Potassium Ion Channels in Glioma: From Basic Knowledge into Therapeutic Applications
Abstract
1. Introduction to Ion Channels
2. The Role of Ion Channels in Cancer
3. The Disease Burden of Glioma
4. Ion Channels in Glioma and the Importance of Potassium Channels
5. Overview of Potassium Channel Topology and Function
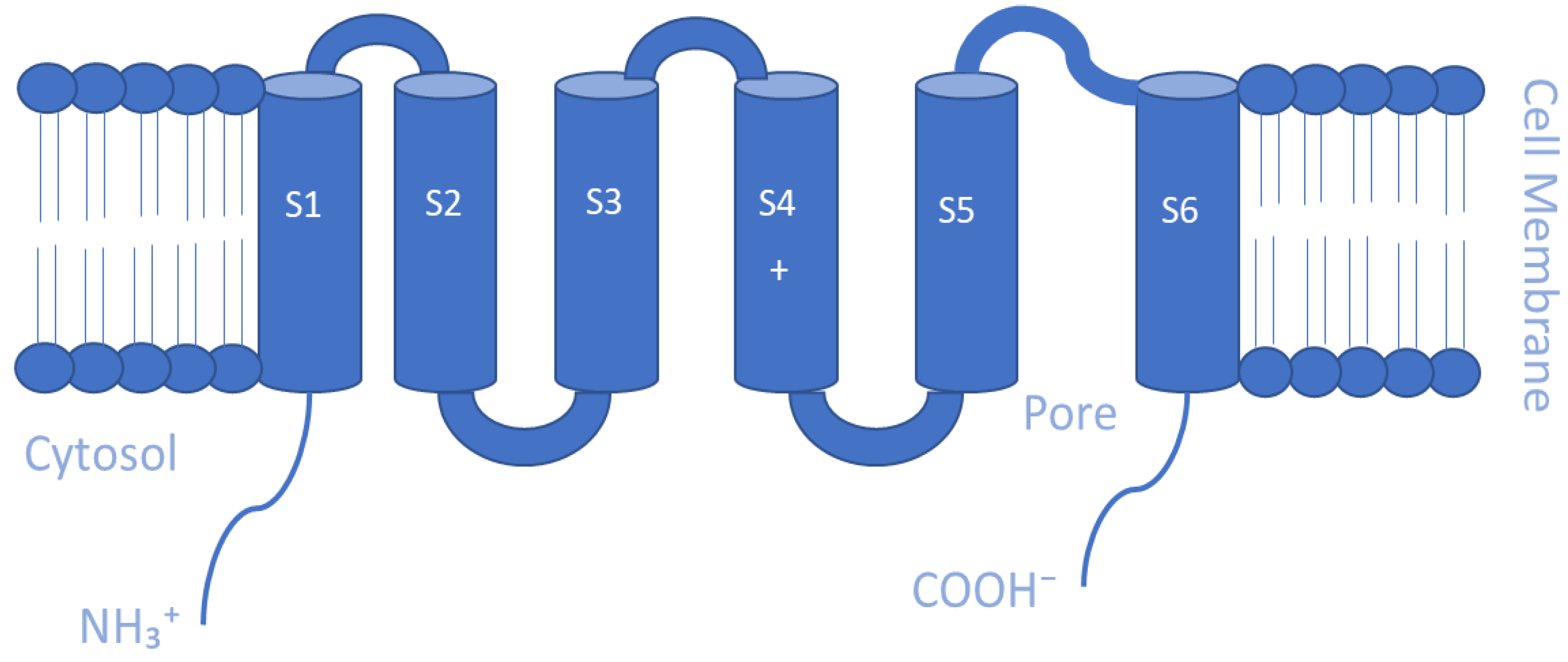
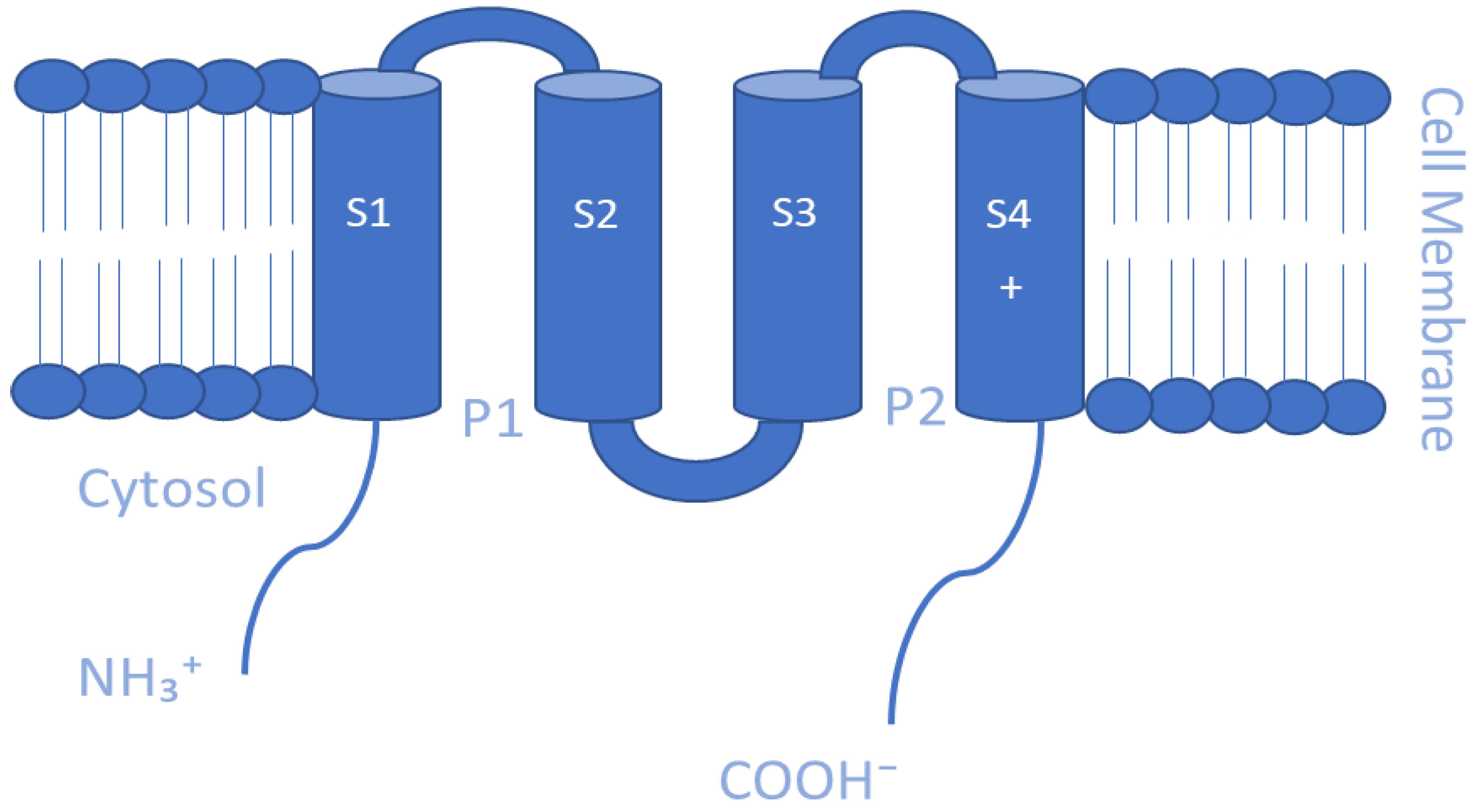
5.1. Voltage-gated K+ Channels (Kv)
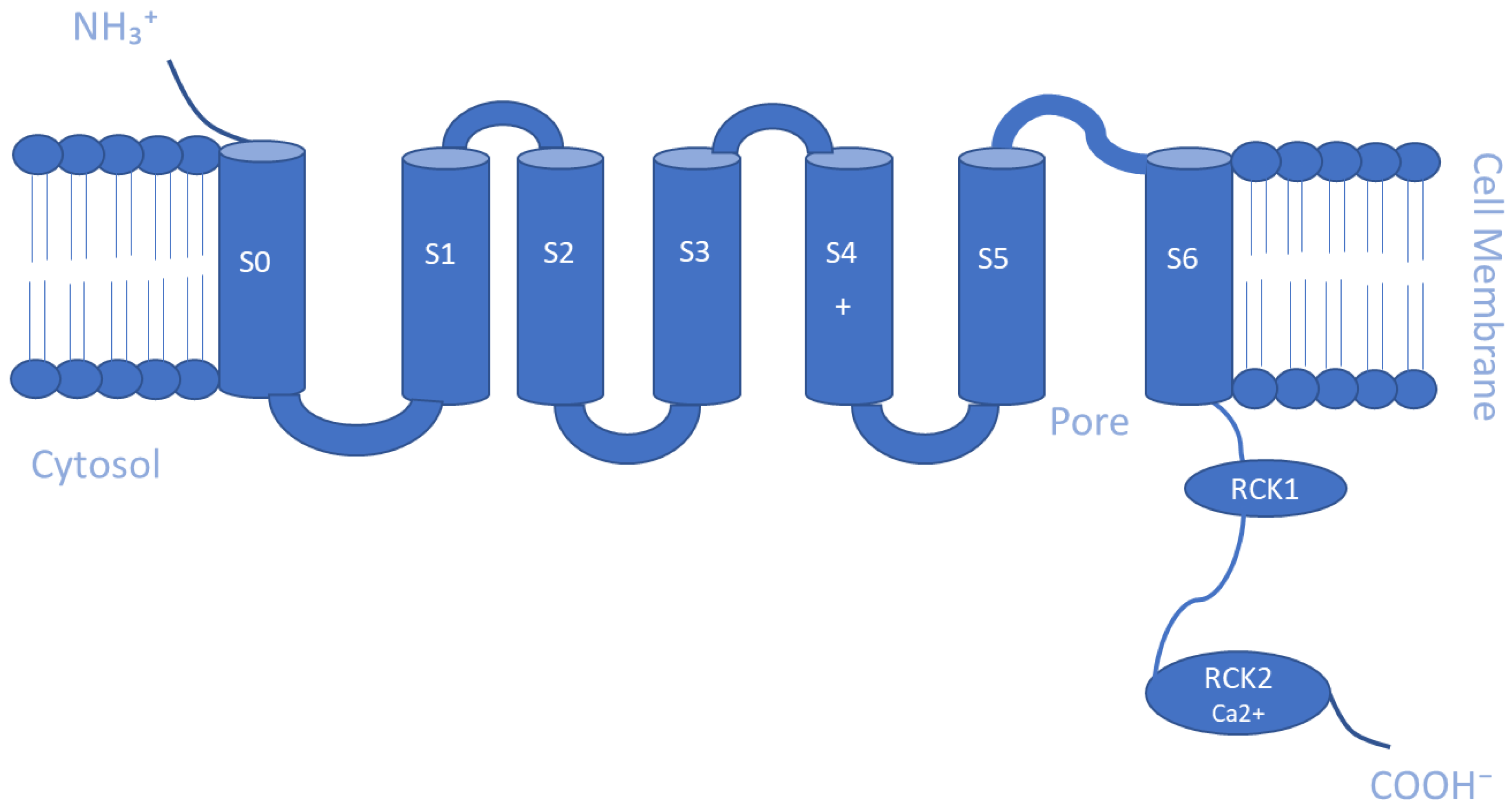
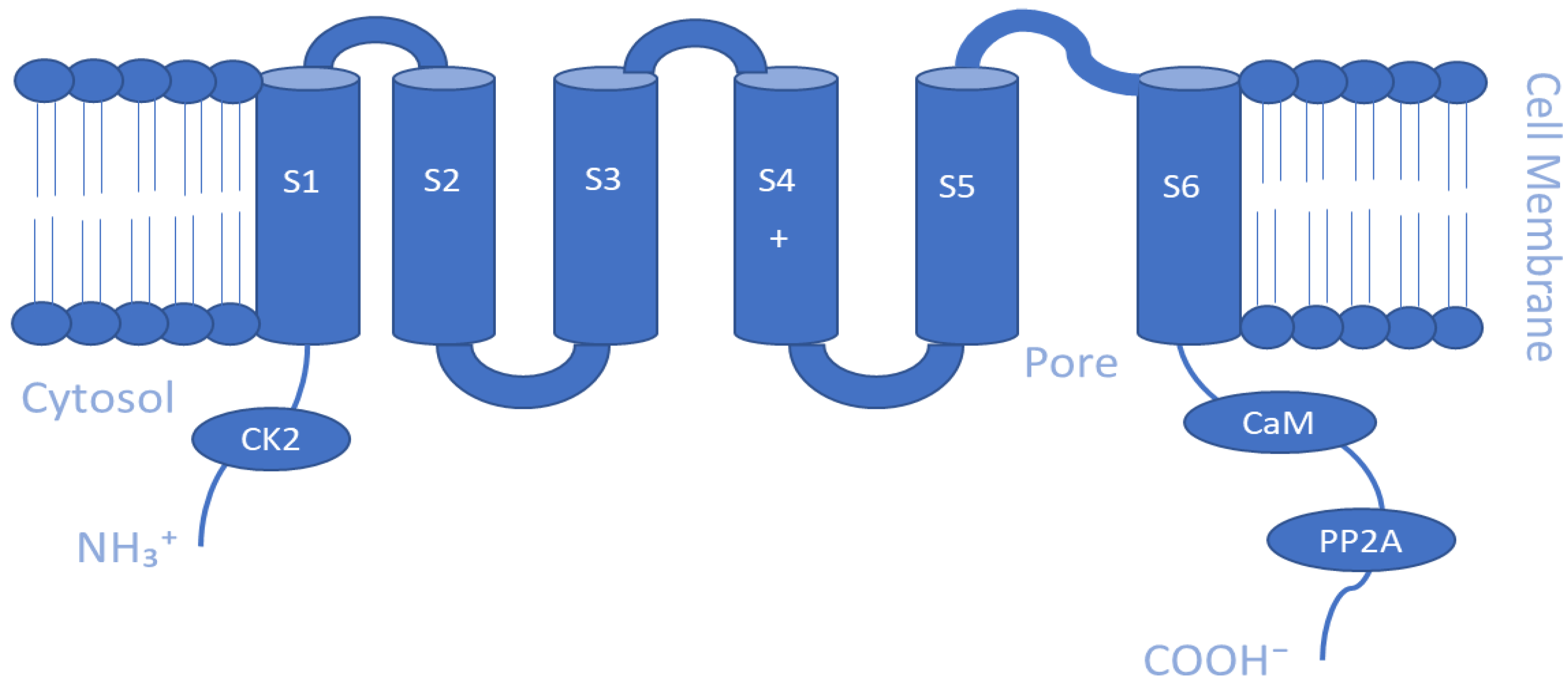
5.2. Ca2+- and Na+-Activated K+ Channels (KCa and KNa)
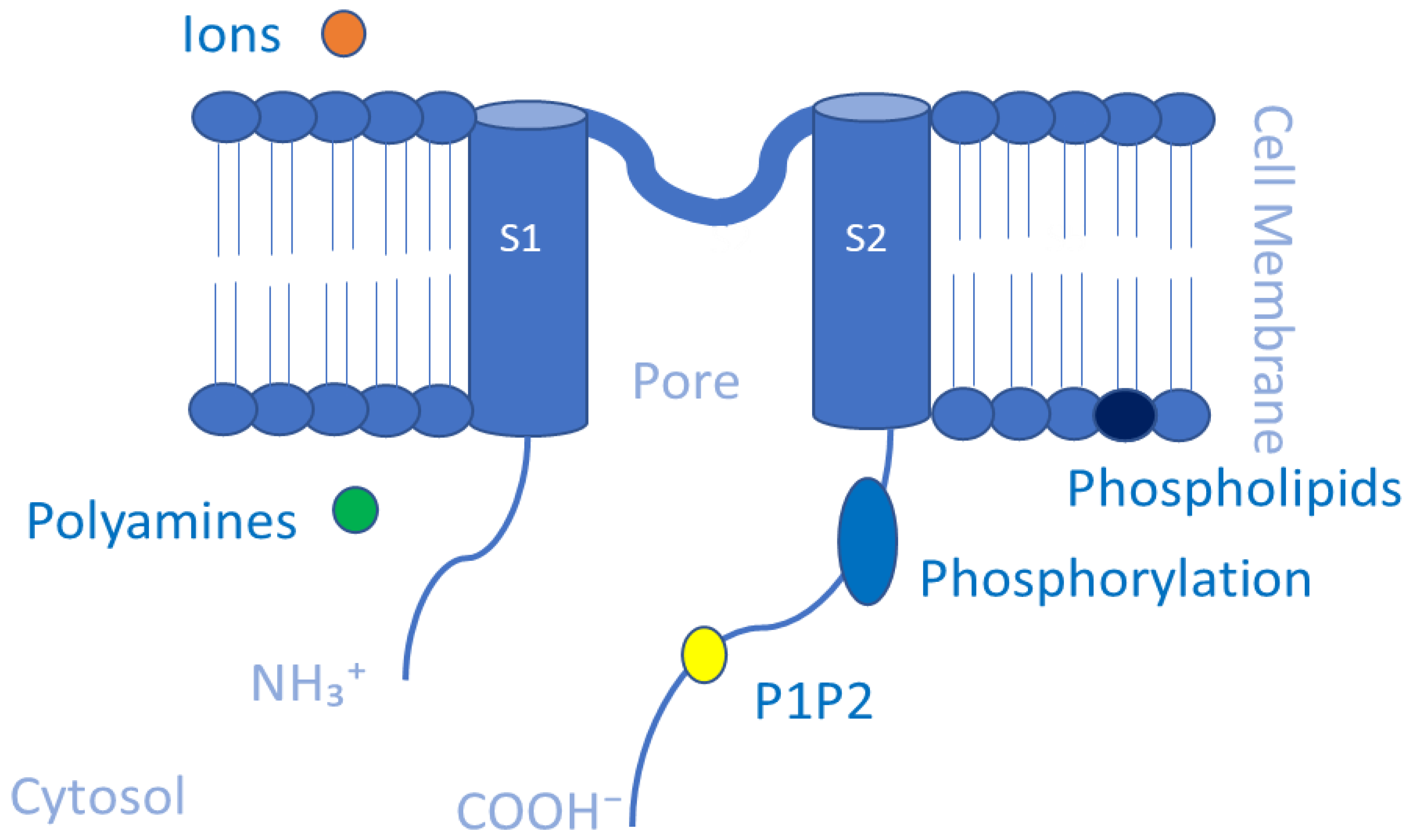
5.3. Inwardly Rectifying K+ Channels (Kir)
5.4. Two-Pore Domain K+ Channels (K2P)
6. Potassium Channels Dysfunction in a Tumor Microenvironment
6.1. Dysfunction in Proliferation and Apoptosis
6.2. Dysfunction in Favor of Migration
7. Targeting Potassium Channels as Potential Therapeutic Adjuvant in Glioma
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moiseenkova-Bell, V.; Delemotte, L.; Minor, D.L. Ion Channels: Intersection of Structure, Function, and Pharmacology. J. Mol. Biol. 2021, 433, 167102. [Google Scholar] [CrossRef] [PubMed]
- Asher, V.; Sowter, H.; Shaw, R.; Bali, A.; Khan, R. Eag and HERG Potassium Channels as Novel Therapeutic Targets in Cancer. World J. Surg. Oncol. 2010, 8, 113. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.H.; Tomaselli, G.F.; Marban, E. Ion Channels: Structure and Function. Heart Dis. Stroke 1993, 2, 75–80. [Google Scholar] [PubMed]
- Catterall, W.A. From Ionic Currents to Molecular Mechanisms: The Structure and Function of Voltage-Gated Sodium Channels. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Sine, S.M.; Engel, A.G. Recent Advances in Cys-Loop Receptor Structure and Function. Nature 2006, 440, 448–455. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Kasianowicz, J.J. Introduction to Ion Channels and Disease. Chem. Rev. 2012, 112, 6215–6217. [Google Scholar] [CrossRef]
- Imbrici, P.; Nicolotti, O.; Leonetti, F.; Conte, D.; Liantonio, A. Ion Channels in Drug Discovery and Safety Pharmacology. Methods Mol. Biol. 2018, 1800, 313–326. [Google Scholar] [CrossRef]
- Zhu, Z.; Deng, Z.; Wang, Q.; Wang, Y.; Zhang, D.; Xu, R.; Guo, L.; Wen, H. Simulation and Machine Learning Methods for Ion-Channel Structure Determination, Mechanistic Studies and Drug Design. Front Pharm. 2022, 13, 939555. [Google Scholar] [CrossRef]
- Sterea, A.M.; Almasi, S.; El Hiani, Y. The Hidden Potential of Lysosomal Ion Channels: A New Era of Oncogenes. Cell Calcium 2018, 72, 91–103. [Google Scholar] [CrossRef]
- Szabo, I.; Zoratti, M. Mitochondrial Channels: Ion Fluxes and More. Physiol. Rev. 2014, 94, 519–608. [Google Scholar] [CrossRef]
- Xiao, B. Levering Mechanically Activated Piezo Channels for Potential Pharmacological Intervention. Annu. Rev. Pharm. Toxicol. 2020, 60, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Fiske, J.L.; Fomin, V.P.; Brown, M.L.; Duncan, R.L.; Sikes, R.A. Voltage-Sensitive Ion Channels and Cancer. Cancer Metastasis Rev. 2006, 25, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Mansur, S.; Bao, Y. Sodium Ion Channels as Potential Therapeutic Targets for Cancer Metastasis. Drug Discov. Today 2021, 26, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Lastraioli, E.; Iorio, J.; Arcangeli, A. Ion Channel Expression as Promising Cancer Biomarker. Biochim. Biophys. Acta 2015, 1848, 2685–2702. [Google Scholar] [CrossRef]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of Ion Channels in Gastrointestinal Cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, A.; Duranti, C.; Iorio, J.; Lastraioli, E. The Role of Potassium Channels in Tumours of the Gastrointestinal Tract: A Focus on the Human Ether-à-Go-Go Related Gene 1 Channels. J. Physiol. 2022. [Google Scholar] [CrossRef]
- Hofschröer, V.; Najder, K.; Rugi, M.; Bouazzi, R.; Cozzolino, M.; Arcangeli, A.; Panyi, G.; Schwab, A. Ion Channels Orchestrate Pancreatic Ductal Adenocarcinoma Progression and Therapy. Front. Pharm. 2020, 11, 586599. [Google Scholar] [CrossRef]
- Bulk, E.; Todesca, L.M.; Schwab, A. Ion Channels in Lung Cancer. Rev. Physiol. Biochem. Pharm. 2021, 181, 57–79. [Google Scholar] [CrossRef]
- Glaser, F.; Hundehege, P.; Bulk, E.; Todesca, L.M.; Schimmelpfennig, S.; Nass, E.; Budde, T.; Meuth, S.G.; Schwab, A. KCa Channel Blockers Increase Effectiveness of the EGF Receptor TK Inhibitor Erlotinib in Non-Small Cell Lung Cancer Cells (A549). Sci. Rep. 2021, 11, 18330. [Google Scholar] [CrossRef]
- Han, Y.; Liu, C.; Zhang, D.; Men, H.; Huo, L.; Geng, Q.; Wang, S.; Gao, Y.; Zhang, W.; Zhang, Y.; et al. Mechanosensitive Ion Channel Piezo1 Promotes Prostate Cancer Development through the Activation of the Akt/MTOR Pathway and Acceleration of Cell Cycle. Int. J. Oncol. 2019, 55, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.G.; Ayan, H. Oleuropein: A Potential Inhibitor for Prostate Cancer Cell Motility by Blocking Voltage-Gated Sodium Channels. Nutr. Cancer 2021, 73, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, C.; Ma, Z.; Wang, H.; Tuo, B.; Cheng, X.; Liu, X.; Li, T. Pathophysiological Role of Ion Channels and Transporters in HER2-Positive Breast Cancer. Cancer Gene 2022, 29, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Rhana, P.; Trivelato, R.R.; Beirão, P.S.L.; Cruz, J.S.; Rodrigues, A.L.P. Is There a Role for Voltage-Gated Na+ Channels in the Aggressiveness of Breast Cancer? Braz. J. Med. Biol. Res. 2017, 50, e6011. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, X.; Wang, L.; Zhang, J.; Pan, X.; Li, Y.; Cao, R.; Li, B.; Lin, H.; Wang, Y.; et al. Recent Advances in Acid-Sensitive Ion Channels in Central Nervous System Diseases. Curr. Pharm. Des. 2022, 28, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, L.; Sezgin, E.C.; Eckert, F.; Huber, S.M. Ion Channels in Brain Metastasis. Int. J. Mol. Sci. 2016, 17, 1513. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Tokay, T.; Zhang, G.; Sun, P.; Hou, S. Eag1 K+ Channel: Endogenous Regulation and Functions in Nervous System. Oxid. Med. Cell Longev. 2017, 2017, 7371010. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Ion Channels and Apoptosis in Cancer. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130104. [Google Scholar] [CrossRef]
- Lang, F.; Shumilina, E.; Ritter, M.; Gulbins, E.; Vereninov, A.; Huber, S.M. Ion Channels and Cell Volume in Regulation of Cell Proliferation and Apoptotic Cell Death. Contrib Nephrol. 2006, 152, 142–160. [Google Scholar] [CrossRef]
- Spitzner, M.; Ousingsawat, J.; Scheidt, K.; Kunzelmann, K.; Schreiber, R. Voltage-Gated K+ Channels Support Proliferation of Colonic Carcinoma Cells. FASEB J. 2007, 21, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Repp, H.; Draheim, H.; Ruland, J.; Seidel, G.; Beise, J.; Presek, P.; Dreyer, F. Profound Differences in Potassium Current Properties of Normal and Rous Sarcoma Virus-Transformed Chicken Embryo Fibroblasts. Proc. Natl. Acad. Sci. USA 1993, 90, 3403–3407. [Google Scholar] [CrossRef] [PubMed]
- Oeggerli, M.; Tian, Y.; Ruiz, C.; Wijker, B.; Sauter, G.; Obermann, E.; Güth, U.; Zlobec, I.; Sausbier, M.; Kunzelmann, K.; et al. Role of KCNMA1 in Breast Cancer. PLoS ONE 2012, 7, e41664. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of Potassium Channels. Cell Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef]
- Boyle, Y.; Johns, T.G.; Fletcher, E.V. Potassium Ion Channels in Malignant Central Nervous System Cancers. Cancers 2022, 14, 4767. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stühmer, W. The Roles of K(+) Channels in Cancer. Nat. Rev. Cancer 2014, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Teisseyre, A.; Gąsiorowska, J.; Michalak, K. Voltage-Gated Potassium Channels Kv1.3--Potentially New Molecular Target in Cancer Diagnostics and Therapy. Adv. Clin. Exp. Med. 2015, 24, 517–524. [Google Scholar] [CrossRef]
- Aissaoui, D.; Mlayah-Bellalouna, S.; Jebali, J.; Abdelkafi-Koubaa, Z.; Souid, S.; Moslah, W.; Othman, H.; Luis, J.; ElAyeb, M.; Marrakchi, N.; et al. Functional Role of Kv1.1 and Kv1.3 Channels in the Neoplastic Progression Steps of Three Cancer Cell Lines, Elucidated by Scorpion Peptides. Int. J. Biol. Macromol. 2018, 111, 1146–1155. [Google Scholar] [CrossRef]
- Rabjerg, M.; Oliván-Viguera, A.; Hansen, L.K.; Jensen, L.; Sevelsted-Møller, L.; Walter, S.; Jensen, B.L.; Marcussen, N.; Köhler, R. High Expression of KCa3.1 in Patients with Clear Cell Renal Carcinoma Predicts High Metastatic Risk and Poor Survival. PLoS ONE 2015, 10, e0122992. [Google Scholar] [CrossRef]
- Abdul, M.; Hoosein, N. Expression and Activity of Potassium Ion Channels in Human Prostate Cancer. Cancer Lett. 2002, 186, 99–105. [Google Scholar] [CrossRef]
- Ortiz, C.S.; Montante-Montes, D.; Saqui-Salces, M.; Hinojosa, L.M.; Gamboa-Dominguez, A.; Hernández-Gallegos, E.; Martínez-Benítez, B.; Del Rosario Solís-Pancoatl, M.; Garcia-Villa, E.; Ramírez, A.; et al. Eag1 Potassium Channels as Markers of Cervical Dysplasia. Oncol. Rep. 2011, 26, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, D.; Wu, X.; Sha, M.; Kang, L.; Ding, Z. Voltage-Gated Potassium Channel Kv1.3 Is Highly Expressed in Human Osteosarcoma and Promotes Osteosarcoma Growth. Int. J. Mol. Sci. 2013, 14, 19245–19256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Wright, C.M.; Kirschner, M.B.; Williams, M.; Sarun, K.H.; Sytnyk, V.; Leshchynska, I.; Edelman, J.J.; Vallely, M.P.; McCaughan, B.C.; et al. KCa1.1, a Calcium-Activated Potassium Channel Subunit Alpha 1, Is Targeted by MiR-17-5p and Modulates Cell Migration in Malignant Pleural Mesothelioma. Mol. Cancer 2016, 15, 44. [Google Scholar] [CrossRef]
- Song, M.S.; Park, S.M.; Park, J.S.; Byun, J.H.; Jin, H.J.; Seo, S.H.; Ryu, P.D.; Lee, S.Y. Kv3.1 and Kv3.4, Are Involved in Cancer Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 1061. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Dai, Y.; Xu, X.; Jiang, Y. HIF-1α Regulates Proliferation and Invasion of Oral Cancer Cells through Kv3.4 Channel. Ann. Clin. Lab. Sci. 2019, 49, 457–467. [Google Scholar]
- Lan, M.; Shi, Y.; Han, Z.; Hao, Z.; Pan, Y.; Liu, N.; Guo, C.; Hong, L.; Wang, J.; Qiao, T.; et al. Expression of Delayed Rectifier Potassium Channels and Their Possible Roles in Proliferation of Human Gastric Cancer Cells. Cancer Biol. 2005, 4, 1342–1347. [Google Scholar] [CrossRef]
- Ibrahim, S.; Dakik, H.; Vandier, C.; Chautard, R.; Paintaud, G.; Mazurier, F.; Lecomte, T.; Guéguinou, M.; Raoul, W. Expression Profiling of Calcium Channels and Calcium-Activated Potassium Channels in Colorectal Cancer. Cancers 2019, 11, 561. [Google Scholar] [CrossRef]
- Ramírez, A.; Vera, E.; Gamboa-Domínguez, A.; Lambert, P.; Gariglio, P.; Camacho, J. Calcium-Activated Potassium Channels as Potential Early Markers of Human Cervical Cancer. Oncol. Lett. 2018, 15, 7249–7254. [Google Scholar] [CrossRef]
- Zhang, G.-M.; Wan, F.-N.; Qin, X.-J.; Cao, D.-L.; Zhang, H.-L.; Zhu, Y.; Dai, B.; Shi, G.-H.; Ye, D.-W. Prognostic Significance of the TREK-1 K2P Potassium Channels in Prostate Cancer. Oncotarget 2015, 6, 18460–18468. [Google Scholar] [CrossRef]
- Palme, D.; Misovic, M.; Ganser, K.; Klumpp, L.; Salih, H.R.; Zips, D.; Huber, S.M. HERG K+ Channels Promote Survival of Irradiated Leukemia Cells. Front Pharm. 2020, 11, 489. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The Epidemiology of Glioma in Adults: A “State of the Science” Review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Aquilanti, E.; Wen, P.Y. Current Therapeutic Options for Glioblastoma and Future Perspectives. Expert Opin. Pharmacother. 2022, 23, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-M.; Cioffi, G.; Patil, N.; Waite, K.A.; Lanese, R.; Ostrom, Q.T.; Kruchko, C.; Berens, M.E.; Connor, J.R.; Lathia, J.D.; et al. Importance of the Intersection of Age and Sex to Understand Variation in Incidence and Survival for Primary Malignant Gliomas. Neuro-Oncology 2022, 24, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Sforna, L.; Esposito, V.; Limatola, C.; Franciolini, F. Ion Channels in Glioma Malignancy. Rev. Physiol. Biochem. Pharm. 2021, 181, 223–267. [Google Scholar] [CrossRef]
- Cohen, A.L.; Colman, H. Glioma Biology and Molecular Markers. Cancer Treat. Res. 2015, 163, 15–30. [Google Scholar] [CrossRef]
- Lester, A.; McDonald, K.L. Intracranial Ependymomas: Molecular Insights and Translation to Treatment. Brain Pathol. 2020, 30, 3–12. [Google Scholar] [CrossRef]
- Yao, M.; Li, S.; Wu, X.; Diao, S.; Zhang, G.; He, H.; Bian, L.; Lu, Y. Cellular Origin of Glioblastoma and Its Implication in Precision Therapy. Cell Mol. Immunol. 2018, 15, 737–739. [Google Scholar] [CrossRef]
- Venturini, E.; Leanza, L.; Azzolini, M.; Kadow, S.; Mattarei, A.; Weller, M.; Tabatabai, G.; Edwards, M.J.; Zoratti, M.; Paradisi, C.; et al. Targeting the Potassium Channel Kv1.3 Kills Glioblastoma Cells. Neurosignals 2017, 25, 26–38. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- He, H.; Yao, M.; Zhang, W.; Tao, B.; Liu, F.; Li, S.; Dong, Y.; Zhang, C.; Meng, Y.; Li, Y.; et al. MEK2 Is a Prognostic Marker and Potential Chemo-Sensitizing Target for Glioma Patients Undergoing Temozolomide Treatment. Cell Mol. Immunol. 2016, 13, 658–668. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Schiff, D.; Lee, E.Q.; Nayak, L.; Norden, A.D.; Reardon, D.A.; Wen, P.Y. Medical Management of Brain Tumors and the Sequelae of Treatment. Neuro-Oncology 2015, 17, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tyler, E.; Lustick, M.; Klein, D.; Walter, K.A. Healthcare Costs for High-Grade Glioma. Anticancer Res. 2019, 39, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.T.; Zhan, J.S.; Xiao, L.M.; Li, L.; Xu, H.X.; Fu, Z.B.; Zhang, Y.H.; Zhang, J.; Jia, X.H.; Ge, G.; et al. The Unwanted Cell Migration in the Brain: Glioma Metastasis. Neurochem. Res. 2017, 42, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Ko, E.A.; Gu, W.; Lim, I.; Bang, H.; Zhou, T. Expression Profiling of Ion Channel Genes Predicts Clinical Outcome in Breast Cancer. Mol. Cancer 2013, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-A.; Kim, Y.-W.; Lee, D.; Choi, J.; Kim, S.; Seo, Y.; Bang, H.; Kim, J.-H.; Ko, J.-H. Expression of Potassium Channel Genes Predicts Clinical Outcome in Lung Cancer. Korean J. Physiol. Pharm. 2019, 23, 529–537. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, Q.; Liang, J.; Zhang, L.; Zhang, H. The Ion Channel Gene KCNAB2 Is Associated with Poor Prognosis and Loss of Immune Infiltration in Lung Adenocarcinoma. Cells 2022, 11, 3438. [Google Scholar] [CrossRef]
- Wang, R.; Gurguis, C.I.; Gu, W.; Ko, E.A.; Lim, I.; Bang, H.; Zhou, T.; Ko, J.-H. Ion Channel Gene Expression Predicts Survival in Glioma Patients. Sci. Rep. 2015, 5, 11593. [Google Scholar] [CrossRef] [PubMed]
- Stock, C. How Dysregulated Ion Channels and Transporters Take a Hand in Esophageal, Liver, and Colorectal Cancer. Rev. Physiol. Biochem. Pharm. 2021, 181, 129–222. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Limatola, C.; Catalano, M. Functional Roles of the Ca2+-Activated K+ Channel, KCa3.1, in Brain Tumors. Curr. Neuropharmacol. 2018, 16, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, X. TRPC Channels and Glioma. Adv. Exp. Med. Biol. 2017, 976, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F. Transient Receptor Potential (TRP) Ion Channels Involved in Malignant Glioma Cell Death and Therapeutic Perspectives. Front. Cell Dev. Biol. 2021, 9, 618961. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Ions. the Movement of Water and the Apoptotic Volume Decrease. Front. Cell Dev. Biol. 2020, 8, 611211. [Google Scholar] [CrossRef] [PubMed]
- Ernest, N.J.; Habela, C.W.; Sontheimer, H. Cytoplasmic Condensation Is Both Necessary and Sufficient to Induce Apoptotic Cell Death. J. Cell Sci. 2008, 121, 290–297. [Google Scholar] [CrossRef]
- Watkins, S.; Sontheimer, H. Hydrodynamic Cellular Volume Changes Enable Glioma Cell Invasion. J. Neurosci. 2011, 31, 17250–17259. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Aiello, F.; Fioretti, B.; Sforna, L.; Castigli, E.; Ruggieri, P.; Tata, A.M.; Calogero, A.; Franciolini, F. Serum-Activated K and Cl Currents Underlay U87-MG Glioblastoma Cell Migration. J. Cell Physiol. 2011, 226, 1926–1933. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef]
- Turner, K.L.; Honasoge, A.; Robert, S.M.; McFerrin, M.M.; Sontheimer, H. A Proinvasive Role for the Ca(2+) -Activated K(+) Channel KCa3.1 in Malignant Glioma. Glia 2014, 62, 971–981. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Grimaldi, A.; Chece, G.; Porzia, A.; Esposito, V.; Santoro, A.; Salvati, M.; Mainiero, F.; Ragozzino, D.; Di Angelantonio, S.; et al. KCa3.1 Channel Inhibition Sensitizes Malignant Gliomas to Temozolomide Treatment. Oncotarget 2016, 7, 30781–30796. [Google Scholar] [CrossRef]
- Serrano-Novillo, C.; Capera, J.; Colomer-Molera, M.; Condom, E.; Ferreres, J.C.; Felipe, A. Implication of Voltage-Gated Potassium Channels in Neoplastic Cell Proliferation. Cancers 2019, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Benjin, X.; Ling, L. Developments, Applications, and Prospects of Cryo-electron Microscopy. Protein Sci. 2020, 29, 872–882. [Google Scholar] [CrossRef]
- Nwanochie, E.; Uversky, V.N. Structure Determination by Single-Particle Cryo-Electron Microscopy: Only the Sky (and Intrinsic Disorder) Is the Limit. Int. J. Mol. Sci. 2019, 20, 4186. [Google Scholar] [CrossRef] [PubMed]
- Vénien-Bryan, C.; Li, Z.; Vuillard, L.; Boutin, J.A. Cryo-Electron Microscopy and X-Ray Crystallography: Complementary Approaches to Structural Biology and Drug Discovery. Acta Cryst. F Struct. Biol. Commun. 2017, 73, 174–183. [Google Scholar] [CrossRef]
- Willegems, K.; Eldstrom, J.; Kyriakis, E.; Ataei, F.; Sahakyan, H.; Dou, Y.; Russo, S.; Petegem, F.V.; Fedida, D. Structural and Electrophysiological Basis for the Modulation of KCNQ1 Channel Currents by ML277. Nat. Commun. 2022, 13, 3760. [Google Scholar] [CrossRef] [PubMed]
- Natale, A.M.; Deal, P.E.; Minor, D.L. Structural Insights into the Mechanisms and Pharmacology of K2P Potassium Channels. J. Mol. Biol. 2021, 433, 166995. [Google Scholar] [CrossRef]
- Liu, J.; Qu, C.; Han, C.; Chen, M.-M.; An, L.-J.; Zou, W. Potassium Channels and Their Role in Glioma: A Mini Review. Mol. Membr. Biol. 2019, 35, 76–85. [Google Scholar] [CrossRef]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-Gated Potassium Channels as Therapeutic Drug Targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar] [CrossRef]
- Tian, C.; Zhu, R.; Zhu, L.; Qiu, T.; Cao, Z.; Kang, T. Potassium Channels: Structures, Diseases, and Modulators. Chem. Biol. Drug Des. 2014, 83, 1–26. [Google Scholar] [CrossRef]
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K(+) Channels: Function-Structural Overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef]
- Del Camino, D.; Kanevsky, M.; Yellen, G. Status of the Intracellular Gate in the Activated-Not-Open State of Shaker K+ Channels. J. Gen. Physiol. 2005, 126, 419. [Google Scholar] [CrossRef]
- Kim, D.M.; Nimigean, C.M. Voltage-Gated Potassium Channels: A Structural Examination of Selectivity and Gating. Cold Spring Harb. Perspect. Biol. 2016, 8, a029231. [Google Scholar] [CrossRef]
- Doyle, D.A.; Morais Cabral, J.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The Structure of the Potassium Channel: Molecular Basis of K+ Conduction and Selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Schow, E.V.; Freites, J.A.; Nizkorodov, A.; White, S.H.; Tobias, D.J. Coupling between the Voltage-Sensing and Pore Domains in a Voltage-Gated Potassium Channel. Biochim. Biophys. Acta 2012, 1818, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Long, S.B.; Campbell, E.B.; Mackinnon, R. Voltage Sensor of Kv1.2: Structural Basis of Electromechanical Coupling. Science 2005, 309, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Combi, R. Potassium Channels and Human Epileptic Phenotypes: An Updated Overview. Front. Cell Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef]
- Jiang, Y.; Lee, A.; Chen, J.; Ruta, V.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-Ray Structure of a Voltage-Dependent K+ Channel. Nature 2003, 423, 33–41. [Google Scholar] [CrossRef]
- Zúñiga, L.; Cayo, A.; González, W.; Vilos, C.; Zúñiga, R. Potassium Channels as a Target for Cancer Therapy: Current Perspectives. Onco. Targets 2022, 15, 783–797. [Google Scholar] [CrossRef]
- Long, S.B.; Campbell, E.B.; Mackinnon, R. Crystal Structure of a Mammalian Voltage-Dependent Shaker Family K+ Channel. Science 2005, 309, 897–903. [Google Scholar] [CrossRef]
- Hernandez-Resendiz, I.; Hartung, F.; Pardo, L.A. Antibodies Targeting KV Potassium Channels: A Promising Treatment for Cancer. Bioelectricity 2019, 1, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Niday, Z.; Tzingounis, A.V. Potassium Channel Gain of Function in Epilepsy: An Unresolved Paradox. Neuroscientist 2018, 24, 368–380. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Voltage-Gated Potassium Channels and the Diversity of Electrical Signalling. J. Physiol. 2012, 590, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Cieślar-Pobuda, A.; Wiechec, E. Role of Ion Channels in Regulating Ca2+ Homeostasis during the Interplay between Immune and Cancer Cells. Cell Death Dis. 2015, 6, e1648. [Google Scholar] [CrossRef]
- Shah, N.H.; Aizenman, E. Voltage-Gated Potassium Channels at the Crossroads of Neuronal Function, Ischemic Tolerance, and Neurodegeneration. Transl. Stroke Res. 2014, 5, 38–58. [Google Scholar] [CrossRef]
- Köhling, R.; Wolfart, J. Potassium Channels in Epilepsy. Cold Spring Harb. Perspect. Med. 2016, 6, a022871. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, R.J. Ion Channels in Glioblastoma. ISRN Neurol. 2011, 2011, 590249. [Google Scholar] [CrossRef]
- Wulff, H.; Köhler, R. Endothelial Small- and Intermediate-Conductance KCa Channels: An Update on Their Pharmacology and Usefulness as Cardiovascular Targets. J. Cardiovasc. Pharm. 2013, 61, 102–112. [Google Scholar] [CrossRef]
- Kaczmarek, L.K.; Aldrich, R.W.; Chandy, K.G.; Grissmer, S.; Wei, A.D.; Wulff, H. International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels. Pharm. Rev. 2017, 69, 1–11. [Google Scholar] [CrossRef]
- Sweet, T.-B.; Cox, D.H. Measuring the Influence of the BKCa {beta}1 Subunit on Ca2+ Binding to the BKCa Channel. J. Gen. Physiol. 2009, 133, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.S.; Cui, J. BK Channel Activation: Structural and Functional Insights. Trends Neurosci. 2010, 33, 415–423. [Google Scholar] [CrossRef]
- Liu, P.; Chen, B.; Wang, Z.-W. SLO-2 Potassium Channel Is an Important Regulator of Neurotransmitter Release in Caenorhabditis Elegans. Nat. Commun. 2014, 5, 5155. [Google Scholar] [CrossRef] [PubMed]
- Kshatri, A.S.; Gonzalez-Hernandez, A.; Giraldez, T. Physiological Roles and Therapeutic Potential of Ca2+ Activated Potassium Channels in the Nervous System. Front. Mol. Neurosci. 2018, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Kyle, B.D. The Large-Conductance, Calcium-Activated Potassium Channel: A Big Key Regulator of Cell Physiology. Front. Physiol. 2021, 12, 750615. [Google Scholar] [CrossRef]
- Orfali, R.; Albanyan, N. Ca2+-Sensitive Potassium Channels. Molecules 2023, 28, 885. [Google Scholar] [CrossRef]
- Hager, N.A.; McAtee, C.K.; Lesko, M.A.; O’Donnell, A.F. Inwardly Rectifying Potassium Channel Kir2.1 and Its “Kir-Ious” Regulation by Protein Trafficking and Roles in Development and Disease. Front. Cell Dev. Biol. 2022, 9, 796136. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Cui, M.; Cantwell, L.; Zorn, A.; Logothetis, D.E. Kir Channel Molecular Physiology, Pharmacology, and Therapeutic Implications. Handb. Exp. Pharm. 2021, 267, 277–356. [Google Scholar] [CrossRef]
- de Boer, T.P.; Houtman, M.J.C.; Compier, M.; van der Heyden, M.A.G. The Mammalian K(IR)2.x Inward Rectifier Ion Channel Family: Expression Pattern and Pathophysiology. Acta Physiol. 2010, 199, 243–256. [Google Scholar] [CrossRef]
- Baronas, V.A.; Kurata, H.T. Inward Rectifiers and Their Regulation by Endogenous Polyamines. Front. Physiol. 2014, 5, 325. [Google Scholar] [CrossRef]
- Jeremic, D.; Sanchez-Rodriguez, I.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Therapeutic Potential of Targeting G Protein-Gated Inwardly Rectifying Potassium (GIRK) Channels in the Central Nervous System. Pharmacol. Ther. 2021, 223, 107808. [Google Scholar] [CrossRef]
- Walsh, K.B. Screening Technologies for Inward Rectifier Potassium Channels: Discovery of New Blockers and Activators. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 420–433. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Ning, Q. Review on Regulation of Inwardly Rectifying Potassium Channels. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 303–311. [Google Scholar] [CrossRef]
- Oliver, D.; Baukrowitz, T.; Fakler, B. Polyamines as Gating Molecules of Inward-Rectifier K+ Channels. Eur. J. Biochem. 2000, 267, 5824–5829. [Google Scholar] [CrossRef]
- Logothetis, D.E.; Lupyan, D.; Rosenhouse-Dantsker, A. Diverse Kir Modulators Act in Close Proximity to Residues Implicated in Phosphoinositide Binding. J. Physiol. 2007, 582, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Sancho, M.; Fletcher, J.; Welsh, D.G. Inward Rectifier Potassium Channels: Membrane Lipid-Dependent Mechanosensitive Gates in Brain Vascular Cells. Front. Cardiovasc. Med. 2022, 9, 650. [Google Scholar] [CrossRef]
- Enyedi, P.; Czirják, G. Molecular Background of Leak K+ Currents: Two-Pore Domain Potassium Channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Gada, K.; Plant, L.D. Two-pore Domain Potassium Channels: Emerging Targets for Novel Analgesic Drugs: IUPHAR Review 26. Br. J. Pharm. 2019, 176, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, J.; Chen, X.-D. General Anesthesia Mediated by Effects on Ion Channels. World J. Crit. Care Med. 2012, 1, 80–93. [Google Scholar] [CrossRef]
- Lamas, J.A.; Fernández-Fernández, D. Tandem Pore TWIK-Related Potassium Channels and Neuroprotection. Neural Regen. Res. 2019, 14, 1293. [Google Scholar] [CrossRef]
- Goldstein, S.A.N.; Bayliss, D.A.; Kim, D.; Lesage, F.; Plant, L.D.; Rajan, S. International Union of Pharmacology. LV. Nomenclature and Molecular Relationships of Two-P Potassium Channels. Pharm. Rev. 2005, 57, 527–540. [Google Scholar] [CrossRef]
- Braun, A.P. Two-Pore Domain Potassium Channels. Channels (Austin) 2012, 6, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, L.; Zúñiga, R. Understanding the Cap Structure in K2P Channels. Front. Physiol. 2016, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Enyedi, P.; Czirják, G. Negative Influence by the Force: Mechanically Induced Hyperpolarization via K2P Background Potassium Channels. Int. J. Mol. Sci. 2021, 22, 9062. [Google Scholar] [CrossRef]
- Feliciangeli, S.; Chatelain, F.C.; Bichet, D.; Lesage, F. The Family of K2P Channels: Salient Structural and Functional Properties. J. Physiol. 2015, 593, 2587–2603. [Google Scholar] [CrossRef]
- Herrera-Pérez, S.; Campos-Ríos, A.; Rueda-Ruzafa, L.; Lamas, J.A. Contribution of K2P Potassium Channels to Cardiac Physiology and Pathophysiology. Int. J. Mol. Sci. 2021, 22, 6635. [Google Scholar] [CrossRef] [PubMed]
- Riel, E.B.; Jürs, B.C.; Cordeiro, S.; Musinszki, M.; Schewe, M.; Baukrowitz, T. The Versatile Regulation of K2P Channels by Polyanionic Lipids of the Phosphoinositide and Fatty Acid Metabolism. J. Gen. Physiol. 2021, 154, e202112989. [Google Scholar] [CrossRef]
- Lee, L.-M.; Müntefering, T.; Budde, T.; Meuth, S.G.; Ruck, T. Pathophysiological Role of K2P Channels in Human Diseases. Cell Physiol. Biochem. 2021, 55, 65–86. [Google Scholar] [CrossRef]
- Felipe, A.; Vicente, R.; Villalonga, N.; Roura-Ferrer, M.; Martínez-Mármol, R.; Solé, L.; Ferreres, J.C.; Condom, E. Potassium Channels: New Targets in Cancer Therapy. Cancer Detect. Prev. 2006, 30, 375–385. [Google Scholar] [CrossRef]
- Ru, Q.; Tian, X.; Pi, M.S.; Chen, L.; Yue, K.; Xiong, Q.; Ma, B.-M.; Li, C.-Y. Voltage-gated K+ Channel Blocker Quinidine Inhibits Proliferation and Induces Apoptosis by Regulating Expression of MicroRNAs in Human Glioma U87-MG Cells. Int. J. Oncol. 2015, 46, 833–840. [Google Scholar] [CrossRef]
- Weiger, T.M.; Colombatto, S.; Kainz, V.; Heidegger, W.; Grillo, M.A.; Hermann, A. Potassium Channel Blockers Quinidine and Caesium Halt Cell Proliferation in C6 Glioma Cells via a Polyamine-Dependent Mechanism. Biochem. Soc. Trans. 2007, 35, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, X.; Li, X.; Hao, X. Overexpression of Tau Downregulated the MRNA Levels of Kv Channels and Improved Proliferation in N2A Cells. PLoS ONE 2015, 10, e0116628. [Google Scholar] [CrossRef] [PubMed]
- Weiger, T.M.; Hermann, A. Cell Proliferation, Potassium Channels, Polyamines and Their Interactions: A Mini Review. Amino Acids 2014, 46, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.B.; Zhao, S.G.; Liu, Y.H.; Hu, E.X.; Liu, B.X. Tetraethylammonium Inhibits Glioma Cells via Increasing Production of Intracellular Reactive Oxygen Species. Chemotherapy 2009, 55, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Szabo, I.; Baltzer, K.; Lang, F. Ceramide-Induced Inhibition of T Lymphocyte Voltage-Gated Potassium Channel Is Mediated by Tyrosine Kinases. Proc. Natl. Acad. Sci. USA 1997, 94, 7661–7666. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Li, W.; Edwards, M.J.; Ahmad, S.A.; Patel, S.; Szabo, I.; Gulbins, E. Voltage-Gated Potassium Channels as Regulators of Cell Death. Front. Cell Dev. Biol. 2020, 8, 611853. [Google Scholar] [CrossRef] [PubMed]
- Sales, T.T.; Resende, F.F.B.; Chaves, N.L.; Titze-De-Almeida, S.S.; Báo, S.N.; Brettas, M.L.; Titze-De-Almeida, R. Suppression of the Eag1 Potassium Channel Sensitizes Glioblastoma Cells to Injury Caused by Temozolomide. Oncol. Lett. 2016, 12, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer, H. An Unexpected Role for Ion Channels in Brain Tumor Metastasis. Exp. Biol. Med. 2008, 233, 779. [Google Scholar] [CrossRef]
- Ruggieri, P.; Mangino, G.; Fioretti, B.; Catacuzzeno, L.; Puca, R.; Ponti, D.; Miscusi, M.; Fabio Franciolini, F.; Giuseppe Ragona, G.; Calogero, C. The Inhibition of KCa3.1 Channels Activity Reduces Cell Motility in Glioblastoma Derived Cancer Stem Cells. PloS ONE 2012, 7, e47825. [Google Scholar] [CrossRef]
- d’Alessandro, G.; Catalano, M.; Sciaccaluga, M.; Chece, G.; Cipriani, R.; Rosito, M.; Grimaldi, A.; Lauro, C.; Cantore, G.; Santoro, A.; et al. KCa3.1 Channels Are Involved in the Infiltrative Behavior of Glioblastoma in Vivo. Cell Death Dis. 2013, 4, e773. [Google Scholar] [CrossRef] [PubMed]
- Sciaccaluga, M.; Fioretti, B.; Catacuzzeno, L.; Pagani, F.; Bertollini, C.; Rosito, M.; Catalano, M.; D’Alessandro, G.; Santoro, A.; Cantore, G.; et al. CXCL12-Induced Glioblastoma Cell Migration Requires Intermediate Conductance Ca2+-Activated K+ Channel Activity. Am. J. Physiol. Cell Physiol. 2010, 299, C175–C184. [Google Scholar] [CrossRef]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. Expression and Role of the Intermediate-Conductance Calcium-Activated Potassium Channel KCa3.1 in Glioblastoma. J. Signal Transduct. 2012, 2012, 421564. [Google Scholar] [CrossRef]
- Bai, Y.; Liao, H.; Liu, T.; Zeng, X.; Xiao, F.; Luo, L.; Guo, H.; Guo, L. MiR-296-3p Regulates Cell Growth and Multi-Drug Resistance of Human Glioblastoma by Targeting Ether-à-Go-Go (EAG1). Eur. J. Cancer 2013, 49, 710–724. [Google Scholar] [CrossRef] [PubMed]
- MiR-133b Contributes to Arsenic-Induced Apoptosis in U251 Glioma Cells by Targeting the HERG Channel | Request PDF. Available online: https://www.researchgate.net/publication/267735161_MiR-133b_Contributes_to_Arsenic-Induced_Apoptosis_in_U251_Glioma_Cells_by_Targeting_the_hERG_Channel (accessed on 6 April 2023).
- Debska-Vielhaber, G.; Godlewski, M.M.; Kicinska, A.; Skalska, J.; Kulawiak, B.; Piwonska, M.; Zablocki, Z.; Kunz, W.S.; Szewczyk, A.; Moty, T. Large-Conductance K+ Channel Openers Induce Death of Human Glioma Cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 27–36. [Google Scholar]
- Rosa, P.; Sforna, L.; Carlomagno, S.; Mangino, G.; Miscusi, M.; Pessia, M.; Franciolini, F.; Calogero, A.; Catacuzzeno, L. Overexpression of Large-Conductance Calcium-Activated Potassium Channels in Human Glioblastoma Stem-Like Cells and Their Role in Cell Migration. J. Cell. Physiol. 2017, 232, 2478–2488. [Google Scholar] [CrossRef]
- Lefranc, F.; Le Rhun, E.; Kiss, R.; Weller, M. Glioblastoma Quo Vadis: Will Migration and Invasiveness Reemerge as Therapeutic Targets? Cancer Treat. Rev. 2018, 68, 145–154. [Google Scholar] [CrossRef]
- Huang, L.; Li, B.; Li, W.; Guo, H.; Zou, F. ATP-Sensitive Potassium Channels Control Glioma Cells Proliferation by Regulating ERK Activity. Carcinogenesis 2009, 30, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.L.; Sontheimer, H. Cl− and K+ Channels and Their Role in Primary Brain Tumour Biology. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 20130095. [Google Scholar] [CrossRef]
- An, J.R.; Seo, M.S.; Jung, H.S.; Heo, R.; Kang, M.; Han, E.-T.; Park, H.; Jung, W.-K.; Choi, I.-W.; Park, W.S. Inhibition by Imipramine of the Voltage-Dependent K+ Channel in Rabbit Coronary Arterial Smooth Muscle Cells. Toxicol. Sci. 2020, 178, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-H.; Kim, S.H.; Kim, Y.; Kim, Y.S.; Lim, Y.; Lee, Y.H.; Shin, S.Y. The Tricyclic Antidepressant Imipramine Induces Autophagic Cell Death in U-87MG Glioma Cells. Biochem. Biophys. Res. Commun. 2011, 413, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rubaiy, H.N. The Therapeutic Agents That Target ATP-Sensitive Potassium Channels. Acta Pharm. 2016, 66, 23–34. [Google Scholar] [CrossRef]
- Sánchez-Alvarez, R.; Paíno, T.; Herrero-González, S.; Medina, J.M.; Tabernero, A. Tolbutamide Reduces Glioma Cell Proliferation by Increasing Connexin43, Which Promotes the up-Regulation of P21 and P27 and Subsequent Changes in Retinoblastoma Phosphorylation. Glia 2006, 54, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Wahl, P.; Holmes, W.E.; Ashcroft, F.M. Effect of Repaglinide on Cloned Beta Cell, Cardiac and Smooth Muscle Types of ATP-Sensitive Potassium Channels. Diabetologia 2001, 44, 747–756. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Chen, R.Q.; Hu, D.X.; Xie, X.Q.; Yu, S.B.; Chen, X.Q. Identification of Repaglinide as a Therapeutic Drug for Glioblastoma Multiforme. Biochem. Biophys. Res. Commun. 2017, 488, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Yatani, A.; Wakamori, M.; Mikala, G.; Bahinski, A. Block of Transient Outward-Type Cloned Cardiac K+ Channel Currents by Quinidine. Circ. Res. 1993, 73, 351–359. [Google Scholar] [CrossRef]
- Undrovinas, A.I.; Burnashev, N.; Eroshenko, D.; Fleidervish, I.; Starmer, C.F.; Makielski, J.C.; Rosenshtraukh, L.V. Quinidine Blocks Adenosine 5’-Triphosphate-Sensitive Potassium Channels in Heart. Am. J. Physiol. Heart Circ. Physiol. 1990, 259, H1609–H1612. [Google Scholar] [CrossRef]
- Vasilev, A.; Sofi, R.; Tong, L.; Teschemacher, A.G.; Kasparov, S. In Search of a Breakthrough Therapy for Glioblastoma Multiforme. Neuroglia 2018, 1, 292–310. [Google Scholar] [CrossRef]
- Griffin, M.; Khan, R.; Basu, S.; Smith, S. Ion Channels as Therapeutic Targets in High Grade Gliomas. Cancers 2020, 12, 3068. [Google Scholar] [CrossRef]
- Ferrer, T.; Aréchiga-Figueroa, I.A.; Shapiro, M.S.; Tristani-Firouzi, M.; Sanchez-Chapula, J.A. Tamoxifen Inhibition of Kv7.2/Kv7.3 Channels. PLoS ONE 2013, 8, e76085. [Google Scholar] [CrossRef]
- He, W.; Liu, R.; Yang, S.-H.; Yuan, F. Chemotherapeutic Effect of Tamoxifen on Temozolomide-Resistant Gliomas. Anticancer Drugs 2015, 26, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-H.; Wang, C.-H.; Lin, S.-M.; Chen, C.-K.; Huang, H.-Y.; Chen, Y. Activation of C-Jun N-Terminal Kinase 1 and Caspase 3 in the Tamoxifen-Induced Apoptosis of Rat Glioma Cells. J. Cancer Res. Clin. Oncol. 2004, 130, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lyne, S.B.; Yamini, B. An Alternative Pipeline for Glioblastoma Therapeutics: A Systematic Review of Drug Repurposing in Glioblastoma. Cancers 2021, 13, 1953. [Google Scholar] [CrossRef]
- Teisseyre, A.; Palko-Labuz, A.; Sroda-Pomianek, K.; Michalak, K. Voltage-Gated Potassium Channel Kv1.3 as a Target in Therapy of Cancer. Front. Oncol. 2019, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Mulkearns-Hubert, E.E.; Torre-Healy, L.A.; Silver, D.J.; Eurich, J.T.; Bayik, D.; Serbinowski, E.; Hitomi, M.; Zhou, J.; Przychodzen, B.; Zhang, R.; et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019, 27, 1062–1072.e5. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.M.; Pressley, B.; Wulff, H. KCa3.1 Channel Modulators as Potential Therapeutic Compounds for Glioblastoma. Curr. Neuropharmacol. 2018, 16, 618–626. [Google Scholar] [CrossRef]
- Xin, P.; Xu, L.; Dong, W.; Mao, L.; Guo, J.; Bi, J.; Zhang, S.; Pei, Y.; Chen, C.-P. Synthetic K+ Channels Constructed by Rebuilding the Core Modules of Natural K+ Channels in an Artificial System. Angew. Chem. Int. Ed. Engl. 2023, 62, e202217859. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; van Beek, L.; Shilliday, F.; Debreczeni, J.É.; Phillips, C. Cryo-EM: The Resolution Revolution and Drug Discovery. SLAS Discov. 2021, 26, 17–31. [Google Scholar] [CrossRef]
- Robertson, M.J.; Meyerowitz, J.G.; Skiniotis, G. Drug Discovery in the Era of Cryo-Electron Microscopy. Trends Biochem. Sci. 2022, 47, 124–135. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Z.; Yang, H. Computer-Aided Drug Discovery and Design Targeting Ion Channels. Curr. Top. Med. Chem. 2016, 16, 1819–1829. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial Intelligence in Drug Discovery and Development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, Y.; Wang, H.; Zhang, X.; Wang, M.; He, J.; Li, S.; Zhang, L.; Li, K.; Cao, L. Advances of Artificial Intelligence in Anti-Cancer Drug Design: A Review of the Past Decade. Pharmaceuticals 2023, 16, 253. [Google Scholar] [CrossRef]
| Type of Channel | Model/Cells | Related Major Findings | References |
|---|---|---|---|
| EAG1 | Glioblastoma (GBM) |
| [154] |
| hERG | Glioblastoma (GBM) |
| [155] |
| BK | a. Glioblastoma Stem-like Cells b. Human Glioma Cell line c. U251 glioma cells |
| [156,157,158] |
| IK | Human GBM Cells |
| [159] |
| KCa3.1 | a. Mouse Glioma Model b. GL261 cells |
| [82] |
| Kir6.2 | Human Glioma Cells |
| [159] |
| Drug | Category | Mechanism | Effect on Glioma | References |
|---|---|---|---|---|
| Imipramine | Tricyclic antidepressant | - Inhibits vascular voltage-dependent K+ channels - Inhibits PI3K/Akt/mTOR signaling | Induces autophagic cell death | [161,162] |
| Tolbutamide | Sulfonylurea (oral-hypoglycemic agent) | - Binds to the beta-cell ATP-sensitive potassium channel resulting in blocking of K+ efflux through the KIR6.2 channel - Increases connexin43, upregulates cyclin-dependent kinase (Cdk) inhibitors p21 and p27, and reduces pRb phosphorylation | Inhibits cell proliferation | [163,164] |
| Repaglinide | Short-acting insulin secretagogue | - Closes ATP-sensitive potassium channels | Exhibits anticancer effects via apoptotic, autophagic, and immune checkpoint signaling | [165,166] |
| Quinidine | Antiarrhythmic drug | - Blocks voltage-gated K+ channels | Exhibits antiproliferative and proapoptosis effect | [141,142] |
| Tamoxifen | Selective estrogen receptor modulator | - Inhibits the Kv7.2/Kv7.3 through preventing PIP2-channel interaction - Exact mechanism unknown | Exerts cytotoxic actions, induces apoptosis, and has direct action on mitochondrial complex I inhibition | [170,171,172,173] |
| Clofazimine | Antimycobacterial agent | - Blocks Kv1.3 channels | Reduces tumor growth, proliferation, and self-renewal | [59,174,175,176] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younes, S.; Mourad, N.; Salla, M.; Rahal, M.; Hammoudi Halat, D. Potassium Ion Channels in Glioma: From Basic Knowledge into Therapeutic Applications. Membranes 2023, 13, 434. https://doi.org/10.3390/membranes13040434
Younes S, Mourad N, Salla M, Rahal M, Hammoudi Halat D. Potassium Ion Channels in Glioma: From Basic Knowledge into Therapeutic Applications. Membranes. 2023; 13(4):434. https://doi.org/10.3390/membranes13040434
Chicago/Turabian StyleYounes, Samar, Nisreen Mourad, Mohamed Salla, Mohamad Rahal, and Dalal Hammoudi Halat. 2023. "Potassium Ion Channels in Glioma: From Basic Knowledge into Therapeutic Applications" Membranes 13, no. 4: 434. https://doi.org/10.3390/membranes13040434
APA StyleYounes, S., Mourad, N., Salla, M., Rahal, M., & Hammoudi Halat, D. (2023). Potassium Ion Channels in Glioma: From Basic Knowledge into Therapeutic Applications. Membranes, 13(4), 434. https://doi.org/10.3390/membranes13040434








