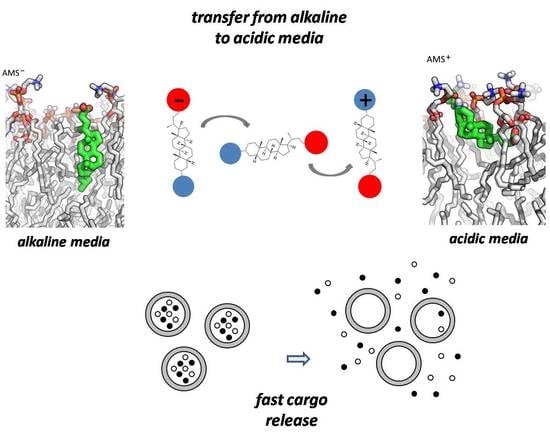
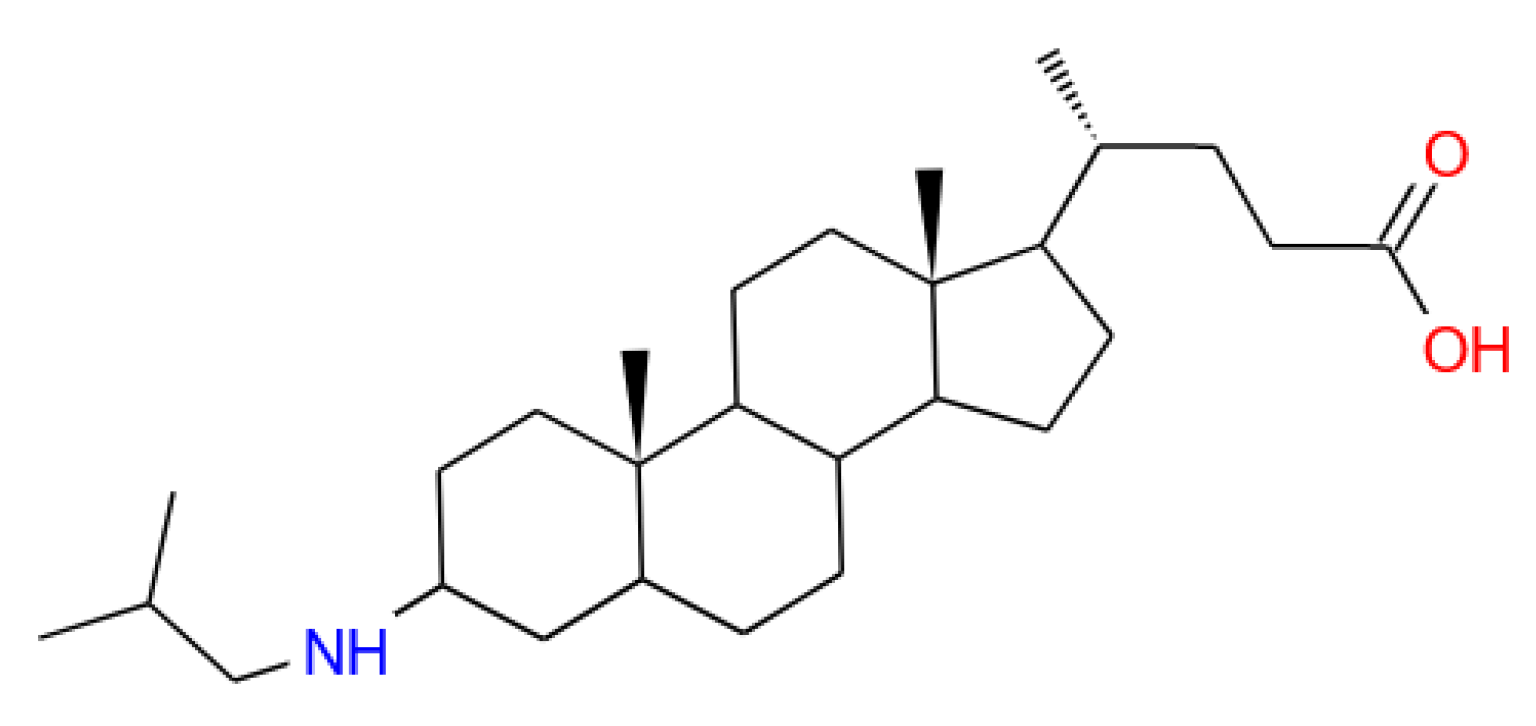
pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release?
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Liposomes
2.3. Methods
2.4. Molecular Dynamic (MD) Simulations
2.5. ATR-FTIR Spectroscopy Experiments
3. Results and Discussion
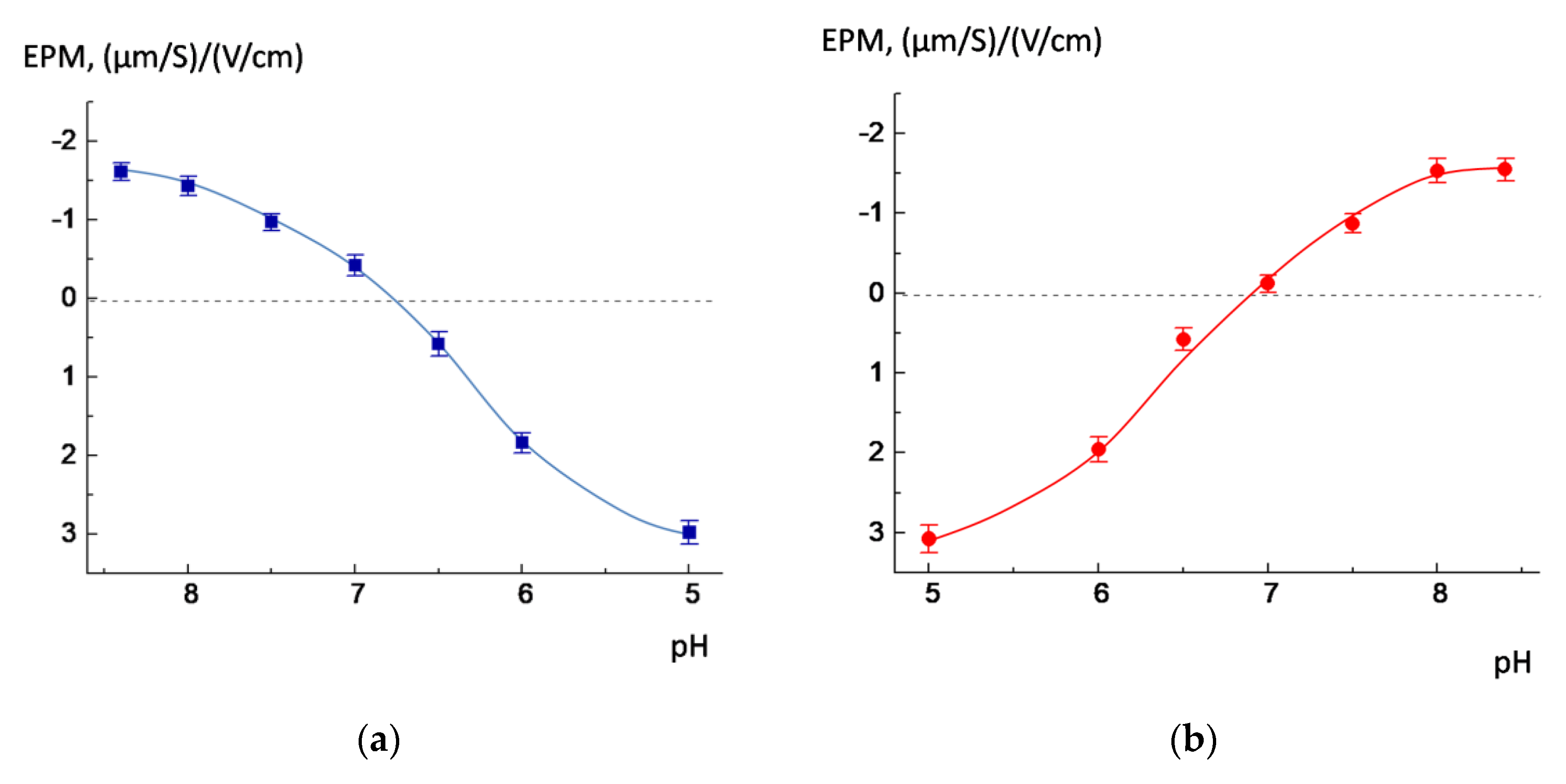
3.1. POPC/AMS Liposomes
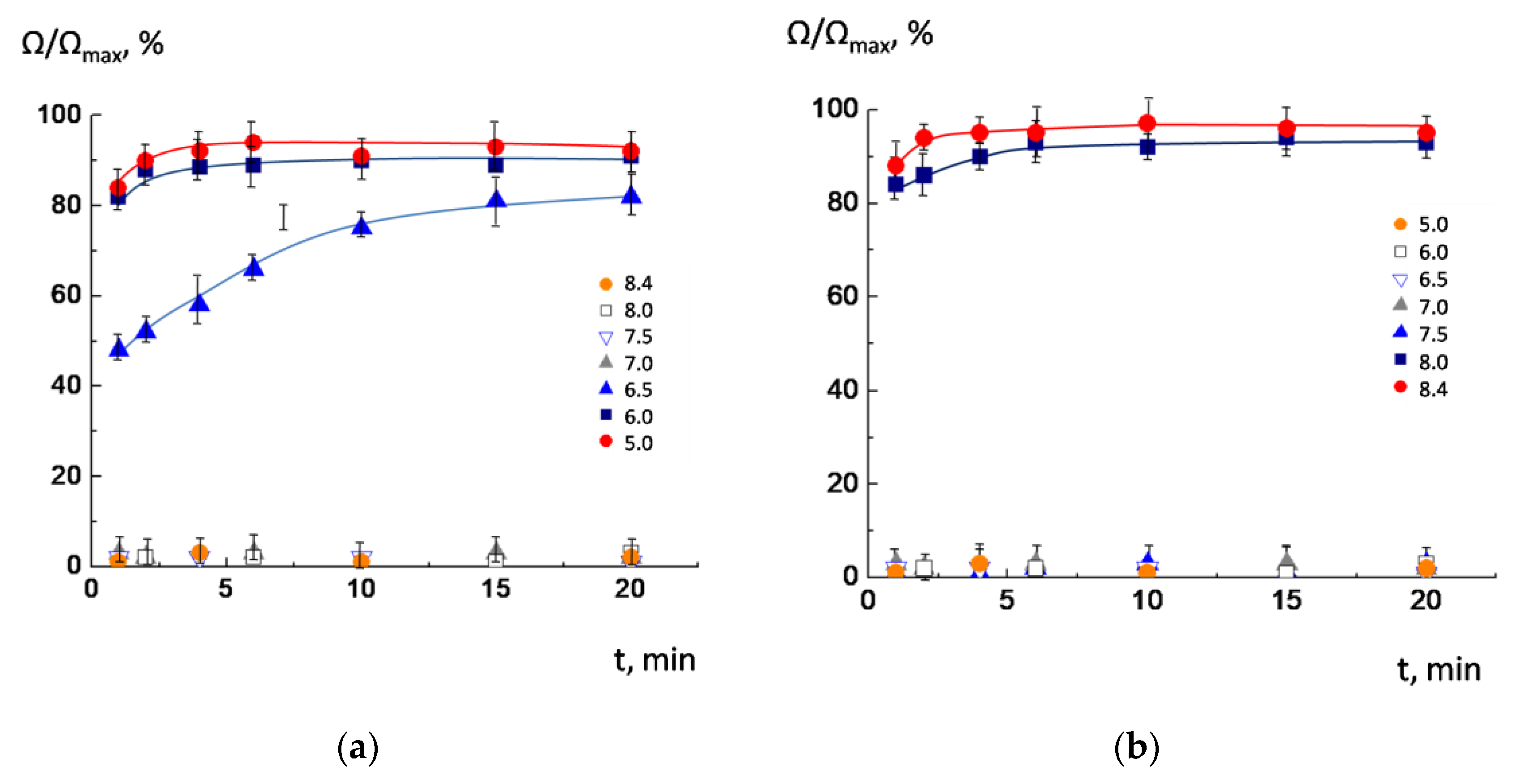
3.2. Release of the Cargo by POPC/AMS Liposomes under the Changes in the pH
3.3. Molecular Modeling of the Switch
3.4. ATR-FTIR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M.; Whitehead, K.A. In pursuit of a moving target: Nanotherapeutics for the treatment of non-Hodgkin B-cell lymphoma. Expert Opin. Drug Deliv. 2014, 11, 1923–1937. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Carvalho, M.A.; Castanheira, E.M.S. Functionalized Liposome and Albumin-Based Systems as Carriers for Poorly Water-Soluble Anticancer Drugs: An Updated Review. Biomedicines 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Europ. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofrè, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Pereira, F.H.; Batalhão, M.E.; Cárnio, E.C. Correlation between body temperature, blood pressure and plasmatic nitric oxide in septic patients. Rev. Lat. Am. Enfermagem 2014, 22, 123–128. [Google Scholar] [CrossRef]
- Helmlinger, G.; Sckell, A.; Dellian, M.; Forbes, N.S.; Jain, R.K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 2002, 8, 1284–1291. [Google Scholar] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annual Rev. Biomed. Eng. 2006, 8, 343–375. [Google Scholar] [CrossRef] [PubMed]
- Karanth, H.; Murthy, R.S. pH-sensitive liposomes--principle and application in cancer therapy. J. Pharm. Pharmacol. 2007, 59, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.D.S.; Lopes, S.C.; Franco, M.S.; Oliveira, M.C. pH-sensitive liposomes for drug delivery in cancer treatment. Ther. Deliv. 2013, 4, 1099–1123. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef]
- Aryasomayajula, B.; Salzano, G.; Torchilin, V.P. Multifunctional Liposomes. Methods Mol. Biol. 2017, 1530, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Gagne, L.; Chen, H.; Szoka, F.C. Novel ortho ester-based, pH-sensitive cationic lipid for gene delivery in vitro and in vivo. J. Liposome Res. 2014, 24, 90–98. [Google Scholar] [CrossRef]
- Viricel, W.; Mbarek, A.; Leblond, J. Switchable Lipids: Conformational Change for Fast pH-Triggered Cytoplasmic Delivery. Angew. Chem. Int. Ed. 2015, 54, 12743–12747. [Google Scholar] [CrossRef]
- Samoshina, N.M.; Liu, X.; Brazdova, B.; Franz, A.H.; Samoshin, V.V.; Guo, X. Fliposomes: pH-Sensitive Liposomes Containing a trans-2-morpholinocyclohexanol-Based Lipid That Performs a Conformational Flip and Triggers an Instant Cargo Release in Acidic Medium. Pharmaceutics 2011, 3, 379–405. [Google Scholar] [CrossRef]
- Yaroslavov, A.; Efimova, A.; Smirnova, N.; Erzunov, D.; Lukashev, N.; Grozdova, I.; Melik-Nubarov, N. A novel approach to a controlled opening of liposomes. Colloids Surf. B Biointerfaces 2020, 190, 110906. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.S.; Efimova, A.A.; Kazantsev, A.V.; Erzunov, D.A.; Lukashev, N.V.; Grozdova, I.D.; Melik-Nubarov, N.S.; Yaroslavov, A.A. pH-Sensitive liposomes with embedded ampholytic derivatives of cholan-24-oic acid. Mendeleev Commun. 2021, 31, 827–829. [Google Scholar] [CrossRef]
- Gurtovenko, A.A. Molecular-Level Insight into the Interactions of DNA/Polycation Complexes with Model Cell Membranes. J. Phys. Chem. B 2019, 123, 6505–6514. [Google Scholar] [CrossRef]
- Semenyuk, P.I.; Efimova, A.A.; Lentin, I.I.; Le-Deygen, I.M.; Izumrudov, V.A. Interaction of Ionenes with Lipid Membrane: Unusual Impact of Charge Density. Langmuir 2020, 36, 14717–14727. [Google Scholar] [CrossRef] [PubMed]
- Kostritskii, A.Y.; Kondinskaia, D.A.; Nesterenko, A.M.; Gurtovenko, A.A. Adsorption of Synthetic Cationic Polymers on Model Phospholipid Membranes: Insight from Atomic-Scale Molecular Dynamics Simulations. Langmuir 2016, 32, 10402–10414. [Google Scholar] [CrossRef]
- Trosheva, K.S.; Sorokina, S.A.; Efimova, A.A.; Semenyuk, P.I.; Berkovich, A.K.; Yaroslavov, A.A.; Shifrina, Z.B. Interaction of multicomponent anionic liposomes with cationic pyridylphenylene dendrimer: Does the complex behavior depend on the liposome composition? Biochim. Biophys. Acta Biomembr. 2021, 1863, 183761. [Google Scholar] [CrossRef]
- Wilkosz, N.; Jamróz, D.; Kopeć, W.; Nakai, K.; Yusa, S.I.; Wytrwal-Sarna, M.; Bednar, J.; Nowakowska, M.; Kepczynski, M. Effect of Polycation Structure on Interaction with Lipid Membranes. J. Phys. Chem. B 2017, 121, 7318–7326. [Google Scholar] [CrossRef]
- Awasthi, N.; Kopec, W.; Wilkosz, N.; Jamróz, D.; Hub, J.S.; Zatorska, M.; Petka, R.; Nowakowska, M.; Kepczynski, M. Molecular Mechanism of Polycation-Induced Pore Formation in Biomembranes. ACS Biomater. Sci. Eng. 2019, 5, 780–794. [Google Scholar] [CrossRef]
- Goormaghtigh, E.; Raussens, V.; Ruysschaert, J.-M. Attenuated Total Reflection Infrared Spectroscopy of Proteins and Lipids in Biological Membranes. Biochim. Biophys. Acta Rev. Biomembr. 1999, 1422, 105–185. [Google Scholar] [CrossRef]
- Biruss, B.; Dietl, R.; Valenta, C. The Influence of Selected Steroid Hormones on the Physicochemical Behaviour of DPPC Liposomes. Chem. Phys. Lipids. 2007, 148, 84–90. [Google Scholar] [CrossRef]
- Deygen, I.M.; Seidl, C.; Kölmel, D.K.; Bednarek, C.; Heissler, S.; Kudryashova, E.V.; Bräse, S.; Schepers, U. Novel Prodrug of Doxorubicin Modified by Stearoylspermine Encapsulated into PEG-Chitosan-Stabilized Liposomes. Langmuir 2016, 32, 10861–10869. [Google Scholar] [CrossRef] [PubMed]
- Le-Deygen, I.M.; Vlasova, K.Y.; Kutsenok, E.O.; Usvaliev, A.D.; Efremova, M.V.; Zhigachev, A.O.; Rudakovskaya, P.G.; Golovin, D.Y.; Gribanovsky, S.L.; Kudryashova, E.V.; et al. Magnetic nanorods for remote disruption of lipid membranes by non-heating low frequency magnetic field. Nanomedicine 2019, 21, 102065. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Pastor, R.W.; Mackerell, A.D. Development of the CHARMM Force Field for Lipids. J. Phys. Chem. Lett. 2011, 2, 1526–1532. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of bonded parameters and partial atomic charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Manrique-Moreno, M.; Garidel, P.; Suwalsky, M.; Howe, J.; Brandenburg, K. The membrane-activity of Ibuprofen, Diclofenac, and Naproxen: A physico-chemical study with lecithin phospholipids. Biochim. Biophys. Acta 2009, 1788, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Disalvo, E.A.; Frias, M.A. Water state and carbonyl distribution populations in confined regions of lipid bilayers observed by FTIR spectroscopy. Langmuir 2013, 29, 6969–6974. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N.; Pohle, W.; Mantsch, H.H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: A reevaluation. Biophys. J. 1994, 67, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Bobroff, V.; Rubio, C.; Vigier, V.; Petibois, C. FTIR spectroscopy characterization of fatty-acyl-chain conjugates. Anal. Bioanal. Chem. 2016, 408, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]

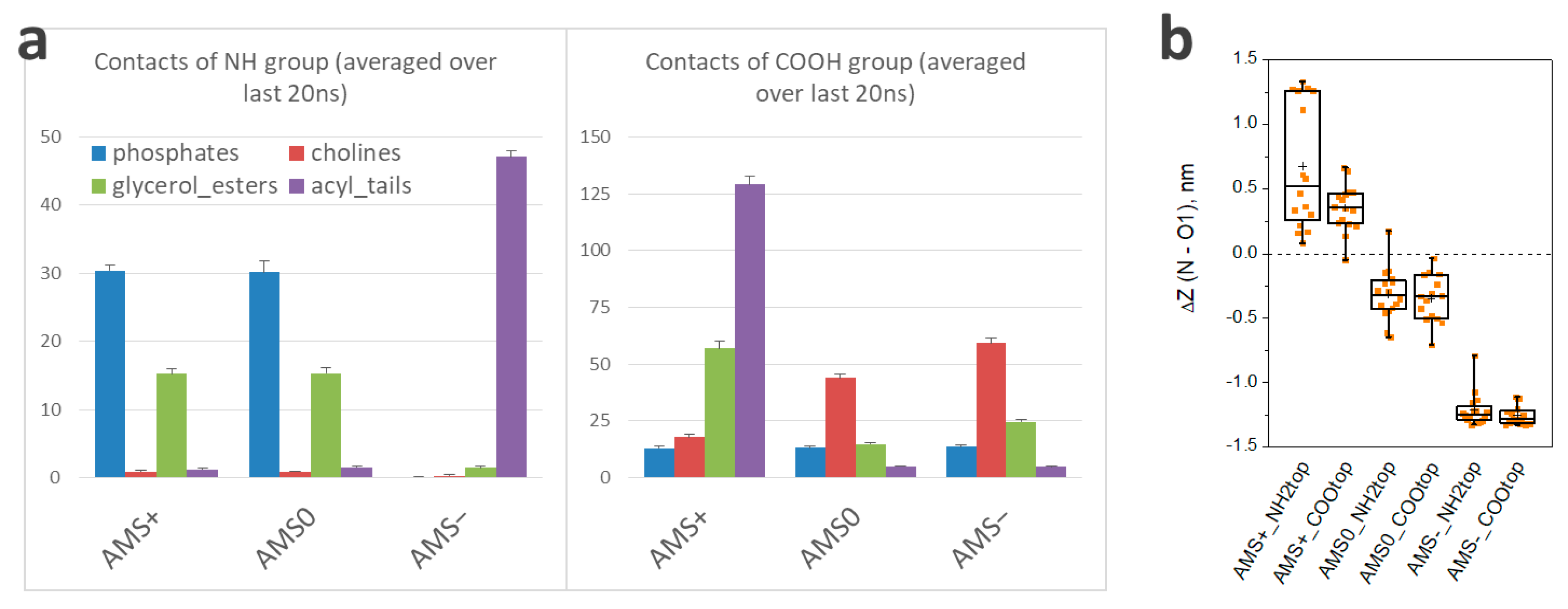
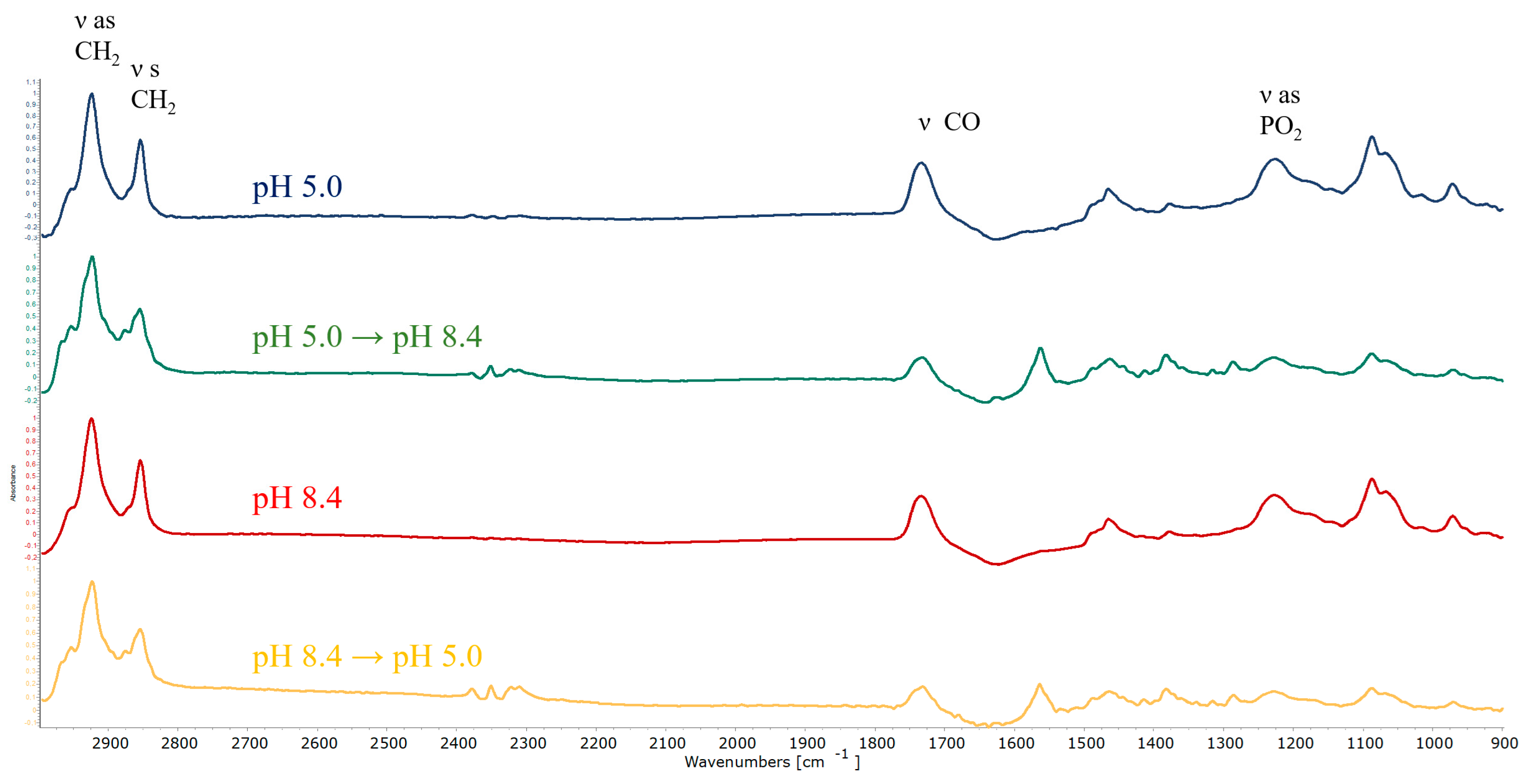
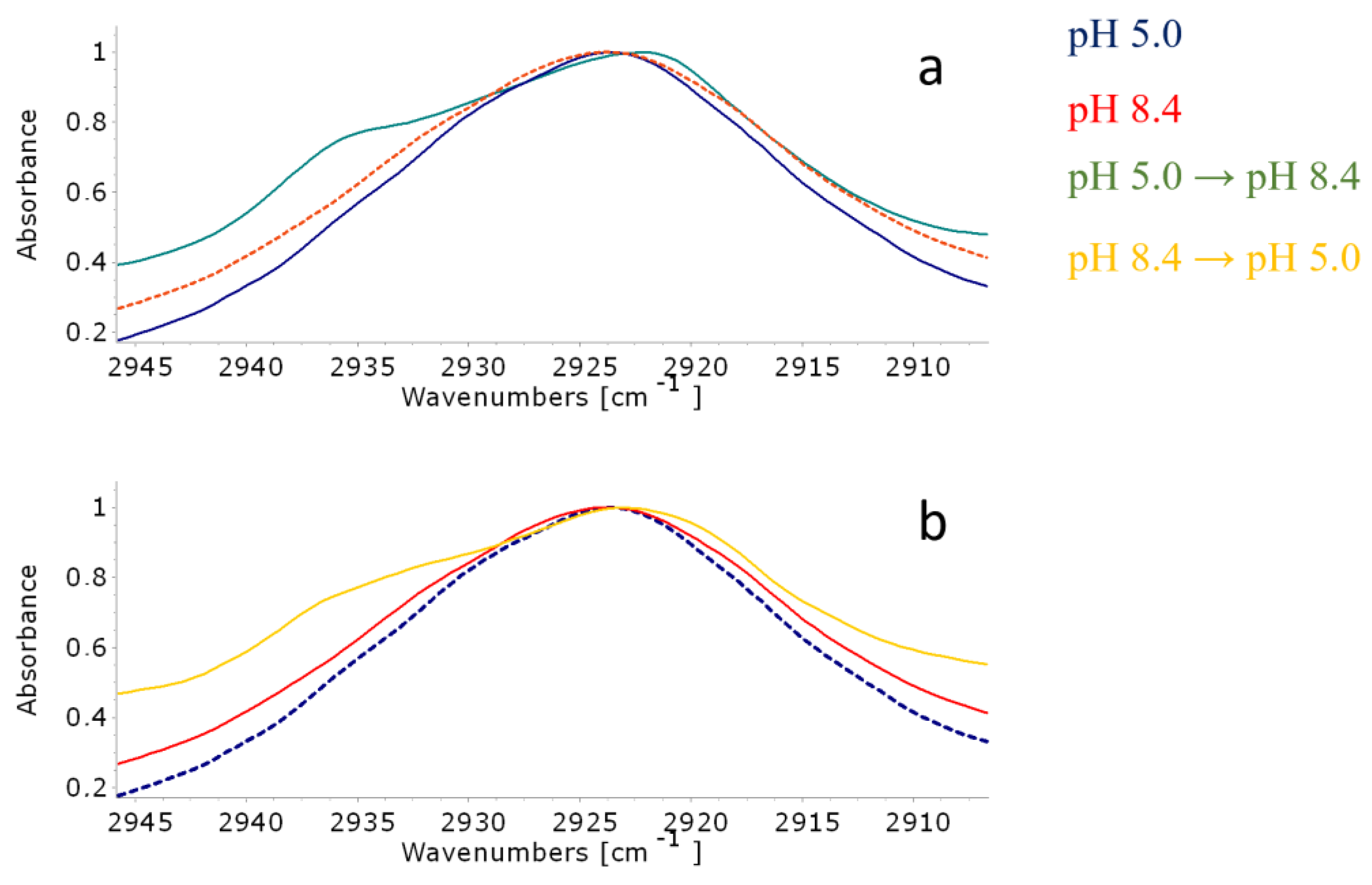
| Amino Group | Carboxylic Group | Counterions | |
|---|---|---|---|
| AMS+_NH2top | >NH2+ | –COOH | 4x Na+, 20x Cl– |
| AMS+_COOtop | >NH2+ | –COOH | 4x Na+, 20x Cl– |
| AMS0_NH2top | >NH2+ | –COO– | 20x Na+, 20x Cl– |
| AMS0_COOtop | >NH2+ | –COO– | 20x Na+, 20x Cl– |
| AMS−_NH2top | >NH | –COO– | 20x Na+, 4x Cl– |
| AMS−_COOtop | >NH | –COO– | 20x Na+, 4x Cl– |
| Total Lipid Concentration, mg/mL | ||||||
|---|---|---|---|---|---|---|
| 2 | 1 | 0.8 | 0.6 | 0.4 | 0.2 | |
| EPM, (μm/s)/(V/cm) | −1.61 ± 0.11 | −1.61 ± 0.11 | −1.58 ± 0.08 | −1.59 ± 0.07 | −1.62 ± 0.08 | −1.63 ± 0.06 |
| Hydrodynamic diameter, nm | 45 ± 5 | 42 ± 4 | 39 ± 2 | 44 ± 2 | 39 ± 4 | 43 ± 5 |
| Total Lipid Concentration, mg/mL | ||||||
|---|---|---|---|---|---|---|
| 2 | 1 | 0.8 | 0.6 | 0.4 | 0.2 | |
| EPM, (μm/s)/(V/cm) | 2.97 ± 0.07 | 2.98 ± 0.05 | 2.98 ± 0.06 | 2.98 ± 0.07 | 2.99 ± 0.08 | 3.00 ± 0.07 |
| Hydrodynamic diameter, nm | 36 ± 6 | 39 ± 5 | 40 ± 3 | 35 ± 3 | 47 ± 3 | 36 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efimova, A.A.; Popov, A.S.; Kazantsev, A.V.; Semenyuk, P.I.; Le-Deygen, I.M.; Lukashev, N.V.; Yaroslavov, A.A. pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes 2023, 13, 407. https://doi.org/10.3390/membranes13040407
Efimova AA, Popov AS, Kazantsev AV, Semenyuk PI, Le-Deygen IM, Lukashev NV, Yaroslavov AA. pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes. 2023; 13(4):407. https://doi.org/10.3390/membranes13040407
Chicago/Turabian StyleEfimova, Anna A., Anton S. Popov, Alexey V. Kazantsev, Pavel I. Semenyuk, Irina M. Le-Deygen, Nikolay V. Lukashev, and Alexander A. Yaroslavov. 2023. "pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release?" Membranes 13, no. 4: 407. https://doi.org/10.3390/membranes13040407
APA StyleEfimova, A. A., Popov, A. S., Kazantsev, A. V., Semenyuk, P. I., Le-Deygen, I. M., Lukashev, N. V., & Yaroslavov, A. A. (2023). pH-Sensitive Liposomes with Embedded 3-(isobutylamino)cholan-24-oic Acid: What Is the Possible Mechanism of Fast Cargo Release? Membranes, 13(4), 407. https://doi.org/10.3390/membranes13040407