Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles
Abstract
1. Introduction
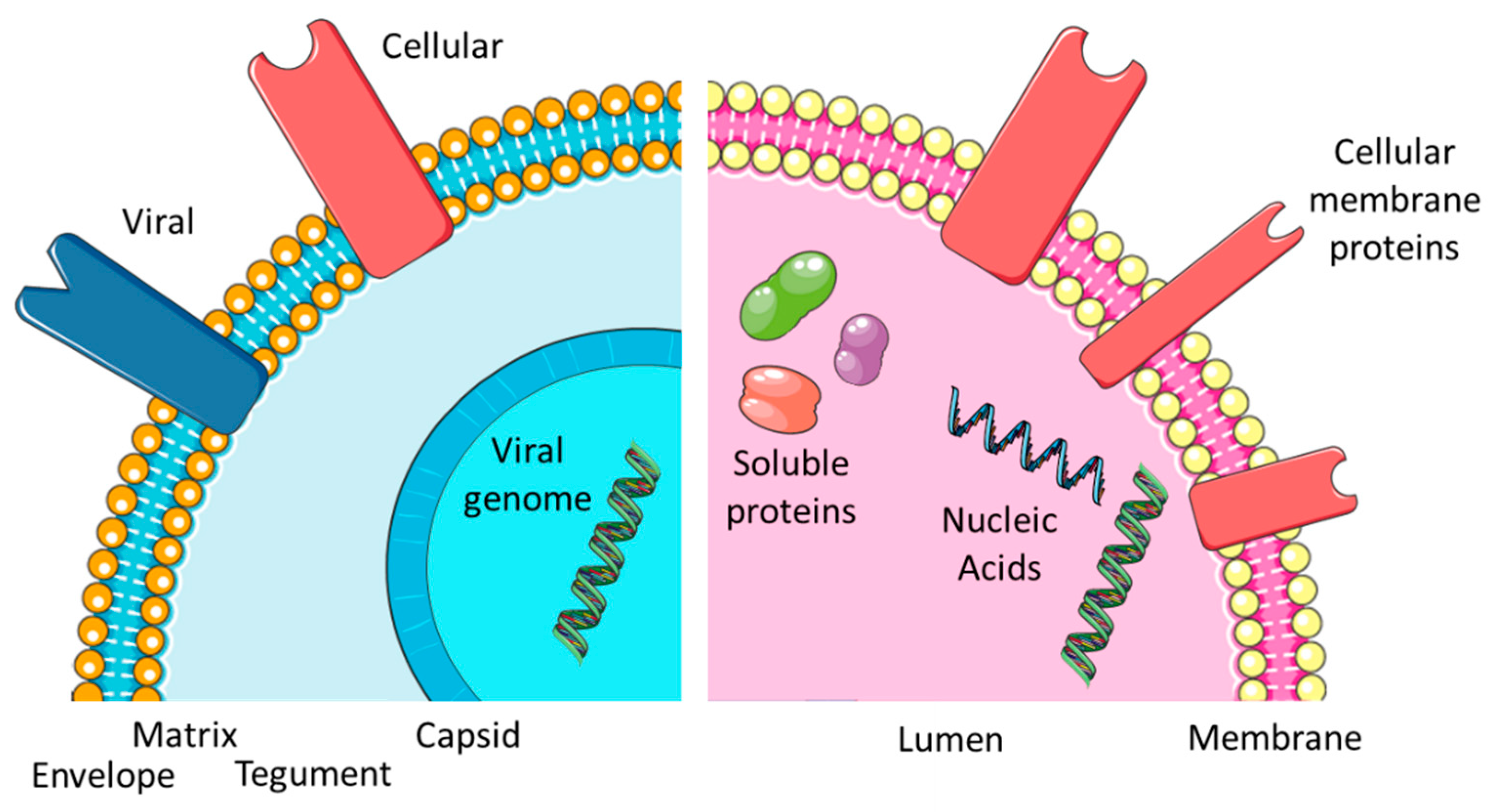
2. The Biogenesis of EXO and EVI
3. Viral Envelopes and Exosomal Membranes
3.1. Lipid Content and Characteristics
3.2. Protein Content
4. Discussion—Implications for Applications
5. Conclusions
- Physical: size, density, charge;
- Membrane structures: lipid composition and membrane proteins;
- Content and cargo: enrichment and exclusion;
- Lifecycle: cellular contributions and functions of membrane vs. initiation and perspectives;
- Functions: reprogramming of cell gene expression and metabolism.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Metzner, C.; Zaruba, M. On the Interplay of Extracellular vesicles and Viral Infections. Trillium Extracell. Vesicles 2020, 1, 12. [Google Scholar] [CrossRef]
- Zaruba, M.; Roschitz, L.; Sami, H.; Ogris, M.; Gerner, W.; Metzner, C. Surface Modification of E. coli Outer Membrane Vesicles with Glycosylphosphatidylinositol-Anchored Proteins: Generating Pro/Eukaryote Chimera Constructs. Membranes 2021, 11, 428. [Google Scholar] [CrossRef]
- Couch, Y.; Buzas, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lotvall, J.; Raposo, G.; Stahl, P.D.; Thery, C.; et al. A brief history of nearly EV-erything-The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Cocucci, E.; Meldolesi, J. Ectosomes and exosomes: Shedding the confusion between extracellular vesicles. Trends Cell. Biol. 2015, 25, 364–372. [Google Scholar] [CrossRef]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [CrossRef]
- Laulagnier, K.; Grand, D.; Dujardin, A.; Hamdi, S.; Vincent-Schneider, H.; Lankar, D.; Salles, J.P.; Bonnerot, C.; Perret, B.; Record, M. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004, 572, 11–14. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzas, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V.; et al. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef]
- Metzner, C.; Zaruba, M. On the Relationship of Viral Particles and Extracellular Vesicles: Implications for Viral Vector Technology. Viruses 2021, 13, 1238. [Google Scholar] [CrossRef]
- Badierah, R.A.; Uversky, V.N.; Redwan, E.M. Dancing with Trojan horses: An interplay between the extracellular vesicles and viruses. J. Biomol. Struct. Dyn. 2020, 39, 3034–3060. [Google Scholar] [CrossRef]
- Nolte’t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular vesicles and viruses: Are they close relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef]
- Raab-Traub, N.; Dittmer, D.P. Viral effects on the content and function of extracellular vesicles. Nat. Rev. Microbiol. 2017, 15, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ketter, E.; Randall, G. Virus Impact on Lipids and Membranes. Annu. Rev. Virol. 2019, 6, 319–340. [Google Scholar] [CrossRef]
- Chaudhari, P.; Ghate, V.; Nampoothiri, M.; Lewis, S. Multifunctional role of exosomes in viral diseases: From transmission to diagnosis and therapy. Cell. Signal. 2022, 94, 110325. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Tashak Golroudbari, H.; Banikarimi, S.P.; Ayati, A.; Hadizadeh, A.; Khorasani Zavareh, Z.; Hajikhani, K.; Heirani-Tabasi, A.; Ahmadi Tafti, M.; Davoodi, S.; Ahmadi Tafti, H. Advanced micro-/nanotechnologies for exosome encapsulation and targeting in regenerative medicine. Clin. Exp. Med. 2023. [Google Scholar] [CrossRef]
- Riedel, C.; Lamp, B.; Heimann, M.; Konig, M.; Blome, S.; Moennig, V.; Schuttler, C.; Thiel, H.J.; Rumenapf, T. The core protein of classical Swine Fever virus is dispensable for virus propagation in vitro. PLoS Pathog. 2012, 8, e1002598. [Google Scholar] [CrossRef]
- Hie, B.; Zhong, E.D.; Berger, B.; Bryson, B. Learning the language of viral evolution and escape. Science 2021, 371, 284–288. [Google Scholar] [CrossRef]
- Has, C.; Pan, S. Vesicle formation mechanisms: An overview. J. Liposome Res. 2021, 31, 90–111. [Google Scholar] [CrossRef]
- Hurley, J.H.; Boura, E.; Carlson, L.A.; Rozycki, B. Membrane budding. Cell 2010, 143, 875–887. [Google Scholar] [CrossRef]
- Hurley, J.H.; Hanson, P.I. Membrane budding and scission by the ESCRT machinery: It’s all in the neck. Nat. Rev. Mol. Cell. Biol. 2010, 11, 556–566. [Google Scholar] [CrossRef]
- Bremaud, E.; Favard, C.; Muriaux, D. Deciphering the Assembly of Enveloped Viruses Using Model Lipid Membranes. Membranes 2022, 12, 441. [Google Scholar] [CrossRef] [PubMed]
- Votteler, J.; Sundquist, W.I. Virus budding and the ESCRT pathway. Cell. Host Microbe 2013, 14, 232–241. [Google Scholar] [CrossRef]
- Veesler, D.; Johnson, J.E. Virus maturation. Annu. Rev. Biophys. 2012, 41, 473–496. [Google Scholar] [CrossRef]
- Veesler, D.; Quispe, J.; Grigorieff, N.; Potter, C.S.; Carragher, B.; Johnson, J.E. Maturation in action: CryoEM study of a viral capsid caught during expansion. Structure 2012, 20, 1384–1390. [Google Scholar] [CrossRef]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell. Res. 2021, 31, 157–177. [Google Scholar] [CrossRef]
- Schirrmacher, V. Molecular Mechanisms of Anti-Neoplastic and Immune Stimulatory Properties of Oncolytic Newcastle Disease Virus. Biomedicines 2022, 10, 562. [Google Scholar] [CrossRef]
- Krijnse Locker, J.; Chlanda, P.; Sachsenheimer, T.; Brugger, B. Poxvirus membrane biogenesis: Rupture not disruption. Cell. Microbiol. 2013, 15, 190–199. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell. Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Laulagnier, K.; Motta, C.; Hamdi, S.; Roy, S.; Fauvelle, F.; Pageaux, J.F.; Kobayashi, T.; Salles, J.P.; Perret, B.; Bonnerot, C.; et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 2004, 380, 161–171. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Angelini, D.F.; Di Raimo, R.; Falchi, M.; Battistini, L.; Fais, S. Microenvironmental pH and Exosome Levels Interplay in Human Cancer Cell Lines of Different Histotypes. Cancers 2018, 10, 370. [Google Scholar] [CrossRef]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef]
- Hantak, M.P.; Qing, E.; Earnest, J.T.; Gallagher, T. Tetraspanins: Architects of Viral Entry and Exit Platforms. J. Virol. 2019, 93, e01429-17. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Savelyeva, E.E.; Tchevkina, E.M. Lipid Rafts in Exosome Biogenesis. Biochemistry 2020, 85, 177–191. [Google Scholar] [CrossRef]
- Metzner, C.; Salmons, B.; Gunzburg, W.H.; Dangerfield, J.A. Rafts, anchors and viruses—A role for glycosylphosphatidylinositol anchored proteins in the modification of enveloped viruses and viral vectors. Virology 2008, 382, 125–131. [Google Scholar] [CrossRef]
- Weissenhorn, W.; Poudevigne, E.; Effantin, G.; Bassereau, P. How to get out: ssRNA enveloped viruses and membrane fission. Curr. Opin. Virol. 2013, 3, 159–167. [Google Scholar] [CrossRef]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-kappaB Signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Ding, J.; Guo, X.; Zhu, X.Q.; Zheng, Y. Exosomes in virus-associated cancer. Cancer Lett. 2018, 438, 44–51. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Ipinmoroti, A.O.; Matthews, Q.L. Extracellular Vesicles: Roles in Human Viral Infections, Immune-Diagnostic, and Therapeutic Applications. Pathogens 2020, 9, 1056. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Luo, B. Effects of Exosomal Viral Components on the Tumor Microenvironment. Cancers 2022, 14, 3552. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Sanyal, S.; Menon, A.K. Flipping lipids: Why an’ what’s the reason for? ACS Chem. Biol. 2009, 4, 895–909. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, N.; Yan, T.; Shi, Y.N.; Chen, J.; Zhang, C.J.; Xie, X.J.; Liao, D.F.; Qin, L. The crosstalk: Exosomes and lipid metabolism. Cell. Commun. Signal. 2020, 18, 119. [Google Scholar] [CrossRef]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- Omasta, B.; Tomaskova, J. Cellular Lipids-Hijacked Victims of Viruses. Viruses 2022, 14, 1896. [Google Scholar] [CrossRef]
- Aloia, R.C.; Tian, H.; Jensen, F.C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA 1993, 90, 5181–5185. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Ivanova, P.T.; Myers, D.S.; Milne, S.B.; McClaren, J.L.; Thomas, P.G.; Brown, H.A. Lipid composition of viral envelope of three strains of influenza virus-not all viruses are created equal. ACS Infect. Dis. 2015, 1, 399–452. [Google Scholar] [CrossRef]
- Lai, R.C.; Lim, S.K. Membrane lipids define small extracellular vesicle subtypes secreted by mesenchymal stromal cells. J. Lipid Res. 2019, 60, 318–322. [Google Scholar] [CrossRef]
- Marzec, M.E.; Rzaca, C.; Moskal, P.; Stepien, E.L. Study of the influence of hyperglycemia on the abundance of amino acids, fatty acids, and selected lipids in extracellular vesicles using TOF-SIMS. Biochem. Biophys. Res. Commun. 2022, 622, 30–36. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Ortega-Deballon, P.; Hermite, L.; Pais-De-Barros, J.P.; Gobbo, J.; Garrido, C. Lipidomic profiling of exosomes from colorectal cancer cells and patients reveals potential biomarkers. Mol. Oncol. 2022, 16, 2710–2718. [Google Scholar] [CrossRef]
- Suga, K.; Matsui, D.; Watanabe, N.; Okamoto, Y.; Umakoshi, H. Insight into the Exosomal Membrane: From Viewpoints of Membrane Fluidity and Polarity. Langmuir 2021, 37, 11195–11202. [Google Scholar] [CrossRef]
- Mironov, A.A.; Mironov, A.; Derganc, J.; Beznoussenko, G.V. Membrane Curvature, Trans-Membrane Area Asymmetry, Budding, Fission and Organelle Geometry. Int. J. Mol. Sci. 2020, 21, 7594. [Google Scholar] [CrossRef]
- Jing, H.; Wang, Y.; Desai, P.R.; Ramamurthi, K.S.; Das, S. Lipid flip-flop and desorption from supported lipid bilayers is independent of curvature. PLoS ONE 2020, 15, e0244460. [Google Scholar] [CrossRef]
- Markosyan, R.M.; Cohen, F.S. The transmembrane domain and acidic lipid flip-flop regulates voltage-dependent fusion mediated by class II and III viral proteins. PLoS ONE 2013, 8, e76174. [Google Scholar] [CrossRef]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010, 235, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Qing, Y.; Li, D.; Cui, M.; Jin, P.; Xu, T. Proteomic and lipidomic analysis of exosomes derived from ovarian cancer cells and ovarian surface epithelial cells. J. Ovarian Res. 2020, 13, 9. [Google Scholar] [CrossRef]
- Munoz, O.; Banga, R.; Perreau, M. Host Molecule Incorporation into HIV Virions, Potential Influences in HIV Pathogenesis. Viruses 2022, 14, 2523. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.L.; Frappier, L. Viral proteomics. Microbiol. Mol. Biol. Rev. 2007, 71, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Mardi, N.; Haiaty, S.; Rahbarghazi, R.; Mobarak, H.; Milani, M.; Zarebkohan, A.; Nouri, M. Exosomal transmission of viruses, a two-edged biological sword. Cell. Commun. Signal. 2023, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, S.; Shokri, S.; Nakhaie, M.; Jalilian, F.A.; Mehri-Ghahfarrokhi, A.; Yarani, R.; Shojaeian, A. Small extracellular vesicles as key players in cancer development caused by human oncogenic viruses. Infect. Agent. Cancer 2022, 17, 58. [Google Scholar] [CrossRef]
- Duan, L.; Xu, L.; Xu, X.; Qin, Z.; Zhou, X.; Xiao, Y.; Liang, Y.; Xia, J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale 2021, 13, 1387–1397. [Google Scholar] [CrossRef]
- Metzner, C.; Kochan, F.; Dangerfield, J.A. Postexit surface engineering of retroviral/lentiviral vectors. Biomed. Res. Int. 2013, 2013, 253521. [Google Scholar] [CrossRef]
- Heider, S.; Dangerfield, J.A.; Metzner, C. Biomedical applications of glycosylphosphatidylinositol-anchored proteins. J. Lipid Res. 2016, 57, 1778–1788. [Google Scholar] [CrossRef]
- Heider, S.; Kleinberger, S.; Kochan, F.; Dangerfield, J.A.; Metzner, C. Immune Protection of Retroviral Vectors Upon Molecular Painting with the Complement Regulatory Protein CD59. Mol. Biotechnol. 2016, 58, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Metzner, C.; Kochan, F.; Dangerfield, J.A. Fluorescence molecular painting of enveloped viruses. Mol. Biotechnol. 2013, 53, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lu, L.; Liu, Y.; Li, Z.; Fang, Y.; Chen, Z.; Zhou, J. Native and engineered extracellular vesicles for wound healing. Front. Bioeng. Biotechnol. 2022, 10, 1053217. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.Y.; Tam, K.; Cheyyatraivendran, S.; Gan, S.U.; Gauthaman, K.; Armugam, A.; Jeyaseelan, K.; Choolani, M.; Biswas, A.; Bongso, A. Human Wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J. Cell. Biochem. 2014, 115, 290–302. [Google Scholar] [CrossRef]
- Raj, V.; Claudine, S.; Subramanian, A.; Tam, K.; Biswas, A.; Bongso, A.; Fong, C.Y. Histological, immunohistochemical, and genomic evaluation of excisional and diabetic wounds treated with human Wharton’s jelly stem cells with and without a nanocarrier. J. Cell. Biochem. 2019, 120, 11222–11240. [Google Scholar] [CrossRef]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal. Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid biopsy: Current technology and clinical applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef]


| Collective Term | Abbr. | Types | Abbr. | Subtypes | Variants | Relevance |
| Extracellular vesicles a | EVE | Ectosomes b | ECT | Microvesicles (MIVs) | Signaling | |
| Apoptotic bodies (ABOs) | Apoptosis | |||||
| Exosomes c | EXO | Based on size, cell type | Signaling | |||
| Enveloped virus d | EVI | Replication competent | Functional pathogen | |||
| Replication incompetent | Naturally | e.g., immune decoy | ||||
| Artificial | e.g., gene therapy vectors | |||||
| Substructures | Abbr | Definition | ||||
| Exosomal membrane | EXM | The protein-rich lipid bilayer surrounding an exosome | ||||
| Capsid | CAP | The highly organized protein lattice surrounding the viral genome | ||||
| Matrix | MAT | A protein-rich area found in some viruses connecting CAP and VEN | ||||
| Tegument | TEG | A protein and RNA containing structure found in some viruses, located between CAP and VEN | ||||
| Viral envelope | VEN | The protein-rich lipid bilayer surrounding a subset of virus species | ||||
| GENERAL PROPERTIES AND MEMBRANE CHARACTERISTICS | |||||
|---|---|---|---|---|---|
| Exosomes | HIV | IAV | VACV | SARS-CoV | |
| Diameter | 30–300 nm | 80–100 nm | 80–120 nm | 220–450 nm long 140–260 nm wide | 120 nm |
| Marker proteins | Tetraspanins (CD9, CD63, CD81) | gp120 (ENV), p24 (CA) | HA, NA, | NC | S(pike) or N(ucleocapsid) |
| Cargo | Cellular RNAs and proteins | Viral genome (ssRNA) | Viral genome | Viral genome | Viral genome |
| Membrane origin | Late endosome, multivesicular body | Plasma membrane | Plasma membrane | Endoplasmic reticulum, trans-Golgi | From Endoplasmic reticulum to Golgi-apparatus |
| Biogenesis mechanism(s) | ESCRT-dependent or independent | MA, (partially) ESCRT-dependent, alternatives | MA2, ESCRT-independent, alternatives | unusual, de novo lipogeneses | M, N and E, |
| Difference to source membrane | Increase in cholesterol, sphingomyelin, glycosphingolipids, phosphatidylserine. Decrease in phosphatiylcholine, phosphatidylinositol | Increase in cholesterol, decrease in phosphatidylcholine | Increase in cholesterol and sphingolipids; decrease in glycerophospholipids | Decrease in cholesterol; increase in phosphatidic acid and phosphatidylinositol | Decrease in cholesterol, increase in phospholipids |
| Asymmetry | unclear | Lost | Maintained | n.d. | n.d. |
| Proteins, viral | Facultative * | gp 120 or Env: SU and TM) | HA, NA, M2 | Virion membrane proteins | S, M(embrane), E(nvelope) |
| Proteins, cellular | Tetraspanins (CD9, CD63, CD81) | excluded: CD45, CD4 enriched: HLA-DR, ICAM-1, | Yes | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortes-Galvez, D.; Dangerfield, J.A.; Metzner, C. Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles. Membranes 2023, 13, 397. https://doi.org/10.3390/membranes13040397
Cortes-Galvez D, Dangerfield JA, Metzner C. Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles. Membranes. 2023; 13(4):397. https://doi.org/10.3390/membranes13040397
Chicago/Turabian StyleCortes-Galvez, Daniela, John A. Dangerfield, and Christoph Metzner. 2023. "Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles" Membranes 13, no. 4: 397. https://doi.org/10.3390/membranes13040397
APA StyleCortes-Galvez, D., Dangerfield, J. A., & Metzner, C. (2023). Extracellular Vesicles and Their Membranes: Exosomes vs. Virus-Related Particles. Membranes, 13(4), 397. https://doi.org/10.3390/membranes13040397






