L.D.G.; Lonardoni, M.V.C.; Teixeira, J.J.V.; Bonfim-Mendonça, P.S.; Kioshima, E.S. A systematic review of photodynamic therapy as an antiviral treatment: Potential guidance for dealing with SARS-CoV-2. Photodiagnosis Photodyn. Ther. 2021, 34, 102221. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Lieleg, C.; Bloom, J.; Buck, C.B.; Ribbeck, K. Mucin biopolymers as broad-spectrum antiviral agents. Biomacromolecules 2012, 13, 1724. [Google Scholar] [CrossRef]
- de Castro, K.C.; Costa, J.M. Polymeric surfaces with biocidal action: Challenges imposed by the SARS-CoV-2, technologies employed, and future perspectives. J. Polym. Res. 2021, 28, 230. [Google Scholar] [CrossRef]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Rani, P.; Kapoor, B.; Gulati, M.; Atanasov, A.G.; Alzahrani, Q.; Gupta, R. Antimicrobial peptides: A plausible approach for COVID-19 treatment. Expert Opin. Drug Discov. 2022, 17, 473–487. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef]
- Currie, S.M.; Findlay, E.G.; McFarlane, A.J.; Fitch, P.M.; Böttcher, B.; Colegrave, N.; Paras, A.; Jozwik, A.; Chiu, C.; Schwarze, J.; et al. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J. Immunol. Author Choice 2016, 196, 2699. [Google Scholar] [CrossRef] [PubMed]
- Kurpe, S.R.; Grishin, S.Y.; Surin, A.K.; Panfilov, A.V.; Slizen, M.V.; Chowdhury, S.D.; Galzitskaya, O.V. Antimicrobial and Amyloidogenic Activity of Peptides. Can Antimicrobial Peptides Be Used against SARS-CoV-2? Int. J. Mol. Sci. 2020, 21, 9552. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Jian, Z.; Fei, G.; Qian, W.; Luo, G.; Wang, Z.; Xia, H. Novel Poly(vinyl alcohol)/Chitosan/Modified Graphene Oxide Biocomposite for Wound Dressing Application. Macromol. Biosci. 2020, 20, 1900385. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Nayak, V.; Singh, K.R.; Jain, B.; Baid, M.; Alexis, F.; Singh, A.K. Potentialities of graphene and its allied derivatives to combat against SARS-CoV-2 infection. Mater. Today Adv. 2022, 13, 100208. [Google Scholar] [CrossRef] [PubMed]
- De Maio, F.; Palmieri, V.; Babini, G.; Augello, A.; Palucci, I.; Perini, G.; Salustri, A.; Spilman, P.; De Spirito, M.; Sanguinetti, M.; et al. Graphene nanoplatelet and graphene oxide functionalization of face mask materials inhibits infectivity of trapped SARS-CoV-2. iScience 2021, 24, 102788. [Google Scholar] [CrossRef]
- Health-Tech Startup Flextrapower (Formerly Bonbouton) Launches GO-Enhanced Protective Face Masks|Graphene-Info. Available online: https://www.graphene-info.com/health-tech-startup-bonbouton-launches-go-enhanced-protective-face-masks (accessed on 10 July 2022).
- Han, J.; Chen, L.; Duan, S.M.; Yang, Q.X.; Yang, M.; Gao, C.; Zhang, B.Y.; He, H.; Dong, X.P. Efficient and quick inactivation of SARS coronavirus and other microbes exposed to the surfaces of some metal catalysts. Biomed. Environ. Sci. 2005, 18, 176–180. [Google Scholar]
- Bright, K.R.; Sicairos-Ruelas, E.E.; Gundy, P.M.; Gerba, C.P. Assessment of the Antiviral Properties of Zeolites Containing Metal Ions. Food Environ. Virol. 2009, 1, 37–41. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Akbari, A.; Bigham, A.; Rahimkhoei, V.; Sharifi, S.; Jabbari, E. Antiviral Polymers: A Review. Polymers 2022, 14, 1634. [Google Scholar] [CrossRef] [PubMed]
- Grzeczkowicz, A.; Lipko, A.; Kwiatkowska, A.; Strawski, M.; Bącal, P.; Więckowska, A.; Granicka, L.H. Polyelectrolyte Membrane Nanocoatings Aimed at Personal Protective and Medical Equipment Surfaces to Reduce Coronavirus Spreading. Membranes 2022, 12, 946. [Google Scholar] [CrossRef]
- Baselga, M.; Uranga-Murillo, I.; de Miguel, D.; Arias, M.; Sebastián, V.; Pardo, J.; Arruebo, M. Silver Nanoparticles-Polyethyleneimine-Based Coatings with Antiviral Activity against SARS-CoV-2: A New Method to Functionalize Filtration Media. Materials 2022, 15, 4742. [Google Scholar] [CrossRef] [PubMed]
- Ucar, B.; Acar, T.; Arayici, P.P.; Derman, S. A nanotechnological approach in the current therapy of COVID-19: Model drug oseltamivir-phosphate loaded PLGA nanoparticles targeted with spike protein binder peptide of SARS-CoV-2. Nanotechnology 2021, 32, 485601. [Google Scholar] [CrossRef] [PubMed]
- Lorena Cortez, M.; De Matteis, N.; Ceolín, M.; Knoll, W.; Battaglini, F.; Azzaroni, O. Hydrophobic interactions leading to a complex interplay between bioelectrocatalytic properties and multilayer meso-organization in layer-by-layer assemblies. Phys. Chem. Chem. Phys. 2014, 16, 20844–20855. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Harbaugh, S.; Drachuk, I.; Shchepelina, O.; Kelley-Loughnane, N.; Stone, M.; Tsukruk, V.V. Hydrogen-bonded LbL shells for living cell surface engineering. Soft Matter 2011, 7, 2364–2372. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Zavgorodnya, O.; Chen, Y.; Ellis, K.; Tse, H.M.; Cui, W.; Thompson, J.A.; Kharlampieva, E. Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv. Funct. Mater. 2012, 22, 3389. [Google Scholar] [CrossRef]
- Wong, J.E.; Zastrow, H.; Jaeger, W.; Von Klitzing, R. Specific ion versus electrostatic effects on the construction of polyelectrolyte multilayers. Langmuir 2009, 25, 14061–14070. [Google Scholar] [CrossRef]
- Carter, J.L.; Drachuk, I.; Harbaugh, S.; Kelley-Loughnane, N.; Stone, M.; Tsukruk, V.V. Truly Nonionic Polymer Shells for the Encapsulation of Living Cells. Macromol. Biosci. 2011, 11, 1244–1253. [Google Scholar] [CrossRef]
- Burke, S.E.; Barrett, C.J. pH-responsive properties of multilayered poly(L-lysine)/hyaluronic acid surfaces. Biomacromolecules 2003, 4, 1773–1783. [Google Scholar] [CrossRef]
- Mauser, T.; Déjugnat, C.; Sukhorukov, G.B. Reversible pH-Dependent Properties of Multilayer Microcapsules Made of Weak Polyelectrolytes. Macromol. Rapid Commun. 2004, 25, 1781–1785. [Google Scholar] [CrossRef]
- Jain, S.; Kumar, D.; Swarnakar, N.K.; Thanki, K. Polyelectrolyte stabilized multilayered liposomes for oral delivery of paclitaxel. Biomaterials 2012, 33, 6758–6768. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Kim, H. Il Control of release characteristics in pH-sensitive poly(vinyl alcohol)/poly(acrylic acid) microcapsules containing chemically treated alumina core. J. Appl. Polym. Sci. 2010, 115, 1853–1858. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, B. pH-controlled drug loading and release from biodegradable microcapsules. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 302–310. [Google Scholar] [CrossRef]
- Sukhishvili, S.A.; Granick, S. Layered, erasable, ultrathin polymer films. J. Am. Chem. Soc. 2000, 122, 9550–9551. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Sukhishvili, S.A. Layer-by-Layer Hydrogen-Bonded Polymer Films: From Fundamentals to Applications. Adv. Mater. 2009, 21, 3053–3065. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Shuttleworth, P.S. Layer-by-Layer Assembly of Biopolyelectrolytes onto Thermo/pH-Responsive Micro/Nano-Gels. Materials 2014, 7, 7472–7512. [Google Scholar] [CrossRef]
- Idoine, J.B.; Wachter, R.F.; Costlow, R.D. Effects of Poly-l-Lysine on Infectious Viral Nucleic Acid. J. Virol. 1971, 7, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Neyts, J.; Yamamoto, N.; Schols, D.; Smoeck, R.; Pauwels, R.; De Clercq, E. Inhibitory effects of polycations on the replication of enveloped viruses (HIV, HSV, CMV, RSV, influenza A virus and togaviruses) in vitro. Antivir. Chem. Chemother. 1991, 2, 243–248. [Google Scholar] [CrossRef]
- Stagi, L.; de Forni, D.; Innocenzi, P. Blocking viral infections with lysine-based polymeric nanostructures: A critical review. Biomater. Sci. 2022, 10, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Mettenleiter, T.C.; Sugg, N.; Ben-Porat, T. Effect of polylysine on the early stages of infection of wild type pseudorabies virus and of mutants defective in gIII. Virology 1990, 179, 330–338. [Google Scholar] [CrossRef]
- Stagi, L.; De Forni, D.; Malfatti, L.; Caboi, F.; Salis, A.; Poddesu, B.; Cugia, G.; Lori, F.; Galleri, G.; Innocenzi, P. Effective SARS-CoV-2 antiviral activity of hyperbranched polylysine nanopolymers. Nanoscale 2021, 13, 16465–16476. [Google Scholar] [CrossRef] [PubMed]
- Spoden, G.A.; Besold, K.; Krauter, S.; Plachter, B.; Hanik, N.; Kilbinger, A.F.M.; Lambert, C.; Florin, L. Polyethylenimine is a strong inhibitor of human papillomavirus and cytomegalovirus infection. Antimicrob. Agents Chemother. 2012, 56, 75–82. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, N.; Ji, Q.; Li, M.; Nan, Y.; Zhou, E.M.; Zhang, Y.; Wu, C. The 40 kDa linear polyethylenimine inhibits porcine reproductive and respiratory syndrome virus infection by blocking its attachment to permissive cells. Viruses 2019, 11, 876. [Google Scholar] [CrossRef]
- Haldar, J.; An, D.; De Cienfuegos, L.Á.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic acid: The influence of molecular weight on structural, physical, physico-chemical, and degradable properties of biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Cermelli, C.; Cuoghi, A.; Scuri, M.; Bettua, C.; Neglia, R.G.; Ardizzoni, A.; Blasi, E.; Iannitti, T.; Palmieri, B. In vitro evaluation of antiviral and virucidal activity of a high molecular weight hyaluronic acid. Virol. J. 2011, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Mohamed, A.; Taher, F.; Kamel, M. Antimicrobial activities of chitosan nanoparticles prepared from lucilia cuprina maggots (diptera: Calliphoridae). J. Egypt. Soc. Parasitol. 2016, 46, 563–570. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Ejeromedoghene, O.; Orege, J.I.; Onwuka, J.U.; Adebule, P.A.; Ehianeta, T.; Okonkwo, B.O.; Akinyeye, R.O. The Role of Nanoparticles as Nanocarriers for the Controlled Release of some Potential Existing Antiviral Drugs for SARS-CoV-2 Management: A Review. Coronaviruses 2020, 2, 7–18. [Google Scholar] [CrossRef]
- Tatlow, D.; Tatlow, C.; Tatlow, S.; Tatlow, S. A novel concept for treatment and vaccination against Covid-19 with an inhaled chitosan-coated DNA vaccine encoding a secreted spike protein portion. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1874–1878. [Google Scholar] [CrossRef]
- Ding, J.; Shi, H.; Wu, J.; Hathout, R.M.; Kassem, D.H. Positively Charged Electroceutical Spun Chitosan Nanofibers Can Protect Health Care Providers From COVID-19 Infection: An Opinion. Front. Bioeng. Biotechnol. 2020, 1, 885. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of Poly(lactic- co-glycolic Acid) (PLGA) Nanoparticles for Vaginal Co-Delivery of Griffithsin and Dapivirine and Their Synergistic Effect for HIV Prophylaxis. Pharmaceutics 2019, 11, 184. [Google Scholar] [CrossRef]
- Duvvuri, S.; Janoria, K.G.; Mitra, A.K. Development of a novel formulation containing poly(D,L-lactide-co- glycolide) microspheres dispersed in PLGA-PEG-PLGA gel for sustained delivery of ganciclovir. J. Control. Release 2005, 108, 282–293. [Google Scholar] [CrossRef]
- Patel, B.K.; Parikh, R.H.; Patel, N. Targeted delivery of mannosylated-PLGA nanoparticles of antiretroviral drug to brain. Int. J. Nanomedicine 2018, 13, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Honko, A.; Zhou, J.; Gong, H.; Downs, S.N.; Vasquez, J.H.; Fang, R.H.; Gao, W.; Griffiths, A.; Zhang, L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020, 20, 5570–5574. [Google Scholar] [CrossRef] [PubMed]
- Pavlukhina, S.; Sukhishvili, S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv Drug Deliv Rev 2011, 63, 822–836. [Google Scholar] [CrossRef] [PubMed]
- De Temmerman, M.L.; Demeester, J.; De Smedt, S.C.; Rejman, J. Tailoring layer-by-layer capsules for biomedical applications. Nanomedicine 2012, 7, 771–788. [Google Scholar] [CrossRef]
- Schanze, K.S.; Shelton, A.H. Functional polyelectrolytes. Langmuir 2009, 25, 13698–13702. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Wang, S.; Jiang, L. Cytophilic/cytophobic design of nanomaterials at biointerfaces. Small 2013, 9, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.K.; Murphy, W.L.; Gopalan, P. Crosslinked PEG mats for peptide immobilization and stem cell adhesion. J. Mater. Chem. B 2013, 1, 1349–1360. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Wang, H.; Zhao, R.; Ji, T.; Ren, H.; Anderson, G.J.; Nie, G.; Hao, J. Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved folfirinox chemotherapy in pancreatic tumor models. Adv. Funct. Mater. 2015, 25, 788–798. [Google Scholar] [CrossRef]
- Liu, G.; Luo, Q.; Gao, H.; Chen, Y.; Wei, X.; Dai, H.; Zhang, Z.; Ji, J. Cell membrane-inspired polymeric micelles as carriers for drug delivery. Biomater. Sci. 2015, 3, 490–499. [Google Scholar] [CrossRef]
- Deokar, A.R.; Perelshtein, I.; Saibene, M.; Perkas, N.; Mantecca, P.; Nitzan, Y.; Gedanken, A. Antibacterial and In Vivo Studies of a Green, One-Pot Preparation of Copper/Zinc Oxide Nanoparticle-Coated Bandages. Membranes 2021, 11, 462. [Google Scholar] [CrossRef]
- Sinclair, T.R.; Robles, D.; Raza, B.; van den Hengel, S.; Rutjes, S.A.; de Roda Husman, A.M.; de Grooth, J.; de Vos, W.M.; Roesink, H.D.W. Virus reduction through microfiltration membranes modified with a cationic polymer for drinking water applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 33–41. [Google Scholar] [CrossRef]
- Sinclair, T.; Patil, A.; Raza, B.; Reurink, D.; van Den Hengel, S.; Rutjes, S.; de Roda Husman, A.; Roesink, H.; De Vos, W. Cationically modified membranes using covalent layer-by-layer assembly for antiviral applications in drinking water. J. Membr. Sci. 2019, 570, 494–503. [Google Scholar] [CrossRef]
- Durmaz, E.N.; Sahin, S.; Virga, E.; De Beer, S.; De Smet, L.C.P.M.; De Vos, W.M. Polyelectrolytes as Building Blocks for Next-Generation Membranes with Advanced Functionalities. ACS Appl. Polym. Mater. 2021, 3, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Abad, A.; Ocio, M.J.; Lagarón, J.M.; Sánchez, G. Evaluation of silver-infused polylactide films for inactivation of Salmonella and feline calicivirus in vitro and on fresh-cut vegetables. Int. J. Food Microbiol. 2013, 162, 89–94. [Google Scholar] [CrossRef]
- Scheller, C.; Krebs, F.; Minkner, R.; Astner, I.; Gil-Moles, M.; Wätzig, H. Physicochemical properties of SARS-CoV-2 for drug targeting, virus inactivation and attenuation, vaccine formulation and quality control. Electrophoresis 2020, 41, 1137–1151. [Google Scholar] [CrossRef]
- Pawłowski, P.H. Additional positive electric residues in the crucial spike glycoprotein s regions of the new SARS-CoV-2 variants. Infect. Drug Resist. 2021, 14, 5099–5105. [Google Scholar] [CrossRef]
- Arnáiz, E.; Vacas-Cõrdoba, E.; Galán, M.; Pion, M.; Gõmez, R.; Muñoz-Fernández, M.Á.; De La Mata, F.J. Synthesis of anionic carbosilane dendrimers via “click chemistry” and their antiviral properties against HIV. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1099–1112. [Google Scholar] [CrossRef]
- Stein, A. Induced Interference by Synthetic Polyanions with the Infection of Tobacco Mosaic Virus. Phytopathology 1972, 62, 1461–1466. [Google Scholar] [CrossRef]
- Merigan, T.C.; Finkelstein, M.S. Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology 1968, 35, 363–374. [Google Scholar] [CrossRef]
- Mohan, P.; Schols, D.; Baba, M.; De Clercq, E. Sulfonic acid polymers as a new class of human immunodeficiency virus inhibitors. Antiviral Res. 1992, 18, 139–150. [Google Scholar] [CrossRef]
- Groß, R.; Dias Loiola, L.M.; Issmail, L.; Uhlig, N.; Eberlein, V.; Conzelmann, C.; Olari, L.R.; Rauch, L.; Lawrenz, J.; Weil, T.; et al. Macromolecular Viral Entry Inhibitors as Broad-Spectrum First-Line Antivirals with Activity against SARS-CoV-2. Adv. Sci. 2022, 9, 2201378. [Google Scholar] [CrossRef] [PubMed]
- Paull, J.R.A.; Castellarnau, A.; Luscombe, C.A.; Fairley, J.K.; Heery, G.P. Astodrimer sodium, dendrimer antiviral, inhibits replication of SARS-CoV-2 in vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Córdoba, E.V.; Arnaiz, E.; Relloso, M.; Sánchez-Torres, C.; García, F.; Pérez-Álvarez, L.; Gómez, R.; De La Mata, F.J.; Pion, M.; Muñoz-Fernández, M.Á. Development of sulphated and naphthylsulphonated carbosilane dendrimers as topical microbicides to prevent HIV-1 sexual transmission. AIDS 2013, 27, 1219–1229. [Google Scholar] [CrossRef]
- Asaftei, S.; De Clercq, E. “viologen”dendrimers as antiviral agents: The effect of charge number and distance. J. Med. Chem. 2010, 53, 3480–3488. [Google Scholar] [CrossRef]
- Christensen, N.D.; Reed, C.A.; Culp, T.D.; Hermonat, P.L.; Howett, M.K.; Anderson, R.A.; Zaneveld, L.J.D. Papillomavirus microbicidal activities of high-molecular-weight cellulose sulfate, dextran sulfate, and polystyrene sulfonate. Antimicrob. Agents Chemother. 2001, 45, 3427–3432. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Dabrowska, A.; Botwina, P.; Obloza, M.; Liu, K.; Liu, D.; Guo, X.; et al. HTCC as a Polymeric Inhibitor of SARS-CoV-2 and MERS-CoV. J. Virol. 2021, 95, e01622-20. [Google Scholar] [CrossRef]
- Pyrc, K.; Milewska, A.; Duran, E.B.; Botwina, P.; Lopes, R.; Arenas-Pinto, A.; Badr, M.; Mellor, R.; Kalber, T.; Fernandez-Reyes, D.; et al. SARS-CoV-2 inhibition in human airway epithelial cells using a mucoadhesive, amphiphilic chitosan that may serve as an anti-viral nasal spray. bioRXiv 2020. [Google Scholar] [CrossRef]
- Jicsinszky, L.; Martina, K.; Cravotto, G. Cyclodextrins in the antiviral therapy. J. Drug Deliv. Sci. Technol. 2021, 64, 102589. [Google Scholar] [CrossRef]
- Paull, J.R.A.; Heery, G.P.; Bobardt, M.D.; Castellarnau, A.; Luscombe, C.A.; Fairley, J.K.; Gallay, P.A. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antiviral Res. 2021, 191, 105089. [Google Scholar] [CrossRef]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef] [PubMed]
- Venosi, S.; Ceccarelli, G.; De Angelis, M.; Laghi, L.; Bianchi, L.; Martinelli, O.; Maruca, D.; Cavallari, E.N.; Toscanella, F.; Vassalini, P.; et al. Infected chronic ischemic wound topically treated with a multi-strain probiotic formulation: A novel tailored treatment strategy. J. Transl. Med. 2019, 17, 364. [Google Scholar] [CrossRef]
- Jiang, H.; Manolache, S.; Somers, E.; Wong, A.C.L.; Denes, F.S. Plasma-Enhanced Generation of Stable PAA-and PVP-based Multi-layer Structures. Polym. Bull. 2008, 60, 837–845. [Google Scholar] [CrossRef]
- Yoo, P.J.; Nam, K.T.; Qi, J.; Lee, S.K.; Park, J.; Belcher, A.M.; Hammond, P.T. Spontaneous assembly of viruses on multilayered polymer surfaces. Nat. Mater. 2006, 5, 234–240. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.; Tai, S.; Pedowitz, M.; Rzasa, J.R.; Pennachio, D.J.; Hajzus, J.R.; Milton, D.K.; Myers-Ward, R.; Daniels, K.M. Real-time ultra-sensitive detection of SARS-CoV-2 by quasi-freestanding epitaxial graphene-based biosensor. Biosens. Bioelectron. 2022, 197, 113803. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-based field-effect transistor biosensors for the rapid detection and analysis of viruses: A perspective in view of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Gao, J.; Wang, C.; Chu, Y.; Han, Y.; Gao, Y.; Wang, Y.; Wang, C.; Liu, H.; Han, L.; Zhang, Y. Graphene oxide-graphene Van der Waals heterostructure transistor biosensor for SARS-CoV-2 protein detection. Talanta 2022, 240, 123197. [Google Scholar] [CrossRef]
- Gao, B.; Rojas Chavez, A.A.; Malkawi, W.I.; Keefe, D.W.; Smith, R.; Haim, H.; Salem, A.K.; Toor, F. Sensitive detection of SARS-CoV-2 spike protein using vertically-oriented silicon nanowire array-based biosensor. Sens. Bio-Sens. Res. 2022, 36, 100487. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Volpe, J.; Bach-Toledo, L.; Kurpel, K.C.; Deller, A.E.; Soares, A.L.; Nardin, J.M.; Marchesi, L.F.; Simas, F.F.; Oliveira, C.C.; et al. Development of polypyrrole (nano)structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater. Today Chem. 2022, 24, 100817. [Google Scholar] [CrossRef]
- Tian, J.; Liang, Z.; Hu, O.; He, Q.; Sun, D.; Chen, Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta 2021, 387, 138553. [Google Scholar] [CrossRef]
- Karakuş, E.; Erdemir, E.; Demirbilek, N.; Liv, L. Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal. Chim. Acta 2021, 1182, 338939. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Ferhan, A.R.; Yoon, B.K.; Sut, T.N.; Jeon, W.Y.; Koo, D.J.; Jackman, J.A.; Cho, N.J. Surface engineering of plasmonic gold nanoisland platforms for high-sensitivity refractometric biosensing applications. Appl. Mater. Today 2022, 26, 101280. [Google Scholar] [CrossRef]
- Ramakrishnan, S.G.; Robert, B.; Salim, A.; Ananthan, P.; Sivaramakrishnan, M.; Subramaniam, S.; Natesan, S.; Suresh, R.; Rajeshkumar, G.; Maran, J.P.; et al. Nanotechnology based solutions to combat zoonotic viruses with special attention to SARS, MERS, and COVID 19: Detection, protection and medication. Microb. Pathog. 2021, 159, 105133. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kang, S.W.; Oh, S.; Lee, J.; Neethirajan, S. Chiral zirconium quantum dots: A new class of nanocrystals for optical detection of coronavirus. Heliyon 2018, 4, e00766. [Google Scholar] [CrossRef] [PubMed]
- Jermy, B.R.; Ravinayagam, V.; Almohazey, D.; Alamoudi, W.A.; Dafalla, H.; Akhtar, S.; Tanimu, G. PEGylated green halloysite/spinel ferrite nanocomposites for pH sensitive delivery of dexamethasone: A potential pulmonary drug delivery treatment option for COVID-19. Appl. Clay Sci. 2022, 216, 106333. [Google Scholar] [CrossRef]
- Anand, K.; Vadivalagan, C.; Joseph, J.S.; Singh, S.K.; Gulati, M.; Shahbaaz, M.; Abdellattif, M.H.; Prasher, P.; Gupta, G.; Chellappan, D.K.; et al. A novel nano therapeutic using convalescent plasma derived exosomal (CPExo) for COVID-19: A combined hyperactive immune modulation and diagnostics. Chem. Biol. Interact. 2021, 344, 109497. [Google Scholar] [CrossRef]
- Palmieri, V.; De Maio, F.; De Spirito, M.; Papi, M. Face masks and nanotechnology: Keep the blue side up. Nano Today 2021, 37, 101077. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Merkl, P.; Long, S.; Mcinerney, G.M.; Sotiriou, G.A.; Ahonen, M.; Kogermann, K.; Pestryakov, A.; Mihailescu, I.N.; Papini, E. Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312. [Google Scholar] [CrossRef]
- Hamouda, T.; Kafafy, H.; Mashaly, H.M.; Aly, N.M. Breathability performance of antiviral cloth masks treated with silver nanoparticles for protection against COVID-19. J. Ind. Text. 2021, 51, 1494–1523. [Google Scholar] [CrossRef]
- Seidi, F.; Deng, C.; Zhong, Y.; Liu, Y.; Huang, Y.; Li, C.; Xiao, H.; Seidi, F.; Deng, C.; Zhong, Y.; et al. Functionalized Masks: Powerful Materials against COVID-19 and Future Pandemics. Small 2021, 17, 2102453. [Google Scholar] [CrossRef]
- Dika, C.; Ly-Chatain, M.H.; Francius, G.; Duval, J.F.L.; Gantzer, C. Non-DLVO adhesion of F-specific RNA bacteriophages to abiotic surfaces: Importance of surface roughness, hydrophobic and electrostatic interactions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 178–187. [Google Scholar] [CrossRef]
- Li, B.; Huang, Y.; Guo, D.; Liu, Y.; Liu, Z.; Han, J.C.; Zhao, J.; Zhu, X.; Huang, Y.; Wang, Z.; et al. Environmental risks of disposable face masks during the pandemic of COVID-19: Challenges and management. Sci. Total Environ. 2022, 825, 153880. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef]
- Schio, A.L.; Michels, A.F.; Fongaro, G.; Figueroa, C.A. Trends in the Antiviral Chemical Activity of Material Surfaces Associated With the SARS-CoV-2 Outbreak. Front. Chem. Eng. 2021, 3, 636075. [Google Scholar] [CrossRef]
- Basak, S.; Packirisamy, G. Nano-based antiviral coatings to combat viral infections. Nano-Struct. Nano-Objects 2020, 24, 100620. [Google Scholar] [CrossRef]
- Mohite, V.S.; Darade, M.M.; Sharma, R.K.; Pawar, S.H. Nanoparticle Engineered Photocatalytic Paints: A Roadmap to Self-Sterilizing against the Spread of Communicable Diseases. Catalysts 2022, 12, 326. [Google Scholar] [CrossRef]
- Bregnocchi, A.; Jafari, R.; Momen, G. Design strategies for antiviral coatings and surfaces: A review. Appl. Surf. Sci. Adv. 2022, 8, 100224. [Google Scholar] [CrossRef]
- Dahanayake, M.H.; Athukorala, S.S.; Jayasundera, A.C.A. Recent breakthroughs in nanostructured antiviral coating and filtration materials: A brief review. RSC Adv. 2022, 12, 16369. [Google Scholar] [CrossRef]
- Chithra, P.G.; Abraham, P.; George, J.S.; Maria, H.J.; Sreedevi, T.; Thomas, S. Nanocoatings: Universal antiviral surface solution against COVID-19. Prog. Org. Coat. 2022, 163, 106670. [Google Scholar] [CrossRef]
- Das Jana, I.; Kumbhakar, P.; Banerjee, S.; Gowda, C.C.; Kedia, N.; Kuila, S.K.; Banerjee, S.; Das, N.C.; Das, A.K.; Manna, I.; et al. Copper Nanoparticle-Graphene Composite-Based Transparent Surface Coating with Antiviral Activity against Influenza Virus. ACS Appl. Nano Mater. 2021, 4, 352–362. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine polysaccharide nanofibrous membranes for water purification. Biomacromolecules 2011, 12, 970–976. [Google Scholar] [CrossRef] [PubMed]
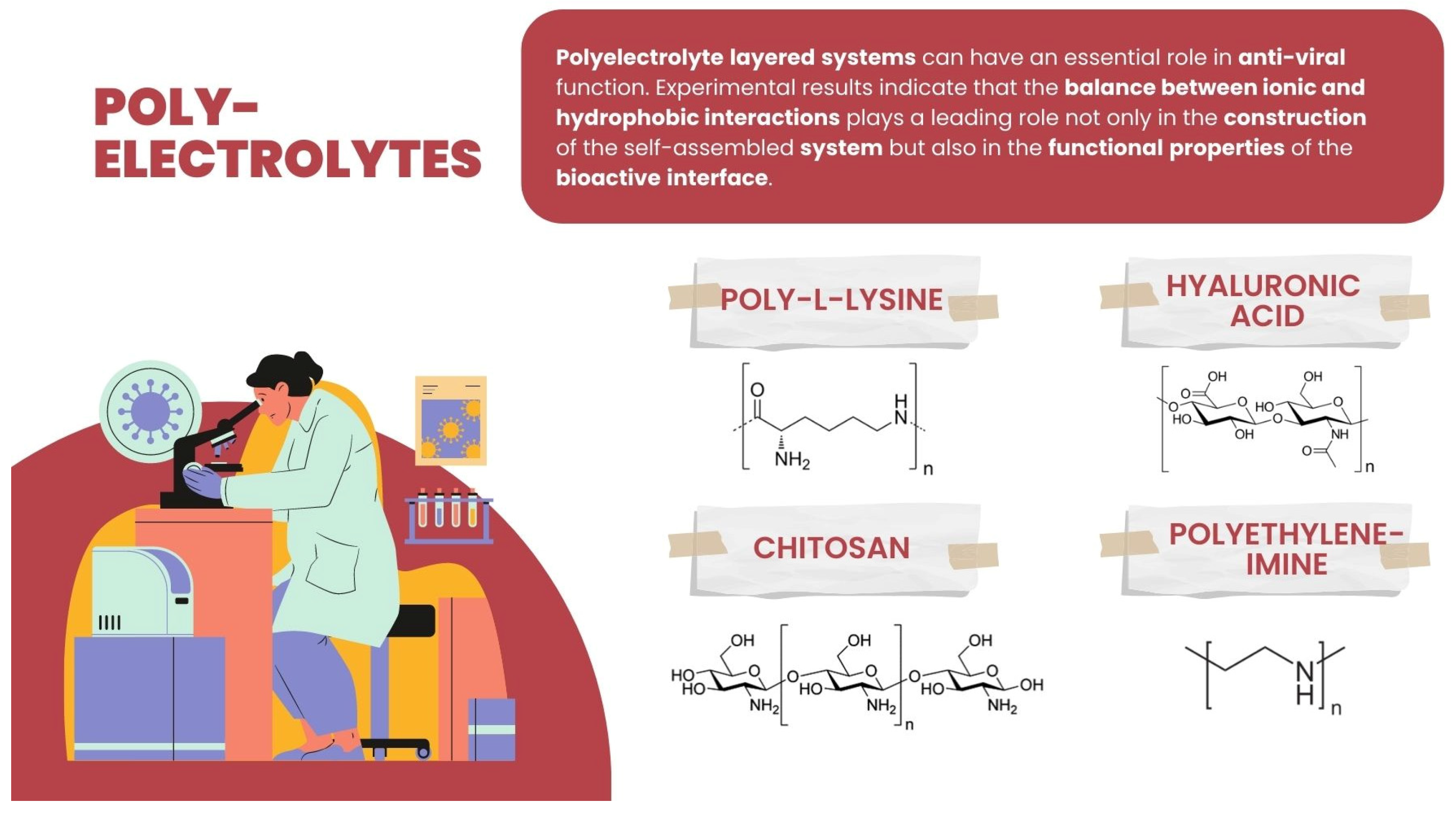
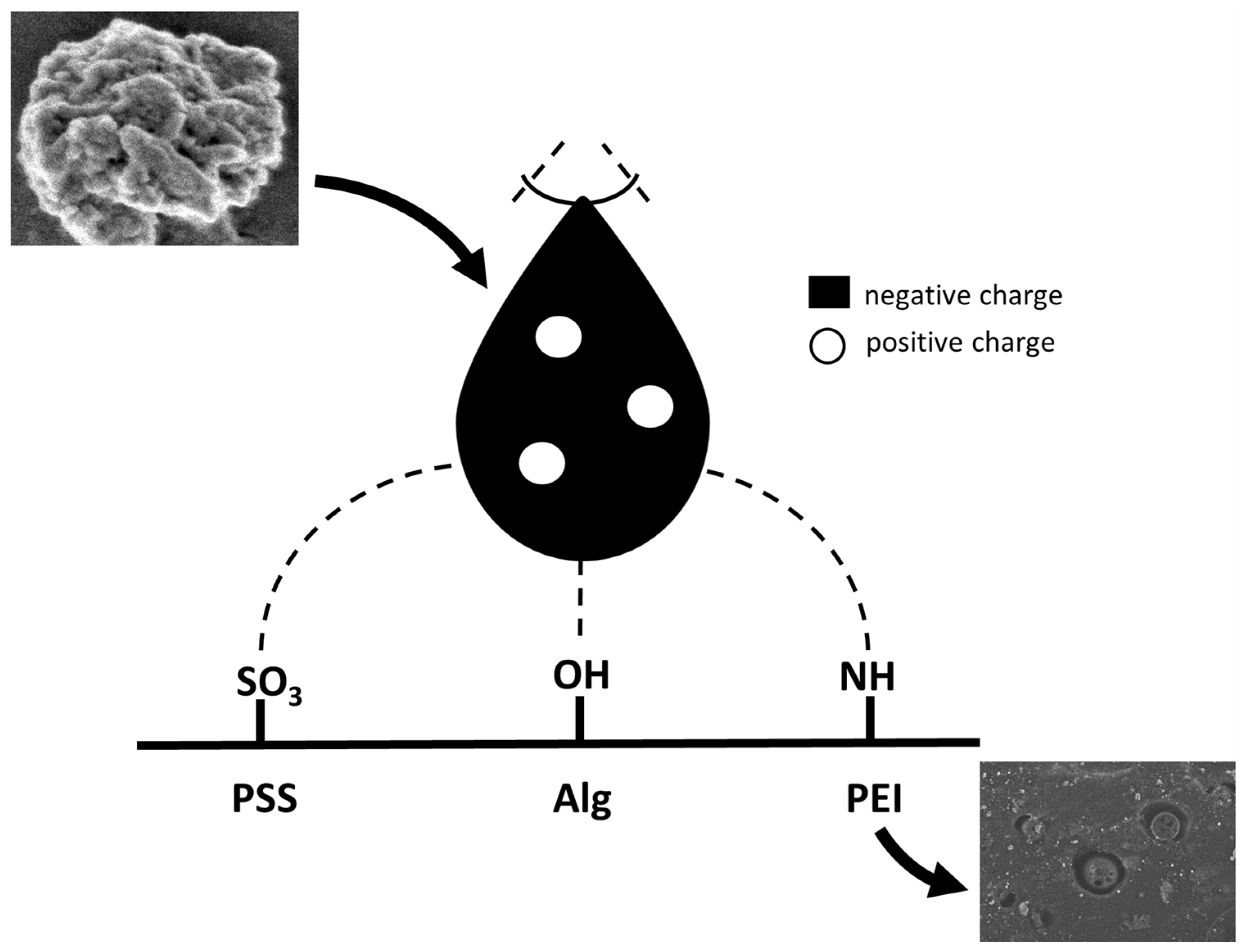
| Polyelectrolyte (PE) or PE-Based Composite | Virus | Ref. |
|---|---|---|
| Polystyrene sulfonate | Bovine papillomavirus type 1 (BPV-1), human papillomavirus type 11 (HPV-11) and type 40 (HPV-40) | [152] |
| Poly-L-lysine | Venezuelan equine encephalitis, Eastern equine encephalitis, human immunodeficiency virus type 1 and type 2 (HIV-1 and HIV-2), respiratory syncytial virus (RSV), Influenza A virus, Sindbis virus, Semliki Forest virus, vesicular stomatitis virus (VSV), herpes simplex virus type 1 (HSV-1), human cytomegalovirus (CMV). | [108,109] |
| Polyethyleneimine | Human papillomaviruses, human cytomegaloviruses, porcine reproductive, and respiratory syndrome virus (PRRSV) | [113,114] |
| Chitosan | Human H1N1 influenza A virus, Rift Valley Fever virus (RVFV), Herpes Simplex-1 virus (HSV-1), and Coxsackie virus | [121,122] |
| Hyaluronic acid | Coxsackievirus B5 (COXB5), mumps virus (MV), herpes simplex virus (HSV-1) | [119] |
| No | Polymer | Ref. |
|---|---|---|
| 1 | Polystyrene sulfonate (PSS) | [148] |
| 2 | Poly-L-lysine (PLL) | [112] |
| 3 | Alginates | [153] * |
| 4 | Chitosan derivatives | [154,155] |
| 5 | Cyclodextrin | [156] |
| 6 | Dendrimers | [157] |
| 7 | Carrageenans | [158,159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwiatkowska, A.; Granicka, L.H. Anti-Viral Surfaces in the Fight against the Spread of Coronaviruses. Membranes 2023, 13, 464. https://doi.org/10.3390/membranes13050464
Kwiatkowska A, Granicka LH. Anti-Viral Surfaces in the Fight against the Spread of Coronaviruses. Membranes. 2023; 13(5):464. https://doi.org/10.3390/membranes13050464
Chicago/Turabian StyleKwiatkowska, Angelika, and Ludomira H. Granicka. 2023. "Anti-Viral Surfaces in the Fight against the Spread of Coronaviruses" Membranes 13, no. 5: 464. https://doi.org/10.3390/membranes13050464
APA StyleKwiatkowska, A., & Granicka, L. H. (2023). Anti-Viral Surfaces in the Fight against the Spread of Coronaviruses. Membranes, 13(5), 464. https://doi.org/10.3390/membranes13050464







