Graphene-Oxide-Grafted Natural Phosphate Support as a Low-Cost Ceramic Membrane for the Removal of Anionic Dyes from Simulated Textile Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization Techniques
2.2. Synthesis of GO
2.3. Fabrication of Ceramic Supports
2.4. Surface Modification of the Ceramic Membrane
2.5. Filtration Test
3. Results and Discussion
3.1. Thermal Behavior of the Moroccan Natural Phosphate
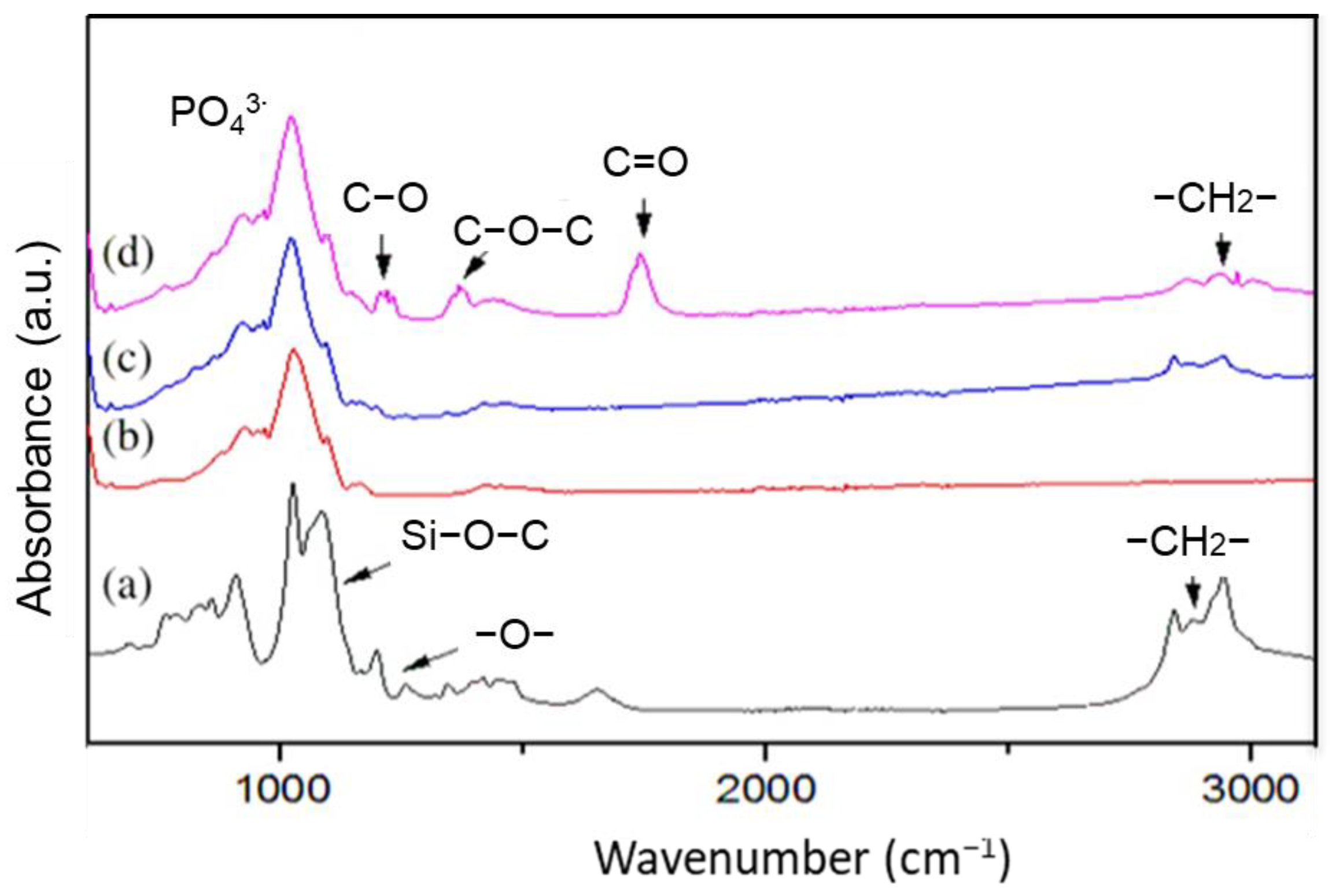
3.2. GO Properties
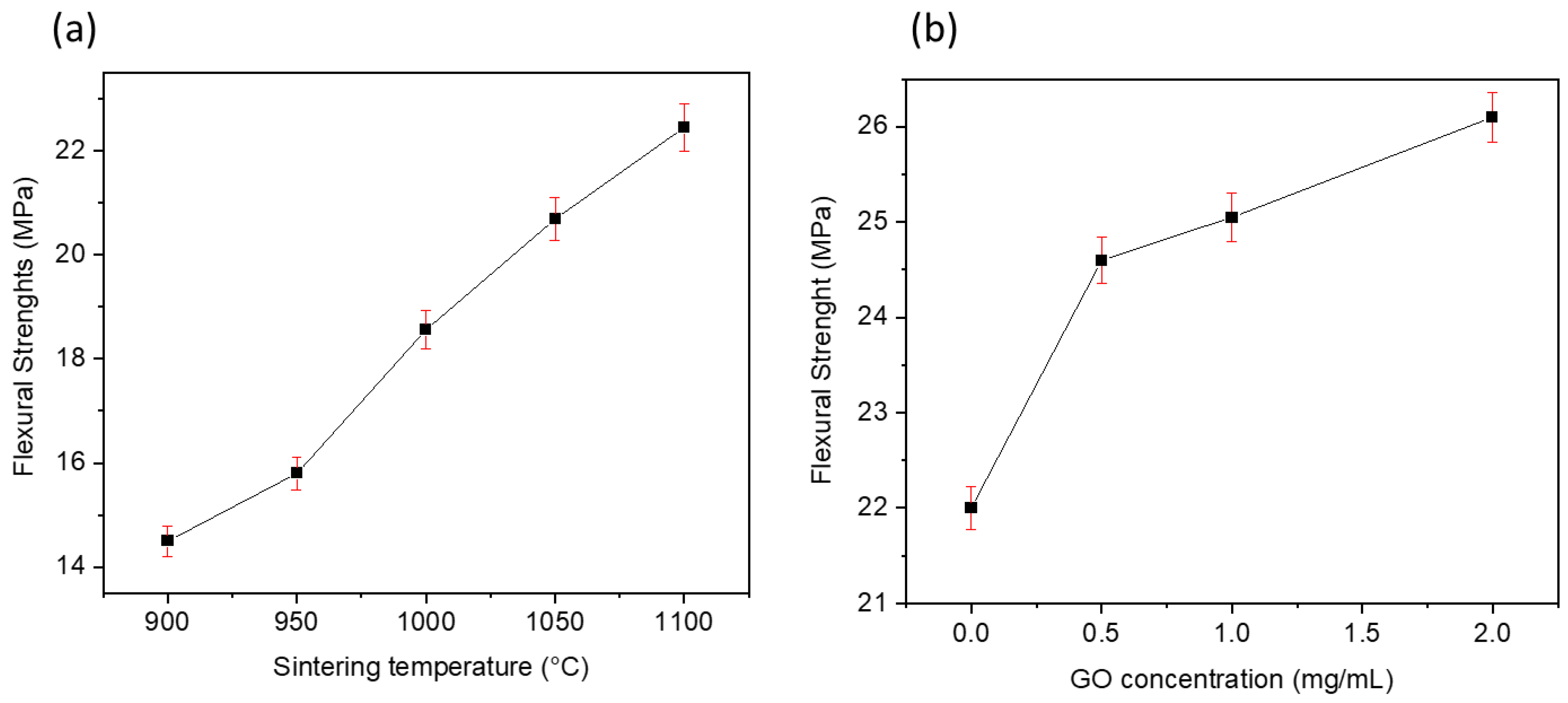
3.3. Flexural Strength
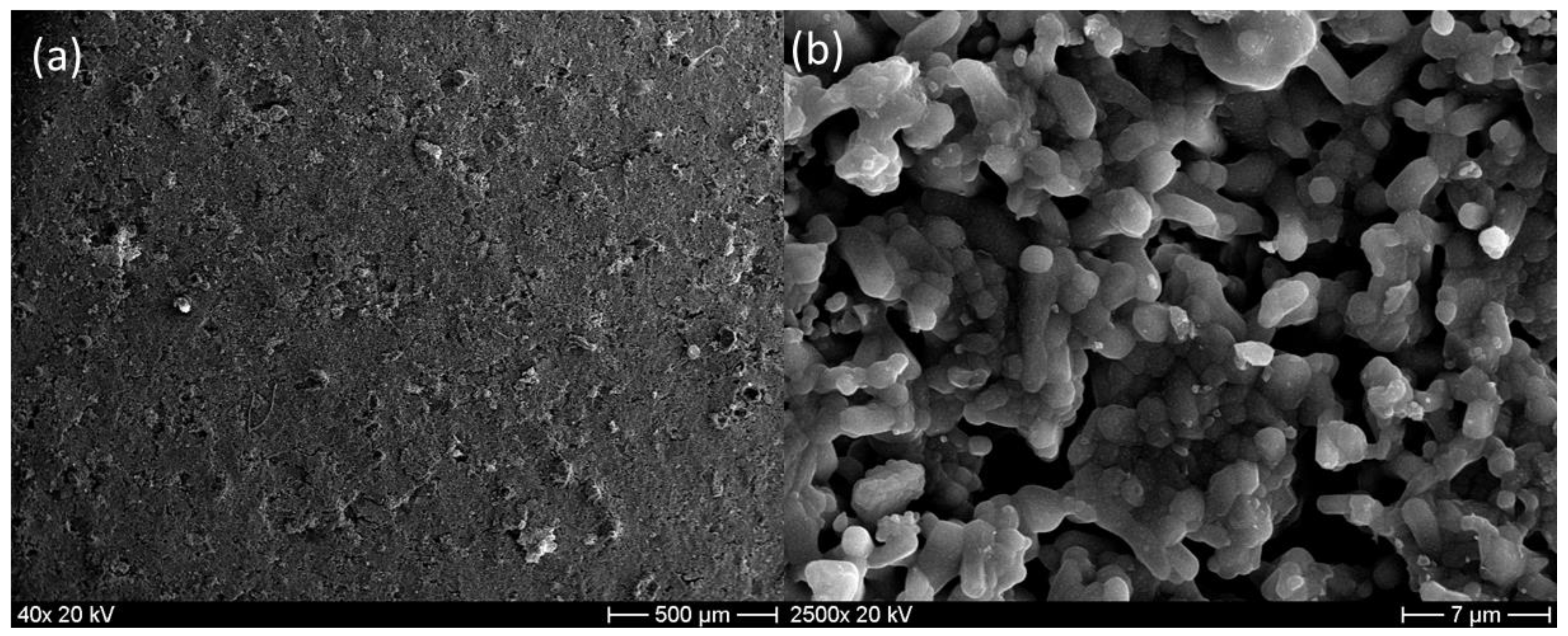
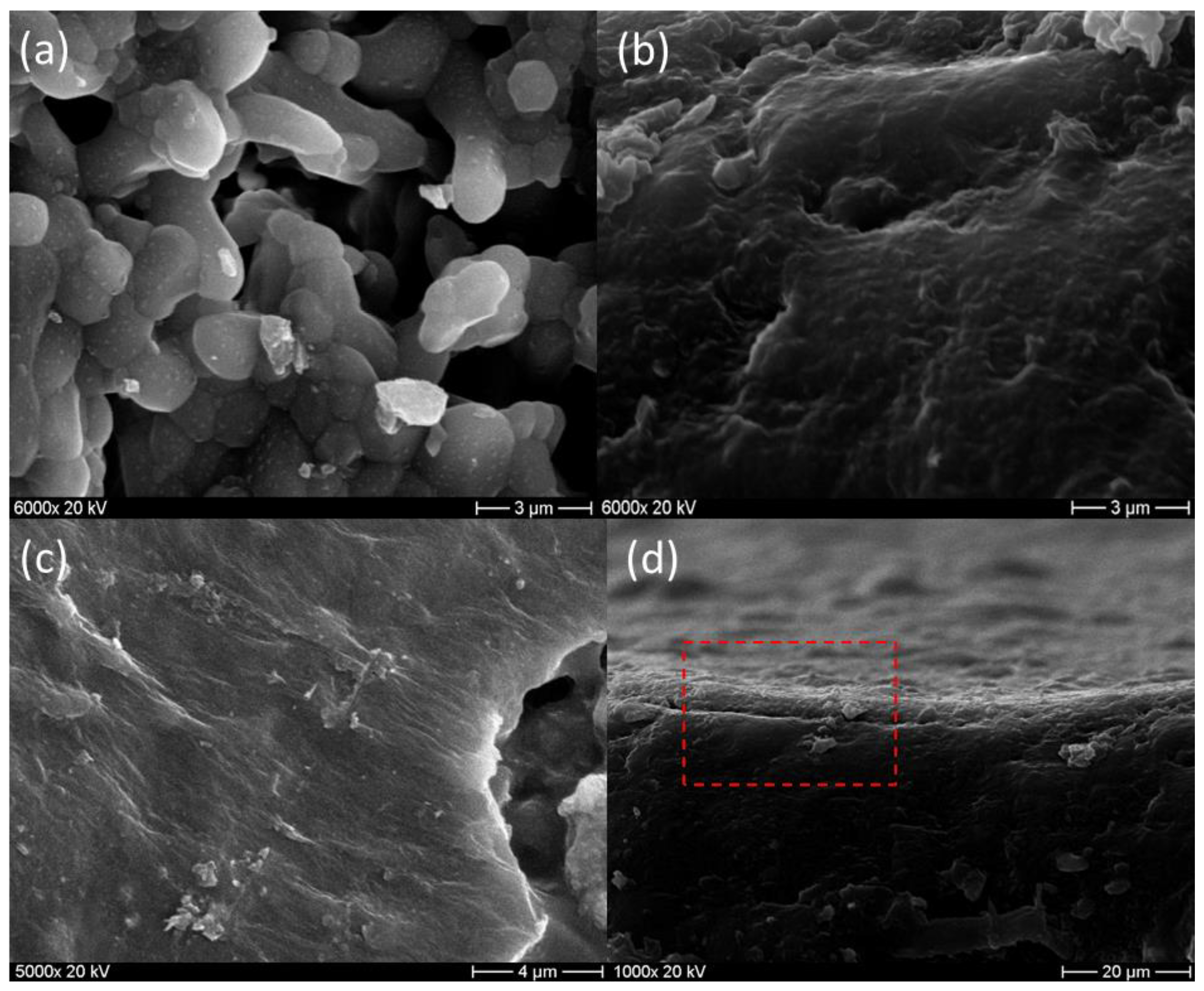
3.4. Morphology and Surface Analysis
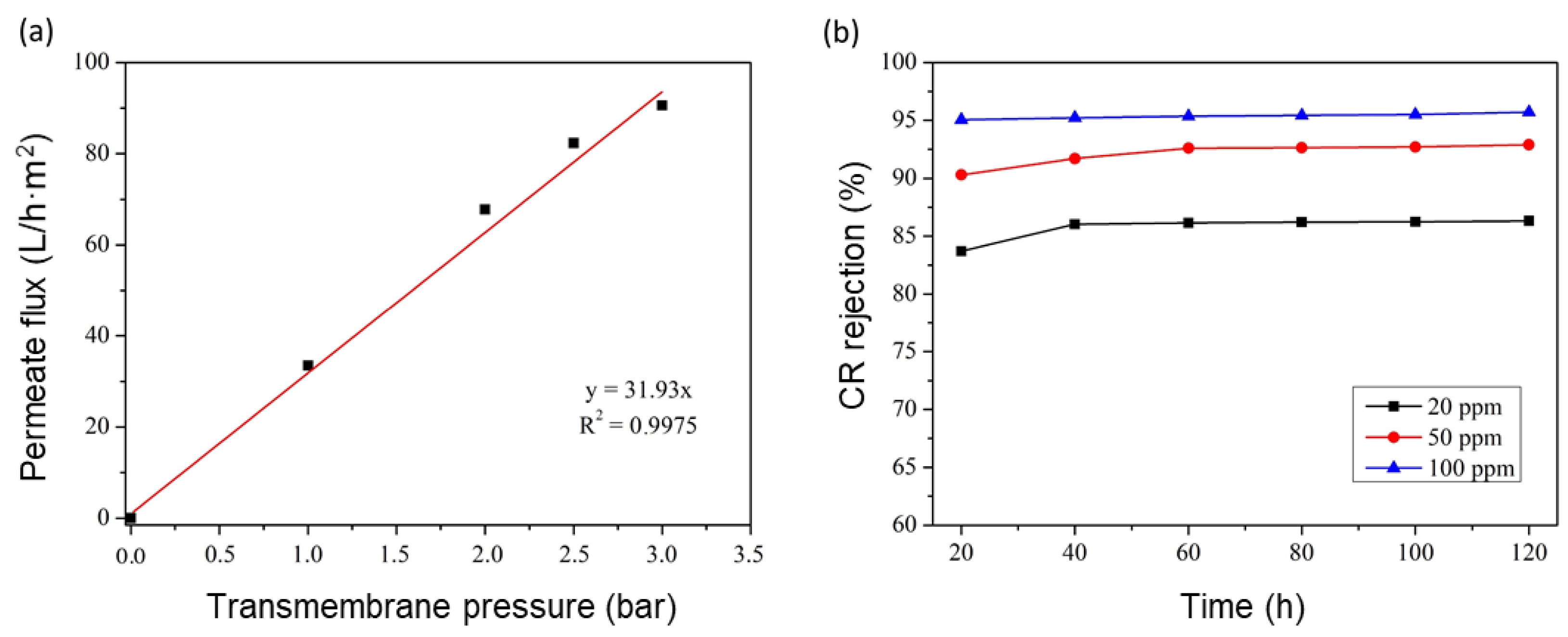
3.5. Phosphate Support Performance
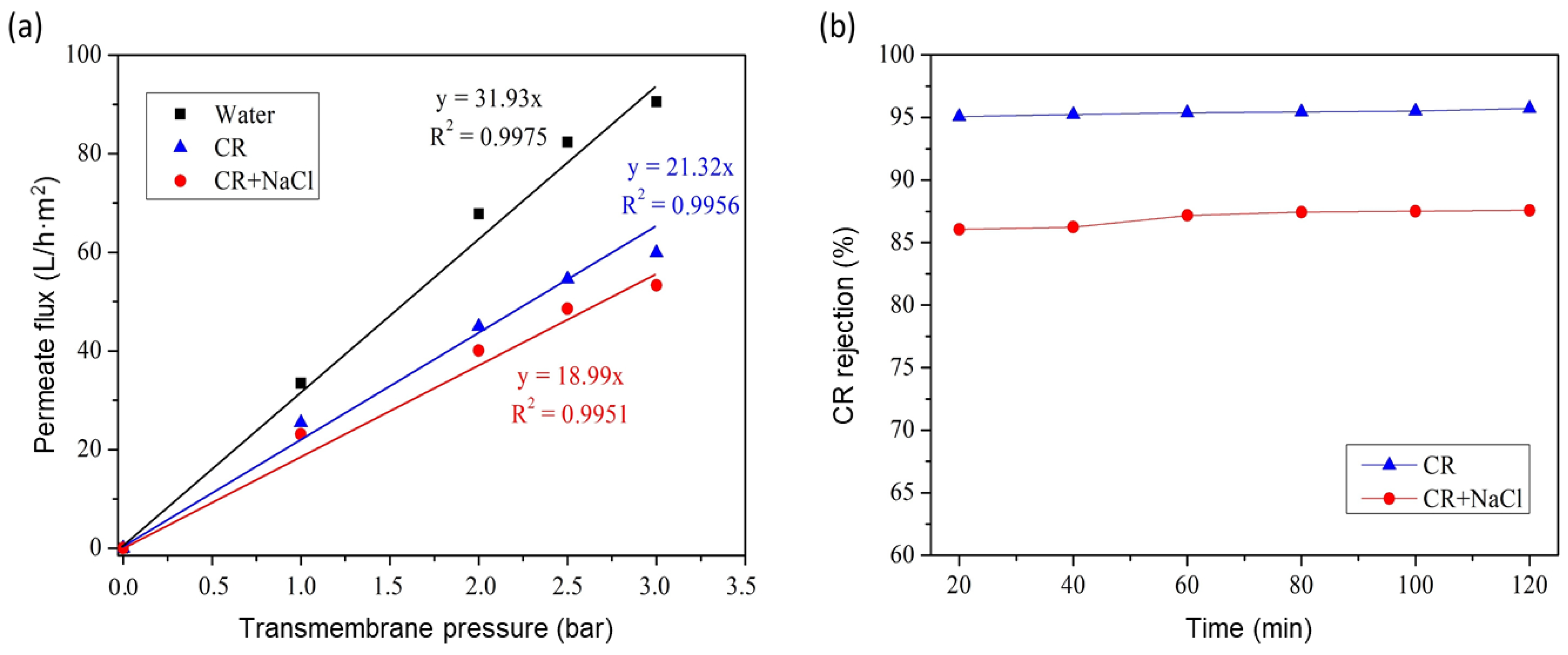
3.6. GO/Phosphate Composite Membrane Performance
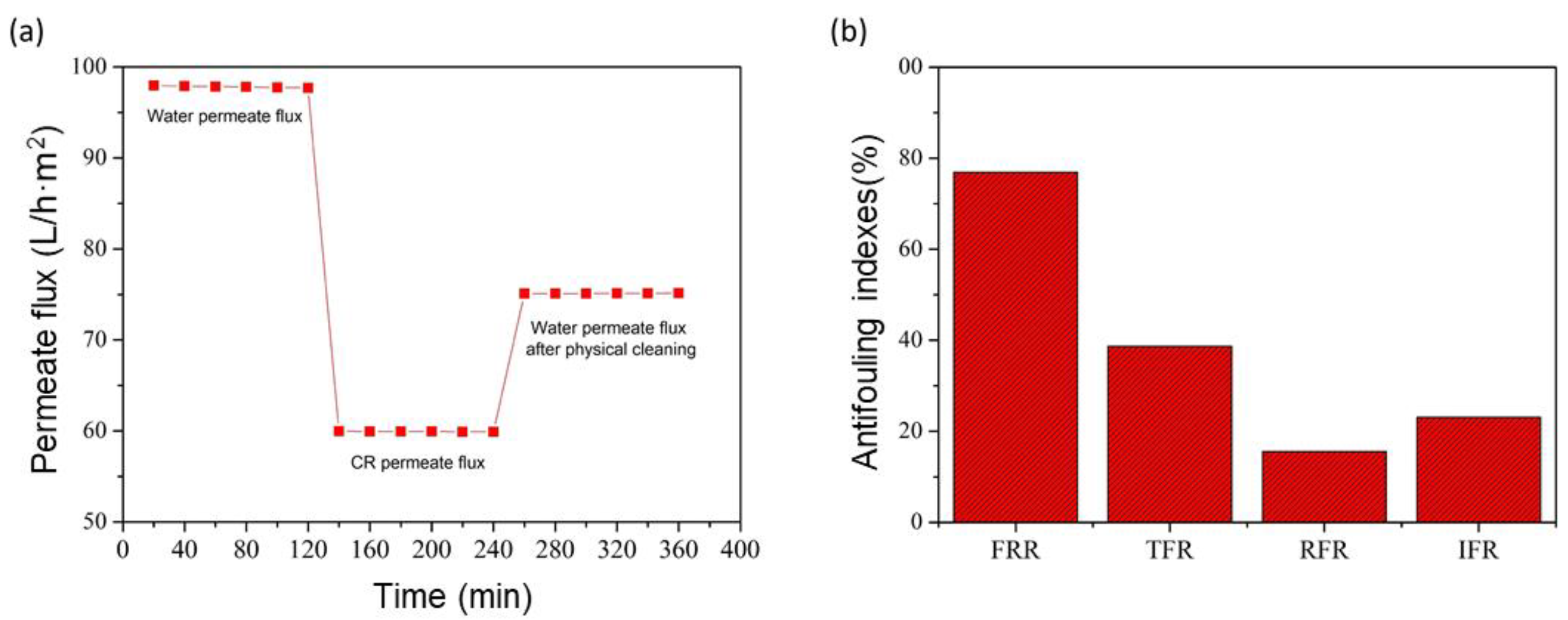
3.7. Antifouling Performance of the GO/Phosphate Membrane
3.8. Recyclability Studies of GO/Phosphate Membrane
3.9. Rejection of Simulated Dye Effluent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Yue, C.; Dong, H.; Chen, Y.; Shang, B.; Wang, Y.; Wang, S.; Zhu, Z. Direct Purification of Digestate Using Ultrafiltration Membranes: Influence of Pore Size on Filtration Behavior and Fouling Characteristics. Membranes 2021, 11, 179. [Google Scholar] [CrossRef]
- Li, J.; Ren, S.; Qiu, X.; Zhao, S.; Wang, R.; Wang, Y. Electroactive Ultrafiltration Membrane for Simultaneous Removal of Antibiotic, Antibiotic Resistant Bacteria, and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2022, 56, 15120–15129. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Ganesan, P.; Park, S.; Popov, B.N. Development of a Titanium Dioxide-Supported Platinum Catalyst with Ultrahigh Stability for Polymer Electrolyte Membrane Fuel Cell Applications. J. Am. Chem. Soc. 2009, 131, 13898–13899. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.; Park, H.; Choi, H.; Choi, H. Carbon nanotube blended polyethersulfone membranes for fouling control in water treatment. Water Res. 2011, 45, 274–282. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Guo, X.; Liu, W.; Cao, L.; Su, G.; Xu, H.; Liu, B. Graphene incorporated nanocrystalline TiO2 films for the photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 2013, 283, 498–504. [Google Scholar] [CrossRef]
- Dong, X.; Long, Q.; Wang, J.; Chan-Park, M.B.; Huang, Y.; Huang, W.; Chen, P. A graphene nanoribbon network and its biosensing application. Nanoscale 2011, 3, 5156–5160. [Google Scholar] [CrossRef] [PubMed]
- El-Maghrabi, H.H.; Nada, A.A.; Roualdes, S.; Bekheet, M.F. Design of Ni/NiO–TiO2/rGO nanocomposites on carbon cloth conductors via PECVD for electrocatalytic water splitting. Int. J. Hydrogen Energy 2020, 45, 32000–32011. [Google Scholar] [CrossRef]
- Lee, J.; Aluru, N.R. Water-solubility-driven separation of gases using graphene membrane. J. Membr. Sci. 2013, 428, 546–553. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef]
- Huang, H.-D.; Ren, P.-G.; Chen, J.; Zhang, W.-Q.; Ji, X.; Li, Z.-M. High barrier graphene oxide nanosheet/poly (vinyl alcohol) nanocomposite films. J. Membr. Sci. 2012, 409, 156–163. [Google Scholar] [CrossRef]
- Henning, L.M.; Simon, U.; Gurlo, A.; Smales, G.J.; Bekheet, M.F. Grafting and stabilization of ordered mesoporous silica COK-12 with graphene oxide for enhanced removal of methylene blue. RSC Adv. 2019, 9, 36271–36284. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Shao, G.; Wang, J.; Gurlo, A.; Bekheet, M.F. Revealing Nanodomain Structures of Bottom-Up-Fabricated Graphene-Embedded Silicon Oxycarbide Ceramics. Polymers 2022, 14, 3675. [Google Scholar] [CrossRef] [PubMed]
- Henning, L.M.; Abdullayev, A.; Vakifahmetoglu, C.; Simon, U.; Bensalah, H.; Gurlo, A.; Bekheet, M.F. Review on Polymeric, Inorganic, and Composite Materials for Air Filters: From Processing to Properties. Adv. Energy Sustain. Res. 2021, 2, 2100005. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.-P.; Maddy, J.; Hilal, N. Ceramic Microfiltration Membranes in Wastewater Treatment: Filtration Behavior, Fouling and Prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Abdullayev, A.; Klimm, D.; Kamutzki, F.; Gurlo, A.; Bekheet, M.F. AlF3-assisted flux growth of mullite whiskers and their application in fabrication of porous mullite-alumina monoliths. Open Ceram. 2021, 7, 100145. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, A.; Avcioglu, C.; Fey, T.; Hilger, A.; Osenberg, M.; Manke, I.; Henning, L.M.; Gurlo, A.; Bekheet, M.F. Fabrication and characterization of porous mullite ceramics derived from fluoride-assisted Metakaolin-Al(OH)3 annealing for filtration applications. Open Ceram. 2022, 9, 100240. [Google Scholar] [CrossRef]
- Henning, L.M.; Müller, J.T.; Smales, G.J.; Pauw, B.R.; Schmidt, J.; Bekheet, M.F.; Gurlo, A.; Simon, U. Hierarchically porous and mechanically stable monoliths from ordered mesoporous silica and their water filtration potential. Nanoscale Adv. 2022, 4, 3892–3908. [Google Scholar] [CrossRef]
- Abdullayev, A.; Kamm, P.H.; Bekheet, M.F.; Gurlo, A. Fabrication and Characterization of Ice Templated Membrane Supports from Portland Cement. Membranes 2020, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, A.; Breida, M.; Achiou, B.; Ouammou, M.; Calvo, J.I.; Aaddane, A.; Younssi, S.A. Removal of dyes by a new nano–TiO2 ultrafiltration membrane deposited on low-cost support prepared from natural Moroccan bentonite. Appl. Clay Sci. 2017, 149, 127–135. [Google Scholar] [CrossRef]
- Derouich, G.; Alami Younssi, S.; Bennazha, J.; Cody, J.A.; Ouammou, M.; El Rhazi, M. Development of low-cost polypyrrole/sintered pozzolan ultrafiltration membrane and its highly efficient performance for congo red dye removal. J. Environ. Chem. Eng. 2020, 8, 103809. [Google Scholar] [CrossRef]
- Belgada, A.; Achiou, B.; Alami Younssi, S.; Charik, F.Z.; Ouammou, M.; Cody, J.A.; Benhida, R.; Khaless, K. Low-cost ceramic microfiltration membrane made from natural phosphate for pretreatment of raw seawater for desalination. J. Eur. Ceram. Soc. 2021, 41, 1613–1621. [Google Scholar] [CrossRef]
- Karim, A.; Achiou, B.; Bouazizi, A.; Aaddane, A.; Ouammou, M.; Bouziane, M.; Bennazha, J.; Alami Younssi, S. Development of reduced graphene oxide membrane on flat Moroccan ceramic pozzolan support. Application for soluble dyes removal. J. Environ. Chem. Eng. 2018, 6, 1475–1485. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Zerafat, M.M.; Mohammadi, Y.; Asadnia, M. Fabrication of tubular ceramic membranes as low-cost adsorbent using natural clay for heavy metals removal. Clean. Eng. Technol. 2022, 10, 100550. [Google Scholar] [CrossRef]
- Barrouk, I.; Alami Younssi, S.; Kabbabi, A.; Persin, M.; Albizane, A.; Tahiri, S. New ceramic membranes from natural Moroccan phosphate for microfiltration application. Desalination Water Treat. 2015, 55, 53–60. [Google Scholar] [CrossRef]
- Wei, W.; Xia, S.; Liu, G.; Gu, X.; Jin, W.; Xu, N. Interfacial adhesion between polymer separation layer and ceramic support for composite membrane. AIChE J. 2010, 56, 1584–1592. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Wu, H.; Yin, X.; Chen, J.; Jiang, Z. Mussel-inspired fabrication of structurally stable chitosan/polyacrylonitrile composite membrane for pervaporation dehydration. J. Membr. Sci. 2010, 348, 150–159. [Google Scholar] [CrossRef]
- Bensalah, H.; Bekheet, M.F.; Younssi, S.A.; Ouammou, M.; Gurlo, A. Removal of cationic and anionic textile dyes with Moroccan natural phosphate. J. Environ. Chem. Eng. 2017, 5, 2189–2199. [Google Scholar] [CrossRef]
- Wang, X.; Mera, G.; Morita, K.; Ionescu, E. Synthesis of polymer-derived graphene/silicon nitride-based nanocomposites with tunable dielectric properties. J. Ceram. Soc. Jpn. 2016, 124, 981–988. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A. Elaboration and characterization of low-cost ceramic membrane made from natural Moroccan perlite for treatment of industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 451–458. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
- Abbes, N.; Bilal, E.; Hermann, L.; Steiner, G.; Haneklaus, N. Thermal Beneficiation of Sra Ouertane (Tunisia) Low-Grade Phosphate Rock. Minerals 2020, 10, 937. [Google Scholar] [CrossRef]
- Faniyi, I.O.; Fasakin, O.; Olofinjana, B.; Adekunle, A.S.; Oluwasusi, T.V.; Eleruja, M.A.; Ajayi, E.O.B. The comparative analyses of reduced graphene oxide (RGO) prepared via green, mild and chemical approaches. SN Appl. Sci. 2019, 1, 1181. [Google Scholar] [CrossRef]
- Elomari, H.; Achiou, B.; Karim, A.; Ouammou, M.; Albizane, A.; Bennazha, J.; Alami. Younssi, S.; Elamrani, I. Influence of starch content on the properties of low cost microfiltration membranes. J. Asian Ceram. Soc. 2017, 5, 313–319. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Hosny, M.; El-Maghrabi, N.; Fawzy, M. Facile synthesis of reduced graphene oxide by Tecoma stans extracts for efficient removal of Ni (II) from water: Batch experiments and response surface methodology. Sustain. Environ. Res. 2022, 32, 22. [Google Scholar] [CrossRef]
- Galata, E.; Veziri, C.M.; Theodorakopoulos, G.V.; Romanos, G.E.; Pavlatou, E.A. Composite GO/Ceramic Membranes Prepared via Chemical Attachment: Characterisation and Gas Permeance Properties. Membranes 2022, 12, 1181. [Google Scholar] [CrossRef]
- Kim, J.E.; Han, T.H.; Lee, S.H.; Kim, J.Y.; Ahn, C.W.; Yun, J.M.; Kim, S.O. Graphene Oxide Liquid Crystals. Angew. Chem. Int. Ed. 2011, 50, 3043–3047. [Google Scholar] [CrossRef]
- Beqqour, D.; Taanaoui, W.; Derouich, G.; Ouammou, M.; Alami Younssi, S.; Bennazha, J.; Cody, J.A. Preparation of a composite membrane made of PoPD/PVA ultrafiltration layer on ceramic pozzolan/micronized phosphate support for removal of Congo red dye. DWT 2022, 257, 23–33. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z. A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J. Membr. Sci. 2017, 524, 214–224. [Google Scholar] [CrossRef]
- Beqqour, D.; Derouich, G.; Taanaoui, W.; Essate, A.; Ouammou, M.; Allami Younssi, S.; Bennazha, J.; Cody, J.A.; El Rhazi, M. Development of composite ultrafiltration membrane made of PmPD/PVA layer deposited on ceramic pozzolan/micronized phosphate support and its application for Congo red dye removal. DWT 2021, 240, 152–164. [Google Scholar] [CrossRef]
- Lavanya, C.; Kusuma, J.; Geetha Balakrishna, R. Pyrochlores: Oxygen-rich moieties as ceramic fillers in uplifting the antifouling property and dye removal capacity of polymeric membranes. Sep. Purif. Technol. 2021, 272, 118946. [Google Scholar] [CrossRef]
- Chen, P.; Ma, X.; Zhong, Z.; Zhang, F.; Xing, W.; Fan, Y. Performance of ceramic nanofiltration membrane for desalination of dye solutions containing NaCl and Na2SO4. Desalination 2017, 404, 102–111. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Chen, M.; Huang, P.; Li, T.; Zhang, X.; Jiang, K. Efficient removal and fouling-resistant of anionic dyes by nanofiltration membrane with phosphorylated chitosan modified graphene oxide nanosheets incorporated selective layer. J. Water Process. Eng. 2020, 34, 101086. [Google Scholar] [CrossRef]
- Makhetha, T.A.; Moutloali, R.M. Antifouling properties of Cu(tpa)@GO/PES composite membranes and selective dye rejection. J. Membr. Sci. 2018, 554, 195–210. [Google Scholar] [CrossRef]
- Amin, M.S.A.; Stüber, F.; Giralt, J.; Fortuny, A.; Fabregat, A.; Font, J. Ceramic-supported graphene oxide membrane bioreactor for the anaerobic decolorization of azo dyes. J. Water Process. Eng. 2022, 45, 102499. [Google Scholar] [CrossRef]
- Homem, N.C.; de Camargo Lima Beluci, N.; Amorim, S.; Reis, R.; Vieira, A.M.S.; Vieira, M.F.; Bergamasco, R.; Amorim, M.T.P. Surface modification of a polyethersulfone microfiltration membrane with graphene oxide for reactive dyes removal. Appl. Surf. Sci. 2019, 486, 499–507. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef]
- Gholami, N.; Mahdavi, H. Nanofiltration composite membranes of polyethersulfone and graphene oxide and sulfonated graphene oxide. Adv. Polym. Technol. 2018, 37, 3529–3541. [Google Scholar] [CrossRef]
- Zeng, G.; He, Y.; Ye, Z.; Yang, X.; Chen, X.; Ma, J.; Li, F. Novel Halloysite Nanotubes Intercalated Graphene Oxide Based Composite Membranes for Multifunctional Applications: Oil/Water Separation and Dyes Removal. Ind. Eng. Chem. Res. 2017, 56, 10472–10481. [Google Scholar] [CrossRef]







| Composite Membrane | Modification Method | PWP (L/h·m2·bar) | Dyes | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| GO-PCS | Surface coating | NA | Direct black (100 mg/L) | 99.80 | [46] |
| Cu(tpa)@GO/PES | Phase inversion | 0.7867 | Methyl blue Methyl orange Congo red | 15 65 90 | [47] |
| rGO/Pozzolan | Spin coating | 2 | Bromothymol blue Methyl orange Murexide | 94 93 97 | [26] |
| GO/commercial ZrO2-TiO2 | Vacuum filtration | 5.1 | Acid Orange 7 Reactive Black 5 Direct Blue 71 | 99 96 92 | [48] |
| MPES | Pressure-assisted filtration | 99.40 | Blue Corazol | 97.80 | [49] |
| GO/PES | Non-solvent-induced phase separation | 13 | Sunset yellow Acridine orange | 62.3 35.4 | [50] |
| Sulfonated (s-GO)/PES | Phase inversion | 9.1 | Acid blue Bismark Brown | 83.9 83.5 | [51] |
| GO/cellulose acetate | Vacuum-assisted filtration | NA | Methylene blue | 99 | [52] |
| GO/natural phosphate | Silane grafting + dip coating | 31.93 | Congo red | 95.2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensalah, H.; Derouich, G.; Wang, X.; Alami Younssi, S.; Bekheet, M.F. Graphene-Oxide-Grafted Natural Phosphate Support as a Low-Cost Ceramic Membrane for the Removal of Anionic Dyes from Simulated Textile Effluent. Membranes 2023, 13, 345. https://doi.org/10.3390/membranes13030345
Bensalah H, Derouich G, Wang X, Alami Younssi S, Bekheet MF. Graphene-Oxide-Grafted Natural Phosphate Support as a Low-Cost Ceramic Membrane for the Removal of Anionic Dyes from Simulated Textile Effluent. Membranes. 2023; 13(3):345. https://doi.org/10.3390/membranes13030345
Chicago/Turabian StyleBensalah, Hiba, Ghizlane Derouich, Xifan Wang, Saad Alami Younssi, and Maged F. Bekheet. 2023. "Graphene-Oxide-Grafted Natural Phosphate Support as a Low-Cost Ceramic Membrane for the Removal of Anionic Dyes from Simulated Textile Effluent" Membranes 13, no. 3: 345. https://doi.org/10.3390/membranes13030345
APA StyleBensalah, H., Derouich, G., Wang, X., Alami Younssi, S., & Bekheet, M. F. (2023). Graphene-Oxide-Grafted Natural Phosphate Support as a Low-Cost Ceramic Membrane for the Removal of Anionic Dyes from Simulated Textile Effluent. Membranes, 13(3), 345. https://doi.org/10.3390/membranes13030345







